Abstract
NZW “ x ” BXSB F1 mice develop SLE that is associated with an anti-phospholipid syndrome characterized by anti-cardiolipin antibodies, thrombocytopenia and small coronary artery thrombosis. This syndrome is immune mediated and, dependent, on CD4+T cells. To determine whether disease in these mice can be treated with blockade of T cell costimulation we treated them with the CD28 antagonist CTLA4Ig at 9 or 12 weeks of age. CTLA4Ig completely prevented both SLE nephritis and myocardial infarcts if it was given at 9 weeks of age, before anti-cardiolipin antibodies could be detected in the serum and prevented both B cell expansion and activation and the development of peripheral monocytosis. If treatment was delayed until 12 weeks of age after cardiolipin antibodies had arisen but before the onset of clinical disease, CTLA4Ig had very little effect on disease progression. These findings indicate that CD4+T cell activation through CD28 is critical for disease initiation in this model but plays little role in disease progression or tissue damage. These findings have relevance to the treatment of anti-phospholipid syndrome in humans.
Keywords: CTLA4Ig, Anti-phospholipid syndrome, SLE, NZW/BXSB mice
INTRODUCTION
Male NZW/BXSB F1 mice develop a lupus like syndrome characterized by the production of autoantibodies, formation of immune complexes and development of aggressive inflammatory glomerulonephritis. These mice also produce anti-cardiolipin and platelet antibodies and manifest an anti-phospholipid syndrome characterized by thrombocytopenia and bland thromboses affecting the small coronary vessels that cause myocardial micro-infarcts leading to a dilated cardiomyopathy.[1-3] Both glomerular disease and myocardial infarcts in these mice can be prevented by transplantation of bone marrow or T cell depleted bone marrow,[4] or by depletion of CD4+T cells[3] early in life, indicating that both the glomerulonephritis and the cardiac thromboses are immune mediated and require CD4+T cells for initiation.
In order to be activated, CD4+T cells need to receive two signals. The first signal occurs when specific antigen is recognized by the T cell receptor. The second signal is triggered when costimulatory receptors on the T cell surface are activated by the appropriate costimulatory ligand.[5,6] Drugs based on modulating the effects of costimulation are currently being developed for clinical use. One such drug, CTLA4Ig, is a potent blocker of the costimulatory interaction of CD28 with CD80 and CD86 and has been used safely in human clinical trials for psoriasis and rheumatoid arthritis.[7,8] In several mouse SLE models, nephritis can be prevented by costimulatory blockade with CTLA4Ig[9,10] and remission of active nephritis can be achieved by the combination of CTLA4Ig and the conventional immunosuppressive agent cyclophosphamide.[11,12]
Anti-phospholipid syndrome in patients with SLE is generally not responsive to conventional immunosuppression and is currently treated with life-long anti-coagulation. Catastrophic cases are also treated with glucocorticoids, IVIg, cyclophosphamide and plasma-pheresis to remove pathogenic antibodies but this is not always successful.[13,14] The NZW/BXSB model offers a murine model of the anti-phospholipid syndrome in the setting of SLE that can be used to explore disease mechanisms and potential new therapies. Since disease initiation in this model requires the presence of CD4+T cells we asked whether disease could be prevented or treated with costimulatory blockade using CTLA4Ig. We found that both nephritis and myocardial infarcts can be prevented with CTLA4Ig administered before any anti-phospholipid antibodies are detected, but disease progresses despite CD28/B7 blockade once these antibodies begin to appear in the serum, suggesting that late T cell activation and B cell proliferation in this mouse are probably driven by other costimulatory molecules or soluble mediators.
METHODS
Mice
NZW female and BXSB male mice were purchased from Jackson laboratories and bred in our institution. Male F1 progeny were treated with a single dose of adenovirus expressing CTLA4Ig at the age of 9 weeks (9 mice), 10 weeks (9 mice) or 12 weeks (11 mice). Control mice (22 mice) were untreated. Mice were bled every 2-4 weeks. Urine was tested for proteinuria by dipstick (Multistick, Fisher, Pittsburg PA) every 2 weeks. Some mice were sacrificed at 21-24 weeks of age, when >50% of the controls had developed proteinuria, and the rest were followed and sacrificed at the age of 30 weeks, when 70% of the controls had died.
Antibodies to Cardiolipin
Antibodies to cardiolipin were measured by ELISA. In brief, 96-well Immulon 2HB plates (Fisher, Pittsburgh, PA) were coated with cardiolipin (Sigma, St Louis, MO) 75 ug/ml in ethanol and allowed to dry overnight. Plates were blocked with 5% FCS/3% BSA in PBS for 90 min at room temperature and then incubated with serum 1/500 in PBS/1% BSA for 2 h at 37°C. Fetal calf serum in the blocking solution is a source of β2 glycoprotein-1. After washing the plates were incubated with alkaline phosphatase conjugated goat anti-mouse IgG (Southern Biotech, Birmingham, AL) or IgM 1/1000 in PBS/1%BSA followed by BCIP substrate (Sigma).
Flow Cytometry and Immunofluorescence Analysis of Spleens
Spleen and peripheral blood cells were analyzed for B and T cell markers using antibodies to CD4 (Caltag, Burlingham, CA), CD8 (Caltag), and CD19. T cell subsets were identified using FITC-anti-CD4, PE-anti-CD69, Cy-anti-CD44 and PE-anti-CD62L. Spleen and peripheral blood dendritic cells were identified using PE-anti-CD11b and FITC anti-CD11c. Except where indicated, all antibodies were purchased from BD Pharmingen, San Diego, CA.
ELISpot Analysis
ELISpot assays for anti-dsDNA secreting B cells were performed as previously described[10] using 4-5 mice per group. ELISpot assay for anti-cardiolipin antibody producing B cells were performed in a similar fashion. In brief 3 fold serial dilutions of spleen cells were plated in duplicates on cardiolipin-coated plates starting at 1 × 106 cells per well. The cells were incubated at 37°C for 2 h and then an equal volume of biotin conjugated anti-IgM or IgG (Southern Biotechnology, Birmingham, AL) 1/500 in DME/10%FCS was added. Plates were incubated overnight at 37°C, washed and incubated with streptavidin alkaline phosphatase (Southern Biotech) 1/1000 in PBS/1% BSA for 45 min. Plates were then developed with BCIP (Sigma) 1 mg/ml in AMP buffer (0.75 mM MgCl/0.01% Triton-X/9.58% 2-amino-methyl-1propanol, pH 10.25). Spots were counted using a dissecting microscope. Total numbers of immunoglobulin secreting cells were measured the same way using anti-mouse immunoglobulins (Cappel, Westchester, PA) to coat the plates.
Generation of Hybridomas
Hybridomas were generated from spleen cells by standard techniques using NSO cells as the fusion partner. Hybridomas were screened for anti-cardiolipin activity and positive hybridomas were isotyped as previously described.[15]
Immunohistochemical Analysis of Kidneys and Hearts
Examination and scoring of the kidneys was performed using a 1 to 4 scale for glomerular damage and interstitial inflammation as previously described.[16] Hearts were sectioned horizontally into 4 slices and each slice was examined for ischemia, necrosis, scarring and inflammation. Myocardial damage was scored on a 1 to 4 scale where 1 was a single area of necrosis or scarring, 2 was several areas of necrosis or fibrosis, 3 was extensive focal areas of necrosis and fibrosis and 4 was full thickness myocardial infarct.
Statistics
Proteinuria and survival data shown in Fig. 1 were analyzed using Kaplan Meier curves and Log Rank test. Comparisons shown in Figs. 3-5 and 7-9 were performed using Wilcoxon Rank Sum Test. Only significant p values are shown.
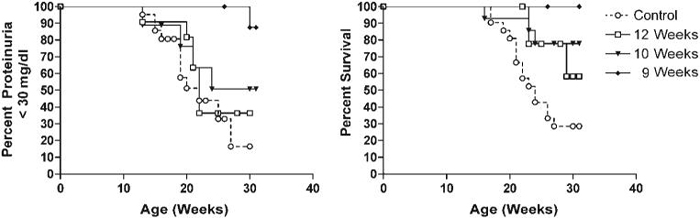
FIGURE 1.
Clinical outcomes of mice treated with CTLA4Ig. Kaplan Meier plots of proteinuria free survival (A) and overall survival (B) in the 3 groups of NZW/BXSB mice described in the Materials and Methods section. Mice treated with CTLA4Ig all had prolonged survival (Ad-CTLA4Ig vs. untreated: 9 weeks, p < 0.002; 10 weeks, p < 0.02; 12 weeks, p < 0.05), but only mice treated at 9 weeks had a significant delay until the onset of proteinuria (p <0.002).
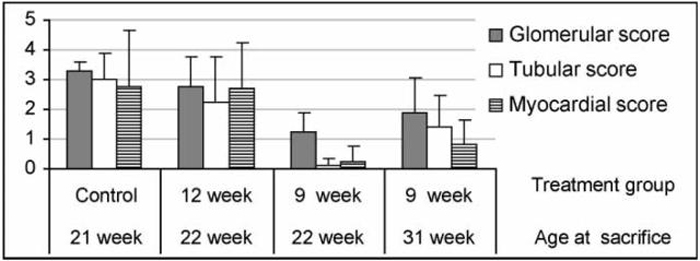
FIGURE 3.
Analysis of renal and cardiac damage scores at 22 and 30 weeks. Mice treated with CTLA4Ig at 9 weeks had significantly less renal (p <0.03 for glomerular and tubular scores) and cardiac damage (p < 0.05) than untreated controls. Mice treated with CTLA4Ig at 12 weeks were not different from untreated controls. Histologic damage scores were assessed as described in the materials and methods using 6 untreated controls, 6 mice treated at 12 weeks, and 4 and 5 mice treated at 9 weeks examined at 22 and 30 weeks, respectively.
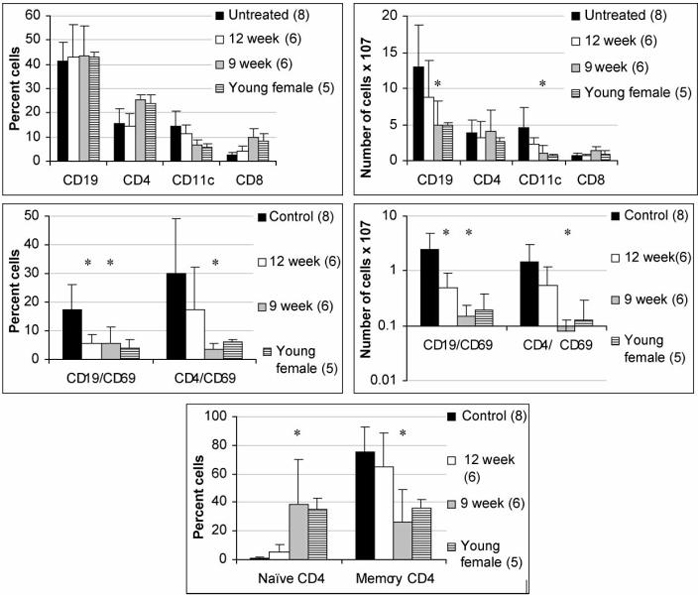
FIGURE 5.
Phenotypic analysis of spleen cells at 22 weeks. Flow cytometry data showing the percentage of cells mice (left panels) and the total number of cells (right panels) in the spleens of treated and control mice. Young female NZW/BXSB mice are used as a negative control. Results for mice treated at 9 weeks of age were similar in spleens harvested at 22 and 30 weeks and are pooled. Untreated mice had an increased number of CD19 + B cells and of CD11c+/CD11b+dendritic cells, an increased number of activated (CD69+) CD4+T and B cells and an increased number of memory (CD44+/CD62L-) CD4+T cells compared with young female NZW/BXSB. Mice treated with CTLA4Ig at 9 weeks had a similar phenotype as young females, whereas mice treated with CTLA4Ig at 12 weeks were intermediate between untreated and 9 week treated mice. (* indicates p < 0.03 treated vs. untreated age matched control. Only significant p values are shown).
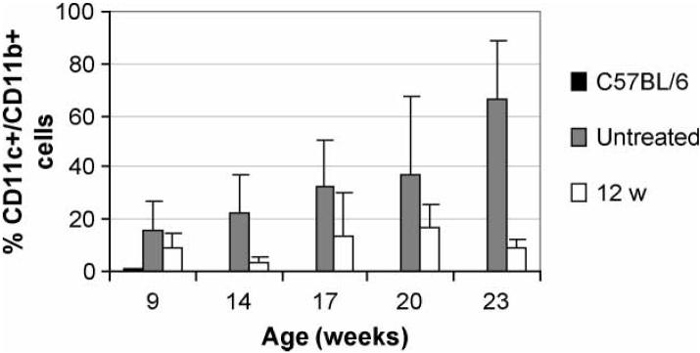
FIGURE 7.
Analysis of peripheral blood for dendritic cells. A peripheral monocytosis is noted even as early as 9 weeks of age in NZW/BXSB mice compared with non-autoimmune C57BL/6 mice. Disease progression in untreated mice was associated with a further accumulation of peripheral blood monocytes. This age related expansion of CD11c+/CD11b+cells was prevented in mice treated with CTLA4Ig either at 12 weeks (p < 0.01 for all times after 14 weeks) or at 9 weeks (not shown).
FIGURE 9.
Enumeration of spontaneous hybridomas. Frequency of anti-cardiolipin hybridomas in untreated and treated mice. Mice treated at 9 and 12 weeks had a significantly lower frequency of anti-cardiolipin hybridomas compared with untreated controls (p < 0.02). Mice treated at 9 weeks had significantly fewer IgG anti-cardiolipin hybridomas than untreated mice (p < 0.002) or mice treated at 12 weeks (p < 0.05).
RESULTS
As we have previously reported in NZB/W F1 mice,[10] CTLA4Ig was expressed in the serum of treated mice at levels >20 ug/ml for the duration of the 30 week experiment (not shown). Mice treated with CTLA4Ig at the age of 9 weeks had a significant delay in the onset of proteinuria and prolonged survival compared with controls. In contrast, mice treated at 12 weeks had no delay in the onset of proteinuria and had only a modest increase in survival. Mice treated at 10 weeks had an outcome intermediate between the 9- and 12-week treatment groups and were not further studied (Fig. 1).
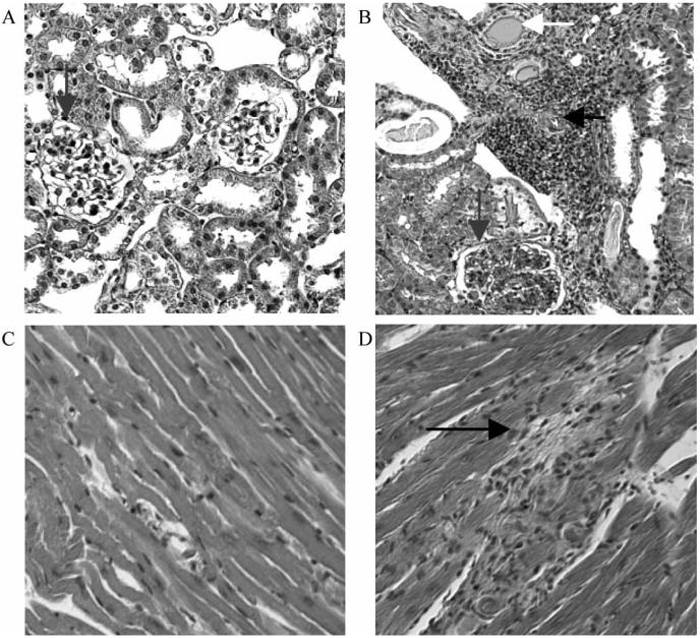
Untreated mice developed severe glomerulonephritis with inflammatory infiltrates and vasculitis. In addition most of these mice had cardiac enlargement with evidence of focal ischemia and, in some cases, full thickness infarcts of the right ventricle. Commensurate with the survival data, mice treated at 9 weeks had significantly less renal and cardiac damage than untreated controls, whereas mice treated at 12 weeks were not significantly different from controls (Figs. 2 and 3).
FIGURE 2.
Histologic evaluation of kidneys and hearts. Histologic analysis of kidneys (A, B) and hearts (C, D) from an untreated control (B, D) and from a 9 week CTLA4Ig treated mouse (A, C). Note the presence of vasculitis (horizontal arrow, B) tubular casts (white arrow, B) and glomerulonephritis (vertical arrow, B) in the kidney of the untreated control. Small myocardial infarcts are noted in the control (black arrow) but not in the treated mouse.
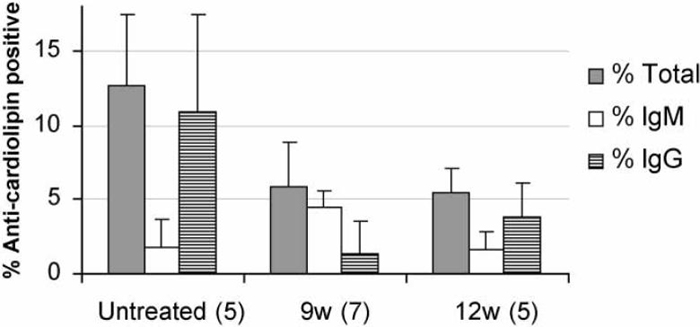
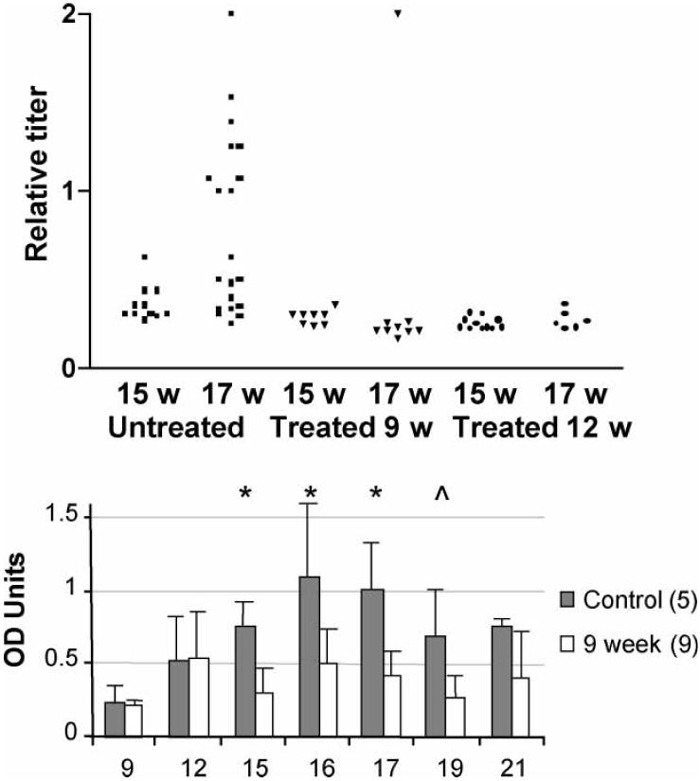
To determine whether CTLA4Ig treatment inhibited the production of anti-cardiolipin antibodies, titers of these antibodies were measured serially from the age of 9 weeks. The mice which were 9-week-old had negligible titers of anti-cardiolipin antibodies, whereas they were readily detected in 12-week-old mice. In untreated mice anti-cardiolipin antibodies increased in titer over time with a peak at 16-17 weeks of age, after which they stabilized or declined. In mice treated at 9 or 12 weeks, anti-cardiolipin titers did not increase over the 12-week level (Fig. 4).
FIGURE 4.
Serum levels of anti-cardiolipin antibodies. Upper panel: Serum anti-cardiolipin antibody titers at 15 and 17 weeks of age. Titers are calculated relative to an untreated mouse with the highest titer of anti-cardiolipin antibodies. Approximately 50% of untreated mice had an increase in titers between 15 and 17 weeks that was not observed in treated mice (Untreated vs. either 9 or 12 week treated mice p < 0.005 at 17 weeks). Lower panel: Serial measurement of IgG anti-cardiolipin levels in 9 week treated mice showed that they could be detected at low levels at 12 weeks of age but failed to increase further over time (*p < 0.01; ^p < 0.02). A similar pattern was observed in mice treated at 12 weeks (not shown).
To determine whether there were phenotypic differences between 9 and 12 week old NZW/BXSB mice that could account for the differences in response to CTLA4Ig treatment, we examined spleen cells of a group of five 9 week and five 12 week untreated mice. Analysis of spleen cells showed subtle phenotypic differences between untreated 9 and 12 week old mice that did not reach statistical significance including increases in the absolute number of total B cells, mature B cells and memory T cells in the 12 week mice (data not shown). These phenotypic changes are characteristic of disease progression in NZW/BXSB mice and became more pronounced with age (see, Fig. 5).
CTLA4Ig treated and untreated mice were sacrificed or splenectomized at 22 weeks. Spleens from some mice in the 9-week treatment group were also harvested at 30 weeks of age. The spleens of untreated mice were markedly enlarged, sometimes reaching 5 g in weight and there was marked fibrosis and extramedullary hematopoiesis. Spleens from CTLA4Ig treated mice were smaller and none weighed more than 0.75 g. The number of cells per spleen in the untreated mice was significantly higher in untreated controls than in young controls or CTLA4Ig treated mice (Fig. 5).
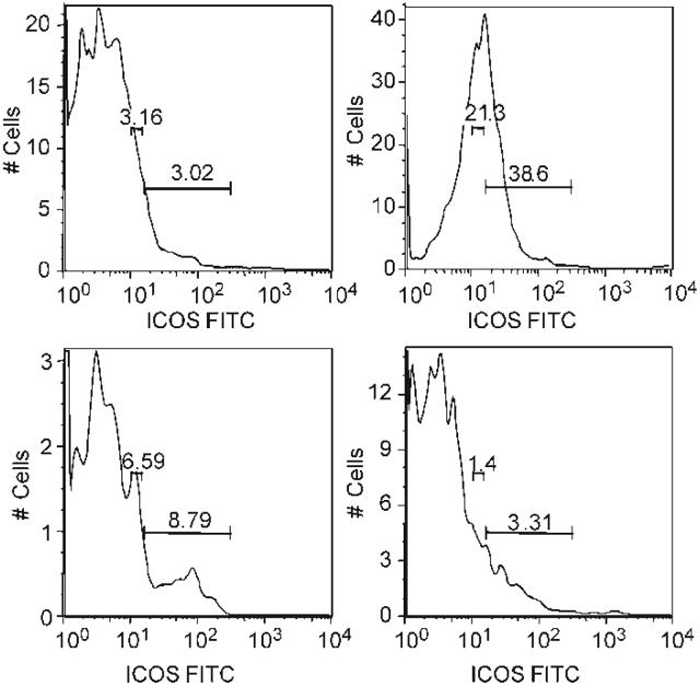
Flow cytometry was performed at the age of 22 weeks to phenotype lymphoid cell populations in the spleen. Untreated NZW/BXSB mice had increased numbers of B cells and of myeloid dendritic cells. The total number of T cells was not increased, but there was increased expression of T cell activation markers and an accumulation of T cells bearing memory markers as the mice aged. Treatment with CTLA4Ig at the age of 9 weeks almost completely prevented the acquisition of all phenotypic abnormalities in the spleen. In contrast, mice treated at 12 weeks had only partial reversal of the activated splenic phenotype (Fig. 5). Since ICOS expression is regulated by CTLA4, we asked whether CD4 T cells from NZW/BXSB mice expressed ICOS and whether this was affected by CTLA4Ig. Untreated 22-week-old NZW/BXSB mice expressed very high levels of ICOS on splenic CD4+T cells and this phenotype was acquired with age. Interestingly, ICOS up regulation was completely abrogated in mice treated with CTLA4Ig at 9 weeks but only partially abrogated in mice treated at 12 weeks (Fig. 6). As previously reported,[17] untreated mice also accumulated large numbers of CD11c+/CD11b+immature myeloid dendritic cells in the peripheral blood with age. Treatment with CTLA4Ig at either 9 or 12 weeks prevented this abnormality (Fig. 7).
FIGURE 6.
Analysis of ICOS expression on splenic CD4+T cells. Histograms represent CD4+gated cells. CD4+T cells in untreated mice at 22 weeks expressed high levels of the costimulatory molecule ICOS compared with young (9 week old) mice (B vs. A). Increased ICOS expression was partially prevented in mice treated at 12 weeks (C) and completely prevented in mice treated at 9 weeks (D). Data are representative of 3-4 mice per group.
To further examine the effect of CTLA4Ig treatment on autoreactive B cells, the frequencies of immunoglobulin producing B cells and of autoantibody producing B cells were enumerated using an ELISpot assay. Untreated control mice had a marked increase in the frequency of IgG secreting splenic B cells compared with nonautoimmune C57BL/6 mice (IgG 41.8 ± 24.2 vs. 2.7 ± 0.2 per 1000, p < 0.01). This increase was attenuated in the CTLA4Ig treated mice treated at 9 weeks, but not in those treated at 12 weeks. Similarly, control NZW/BXSB mice had a high frequency of anti-cardiolipin antibody secreting B cells that was significantly decreased in mice treated with CTLA4Ig at 9 weeks. Anti-dsDNA antibody secreting B cells of the IgM and IgG isotypes were detected in the untreated mice but the frequency of IgG anti-dsDNA secreting cells was decreased in the mice treated with CTLA4Ig at 9 weeks (Fig. 8).
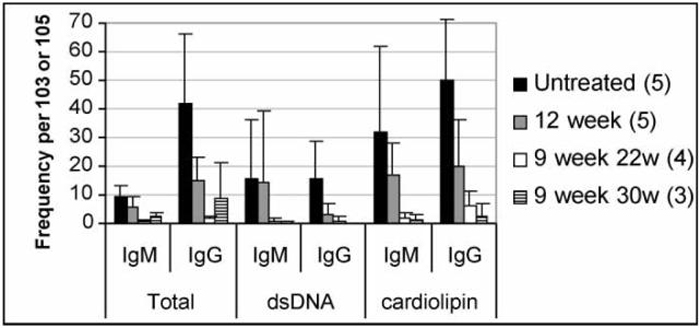
FIGURE 8.
Frequency of Ig secreting cells analyzed by ELISpot. Mean + SD frequency of antibody and autoantibody secreting B cells of the IgM and IgG isotypes from spleens of untreated and treated mice. Spleens from untreated and 12 week treated mice were analyzed at 22 weeks. Frequency is per 103 cells for total Ig and per 105 cells for anti-dsDNA Ig and anti-cardiolipin Ig. Mice treated with CTLA4Ig at 12 weeks were not different from untreated mice. Mice treated with CTLA4Ig at 9 weeks had a significantly lower frequency of Ig and autoantibody-secreting cells than untreated controls (p <0.02 for all analyses). Mice treated at 12 weeks had a lower frequency of IgG secreting cells and of anti-cardiolipin secreting cells than untreated controls (p < 0.05).
In order to detect activated autoreactive B cells, we generated spontaneous hybridomas from the spleens of treated and untreated mice; only highly activated or dividing B cells will form spontaneous hybridomas. There was a very high frequency of activated anti-cardiolipin secreting B cells in untreated mice. The frequency of positive hybridomas was significantly decreased in CTLA4Ig treated mice treated at both 9 and 12 weeks, but in the mice treated at 9 weeks most of the hybridomas were of the IgM isotype, whereas in the mice treated at 12 weeks most were of the IgG isotype (Fig. 9).
DISCUSSION
Murine models of SLE have been under-utilized for the study of SLE vasculopathy. Although most mice that spontaneously develop SLE also develop anti-phospholipid antibodies, spontaneous thrombosis and vasculopathy is relatively uncommon in most strains and only occurs with high frequency in the NZW/BXSB model where it is associated with myocardial infarctions.[1] This appears to be because there is a genetic component of the disease that maps to a chromosome different from that associated with autoantibody production.[18]
Since disease initiation in this model requires the presence of CD4+T cells, we asked whether disease could be prevented or treated with costimulatory blockade using CTLA4Ig. IgG anti-cardiolipin antibodies begin to appear in the serum of the mice at 11-12 weeks of age and myocardial infarcts become evident between 12 and 16 weeks of age.[19] Administration of adenovirus expressing CTLA4Ig at the age of 9 weeks, before IgG anti-cardiolipin antibodies are detected in the serum, prevented both renal disease and myocardial infarcts and all the mice were still alive at the time of termination of the experiment at 30 weeks. In contrast, administration of CTLA4Ig at the age of 12 weeks, when anti-phospholipid antibodies could be detected, but before the onset of clinical disease, did not substantially delay the onset of proteinuria or alter the prevalence of myocardial infarcts. However, the time to death was modestly delayed in these mice.
Using flow cytometry we found that the NZW/BXSB mouse has a different phenotype from the NZB/W that we have previously studied in detail.[10,15] There is no expansion of the marginal zone B cell subset or polyclonal activation of IgM producing B cells (data not shown). However, there is a profound hyperplasia of the spleen that is associated with the accumulation of large numbers of B cells and of myeloid dendritic cells and with the activation, but not expansion, of CD4 T cells. Despite only subtle differences in the spleen cell phenotype between 9 and 12 week old mice, CTLA4Ig given at 9 weeks almost completely prevented the accumulation of activated B cells, T cells and dendritic cells in the spleen, whereas delay of treatment until 12 weeks resulted in only a modest effect. Activation of class switched anti-phospholipid producing B cells was prevented by CTLA4Ig given at 9 weeks but only partially prevented by CTLA4Ig given at 12 weeks (Fig. 9).
Our data show that the mechanisms that initiate disease in this model are different from those that mediate disease perpetuation and tissue damage. Disease initiation clearly requires the presence of CD4+T cells and costimulation through B7/CD28. Disease prevention using CTLA4Ig results in abrogation of other features of the disease including B cell hyperplasia, class switching of the autoimmune response, and the expansion of a population of CD11c/CD11b dendritic cells. In addition, CTLA4Ig given before autoantibodies arise prevents the up regulation of ICOS expression on T cells, suggesting that expression of ICOS is dependent upon T cell activation through CD28.
Once autoantibody producing B cells have been generated however, the disease is no longer highly responsive to blockade of CD28 costimulation. There are several possible reasons for this. One is that some autoreactive memory T cells may already have arisen by 12 weeks of age and these may be less susceptible than naïve cells to CD28 blockade.[20] Costimulation of these cells may be mediated through other cell surface molecules such as ICOS.[21] Second, once autoreactive B cells have arisen and have been activated they can act as antigen presenting cells to memory T cells in a manner that may be CD28 independent.[22] Furthermore, formation of immune complexes is a stimulus for the maturation of dendritic cells[23,24] and release of the TNF like molecule BAFF from dendritic cells may in turn directly enhance B cell survival; this does not require help from T cells.[25]
Our data also show that there are certain aspects of the disease that are still CD28, dependent, after the age of 12 weeks. First, between 15 and 17 weeks of age, about 50% of untreated mice manifested a 2-3-fold increase in the titer of anti-cardiolipin antibodies. This did not occur in CTLA4Ig treated mice. Second, all the untreated mice developed an extreme monocytosis with age with these cells accounting for 30-80% of peripheral lymphocytes by the age of 26 weeks. These cells are CD11c+/CD11b+and have the phenotype of immature dendritic cells.[17] This peripheral monocytosis was prevented in CTLA4Ig treated mice despite an expansion of CD11c+/CD11b+dendritic cells in the spleen. These effects of CTLA4Ig were, however, not sufficient to prevent disease in the mice treated at 12 weeks, although, together with a modest decrease in the number of activated lymphocytes in the spleen, they may help to account for the delay in the time to death.
Our findings may have clinical implications for the treatment of human disease. Our data suggest that there is only a narrow window of opportunity for prevention of the anti-phospholipid syndrome using CTLA4Ig and that, once autoantibodies arise, suppression of T cell function may not be effective. These findings are in accord with observations in humans in whom disease is not amenable to T cell immunosuppression and in whom catastrophic disease is treated with therapies that clear autoantibodies or kill autoantibody producing B cells.[13] Further study of the NZW/BXSB model should allow clarification of the types of therapeutic intervention, including B cell directed therapies that may be useful for the anti-phospholipid syndrome.
Acknowledgements
We thank Dr Harold Keiser for critical reading of the manuscript. This work was supported by the NY SLE Foundation (LS and MR) the National Arthritis Foundation (MR) and by NIAID (AD).
References
- [1].Hang LM, Izui S, Dixon FJ. J. Exp. Med. 1981;154:216–221. doi: 10.1084/jem.154.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hashimoto Y, Kawamura M, Ichikawa K, Suzuki T, Sumida T, Yoshida S, Matsuura E, Ikehara S, Koike T. J. Immunol. 1992;149:1063–1068. [PubMed] [Google Scholar]
- [3].Adachi Y, Inaba M, Sugihara A, Koshiji M, Sugiura K, Amoh Y, Mori S, Kamiya T, Genba H, Ikehara S. Immunobiology. 1998;198:451–464. doi: 10.1016/s0171-2985(98)80052-1. [DOI] [PubMed] [Google Scholar]
- [4].Kirzner RP, Engelman RW, Mizutani H, Specter S, Good RA. Biol. Blood Marrow Transplant. 2000;6:513–522. doi: 10.1016/s1083-8791(00)70022-x. [DOI] [PubMed] [Google Scholar]
- [5].Howland KC, Ausubel LJ, London CA, Abbas AK. J. Immunol. 2000;164:4465–4470. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- [6].Appleman LJ, Boussiotis VA. Immunol. Rev. 2003;192:161–180. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- [7].Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, Weiner RS, Birkhofer MJ, Warner GL, Berry KK, Linsley PS, Krueger JG, Ochs HD, Kelley SL, Kang S. J. Clin. Investig. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moreland LW, Alten R, Van den Bosch F, Appelboom T, Leon M, Emery P, Cohen S, Luggen M, Shergy W, Nuamah I, Becker JC. Arthritis Rheum. 2002;46:1470–1479. doi: 10.1002/art.10294. [DOI] [PubMed] [Google Scholar]
- [9].Finck BK, Linsley PS, Wofsy D. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- [10].Mihara M, Tan I, Chuzhin Y, Reddy B, Budhai L, Holzer A, Gu Y, Davidson A. J. Clin. Investig. 2000;106:91–101. doi: 10.1172/JCI9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Daikh DI, Wofsy D. J. Immunol. 2001;166:2913–2916. doi: 10.4049/jimmunol.166.5.2913. [DOI] [PubMed] [Google Scholar]
- [12].Schiffer L, Sinha J, Wang X, Davidson A. SLE Therapeutics Meeting; Washngton DC. 2002. [Google Scholar]
- [13].Erkan D, Cervera R, Asherson RA. Arthritis Rheum. 2003;48:3320–3327. doi: 10.1002/art.11359. [DOI] [PubMed] [Google Scholar]
- [14].Hanly JG. CMAJ. 2003;168:1675–1682. [PMC free article] [PubMed] [Google Scholar]
- [15].Wang X, Huang W, Mihara M, Sinha J, Davidson A. J. Immunol. 2002;168:2046–2053. doi: 10.4049/jimmunol.168.4.2046. [DOI] [PubMed] [Google Scholar]
- [16].Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A. J. Immunol. 2003;171:489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- [17].Adachi Y, Taketani S, Toki J, Ikebukuro K, Sugiura K, Oyaizu H, Yasumizu R, Tomita M, Kaneda H, Amoh Y, Ito T, Okigaki M, Inaba M, Ikehara S. Stem Cells. 2002;20:61–72. doi: 10.1634/stemcells.20-1-61. [DOI] [PubMed] [Google Scholar]
- [18].Ida A, Hirose S, Hamano Y, Kodera S, Jiang Y, Abe M, Zhang D, Nishimura H, Shirai T. Eur. J. Immunol. 1998;28:2694–2703. doi: 10.1002/(SICI)1521-4141(199809)28:09<2694::AID-IMMU2694>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [19].Yoshida H, Fujiwara H, Fujiwara T, Ikehara S, Hamashima Y. Am. J. Pathol. 1987;129:477–485. [PMC free article] [PubMed] [Google Scholar]
- [20].Reddy B, Gupta S, Chuzhin Y, Kalergis AM, Budhai L, Zhang M, Droguett G, Horwitz MS, Chowdhury JR, Nathenson SG, Davidson A. Transplantation. 2001;71:801–811. doi: 10.1097/00007890-200103270-00020. [DOI] [PubMed] [Google Scholar]
- [21].Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, et al. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- [22].Shlomchik MJ, Craft JE, Mamula MJ. Nat. Rev. Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- [23].Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. J. Exp. Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- [25].Schneider P, Tschopp J. Immunol. Lett. 2003;88:57–62. doi: 10.1016/s0165-2478(03)00050-6. [DOI] [PubMed] [Google Scholar]