Abstract
Duchenne muscular dystrophy (DMD) is a fatal X-linked genetic disorder of skeletal muscle caused by mutation in dystrophin gene. Although the degradation of skeletal muscle extracellular matrix, inflammation and fibrosis are the common pathological features in DMD, the underlying mechanisms remain poorly understood. In this study, we have investigated the role and the mechanisms by which increased levels of matrix metalloproteinase-9 (MMP-9) protein causes myopathy in dystrophin-deficient mdx mice. The levels of MMP-9 but not tissue inhibitor of MMPs were drastically increased in skeletal muscle of mdx mice. Besides skeletal muscle, infiltrating macrophages were found to contribute significantly to the elevated levels of MMP-9 in dystrophic muscle. In vivo administration of a nuclear factor-kappa B inhibitory peptide, NBD, blocked the expression of MMP-9 in dystrophic muscle of mdx mice. Deletion of Mmp9 gene in mdx mice improved skeletal muscle structure and functions and reduced muscle injury, inflammation and fiber necrosis. Inhibition of MMP-9 increased the levels of cytoskeletal protein β-dystroglycan and neural nitric oxide synthase and reduced the amounts of caveolin-3 and transforming growth factor-β in myofibers of mdx mice. Genetic ablation of MMP-9 significantly augmented the skeletal muscle regeneration in mdx mice. Finally, pharmacological inhibition of MMP-9 activity also ameliorated skeletal muscle pathogenesis and enhanced myofiber regeneration in mdx mice. Collectively, our study suggests that the increased production of MMP-9 exacerbates dystrophinopathy and MMP-9 represents as one of the most promising therapeutic targets for the prevention of disease progression in DMD.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is one of the most common and annihilating form of muscular dystrophy, which afflicts 0.03% of all male birth (1). Inactivation of the dystrophin gene is the primary cause of DMD in humans and in mdx mice (a mouse model of DMD) (2–4). Dystrophin is an integral component of the transmembrane protein network known as dystrophin–glycoprotein complex (DGC), which not only provides mechanical stability to muscle fibers during contraction but also serves as an important signaling link from the extracellular matrix (ECM) to the cytoskeleton (5). Loss of functional dystrophin protein leads to greater mechanical instability of the sarcolemma and aberrant intracellular signaling leading to progressive muscle degeneration and weakness (5–8). Although skeletal muscles have remarkable ability to regenerate after injury, myofiber regeneration is significantly impaired in DMD (2,9). Accumulating evidence strongly suggests that the primary deficiency of the dystrophin results in the activation of several secondary processes, such as inflammation, ECM degradation, chronic degeneration and regeneration of fibers and fibrosis, which exacerbate disease progression in DMD (1). A better understanding and management of these secondary processes have enormous potential to improving the quality of life and extending the life expectancy in DMD patients (10).
Matrix metalloproteinases (MMPs) are a family of zinc-containing enzymes that are implicated in degradation and remodeling of the components of the ECM in both physiological and pathophysiological conditions (11,12). These proteases are synthesized as secreted or transmembrane proenzymes and processed to an active form by the removal of an amino-terminal propeptide. The expression of MMPs is rapidly increased upon tissue injury suggesting their possible role in repair process, release of growth factors and modulation of ECM for cell migration (11–13). However, the presence of large amounts of MMPs, and in particular of MMP-9 (gelatinase B), has been found to contribute to tissue destruction in many pathological conditions including chronic wounds, heart failure, rheumatic arthritis, fibrotic lung disease, dilated cardiomyopathy, gastric ulcer, multiple sclerosis, asthma and cancer (13–18). The proteolytic activity of MMPs is tightly controlled by their interaction with endogenous tissue inhibitors of matrix metalloproteinases (TIMPs), which specifically inhibit active form of MMPs and in some cases latent MMPs (11–13). The balance between MMPs and TIMPs plays an important role in maintaining tissue integrity (13).
The potential function of MMPs in myopathy is supported by several published reports demonstrating increased expression of MMP-9 in skeletal muscle of patients and animal models of muscle-wasting diseases (19). Elevated levels of MMP-9 have been observed in skeletal muscle tissues after nerve injury, heart failure and inflammatory myopathy (19–22). Furthermore, pharmacological inhibition of MMPs activity has been found to attenuate pathogenesis in a mouse model of oculopharyngeal muscular dystrophy (23). Although MMP-9 appears to play an important role in skeletal muscle remodeling in various pathological conditions, the direct evidence regarding the role of MMP-9 in skeletal muscle pathology is still lacking. In this study, using genetic and pharmacological approaches, we have investigated the role and underpinning mechanisms by which the increased production of MMP-9 causes muscle pathogenesis in mdx mice. Our study demonstrates that the inhibition of MMP-9 activity drastically reduces skeletal muscle structural deterioration, inflammation, necrosis and fibrosis and improves skeletal muscle contractile functions in mdx mice. Furthermore, the inhibition of MMP-9 activity also significantly improves skeletal muscle regeneration in mdx mice.
RESULTS
Deregulation of MMP-9 expression during postnatal development of mdx mice
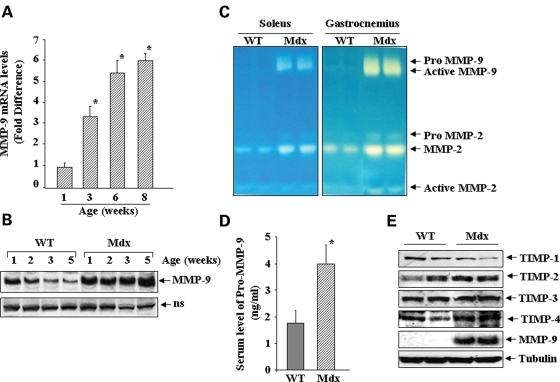
We first studied the mRNA and protein levels of MMP-9 in diaphragm muscle of normal and mdx mice by quantitative real-time PCR (QRT-PCR) and western blotting, respectively. The mRNA levels of MMP-9 were found to be significantly higher in diaphragm muscle from 3-, 6- and 8-week-old mdx mice compared with age-matched C57BL/10 control mice (Fig. 1A). Although elevated levels of MMP-9 protein were observed in both control and mdx mice of 1 or 2 weeks post birth, the amounts of MMP-9 proteins were significantly reduced after 2 weeks in control mice. In contrast, the levels of MMP-9 protein remained abnormally high in diaphragm of 3- and 5-week mdx mice (Fig. 1B). To evaluate whether MMP-9 protein present in skeletal muscle of mdx mice was enzymatically active, gelatin zymography was performed. A drastic increase in gelatinolytic activity of MMP-9 was observed in soleus and gastrocnemius muscle of 8-week-old mdx mice compared with age-matched control mice (Fig. 1C). Similar increase in MMP-9 protein level and gelatinolytic activity was observed in diaphragm, soleus, gastrocnemius and quadriceps muscle (data not shown). In addition, the amount of MMP-2 was also found to be slightly increased in skeletal muscle of mdx mice (Fig. 1C). These data are consistent with other published reports demonstrating increased levels of MMP-9 in skeletal muscle of mdx mice (24,25). Since MMP-9 is a secreted protein, we also compared the levels of MMP-9 in serum of control and mdx mice using a commercially available ELISA kit (R&D Systems). As shown in Figure 1D, there was approximately 2-fold increase in serum level of MMP-9 in 6-week mdx mice compared with age-matched control mice (Fig. 1D). Furthermore, because the proteolytic activity of MMP-9 is regulated via their interaction with TIMPs (11–13), we also investigated whether the expression of TIMPs was altered in skeletal muscle of mdx mice compared with control mice. There was no significant difference in the levels of TIMP-1, -2, -3 or -4 protein between 8-week-old control and mdx mice (Fig. 1E), suggesting a disruption of balance between MMP-9 and TIMPs in skeletal muscle of mdx mice.
Figure 1.
Elevated levels of MMP-9 in skeletal muscle of mdx mice. (A) Diaphragm from control and mdx mice was analyzed for the expression of MMP-9 by QRT-PCR. The fold increase in MMP-9 mRNA levels in diaphragm of mdx mice (n = 3, each point) compared with age-matched control (n = 4) is presented here. *P < 0.01, values significantly different from age-matched control mice. (B) Levels of MMP-9 protein measured in diaphragm of control and mdx mice at different stages of development suggest deregulation of MMP-9 activity in mdx mice. (C) Soleus and gastrocnemius muscle isolated from 8-week-old control and mdx mice were analyzed by gelatin zymography. (D) Amount of MMP-9 protein in serum of control (n = 4) and mdx (n = 4) mice. *P < 0.01, values significantly different from control mice. (E) No significant difference was observed in the levels of TIMP-1, -2, -3 and -4 protein in diaphragm of 8-week-old control and mdx mice. ns, non-specific (loading control).
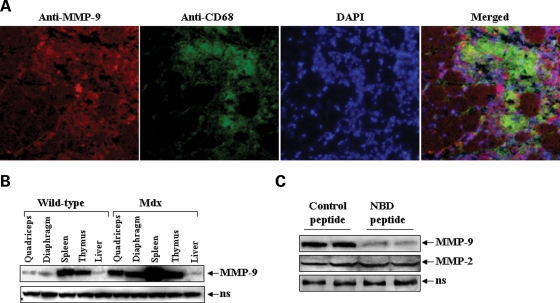
Because dystrophic muscle of mdx mice contains infiltrating immune cells especially macrophages (26,27), we investigated whether MMP-9 is produced by skeletal muscle, macrophages or both. Skeletal muscle cryosections made from gastrocnemius muscle of mdx mice were immunostained for MMP-9 and CD68 protein (a cell surface marker for activated macrophages). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Being a secreted protein, MMP-9 was present in and around myofibers in skeletal muscle of mdx (Fig. 2A). The amounts of MMP-9 were found to be increased in the areas where the concentration of macrophages was higher (Fig. 2A). To further confirm the role of immunocytes in MMP-9 expression in mdx mice, we measured MMP-9 protein levels in other immune (e.g. spleen and thymus) and non-immune (e.g. liver) organs of 8-week-old control and mdx mice. Interestingly, in addition to skeletal muscle, the level of MMP-9 protein was also significantly increased in the spleen and thymus, but not in the liver of mdx mice (Fig. 2B).
Figure 2.
Skeletal muscle and immune cells are the major source of MMP-9 in mdx mice. (A) Gastrocnemius muscle cryosections (10 µm) of 8-week-old mdx mice were immunostained for MMP-9 and CD68 proteins. Nucleus was stained with DAPI and representative pictures taken were merged using Nikon NIS Elements BR 3.00 software. Scale bar, 50 µm. (B) Levels of MMP-9 protein in quadriceps muscle, diaphragm, spleen, thymus and liver of normal and mdx mice measured by western blotting. (C) Four-week-old mdx mice were given three intraperitoneal injection of control or NBD peptide (200 µg/mice) every third day. Twenty-four hours after the final injection, the mice were sacrificed and the amounts of MMP-2 and MMP-9 protein were measured in diaphragm muscle by western blotting. ns, non-specific (loading control).
We have previously reported that the activity of nuclear factor-kappa B (NF-κB) transcription factor is highly up-regulated in skeletal muscle of mdx mice (6). Because the promoter region of MMP-9 contains consensus binding sequence for NF-κB and the activation of NF-κB is prerequisite for the expression of MMP-9 in response to various catabolic stimuli (28–30), we investigated whether the activation of NF-κB is responsible for the increased expression of MMP-9 in skeletal muscle of mdx mice. As shown in Figure 2C, in vivo administration of a cell permeable NF-κB essential modifier (NEMO)-binding domain (NBD) peptide, a selective inhibitor of NF-κB (27,31), significantly reduced the amounts of MMP-9 protein in diaphragm of mdx mice. In contrast, administration of NBD peptide did not affect the level of MMP-2 protein in skeletal muscle of mdx mice (Fig. 2C). Taken together, these results suggest that both skeletal muscle and immunocytes (e.g. macrophages) contribute to the elevated levels of MMP-9 and the expression of MMP-9 is regulated through the activation of NF-κB in skeletal muscle of mdx mice.
Genetic ablation of MMP-9 inhibits the activation of activator protein-1 (AP-1) and NF-κB transcription factors, reduces the serum level of creatine kinase (CK) and improves skeletal muscle structure in mdx mice
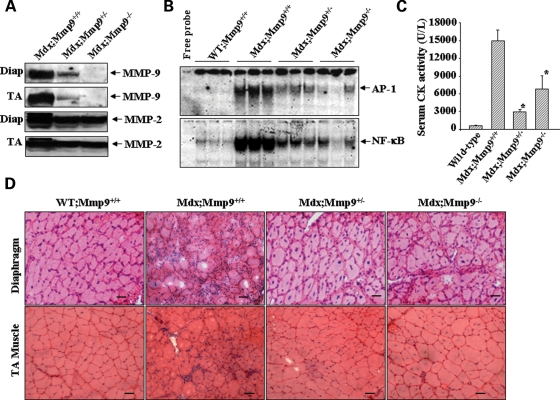
To understand the role of MMP-9 in pathogenesis of mdx mice, male Mmp9-knockout mice were crossed with female mdx mice to generate mdx+/−;Mmp9+/− and mdx;Mmp9+/− mice. Breeding of mdx+/−;Mmp9+/− and mdx;Mmp9+/− mice generated littermate wild-type (WT);Mmp9+/+, mdx;Mmp9+/+, mdx;Mmp9+/− and mdx;Mmp9−/− mice in expected Mendelian ratios. By performing western blotting, we first confirmed that the deletion of single allele of Mmp9 gene (i.e. in Mdx;Mmp9+/−) reduced the protein levels of MMP-9 in skeletal muscle of mdx mice and there was no detectable level of MMP-9 protein in mdx;Mmp9−/− mice (Fig. 3A). Interestingly, instead of expected 50% decrease, deletion of single allele of MMP-9 (i.e. mdx;Mmp9+/−) more drastically (>80%) reduced the level of MMP-9 protein in skeletal muscle of mdx mice. Furthermore, the level of MMP-2 protein was also found to reduce in mdx;Mmp9+/− or mdx;Mmp9−/− mice compared with mdx;Mmp9+/+ mice (Fig. 3A), indicating that the increased production of MMP-9 also modulates the expression/activity of other MMPs in skeletal muscle of mdx mice. Published reports suggest that besides NF-κB, the transcriptional activation of MMP-9 promoter also involves the activation of activator protein-1 (AP-1) transcription factor (28–30). We investigated the possibility whether MMP-9 affects its own expression in skeletal muscle of mdx mice through a positive feed-back mechanism which might involve the activation of AP-1 and NF-κB. Interestingly, the activation of both AP-1 and NF-κB was significantly reduced in tibial anterior (TA) muscle of mdx;Mmp9+/− or mdx;Mmp9−/− mice compared with mdx;Mmp9+/+ mice (Fig. 3B).
Figure 3.
Genetic deletion of MMP-9 inhibits AP-1 and NF-κB and reduces the serum level of CK and improves muscle structure in mdx mice. (A) Representative immunoblots presented here demonstrate reduced levels of MMP-9 and MMP-2 protein in diaphragm and TA muscle of mdx;Mmp9+/− (n = 3), mdx;Mmp9−/− (n = 3) compared with littermate mdx;Mmp9+/+ (n = 4) mice. (B) Representative EMSA gels from two independent experiments presented here demonstrate that the activation of AP-1 and NF-κB is significantly reduced in TA muscle of 8-week-old mdx;Mmp9+/− and mdx/Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice. (C) Serum level of CK measured using a commercially available kit (Stanbio Laboratory, TX, USA) in 8-week-old littermate WT (n = 4), mdx;Mmp9+/+ (n = 6), mdx;Mmp9+/− (n = 7) and mdx;Mmp9+/+ (n = 6) suggest that heterozygous and homozygous deletion of Mmp9 gene significantly reduces the serum level of CK in mdx mice. Data presented are mean ± SD from two independent experiments. *P < 0.01 values significantly different from littermate mdx;Mmp9+/+ mice. (D) Frozen cross-sections made from diaphragm and TA muscle of 7-week-old WT;Mmp9+/+ (n = 3), mdx;Mmp9+/+ (n = 4), mdx;Mmp9+/− (n = 7) and mdx;Mmp9−/− (n = 6) mice were stained with H&E and photomicrograph. Scale bar: 50 µm. Data presented here demonstrate that the deletion of Mmp9 gene in mdx mice improves skeletal muscle structure.
To study the effect of inhibition of MMP-9 on skeletal muscle pathogenesis in mdx mice, we measured serum level of creatine kinase (CK) in 8-week-old littermate WT, mdx;Mmp9+/+, mdx;Mmp9+/− and mdx;Mmp9−/− mice. As shown in Figure 3C, the CK levels were significantly lower in mdx;Mmp9+/− and mdx;Mmp9−/− compared with littermate mdx;Mmp9+/+ mice, thus providing the initial evidence that the inhibition of MMP-9 activity inhibits skeletal muscle injury in mdx mice. Because myopathy in mdx mice is associated with significant ECM abnormalities, we studied the effects of inhibition of MMP-9 activity on skeletal muscle structure in mdx mice. Diaphragm and TA muscle of WT/Mmp9+/+, mdx;Mmp9+/+, mdx;Mmp9+/− and mdx;Mmp9−/− mice were isolated and cryosections (10 µm) made were subjected to Hematoxylin and Eosin (H&E) staining. WT;Mmp9+/+ mice showed fibers of uniform diameter and no clustering of inflammatory cells between adjacent fibers (Fig. 3D). However, mdx;Mmp9+/+ mice showed typical features of muscular dystrophy characterized by fiber population of variable diameters, central nucleation and appearance of darkly stained nuclei of inflammatory cells between adjacent fibers (Fig. 3D). In contrast, skeletal muscle of mdx;Mmp9+/− and mdx;Mmp9−/− mice showed significantly improved muscle structure; the fiber diameter was more uniform and the number of inflammatory cells between adjacent fibers was drastically reduced (Fig. 3D).
Heterozygous or homozygous deletion of MMP-9 attenuates accumulation of macrophages, fiber necrosis and improves force production in skeletal muscle of mdx mice
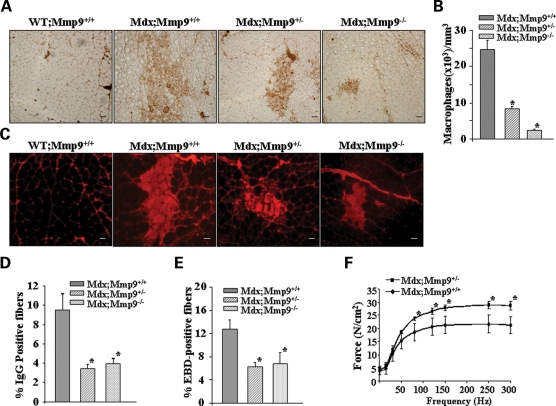
Since MMP-9 is a major mediator of inflammatory response, we next investigated whether the inhibition of MMP-9 affects the infiltration of macrophages in skeletal muscle of mdx mice. Gastrocnemius muscle from 7- to 8-week-old WT;Mmp9+/+, mdx;Mmp9+/+, mdx;Mmp9+/− and mdx;Mmp9−/− mice were stained for macrophages using rat anti-mouse F4/80 (ABD Serotec), the number of labeled cells (brown colored) within two entire sections for each muscle was manually counted and the total area of each section was assessed using a calibrated square grid. The volume of muscle sample was calculated as the product of the section area and thickness (10 µm). Concentrations of macrophages were expressed as number of cells per cubic millimeter (mm3). Interestingly, the concentration of F4/80 positive cells was drastically reduced in gastrocnemius muscle of mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with mdx;Mmp9+/+ mice (Fig. 4A and B).
Figure 4.
Genetic ablation of MMP-9 attenuates muscle pathology in mdx mice. (A) Representative photomicrographs of gastrocnemius muscle sections immunostained for F4/80 antigen suggest that the accumulation of macrophages in skeletal muscle is decreased in mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with littermate mdx/Mmp9+/+ mice. Scale bar, 50 µm. (B) Quantification of F4/80 positive cells revealed significantly (*P < 0.001) reduced concentration of F4/80 stained cells in mdx;Mmp9+/− (n = 4) and mdx;Mmp9−/− (n=5) compared with mdx;Mmp9+/+ (n = 3) mice. (C) Gastrocnemius muscle cross-sections from 7- to 8-week mdx;Mmp9+/+, mdx;Mmp9+/− and mdx;Mmp9−/− mice were immunostained with Cy3-labelled goat anti-mouse IgG to detect necrotic fibers. Scale bar: 50 µm. (D) Percentage of IgG positive (filled with red color) fibers was significantly (*P < 0.05) lower in mdx;Mmp9+/− (n = 5) and mdx;Mmp9−/− (n = 6) mice compared with littermate mdx;Mmp9+/+ (n = 4) mice. (E) Percentage of EBD-positive cells were also found to be decreased in gastrocnemius muscle of mdx;Mmp9+/− or mdx;Mmp9−/− compared with littermate mdx;Mmp9+/+ mice. *P < 0.05, values significantly different from mdx;Mmp9+/+ mice. (F) Force–frequency curves. Data presented here are the average force produced by soleus muscle of mdx;Mmp9+/+ (n = 4) and mdx;Mmp9+/− mice (n = 4). *P < 0.05, values significantly different from corresponding mdx;Mmp9+/+ mice at indicated frequency.
Because necrosis is the major mechanism of skeletal muscle fiber death in mdx mice and in DMD patients (32), we investigated whether genetic ablation of MMP-9 affects fiber necrosis in skeletal muscle of mdx mice. WT control mice did not show any intracellular fiber staining with anti-mouse IgG (Fig. 4C). In contrast, mdx;Mmp9+/+ mice showed intracellular staining for IgG within a part of their muscle fibers (Fig. 4C). However, the number of necrotic (filled) fibers in gastrocnemius muscle was significantly reduced in mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with mdx;Mmp9+/+ mice (Fig. 4C and D).
We also employed Evans blue dye (EBD) to identify permeable skeletal muscle that became damaged as a result of muscular dystrophy (26). Similar to necrosis, the percentage of EBD-positive fibers was significantly reduced in skeletal muscle of mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice (Fig. 4E).
Finally, we investigated the effect of genetic ablation of MMP-9 on skeletal muscle force production in mdx mice. Force produced by soleus muscle in isometric contractions was significantly higher in mdx;Mmp9+/− compared with littermate mdx;Mmp9+/+ mice (Fig. 4F). Taken together, these data strongly suggest that the inhibition of MMP-9 activity ameliorates skeletal muscle pathogenesis and improves muscle contractile function in mdx mice.
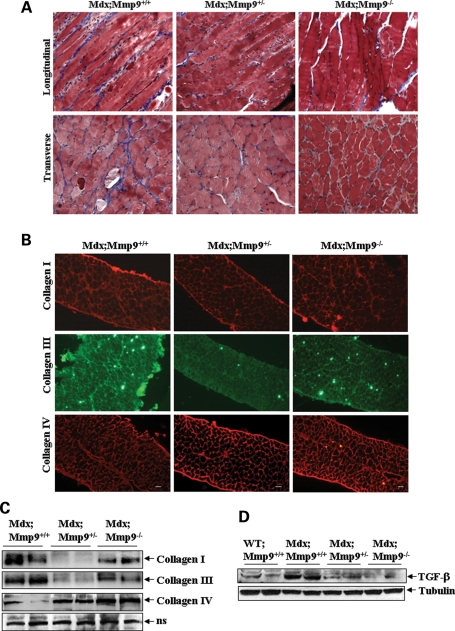
Genetic deletion of MMP-9 attenuates fibrosis in diaphragm of mdx mice
Fibrosis is an important pathological feature of dystrophic muscle of mdx mice and DMD patients (2–4). To understand the role of MMP-9 in fibrosis in mdx mice, we studied the accumulation of collagen fibers in diaphragm using Masson's Trichrome staining method. As shown in Figure 5A, the level of collagens (blue colored) was significantly lower in mdx;Mmp9+/− or mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice. Although skeletal muscle may contain many types of collagens, collagens I and III are the major one, which are present in ECM of skeletal muscle (33) and drastically increased in dystrophic muscle of mdx mice (34). In contrast, collagen IV is present mainly in the basement membrane of muscle fibers (33). Immunohistochemical and western blot analyses of diaphragm revealed that the levels of type I and type III collagens were significantly reduced in mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice (Fig. 5B and C). Interestingly, among mdx;Mmp9+/− and mdx;Mmp9−/− mice, the levels of collagens I and III were significantly lower in mdx;Mmp9+/− mice when compared with mdx;Mmp9−/− mice (Fig. 5B and C). Although the exact mechanisms remain unknown, it is possible that small amount of MMP-9 may be essential for collagen metabolism in order to prevent fibrosis in skeletal muscle of mdx mice. Nevertheless, the reduced levels of collagens I and III in diaphragm of mdx;Mmp9+/− and mdx;Mmp9−/− mice clearly suggest that MMP-9 contributes to fibrosis. In contrast to collagens I and III, the level of collagen IV was found to be significantly increased in mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice (Fig. 5B and C).
Figure 5.
Heterozygous or homozygous deletion of MMP-9 reduces fibrosis in mdx mice. (A) Diaphragm muscle isolated from 14-week-old mdx;Mmp9+/+ (n = 3), mdx;Mmp9+/− (n = 3) and mdx;Mmp9−/− (n = 3) were fixed in formalin, paraffin-embedded and sectioned longitudally and transversely and Mason's Trichrome staining was performed. (B) Diaphragm muscle cross-sections from 10-week-old mdx;Mmp9+/+ (n = 5), mdx;Mmp9+/− (n = 6) and mdx;Mmp9−/− (n = 6) mice were immunostained for collagen I, III and IV. Scale bar: 50 µm. (C) Western blot analyses for collagens I, III and IV and mature TGF-β in diaphragm muscle of 10-week-old mdx;Mmp9+/+, mdx;Mmp9+/− and mdx;Mmp9−/− mice. Data presented here show that the levels of collagen I and collagen III protein was reduced, whereas the level of collagen IV was increased in diaphragm of mdx;Mmp9+/− or mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice. (D) The level of active TGF-β protein was also found to be significantly reduced in diaphragm of mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice.
TGF-β is a predominant mediator of fibrosis (35). Increased levels of TGF-β have been observed in dystrophic muscle of mdx mice (36,37) and DMD patients (38). MMP-9 is known to cause the activation of latent TGF-β into mature form by proteolytically removing its inhibitory domain (13,39). To determine the mechanisms by which the increased levels of MMP-9 lead to fibrosis in mdx mice, we determined the level of active TGF-β protein in diaphragm muscle by western blotting. There was negligible amount of active TGF-β protein in skeletal muscle of WT control mice (data not shown). However, the diaphragm of mdx;Mmp9+/+ mice contained high amounts of TGF-β. Interestingly, compared with mdx;Mmp9+/+ mice, the levels of TGF-β protein were significantly reduced in mdx;Mmp9+/− or mdx;Mmp9−/− mice (Fig. 5B). These results suggest that MMP-9 might be promoting fibrosis by augmenting the amounts of active TGF-β in skeletal muscle of mdx mice.
Genetic ablation of MMP-9 improves sarcolemmal structure and augments the cellular levels of β-dystroglycan and nNOS in skeletal muscle of mdx mice
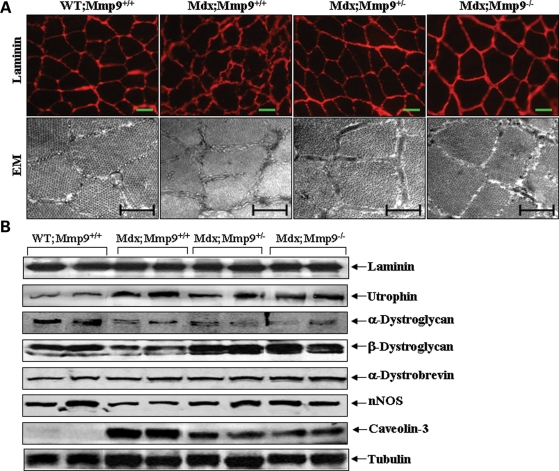
To further understand the mechanisms of action of MMP-9 in skeletal muscle of mdx mice, we studied the effect of inhibition of MMP-9 activity on skeletal muscle membrane by immunostaining with laminin antibody (29). Compared with a well-defined membrane bordering of each myofiber in gastrocnemius muscle of WT mice, sarcolemma in mdx mice appeared wrinkled and irregular (Fig. 6A). Interestingly, these abnormalities in muscle membrane were significantly attenuated in mdx;Mmp9+/− or mdx;Mmp9−/− mice (Fig. 6A, upper panels). This phenomenon was also confirmed by electron microscopy analysis of gastrocnemius muscle cross-sections. As shown in Figure 6A, there was a significant improvement in sarcolemmal as well as myofibril structure in mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with littermate mdx/Mmp9+/+ mice. These data, thus, indicate that the increased production of MMP-9 contributes to sarcolemmal abnormalities in mdx mice.
Figure 6.
Effect of genetic ablation of MMP-9 on membrane structure and levels of DGC protein in mdx mice. (A) Representative photomicrographs (upper panel) after immunostaining with laminin antibody demonstrate that sarcolemmal integrity was significantly improved in gastrocnemius muscle of mdx;Mmp9+/− (n = 5) and mdx;Mmp9−/− (n = 4) compared with littermate mdx;Mmp9+/+ (n = 5) mice. Scale bars: 50 µm. Analysis of gastrocnemius muscle with electron microscopy (lower panel) also revealed improved sarcolemmal and myofibril structure in mdx;Mmp9+/− (n = 3) and mdx;Mmp9−/− (n = 3) mice compared with littermate mdx/Mmp9+/+ (n = 3) mice. Scale bar: 0.5 µm. (B) Western blot analysis showed that the levels of β-dystroglycan and nNOS were increased, whereas the level of caveolin-3 was reduced in gastrocnemius muscle of mdx;Mmp9+/− (n = 5) and mdx;Mmp9−/− (n = 4) mice compared with littermate mdx;Mmp9+/+ (n = 5) mice. EM, electron microscopy.
Available literature suggests that besides dystrophin, the levels of several other proteins of the DGC are affected in skeletal muscle of mdx mice (40). Interestingly, it was recently reported that β-dystroglycan is one of the most important proteolytic targets of MMP-9 (41,42). Furthermore, MMP-9 has also been suggested to induce the degradation of laminin-2 (13). Consistent with published reports, we found increased protein levels of utrophin (a homologue of dystrophin) and caveolin-3, and reduced levels of α-dystroglycan, β-dystroglycan and neural nitric oxide synthase (nNOS) in mdx mice compared with WT mice (Fig. 6B). However, there was no difference in level of laminin, utrophin, α-dystroglycan and α-dystrobrevin in skeletal muscle of mdx;Mmp9+/+, mdx;Mmp9+/− and mdx;Mmp9−/− mice (Fig. 6B, upper panel). In contrast, the protein level of β-dystroglycan and nNOS was enhanced, whereas the levels of caveolin-3 were reduced in gastrocnemius muscle of mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice (Fig. 6B).
Genetic ablation of MMP-9 augments myofiber regeneration in mdx mice
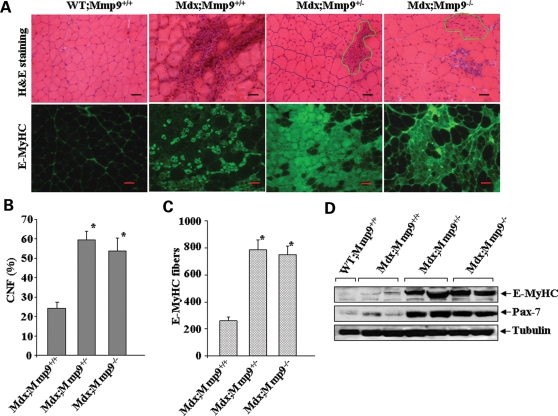
We next investigated whether MMP-9 has any role in skeletal muscle regeneration in mdx mice. The regeneration of myofiber was monitored by counting the number of centrally nucleated fibers (CNF) in H&E-stained gastrocnemius muscle sections and by immunostaining with antibody that recognizes embryonic (developmental) myosin heavy chain (E-MyHC) (Clone F1.652, Development Studies Hybridoma Bank, University of Iowa). Interestingly, deletion of either single or both alleles of Mmp9 gene significantly increased the count of CNF in gastrocnemius muscle of 8-week-old mdx mice (Fig. 7A and B). Gross analysis of H&E-stained muscle sections further revealed that while mdx/Mmp9+/+ contained significant amount of area having necrotic fibers, the necrotic area appeared to be filled with newly formed myofibers which were smaller in size and had central nucleation in mdx/Mmp9+/− and mdx/Mmp9−/− mice (Fig. 7A, highlighted area) suggesting that the inhibition of MMP-9 accelerates regeneration of injured myofibers in mdx mice. Furthermore, the number of E-MyHC-stained myofibers was also found to be drastically increased in both mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice (Fig. 7A and C). In addition, we found that the level of Pax-7 protein, a marker for satellite cells (43), was also increased in gastrocnemius muscle of mdx;Mmp9+/− or mdx;Mmp9−/− mice (Fig. 7D).
Figure 7.
Genetic ablation of MMP-9 improves skeletal muscle regeneration in mdx mice. (A) Gastrocnemius muscle from 8-week-old WT, mdx;Mmp9+/+, mdx;Mmp9+/− and mdx;Mmp9−/− mice were processed for H&E (top panel) or E-MyHC (bottom) staining. Highlighted area represents the newly formed centrally nucleated myofibers. (B) Number of CNF were significantly (*P < 0.01) increased in gastrocnemius muscle of 7-week-old mdx;Mmp9+/− (n = 7) or mdx;Mmp9−/− (n = 8) mice compared with littermate mdx;Mmp9+/+ (n = 6) mice. Scale bars: 50 µm. (C) Number of E-MyHC-positive fibers were significantly increased in gastrocnemius muscle of mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ mice. (D) Western blot analysis of gastrocnemius muscle extracts revealed increased levels of E-MyHC and Pax-7 protein in mdx;Mmp9+/− or mdx;Mmp9−/− mice compared with littermate mdx;Mmp9+/+ or WT mice.
Pharmacological inhibition of MMP-9 alleviates skeletal muscle pathogenesis and augments regeneration in mdx mice
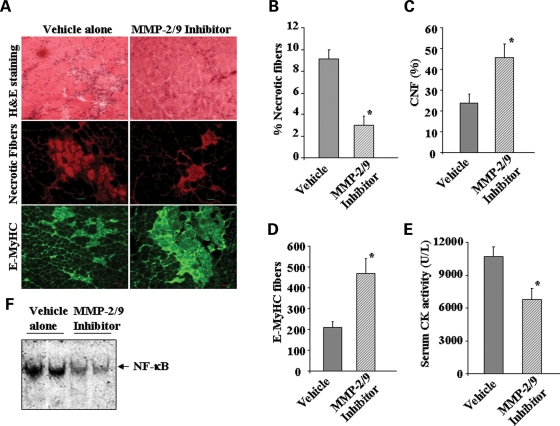
Although our experiments with knockout mice are highly suggestive of the role of MMP-9 in muscle pathogenesis in mdx mice, there is a possibility that some of the apparent effects of MMP-9 deficiency in mdx mice may be reflective of altered physiology that developed as a way to circumvent MMP-9 deficiency. In general, the results obtained with genetically deficient animals that can be recapitulated with pharmacological agents offer the strongest evidence for involvement of any specific protein in a particular disease process. (2R)-2-[(4-Biphenylylsulfonyl) amino]-3-phenylpropionic acid (also known as MMP-2/MMP-9 inhibitor I) is known to specifically inhibits MMP-9 activity and at higher concentration MMP-2 too (44). It has been shown to provide protective effects in number of disease states involving MMP-9 (44,45). To further confirm the role of MMP-9 in myopathy, we investigated the effect of chronic administration of MMP-2/MMP-9 inhibitor I on skeletal muscle pathogenesis in mdx mice. Interestingly, treatment with MMP-2/MMP-9 inhibitor I improved muscle structure (Fig. 8A, upper panels) and reduced fiber necrosis (Fig. 8B, middle panel) in gastrocnemius muscle of mdx mice. Furthermore, skeletal muscle regeneration, evident by the increased number of CNF (Fig. 8C) and E-MyHC-positive fibers (Fig. 8B, lower panel and Fig. 8D), was also significantly increased in MMP-2/MMP-9 inhibitor I-treated mdx mice. The improved muscle pathology was also reflected by a significant reduction in the serum levels of CK in mdx mice upon treatment with MMP-2/MMP-9 inhibitor I (Fig. 8E). Finally, treatment with MMP-2/MMP-9 inhibitor was also found to significantly block the activation of NF-κB transcription factor in mdx mice (Fig. 8F).
Figure 8.
Effect of chronic administration of MMP inhibitor I on skeletal muscle pathology and regeneration in mdx mice. Mdx mice were given daily intraperitoneal injection of vehicle alone or MMP-2/MMP-9 inhibitor I following a protocol as described in the ‘Materials and Methods’ section. (A) Data presented here show that muscle pathology (top panel) and fiber necrosis (middle panel) were reduced, whereas E-MyHC-positive fibers were increased in gastrocnemious muscle of MMP-2/MMP-9 inhibitor I-treated mdx mice (n = 3) compared with untreated mice (n = 3). Scale bars: 50 µm. Quantification of (B) necrotic fibers; (C) CNF; (D) E-MyHC-positive fibers; and (E) serum CK levels in untreated and MMP-2/MMP-9 inhibitor I-treated mdx mice. *P < 0.05, values significantly different from vehicle alone-treated mdx mice. (F) Treatment with MMP-2/MMP-9 inhibitor I blocked the DNA-binding activity of NF-κB transcription factor in mdx mice determined by EMSA.
DISCUSSION
Although dystrophin is absent at birth, clinical symptoms are not evident until 2–3 years after birth in DMD patients and 2.5 weeks in mdx mice (2–4) suggesting that the loss of dystrophin leads to specific biochemical changes in skeletal muscle, which ultimately result in pathogenesis especially when physical activity is increased. Accumulating evidence strongly suggests that chronic inflammatory response and fibrosis in skeletal muscle are some of the important pathological consequences for progressive dysfunction and weakness in DMD patients (5,10,46). In this study, we have identified MMP-9 as an important mediator of myopathy in mdx mice. Our data clearly demonstrate that the increased levels of MMP-9 exacerbate dystrophinopathy by augmenting fiber necrosis, ECM degradation, inflammation and fibrosis. Furthermore, our study also suggests that the excessive production of MMP-9 blocks the regeneration of myofibers in mdx mice.
MMP-9 represents one of the most important extracellular proteases, the increased production of which can drastically alter skeletal muscle microenvironment in vivo (19,29,30). Consistent with the established role of MMP-9 in inflammation and ECM remodeling, we found that the inhibition of MMP-9 activity significantly improves skeletal muscle structure in mdx mice (Fig. 3D). Furthermore, serum level of CK, accumulation of macrophages, fiber necrosis and sarcolemmal injury were also drastically reduced upon heterozygous and homozygous deletion of Mmp9 gene in mdx mice (Figs 3 and 4) suggesting that the elevated levels of MMP-9 protein contribute to skeletal muscle pathogenesis in mdx mice by multiple mechanisms (Fig. 4). One of the intriguing aspects of our study is that the deletion of a single allele of Mmp9 gene was sufficient to reduce the protein levels of MMP-9 in skeletal muscle (Fig. 3A) and attenuated muscle pathogenesis to a greater extent (Figs 3 and 4). A drastic reduction in MMP-9 levels in skeletal muscle tissues on deletion of a single allele of Mmp9 in mdx mice (Fig. 3A) suggests that MMP-9 might be regulating its own expression possibly by exaggerating the accumulation of inflammatory cells and/or augmenting the activity of various proinflammatory molecules. This contention is supported by our data which demonstrate that heterozygous deletion of Mmp9 gene significantly inhibited the activation of proinflammatory transcription factors AP-1 and NF-κB (Fig. 3B) and reduced the accumulation of macrophages (Fig. 4A and B) in dystrophic muscle of mdx mice. Furthermore, along with MMP-9, the levels of MMP-2 proteins were also decreased in skeletal muscle of mdx;Mmp9+/− and mdx;Mmp9−/− mice (Fig. 3A) indicating that MMP-9 also contributes to the increased expression of MMP-2. Indeed, accumulating evidence strongly suggests that there is a cooperative interaction between various MMPs to promote effective tissue degradation (11–13). However, it is also possible that the reduced amount of MMP-2 in mdx;Mmp9+/− or mdx;Mmp9−/− mice is a result of the reduced concentration of inflammatory cells (Fig. 4A and B) in dystrophic muscle of mdx mice upon inhibition of MMP-9 activity.
Skeletal muscles of DMD patients and mdx mice show an increase in connective tissues between muscle fibers (fibrosis) and fatty infiltration (36–38). The diaphragm of the mdx mice is the first muscle to exhibit progressive degeneration, fibrosis and functional insufficiency similar to that seen in DMD muscles (47,48). However, the underlying mechanisms leading to the accumulation of matrix components in dystrophic muscle remain poorly understood. A common view is that the decrease in muscle fiber stability, due to lack of dystrophin, leads to degeneration of muscle fibers followed by invasion of muscle tissues by inflammatory cells such as macrophages, neutrophils and T lymphocytes, which increases fibrosis (48,49). In fact, depletion of macrophages and lymphocytes has been found to reduce muscle degeneration and fibrosis in mdx mice (34,49,50). Interestingly, MMP-9 is one of the most important mediators of inflammation and is strongly implicated in ECM remodeling leading to fibrosis in several other tissues (51,52). MMP-9 is also involved in proteolytic processing of many proinflammatory cytokines including the focal release and activation of TGF-β (13,39), a major mediator of fibrosis in dystrophinopathy (38). Our results demonstrating a significant decrease in the activity of AP-1 and NF-κB transcription factors and reduced concentration of inflammatory cells (Figs 3C and 4A and B) with concomitant decrease in fibrosis (Fig. 5A–C) in mdx;Mmp9+/− and mdx;Mmp9−/− mice strongly suggest that MMP-9 contributes to the accumulation of fibrotic tissues in mdx mice. Furthermore, the levels of active TGF-β protein were also significantly reduced in skeletal muscle of mdx;Mmp9+/− or mdx;Mmp9−/− mice (Fig. 5D) indicating that MMP-9 is involved in proteolytic processing of latent TGF-β into active form in dystrophic muscles.
Although the exact mechanisms by which MMP-9 causes muscle pathology could be quite complex, the most important proteolytic targets of MMP-9 might be the components of skeletal muscle cytoskeleton and interacting proteins in ECM (12,13). It has also been reported that besides dystrophin, the levels of several other proteins of the DGC (e.g. α-, β- and γ-sarcoglycan, β-dystroglycan and nNOS) are reduced in skeletal muscle of mdx mice (40). Our results suggest that the enhanced proteolysis of the specific components of the ECM-cytoskeleton network is one of the potential mechanisms by which MMP-9 causes muscle pathogenesis. This contention is supported by our data demonstrating that sarcolemmal structure was significantly improved in skeletal muscle of mdx;Mmp9+/− or mdx;Mmp9−/− mice compared with mdx;Mmp9+/+ mice (Fig. 6A). Furthermore, genetic ablation of MMP-9 in mdx mice also increased the protein levels of collagen IV (Fig. 5C), β-dystroglycan and nNOS (Fig. 6B) without affecting the levels of several other DGC-related proteins (Fig. 6B). Since collagen IV and b-dystroglycan are the known physiological substrates of MMP-9 (12,13,41), their increased level in mdx;Mmp9+/− and mdx;Mmp9−/− mice is on the expected lines. However, it remains enigmatic how MMP-9 inhibition increases the protein levels of nNOS in myofibers of mdx mice. In addition, we found that genetic ablation of MMP-9 reduces the level of caveolin-3 (a small molecular weight protein localized to the sarcolemma) in skeletal muscle of mdx mice (Fig. 6B). Interestingly, caveolin-3 directly interacts with nNOS and inhibits its catalytic activity (53). Furthermore, both reduced protein levels of nNOS (26) and increased expression of caveolin-3 (54,55) are linked to skeletal muscle pathogenesis in mdx mice. Collectively, these results suggest that besides directly inducing the proteolysis of specific ECM-cytoskeletal proteins, the elevated levels of MMP-9 may also induce pathogenesis by indirect mechanisms which include the alteration in the protein level/activity of nNOS and caveolin-3 protein in dystrophic muscle.
An interesting observation of the present study was that genetic ablation or pharmacological inhibition of MMP-9 significantly improved skeletal muscle regeneration in mdx mice (Figs 7 and 8). Furthermore, the expression of Pax-7, a specific cell surface marker for satellite cells (43), was significantly increased in skeletal muscle of mdx;Mmp9+/− and mdx;Mmp9−/− mice compared with mdx;Mmp9+/+ mice (Fig. 7D) indicating that the enhanced myofiber regeneration was a result of the increased satellite cell proliferation in mdx mice upon inhibition of MMP-9 activity. Although the exhaustion of satellite cells due to excessive degeneration and regeneration cycles has been suggested as the major reason for insufficient myofiber regeneration in DMD (4,56), the loss of regenerative capacity in DMD could also be attributed to other factors such as a progressive increase in muscle interstitial fibrosis, which may prevent the availability of growth factors and migration of myogenic cells required for myotube formation (57,58). Increased amount of proinflammatory cytokines such as TNF-α and IL-1β, which are present in dystrophic muscle (6,27), may also inhibit the fusion of myoblasts into injured myofibers. Furthermore, since basement membrane is essential for skeletal muscle regeneration and formation of neuromuscular junctions (59), extensive degradation of basement membrane in dystrophic myofibers might also interfere with the ability of skeletal muscle to regenerate. A recent report also suggests that the increased activation of NF-κB interferes with the skeletal muscle regeneration in mdx mice (27). Because the inhibition of MMP-9 activity significantly inhibits inflammatory response (Figs 3 and 4A and B), fibrosis (Fig. 5) and the activation of NF-κB transcription factor (Figs 3B and 8F), and attenuates myofiber membrane abnormalities (Fig. 6A), the increased regeneration of skeletal muscle in mdx;Mmp9+/− and mdx;Mmp9−/− mice could be the result of some of these effects of the inhibition of MMP-9.
Recent reports from our and other groups suggest that the activity of various proinflammatory transcription factors such as NF-κB and AP-1 is significantly elevated in skeletal muscle of mdx mice (6,7,27). Furthermore, pharmacological or genetic inhibition of NF-κB has been reported to attenuate skeletal muscle pathogenesis in mdx mice (27). We have also shown that the expression of MMP-9 in response to various proinflammatory cytokines is controlled through the transcriptional activation of NF-κB and AP-1 in skeletal muscle (29,30). In addition, our data show that the in vivo administration of NF-κB inhibitory peptide (i.e. NBD) reduces the amount of MMP-9 protein in skeletal muscle of mdx mice (Fig. 2C). Interestingly, Acharyya et al. (27) have recently reported that in vivo administration of NBD peptide prevents muscle pathology and improves skeletal muscle regeneration in mdx mice. Because MMP-9 is a prominent NF-κB-regulated gene (28), it is possible that NF-κB induces muscle pathology in mdx mice through the increased expression of MMP-9 protein. The following line of evidence is further suggestive of a cooperative interaction between NF-κB and MMP-9 to induce muscle pathogenesis in mdx mice. (i) NF-κB activation and expression of MMP-9 follow similar pattern in skeletal muscle of mdx mice. The activation of NF-κB (6,27) and levels of MMP-9 protein (Fig. 1B) remain elevated after 2 weeks in mdx mice; (ii) genetic or pharmacological inhibition of either NF-κB (27) or MMP-9 (Figs 3, 4 and 8) inhibits various parameters of skeletal muscle pathology such as fiber necrosis and inflammation in mdx mice; and (iii) inhibition of either NF-κB (27) or MMP-9 (Figs 7 and 8) improves skeletal muscle regeneration in mdx mice. In an earlier study, Chen et al. (60) reported that NF-κB is strongly induced in skeletal muscle of DMD patients immediately after birth. However, at later stages of disease progression, the activation of TGF-β pathway is increased (60) indicating that the activated NF-κB might be inducing TGF-β pathways through enhancing the expression of MMP-9, which converts latent TGF-β into active form (13).
In summary, our study provides strong evidence that the increased production of MMP-9 contributes to the skeletal muscle pathogenesis in dystrophin-deficient skeletal muscle. Based on the results of this study, which suggest that even partial inhibition of MMP-9 activity effectively reduces skeletal muscle pathogenesis in mdx mice, we believe that MMP-9 will serve as an important molecular target to mitigate skeletal muscle pathogenesis in DMD patients in future therapies.
MATERIALS AND METHODS
Mice
Control (strain: C57BL10/ScSn), mdx (strain: C57BL/10ScSn DMDmdx) and Mmp9-knockout (strain: FVB.Cg-Mmp9tm1Tvu) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Mmp9-knockout mice were first crossed with C57BL10/ScSn mice for four to five generations and then with mdx mice to generate littermate WT, mdx;Mmp9+/+, mdx;Mmp9+/− and mdx;Mmp9−/−. All genotypes were determined by PCR analysis from tail DNA. Amplification-resistant mutation system assay was used to identify control and mdx mice (61). Mmp9-knockout and WT mice were identified using the primer sets suggested by the Jackson Laboratory. Mice were housed in the animal facility of the University of Louisville School of Medicine under conventional conditions with constant temperature and humidity and fed a standard diet.
For pharmacological studies in vivo, (2R)-2-[(4-Biphenylylsulfonyl) amino]-3-phenylpropionic acid (also known as MMP-2/9 inhibitor I) (from CalBiochem, cat no. 444241) was first dissolved in dimethyl sulfoxide (DMSO) and diluted in phosphate buffered saline (PBS) (final, 0.05% DMSO). Three-week-old mdx mice were given daily intraperitoneal injections of the inhibitor (5 mg/Kg body weight) or vehicle (i.e. 0.05% DMSO in PBS) alone before euthanizing mice and analysis of skeletal muscle pathology at 6 weeks.
To study the role of NF-κB in MMP-9 expression in skeletal muscle, 4-week-old mdx mice were given three intraperitoneal injection of 200 µg either control or NBD peptide (Imgenex, San Diego, CA, USA) every third day. Twenty-four hours after the final injection, the mice were sacrificed and muscle tissues were isolated for biochemical analysis.
To detect sarcolemmal damage, mice were given intraperitoneal injection of 1% EBD in PBS at a dose of 100 µl per 10 gm body weight. The mice were visually inspected for dye uptake. Discoloration of all mice was observed within 50–60 min after EBD injection and the successful injection of the dye was indicated by the blue colors of ears and paws. Mice were sacrificed 24 h post-EBD injection and uptake of EBD by injured myofibers was examined in muscle cryosections using a fluorescent microscope. All experiments with animals were approved by the Institutional Animal Care and Use Committee of the University of Louisville.
Gelatin zymography
To determine MMP-9 activity in skeletal muscle of control and mdx mice, muscle extracts were prepared in non-reducing lysis buffer [50 mm Tris-Cl (pH 8.0), 200 mm NaCl, 50 mm NaF, 0.3% IGPAL CA-630 and protease inhibitors]. Equal amount of proteins (100 µg/sample) was separated on 8% SDS–PAGE containing 1 mg/ml gelatin B (Fisher Scientific) under non-reducing conditions. Gels were washed in 2.5% Triton X-100 for 1 h at room temperature followed by incubation in reaction buffer [50 mm Tris–HCl (pH 8.0), 50 mm NaCl, 5 mm CaCl2 and 0.02% sodium azide] for 48 h at 37°C. To visualize gelatinolytic bands, gels were stained with Coomassie Brilliant Blue dye at room temperature followed by extensive washing in destaining buffer (10% methanol and 10% acetic acid in distilled water). The gels were photographed for determination of gelatinolytic activity.
Western blotting
Tissue levels of different proteins were determined using a method as described (6). Briefly, tissues were washed with phosphate-buffered saline (PBS) and homogenized in lysis buffer A [50 mm Tris-Cl (pH 8.0), 200 mm NaCl, 50 mm NaF, 1 mm dithiotheritol (DTT), 1 mm sodium orthovanadate, 0.3% IGEPAL, and protease inhibitors]. Approximately, 100 µg protein was resolved on each lane on 8–12% SDS–PAGE, electrotransferred onto nitrocellulose membrane and probed using anti-MMP-9 (1:2000; R&D Systems), anti-MMP-2 (1:3000, R&D Systems), anti-β-dystroglycan (1:100, Novacastra), laminin (1:1000; Sigma), anti-TIMP-1 (1:1000, Santa Cruz), anti-TIMP-2 (1:500, CalBiochem), anti-TIMP-3 (1:1000; Santa Cruz), anti-TIMP-4 (1:1000, Santa Cruz), anti-E-MyHC (1:100, Developmental Studies Hybridoma Bank), anti-Pax-7 (1:500; Developmental Studies Hybridoma Bank), collagen I (1:1000; Abcam), anti-collagen III (1:1000; Abcam), anti-collagen IV (1:500; Abcam), anti-utrophin (1:100; Developmental Studies Hybridoma Bank), anti-alpha-dystroglycan (Santa Cruz), anti-α-dystrobrevin (1:500; Santa Cruz), anti-nNOS (1:500; Santa Cruz), anti-caveolin-3 (1:500, Santa Cruz), anti-TGF-β (1:1000, Cell Signaling Technology) and anti-tubulin (1:5000, Abcam) and detected by chemiluminescence. To determine the levels of collagens I and III, muscle extracts were prepared in lysis buffer lacking DTT and separated on SDS–PAGE under non-reducing conditions.
Quantitative real-time PCR
RNA isolation and QRT-PCR were performed to measure the mRNA level of MMP-9 using a method as described (30).
Electrophoretic mobility shift assay (EMSA)
Activation of NF-κB and AP-1 transcription factors was studied using electrophoretic mobility shift assay (EMSA) as previously described (6,7).
Creatine kinase assay
The serum level of CK was determined using a commercially available kit (Stanbio Laboratory, TX, USA). CK activity was expressed as Units/liter.
Histology and immunohistochemistry
For immunofluorescent staining, serial cross sections (10 µm thick) from mid-belly of frozen skeletal muscle tissues were mounted on glass slides and fixed in acetone for 10 min. The sections were blocked in 1% bovine serum albumin in PBS for 1 h, and incubated with primary antibodies in blocking solution at 4°C overnight under humidified conditions. The sections were washed briefly with PBS before incubation with secondary antibodies for 1 h at room temperature and then washed three times for 30 min with PBS. The slides were mounted using fluorescence medium with DAPI (Vector Laboratories), visualized with a fluorescent microscope (Nikon) and images were captured using Nikon DS Fi1 camera (Nikon). The primary antibodies dilution and source are as follows: anti-MMP9 (1:100; Millipore), CD-68 (1:50; Serotec), anti-laminin (1:100, Sigma), anti-collagen I (1:300, Abcam), anti-collagen III (1:300, Abcam), anti-collagen IV (1:500), anti-E-MyHC (1:50, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA). Alexa Fluor® 488 or Alexa Fluor®596-conjugated secondary antibodies were obtained from Invitrogen and used at 1:3000 dilution. Anti-F4/80 (1:50, AbD Serotec) was used in conjunction with the VECSTAIN ABC staining kit (Vector) with DAB substrate according to the manufacturer's protocol. Necrotic fibers in muscle cryosections were identified by immunostaining with Cy3-labelled goat anti-mouse IgG (1:3000, Invitrogen). Amount of fibrosis in paraffin-embedded diaphragm muscle sections was determined using Mason's Trichrome staining kit (American Master Tech) following a protocol suggested by manufacturers.
Transmission electron microscopy
Gastrocnemius muscle isolated were fixed in 3% glutaraldehyde in cocodylate buffer (0.1 m) overnight followed by fixing in 1% cocodylate buffered Osmium tetroxide. The tissue was dehydrated through a series of graded alcohols, and embedded in LX-112 plastic (Ladd Research Industries, Burlington, VT, USA). Transverse sections (80 nm) were cut using an LKB ultramicrotome and stained with uranium acetate and lead citrate. Samples were analyzed using a Philips CM 12 transmission electron microscope operating at 60 kV. The pictures were captured at 4400× magnification using a 3.2 mega pixel digital camera (Sia-7C).
Skeletal muscle functional studies
Soleus muscle was rapidly excised and placed in Krebs-Ringer solution. The muscle was mounted between a Fort25 force transducer (World Precision Instrumentation) and a micromanipulator device in a temperature-controlled myobath (World Precision Instrumentation). The muscle was positioned between platinum wire stimulating electrodes and stimulated to contract isometrically using electrical field stimulation (supramaximal voltage, 1.2 ms pulse duration) using a Grass S88 stimulator. In each experiment, muscle length was adjusted to optimize twitch force (optimal length, Lo). The muscle was rested for 15 min before the tetanic protocol was started. The output of the force transducer was recorded in computer using LAB-TRAX-4 software. Maximal tetanic contraction was assessed at 250 Hz for 500 ms duration. To investigate a potentially different frequency response between groups, tetanic were assessed by sequential stimulation at 5, 15, 30, 50, 80, 120, 150 and 250 Hz with 2 min rest in between. The cross-sectional area for each muscle was determined by dividing muscle weight by its length and tissue density (1.06 g/l), and muscle force was compared after correction for cross-sectional area.
Statistical significance
Results are expressed as mean ± standard deviation (SD). Statistical analysis used Student's t-test or ANOVA to compare quantitative data populations with normal distribution and equal variance. A value of P < 0.05 was considered statistically significant unless otherwise specified.
Conflict of Interest statement. None declared.
FUNDING
This work was supported, in parts, by institutional start-up funds and a National Institute of Health grant (RO1 AG129623) to A.K.
REFERENCES
- 1.Emery A.E. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 2.Cohn R.D., Campbell K.P. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Allamand V., Campbell K.P. Animal models for muscular dystrophy: valuable tools for the development of therapies. Hum. Mol. Genet. 2000;9:2459–2467. doi: 10.1093/hmg/9.16.2459. [DOI] [PubMed] [Google Scholar]
- 4.Dalkilic I., Kunkel L.M. Muscular dystrophies: genes to pathogenesis. Curr. Opin. Genet. Dev. 2003;13:231–238. doi: 10.1016/s0959-437x(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 5.Rando T.A. The dystrophin–glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve. 2001;24:1575–1594. doi: 10.1002/mus.1192. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A., Boriek A.M. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17:386–396. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A., Khandelwal N., Malya R., Reid M.B., Boriek A.M. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004;18:102–113. doi: 10.1096/fj.03-0453com. [DOI] [PubMed] [Google Scholar]
- 8.Judge L.M., Haraguchiln M., Chamberlain J.S. Dissecting the signaling and mechanical functions of the dystrophin–glycoprotein complex. J. Cell. Sci. 2006;119:1537–1546. doi: 10.1242/jcs.02857. [DOI] [PubMed] [Google Scholar]
- 9.Blake D.J., Weir A., Newey S.E., Davies K.E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 10.Engvall E., Wewer U.M. The new frontier in muscular dystrophy research: booster genes. FASEB J. 2003;17:1579–1584. doi: 10.1096/fj.02-1215rev. [DOI] [PubMed] [Google Scholar]
- 11.Mott J.D., Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu T.H., Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 13.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandler S., Miller K.M., Clements J.M., Lury J., Corkill D., Anthony D.C., Adams S.E., Gearing A.J. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J. Neuroimmunol. 1997;72:155–161. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 15.Hu J., Van den Steen P.E., Sang Q.X., Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 16.Waubant E., Goodkin D.E., Gee L., Bacchetti P., Sloan R., Stewart T., Andersson P.B., Stabler G., Miller K. Serum MMP-9 and TIMP-1 levels are related to MRI activity in relapsing multiple sclerosis. Neurology. 1999;53:1397–1401. doi: 10.1212/wnl.53.7.1397. [DOI] [PubMed] [Google Scholar]
- 17.Hoyhtya M., Hujanen E., Turpeenniemi-Hujanen T., Thorgeirsson U., Liotta L.A., Tryggvason K. Modulation of type-IV collagenase activity and invasive behavior of metastatic human melanoma (A2058) cells in vitro by monoclonal antibodies to type-IV collagenase. Int. J. Cancer. 1990;46:282–286. doi: 10.1002/ijc.2910460224. [DOI] [PubMed] [Google Scholar]
- 18.Turpeenniemi-Hujanen T., Thorgeirsson U.P., Hart I.R., Grant S.S., Liotta L.A. Expression of collagenase IV (basement membrane collagenase) activity in murine tumor cell hybrids that differ in metastatic potential. J. Natl Cancer Inst. 1985;75:99–103. [PubMed] [Google Scholar]
- 19.Carmeli E., Moas M., Reznick A.Z., Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. 2004;29:191–197. doi: 10.1002/mus.10529. [DOI] [PubMed] [Google Scholar]
- 20.Choi Y.C., Dalakas M.C. Expression of matrix metalloproteinases in the muscle of patients with inflammatory myopathies. Neurology. 2000;54:65–71. doi: 10.1212/wnl.54.1.65. [DOI] [PubMed] [Google Scholar]
- 21.Koskinen S.O., Kjaer M., Mohr T., Sorensen F.B., Suuronen T., Takala T.E. Type IV collagen and its degradation in paralyzed human muscle: effect of functional electrical stimulation. Muscle Nerve. 2000;23:580–589. doi: 10.1002/(sici)1097-4598(200004)23:4<580::aid-mus18>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Schiotz Thorud H.M., Stranda A., Birkeland J.A., Lunde P.K., Sjaastad I., Kolset S.O., Sejersted O.M., Iversen P.O. Enhanced matrix metalloproteinase activity in skeletal muscles of rats with congestive heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R389–R394. doi: 10.1152/ajpregu.00067.2005. [DOI] [PubMed] [Google Scholar]
- 23.Davies J.E., Wang L., Garcia-Oroz L., Cook L.J., Vacher C., O'Donovan D.G., Rubinsztein D.C. Doxycycline attenuates and delays toxicity of the oculopharyngeal muscular dystrophy mutation in transgenic mice. Nat. Med. 2005;11:672–677. doi: 10.1038/nm1242. [DOI] [PubMed] [Google Scholar]
- 24.Hnia K., Hugon G., Rivier F., Masmoudi A., Mercier J., Mornet D. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient Mdx mice. Am. J. Pathol. 2007;170:633–643. doi: 10.2353/ajpath.2007.060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kherif S., Lafuma C., Dehaupas M., Lachkar S., Fournier J.G., Verdiere-Sahuque M., Fardeau M., Alameddine H.S. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev. Biol. 1999;205:158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- 26.Wehling M., Spencer M.J., Tidball J.G. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acharyya S., Villalta S.A., Bakkar N., Bupha-Intr T., Janssen P.M., Carathers M., Li Z.W., Beg A.A., Ghosh S., Sahenk Z., et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakraborti S., Mandal M., Das S., Mandal A., Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol. Cell. Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 29.Li H., Mittal A., Paul P.K., Kumar M., Srivastava D.S., Tyagi S.C., Kumar A. Tumor necrosis factor-related weak inducer of apoptosis augments matrix metalloproteinase-9 production (MMP-9) in skeletal muscle through the activation of nuclear factor-kappa B-inducing kinase and p38 mitogen-activated protein kinase: a potential role of MMP-9 in myopathy. J. Biol. Chem. 2008;284:4439–4450. doi: 10.1074/jbc.M805546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava A.K., Qin X., Wedhas N., Arnush M., Linkhart T.A., Chadwick R.B., Kumar A. Tumor necrosis factor-alpha augments matrix metalloproteinase-9 production in skeletal muscle cells through the activation of transforming growth factor-beta-activated kinase 1 (TAK1)-dependent signaling pathway. J. Biol. Chem. 2007;282:35113–35124. doi: 10.1074/jbc.M705329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May M.J., D'Acquisto F., Madge L.A., Glockner J., Pober J.S., Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 32.Straub V., Campbell K.P. Muscular dystrophies and the dystrophin–glycoprotein complex. Curr. Opin. Neurol. 1997;10:168–175. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 34.Wehling-Henricks M., Sokolow S., Lee J.J., Myung K.H., Villalta S.A., Tidball J.G. Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum. Mol. Genet. 2008;17:2280–2292. doi: 10.1093/hmg/ddn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreetta F., Bernasconi P., Baggi F., Ferro P., Oliva L., Arnoldi E., Cornelio F., Mantegazza R., Confalonieri P. Immunomodulation of TGF-beta 1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: implications for antifibrotic therapy. J. Neuroimmunol. 2006;175:77–86. doi: 10.1016/j.jneuroim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Gosselin L.E., Williams J.E., Personius K., Farkas G.A. A comparison of factors associated with collagen metabolism in different skeletal muscles from dystrophic (mdx) mice: impact of pirfenidone. Muscle Nerve. 2007;35:208–216. doi: 10.1002/mus.20681. [DOI] [PubMed] [Google Scholar]
- 38.Bernasconi P., Torchiana E., Confalonieri P., Brugnoni R., Barresi R., Mora M., Cornelio F., Morandi L., Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J. Clin. Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Q., Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 40.Ohlendieck K., Campbell K.P. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J. Cell. Biol. 1991;115:1685–1694. doi: 10.1083/jcb.115.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michaluk P., Kolodziej L., Mioduszewska B., Wilczynski G.M., Dzwonek J., Jaworski J., Gorecki D.C., Ottersen O.P., Kaczmarek L. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J. Biol. Chem. 2007;282:16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- 42.Zhong D., Saito F., Saito Y., Nakamura A., Shimizu T., Matsumura K. Characterization of the protease activity that cleaves the extracellular domain of beta-dystroglycan. Biochem. Biophys. Res. Commun. 2006;345:867–871. doi: 10.1016/j.bbrc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Le Grand F., Rudnicki M.A. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura Y., Watanabe F., Nakatani T., Yasui K., Fuji M., Komurasaki T., Tsuzuki H., Maekawa R., Yoshioka T., Kawada K., et al. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J. Med. Chem. 1998;41:640–649. doi: 10.1021/jm9707582. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi M., Jadhav V., Obenaus A., Colohan A., Zhang J.H. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery. 2007;61:1067–1075. doi: 10.1227/01.neu.0000303203.07866.18. [DOI] [PubMed] [Google Scholar]
- 46.Khurana T.S., Davies K.E. Pharmacological strategies for muscular dystrophy. Nat. Rev. Drug. Discov. 2003;2:379–390. doi: 10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- 47.Stedman H.H., Sweeney H.L., Shrager J.B., Maguire H.C., Panettieri R.A., Petrof B., Narusawa M., Leferovich J.M., Sladky J.T., Kelly A.M. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 48.Morrison J., Lu Q.L., Pastoret C., Partridge T., Bou-Gharios G. T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab. Invest. 2000;80:881–891. doi: 10.1038/labinvest.3780092. [DOI] [PubMed] [Google Scholar]
- 49.Morrison J., Palmer D.B., Cobbold S., Partridge T., Bou-Gharios G. Effects of T-lymphocyte depletion on muscle fibrosis in the mdx mouse. Am. J. Pathol. 2005;166:1701–1710. doi: 10.1016/S0002-9440(10)62480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spencer M.J., Montecino-Rodriguez E., Dorshkind K., Tidball J.G. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin. Immunol. 2001;98:235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- 51.Ducharme A., Frantz S., Aikawa M., Rabkin E., Lindsey M., Rohde L.E., Schoen F.J., Kelly R.A., Werb Z., Libby P., et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim D.H., Cho J.Y., Miller M., McElwain K., McElwain S., Broide D.H. Reduced peribronchial fibrosis in allergen-challenged MMP-9-deficient mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L265–L271. doi: 10.1152/ajplung.00305.2005. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Cardena G., Martasek P., Masters B.S., Skidd P.M., Couet J., Li S., Lisanti M.P., Sessa W.C. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 54.Galbiati F., Volonte D., Chu J.B., Li M., Fine S.W., Fu M., Bermudez J., Pedemonte M., Weidenheim K.M., Pestell R.G., et al. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc. Natl Acad. Sci. USA. 2000;97:9689–9694. doi: 10.1073/pnas.160249097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaghy P.L., Fang J., Wu W., Vaghy L.P. Increased caveolin-3 levels in mdx mouse muscles. FEBS Lett. 1998;431:125–127. doi: 10.1016/s0014-5793(98)00738-8. [DOI] [PubMed] [Google Scholar]
- 56.Heslop L., Morgan J.E., Partridge T.A. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J. Cell. Sci. 2000;113:2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- 57.Irintchev A., Zweyer M., Wernig A. Impaired functional and structural recovery after muscle injury in dystrophic mdx mice. Neuromuscul. Disord. 1997;7:117–125. doi: 10.1016/s0960-8966(96)00422-1. [DOI] [PubMed] [Google Scholar]
- 58.Duance V.C., Stephens H.R., Dunn M., Bailey A.J., Dubowitz V. A role for collagen in the pathogenesis of muscular dystrophy? Nature. 1980;284:470–472. doi: 10.1038/284470a0. [DOI] [PubMed] [Google Scholar]
- 59.Sanes J.R. The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 2003;278:12601–12604. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y.W., Nagaraju K., Bakay M., McIntyre O., Rawat R., Shi R., Hoffman E.P. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005;65:826–834. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- 61.Amalfitano A., Chamberlain J.S. The mdx-amplification-resistant mutation system assay, a simple and rapid polymerase chain reaction-based detection of the mdx allele. Muscle Nerve. 1996;19:1549–1553. doi: 10.1002/(SICI)1097-4598(199612)19:12<1549::AID-MUS4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]