Abstract
BACKGROUND
Cardiovascular disease causes severe morbidity and mortality in type 1 diabetes, although the specific risk factors and whether chronic hyperglycemia has a role are unknown. We examined the progression of carotid intima–media thickness, a measure of atherosclerosis, in a population with type 1 diabetes.
METHODS
As part of the Epidemiology of Diabetes Interventions and Complications (EDIC) study, the long-term follow-up of the Diabetes Control and Complications Trial (DCCT), 1229 patients with type 1 diabetes underwent B-mode ultrasonography of the internal and common carotid arteries in 1994–1996 and again in 1998–2000. We assessed the intima–media thickness in 611 subjects who had been randomly assigned to receive conventional diabetes treatment during the DCCT and in 618 who had been assigned to receive intensive diabetes treatment.
RESULTS
At year 1 of the EDIC study, the carotid intima–media thickness was similar to that in an age- and sex-matched nondiabetic population. After six years, the intima–media thickness was significantly greater in the diabetic patients than in the controls. The mean progression of the intima–media thickness was significantly less in the group that had received intensive therapy during the DCCT than in the group that had received conventional therapy (progression of the intima–media thickness of the common carotid artery, 0.032 vs. 0.046 mm; P=0.01; and progression of the combined intima–media thickness of the common and internal carotid arteries, −0.155 vs. 0.007; P=0.02) after adjustment for other risk factors. Progression of carotid intima–media thickness was associated with age, and the EDIC base-line systolic blood pressure, smoking, the ratio of low-density lipoprotein to high-density lipoprotein cholesterol, and urinary albumin excretion rate and with the mean glycosylated hemoglobin value during the mean duration (6.5 years) of the DCCT.
CONCLUSIONS
Intensive therapy during the DCCT resulted in decreased progression of intima–media thickness six years after the end of the trial.
Diabetes Mellitus is Accompanied by a substantial increase in the risk of cardiovascular disease.1–5 Most epidemiologic and clinical-trial data have derived from the study of type 2 diabetes, in which cardiovascular disease accounts for 70 percent of all deaths.1–3 Much less is known about cardiovascular disease in type 1 diabetes. Although the absolute risk of cardiovascular disease is lower in patients with type 1 diabetes than in those with type 2 diabetes, owing in part to their younger age, the relative risk, as compared with that of nondiabetic persons of similar age, may be increased by a factor of 10.4,5 Much of the risk of cardiovascular disease in patients with type 1 diabetes has been attributed to the development of renal disease.6 In addition to renal disease, autonomic neuropathy, dyslipidemia, and microvascular cardiac disease have been suggested as cardiovascular risk factors.7 Interestingly, glycemia has not been documented to be a risk factor for heart disease in patients with type 1 diabetes.
During the Diabetes Control and Complications Trial (DCCT), patients with type 1 diabetes were randomly assigned either to receive intensive diabetes therapy, subsequently maintaining a mean glycosylated hemoglobin value of 7.2 percent during the mean follow-up of 6.5 years, or to receive standard therapy, subsequently maintaining a mean glycosylated hemoglobin value of 9 percent.8 Although intensive therapy reduced the risk of development and progression of microvascular and neuropathic complications by 35 to 76 percent, the incidence of cardiovascular disease events was not significantly different between the two treatment groups.9 After completion of the DCCT, long-term follow-up of the DCCT cohort, called the Epidemiology of Diabetes Interventions and Complications (EDIC) study,10 included B-mode ultrasonography to measure the thickness of the intima–media wall of the carotid artery on two occasions. Carotid intima–media thickness is a well-established index of atherosclerosis that correlates with prevalent and incident coronary heart disease11–14 and stroke.12,14,15 We analyzed the changes in the intima–media thickness over time and associated risk factors, according to the original intention-to-treat assignment during the DCCT.
METHODS
PATIENTS
The 1441 patients enrolled in the DCCT between 1983 and 1989 were 13 to 39 years old, had had type 1 diabetes for 1 to 15 years, and were in generally good health at base line.8 After a mean of 6.5 years of follow-up, 1375 of the 1425 surviving members volunteered to participate in the EDIC study, an observational follow-up of the DCCT cohort.10 During the EDIC study, all therapy was provided by the patients’ own physicians and intensive therapy was recommended for all patients. A detailed description of the study procedures and base-line characteristics has been published.10 Carotid ultrasonography was performed between June 1994 and April 1996 (1 to 2 years after the initiation of the EDIC study and approximately 8 years after the beginning of the DCCT; range, 4 to 11). It was repeated between October 1998 and November 2000 in 1229 participants, who are the subjects of this study (Table 1).
Table 1.
Clinical Characteristics of the Epidemiology of Diabetes Interventions and Complications (EDIC) Participants, According to Sex and Treatment Assignment in the Diabetes Control and Complications Trial (DCCT).*
| Characteristic | Female Patients | Male Patients | ||
|---|---|---|---|---|
| Intensive Treatment (N=295) |
Conventional Treatment (N=289) |
Intensive Treatment (N=323) |
Conventional Treatment (N=322) |
|
| Demographic, year 1 | ||||
| Age (yr) | 35±7 | 34±7 | 36±7 | 36±7 |
| Current smoker (%) | 20 | 19 | 20 | 17 |
| Duration of diabetes (yr) | 13.9±4.8 | 14.2±5.2 | 13.9±4.8 | 13.3±4.6 |
| Medical, year 1 | ||||
| Body-mass index | 26.5±4.5† | 25.0±3.5 | 26.7±3.9‡ | 26.0±3.2 |
| Natural waist:hip circumference | 0.76±0.07 | 0.76±0.07 | 0.88±0.08 | 0.87±0.09 |
| Ankle:arm blood pressure <0.9 (%) | 8.8 | 8.7 | 4.6 | 6.9 |
| Systolic blood pressure (mm Hg) | 114±12 | 114±13 | 119±11 | 120±12 |
| Diastolic blood pressure (mm Hg) | 74±9 | 72±9 | 77±9 | 77±8 |
| Hypertension (%)§ | 10.4 | 14.0 | 22.9 | 17.9 |
| Lipids, year 1 or 2 | ||||
| Total cholesterol (mg/dl)¶ | 188±36 | 188±38 | 187±35 | 182±36 |
| HDL cholesterol (mg/dl)¶ | 59±14 | 59±14 | 49±13 | 50±11 |
| LDL cholesterol (mg/dl)¶ | 112±29 | 112±30 | 119±30‡ | 114±32 |
| LDL:HDL ratio | 2.0 | 2.0 | 2.6 | 2.4 |
| Triglycerides (mg/dl)‖ | 83±76 | 83±76 | 96±72 | 96±79 |
| Hyperlipidemia (%)** | 27.1 | 26.8 | 35.3 | 30.0 |
| Albumin excretion rate, year 1 or 2 | ||||
| Value (mg/24 hr) | 22±67†† | 67±330 | 30±118 | 43±117 |
| >40 mg/24 hr (%) | 6.8† | 15.7 | 7.7† | 16.5 |
| Glycosylated hemoglobin (%) | ||||
| During DCCT | 7.3±0.9† | 9.1±1.3 | 7.2±0.9† | 9.0±1.1 |
| Year 1 EDIC | 7.9±1.4‡ | 8.1±1.4 | 7.8±1.2† | 8.3±1.2 |
| Intima–media thickness, year 1 or 2 (mm) | ||||
| Common carotid artery | 0.566±0.077 | 0.557±0.076 | 0.597±0.082 | 0.604±0.097 |
| Internal carotid artery | 0.608±0.165 | 0.628±0.251 | 0.668±0.220 | 0.681±0.268 |
Plus–minus values are means ±SD. All data are from EDIC year 1 or year 2 unless otherwise noted. Comparisons between intensive-treatment and conventional-treatment groups are based on the chi-square test or Wilcoxon rank-sum test. The body-mass index is the weight in kilograms divided by the square of the height in meters.
P<0.001 for the comparison of intensive treatment with conventional treatment.
P<0.05 for the comparison of intensive treatment with conventional treatment.
Hypertension was defined by a systolic blood pressure of at least 140 mm Hg, a diastolic blood pressure of at least 90 mm Hg, the presence of documented hypertension, or the use of antihypertensive agents.
To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. HDL denotes high-density lipoprotein, and LDL low-density lipoprotein.
To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
Hyperlipidemia was defined by an LDL cholesterol level of at least 130 mg per deciliter (3.36 mmol per liter) or by the use of lipid-lowering agents.
P<0.01 for the comparison of intensive treatment with conventional treatment.
CONTROL SUBJECTS
Healthy age- and sex-matched subjects without diabetes were recruited from each of the 28 EDIC centers to serve as contemporaneous controls to determine carotid intima–media thickening. One group of eight controls from each center was selected in 1994–1996,16 and a second group of eight was selected in 1998–2000. In 1998–2000, 222 healthy volunteers with a mean (±SD) age of 39±11 years were studied. Fifty percent were female. Mean systolic and diastolic blood pressures were 117±11 and 75±9 mm Hg, respectively, similar to those of the DCCT cohort. The prevalence of smoking, however, was much lower: 5.9 percent, as compared with 16.8 percent in the DCCT cohort (P<0.001). The mean glycosylated hemoglobin value was 5.0±0.35 percent.
ASSESSMENT OF CAROTID INTIMA–MEDIA THICKNESS
The measurement of intima–media thickness has been described in detail.16 A single longitudinal lateral view of the distal 10 mm of the right and left common carotid arteries and three longitudinal views in different imaging planes of each internal carotid artery were obtained. The internal carotid artery was defined as including both the carotid bulb and the 10-mm segment distal to the tip of the flow divider that separates the internal from the external carotid artery. Studies were performed by certified technicians at the clinical centers, recorded on videotapes, and read in a central unit (Tufts University, Boston) by a single reader, who was unaware of the subjects’ diagnostic groups, treatment assignments and the time of the studies (year 1 as compared with year 6).
QUALITY-CONTROL PROCEDURES
Reproducibility analysis of 50 replicate measures of year 1 and year 6 carotid studies resulted in absolute mean differences of 0.03 and 0.02 mm for the year 1 common carotid artery and internal carotid artery, respectively, and 0.03 and 0.04 mm for the year 6 common carotid artery and internal carotid artery, respectively. The respective intraclass correlations between the original and maximal wall thickness and the measurement obtained on rereading were 0.87 and 0.99 for year 1 common and internal carotid arteries and 0.99 and 0.99 for year 6 common and internal carotid arteries.
OTHER PROCEDURES
Each subject in the EDIC study underwent an annual history-taking, physical examination, electrocardiography, and laboratory testing, including measurements of serum creatinine and glycosylated hemoglobin, determined as they were in the DCCT.8,17 Lipid profiles and four-hour urine collections for the measurement of the albumin excretion rate and creatinine clearance were obtained in alternate years during the EDIC study.10
MEASUREMENTS
Base-line covariates were obtained from the year 1 history and physical examination and from the laboratory data (lipid levels measured after an overnight fast and renal-function values) collected in either year 1 or year 2. The maximal intima–media thickness of the common carotid artery was defined as the mean of the maximal value for the near and far walls on both the right and left sides. The internal intima–media thickness was defined as the mean of the maximal value for anterior, lateral, and posterior views on both sides. The combined intima–media thickness was defined as the sum of the standardized intima–media measurements of the common and internal carotid arteries. The standardized intima–media thickness was defined as (variable–mean)÷SD.18 The change in the thickness was defined as the difference between results for year 6 and those for year 1.
STATISTICAL ANALYSIS
Group differences were compared with use of the Wilcoxon rank-sum test for quantitative variables and the chi-square test for categorical variables. A paired t-test was used to test the significance of the change over time. To compare the two treatment groups, we used analysis of covariance of the change in the intima–media thickness (year 6 minus year 1) with the year 1 thickness as an adjusting covariate. To obtain the least-squares means of the change in intima–media thickness, we fitted the model using the change in the thickness as the outcome and adjusting for the year 1 value, age, sex, and the ultrasonography equipment used (12 combinations of ultrasonography equipment were used at 28 clinical centers). A reciprocal transformation of the internal intima–media thickness was used to yield approximately normal residuals.19 The association of each covariate (listed in Table 1) with each intima–media measure was assessed, with adjustment for the year 1 value, sex, attained age, and the ultrasonography equipment used. To study the risk factors, a multiple linear regression model was fitted with the use of the year 6 intima–media thickness as the outcome and the year 1 thickness as a covariate. The most significant factor for the multivariate association among similar variables (e.g., systolic and diastolic blood pressure) was selected. Only covariates known to be unaffected by the DCCT treatment group were included. All two-way interaction terms were assessed, and those nominally significant at a P level of less than 0.10 were retained.20 The final model included the ultrasonography equipment used, attained age, sex, the year 1 intima–media thickness, smoking status, systolic blood pressure, treatment group, and the interaction between attained age and treatment group. The overall effect of the DCCT treatment group was assessed with the use of a general linear test with 2 degrees of freedom for both the main effect and the interaction.21,22
RESULTS
The clinical characteristics of the EDIC study cohort at year 1, according to sex and treatment assignment during the DCCT, are shown in Table 1. Mean blood pressure and lipid levels were not significantly different between the group that had received intensive therapy during the DCCT and the group that had received conventional treatment. On the other hand, mean glycosylated hemoglobin levels in the two groups remained significantly, albeit minimally, different during the first four years of the EDIC study; by year 5 they were no longer different (7.9 percent in the original intensive-treatment group and 8.0 percent in the conventional-treatment group, P=0.075).23 Albumin excretion rates remained significantly lower in the intensive-results treatment group than in the conventional-treatment group during the six years of EDIC follow-up, reflecting the previously described long-lasting beneficial effects of intensive therapy on diabetic nephropathy.8,24 Body-mass index during the EDIC study remained significantly higher in the group that had received intensive treatment during DCCT, as it had been at the end of that study.
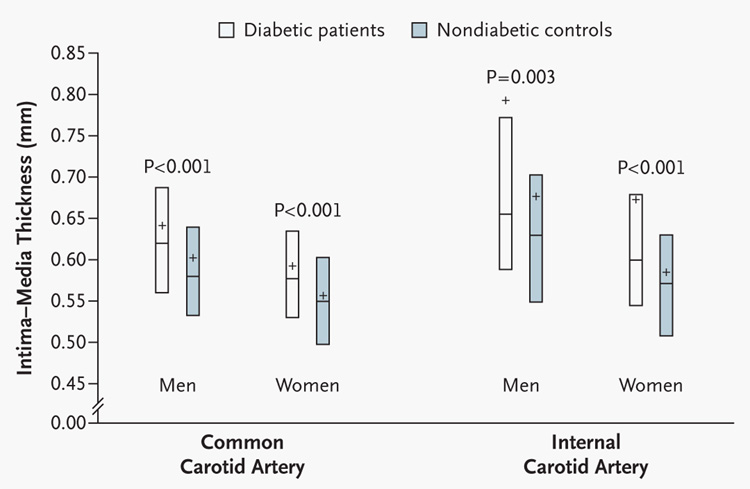
The intima–media measurements in the age- and sex-matched 1998–2000 nondiabetic population were similar to published data in healthy nondiabetic subjects, with a mean intima–media thickness of 0.58±0.10 mm for the common carotid artery and a mean of 0.63±0.18 mm for the internal carotid artery.15,24 Although no significant differences in thickness were demonstrable between the diabetic cohort and nondiabetic controls at year 1,16 by year 6, the intima–media thickness for the common and internal carotid arteries was significantly greater in the diabetic cohort than in the nondiabetic cohort for each sex, even after adjustment for smoking status (Fig. 1).
Figure 1. Intima–Media Thickness of the Common and Internal Carotid Arteries at Year 6 in Diabetic Patients and Age-Matched Nondiabetic Control Subjects.
Box plots represent the second and third quartiles of the distribution, the center line the median, and the plus sign the mean. P values were calculated with use of the Wilcoxon rank-sum test.
There was less progression of the intima–media thickness of the common carotid artery from year 1 to year 6 among the patients who had received intensive treatment during the DCCT than among those who had received conventional treatment. After adjustment for sex, age, the ultrasonography equipment used, and the year 1 intima–media thickness (Table 2), the mean progression was 0.032 mm in the intensive-treatment group and 0.046 mm in the conventional-treatment group, with a difference of 0.013 mm (95 percent confidence interval for the difference, 0.003 to 0.024). The progression of the intima–media thickness of the combined common and internal carotid arteries was also less in the intensive-treatment group, where regression occurred, than in the conventional-treatment group (−0.155 vs. 0.007; a difference of 0.162; 95 percent confidence interval for the difference, 0.031 to 0.293) (Table 2). The differences between treatment groups in the reciprocal values for the intima–media thickness of the internal carotid artery were not significant (P=0.07). There was no significant treatment effect according to sex. Finally, the potential effect of any differences in the use of hypolipidemic or antihypertensive agents between the two treatment groups was analyzed by including terms for medication use in the analyses in Table 2. The results were unchanged.
Table 2.
Least-Squares Mean Change in the Intima–Media Thickness of the Common Carotid Artery and of the Combined Common and Internal Carotid Arteries from Year 1 to Year 6 of the Epidemiology of Diabetes Interventions and Complications Study, According to the Treatment Assignment in the Diabetes Control and Complications Trial.*
| Variable | Change in Intima–Media Thickness of Common Carotid Artery | Change in Combined Intima–Media Thickness | ||
|---|---|---|---|---|
| Least-Squares Mean (95% CI) | P Value | Least-Squares Mean (95% CI) | P Value | |
| mm | mm | |||
| Conventional treatment | 0.046 (0.023 to 0.068) | 0.007 (−0.277 to 0.292) | ||
| Intensive treatment | 0.032 (0.010 to 0.055) | −0.155 (−0.440 to 0.131) | ||
| Difference between treatment groups | 0.013 (0.003 to 0.024) | 0.01 | 0.162 (0.031 to 0.293) | 0.02 |
The change in the intima–media thickness was used as the outcome to fit a general linear model, adjusted for sex, age, ultrasonography equipment used, and the year 1 thickness. CI denotes confidence interval.
The intima–media thickness at year 6 of the EDIC study was associated with smoking status, systolic (but not diastolic) blood pressure, the presence or absence of hypertension, total and high-density lipoprotein cholesterol levels (data not shown), the ratio of low-density lipoprotein to high-density lipoprotein cholesterol, urinary albumin excretion rate, and the mean glycosylated hemoglobin level during the DCCT (Table 3). All of the associations were similar in magnitude and direction for the intima–media thickness of the common carotid artery and the internal carotid artery and the combined intima–media thickness and when the change in thickness was substituted for the year 6 thickness. The association of the mean glycosylated hemoglobin value during the DCCT with the year 6 intima–media thickness of the common carotid artery remained significant (P<0.001) after adjustment for age, sex, year 1 intima–media thickness of the common carotid artery, and the ultrasonography equipment used.
Table 3.
Univariate Associations of Carotid Intima–Media Thickness, with Risk Factors Adjusted for Age, Sex, Ultrasonography Equipment Used, and Year 1 Intima–Media Thickness Measurement.*
| Risk Factor | Common Carotid Intima–Media Thickness | Internal Carotid Intima–Media Thickness (Reciprocal) | Combined Intima–Media Thickness | |||
|---|---|---|---|---|---|---|
| Estimate of β Coefficient ±SE | P Value | Estimate of β Coefficient ±SE | P Value | Estimate of β Coefficient ±SE | P Value | |
| mm | ||||||
| Treatment group (conventional vs. intensive) | 0.0135±0.0053 | 0.02 | −0.0330±0.0183 | 0.07 | 0.1619±0.0669 | 0.02 |
| Body-mass index (per unit) | 0.0016±0.0007 | 0.03 | −0.0032±0.0025 | 0.20 | 0.0136±0.0089 | 0.13 |
| Smoking (yes vs. no) | 0.0247±0.0068 | <0.001 | −0.0822±0.0231 | <0.001 | 0.3468±0.0850 | <0.001 |
| Systolic blood pressure (per mm Hg) | 0.0008±0.0002 | <0.001 | −0.0023±0.0008 | 0.004 | 0.0088±0.0029 | 0.002 |
| Hypertension (yes vs. no)† | 0.0189±0.0074 | 0.01 | −0.1105±0.0258 | <0.001 | 0.3016±0.0939 | 0.001 |
| Low-density lipoprotein:high-density lipoprotein ratio | 0.0072±0.0032 | 0.03 | −0.0505±0.0113 | <0.001 | 0.1427±0.0405 | <0.001 |
| Log albumin excretion rate | 0.0068±0.0025 | 0.006 | −0.0212±0.0084 | 0.01 | 0.0912±0.0308 | 0.003 |
| Glycosylated hemoglobin value during DCCT (per 1 percentage point) | 0.0065±0.0020 | 0.001 | −0.0193±0.0067 | 0.004 | 0.1014±0.0244 | <0.001 |
The multiple regression model used the year 6 intima–media thickness as the outcome and fit the covariates one at a time after adjustment for age, sex, ultrasonography equipment used, and year 1 intima–media thickness. The R2, based on a general linear model (PROC GLM) in which the year 6 intima–media thickness was the outcome, adjusted for age, sex, ultrasonography equipment used, and year 1 intima–media measurement, is 37.76 percent, 39.58 percent, and 50.60 percent for the common carotid, internal carotid, and combined intima–media thickness, respectively. DCCT denotes Diabetes Control and Complications Trial.
Hypertension was defined by a systolic blood pressure of at least 140 mm Hg, a diastolic blood pressure of at least 90 mm Hg, documented hypertension, or the use of antihypertensive agents.
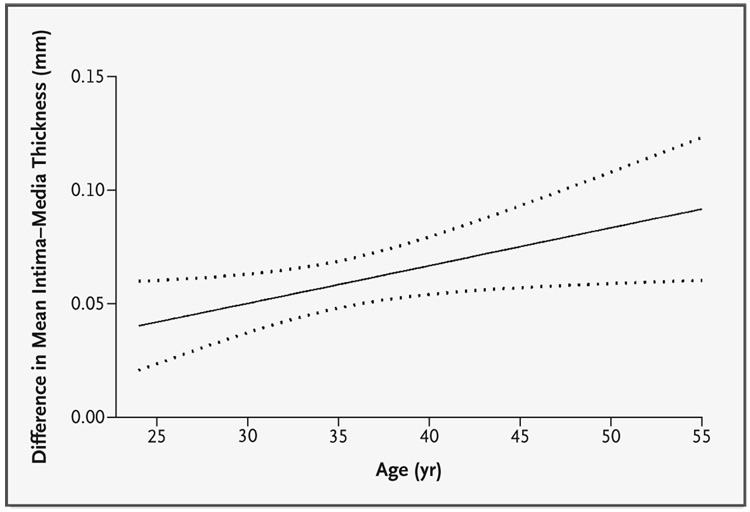
Since many of the univariate risk factors differed between groups at year 1 of the EDIC study, reflecting the effects of treatment during the DCCT, multivariate regression modeling adjusted only for the covariates not affected by treatment at year 1 of the EDIC study. These models revealed that, for each measure, the benefits of intensive treatment increased with age (Table 4). Furthermore, the overall treatment effect with 2 degrees of freedom was significant for all these measures (P=0.004 for the intima–media thickness of the common carotid artery and P=0.005 for the combined thickness), including the reciprocal internal intima–media thickness (P=0.049) (data not shown). The complete model explained approximately 40 percent of the variation in the intima–media thickness of the common carotid artery and 52 percent of the variation in the combined thickness.Figure 2 shows the difference between the conventional-treatment and intensive-treatment groups in the change in the intima–media thickness of the common carotid artery as a function of attained age. The intensive therapy during 6.5 years of the DCCT resulted in a significantly slower rate of progression of intima–media thickness during the 6 years of the EDIC study (P=0.004).
Table 4.
Multivariate Association of Carotid Intima–Media Thickness with Various Risk Factors.*
| Risk Factor | Common Carotid Intima–Media Thickness | Combined Intima–Media Thickness | ||||
|---|---|---|---|---|---|---|
| Semipartial R2 | P Value | Semipartial R2 | P Value | |||
| Estimate of β Coefficient ±SE | % | Estimate of β Coefficient ±SE | % | |||
| Age (per year of age) | 0.0029±0.0006 | 1.32 | <0.001 | 0.0248±0.0071 | 0.49 | <0.001 |
| Sex (female vs. male) | −0.0173±0.0055 | 0.50 | 0.002 | −0.2506±0.0689 | 0.53 | <0.001 |
| Year 1 intima–media thickness | 0.6188±0.0361 | 14.79 | <0.001 | 0.5961±0.0238 | 24.90 | <0.001 |
| Smoking (yes vs. no) | 0.0265±0.0068 | 0.77 | <0.001 | 0.3737±0.0851 | 0.77 | <0.001 |
| Systolic blood pressure (per mm Hg) | 0.0008±0.0002 | 0.61 | <0.001 | 0.0092±0.0029 | 0.42 | 0.001 |
| Ultrasonography equipment used (12 combinations)† | — | 1.64 | <0.001 | — | 1.92 | <0.001 |
| Treatment group (conventional vs. intensive) | −0.0453±0.0274 | 0.14 | 0.10 | −0.5433±0.3435 | 0.10 | 0.12 |
| Interaction between age and treatment group | 0.0017±0.0008 | 0.24 | 0.03 | 0.0201±0.0096 | 0.18 | 0.04 |
| Overall treatment effect (treatment effect as a function of age)‡ | § | 0.57 0.40 |
0.004 | ‖ | 0.42 0.52 |
0.005 |
| Total R2 (%) | 40 | 52 | ||||
The multiple regression model used the year 6 intima–media thickness as the outcome and fit the listed covariates simultaneously.
Eleven estimates are omitted.
The overall treatment effect was determined by two regression coefficients: treatment group and the interaction between age and treatment group. The use of either coefficient alone did not reflect the overall treatment effect. The overall treatment effect was based on the general linear test with 2 degrees of freedom for the numerator.
For the common carotid artery, β = (−0.0453) + (0.0017) × age, and the standard error = (7.5×10−4) − (4.1×10−5) × age + (6.4×10−7) × age2.
For the combined value, β = (−0.5433) + (0.0201) × age, and the standard error = (0.1180) − (0.0065) × age + (9.2×10−5) × age2.
Figure 2. Mean Treatment-Related Difference in the Relation between the Estimated Mean Intima–Media Thickness and Age.
Dotted lines represent 95 percent confidence intervals. The overall difference (conventional treatment minus intensive treatment) was significant (P=0.004).
DISCUSSION
We assessed the long-term effect of intensive treatment of type 1 diabetes, presumably mediated through improved glycemic control, on the thickness of the carotid-artery wall over time. Using a multivariate linear regression model incorporating important covariates that were not affected or confounded by treatment, as well as the interaction between age and treatment group, we found a significant effect of intensive therapy, as compared with conventional treatment, during the DCCT on the subsequent change in the intima–media thickness with age. The intensively treated group had a smaller increase in the thickness with age than did the conventionally treated group. The differences in intima–media thickness between these treatment groups could be due to the less atherogenic lipid profile and decreased level of microalbuminuria seen with intensive therapy during the DCCT. However, even after adjustment for these variables in additional models, intensive therapy (and the lower mean glycosylated hemoglobin value during the DCCT) continued to be associated with a decrease in the progression of the intima–media thickness. The differences in the glycosylated hemoglobin value during the DCCT explained 96 percent of the long-term differences between groups (sum of squares) in the intima–media thickness of the common carotid artery at year 6.
Although the rate of cardiovascular disease is increased among patients with diabetes,1,5 the role of glycemia in this process remains uncertain.1,25 Intervention trials achieving variable degrees of glycemic control in patients with type 1 and type 2 diabetes have found either no statistically significant beneficial effect on cardiovascular end points9,26,27 or a positive effect that was not consistent in all groups and analyses.28–30 A meta-regression analysis, including predominantly patients with type 2 diabetes and subjects without diabetes, found a progressive relation between initial fasting and postprandial glucose levels and the subsequent occurrence of cardiovascular events over a 12-year period.31 The increased relative risk extended to subjects with glucose levels below the threshold for the diagnosis of diabetes. These studies have generally focused on cardiovascular disease events. However, several epidemiologic analyses in patients with type 2 diabetes have shown associations between intima–media thickness — as an early indicator of atherosclerosis — and glycemia.32,33
Our results demonstrate an association between glycemia and intima–media thickness, a sensitive marker for coronary and cerebral vascular disease, in patients with type 1 diabetes. The explanation for the apparently delayed effect of diabetes interventions on intima–media thickness — at year 1 of the EDIC study there was no effect of intensive therapy and no significant associations between carotid intima–media thickness and the mean glycosylated hemoglobin value during the DCCT16 — may lie in the putative pathogenic mechanism of atherosclerosis and in the demographics of the DCCT cohort. The accelerated development of atherosclerotic lesions in patients with diabetes may be the result of a gradual accumulation of advanced glycosylation end products.34,35 Thus, it may take years for atherosclerosis caused by various levels of hyperglycemia to develop, especially in a relatively young population, such as the DCCT cohort.
We16 and others36–38 have found the conventional cardiovascular disease risk factors of hypertension, dyslipidemia, and smoking to be related to intima–media thickness in patients with type 1 diabetes. Urinary albumin excretion was also associated with atherosclerosis, as suggested in other studies.39
The differences in intima–media thickness that we observed at year 6 between the diabetic cohort and the age- and sex-matched nondiabetic controls confirm and extend the results of several earlier, smaller studies. Increased carotid intima–media thickness has been reported in 105 Japanese patients with type 1 diabetes, as compared with those in age- and sex-matched controls,40 and in 60 Italian patients with type 1 diabetes.41 Studies of patients with type 2 diabetes have also demonstrated a difference in intima–media thickness between diabetic patients and nondiabetic subjects32,42; however, the relevance of these findings in the generally younger patients with type 1 diabetes who have a lower burden of cardiovascular disease and risk factors for cardiovascular disease is uncertain.
Our study has some limitations. The entire EDIC cohort did not participate in the carotid ultrasonographic measurements. However, the proportion of subjects who did not participate was small (10.6 percent), and the clinical characteristics of the non-participants and participants were generally similar. The unequal prevalence of smoking in the EDIC cohort and the age-matched nondiabetic controls could account for some of the differences in carotid intima–media thickness between these two groups; however, analyses that controlled for smoking yielded similar results. Differential use between treatment groups of medications known to ameliorate risk factors for cardiovascular disease and atherogenesis might explain, or confound, these results. However, here again, analyses that controlled for medication use and elevated blood pressure or low-density lipoprotein level yielded the same results. In fact, the more frequent use of such medications by the conventional-treatment group would be expected to decrease the differences in intima–media thickness that we found.
The results for intima–media thickness in the DCCT cohort, which was carefully selected to exclude patients with several other risk factors for atherosclerosis,9 might not extend to all patients with type 1 diabetes. However, as noted previously,43 at base line there were few differences between the DCCT cohort and the unselected population-based cohort with type 1 diabetes in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Therefore, the current results can probably be applied to the general population of patients with type 1 diabetes.
Finally, although cross-sectional intima–media measurements have been convincingly shown to correlate with the risk of cardiovascular disease events, data to support an association between the progression of intima–media thickness and such events are scarce.44 The clinical manifestations of atherosclerosis will increase as the DCCT cohort ages,5,45 increasing the likelihood of detecting a difference in cardiovascular disease event rates between treatment groups, should one exist. Longer follow-up of this cohort will reveal whether the decrease in the progression of intima–media thickness with intensive diabetes therapy translates into a clinically meaningful reduction in cardiovascular disease events.
Acknowledgments
Supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases and by the General Clinical Research Centers Program, National Center for Research Resources, and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Orchard reports having received honorariums from Merck, Schering-Plough, and AstraZeneca and having equity in Bristol-Myers Squibb, and Dr. Brillon honorariums from Aventis and Novo-Nordisk and equity in Aventis, Bristol-Myers Squibb, Lilly, and GlaxoSmithKline.
APPENDIX
The following persons and institutions participated in the DCCT/EDIC Research Group: Albert Einstein College of Medicine — S. Engel, H. Martinez, H. Shamoon, H. Engel; Case Western Reserve University — W. Dahms, L. Mayer, S. Pendegras, H. Zegarra, D. Miller, L. Singerman, S. Smith-Brewer, S. Genuth (past); Cornell University Medical Center — D. Brillon, M. Lackaye, M. Heinemann, V. Reppuci, T. Lee; Henry Ford Health System — F. Whitehouse, D. Kruger, A. Galpern, J.D. Carey; International Diabetes Center — R. Bergenstal, M. Johnson, D. Kendall, M. Spencer, D. Noller, K. Morgan, D. Etzwiler (past); Joslin Diabetes Center — A. Jacobson, E. Golden, G. Sharuk, P. Arrigg, R. Baeser, O. Ganda, J. Rosenzweig, H. Wolpert, P. Economides, O. Handy, L. Rand (past); Massachusetts General Hospital — D. Nathan, S. Fritz, J. Godine, C. McKitrick, P. Lou; Mayo Foundation — F.J. Service, G. Ziegler, J. Pach, J. Lindsey; Medical University of South Carolina — J. Colwell, D. Wood, R. Mayfield, K. Hermayer, M. Szpiech, T. Lyons, J. Parker, A. Farr, S. Elsing, T. Thompson, J. Selby, M. Bracey; Northwestern University — M. Molitch, B. Schaefer, L. Jampol, D. Weinberg, A. Lyon, Z. Strugula, J. Shankle, P. Astlesford; University of California, San Diego — O. Kolterman, G. Lorenzi, M. Goldbaum; University of Iowa — W. Sivitz, M. Bayless, R. Zeither (past), T. Weingeist, E. Stone, H. Culver Boidt, K. Gehres, S. Russell; University of Maryland School of Medicine — D. Counts, A. Kowarski (past), D. Ostrowski, T. Donner, S. Steidl, B. Jones; University of Michigan — W. Herman, D. Greene (past), C. Martin, M.J. Stevens, A.K. Vine, S. Elner; University of Minnesota — J. Bantle, B. Rogness, T. Olsen, E. Steuer; University of Missouri — D. Goldstein, S. Hitt, J. Giangiacomo, D. Hainsworth; University of New Mexico — D. Schade, M. Burge, J. Canady, M. Schluter, A. Das, D. Hornbeck (past); University of Pennsylvania — S. Schwartz, P.A. Bourne, B.J. Maschak-Carey (past), L. Baker (deceased), S. Braunstein, A. Brucker; University of Pittsburgh — T. Orchard, N. Silvers, T. Songer, B. Doft, S. Olson, R.L. Bergren, L. Lobes, M. Fineman, A. Drash (past); University of South Florida — J. Malone, J. Vaccaro-Kish, C. Berger, R. Gstalder, P.R. Pavan, A. Morrison; University of Tennessee — S. Dagogo-Jack, S. Schussler, A. Kitabchi, H. Lambeth, M.B. Murphy, S. Moser, D. Meyer, A. Iannacone, M. Bryer-Ash (past); University of Texas Southwestern Medical Center — P. Raskin, S. Strowig, A. Edwards, J. Alappatt (past), C. Wilson (past), S. Park (past), Y. He; University of Toronto — B. Zinman, A. Barnie, S. MacLean, R. Devenyi, M. Mandelcorn, M. Brent; University of Washington — J. Palmer, S. Catton, J. Kinyoun, L. Van Ottingham (past), J. Ginsberg (past); University of Western Ontario — J. Dupre, J. Harth, C. Canny (past), D. Nicolle; Vanderbilt University — M. May, R. Lorenz (past), J. Lipps, L. Survant, S. Feman (past), K. Tawansy, A. Agarwal, T. Adkins; Washington University, St. Louis — N. White, J. Santiago (deceased), L. Levandoski, I. Boniuk, G. Grand, M. Thomas, D. Burgess, D. Joseph, K. Blinder, G. Shah; Yale University School of Medicine — W. Tamborlane, P. Gatcomb, K. Stoessel, K. Taylor; Clinical Coordinating Center (Case Western Reserve University) — B. Dahms, R. Trail, J. Quin; Data Coordinating Center (George Washington University, Biostatistics Center) — J. Lachin, P. Cleary, D. Kenny, J. Backlund, L. Diminick, A. Determan, K. Klump, M. Hawkins; National Institute of Diabetes and Digestive and Kidney Diseases Program Office — C. Cowie, J. Fradkin, C. Siebert (past), R. Eastman (past); Central Fundus Photograph Reading Center (University of Wisconsin) — M. Davis, L. Hubbard, P. Geithman, L. Kastorff, M. Neider, D. Badal, B. Esser, K. Miner, H. Wabers, K. Glander, J. Joyce, N. Robinson, C. Hurtenbach, C. Hannon; Central Biochemistry Laboratory (University of Minnesota) — M. Steffes, J. Bucksa, B. Chavers; Central Carotid Ultrasound Unit (New England Medical Center) — D. O’Leary, L. Funk, J. Polak; Central Electrocardiographic Reading Unit (University of Minnesota) — R. Crow, C. O’Donnell (past), B. Gloeb, S. Thomas; Computed Tomography Reading Center (Harbor–UCLA Research and Education Institute) — R. Detrano, N. Wong, M. Fox, L. Kim, R. Oudiz; External Advisory Committee — G. Weir (chair), C. Clark, R. D’Agostino, M. Espeland, B. Klein, T. Manolio, L. Rand, D. Singer, M. Stern; Molecular Risk Factors Program Project (Medical University of South Carolina) — W.T. Garvey, T.J. Lyons, A. Jenkins, R. Klein, M. Lopes-Virella, G. Virella, A.A. Jaffa, D. Zheng, D. Lackland, D. McGee, R.K. Mayfield, M. Brabham; Genetic Studies Group (Hospital for Sick Children) — A. Boright, A. Paterson, S. Scherer, B. Zinman; Lipoprotein Distribution/Obesity Group (University of Washington) — J. Brunzell, J. Hokanson, S. Marcovina, J. Purnell, S. Sibley, S. Deeb, K. Edwards; Editor, EDIC Publications — D. Nathan.
REFERENCES
- 1.Nathan DM, Meigs J, Singer DE. The epidemiology of cardiovascular disease in type 2 diabetes mellitus: how sweet it is … or is it? Lancet. 1997;350 Suppl 1:SI4–SI9. doi: 10.1016/s0140-6736(97)90021-0. [DOI] [PubMed] [Google Scholar]
- 2.Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- 3.Wingard DL, Barrett-Connor E. Heart disease and diabetes. In: Harris M, editor. Diabetes in America. 2nd ed. Bethesda, Md.: National Institute of Diabetes and Digestive and Kidney Diseases; 1995. pp. 429–448. (NIH publication no. 95-1468.) [Google Scholar]
- 4.Dorman JS, Laporte RE, Kuller LH, et al. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study: mortality results. Diabetes. 1984;33:271–276. doi: 10.2337/diab.33.3.271. [DOI] [PubMed] [Google Scholar]
- 5.Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset insulindependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 6.Borch-Johnsen K, Andersen PK, Deckert T. The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1985;28:590–596. doi: 10.1007/BF00281993. [DOI] [PubMed] [Google Scholar]
- 7.Maser RE, Wolfson SK, Jr, Ellis D, et al. Cardiovascular disease and arterial calcification in insulin-dependent diabetes mellitus: interrelationships and risk factor profiles: Pittsburgh Epidemiology of Diabetes Complications Study-V. Arterioscler Thromb. 1991;11:958–965. doi: 10.1161/01.atv.11.4.958. [DOI] [PubMed] [Google Scholar]
- 8.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 9.Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 10.Epidemiology of Diabetes Interventions and Complications (EDIC): design, implementation, and preliminary results of a longterm follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke GL, Evans GW, Riley WA, et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middleaged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26:386–391. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 12.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 13.Wofford JL, Kahl FR, Howard GR, McKinney WM, Toole JF, Crouse JR. Relation of extent of extracranial carotid artery atherosclerosis as measured by B-mode ultrasound to the extent of coronary atherosclerosis. Arterioscler Thromb. 1991;11:1786–1794. doi: 10.1161/01.atv.11.6.1786. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotidartery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 15.Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 16.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Effect of intensive diabetes treatment on carotid artery wall thickness in the Epidemiology of Diabetes Interventions and Complications. Diabetes. 1999;48:383–390. doi: 10.2337/diabetes.48.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The DCCT Research Group. Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. Clin Chem. 1987;33:2267–2271. [PubMed] [Google Scholar]
- 18.O’Leary DH, Polak JF, Kronmal RA, et al. Thickening of the carotid wall: a marker for atherosclerosis in the elderly? Stroke. 1996;27:224–231. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 19.version 6. 4th ed. Cary, N.C.: SAS Institute; 1990. SAS/STAT user’s guide. [Google Scholar]
- 20.Lachin JM. Biostatistical methods: the assessment of relative risks. New York: John Wiley; 2000. [Google Scholar]
- 21.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical methods. 4th ed. Chicago: Irwin; 1996. [Google Scholar]
- 22.Draper NR, Smith H. Applied regression analysis. 2nd ed. New York: John Wiley; 1981. [Google Scholar]
- 23.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [Erratum, N Engl J Med 2000;342:1376.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haffner SM, Agostino RD, Jr, Saad MF, et al. Carotid artery atherosclerosis in type-2 diabetic and nondiabetic subjects with and without symptomatic coronary artery disease (the Insulin Resistance Atherosclerosis Study) Am J Cardiol. 2000;85:1395–1400. doi: 10.1016/s0002-9149(00)00784-0. [DOI] [PubMed] [Google Scholar]
- 25.Barrett-Connor E. Does hyperglycemia really cause coronary heart disease? Diabetes Care. 1997;20:1620–1623. doi: 10.2337/diacare.20.10.1620. [DOI] [PubMed] [Google Scholar]
- 26.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with noninsulindependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 27.Abraira C, Colwell J, Nuttall F, et al. Cardiovascular events and correlates in the Veterans Affairs Diabetes Feasibility Trial: Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes. Arch Intern Med. 1997;157:181–188. [PubMed] [Google Scholar]
- 28.Jensen-Urstad KJ, Reichard PG, Rosfors JS, Lindblad LEL, Jensen-Urstad MT. Early atherosclerosis is retarded by improved longterm blood glucose control in patients with IDDM. Diabetes. 1996;45:1253–1258. doi: 10.2337/diab.45.9.1253. [DOI] [PubMed] [Google Scholar]
- 29.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [Erratum, Lancet 1999;354:602.] [PubMed] [Google Scholar]
- 30.Idem. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [Erratum, Lancet 1998;352:1557.] [PubMed] [Google Scholar]
- 31.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 32.Folsom AR, Eckfeldt JH, Weitzman S, et al. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Stroke. 1994;25:66–73. doi: 10.1161/01.str.25.1.66. [DOI] [PubMed] [Google Scholar]
- 33.Bonora E, Kiechl S, Oberhollenzer F, et al. Impaired glucose tolerance, Type II diabetes and carotid atherosclerosis: prospective results from the Bruneck Study. Diabetologia. 2000;43:156–164. doi: 10.1007/s001250050024. [DOI] [PubMed] [Google Scholar]
- 34.Lyons TJ, Lopes-Virella MF. Glycosylation-related mechanisms. In: Draznin B, Eckel RH, editors. Diabetes and atherosclerosis: molecular basis and clinical aspects. New York: Elsevier; 1993. pp. 169–189. [Google Scholar]
- 35.Vlassara H, Bucala R. Recent progress in advanced glycation and diabetic vascular disease: role of advanced glycation end product receptors. Diabetes. 1996;45 Suppl 3:S65–S66. doi: 10.2337/diab.45.3.s65. [DOI] [PubMed] [Google Scholar]
- 36.Kawamori R, Yamasaki Y, Matsushima H, et al. Prevalence of carotid atherosclerosis in diabetic patients: ultrasound high-resolution B-mode imaging on carotid arteries. Diabetes Care. 1992;15:1290–1294. doi: 10.2337/diacare.15.10.1290. [DOI] [PubMed] [Google Scholar]
- 37.Puija A, Gnasso A, Irace C, et al. Common carotid arterial wall thickness in NIDDM subjects. Diabetes Care. 1994;17:1330–1336. doi: 10.2337/diacare.17.11.1330. [DOI] [PubMed] [Google Scholar]
- 38.Bonora E, Tessari R, Micciolo R, et al. Intimal-medial thickness of the carotid artery in nondiabetic and NIDDM patients: relationship with insulin resistance. Diabetes Care. 1997;20:627–631. doi: 10.2337/diacare.20.4.627. [DOI] [PubMed] [Google Scholar]
- 39.Mann JFE, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 40.Yamasaki Y, Kawamori R, Matsushima H, et al. Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes. 1994;43:634–639. doi: 10.2337/diab.43.5.634. [DOI] [PubMed] [Google Scholar]
- 41.Giannattasio C, Failla M, Grappiolo A, Gamba PL, Paleari F, Mancia G. Progression of large artery structural and functional alterations in Type I diabetes. Diabetologia. 2001;44:203–208. doi: 10.1007/s001250051600. [DOI] [PubMed] [Google Scholar]
- 42.Wagenknecht LE, D’Agostino RB, Haffner SM, Savage PJ, Rewers M. Impaired glucose tolerance, type 2 diabetes, and carotid wall thickness: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 1998;21:1812–1818. doi: 10.2337/diacare.21.11.1812. [DOI] [PubMed] [Google Scholar]
- 43.Klein R, Moss S. A comparison of the study populations in the Diabetes Control and Complications Trial and the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med. 1995;155:745–754. [PubMed] [Google Scholar]
- 44.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 45.Donahue RP, Orchard TJ. Diabetes mellitus and macrovascular complications: an epidemiological perspective. Diabetes Care. 1992;15:1141–1155. doi: 10.2337/diacare.15.9.1141. [DOI] [PubMed] [Google Scholar]