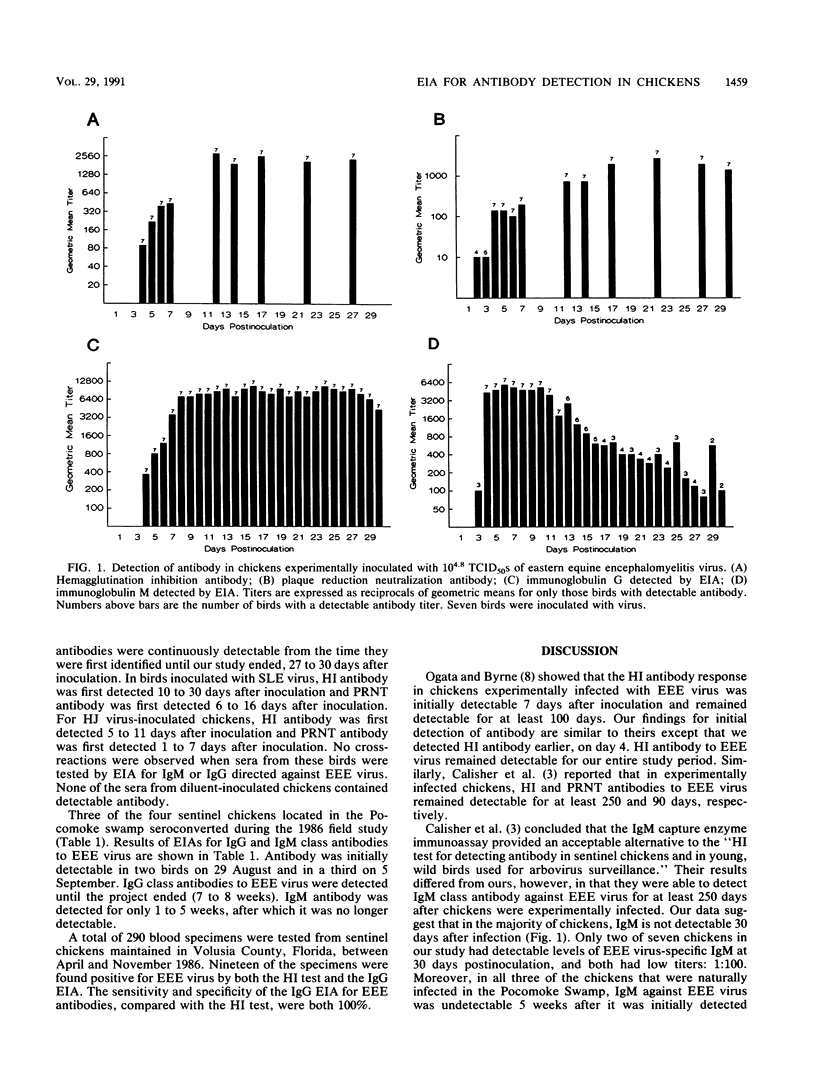
Abstract
We developed an enzyme immunoassay (EIA) for the detection of immunoglobulin M (IgM) and IgG subclass antibodies directed against eastern equine encephalomyelitis (EEE) virus in chickens. The assays were compared with the serum plaque reduction neutralization test (PRNT) and the hemagglutination inhibition (HI) test for ability to detect antibodies against EEE virus in laboratory-infected birds. No cross-reactivity was detected in serum from chickens inoculated with St. Louis encephalitis or Highlands J virus. The interval after infection when EEE virus-specific antibodies were first detected by IgM and IgG EIAs was found to be similar to that determined by the PRNT and HI tests: 2 to 4 days. The IgG EIA, PRNT, and HI test detected antibodies to EEE virus for at least 27 to 30 days after inoculation. In contrast, serum from five of seven chickens did not contain detectable IgM 30 days after infection. Similarly, in all three naturally infected sentinel chickens from Maryland, IgM class antibody was undetectable 1 to 5 weeks after IgM was initially detected. EIAs provide simple and rapid alternatives to traditional tests for monitoring EEE virus infections in sentinel chicken flocks. Moreover, the IgM EIA provides a means to separate recently infected chickens from those infected greater than or equal to 1 month earlier.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke D. S., Tingpalapong M., Elwell M. R., Paul P. S., Van Deusen R. A. Japanese encephalitis virus immunoglobulin M antibodies in porcine sera. Am J Vet Res. 1985 Oct;46(10):2054–2057. [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Calisher C. H., Fremount H. N., Vesely W. L., el-Kafrawi A. O., Mahmud M. I. Relevance of detection of immunoglobulin M antibody response in birds used for arbovirus surveillance. J Clin Microbiol. 1986 Nov;24(5):770–774. doi: 10.1128/jcm.24.5.770-774.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crans W. J. Failure of chickens to act as sentinels during an epizootic of eastern equine encephalitis in southern New Jersey, USA. J Med Entomol. 1986 Dec 4;23(6):626–629. doi: 10.1093/jmedent/23.6.626. [DOI] [PubMed] [Google Scholar]
- Hildreth S. W., Beaty B. J. Detection of eastern equine encephalomyelitis virus and Highlands J virus antigens within mosquito pools by enzyme immunoassay (EIA). I. A laboratory study. Am J Trop Med Hyg. 1984 Sep;33(5):965–972. doi: 10.4269/ajtmh.1984.33.965. [DOI] [PubMed] [Google Scholar]
- Hildreth S. W., Beaty B. J., Maxfield H. K., Gilfillan R. F., Rosenau B. J. Detection of eastern equine encephalomyelitis virus and Highlands J virus antigens within mosquito pools by enzyme immunoassay (EIA). II. Retrospective field test of the EIA. Am J Trop Med Hyg. 1984 Sep;33(5):973–980. doi: 10.4269/ajtmh.1984.33.973. [DOI] [PubMed] [Google Scholar]
- OGATA M., BYRNE R. J. Relationships between eastern and western equine encephalomyelitis viruses as demonstrated by the hemagglutination-inhibition antibody response of experimentally infected chickens. Am J Vet Res. 1961 Mar;22:266–270. [PubMed] [Google Scholar]
- SEVER J. L., CASTELLANO G. A., PELON W., HUEBNER R. J., WOLMAN F. INACTIVATION OF THE INFECTIVITY OF VIRAL HEMAGGLUTINATING ANTIGENS WITH THE USE OF BETAPRONE. J Lab Clin Med. 1964 Dec;64:983–988. [PubMed] [Google Scholar]
- Saugstad E. S., Dalrymple J. M., Eldridge B. F. Ecology of arboviruses in a Maryland freshwater swamp. I. Population dynamics and habitat distribution of potential mosquito vectors. Am J Epidemiol. 1972 Aug;96(2):114–122. doi: 10.1093/oxfordjournals.aje.a121437. [DOI] [PubMed] [Google Scholar]
- Scott T. W., McLean R. G., Francy D. B., Mitchell C. J., Card C. S. Experimental infections of birds with Turlock virus. J Wildl Dis. 1983 Apr;19(2):82–85. doi: 10.7589/0090-3558-19.2.82. [DOI] [PubMed] [Google Scholar]
- Scott T. W., Olson J. G., All B. P., 3rd, Gibbs E. P. Detection of eastern equine encephalomyelitis virus antigen in equine brain tissue by enzyme-linked immunosorbent assay. Am J Vet Res. 1988 Oct;49(10):1716–1718. [PubMed] [Google Scholar]
- Scott T. W., Olson J. G., Lewis T. E., Carpenter J. W., Lorenz L. H., Lembeck L. A., Joseph S. R., Pagac B. B. A prospective field evaluation of an enzyme immunoassay: detection of eastern equine encephalomyelitis virus antigen in pools of Culiseta melanura. J Am Mosq Control Assoc. 1987 Sep;3(3):412–417. [PubMed] [Google Scholar]
- Vernon S. D., Webb P. A. Recent vesicular stomatitis virus infection detected by immunoglobulin M antibody capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1985 Oct;22(4):582–586. doi: 10.1128/jcm.22.4.582-586.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. M., Clark G. G., Crabbs C. L., Rossi C. A., Olin T. R., Bailey C. L. Ecological evidence against vertical transmission of eastern equine encephalitis virus by mosquitoes (Diptera: Culicidae) on the Delmarva Peninsula, USA. J Med Entomol. 1987 Jan;24(1):91–98. doi: 10.1093/jmedent/24.1.91. [DOI] [PubMed] [Google Scholar]


