Abstract
Cell to cell communication is essential for the organization/coordination of multicellular systems and cellular development. Cellular communication is mediated by soluble factors, including growth factors, neurotransmitters, cytokines/chemokines, gap junctions, and the recently described tunneling nanotubes (TNT). TNT are long cytoplasmatic bridges that enable long range directed communication between cells. The proposed function for TNT is the cell-to-cell transfer of large cellular structures such as vesicles and organelles. We demonstrate that HIV-infection of human macrophages results in an increased number of TNT, and show HIV particles within these structures. We propose that HIV “highjacks” TNT communication to spread HIV through an intercellular route between communicated cells, contributing to the pathogenesis of AIDS.
Keywords: Filopodia, Communication, AIDS, Inflammation, Infectivity, HIV
1. Introduction
Cell-to-cell communication is essential in all biological processes. Among these systems that play critical roles in regulating complex processes [1-3] is the recently described communication system, tunneling nanotubes (TNT). The proposed functions of TNT are the long range exchange of organelles and vesicles and the coordination of signaling between TNT connected cells [1].
TNT formation has been observed in immune cells, including B, T and NK cells, neutrophils and monocytes, as well as in neurons and glial cells (see review by [1]). In vivo, TNT like formations have been observed in Drosophila, where they are named cytonemes [4,5]. TNT like structures have been demonstrated between immune cells in lymph nodes (see review by [1,2]) and in the dendritic cells (DC) of the gut, where long DC processes, similar to TNT, cross the epithelial layer into the gut lumen to access bacteria [6,7]. In some pathological conditions, it has been described that the causative agent, such as viruses, protease resistant prion protein (PrP-res) or bacteria can be transported along the long TNT like processes of infected cells, allowing trafficking of these pathogens between TNT connected cells [2,3,8-15]. After the identification of TNT as a new communication system, re-analyses of previous studies demonstrated the formation of TNT-like structures in several cell types after infection with Listeria monocytogenes or mycobacterium bovis, to facilitate the transfer of bacteria to neighboring cells [11,16,17]. In conjunction with gap junctions, TNT like structures have been identified in astrocytes treated with H2O2 [18], apoptotic Hela cells [19], microglia activated with PMA and calcium ionophore [20], and microglia/monocyte/macrophages treated with LPS plus IFN-γ [21,22].
In HIV-infection, monocyte/macrophages play a critical role as they can be productively infected within tissues. This tissue infection is mediated by transmigration of HIV-infected monocytes from blood into tissues, including the brain, and is an early and key mechanism to maintain viral reservoirs within the brain [23-25]. The presence of HIV-infected monocytes/macrophages in the brain results in activation and infection of brain macrophages/microglia, and astrocytes enhancing inflammation and often resulting in cognitive impairment and dementia [23-26]. This mechanism of tissue inflammation and expansion of infection underscores the importance of macrophages in the pathology of HIV. Thus, we examined mechanism of infection of this cell type.
In this report, we demonstrate that HIV-infection of primary human macrophages induces the formation of TNT, both short and long range, and that the formation of TNT correlates with the time course of viral replication. We also show HIV particles within TNT structures. We propose that the increased numbers of TNT induced by HIV-infection allows HIV particles to spread between connected cells intercellularly, as an additional mechanism of viral infection in concert with the classically described extracellular receptor-mediated component.
2. Materials and methods
2.1. Materials
RPMI, fetal bovine serum (FBS), penicillin/streptomycin (P/S), and trypsin-EDTA were from GibcoBRL (Grand Island, NY). FITC antimouse IgG antibodies were from Sigma (St. Louis, MO). Phalloidin-conjugate to Texas red and antifade with DAPI were obtained from Molecular Probes (Eugene, OR). HIV-p24 antibody was from the NIH repository (NIH, Germantown, MA). Purified mouse IgG2B and IgG1 myeloma protein were from Cappel Pharmaceuticals, Inc.
2.2. HIV-infection of primary cultures of monocytes/macrophages
Blood was obtained from healthy volunteers and PBMC were isolated by Ficoll-Paque (GE Healthcare, Uppsala, Sweden). Using CD11b magnetic beads and columns, monocytes were isolated according to the manufacturer protocol (Miltenyi Biotec, Auburn, CA). After isolation, cells (100,000/well), and cultured for 6 days in adherent conditions in the presence of M-CSF (10 ng/ml, Peprotech, Rocky Hill, NJ). At day 6, the cells were exposed to 20 ng/ml of p24 of HIVADA derived from stock virus generated in CEM-SS cells (NIH repository, Germantown, MA). After 24 h of exposure to the virus, cells were washed extensively to eliminate the unbound virus before addition of fresh medium and then supernatants were collected every day to assess viral replication by HIV-p24 ELISA. Immunofluorescence analyses of these cultures for CD68 (Abcam) indicate that all cells were macrophages. No contamination with another cell type was detected.
2.3. Immunofluorescence
Human monocyte-derived macrophages, HIV-infected and uninfected, were grown on plastic permanox chambers slides (Lab-Tek, Naperville, IL), fixed and permeabilized in 70% ethanol for 20 min at -20 °C. Cells were incubated in blocking solution for 30 min at room temperature and then in primary antibody (anti-p24 or isotype controls: both 1:50) overnight at 4 °C. Cells were washed several times with PBS at room temperature and incubated with phalloidin conjugated to Texas Red (Invitrogen, Carlsbad, CA) to identify actin filaments and/or the appropriate secondary antibody conjugated to FITC (Sigma, St. Louis, MO) for 1 h at room temperature, followed by another wash in PBS for 1 h. Plastic chambers were then mounted using antifade reagent with DAPI, and cells were examined by confocal microscopy using a Leica microscope (Wetzlar, Germany). To analyze the processes, serial Z-sections from the surface to the bottom of the macrophages were obtained (70-180 optical sections) and representative optical reconstruction of those macrophages or optical sections of bottom and top sections were examined. The numbers of macrophages containing filopodia, short, long TNT, and their length were quantified using imaging software, NIS Elements, from Nikon Instruments (Nikon, Japan). To measure the length of the processes, a line was drawn from the beginning of the processes to the end on the process in the connected cells, and the NIS Elements program calculated the length based on the length of the line and the amplification of the pictures taken using the confocal microscope.
2.4. Statistical analysis
Student’s one-tailed, paired t test was used to compare the numbers and length of processes and the p24 values. A value of p < 0.05 was considered significant.
3. Results
3.1. Characterization and kinetics of HIV-infection of primary human macrophages
Human monocytes were isolated using CD11b magnetic beads and cultured in M-CSF for 6 days as described in the Methods. Cultures of human monocyte-derived macrophages were then infected with HIVADA (an R5 virus, 20 ng/ml of p24) for 24 h, and after extensive washing to eliminate unbound virus, medium was replaced and p24 production was quantified every 24 h for 8 days by HIV-p24 ELISA (Fig. 1A). Our results indicate that HIV replication reached the maximum after 2 days post-infection (Fig. 1A, n = 8). After the peak of infection, viral replication declined and remained stable for up to 8 days post-infection, the last time point assayed (Fig. 1A). No p24 production was detected in uninfected macrophage cultures (Fig. 1A). HIV-infection also was demonstrated by immune-staining and subsequent confocal microscopy of the HIV-infected cultures of macrophages using DAPI (blue), actin (see small inset, red) and HIV-p24 (see small inset, green) (colocalization of all colors is illustrated in Fig. 1B). An example of HIV-infection of macrophage cultures after 3 days is shown in Fig. 1B. No p24 immunoreactivity was detected in uninfected cultures (data not shown). No background or nonspecific staining was detected using irrelevant isotype-matched antibodies (Fig. 1C).
Fig. 1.
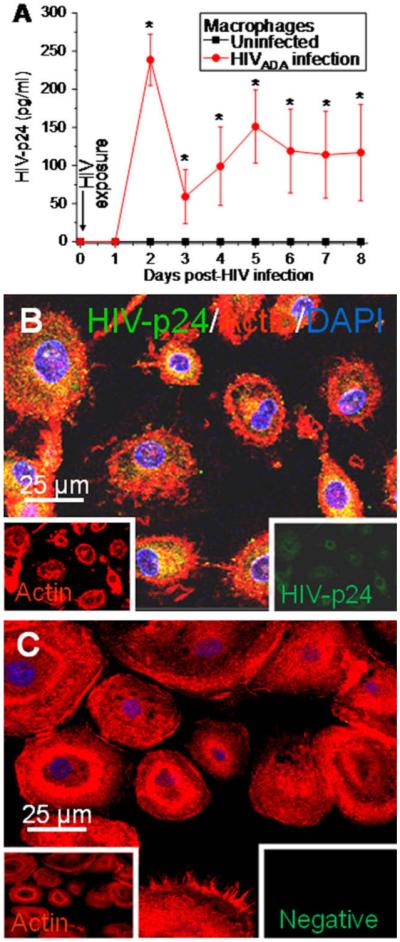
HIV-infection of macrophages results in HIV-p24 production as detected by ELISA and immunofluorescence. Cultures of human macrophages were exposed to HIVADA (20 ng/ml p24) for 24 h and then washed extensively to eliminate unbound virus. Medium was collected every day to assay p24 production by ELISA. (A) HIV-infected macrophages produce significant amounts of p24, after 1-3 days that decrease after 4-8 days post-infection (red line, ●) (n = 8 independent experiments, mean ± SD). No p24 was detected in uninfected cultures (black line, ■). (B) HIV-infected macrophages were stained at different time points with antibodies for HIV-p24 (green staining, see inset), actin (Texas Red-phalloidin, red staining, see inset) and with DAPI (blue staining). A representative picture of 2 days post-infection is shown to illustrate intracellular vesicular staining as well as HIV-p24 staining inside long processes (B). No background or nonspecific staining was detected using isotype-matched irrelevant antibodies (C). Bar = 25 μm.
3.2. Uninfected and HIV-infected macrophages have three kinds of processes, filopodia, short, and long range TNT
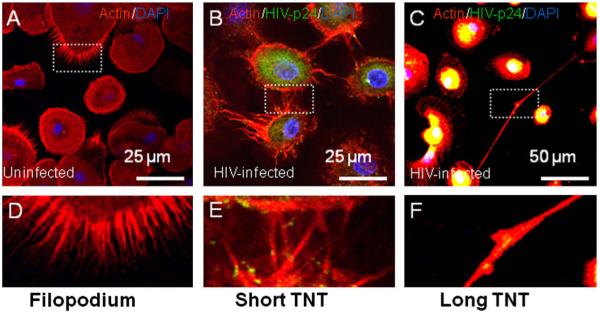
To identify different types of processes in macrophages, we performed immunofluorescence analyses of uninfected and HIV-infected macrophage cultures for HIV-p24 (viral protein, green staining), actin (Phalloidin-Texas Red, red staining) and nuclear staining (DAPI). Using confocal microscopy, 3D reconstruction and an NIS-Element-Advanced Research program (Nikon, Japan), we categorized the different kinds of processes expressed by uninfected and HIV-infected macrophages. TNT differ from filopodia in two ways. First, TNT structures establish a bridge between the cytoplasm of connected cells, while filopodia do not. Second, the length of these two structures differs; filopodia are ∼2.37-5.8 μm while TNT can be up to 100 μm. For both TNT and filopodia, the diameters of the processes range from 50 to 200 nm [3]. We were able to categorize three different types of macrophage processes based on intercellular communication and length. Some examples of these different processes are shown in Fig. 2. The length of filopodia in uninfected and HIV-infected macrophages was ∼5 μm, (Fig. 2A and D), while the length of short TNT was ∼30 μm (Fig. 2B and E), and of long TNT was ∼150 μm (Fig. 2C and F). The length of the processes, filopodia and TNT, was similar to the length of these kind of processes reported for other cells types [3].
Fig. 2.
Uninfected and HIV-infected macrophages have three types of processes, filopodia, short and long TNT. Based on the length of the processes formed by macrophages, we categorized them into three groups, filopodia, short and long TNT. The criteria for these groups were based on the length of the processes as well as on whether the process connected one or more cells, TNT. (A-F) Macrophages stained for HIV-p24 (green staining), actin (Texas Red-phalloidin, red staining) and DAPI (blue staining). Panel (A) shows filopodia in uninfected macrophages and (D), is the enlargement of the boxed area denoted in (A). Panel (B) shows a representative picture 3 days post-HIV-infection of short TNT, and E, is the enlargement of the boxed area denoted in (E). Panel (C) shows a representative picture 3 days post-HIV-infection of long TNT, and (F), is the enlargement of the boxed area denoted in (C). Bars = 25 μm for (A and B) and 50 μm for (C).
3.3. HIV-infection of human macrophages results in enhanced number of TNT
Immunofluorescence analyses of macrophage cultures infected with HIV for HIV-p24 (viral protein, green staining), actin (Phalloidin-Texas Red, red staining) and nuclear staining (DAPI) demonstrated that HIV-p24 protein accumulates in intracellular vesicles, as well as in long actin-positive processes, that we believe are TNT. To examine whether HIV-infection of macrophages induces the formation of short and long TNT, we used three dimensional reconstructions of these fields and the percentage of cells with TNT and filopodia was quantified using an NIS-Element-Advanced Research program (Nikon, Japan).
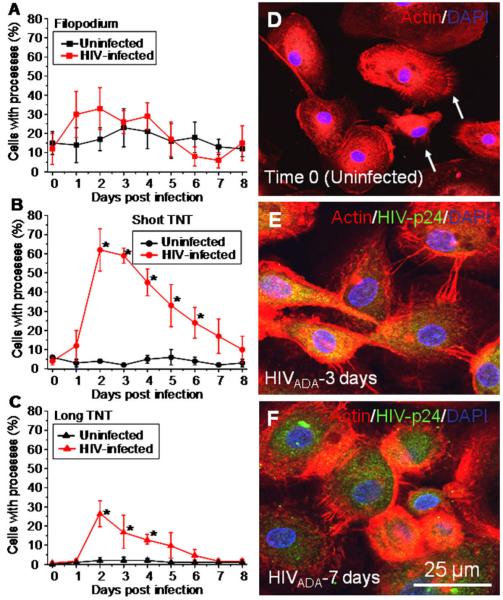
A few macrophages in uninfected cultures have filopodia and TNT, in agreement with other groups (Fig. 3). HIV-infection of macrophages did not alter the number of cells with filopodia as compared to uninfected cells (Fig. 3A and D). However, HIV-infection correlated with a significant increase in the number of macrophages forming short (∼60-70% compared to uninfected cells, Fig.3B and E) and long TNT (∼20-25% compared to uninfected cells, Fig. 3C and F, representative pictures are shown in (E and F)) after 2-3 days post-HIV-infection. After the peak of TNT formation (2-3 days post-infection), a reduction in the number of cells with TNT was detected until it reached similar numbers as uninfected macrophages by 8 days post-infection (Fig. 3B and C, representative pictures are shown in (E and F)). The time course of formation of short and long TNT correlated with active viral replication (compare Fig. 3B and C to Fig. 1A), suggesting that viral replication is important for additional TNT formation. We propose that this increased number of TNT may facilitate transport/exchange of viral particles between connected cells.
Fig. 3.
HIV-infection induces the transient formation of short and long range TNT. The percentage of cells with processes was quantified using confocal microscopy to detect staining for HIV-p24 (green staining), actin (Texas Red-phalloidin, red staining) and DAPI (blue staining). Three dimensional reconstruction, using the imaging program NIS, was performed to visualize all TNTs and filopodia in these cultures. (A) In uninfected (■, black line) and HIV-infected ( , red line) macrophage cultures, ∼15-25% of the cells have filopodia. (B) HIV-infection of macrophages induces the formation of additional short TNT (
, red line) macrophage cultures, ∼15-25% of the cells have filopodia. (B) HIV-infection of macrophages induces the formation of additional short TNT ( , red line, *p < 0.001, n = 4) as compared to uninfected cultures of macrophages (■, black line). (C) HIV-infection of macrophages induces the formation of an increased number of long TNT (
, red line, *p < 0.001, n = 4) as compared to uninfected cultures of macrophages (■, black line). (C) HIV-infection of macrophages induces the formation of an increased number of long TNT ( , red line, *p < 0.003, n = 4) as compared to uninfected cultures (■, black line). Statistical significance in (B and C) graphs, between uninfected and HIV-infected macrophages, was found at days 2-6 and 2-4, respectively, and returned to control levels (uninfected cells) after 5 and 6 days post-infection. (D) An example of filopodia at time 0 in uninfected cells stained for actin and DAPI. (E) An example of short range TNT, 3 days post-infection with HIV stained for HIV-p24 (green staining), actin (Texas Red-phalloidin, red staining) and DAPI (blue staining). (F) An example of a decrease in short and long TNT, 7 days post-infection. Arrow in (D) denotes the filopodia structures at time 0. Bar = 25 μm.
, red line, *p < 0.003, n = 4) as compared to uninfected cultures (■, black line). Statistical significance in (B and C) graphs, between uninfected and HIV-infected macrophages, was found at days 2-6 and 2-4, respectively, and returned to control levels (uninfected cells) after 5 and 6 days post-infection. (D) An example of filopodia at time 0 in uninfected cells stained for actin and DAPI. (E) An example of short range TNT, 3 days post-infection with HIV stained for HIV-p24 (green staining), actin (Texas Red-phalloidin, red staining) and DAPI (blue staining). (F) An example of a decrease in short and long TNT, 7 days post-infection. Arrow in (D) denotes the filopodia structures at time 0. Bar = 25 μm.
3.4. HIV-infection of macrophages did not alter the length of TNT and filopodia
To determine whether HIV-infection of macrophages changes the length and diameter of the processes induced during HIV-infection, measurements of the length and diameter of the processes were taken using an NIS-Element-Advanced Research program (Nikon, Japan). As there are only a few processes in uninfected cells, we examined a large number of macrophages (15 fields per experimental condition, n = 5) to assess whether HIV-infection alters the baseline length of macrophage processes. The length of filopodia in uninfected macrophages was ∼5 μm (Fig. 4). Two kinds of TNT were detected, based on the length of the TNT processes, a ∼30 μm, termed “short” (Fig. 4) and a ∼150 μm, called “long” TNT (Fig. 4). In HIV-infected macrophages, the length of filopodia, short, and long TNT, was not altered as compared to uninfected cells during the time course examined (Fig. 4). Thus, we did not detect changes in the length of the filopodia and TNT in HIV-infected cells as compared to uninfected cells.
Fig. 4.
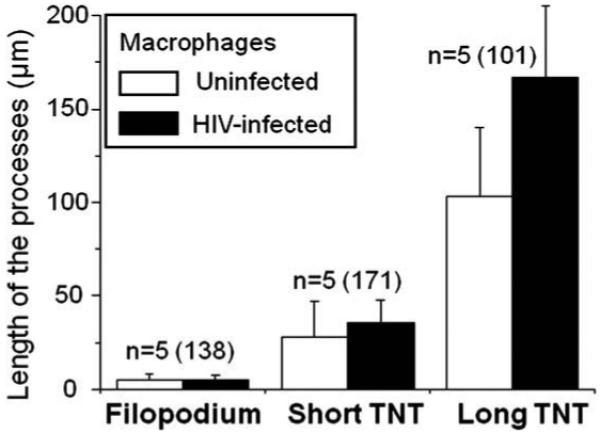
HIV-infection, although alter the numbers of TNT, did not alter the length of the processes. Using the process classification described in Fig. 2, we determined whether HIV-infection alters the length of the processes induced. Length of the processes (μm) in uninfected cells (white bars) and HIV-infected cells (black bars) at days 2 and 3 post-infection. No changes in the length of the processes were detected at any other time points (data not shown). No significant changes in the length of the processes were found between uninfected and HIV-infected macrophages, suggesting that the length of the processes is not altered (day 3, p < 0.7, n = 5) by HIV-infection.
3.5. Differential compartmentalization of HIV in filopodia and TNT
In addition to the changes in TNT formation induced by HIV-infection of macrophages and the potential transfer/movement of HIV particles across TNT, we also detected distinctive compartmentalization in the types of processes induced by HIV-infection. First, one individual cell can have TNT and filopodia concomitantly (Fig. 5A and B, top and bottom optical section of the same field). However, these two processes are localized in different areas of the same cell. Representative top (Fig. 5A) and bottom (Fig. 5B) optical sections of the same field demonstrated that TNT formation occurs preferentially in the top optical sections of the HIV-infected cells, while filopodia are mainly localized in the bottom optical sections of these cells (Fig. 5A and B). These data suggest a regional compartmentalization of these processes within the same cell. This compartmentalization of filopodia and TNT has been observed as well in macrophages infected with Mycobacterium bovis [11].
Fig. 5.
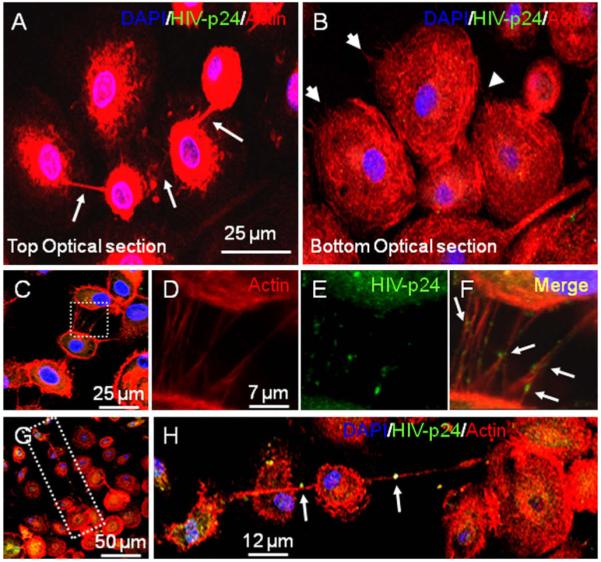
Distinct HIV vesicles localization in TNT processes. Staining of macrophages infected with HIV for HIV-p24 (green staining), actin (Texas Red-phalloidin, red staining) and DAPI (blue staining) and subsequent 3D reconstruction demonstrated that one cell can have both filopodia and TNT in different optical planes. It also demonstrated HIV particles between connected cells. (A and B) These pictures correspond to the same field showing that the same cell can have both TNT (top optical section) and filopodia (bottom optical section) (see arrows). Panel (C) represents a picture of short TNT in HIV-infected macrophages at day 3 post-infection stained for HIV-p24 (green staining), actin (Texas Red-phalloidin, red staining) and DAPI (blue staining). The white box in (C), enlarged in (D-F), corresponds to short range TNT, stained for actin (Texas Red-phalloidin, red staining, (D)), HIV-p24 (green staining, E), and DAPI (blue staining, no shown). Panel (F) corresponds to the merge of (D and E), showing HIV-p24 positive vesicles in short TNT (see arrows). The white box in (G), as enlarged in (H), corresponds to long range TNT, stained for actin (Texas Red-phalloidin, red staining), HIV-p24 (green staining), and DAPI (blue staining). Arrows denote HIV-p24 positive vesicles being transported across long range TNT.
We also observed differences in localization and size of viral particles associated with TNT. In short TNT, the viral particles, (p24 positive signals, green staining) appear inside the TNT (Fig. 5C-F). However, in long range TNT, the viral particle appeared to be bigger than the diameter of the long TNT (Fig. 5G and H). This could be because the internal cytoplasm was stretched, or it could represent extracellular virus “surfing” on the TNT. These two findings, intracellular as well as extracellular surfing, have been described in the context of other viruses and endocytic vesicles (see review by [27]). Further investigation is required to characterize these kinds of viral vesicles. Our data suggest that viral movement may be altered by the length of the TNT or that short and long TNT have different cargo mechanisms for carrying virus or viral particles between connected TNT cells.
4. Discussion
This report characterizes a novel communication system in human primary macrophages, named TNT that can be induced and perhaps utilized by HIV to spread viral particles by an intercellular route between connected cells. Although we also detected these processes in uninfected macrophage cultures in low numbers, HIV-infection resulted in a significant, transient increase in the numbers of short and long range TNT that correlated with viral replication.
TNT are long processes that fuse the membranes of two or more cells, resulting in a bridge that allows direct communication between the cytoplasm of connected cells. In uninfected cells, TNT enable trafficking of cargo vesicles and organelles, as well as small molecules such as calcium. Although gap junctions allow similar communication, these channels do not enable long range communication and only facilitate trafficking of small molecules, up to 1.2 kDa [3,15]. Characterization of TNT in normal, uninfected cells indicated that TNT are actin-positive and have low expression of tubulin [11]. In PC12 cells, immunocytochemical analysis demonstrated that synaptophysin, a marker of synaptic vesicles, as well as myosin-Va, a motor protein, were present inside nanotubes [3]. TNT also mediates the exchange of surface membrane proteins [28] such as receptors. A recent report indicates that TNT are not continuous cytoplasmic bridges in lymphocytes and are almost impermeable to calcium [14], suggesting that there may be many types of TNT with different permeabilities and abilities to facilitate communication between connected cells and/or suggests that TNT formation may be cell type specific. Previous reports, before the discovery of TNT, indicated that viruses, such as herpes [13,29] and pseudo-rabies viruses [30], can be transmitted through long extensions without contact with the extracellular environment, suggesting that viruses may have evolved in a way to use TNT to spread efficiently between connected cells. Our current report suggests the possibility that HIV also can be spread to connected cells through TNT, as an additional and complementary mechanism to the classic extracellular binding to receptors and subsequent entry into susceptible cells.
In our cell culture system, we demonstrated that HIV-infection of human macrophages resulted in significantly increased numbers of TNT. We detected p24 viral protein within TNT. The presence of these viral proteins in the TNT was correlated with viral replication, supporting our hypothesis that the virus is an important component in the formation of these processes, perhaps to facilitate the spread of viral particles to other cells connected by TNT. We hypothesize that early during viral infection, the virus may use two separate mechanisms to assure that cells become infected. These are TNT and the classic receptor binding/fusion mechanism. In our system, initial entry is mediated by the classical and well characterized use of CD4 and CCR5. However, this route requires several steps to spread infection, including reverse transcription, viral DNA nuclear insertion, viral transcription, viral assembly and budding. We propose that during this process, TNT help to facilitate the expanded infection of cells more rapidly than by receptor-mediated fusion alone. Once an important percentage of cells are infected (5-7 days post-HIV-infection), we postulate that TNT are no longer necessary to amplify infection. The intercellular spread of viral particles may also be a viral escape mechanism to evade potential viral targeting by host defense systems, or from therapies based on blocking entry steps, such as gp120 based vaccines. Additional studies are needed to examine these hypotheses.
The TNT lengths that we detected were consistent with previous data obtained in PC12 cells and other cell types (see review by [1]). We did not detect any difference in the length of processes between uninfected and HIV-infected macrophages. However, the numbers of cells with short and long range TNT increased significantly. We were not able to quantify the numbers of TNT per individual cell, because many cells share one TNT and it was not always clear specifically where one TNT began or ended. However, it did appear that there is an increase in the number of short TNT per cell. These observations suggest that HIV-infection, by an unknown mechanism, induces the formation of TNT to establish cell to cell communication with other macrophages, to allow viral exchange between connected cells.
We demonstrated that HIV-infection increases the numbers of TNT in primary human macrophages in correlation with active viral replication. Our results support TNT as a novel mechanism for viral spread and therefore significant in the pathogenesis of AIDS.
Acknowledgments
We thank the Analytical Imaging Facilities at The Albert Einstein College of Medicine. The authors are very thankful to Ms. Aimee Luers for editing the manuscript.
Footnotes
Grant Support. This work was supported by the National Institutes of Mental Health grants (MH075679 and MH070297 to J.W.B.), a Ruth L. Kirschstein Posdoctoral Fellowship from National Institute of Drug Abuse (F32DA024965 to P.J.G.), NIH Centers for AIDS Research (CFAR), especially the Immunology/Pathology Core, Grant AI-051519, a KO1 grant from the National Institute of Mental Health (MH076679 to E.A.E.) and the National Institute of Health Experimental Neuropathology Training Grant to P.J.G. (NS07098).
References
- [1].Gerdes HH, Bukoreshtliev NV, Barroso JF. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–2201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- [2].Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: membrane nanotubes connect immune cells. J. Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- [3].Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- [4].Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- [5].Kornberg T. Pictures in cell biology. Cytonemes. Trends Cell Biol. 1999;9:434. doi: 10.1016/s0962-8924(99)01653-0. [DOI] [PubMed] [Google Scholar]
- [6].Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- [7].Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- [8].Davis DM. Intrigue at the immune synapse. Sci. Am. 2006;294:48–55. doi: 10.1038/scientificamerican0206-48. [DOI] [PubMed] [Google Scholar]
- [9].Hope TJ. Bridging efficient viral infection. Nat. Cell Biol. 2007;9:243–244. doi: 10.1038/ncb0307-243. [DOI] [PubMed] [Google Scholar]
- [10].Magalhaes AC, Baron GS, Lee KS, Steele-Mortimer O, Dorward D, Prado MA, Caughey B. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J. Neurosci. 2005;25:5207–5216. doi: 10.1523/JNEUROSCI.0653-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, Seabra MC, Neil MA, French PM, Davis DM. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J. Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- [12].Onfelt B, Purbhoo MA, Nedvetzki S, Sowinski S, Davis DM. Long-distance calls between cells connected by tunneling nanotubules. Sci. STKE. 2005;2005:pe55. doi: 10.1126/stke.3132005pe55. [DOI] [PubMed] [Google Scholar]
- [13].Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- [15].Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- [16].Dramsi S, Cossart P. Intracellular pathogens and the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 1998;14:137–166. doi: 10.1146/annurev.cellbio.14.1.137. [DOI] [PubMed] [Google Scholar]
- [17].Wehland J, Carl UD. The sophisticated survival strategies of the pathogen Listeria monocytogenes. Int. Microbiol. 1998;1:11–18. [PubMed] [Google Scholar]
- [18].Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J. Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]
- [19].Kalvelyte A, Imbrasaite A, Bukauskiene A, Verselis VK, Bukauskas FF. Connexins and apoptotic transformation. Biochem. Pharmacol. 2003;66:1661–1672. doi: 10.1016/s0006-2952(03)00540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Martinez AD, Eugenin EA, Branes MC, Bennett MV, Saez JC. Identification of second messengers that induce expression of functional gap junctions in microglia cultured from newborn rats. Brain Res. 2002;943:191–201. doi: 10.1016/s0006-8993(02)02621-5. [DOI] [PubMed] [Google Scholar]
- [21].Eugenin EA, Branes MC, Berman JW, Saez JC. TNF-alpha plus IFN-gamma induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J. Immunol. 2003;170:1320–1328. doi: 10.4049/jimmunol.170.3.1320. [DOI] [PubMed] [Google Scholar]
- [22].Eugenin EA, Eckardt D, Theis M, Willecke K, Bennett MV, Saez JC. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc. Natl. Acad. Sci. USA. 2001;98:4190–4195. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J. Neuroimmune Pharmacol. 2006;1:160–181. doi: 10.1007/s11481-006-9017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- [25].Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J. Neurosci. 2007;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gurke S, Barroso JF, Gerdes HH. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem. Cell Biol. 2008 doi: 10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tabiasco J, Vercellone A, Meggetto F, Hudrisier D, Brousset P, Fournie JJ. Acquisition of viral receptor by NK cells through immunological synapse. J. Immunol. 2003;170:5993–5998. doi: 10.4049/jimmunol.170.12.5993. [DOI] [PubMed] [Google Scholar]
- [29].LaBoissiere S, Izeta A, Malcomber S, O’Hare P. Compartmentalization of VP16 in cells infected with recombinant herpes simplex virus expressing VP16-green fluorescent protein fusion proteins. J. Virol. 2004;78:8002–8014. doi: 10.1128/JVI.78.15.8002-8014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc. Natl. Acad. Sci. USA. 2005;102:8990–8995. doi: 10.1073/pnas.0409099102. [DOI] [PMC free article] [PubMed] [Google Scholar]