Abstract
The aryl hydrocarbon receptor (AhR) is part of a powerful signaling system that is triggered by xenobiotic agents such as polychlorinated hydrocarbons (PCHs) and polycyclic aromatic hydrocarbons (PAHs). Although activation of the AhR by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) or certain polycyclic aromatic hydrocarbons (PAHs) can lead to immunosuppression, there is also increasing evidence that the AhR regulates certain normal developmental processes. In this study, we asked whether the AhR plays a role in host resistance using murine listeriosis as an experimental system. Our data clearly demonstrate that AhR null C57BL/6J mice (AhR−/−) are more susceptible to listeriosis than AhR heterozygous (AhR+/−) littermates when inoculated i.v. with log-phase L. monocytogenes. AhR−/− mice exhibited greater numbers of CFU of L. monocytogenes in the spleen and liver, and greater histopathological changes in the liver than AhR+/− mice. Serum levels of IL-6, MCP-1, IFN-γ, and TNF-α were comparable between L. monocytogenes-infected AhR−/− and AhR+/− mice. Increased levels of IL-12 and IL-10 were observed in L. monocytogenes-infected AhR−/− mice. No significant difference was found between AhR+/− and AhR−/− macrophages ex vivo with regard to their ability to ingest and inhibit intracellular growth of L. monocytogenes. Intracellular cytokine staining of CD4+ and CD8+ splenocytes for IFN-γ and TNF-α revealed comparable T-cell mediated responses in AhR−/− and AhR+/− mice. Previously infected AhR−/− and AhR+/− mice both exhibited enhanced resistance to reinfection with L. monocytogenes. These data provide the first evidence that AhR is required for optimal resistance, but is not essential for adaptive immune response to L. monocytogenes infection.
Keywords: aryl hydrocarbon receptor (AhR), L. monocytogenes, innate immunity, adaptive immunity
Introduction
The aryl hydrocarbon receptor (AhR) is a ligand-activated member of the basic helix-loop-helix Per-Arnt-Sim (bHLH-PAS) family of transcriptional regulators (1–3). Together with its bHLH-PAS nuclear partner, the aryl hydrocarbon receptor nuclear transporter (ARNT), it provides a powerful signaling system that plays critical roles in response to several environmental toxins, most notably 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). There is also increasing evidence for the importance of the AhR in normal development, circadian rhythm, response to hypoxia, and hormone signaling (4–6). In the absence of ligands, AhR is inactive and remains in the cytoplasm complexed with a dimer of HSP90 (heat shock protein 90), a src protein kinase, and an immunophilin XAP2 (hepatitis B Virus X-Associated Protein 2) (7–9). Once ligands bind and activate the AhR, these complexes change their conformation and translocate to the nucleus. The cytoplasmic accessory proteins then dissociate from the ligand-AhR complex and ARNT binds to the AhR, forming a heterodimer. This heterodimer binds to xenobiotic response elements (XRE, whose core motif is 5’-GCGTG-3’) present in the upstream regulatory region of target genes (10). A battery of genes (cytochrome P450s, NAD(P)H quinine oxidoreductase, UDP-glucoronosyltransferase-6, AhR repressor, Bax, DNA polymerase κ, and Fas) are up-regulated by the AhR signaling pathway(3, 10, 11). Pro-inflammatory cytokines constitute another important class of genes regulated by the AhR. TCDD treatment causes increased expression of TNF-α, IL-1, IFN-γ, IL-8, IL-6 and CCL1 (12–16), suggesting a role for the AhR in inflammation and immunity.
The immune system is a sensitive target for the toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Many polycyclic aromatic hydrocarbons (PAHs), such as benzo[a]pyrene (B[a]P) and 7,12-dimethylbenz[a]anthracene (DMBA), also act in part via AhR activation. Early studies showed that exposure of mice to TCDD led to bone marrow hypocellularity with depressed colony formation of macrophage-granulocyte progenitor cells and pleuripotent stem cells (17, 18). Similar suppressive effects on the immune response were observed in DMBA-exposed mice (19). Treatment of mice with TCDD suppressed the production of antibodies and the generation of cytotoxic T cells (20, 21), which consequently altered the host resistance to many diseases (22). More recently, it was reported that TCDD treatment led to premature emigration and proliferation arrest of triple negative thymocytes, possibly via an induced ecotopic expression of Kruppel-like factor-2 (KLF2) in these cells (23). AhR−/− mice exhibited decreased accumulation of lymphocytes in the spleen and lymph nodes (24). Conversely, TCDD treatment of mice increased the numbers of peritoneal neutrophils and macrophages in response to SRBC injection (25). These studies suggest a role for the AhR in regulating immune responses.
The cellular and molecular mechanisms involved in PAH- or TCDD-related immunotoxicity remain incompletely understood. T cells are a possible direct target for TCDD, as evidenced by the presence of the AhR in T cells (26), and inhibition of T cell growth by the expression of a constitutively active AhR mutant in AhR-null Jurkat T cells (27) or following TCDD treatment (28, 29). Exposure to PAHs such as B[a]P, DMBA, and 3-methylcholanthrene (3-MC) inhibited differentiation of human monocytes into macrophages (30) as well as differentiation and maturation of human monocyte-derived dendritic cells (DCs) (31) in an AhR-dependent manner. Kerkvliet and colleagues showed that expression of AhR in both CD4+ and CD8+ T cells is required for a full suppression of an allospecific CTL response by TCDD (32), indicating a direct role for AhR in these TCDD-induced immunosuppressive effects. More recently, they identified a group of T-regulatory cells (CD4+CD25+) after TCDD treatment (33). By adoptively transferring AhR−/− CD8+ cells into AhR+/+ mice, Lawrence and colleagues demonstrated that CD8+ T cells are not directly affected by TCDD. Rather TCDD appears to mediate its effects by altering hemopoiesis (34).
Listeria monocytogenes is a gram-positive, facultative intracellular bacterium that causes food-borne disease (listeriosis) that results in considerable morbidity and a relatively high mortality rate (35). It is estimated that L. monocytogenes causes approximately 2,500 cases of serious illness and as many as 500 deaths per year in the United States (36). Risk factors for listeriosis include age (>65 years), pregnancy, HIV infection, immunosuppressive therapy, diabetes, kidney disease, and cancer (37). L. monocytogenes is also widely studied as a model intracellular pathogen, which can multiply to great numbers within infected cells (i.e. hepatocytes and macrophages ) and pass directly from one cell to the next (38). Because activation of the AhR by TCDD or PAHs frequently causes immunosuppression, one might expect that AhR activation would increase susceptibility to infection with L. monocytogenes. However, prior studies have yielded inconsistent results (17–19, 39, 40). These disparate findings could reflect differences in the AhR agonists used or their routes of administration. There have been no reports on the constitutive role of the AhR in resistance to L. monocytogenes or other intracellular bacterial pathogens.
In this study, we compared the susceptibility of AhR−/− and AhR+/− mice to i.v. challenge with L. monocytogenes. Our data convincingly demonstrate that AhR−/− mice are more susceptible than AhR+/− mice to a primary L. monocytogenes infection. However, AhR−/− mice exhibit no defect in acquired resistance to re-infection, nor significant differences in T-cell mediated production of cytokines. These findings demonstrate a heretofore unrecognized role for the AhR in host defense against listeriosis in mice.
Materials and Methods
Materials
RPMI medium, L-glutamine, penicillin/streptomycin, Hanks Balanced Salt Solutions (HBSS) with or without calcium and magnesium, DMEM (high glucose) medium, and non-essential amino acids were purchased from Mediatech, Inc. (Herndon, VA); FBS was obtained from Atlanta Biologicals (Lawrenceville, GA); Ammonium chloride (NH4Cl), sodium azide, trypan blue working solution, EDTA, proteose peptone, and paraformaldehyde were obtained from Sigma (St Louis, MO); Bovine serum albumin (BSA) was from Calbiochem (San Diego, CA); Recombinant mouse macrophage colony stimulating factor (rm M-CSF) was obtained from R&D Systems (Minneapolis, Minnesota); 10 × PBS stock was from Fisher Bioreagents (Fair Lawn, NJ); Listerial peptides (LLO190, LLO253, LLO296) were synthesized by Biosynthesis Inc. (Lewisville, TX); Golgi plug, interleukin-2, anti-CD8-APC, anti-CD4-PE, anti-CD44-FITC, anti-IFN-γ-FITC, anti-TNF-PeCy7, cytofix/cytoperm solution, perm/wash buffer, and mouse inflammation kit were purchased from BD Biosciences (San Diego, CA)
Animal care and breeding
C57B1/6J-AhR-deficient (AhR−/−) mice, created by deletion of exon 2 of the AhR gene (3), were obtained from Dr. C. Bradfield (Madison, WI) and maintained as a breeding colony in the animal care facility at the University of Wisconsin School of Veterinary Medicine. The AhR−/− mice could not be bred efficiently by homozygous mating. Instead, AhR+/+, AhR+/−, and AhR−/− littermates were generated by breeding AhR+/− females with AhR−/− males or AhR+/− females with AhR+/− males. The offspring were genotyped by PCR. All mice were provided either regular chow or breeder chow (Harlan Teklad 8604 rodent diet, Madison, WI) and water ad libitum. All animal handling and experimental processes were performed in accordance with an IACUC approved protocol.
Infection of mice with L. monocytogenes (EGD)
Frozen overnight cultures of L. monocytogenes EGD (a serotype 1/2a strain) were diluted 1:50 in brain heart infusion broth (BHI) and grown to mid-logarithmic phase (4.5–7 h at 37 °C on a rotating platform). The bacterial cells were pelleted by centrifugation at 1500× g for 10 min, washed twice with sterile PBS, and diluted in PBS to the appropriate concentration for inoculation. The number of viable L. monocytogenes in the inoculum was confirmed by plating serial dilutions on blood agar. Seven to twelve week old mice were placed in a cage on a heating pad for ~2 min and then put in a plastic mouse restrainer. One of the lateral tail veins was chosen for i.v. injection of L. monocytogenes at the dose indicated in each experiment.
Bacterial burden in tissues
The listerial burden in each tissue was determined as described previously (41). Briefly, mice were euthanized with carbon dioxide overdose at the specified times. The abdominal cavity was aseptically opened and the gallbladder and portions of the spleen and liver were removed, weighed in sterile weigh boats, and placed in separate sterile tissue grinders containing 1ml cold PBS. The tissues were homogenized, serially diluted in sterile saline and plated onto blood agar. Similarly, the cecum and duodenum from each mouse were removed to separate tissue grinders, homogenized, serially diluted and plated on modified Oxoid agar plates. The plates were incubated at 37°C and the colonies were counted after 24–48 h. The plates with CFU counts in the range of 30–300 were selected.
Cytometric bead assay (CBA)
Serum cytokine levels were quantified using a CBA assay kit purchased from BD Biosciences, which is capable of detecting IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1), IFN-γ, TNF, and IL-12p70. The samples were read using a FACSCalibur flow cytometer at the University of Wisconsin-Madison Comprehensive Cancer Center. The results were then analyzed with CBA software from BD Biosciences.
Infection of peritoneal and bone marrow-derived macrophages
Peritoneal exudate macrophages were prepared as described previously (42). In brief, age-matched AhR+/− and AhR−/− mice were injected i.p. with 1mL of 10% sterile proteose peptone. At 72 hrs after injection, the mice were euthanized and peritoneal exudate cells (PECs) were recovered by flushing the peritoneal cavity with Hanks Balanced Salt Solution (Ca- and Mg-free) containing 0.01M EDTA. Cells were washed twice with HBSS containing Ca2+ and Mg2+ and re-suspended in 1ml of DMEM containing 10% FBS, glucose, sodium pyruvate, HEPES, and penicillin/streptomycin. Cells were quantified and seeded onto a 24-well plate at an initial concentration of 6.0 × 105 cells/well at 37°C in a 5% CO2 incubator. Non-adherent cells were removed 2 h later by washing 3 × with serum free DMEM (no antibiotics). Macrophage monolayers were infected the next day with L. monocytogenes as described below.
To obtain bone marrow derived macrophages, age-matched AhR+/− and AhR−/− mice were euthanized, their femurs were removed and flushed with 3ml of RPMI containing L-glutamine. Bone marrow cells were washed twice with DMEM and re-suspended in DMEM containing 10% FBS, glucose, sodium pyruvate, HEPES, and penicillin/streptomycin. Cells (5.0 × 106 cells/mL) were placed in a T-25 flask with 10ng/mL recombinant mouse macrophage colony stimulating factor (rm M-CSF) for 24 hrs at 37°C in a 5% CO2 incubator. Non-adherent progenitor cells were transferred to a new T-75 flask containing rm M-CSF and incubated for 4 days, at which point the medium with floating cells were removed and replaced with fresh media with rm M-CSF (no antibiotics). The cells were incubated at 37°C with 5% CO2 for 3 days, and confirmed to be macrophages by esterase staining (>99% positive). Macrophages were harvested and 1.0 × 105 cells were seeded into each well of a 24-well plate. The following day, the cells were infected as described below.
To infect peritoneal and bone marrow-derived macrophages, cell monolayers were incubated with 1 × 106 log phase L. monocytogenes for 2 hrs at 37°C. The uningested listerial cells were removed by washing 3 × with DMEM, and fresh culture media with 5µg/mL gentamicin was added to kill extracellular L. monocytogenes. After a further 6 h incubation at 37°C, the cells were washed 3 × with DMEM to remove gentamicin and lysed with sterile ddH2O. The lysates were diluted in PBS and plated on blood agar. The plates were incubated for 24–48 hrs at 37°C. The colonies were counted to determine CFU of L. monocytogenes.
Surface staining of CD44
To monitor the activation and expansion of CD4+ and CD8+ T cells following infection with L. monocytogenes, freshly isolated splenocytes were stained for 1 hr on ice in the dark with combinations of monoclonal antibodies purchased from BD Pharmingen (San Diego, CA): PE-labeled anti-CD4, APC-labeled anti-CD8, and FITC-labeled anti-CD44 (43). For all samples, data were collected from more than 200,000 events using a BD FACSCalibur flow cytometer and then analyzed using Flowjo software by gating on viable splenocytes.
Quantitation of L. monocytogenes-specific T cells by Intracellular Cytokine Staining
Intracellular cytokine staining to detect antigen-specific CD8 or CD4 T cells was done as previously described (44). Briefly, single cell suspensions of erythrocyte-depleted splenocytes were cultured with MHC I- or MHC II-restricted listerial epitope peptides (LLO296 or LLO190 and LLO253, respectively) for 5 hr in the presence of brefeldin A (1 μL/mL) and IL-2 (50 U/mL). After culture, cells were stained for cell surface CD8 or CD4, and intracellular IFN-γ & TNF-α, using the cytofix/cytoperm kit (BD-Pharmingen). The number of IFN-γ- and TNF-α-producing CD4 or CD8 T cells was analyzed by flow cytometry.
Histopathology
Portions of the liver were removed and fixed in 10% formalin. The fixed samples were thin sectioned and stained with H&E or a tissue gram stain at the histopathology laboratory in the University of Wisconsin School of Veterinary Medicine. Photomicrographs of representative sections were taken with an Olympus microscope equipped with a built-in digital camera.
Statistical methods
All statistical analyses were performed using Prism 4.0 (GraphPad Software, San Diego, CA). Significant differences were determined by a one-way analysis of variance (ANOVA) followed by the Tukey post-test or student t-test. Differences were considered statistically significant when P was < 0.05.
Results
Wild type AhR+/+ and heterozygous AhR+/− mice exhibit similar resistance to listeriosis
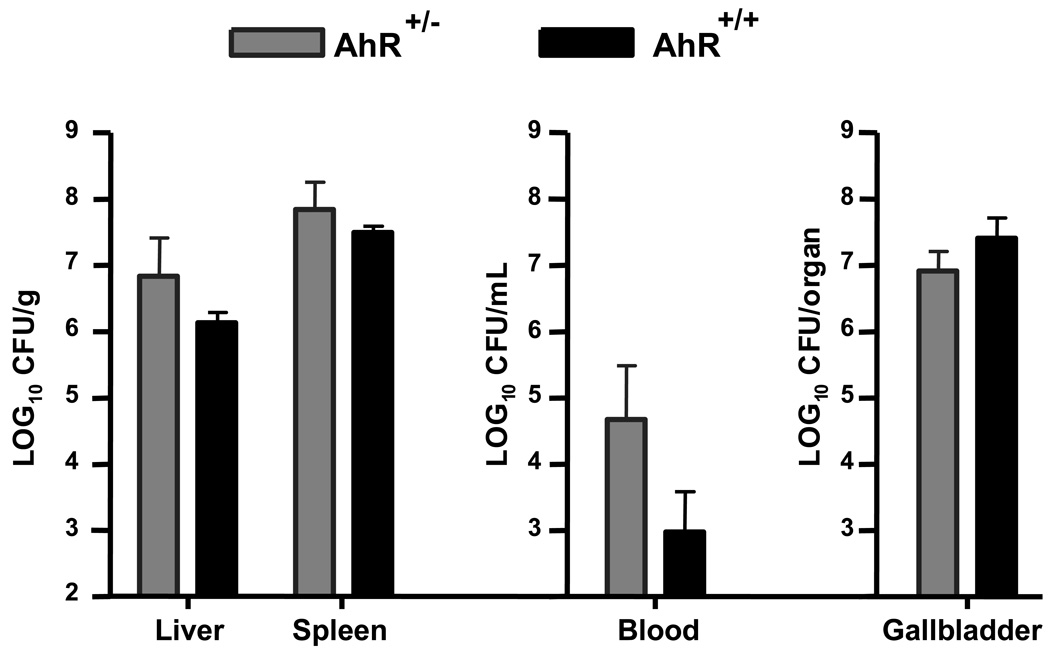
We first determined whether wild type AhR+/+ and heterozygous AhR+/− mice differed in their resistance to listeriosis. AhR+/+ and AhR+/− littermates (5 mice in each group) were injected i.v. with 6.7 × 104 CFU L. monocytogenes via a lateral tail vein. At the peak of the infection (3 days following inoculation), mice were euthanized and tissue burdens of L. monocytogenes in liver, spleen, blood, and gallbladder were determined as described in the Materials and Methods. As shown in Fig. 1, there was no significant difference in the listerial burdens between AhR+/+ and AhR+/− mice. As a result, and because of the lack of AhR+/+ mice resulting from our mating system (AhR+/− × AhR−/−), we used AhR+/− mice as controls in all subsequent experiments.
Figure 1.
There is no difference in susceptibility to listeriosis between AhR+/−( ) and AhR+/+ (▬) mice. Mice were infected by i.v. injection of 6.7 × 104 CFU L. monocytogenes. Three days later, mice were euthanized and the CFU of L. monocytogenes was determined in the liver (per gram), spleen (per gram), blood (per mL), and gallbladder (whole organ). Data represent the mean ± SEM (n=5 mice per group).
) and AhR+/+ (▬) mice. Mice were infected by i.v. injection of 6.7 × 104 CFU L. monocytogenes. Three days later, mice were euthanized and the CFU of L. monocytogenes was determined in the liver (per gram), spleen (per gram), blood (per mL), and gallbladder (whole organ). Data represent the mean ± SEM (n=5 mice per group).
AhR−/− mice are more susceptible than AhR+/− mice to listeriosis
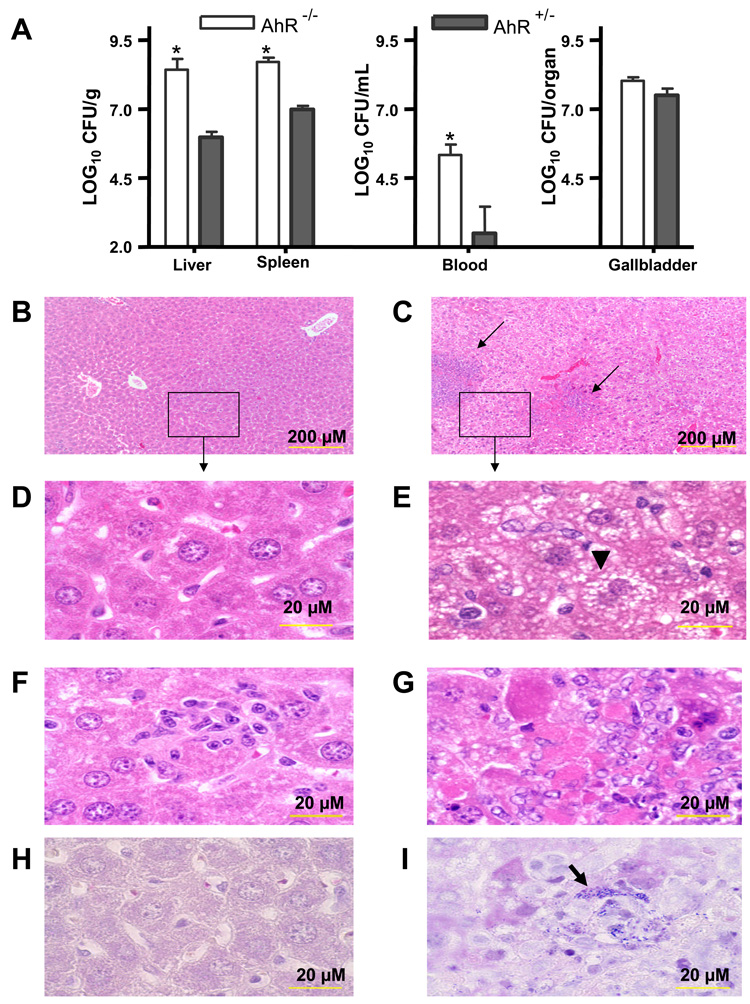
We next compared the susceptibility of AhR−/− and AhR+/− mice to listeriosis. Mice (5 to 8 mice per group) were injected i.v. with 3.0 × 105 CFU L. monocytogenes via a tail vein. Three days later, mice were euthanized and their liver, spleen, blood, and gallbladder were processed and plated on blood agar. Our results show that AhR−/− mice are significantly less resistant to a primary listerial infection than AhR+/− mice (Fig. 2A). Portions of the livers from infected mice were also subjected to histopathology. There were few histopathological changes in the livers (Fig. 2B) or hepatocytes (Fig. 2D) from L. monocytogenes-infected AhR+/− mice. Granulomas were rarely observed and, when present (Fig. 2F), were smaller than in AhR−/− mice (Fig. 2G). Nor were gram-positive rods commonly detected in hepatocytes from AhR+/− mice (Fig. 2H). In contrast, the livers from L. monocytogenes-infected AhR−/− mice exhibited large area of necrosis and granulomas (Fig. 2C and 2G). Their hepatocytes displayed a foamy appearance (Fig. 2E), and gram-positive rods were readily seen within hepatocytes (Fig. 2I).
Figure 2.
AhR−/− (▭) mice are more susceptible than AhR+/− ( ) mice to a primary L. monocytogenes infection. Mice were inoculated i.v. with 3.0 × 105 CFU L. monocytogenes and euthanized 3 days later. (A) The CFU of L. monocytogenes was determined in the liver (per gram), spleen (per gram), blood (per mL), and gallbladder (whole organ). Data represent the mean ± SEM (n=5 to 8 mice per group). *: p<0.05 in comparison to Ah+/− mice. Panels B - I show representative histopathological changes for AhR+/− (B,D,F) and AhR−/− mice (C,E,G) (H&E staining). (E) Note the large mass of necrosis (arrows in C) and the foamy appearance of hepatocytes from AhR−/− mice (black arrowhead). Gram staining (H & I) revealed gram positive rods in hepatocytes of AhR−/− (H) but not AhR+/− mice (I) (black arrow).
) mice to a primary L. monocytogenes infection. Mice were inoculated i.v. with 3.0 × 105 CFU L. monocytogenes and euthanized 3 days later. (A) The CFU of L. monocytogenes was determined in the liver (per gram), spleen (per gram), blood (per mL), and gallbladder (whole organ). Data represent the mean ± SEM (n=5 to 8 mice per group). *: p<0.05 in comparison to Ah+/− mice. Panels B - I show representative histopathological changes for AhR+/− (B,D,F) and AhR−/− mice (C,E,G) (H&E staining). (E) Note the large mass of necrosis (arrows in C) and the foamy appearance of hepatocytes from AhR−/− mice (black arrowhead). Gram staining (H & I) revealed gram positive rods in hepatocytes of AhR−/− (H) but not AhR+/− mice (I) (black arrow).
A similar difference between AhR−/− and AhR+/− mice was observed when they were inoculated with a lower challenge dose of log-phase L. monocytogenes (7.8 × 102 CFU, i.v.). Here too significantly more CFU of L. monocytogenes were recovered from the spleens of AhR−/− than AhR+/− mice at 3 days post-inoculation (data not shown). Furthermore, the effect of AhR absence on resistance to L. monocytogenes infection was seen in both male and female mice (data not shown).
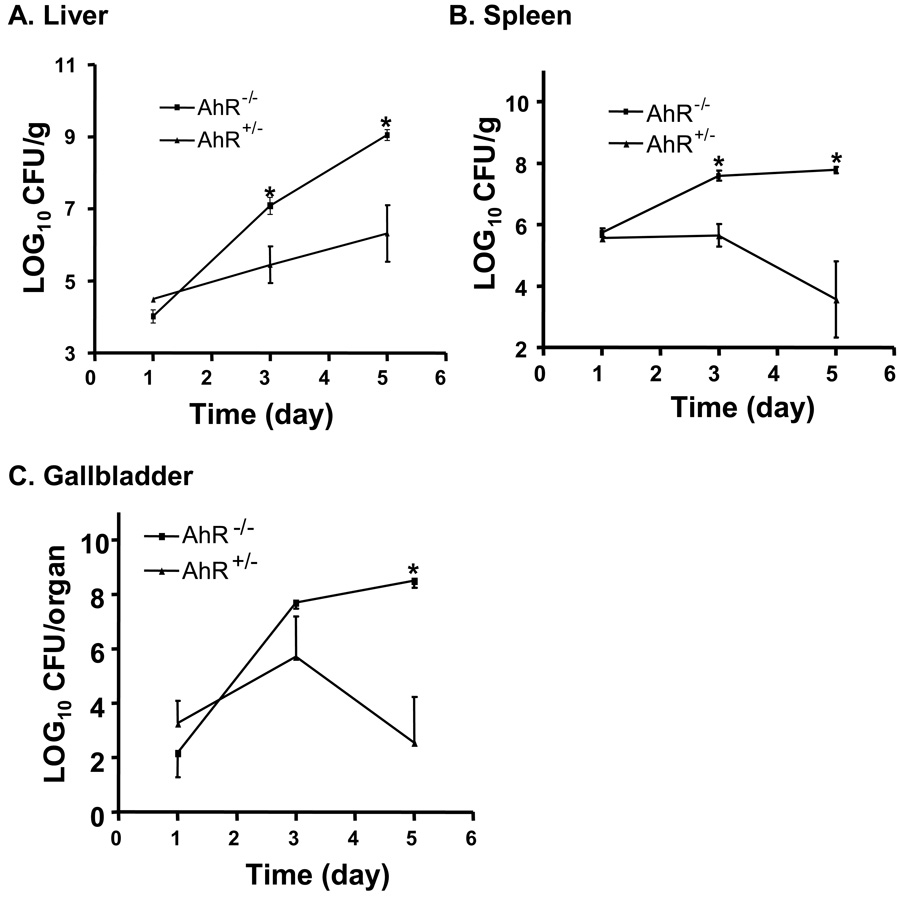
To further examine the role of the AhR in resistance to L. monocytogenes infection, a time-course experiment was performed in which AhR+/− and AhR−/− mice were inoculated i.v. with 7.8 × 103 CFU and euthanized at 1, 3, and 5 days after injection (5 mice per group at each time point). As shown in Fig. 3, there was no significant difference between AhR+/− and AhR−/− mice on day 1. However, there were significantly greater CFU recovered from the spleens and livers of AhR−/− than AhR+/− mice at 3 and 5 days after inoculation. Greater numbers of CFU were also recovered from the gallbladders of AhR−/− mice on day 5.
Figure 3.
Time-course of L monocytogenes infection in AhR−/− (▭) and AhR+/− ( ) mice. Mice were infected i.v. with 7.8 × 103 CFU L. monocytogenes. On days 1, 3, and 5, five mice of each genotype (AhR−/− or AhR+/−) were euthanized and the CFU of L. monocytogenes in the liver (A), spleen (B), and gallbladder (C) were determined. Data represent the mean ± SEM of 5 mice per group. *: p<0.05 as compared to AhR+/− mice at the same time point.
) mice. Mice were infected i.v. with 7.8 × 103 CFU L. monocytogenes. On days 1, 3, and 5, five mice of each genotype (AhR−/− or AhR+/−) were euthanized and the CFU of L. monocytogenes in the liver (A), spleen (B), and gallbladder (C) were determined. Data represent the mean ± SEM of 5 mice per group. *: p<0.05 as compared to AhR+/− mice at the same time point.
AhR−/− mice exhibit a delayed ability to clear a primary L. monocytogenes infection
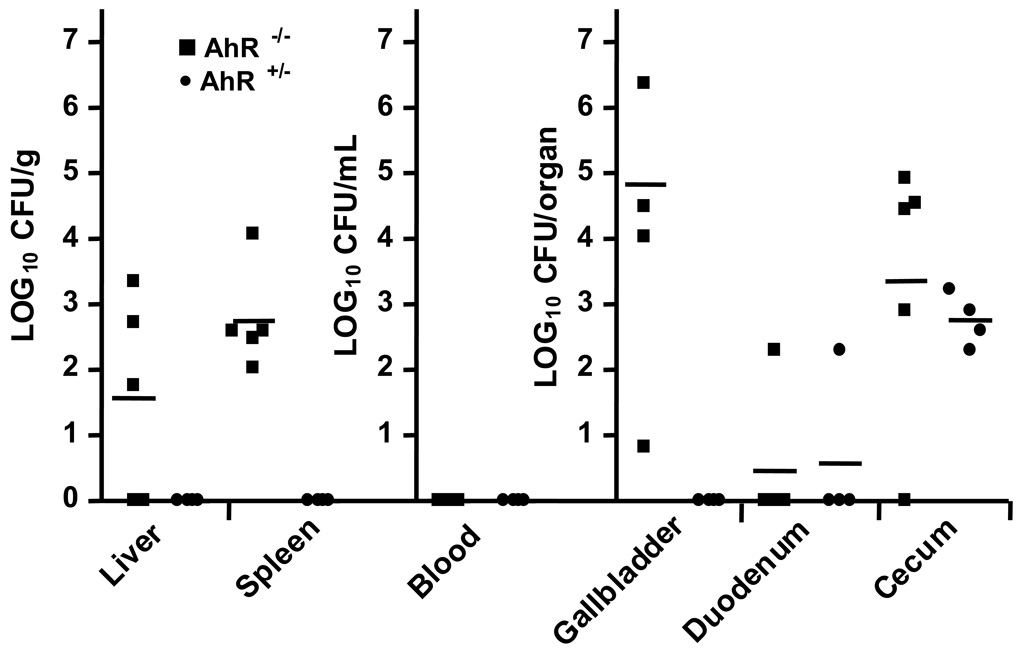
The preceding data showed that AhR−/− mice harbored greater bacterial burden than AhR+/− mice at the peak time of the infection. We next asked whether they were also impaired in their ability to clear the infection. To do so, AhR−/− and AhR+/− mice (Fig. 4) were injected i.v. with low dose of L. monocytogenes (8.2 × 102 CFU/mouse). All mice (both AhR−/− and AhR+/−) survived the infection and did not demonstrate outward signs of clinical illness. On days 7 and 14 post-inoculation, mice were euthanized and portions of their livers, spleens, blood, and whole gallbladders were processed to estimate CFU of L. monocytogenes. The duodenum and cecum from each mouse were also removed to quantify the colonization of L. monocytogenes in the gastrointestinal tract. AhR−/− mice displayed a delayed ability to clear a primary listerial infection, as evidenced by significantly greater numbers of L. monocytogenes in the liver, gallbladder, and spleen on day 7. By day 14, all mice (both AhR−/− and AhR+/−) had cleared L. monocytogenes from their tissues (data not shown). At necropsy we noted that some L. monocytogenes-infected AhR−/− mice had gallbladders that were firm and white in gross appearance at 14 days after inoculation. This observation occurred in the absence of recovery of viable L. monocytogenes from these organs, suggesting that the gross changes were the result of prolonged inflammation. Overall, these data indicate that AhR−/− mice are delayed, but not unable, to clear a primary listerial infection within a 14 day period.
Figure 4.
AhR−/− mice exhibited delayed clearance of L. monocytogenes. AhR−/− (■) and AhR+/− (●) mice were injected i.v. with 8.2 × 102 CFU L. monocytogenes. On day 7 post-inoculation, groups of 4 to 6 mice were euthanized and the numbers of viable L. monocytogenes in the indicated tissues were determined. Symbols represent log10 CFU/g for the liver and spleen, log10 CFU/mL for blood, and log10CFU/whole organ for the gallbladder, duodenum, and cecum in individual mice. The horizontal bars indicate the mean values for groups of mice. P < 0.05 for AhR−/− as compared to AhR+/− mice in the liver, spleen, and gallbladder. On day 14, L. monocytogenes was not recovered from any tissue of any mice (data not shown).
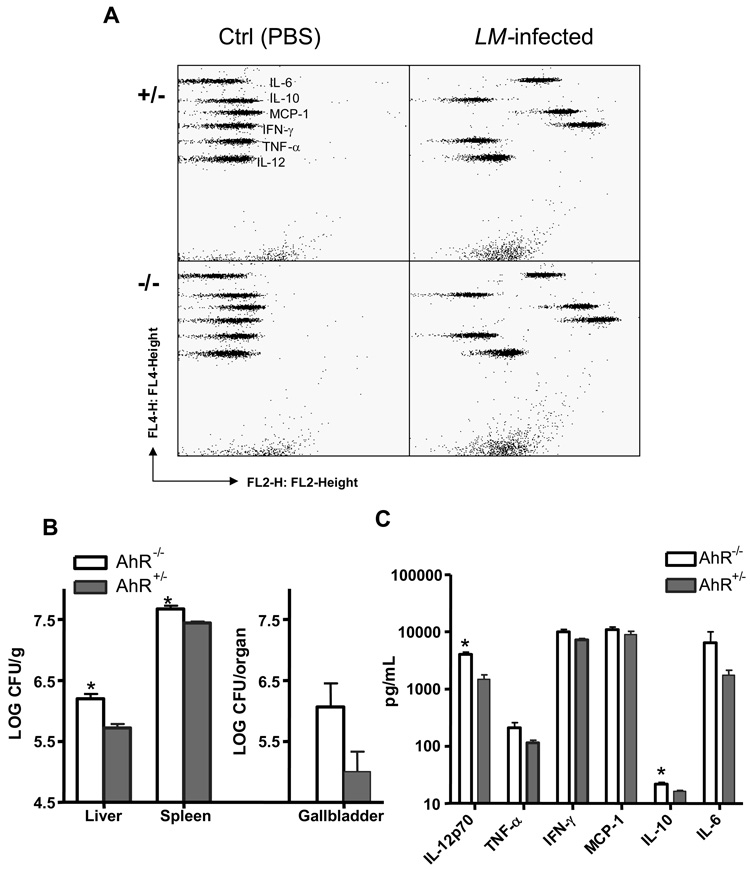
Cytokine production is not impaired in AhR−/− mice
We then examined whether AhR−/− mice were impaired in their ability to produce several inflammatory cytokines in response to L. monocytogenes infection. To elicit a vigorous inflammatory response, mice were injected i.v. with 4.0 × 105 CFU L. monocytogenes (high challenge dose) and euthanized at 24 hrs post-inoculation (early phase of infection). The numbers of viable L. monocytogenes in the liver, spleen, and gallbladder were determined. Serum was prepared from whole blood obtained by cardiac puncture at the time of euthanasia. Cytokine levels (IL-6, IL-10, MCP-1, IFN-γ, TNF-α, and IL-12) in the sera were determined using the CBA mouse inflammation kit from BD Biosciences. Fig. 5A illustrates representative flow diagrams of the CBA assay for control (injected with sterile PBS) and L. monocytogenes-infected mice. Fig. 5B confirms that greater numbers of L. monocytogenes were recovered from AhR−/− than AhR+/− mice in this experiment. Fig. 5C demonstrates that comparable levels of inflammatory cytokines were present in the sera from AhR+/− and AhR−/− mice. The exceptions were IL-12 and IL-10, for which significantly greater amounts were detected in the sera of AhR−/− than AhR+/− mice. Perhaps this observation reflects the more severe infection in AhR−/− than AhR+/− mice.
Figure 5.
Production of cytokines is not impaired in AhR−/− mice. AhR−/− and AhR+/− mice were injected i.v. with 4.0 × 105 CFU L. monocytogenes. Control animals were injected i.v. with sterile PBS. Mice were euthanized 24 hrs later. Panel A shows the representative dot plots of distinct cytometric beads used to specifically detect IL-12, TNF-α, IFN-γ, MCP-1, IL-10, or IL-6. Panel B depicts the CFU of L. monocytogenes recovered from the liver (per gram), spleen (per gram) and gallbladder (per whole organ). Panel C illustrates serum cytokine levels as measured by the CBA assay. Data represent the mean ± SEM of 4 mice per group. *: p<0.05 as compared to the AhR+/− mice.
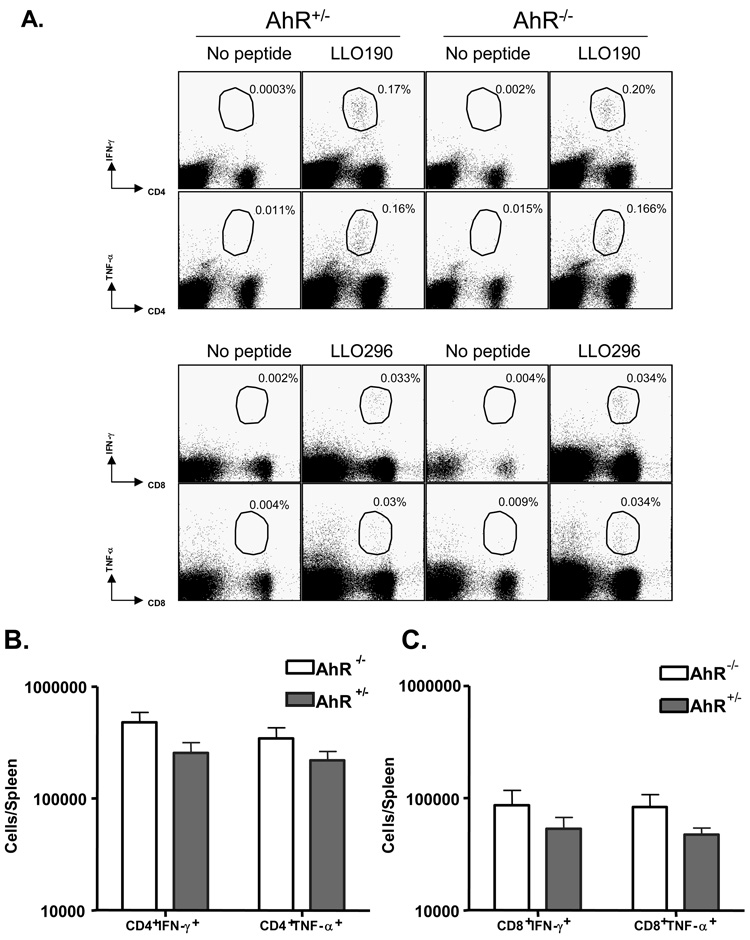
AhR−/− mice generate normal T-cell mediated responses to a primary L. monocytogenes infection
We next compared the ability of AhR−/− and AhR+/− mice to mount T cell responses to L. monocytogenes infection. On day 7 post-inoculation, we quantified the numbers of L. monocytogenes-specific CD8+ and CD4+ T cells in the spleen by intracellular staining for IFN-γ and TNF-α following incubation with LLO peptides ex vivo. As shown in Fig. 6, the frequencies and total numbers of LLO190-specific CD4 T cells (A and B) or LLO-296 specific CD8 T cells (A and C) were comparable between AhR+/− and AhR−/− mice. Likewise, data obtained on day 14 after inoculation showed no significant differences in CD4+IFN-γ+ and CD4+TNF-α+ cells, nor CD8+IFN-γ+ and CD8+TNF-α+ cells, between the AhR+/− and AhR−/− mice (data not shown).
Figure 6.
Activation of CD4+ & CD8+ T cells is similar in AhR−/− and AhR+/− mice on day 7 post-inoculation. The numbers of L. monocytogenes-specific CD4+ and CD8+ T cells were quantified by intracellular cytokine staining. Splenocytes were stimulated with the MHC II-restricted listerial epitope peptide LLO190 or MHC I-restricted epitope peptide LLO-296 and the numbers of IFN-γ- or TNF-producing CD4 or CD8 T cells respectively were determined by flow cytometry. Dot plots in panel A are gated on total splenocytes and the numbers are the % of IFN-γ- or TNF-producing CD8/CD4 T cells amongst splenocytes. Data in panel B & C are total numbers of L. monocytogenes-specific CD4+ and CD8+ T cells, respectively, from 4 to 5 mice/group.
AhR−/− mice develop enhanced resistance to reinfection with L. monocytogenes
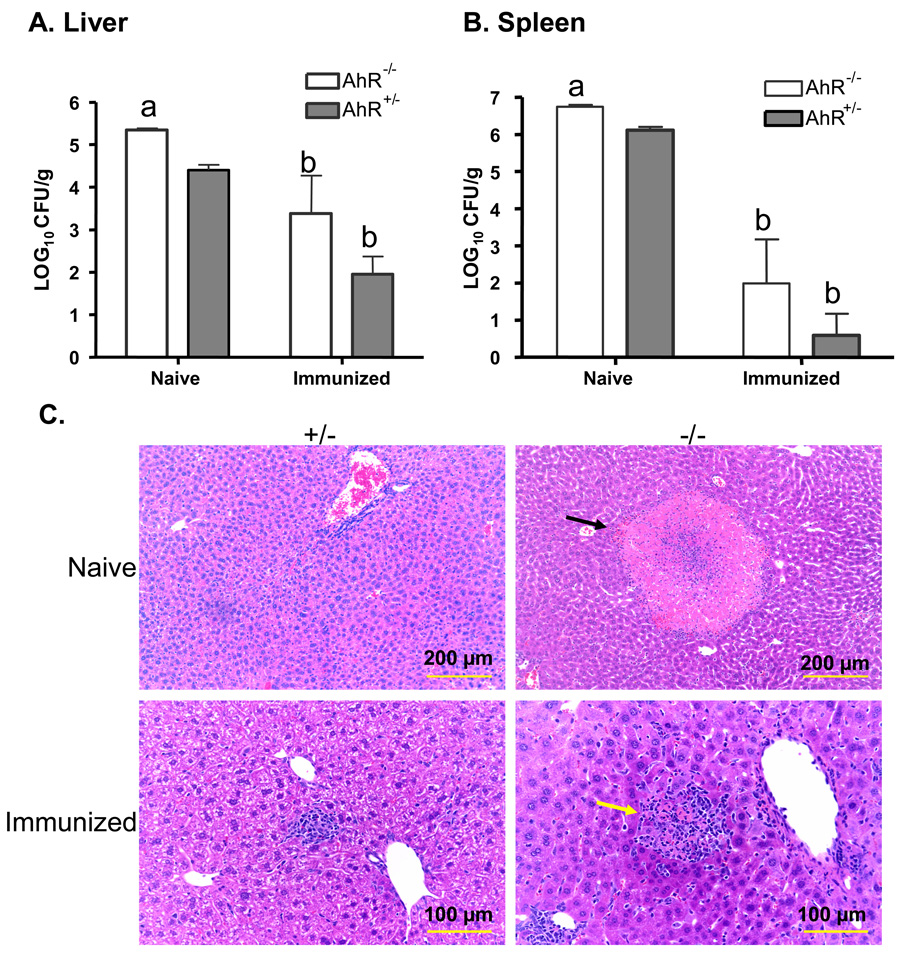
We then asked whether AhR−/− mice could develop enhanced resistance to reinfection following clearance of a primary L. monocytogenes infection. To do so, age-matched AhR+/− and AhR−/− mice were immunized by i.v. injection of a low dose of L. monocytogenes (8.5 × 102 CFU). Non-immunized AhR+/− and AhR−/− mice (injected with sterile PBS) were included as naïve control groups and housed in the same animal room with the immunized animals. Fifteen days later, immunized AhR+/− and AhR−/− mice were re-infected i.v. with 3.0 × 104 CFU L. monocytogenes/mouse. Non-immunized control AhR+/− and AhR−/− mice were injected i.v. with a lower inoculum of L. monocytogenes (3.0 × 103 CFU/mouse) to prevent death of these immunologically naïve mice. Three days later, all mice were euthanized and their tissues removed for bacteriological and histopathological evaluation, as described above. As expected, far fewer CFU were recovered from immunized than naïve mice. There was no significant difference between immunized AhR−/− and AhR+/− mice in the CFU of L. monocytogenes recovered from the liver or spleen (Fig. 7A and Fig. 7B). Likewise, the numbers of viable L. monocytogenes recovered from the blood or gallbladders were not significantly different between immunized AhR−/− and AhR+/− mice, (data not shown). Taken as a whole, these data show that AhR−/− mice are capable of developing an adaptive immune response that protects them against reinfection with L. monocytogenes.
Figure 7.
Immunized AhR−/− mice are protected against a second challenge with L. monocytogenes. Mice were injected i.v. with 8.5 × 102 CFU L. monocytogenes (immunized mice) or with PBS (naïve control mice). Fifteen days later, immunized and naïve mice were challenged i.v. with 3.0 × 104 and 3.0 × 103 CFU L. monocytogenes, respectively. The lower challenge dose for the naïve mice was chosen to reduce deaths in the AhR−/− mice. Three days later, all mice were euthanized and the CFU of L. monocytogenes in the liver (A), and spleen (B) were determined. Data represent the mean ± SEM of 4 mice per group. a: p<0.05 in comparison to AhR+/− mice, b: p<0.05 as compared to the corresponding phenotype of naïve mice. Groups sharing the same letter (a or b) are not significantly different from each other. Panel C shows representative histopathological changes in the livers of immunized and naïve mice. Necrotic foci (indicated by the black arrow) were greater in size and number in AhR−/− naïve mice. Larger inflammatory cell aggregates, surrounding necrotic debris (yellow arrow) were seen in immunized AhR−/− mice as compared to immunized AhR+/− mice.
Histopathological analyses of the livers from these mice revealed relatively normal liver structure, with occasional small foci of necrosis or inflammatory cell aggregates in L. monocytogenes-infected naïve AhR+/− mice (Fig. 7C). Larger areas of necrosis, and inflammatory foci that were surrounded by a mixture of inflammatory cells, were evident in L. monocytogenes-infected naïve AhR−/− mice. Consistent with the reduced bacterial burden illustrated in Fig. 6, the histopathological changes were less in the livers of immunized mice. Occasional inflammatory foci were found in the livers of immunized AhR+/− mice. Larger inflammatory foci, that sometimes contained necrotic debris, were observed in some immunized AhR−/− mice. Histopathological changes in the spleens were minor and did not differ substantially between AhR+/− and AhR−/− mice (data not shown).
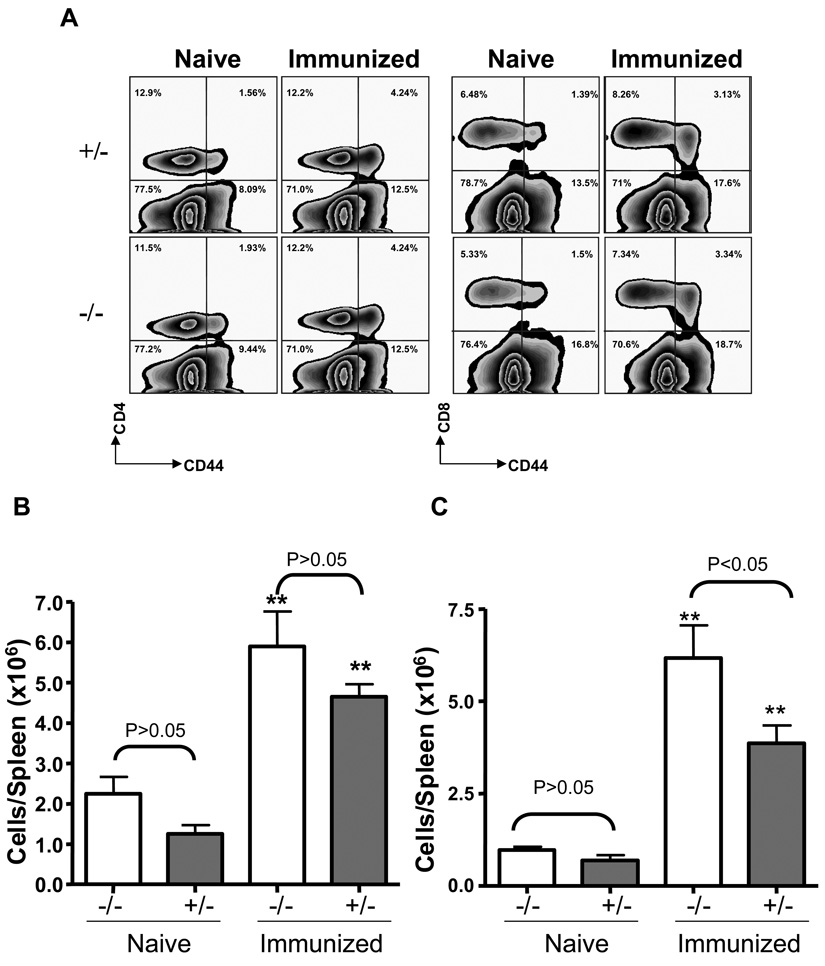
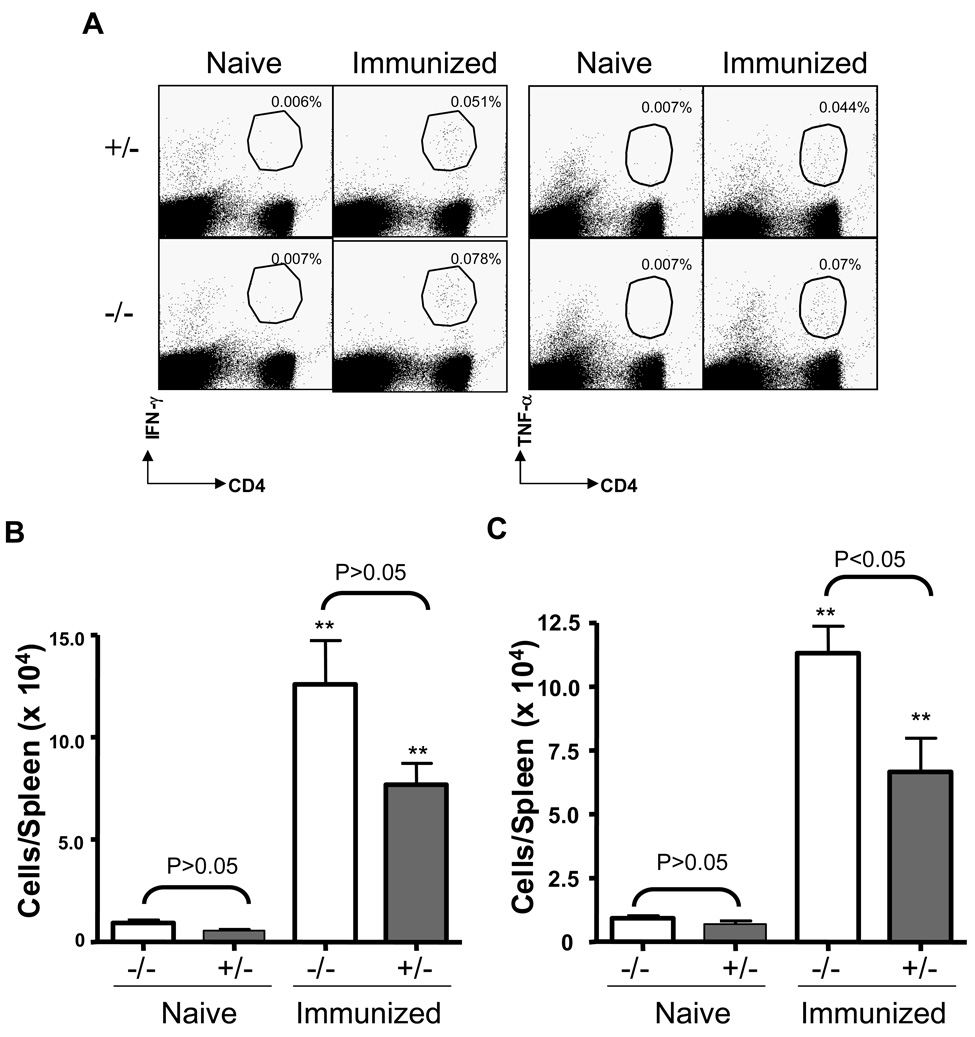
We also investigated the effects of AhR on T cell responses from the spleens of naïve and immunized mice. As indicated in Fig. 8, reinfection resulted in a 3–4 fold increase in the frequencies and total numbers of CD4+CD44hi (A and B) and CD8+CD44hi (A and C) spleen cells from immunized AhR−/− and AhR+/− mice, as compared to naïve mice of the same genotype. The numbers of CD4+CD44hi cells did not differ between immunized AhR+/− and AhR−/− mice. However, fewer CD8+CD44hi cells were seen in the immunized AhR+/− than AhR−/− mice.
Figure 8.
Comparable secondary T cell responses to reinfection with L. monocytogenes in AhR−/− and AhR+/− mice. Immunized or naïve mice were challenged with 3 × 104 and 3 × 103 CFU L. monocytogenes, respectively. Three days later, splenocytes from these mice were prepared and stained with CD4, CD8, and CD44 to quantitative activated memory T cells. Zebra plots (Flowjo) in panel A are gated on total splenocytes and the numbers are the % of CD44 CD8/CD4 T cells amongst splenocytes. Data in B & C are total numbers of L. monocytogenes-specific CD4+CD44hi & CD8+CD44hi T cells, respectively, from 4 to 5 mice per group. **: p<0.01 as compared to the corresponding genotype of naïve mice.
We also evaluated intracellular cytokine expression by spleen cells following ex vivo stimulation with the immunodominant CD4+ T cell-specific peptide LLO190. Similar to the results above, there were significantly increased numbers of LLO190-specific IFN-γ-producing (Fig. 9B) and TNF-α producing (Fig. 9C) CD4 T cells in the spleens of immunized AhR−/− and AhR+/− mice, as compared with naïve mice of the same genotype. We also recovered significantly more L. monocytogenes-specific TNF-α-producing CD4 T cells from the spleens of immunized AhR−/− than AhR+/− mice (Fig. 9C).
Figure 9.
Secondary antigen-specific T cell responses in AhR−/− and AhR+/− mice. The numbers of L. monocytogenes-specific CD4 T cells in the spleens of immunized and naïve mice were quantified by intracellular cytokine staining for IFN-γ or TNF-α. Splenocytes were stimulated with the MHC II-restricted listerial epitope peptide LLO190 and the numbers of IFN-γ- or TNF-producing CD4 T cells respectively were determined by flow cytometry. Dot plots in A are gated on total splenocytes and the numbers are the % of IFN-γ- or TNF-producing CD4+ T cells amongst splenocytes. Data in B & C are total numbers of L. monocytogenes-specific IFN-γ+ (B) and TNF-α+ (C) CD4+ T cells, respectively, from 4 to 5 mice per group. **: p<0.01 as compared to the corresponding genotype of naïve mice.
AhR−/− and AhR+/− peritoneal and bone marrow-derived macrophages do not differ in their ability to ingest and restrict the intracellular growth of L. monocytogenes
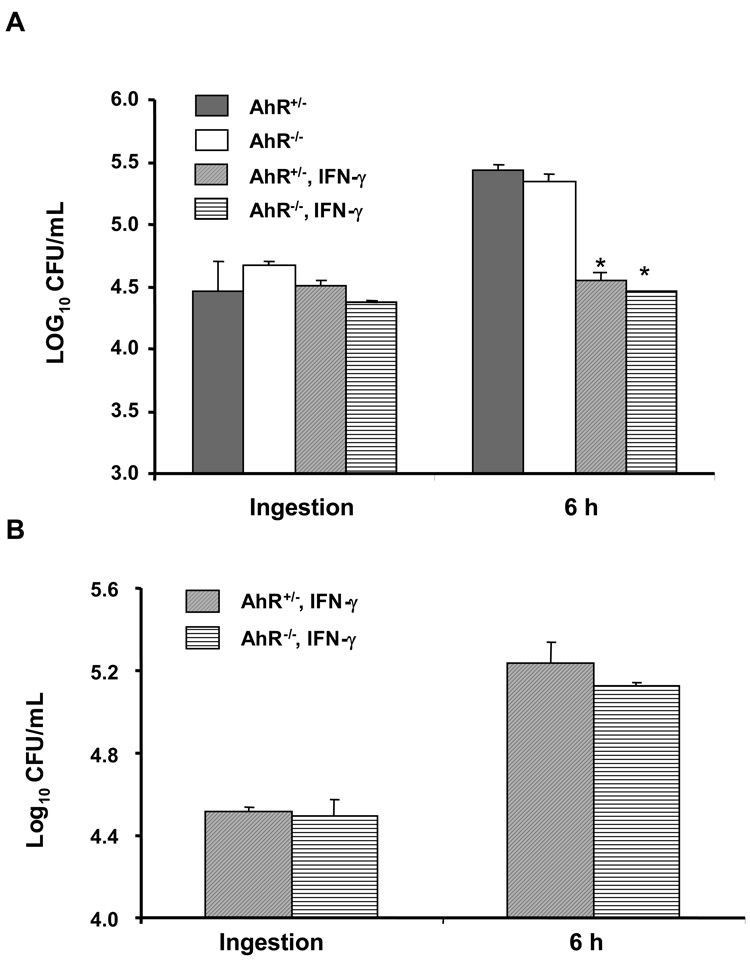
Mononuclear phagocytes are important in controlling the early stage of L. monocytogenes infection. We compared the ability of AhR+/− and AhR−/− macrophages to ingest and inhibit intracellular listerial growth. Fig. 10A shows that ingestion and intracellular growth of L. monocytogenes were similar for peritoneal exudate macrophages from AhR+/− and AhR−/− mice. Pretreatment with 100 U/mL IFN-γ (24 h) augmented the ability of macrophages of both genotypes to control the intracellular growth of L. monocytogenes; AhR+/− and AhR−/− macrophages did not differ in this regard. We performed similar experiments with bone marrow-derived macrophages. As depicted in Fig. 10B, AhR+/− and AhR−/− bone marrow-derived macrophages activated by pretreatment with IFN-γ did not differ in their ability to ingest or restrict the intracellular growth of L. monocytogenes. Thus, we observed no significant differences between AhR+/− and AhR−/− macrophages with regard to their ability to inhibit intracellular L. monocytogenes.
Figure 10.
AhR−/− and AhR+/− peritoneal and bone marrow-derived macrophages do not differ in their ability to ingest and restrict the intracellular growth of L. monocytogenes. A. Peritoneal macrophages were infected with 106 log-phase L. monocytogenes for 2 h (ingestion) and then further incubated for 6 h in the presence of 5 µg/mL of gentamicin (6 h). Macrophages were washed, lysed, and the lysates plated on blood agar to quantify the CFU of L. monocytogenes. Some macrophages were pretreated with IFN-γ (100 U/mL) for 24 h before addition of L. monocytogenes. Results are the mean ± SEM of a representative experiment from four experiments that were performed. *: p < 0.05, in comparison to macrophages without IFN-γ treatment. In panel B, bone marrow-derived macrophages were pretreated with IFN-γ (100 U/mL, 24 h) and then similarly infected with L. monocytogenes as described in panel A. Results are the mean ± SEM of one representative experiment.
Discussion
This study presents the first evidence that AhR−/− mice are more susceptible to L. monocytogenes infection than wild type or heterozygous AhR+/− mice, as quantified by the recovery of viable L. monocytogenes from the spleen, liver and other tissues (Fig. 2–5, 7). Although our experiments were designed to evaluate bacterial burden in internal organs, rather than survival, in preliminary experiments we observed that AhR−/− mice did not survive L. monocytogenes infection when the inoculation dose was ≥104 CFU (i.v.) (data not shown). This is a challenge dose at which deaths would not ordinarily occur in AhR+/+ or AhR+/− mice. Moreover, AhR−/− mice exhibited a delayed ability to clear a sublethal infection with L. monocytogenes (Fig. 3 & 4). Besides harboring a greater burden of L. monocytogenes in their tissues, AhR−/− mice also exhibited more extensive histopathological damage in their livers. Granulomas were greater in number and size (Fig. 2G) and necrotic foci were more evident in AhR−/− mice (Fig. 2C and Fig. 7C). Hepatocytes in AhR−/− mice exhibited a foamy appearance (Fig. 2E) and gram-positive L. monocytogenes were readily seen (Fig. 2I). In contrast, relatively few histopathological changes were seen in the livers of L. monocytogenes-infected AhR+/− mice.
The AhR has been shown to play important roles in regulating the expression of several cytokines. For example, exposure of rats to TCDD led to up-regulation of IL-1β and TNF-α in the liver (12). Interestingly, although TCDD suppressed the production of IFN-γ by mediastinal lymph node (MLN) cells, there was a 10-fold increase in the IFN-γ level in the lungs of TCDD-treated mice (16). A general suppression of IL-12 was seen in both MLN cells and the lung in the same study (16). IL-6 (13), CCL1 (CC-chemokine ligand 1) (14) and IL-8 (15) have also been identified as altered by the AhR signaling pathway. Several cytokines interacting with the AhR signaling pathway (12, 16, 45) (e.g., IL-1, TNF-α, IFN-γ, IL-12) are critically important for innate immunity to listeriosis (46).
Resistance to experimental listeriosis has been shown to be dependent on production of several inflammatory cytokines (i.e. IL-12, TNFα, and IFN-γ). Therefore, we asked whether differential production of inflammatory cytokines by AhR+/− and AhR−/− mice in response to listerial infection was associated with the increased susceptibility of AhR−/− mice to listeriosis. Using a cytometry bead assay, we detected no significant difference in serum levels of TNFα, IFN-γ, MCP-1, and IL-6 between AhR+/− and AhR−/− mice at 24 hrs after L. monocytogenes inoculation. Somewhat to our surprise, serum levels of IL-12 and IL-10 were significantly higher in the AhR−/− mice. When interpreting these data, it should be noted that these mice received a higher challenge dose (4.0 × 105 CFU per mouse i.v.) than in the earlier experiments, to elicit a robust cytokine response at 24 hr after inoculation. As a result significantly greater numbers of CFU were recovered from the spleens and livers of AhR−/− than AhR+/− mice at 24 hrs post-inoculation. Therefore, the increased production of IL-12 and IL-10 in AhR−/− mice could simply reflect the greater bacterial burden in these mice. IL-12 is critical for the expression of a protective Th1 immune response during L. monocytogenes infection (47). On the other hand, IL-10 is generally considered to be anti-inflammatory and an inhibitor of Th1 cellular immunity, as reflected in IL-10−/− mice being more resistant to listeriosis (48, 49). Female mice also produce more IL-10, which correlates with their greater susceptibility to listeriosis than male mice (50). We were surprised to find no difference in serum levels of IFN-γ and TNF-α between L. monocytogenes-infected AhR+/− and AhR−/− mice. These cytokines are essential to controlling listeriosis, especially in the early phase of infection (51). Consistent with these data, we also did not observe any significant difference in serum TNF-α levels between AhR+/− and AhR−/− mice at 3 days post-inoculation as determined by ELISA (data not shown). Overall, these data indicate that production of inflammatory cytokines is not impaired in AhR−/− mice when infected with L. monocytogenes.
The greater susceptibility of AhR−/− mice to listeriosis, despite comparable cytokine responses and numbers of activated T cells, suggests diminished innate immunity. Because we observed no defect in production of inflammatory cytokines in AhR−/− mice, we speculated that there might be quantitative and/or qualitative differences of the cellular components of innate immunity to listeriosis (for instances, neutrophils, macrophages, and NK cells) between the AhR−/− and AhR+/− mice. However, using peritoneal exudate macrophages and bone marrow-derived macrophages, we observed no differences between AhR+/− and AhR−/− macrophages in their ingestion or antimicrobial activity against L. monocytogenes. Nor did we see quantitative difference in the numbers of peritoneal exudate cells, or relative percentages of macrophages, recovered from AhR+/− and AhR−/− mice (data not shown). Transmission electron microscropy (TEM) examination revealed comparable fusion of lysosomes and phagosomes, and cytoplasmic escape of intracellular listerial cells in AhR+/− and AhR−/− macrophages (data not shown). Interestingly, the number of bone marrow cells recovered from AhR−/− mice was only about 50% that obtained from age-matched AhR+/− mice (our observations), which might reflect in part the fact that AhR−/− mice are smaller than age-matched AhR+/− mice. Although our experiments provide no direct evidence for the importance of macrophages in the difference in resistance of AhR+/− and AhR−/− mice, we cannot exclude the possibility that interactions of macrophages with other cells or mediators in vivo contributes in some way to the reduced resistance of AhR−/− mice.
Complete elimination of L. monocytogenes is dependent on an adaptive immune response that involves CD8+ and CD4+ T cells (52). Using intracellular cytokine staining (ICCS), we found equal or greater numbers of IFN-γ- and TNF-α-producing L. monocytogenes-specific CD4 and CD8 cells in AhR−/− mice as compared to AhR+/− mice at 7 and 14 days after the primary infection. These data indicate that AhR−/− mice develop a normal and protective T cell response to L. monocytogenes infection. AhR−/− mice also exhibited a normal contraction of antigen specific CD4 and CD8 cells between 7 and 14 days after inoculation. These findings are consistent with the clearance of L. monocytogenes from AhR−/− mice by 14 days after inoculation. In agreement with these observations, it was reported that AhR−/− mice mount normal humoral and cellular immune responses to injection of SRBC and P815 tumor cells, as compared to AhR+/+ mice (53).
When AhR−/−and AhR+/− mice were immunized with a sublethal challenge does of L. monocytogenes, they both exhibited acquired resistance to reinfection with L. monocytogenes. As expected, there were significant increases in CD4+CD44hi and CD8+CD44hi T cells in the immunized mice as compared to naïve mice of both genotypes. There was no significant difference in the frequency of CD44hi T cells in AhR−/− and AhR+/− mice, thus indicating that AhR is not required for the expansion of memory T cells following L. monocytogenes infection. On the contrary, there were more CD8+CD44hi cells in the AhR−/− than AhR+/− mice. This could indicate that more memory CD8 cells were produced in response to the greater listerial burden in AhR−/− mice during primary infection with L. monocytogenes. An alternative possibility is that increased production of IL-10 in L. monocytogenes-infected AhR−/− mice results in a stronger clonal expansion of CD8+ T cells, since IL-10 is required for optimal CD8+ T cell memory following L. monocytogenes infection (54). There were significantly more IFN-γ and TNF-α producing L. monocytogenes-specific CD4 T cells in immunized than non-immunized AhR−/− and AhR+/− mice. However, there was no significant difference in numbers of L. monocytogenes-specific CD8+IFN-γ+ and CD8+TNF-α+ cells between immunized and AhR−/− and AhR+/− mice in (data not shown). This observation is consistent with adoptive transfer studies using L. monocytogenes-specific CD4+ and CD8+ T cells, which showed that CD4+ T-cell-mediated protective immunity is IFN-γ-dependent, whereas CD8+ T cells can mediate IFN-γ- independent protection (55, 56). Overall, these data indicate no deficiency in the T cell response of AhR−/− mice, suggesting that the AhR is not required for the development of acquired cellular immunity to L. monocytogenes infection.
Although the immunosuppressive effect of TCDD via the AhR signaling pathway is well recognized, we demonstrate here for the first time that constitutive AhR expression is required for optimal resistance to L. monocytogenes infection in mice. A similar beneficial role for the AhR in resistance to pneumoniae infection in mice was recently reported (57). In that study, the authors found that activation of AhR by TCDD protected mice from an otherwise lethal challenge with that extracellular bacterial pathogen. It is curious that, as in the present study, the mechanism responsible for the role of the AhR in resistance to S. pneumoniae infection was not obvious. In that study neither the inflammatory response nor the numbers of neutrophils that accumulated appeared to be responsible for the increased resistance to S. pneumoniae (57). Thus, the AhR appears to play a significant role in host defense against extracellular (S. pneumoniae) and intracellular (L. monocytogenes) bacterial infection by a mechanism as yet to be determined.
In summary, we present here the first evidence that AhR is constitutively required for optimal resistance to murine listeriosis. The AhR was not required for a normal inflammatory cytokine or adaptive T cell response to L. monocytogenes infection, although our data suggest that AhR−/− mice might differ somewhat in the magnitude of the protective adaptive immune response to L. monocytogenes infection. We infer from these results that the AhR may play a novel constitutive role in innate immunity to L. monocytogenes infection in mice, although the underlying mechanisms remain to be elucidated.
Acknowledgments
We also thank Drs. C. Bradfield and C. Jefcoate for their valuable suggestions and critical reading of this manuscript. We thank Lyndsey Berryman for her assistance in establishing the macrophage methods.
This work was supported by funds from the National Public Health Sciences (NIH R21AI059656), the USDA National Research Institute (2005-35201-15313), the National Alliance for Food Safety and Security (58-1935-4450), and the Walter and Martha Renk Endowed Laboratory for Food Safety.
Abbreviations
- AhR
aryl hydrocarbon receptor
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- ARNT
aryl hydrocarbon receptor nuclear transporter
- DMBA
7,12-dimethylbenz[a]anthracene
- PAHs
polycyclic aromatic hydrocarbons
- L. monocytogenes
Listeria monocytogenes
References
- 1.Denison MS, Heath-Pagliuso S. The Ah receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull. Environ. Contam. Toxicol. 1998;61:557–568. doi: 10.1007/pl00002973. [DOI] [PubMed] [Google Scholar]
- 2.Marlowe JL, Puga A. Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J. Cell. Biochem. 2005;96:1174–1184. doi: 10.1002/jcb.20656. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chem. Biol. Interact. 2002;141:3–24. doi: 10.1016/s0009-2797(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 5.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 6.Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen HS, Perdew GH. Subunit composition of the heteromeric cytosolic aryl hydrocarbon receptor complex. J. Biol. Chem. 1994;269:27554–27558. [PubMed] [Google Scholar]
- 8.Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol. Cell. Biol. 1998;18:978–988. doi: 10.1128/mcb.18.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enan E, Matsumura F. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem. Pharmacol. 1996;52:1599–1612. doi: 10.1016/s0006-2952(96)00566-7. [DOI] [PubMed] [Google Scholar]
- 10.Hankinson O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 11.Singh NP, Nagarkatti M, Nagarkatti PS. Role of dioxin response element and nuclear factor-kappaB motifs in 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated regulation of Fas and Fas ligand expression. Mol. Pharmacol. 2007;71:145–157. doi: 10.1124/mol.106.028365. [DOI] [PubMed] [Google Scholar]
- 12.Fan F, Yan B, Wood G, Viluksela M, Rozman KK. Cytokines (IL-1beta and TNFalpha) in relation to biochemical and immunological effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in rats. Toxicology. 1997;116:9–16. doi: 10.1016/s0300-483x(96)03514-7. [DOI] [PubMed] [Google Scholar]
- 13.Jensen BA, Leeman RJ, Schlezinger JJ, Sherr DH. Aryl hydrocarbon receptor (AhR) agonists suppress interleukin-6 expression by bone marrow stromal cells: an immunotoxicology study. Environ. Health. 2003;2:16. doi: 10.1186/1476-069X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.N’Diaye M, Le Ferrec E, Lagadic-Gossmann D, Corre S, Gilot D, Lecureur V, Monteiro P, Rauch C, Galibert MD, Fardel O. Aryl hydrocarbon receptor- and calcium-dependent induction of the chemokine CCL1 by the environmental contaminant benzo[a]pyrene. J. Biol. Chem. 2006;281:19906–19915. doi: 10.1074/jbc.M601192200. [DOI] [PubMed] [Google Scholar]
- 15.Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ. Health Perspect. 2005;113:1536–1541. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren TK, Mitchell KA, Lawrence BP. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza A virus without affecting cytolytic activity in the lung. Toxicol. Sci. 2000;56:114–123. doi: 10.1093/toxsci/56.1.114. [DOI] [PubMed] [Google Scholar]
- 17.Hinsdill RD, Couch DL, Speirs RS. Immunosuppression in mice induced by dioxin (TCDD) in feed. J. Environ. Pathol. Toxicol. 1980;4:401–425. [PubMed] [Google Scholar]
- 18.Luster MI, Boorman GA, Dean JH, Harris MW, Luebke RW, Padarathsingh ML, Moore JA. Examination of bone marrow, immunologic parameters and host susceptibility following pre- and postnatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Int. J. Immunopharmacol. 1980;2:301–310. doi: 10.1016/0192-0561(80)90030-2. [DOI] [PubMed] [Google Scholar]
- 19.Ward EC, Murray MJ, Lauer LD, House RV, Irons R, Dean JH. Immunosuppression following 7,12-dimethylbenz[a]anthracene exposure in B6C3F1 mice. I. Effects on humoral immunity and host resistance. Toxicol. Appl. Pharmacol. 1984;75:299–308. doi: 10.1016/0041-008x(84)90212-6. [DOI] [PubMed] [Google Scholar]
- 20.House RV, Lauer LD, Murray MJ, Thomas PT, Ehrlich JP, Burleson GR, Dean JH. Examination of immune parameters and host resistance mechanisms in B6C3F1 mice following adult exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Toxicol. Environ. Health. 1990;31:203–215. doi: 10.1080/15287399009531449. [DOI] [PubMed] [Google Scholar]
- 21.Kerkvliet NI, Baecher-Steppan L, Shepherd DM, Oughton JA, Vorderstrasse BA, DeKrey GK. Inhibition of TC-1 cytokine production, effector cytotoxic T lymphocyte development and alloantibody production by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Immunol. 1996;157:2310–2319. [PubMed] [Google Scholar]
- 22.Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int. Immunopharmacol. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 23.McMillan BJ, McMillan SN, Glover E, Bradfield CA. 2,3,7,8-tetrachlorodibenzo-P-dioxin induces premature activation of the KLF2 regulon during thymocyte development. J Biol Chem. 2007 doi: 10.1074/jbc.M611446200. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 25.Kerkvliet NI, Oughton JA. Acute inflammatory response to sheep red blood cell challenge in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): phenotypic and functional analysis of peritoneal exudate cells. Toxicol. Appl. Pharmacol. 1993;119:248–257. doi: 10.1006/taap.1993.1066. [DOI] [PubMed] [Google Scholar]
- 26.Doi H, Baba T, Tohyama C, Nohara K. Functional activation of arylhydrocarbon receptor (AhR) in primary T cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chemosphere. 2003;52:655–662. doi: 10.1016/S0045-6535(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Tsukumo S, Suzuki N, Motohashi H, Yamamoto M, Fujii-Kuriyama Y, Mimura J, Lin TM, Peterson RE, Tohyama C, Nohara K. A constitutively active arylhydrocarbon receptor induces growth inhibition of jurkat T cells through changes in the expression of genes related to apoptosis and cell cycle arrest. J. Biol. Chem. 2004;279:25204–25210. doi: 10.1074/jbc.M402143200. [DOI] [PubMed] [Google Scholar]
- 28.Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA, Silverstone AE. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J. Immunol. 2003;171:4582–4591. doi: 10.4049/jimmunol.171.9.4582. [DOI] [PubMed] [Google Scholar]
- 29.Staples JE, Murante FG, Fiore NC, Gasiewicz TA, Silverstone AE. Thymic alterations induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are strictly dependent on aryl hydrocarbon receptor activation in hemopoietic cells. J. Immunol. 1998;160:3844–3854. [PubMed] [Google Scholar]
- 30.van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, Fardel O. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J. Immunol. 2003;170:2374–2381. doi: 10.4049/jimmunol.170.5.2374. [DOI] [PubMed] [Google Scholar]
- 31.Laupeze B, Amiot L, Sparfel L, Le Ferrec E, Fauchet R, Fardel O. Polycyclic aromatic hydrocarbons affect functional differentiation and maturation of human monocyte-derived dendritic cells. J. Immunol. 2002;168:2652–2658. doi: 10.4049/jimmunol.168.6.2652. [DOI] [PubMed] [Google Scholar]
- 32.Kerkvliet NI, Shepherd DM, Baecher-Steppan L. T lymphocytes are direct, aryl hydrocarbon receptor (AhR)-dependent targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): AhR expression in both CD4+ and CD8+ T cells is necessary for full suppression of a cytotoxic T lymphocyte response by TCDD. Toxicol. Appl. Pharmacol. 2002;185:146–152. doi: 10.1006/taap.2002.9537. [DOI] [PubMed] [Google Scholar]
- 33.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 2005;175:4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence BP, Roberts AD, Neumiller JJ, Cundiff JA, Woodland DL. Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8+ T cells in the lung. J. Immunol. 2006;177:5819–5828. doi: 10.4049/jimmunol.177.9.5819. [DOI] [PubMed] [Google Scholar]
- 35.Kathariou S. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 2002;65:1811–1829. doi: 10.4315/0362-028x-65.11.1811. [DOI] [PubMed] [Google Scholar]
- 36.Control CfD. Foodnet: Foodborne diseases active surveilance network. 2000.
- 37.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cossart P, Pizarro-Cerda J, Lecuit M. Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell Biol. 2003;13:23–31. doi: 10.1016/s0962-8924(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 39.Sugita-Konishi Y, Kobayashi K, Naito H, Miura K, Suzuki Y. Effect of lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on the susceptibility to Listeria infection. Biosci. Biotechnol. Biochem. 2003;67:89–93. doi: 10.1271/bbb.67.89. [DOI] [PubMed] [Google Scholar]
- 40.Vos JG, Kreeftenberg JG, Engel HW, Minderhoud A, Van Noorle Jansen LM. Studies on 2,3,7,8-tetrachlorodibenzo-p-dioxin induced immune suppression and decreased resistance to infection: endotoxin hypersensitivity, serum zinc concentrations and effect of thymosin treatment. Toxicology. 1978;9:75–86. doi: 10.1016/0300-483x(78)90033-1. [DOI] [PubMed] [Google Scholar]
- 41.Czuprynski CJ, Faith NG. Sodium bicarbonate enhances the severity of infection in neutropenic mice orally inoculated with Listeria monocytogenes EGD. Clin. Diagn. Lab. Immunol. 2002;9:477–481. doi: 10.1128/CDLI.9.2.477-481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czuprynski CJ, Henson PM, Campbell PA. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J. Leukoc. Biol. 1984;35:193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- 43.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu. Rev. Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 44.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 45.Pande K, Moran SM, Bradfield CA. Aspects of dioxin toxicity are mediated by interleukin 1-like cytokines. Mol. Pharmacol. 2005;67:1393–1398. doi: 10.1124/mol.105.010983. [DOI] [PubMed] [Google Scholar]
- 46.Mocci S, Dalrymple SA, Nishinakamura R, Murray R. The cytokine stew and innate resistance to L. monocytogenes. Immunol. Rev. 1997;158:107–114. doi: 10.1111/j.1600-065x.1997.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 48.Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J. Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 49.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 50.Pasche B, Kalaydjiev S, Franz TJ, Kremmer E, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Lengeling A, Busch DH. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect. Immun. 2005;73:5952–5960. doi: 10.1128/IAI.73.9.5952-5960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackaness GB. Cellular resistance to infection. J. Exp. Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 53.Vorderstrasse BA, Steppan LB, Silverstone AE, Kerkvliet NI. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol. Appl. Pharmacol. 2001;171:157–164. doi: 10.1006/taap.2000.9122. [DOI] [PubMed] [Google Scholar]
- 54.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J. Immunol. 2006;177:2565–2574. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- 55.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 56.Harty JT, Schreiber RD, Bevan MJ. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vorderstrasse BA, Lawrence BP. Protection against lethal challenge with Streptococcus pneumoniae is conferred by aryl hydrocarbon receptor activation but is not associated with an enhanced inflammatory response. Infect. Immun. 2006;74:5679–5686. doi: 10.1128/IAI.00837-06. [DOI] [PMC free article] [PubMed] [Google Scholar]