Abstract
PTL-1, a microtubule-associated protein of the structural MAP2/tau family, is the sole member of this gene family in Caenorhabditis elegans. Sequence analysis of available invertebrate genomes revealed a number of single, putative tau-like genes with high similarity to ptl-1. The ptl-1 gene is expressed in a number of cells, most notably mechanosensory neurons. We examined the role of ptl-1 in Caenorhabditis elegans in adult neurons as well as during development. A ptl-1 knockout strain of worms exhibited an egg hatching defect, as well as a reduced sensitivity to touch stimuli. In addition, the knockout allele ptl-1(ok621) acts as a dominant enhancer of several temperature-sensitive alleles of mec-7 and mec-12, which code the isoforms of β-tubulin and α-tubulin that together form the unusual 15 protofilament microtubules involved in touch sensation. These results demonstrate for the first time a functional role for this microtubule-associated protein in nematodes and suggest that PTL-1 is involved in mechanosensation as well as some aspect of embryogenesis.
Keywords: microtubule-associated protein, tau, Caenorhabditis elegans, tubulin, neuron
Introduction
Structural MAPs of the MAP2/tau family include the vertebrate proteins MAP2, MAP4 and tau, and homologs like PTL-1 (Protein with Tau-like repeats-1) in other animals, including Caenorhabditis elegans (C. elegans) and Drosophila melanogaster (Heidary and Fortini, 2001; Rolls et al., 2007). MAP2 and tau are expressed almost exclusively in neurons in mammals and are thought, by virtue of their microtubule-stabilizing activity in vitro (Goedert et al., 1996), to regulate microtubule networks in dendrites and axons (Sanchez et al., 2000). MAP2, restricted to the somatodendritic compartment of vertebrate neurons, may be involved in the establishment or maintenance of dendritic polarity (Dehmelt and Halpain, 2004). Tau, localized to the axonal subcompartment in vertebrate neurons, may provide a similar function in axons. In addition, this group of proteins may also function in the regulation, formation or maintenance of protein assemblies (Buddle et al., 2003). MAP4 and other members of the structural MAP family are expressed in a larger number of cell types and play diverse roles in microtubule-dependent processes, including microtubule-based organelle movement, cell division and changes in cell morphology (Permana et al., 2005).
There is only a single tau gene in Drosophila melanogaster and C. elegans, two powerful and important invertebrate model organisms. It has been speculated that the tau-like proteins in invertebrate neurons perform the equivalent functions of both MAP2 and tau (Rolls et al., 2007). Examination of the role of tau proteins in invertebrates may shed light on the role of these proteins in the establishment and maintenance of subcellular compartmentalization of proteins and cytoskeletal organization in neurons. PTL-1 (Tau-1), the sole member of the structural MAP family in C. elegans, binds to microtubules and promotes microtubule assembly in vitro (Goedert et al., 1996). When transfected into non-neuronal cells, PTL-1 promotes microtubule assembly and bundling in a manner indistinguishable from human tau (Goedert et al., 1996). Two alternately-spliced isoforms of the PTL-1 protein are expressed at high levels in embryonic epidermal cells and in specific neurons, particularly mechanosensory neurons, from early larval to adult stages (Goedert et al., 1996).
The five mechanosensory neurons (ALML/R, AVM, PLML/R) that express PTL-1 in larval and adult worms sense gentle body touch (Chalfie et al., 1985) and have processes containing bundles of unusual 15 protofilament microtubules, composed of tubulins encoded by the mec-7 gene (Savage et al., 1989; Savage et al., 1994) and the mec-12 gene (Ernstrom and Chalfie, 2002; Fukushige et al., 1999), the only acetylated tubulin in C. elegans. A recent report (O'Hagan et al., 2005) described a mec-7 mutant lacking the 15 protofilament microtubules that exhibited channel activity in response to touch, albeit of a much reduced amplitude, indicating that, while important for the behavioral response to touch (Chalfie and Thomson, 1982), these microtubules are not directly involved in channel gating. One possibility is that the microtubules, via protein interactions with MAPs, might modulate the protein assemblies in the touch cell processes, thereby affecting the sensitivity or functioning of the channel complexes. PTL-1 may play a role in these modulations. While PTL-1 is known to be involved in microtubule assembly in vitro, nothing is known about its function, expression or interaction with other proteins. The goal of this study was to provide a first examination of the invertebrate tau PTL-1 expression and function in vivo in developing and adult nematodes.
Materials and methods
The following worm strains were maintained according to established procedures (Riddle et al., 1997): N2, RB809 (ptl-1(ok621)), CB2217 (mec-7(e1343) lon-2(e678)), CB3270 (mec-7(e1506) lon-2(e678)), CB678 (lon-2(e678)) from the Caenorhabditis elegans Genome Center and TU67 (mec-12(u67)) a kind gift from Martin Chalfie; BC12648 (ptl-1∷GFP, dpy-5(e907), sls11686) was a kind gift from David Baillie. ptl-1(ok621) worms were out-crossed with wild type worms across four separate generations in order to minimize the likelihood of additional background mutations confounding interpretation of results. mec-7(e1343) is a temperature-sensitive, semi-dominant mutation, exhibiting touch-insensitivity at 25°C and wild-type touch sensitivity at 15°C (Savage et al., 1989). mec-7(e1506) is a recessive null allele. mec-12(u67) worms have a temperature-sensitive, dominant allele of mec-12 that is variably touch-insensitive at 20°C. For temperature-sensitive studies worms were grown for two generations at the experimental temperature and all matings occurred at the experimental temperature.
Behavioral touch assay
To examine touch responsiveness worms were moved individually to plates without food. Each worm was touched ten times alternately at the anterior third and posterior third perpendicular to the body axis using an eyebrow hair (Gu et al., 1996). Data, presented as the percentage of touches resulting in a behavioral response (either movement away from the touch or momentary immobility), were analyzed statistically using the Mann-Whitney nonparametric test for two-sample comparisons and the Kruskal-Wallis test for multiple treatment comparisons using Prism (v. 3.0) software for the Macintosh.
Egg hatching assay
The numbers of eggs laid and hatched by wild type (N2) and ptl-1(ok621) hermaphrodite worms were counted by moving individual L4 hermaphrodites to new, seeded plates every 24 hours for 4 days after the appearance of first eggs. The total number of eggs laid and the percentage of eggs hatched were determined for 10–19 worms of each genotype. Data were analyzed using the Mann-Whitney U test for nonparametric comparisons. To determine the developmental stage where arrest occurred, embryos released from dissected adult hermaphrodites were wet-mounted and allowed to develop and visualized using time-lapsed video microscopy.
Competitive PCR for genotyping and RT-PCR
The ptl-1(ok621) mutation is a 1933 base pair deletion encompassing the end of exon 2 through exon 8 (Oklahoma Medical Research Foundation Knockout Group). We developed a PCR-based strategy to determine the ptl-1 genotype of offspring resulting from genetic crosses that were assayed for touch responsiveness (see below). Using three primers for competitive PCR, two flanking the regions deleted within the ptl-1(ok621) mutant [5’TTTTCCGAGGTTGCAGTAG3’; 5’GGCAAATTGTGATCCAGTGT3’] and one within the deleted region [5’GCGTTGAGAGACGAGGGAGTAG3’], we determined whether a worm was homozygous or heterozygous for the ptl-1 mutation. Immediately following the touch assay, individual worms were digested using proteinase K (50 µg/ml) in a lysis buffer (50 mM KCl, 10 mM Tris pH 8.2; 2.5 mM MgCl2, 0.45% Tween 20, 0.45% NP40, 0.01% gelatin) at 60°C and DNA was amplified 40 cycles using PCR with Taq polymerase (USB Scientific, Inc.) with the three primers. Worms homozygous for the ptl-1 deletion generate a PCR product of 464 bp, while wild type worms yield a product of 735 bp. Heterozygotic worms generate both products.
Expression levels of ptl-1 mRNA were determined using RT-PCR. Briefly, mRNA was extracted from mixed stage worms using the Micro-Fast Track Kit from Invitrogen and reverse transcribed to cDNA using Superscript III kit (Invitrogen, Inc.). To detect the presence of ptl-1 mRNA, primers were designed that were specific for the mRNA and compared with expression of actin mRNA as a control. Expression was evaluated using agarose gel electrophoresis and image densitometry of ethidium bromide staining using NIH ImageJ (v.1.39).
Immunocytochemistry
Adult worms were washed off NGM plates with M9 buffer and allowed to settle by gravity for 5 minutes to enrich for adults. The M9 supernatant was washed several times with dH2O to remove any bacterial contamination. 20µl drops of packed worms were placed in the center of freshly prepared poly-L-lysine coated slides according to published procedures (Duerr, 2006) and a second microscope slide was placed on top of the worm drop to sandwich the worms between the two slides. The slide sandwiches were placed on slabs of dry ice for 30 minutes and then the slides were quickly separated, to crack open the worms. Worms were fixed with cold 3% paraformaldehyde, washed and permeabilized with 0.1% triton-X-100, and exposed to primary antibody. Alexa-fluor 488 nm-conjugated goat anti-mouse IgG was then used to visualize immunoreactivity using a confocal microscope (see below). Two different primary antibodies were used. DM1α (Sigma-Aldrich, Inc) is a general tubulin antibody (Duerr, 2006). 6-11-B1 (Sigma-Aldrich, Inc.) is specific for acetylated forms of tubulin, of which only MEC-12 is acetylated in C. elegans (Siddiqui et al., 1989). Thus, 6-11-B1 immunoreactivity is specific for MEC-12 expression, which occurs predominantly in the mechanosensory neurons of adult worms (Fukushige et al., 1999). We verified the specificity of the antibodies both by examining immunoreactivity in the absence of primary antibody as well as in mutant worms lacking MEC-7 (mec-7(u443)) and MEC-12 (mec-12(tu67)).
ptl-1∷GFP expression and confocal microscopy
The expression of GFP under the control of the ptl-1 promoter was examined using a transcription fusion strain, BC12648 (ptl-1∷GFP, dpy-5(e907), sls11686), kindly provided by D. Baillie (McKay et al., 2004). These worms are a stably transformed GFP transcriptional reporter construct. Adult hermaphrodite and larval worms were immobilized on a 2% agarose pad in a drop of buffer containing 10 mM NaN3. Images were acquired with a Nikon PCM2000 confocal laser scanning microscope equipped with an argon laser. Confocal images were processed to adjust contrast using Simple PCI software, as well as Adobe Photoshop (v. 5.0). In all cases, whole images were processed, rather than particular portions of images, to ensure faithful representation of the results obtained.
Results
Sequence comparison of invertebrate tau-like proteins
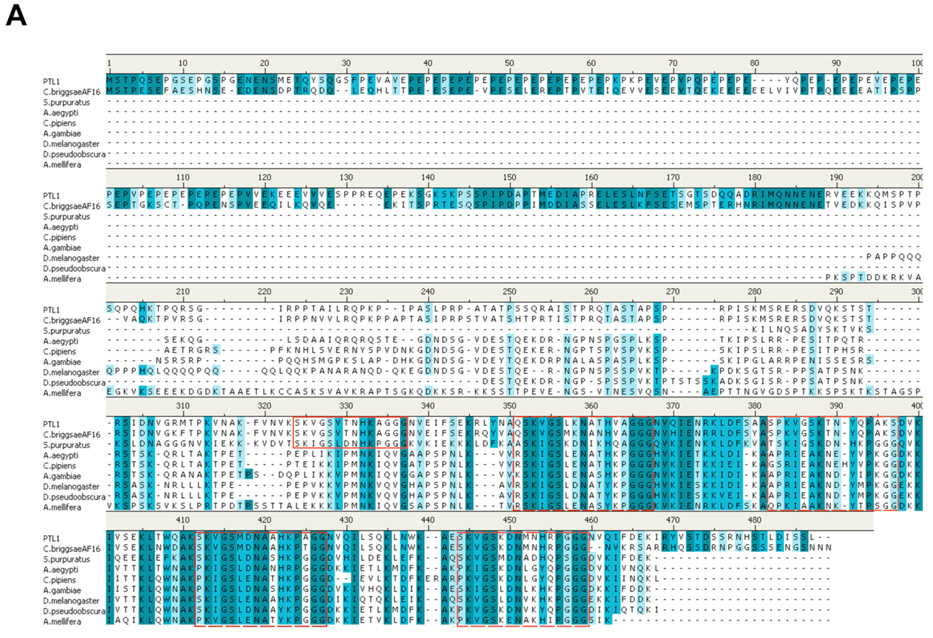
PTL-1 was originally identified based on sequence similarity to the genes encoding the mammalian microtubule-associated proteins tau and MAP4 (Goedert et al., 1996; McDermott et al., 1996). More recently, tau-like genes have been identified in a number of invertebrates. While as a group the invertebrate sequences share considerable identity with vertebrate sequences within the C-terminal, microtubule-binding domain half of the protein, the vertebrate sequences have a much larger N-terminal domain, the first approximately 350 amino acids of which are missing in the invertebrate sequences (data not shown). We focused our comparisons on the invertebrate sequences. In a BLASTp search, we compared the protein sequence information of the eight invertebrate sequences with substantial sequence similarity, several of which have not yet been identified as tau genes. For the sequence comparisons, we used the predicted protein sequence for PTL-1a because that isoform has the largest number of microtubule binding repeats (Goedert et al., 1996); in all other comparisons, the PTL-1 isoforms were identical (data not shown). A putative ptl-1 gene in C. briggsae, gene ID# CBG15240, shares 79% identity with ptl-1, with a virtually identical C-terminal coding region (Fig. 1a). Based on this overall sequence identity, we propose that this hypothetical protein is a tau protein, Cbr-ptl-1. The next most closely related invertebrate tau-like sequence is that of the sea urchin Strongylocentrotus purpuratus (Fig. 1). Of sequenced invertebrate genomes, tau-related proteins are found in the Drosophila genus, as well as several mosquitoes and the honeybee, Apis mellifera (Fig. 1). In all of these cases, there is a single tau-like gene. The sequences show considerable identity and a high degree of similarity in the C-terminal half of the proteins. The N-terminal half of the protein sequences shows divergence among the organisms, except for substantial identity between C. elegans and C. briggsae. Only two proteins outside of the Caenorhabditis genus exhibit significant similarity to PTL-1’s N-terminal domain, the calreticulin P-domain, chain A from mouse (SwissProt ID# 1HHNA) and the luminal domain of calnexin, chain A (SwissProt ID# 1JHNA) from dog. Intriguingly, both of these proteins are thought to act as molecular chaperones (Ellgaard et al., 2001; Hahn et al., 1998).
Figure 1.
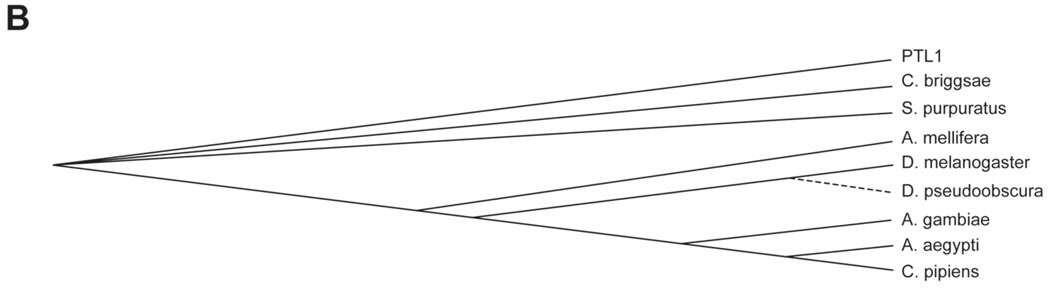
Comparisons of invertebrate tau-like proteins. A) Protein sequence alignments of invertebrate tau-like proteins using Accelyrs Discovery Studio (v. 1.7) and a BLASTp protocol followed by a Clustal W progressive pairwise alignment algorithm. Dark blue indicates identical amino acid residues; lighter blue indicates similar amino acid residues (conservative substitutions). Dashed boxed regions indicate putative microtubule binding regions showing strong similarities among the sequences. Solid line box indicates the fifth putative microtubule binding region found in genus Caenorhabditis. B) Sequence similarity tree based on protein sequences compared and aligned using Accelyrs Discovery Studio multiple sequence alignment protocol followed by an unrooted neighbor joining method (Saitou and Nei, 1987) with bootstrapping (n=1000 iterations). Organisms compared (with species/protein ID): Drosophila melanogaster (NP651575), Drosophila pseudoobscura (XP001357893), Aedes aegypti (XP001655927), Culex pipiens quinquefasciatus (XP001863669), Anopheles gambiae (XP321540), Apis mellifera (XP393045), Strongylocentrotus purpuratus (XP788973), C. elegans (PTL1) and Caenorhabditis briggsae (CAE69200.1).
We aligned the invertebrate tau-related protein sequences using Accelyrs Discovery Studio (Fig. 1a). As predicted from other analyses of tau proteins (Goedert, 2005), the highest degree of sequence identity (dark blue) and similarity (light blue) resides within the C-terminal half of the proteins. The putative microtubule binding domains show particular similarity among the proteins (dashed line boxes in Fig. 1a). Within these highly conserved C-terminal repeat regions, the shorter sequence SKVGS is found three times with almost complete identity and a fourth time with conservative substitutions. In addition, a short distance (approximately 8 amino acids) from the region is the sequence GGG, which is virtually identical among all the organisms. PTL-1, Cb-PTL-1, the putative C. briggsae tau, and the tau-like protein from S. purpuratus contain a fifth repeat region that is not conserved among the other sequences (solid box in Fig. 1A). These corresponding microtubule-binding motifs in human Tau are proposed to target the protein to the microtubule (Mukrasch et al., 2007). From the protein sequence alignments, we contructed a sequence similarity tree using an unrooted neighbor-joining distance matrix phylogenetic algorithm (Saitou and Nei, 1987) and boot-strapping (n=1000 iterations). As expected, sequences within a genus show the closest relatedness. Interestingly (Fig. 1b), there is a closer similarity between nematode sequences and the echinoderm S. purpuratus than with the arthropod sequences.
ptl-1 expression in adult and larval worms
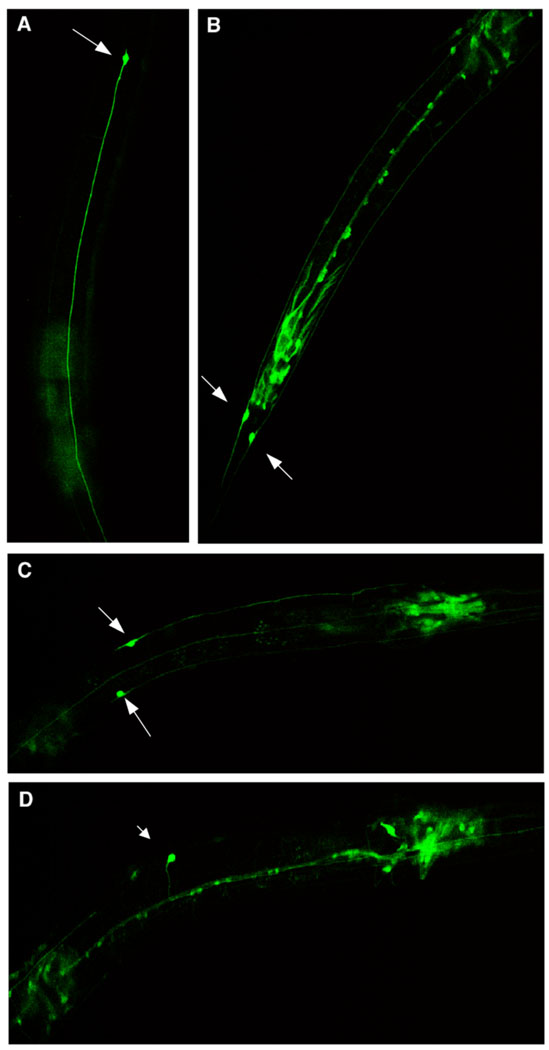
A transcriptional fusion of GFP under the control of the ptl-1 promoter was generated by the C. elegans Expression Consortium (McKay et al., 2004) and was stably incorporated into wild type worms. GFP is expressed in mechanosensory neurons (arrows, Fig. 2). Prominent expression occurs in ALML/R, PLML/R and AVM, while PVM does not express GFP under the control of the ptl-1 promoter in adult worms. This expression pattern agrees with an early report using an antibody to recombinant PTL-1a protein (Goedert et al., 1996). GFP is also expressed in a number of head neurons, neurons along the ventral nerve cord (including DD2, DA3, DB3, DA9, VA3), cells near the vulva (including VC3, VB4) and stomatointestinal muscle, in agreement with more recent reports using this expression construct (McKay et al., 2004).
Figure 2.
Expression of ptl-1 in living adult hermaphrodite touch neurons. Worms stably transformed with ptl-1∷GFP visualized with confocal microscopy at 40X as described in Materials and methods. Anterior is up in A and B and to the left in C and D. A) ALM L or R, B) PLML and PLMR, C) ALML and ALMR, D) AVM. Note other GFP-expressing neurons and cells described in text.
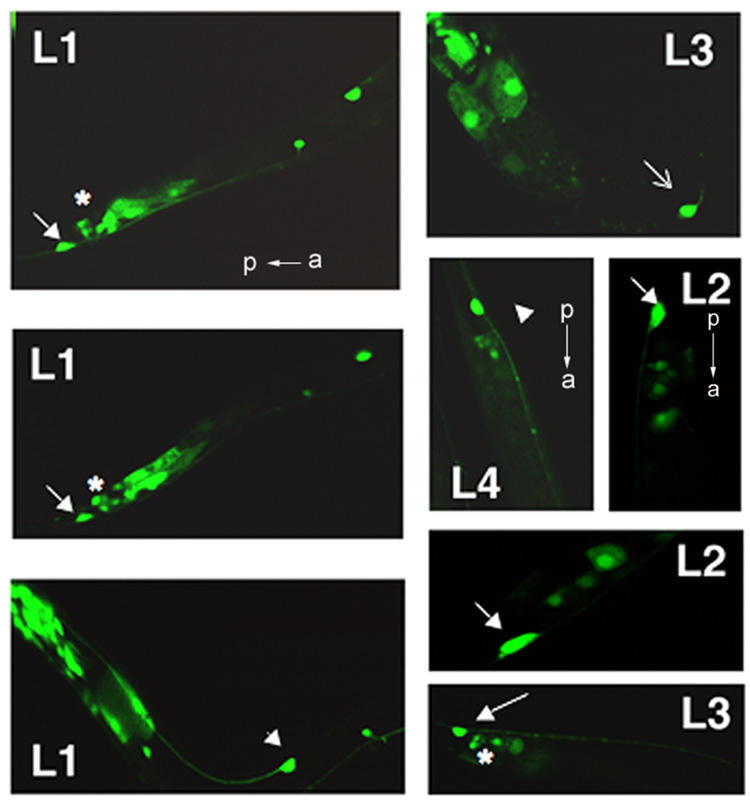
In examining the expression of the ptl-1 gene during larval development, we noted that the gene is expressed in PLML/R (arrows, Fig. 3) and ALML/R (arrowheads, Fig. 3) at all larval stages. In contrast, expression in AVM was more variable. AVM expression was steady by L3 (open arrow in L3, Fig. 3), but was usually not present in L1 and rarely during early L2, which corresponds with the appearance of AVM during development (Altun and Hall, 2006). This indicates that ptl-1 expression initiates within the touch neurons upon the “birth” of the neuron. We also saw reproducible expression at early larval stages (L1, L2) in a number of other cells in the tail region. We believe these cells to be DVA/B interneurons (asterisks, Fig. 3), which are members of the mechanosensory neuron circuit (de Bono and Maricq, 2005). This expression decreased by L3 and was much reduced by L4. We also consistently identified, in 13 out of 15 larval worms examined, a cell expressing ptl-1 as early as L1 that, based on its position, appears to be PVM. The level of expression was variable and we noted no PVM expression in later developmental stages.
Figure 3.
ptl-1 expression in living larval worms. Worms stably transformed with ptl-1∷GFP immobilized as described in Materials and methods were visualized with confocal microscopy. The developmental stage is noted (L1, L2, L3, L4). Mechanosensory neurons are indicated by white arrows. Closed arrows in L1 and L2 show PLML/R; arrowheads indicate PLML/R. * indicates putative DAB interneurons. Open arrow in L3 indicates AVM. All images obtained at 100X oil immersion. For all frames, anterior is to the right, except where indicated.
ptl-1 is involved in egg hatching and responsiveness to gentle touch
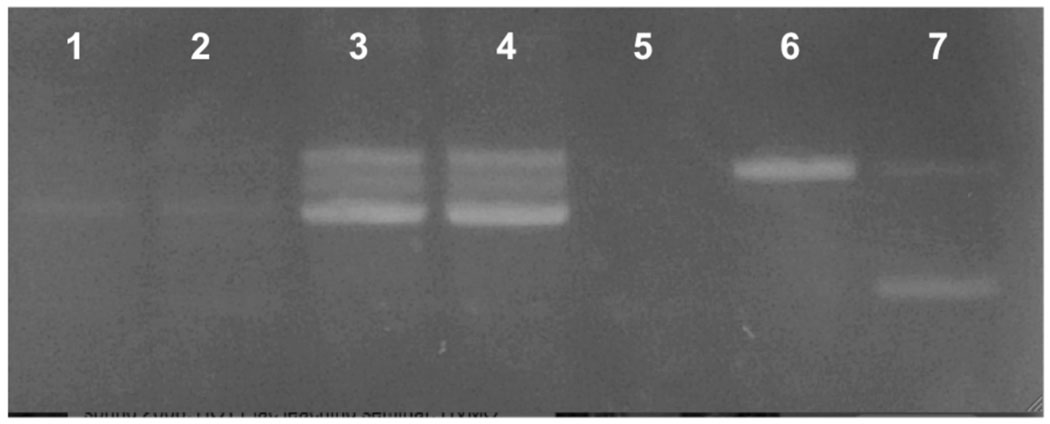
To explore the role of ptl-1 further, we obtained a ptl-1 knockout worm (RB809 (ptl-1(ok621)) from the OMRF C. elegans Gene Knockout group. The ptl-1 gene has a 1.933 kb excision encompassing exons 2–8 (http://www.wormbase.org release WS188, May 2008). We backcrossed these worms over four generations with wild type worms. Genomic DNA extracted from these worms confirms the 1.933 kb deletion (Fig. 4). In addition, mRNA extracted from the null worms revealed negligible expression of the ptl-1 gene, indicating that this is indeed a functional null allele.
Figure 4.
ptl-1 mRNA is not expressed in ptl-1∷(ok621) null mutant worms. mRNA was extracted from mixed stage worms (wild type and ptl-1(ok621) homozygotes) using Invitrogen’s Microfast-track kit (Invitrogen, Inc.) and converted to cDNA using Invitrogen’s Superscript III (Invitrogen, Inc.). Lanes 1,2: cDNA from ptl-1(ok621) worms (1 µg, 0.5µg respectively); Lanes 3,4: cDNA from wild type worms (1 µg, 0.5µg); Lane 5: ptl-1(ok621) single worm genomic DNA amplified with two primers, one of which is within exon 2; Lane 6: wild type genomic DNA with the same two primers as Lane 6; Lane 7: ptl-1(ok621) genomic DNA with three primers, described in Materials and methods.
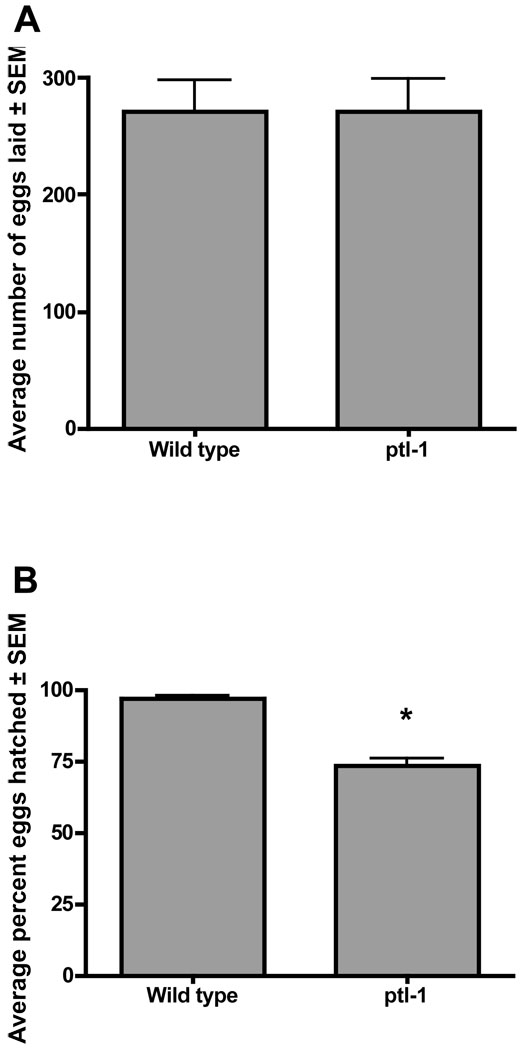
We evaluated the ptl-1(ok621) worms for defects. The worms exhibited normal development and overall larval developmental timing. However, there was a significant reduction in the number of viable progeny (Fig. 5), but no overall effect on the number of eggs laid, when compared with wild type worms. Most often, the developmental arrest occurred at or near the elongation stage of embryogenesis, the time period when PTL-1 immunoreactivity is first detectable (Goedert et al., 1996). A larger proportion of eggs laid later in the egg-laying period appeared arrested and never hatched. These eggs appeared darker and lacked the rounded appearance of healthy eggs.
Figure 5.
ptl-1(ok621) mutants exhibit an egg hatching defect. Wild type (N2) and ptl-1(ok621) knockout worms were plated individually (n= 10–19 worms in each group) at 20°C and eggs counted and larval hatching quantified as described in Materials and methods. Data are presented as the average ± s.e.m. The percentage of eggs that hatched was analyzed using the Mann-Whitney test for nonparametric data. * indicates p< 0.0001.
The worms mated effectively with males, exhibited normal movement on plates and in liquid culture, altered movement on food like wild type worms and responded to nose touch normally (data not shown). However, the null worms exhibited a significant reduction in sensitivity or responsiveness to the gentle stroke of an eyebrow hair perpendicular to the body axis (Table 1). While it is clear the worms are capable of responding to touch, the degree of responsiveness was reduced when compared with wild type worms. Most frequently the worms responded to the first one or two touches, but then ceased responding to subsequent touches.
Table 1.
ptl-1 enhances mec-7 and mec-12 touch insensitivity defects
| Genotype | n | Average percent responses to touch ± SEM | p-value | Notes |
|---|---|---|---|---|
| Wild type (N2) | 60 | 94.3 ± 1.5 | ||
| ptl-1(ok621)III | 63 | 81.1 ± 2.4 | 0.0012 | * |
| mec-7(e1506) X | 19 | 65.8 ± 5.5 | 0.05 | * |
| ptl-1(ok621)III/+; mec-7(e1506) X/0 | 33 | 19.7 ± 5.7 | 0.001 | #, ¶ |
| ptl-1(ok621) III; mec-7(e1506) X | 60 | 24.4 ± 3.5 | 0.01 | #, ¶ |
| mec-7(e1343) X | 16 | 48.8 ± 4.5 | 0.01 | * |
| ptl-1(ok621) III; mec-7(e1343) X | 9 | 22.2 ± 8.1 | 0.05 | # |
| mec-12(u67) III | 20 | 86.5 ± 3.1 | 0.05 | * |
| ptl-1(ok621) mec-12(+) / ptl-1(+) mec-12(u67) | 20 | 67 ± 5.7 | 0.02 | ** |
The percent of responses to gentle touch by an eyebrow hair perpendicular to the body axis was compared using the Kruskal-Wallis test for nonparametric data, followed by the Newman-Keuls post-hoc test for selections indicated. There were no differences in responses to anterior and posterior touches, so data were combined. Genotypes (with indicated allele) for ptl-1 and either the mec-7 or the mec-12 genes are noted. + indicates wild type version of the gene. All crosses and touch assays were conducted at 20° C, except for the mec-12 crosses, which were conducted at 25 °C. There were no differences between male and hermaphrodite touch responsiveness.
when compared with wild type hermaphrodite worms.
when compared with corresponding homozygote mec-7 allele hermaphrodite
when compared with corresponding homozygote ptl-1 (ok621) genotype
when compared with corresponding ptl-1(+)/mec-12(u67) heterozygotes from a cross of wild type worms with mec-12(u67) homozygote hermaphrodites.
We further examined the role of ptl-1 in touch responsiveness using a targeted genetic approach testing for enhancement or suppression of mec-7 and mec-12 mutants that are variably touch-insensitive. mec-7 and mec-12 code for β and α-tubulin respectively, which together form the 15 protofilament microtubules involved in responses to gentle touch (Chalfie and Thomson, 1982; Fukushige et al., 1999; Savage et al., 1989). ptl-1(ok621) males were crossed with several different mec-7 alleles, all having the marker lon-2(e698) X (Table 1). mec-7(e1506) is a recessive mec-7 null allele (Savage et al., 1989). The mec-7 allele is also temperature-sensitive, with homozygotes showing only moderate touch insensitivity at 20°C (Table 1) and almost complete touch insensitivity at 25°C (data not shown; (Savage et al., 1989)). mec-7(e1343) and mec-7(e1527) are both temperature-sensitive alleles, with mec-7(e1343) being a semi-dominant mutation (Gu et al., 1996). mec-12(u67), on the third chromosome like ptl-1, is a recessive, temperature-sensitive allele (Fukushige et al., 1999). Heterozygotes exhibit nearly wild-type sensitivity to touch at 25°C (Table 1) and homozygotes are almost completely touch insensitive at that temperature (data not shown; (Gu et al., 1996)).
For mec-7 crosses with ptl-1(ok621) the resultant F1 male heterozygotes, exhibiting the Lon phenotype, were assayed for touch responsiveness. F1 double heterozygote hermaphrodites were allowed to self-fertilize and F2 Lon offspring were assayed. The ptl-1(ok621) genotype of these worms was determined using competitive PCR (see Materials and methods). ptl-1(ok621) was a dominant enhancer of touch insensitivity of all of the mec-7 and mec-12 mutants (Table 1). Enhancement was not seen for a non-mec mutant (lon-2(e678)). Thus, the genetic interaction is specific for the two tubulin genes, suggesting a functional role for ptl-1 in touch sensation.
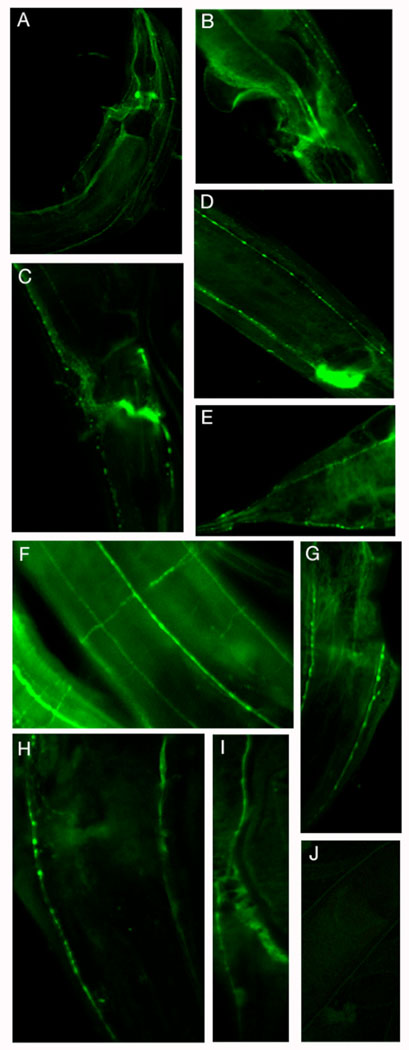
We compared the tubulin immunoreactivity in wild type, ptl-1, mec-7 and ptl-1(ok621) III; mec-7X adult worms using antibodies directed against general microtubules (DM1α, Sigma) and the MEC-12 containing microtubules of the mechanosensory neurons (6-11B1; Fig. 6). General tubulin immunoreactivity (DM1α) was prominent in neurons, in cell bodies as well as neurites. Neurons of the nerve ring, ventral nerve cord, including major branches (Fig. 6f), exhibited immunureactivity (Fig. 6 a, c, f). In contrast, immunoreactivity of MEC-12 containing cells (6-11-B1) is restricted predominantly to mechanosensory neurons. In addition, the immunoreactivity was restricted to the neurites, including the presynaptic swellings along them. Cell body immunoreactivity was absent (Fig. 6 g, h, i). A number of other neurons, including a number of ventral cord neurons, were also immunoreactive. We found no significant difference in either the staining intensity or the pattern of staining in ptl-1 worms, when compared with wild type worms using confocal microscopy. mec-7(u443) and mec-12(u67) worms showed a similar pattern of general tubulin immunoreactivity as wild type worms, but did not exhibit immunoreactivity using the MEC-12 specific antibody (data not shown), reflecting the lack of MEC-12 containing microtubules in these worms. The ptl-1(ok621) III; mec-7(e1506)X double mutant worms exhibited the same immunoreactivity pattern with both antibodies as the mec-7 worms. Thus, at least at a light microscopic level, the ptl-1 does not appear to be directly involved in the formation or overall structure of the microtubules in these cells, which is consistent with the behavioral results.
Figure 6.
The ptl-1 deletion mutation does not affect overall microtubule structures in neurons. Adult wild type (N2) and ptl-1 null mutant worms were fixed and prepared for immunocytochemistry as described in Materials and methods. Freeze-cracked, fixed worms were incubated in mouse monoclonal antibody directed against tubulin (clone DM1α, Sigma) at 1:500, which is a general tubulin antibody (see text), and, following multiple washes, exposed to Alexa-fluor 488 nm goat anti-mouse secondary antibody (Molecular Probes, Inc) at 1:1000. Tubulin immunoreactivity was visualized using a PCM2000 Nikon confocal laser scanning microscope with an argon laser. A, C, F are wild type worms stained with the DM1 α antibody; B, D, E are ptl-1 mutant worms stained with the DM1 α antibody. G) acetylated tubulin (MEC-12; antibody 6-11-B1, 1:10) in ALML/R in ptl-1 mutant worm, H) ALML/R labeled with anti-acetylated tubulin (MEC-12) antibody in wild type neurons. The NR branch of ALM is visible in H and G. I) PLM neuron from ptl-1 mutant worm, labeled with anti-MEC-12 antibody. J) Control ptl-1 mutant worm with no primary antibody. Anterior is up in A, F, G, H, I and J. Anterior is to the right in B and E. Posterior is up in C and D.
Discussion
ptl-1 is the only gene in C. elegans that encodes a tau-related structural MAP. Based on a BLASTp comparison, PTL-1 shares the closest homology with a putative tau-like protein in C. briggsae (gene ID# CBG15240). Outside of the Caenorhabditis genus, the closest homologs are the putative tau-like gene from Strongylocentrotus purpuratus as well as tau-like genes from Drosophila melanogaster (Heidary and Fortini, 2001), D. pseudoobscura, two different genuses of mosquito and Apis mellifera. PTL-1 also shares substantial homology in its microtubule-binding domain with tau-like genes from a wide variety of other organisms, including Macaca fasicularis, Danio rerio, Bos taurus, Mus musculus and Homo sapiens. PTL-1’s N-terminal “projection” domain shares homology with the luminal domain of calnexin and the P-domain of calreticulin chain A, two proteins from mammals that function as molecular chaperones (Ellgaard et al., 2001; Hahn et al., 1998). It has been suggested that the projection domains for tau family members govern several aspects of microtubule function, including the spacing between bundled microtubules (Chen et al., 1992; Goedert et al., 1996), binding to actin and other proteins (Buddle et al., 2003; Dehmelt and Halpain, 2004) and microtubule stability or stiffness (Permana et al., 2005). A chaperone role has not been ascribed to the mammalian forms of tau. However, the N-terminal region of PTL-1 and Cbr-PTL-1 differ substantially from vertebrate taus, suggesting a different function for this domain of the protein in nematodes and possibly other invertebrates. We noted that the N-terminal domains of invertebrate tau proteins are considerably shorter than their vertebrate counterparts. While the evolutionary and functional significance of this difference is not clear, the divergence clearly occurred early, prior to the divergence between echinoderms, nematodes and insects. It is intriguing that such a substantial change occurred while maintaining the high degree of sequence identity in the microtubule-binding C-terminal domain.
All of the known members of this MAP family that have been studied exhibit multiple isoforms derived from alternative splicing and other processing of mRNA. The six putative isoforms of PTL-1 differ in the number of the microtubule-binding repeats, having either 3 or 4 of these regions (Thierry-Mieg and Thierry-Mieg, 2006). Changes in the alternative splicing and alteration in the number of these repeats have been implicated in the etiology of several tauopathies, including myotonic dystrophy, Alzheimer’s disease (Goedert, 2005). It is still uncertain how tau dysfunction is linked to these conditions, but structural changes resulting from improper processing of the mRNA or phosphorylation such that the microtubule-binding motifs exhibit a structural shift from more random coil to β-sheet, resulting in aggregation, have recent experimental support (von Bergen et al., 2001). These structural changes affect not only tau aggregation, but also the structural dynamics of microtubules (Hasegawa et al., 1998).
While much work has been devoted to understanding the role of tau and MAP-2 in the development and organization of vertebrate neurons, very little is known about the functional roles of tau proteins in invertebrate neurons. It is intriguing that invertebrates, including Drosophila (Heidary and Fortini, 2001) and C. elegans, have only one tau-like gene. It is possible that invertebrate taus subserve the functional roles of both tau and MAP-2. Alternatively, it is possible that other novel genes, that do not share sequence similarities, may operate along with the invertebrate tau genes to organize the invertebrate neuron. For example, a recent paper identified a novel MAP in Drosophila, Jupiter, that appears to be a MAP that is restricted to axonal regions in some Drosophila neurons (Rolls et al., 2007). Drosophila tau is expressed in neurons of the developing brain, particularly after stage 14. The expression occurs in a subset of neurons, particularly in the chordotonal organ, which is involved in proprioception (Heidary and Fortini, 2001), as well as adult photoreceptors. Further exploration of the function and subcellular compartmentalization, as well as the protein partners of PTL-1 and Drosophila tau will shed important light on the evolution of neuronal subcellular organization and the regulation of the microtubule-based cytoskeleton in organization. The widespread distribution of tau-like genes throughout the animal kingdom speaks to its evolutionary significance in the development/maintenance of neurons.
We found that ptl-1 deficient worms exhibit a significant decrease in the number of viable progeny, with the developmental arrest coinciding with the elongation phase. PTL-1a and PTL-1b are expressed in C. elegans hypodermal cells during the elongation phase (Goedert et al., 1996). It is possible that PTL-1 influences the stability or stiffness of epidermal microtubules and so plays a role in modulating the elongation process, a developmental period with dynamic and profound cellular reorganization. Drosophila tau is also expressed during embryogenesis (Heidary and Fortini, 2001) and the protein is colocalized with microtubules near the membrane of follicle cells and nurse cells. This, too, is a period of dramatic cellular reorganization and dynamics. Intriguingly, tau mutants in Drosophila and the ptl-1 mutant worms exhibit only mild defects (Doerflinger et al., 2003), indicating that, while microtubule stability and stiffness might play a role in important developmental processes in embryos during development, tau is not essential to viability. This function of invertebrate taus might be redundant with other microtubule-associated proteins.
In agreement with reports on WormBase (Harris et al., 2004), we demonstrated expression of GFP under the control of the ptl-1 promoter predominantly in mechanosensory neurons, with the notable exception of PVM. It is intriguing that ptl-1 is not expressed in PVM in adult worms, a neuron that is not directly responsive to gentle touch, but that forms the unusual 15 protofilament microtubules. However, we do find evidence that PVM neurons in larval worms express ptl-1 transiently in L1. Thus, while PTL-1 is not required for the maintenance of the large microtubule bundles in adult mechanosensory neurons, a role for PTL-1 in the initial formation of the 15 protofilament microtubules during development cannot be ruled out. That PTL-1 is not required for the maintenance of microtubules in adult mechanosensory neurons is also supported by our immunocytochemical analysis of tubulin and acetylated tubulin.
In addition to the mechanosensory neurons, we found ptl-1-directed GFP expression in a number of other neurons and non-neuronal cells. This agrees with recent reports using this reporter construct worm (McKay et al., 2004). Whether this is due to the particular technique used to transform the worms or is a general aspect of C. elegans gene expression regulation remains unknown. For example, the ptl-1 promoter region driving GFP expression in our study is 2857 bp, which may be a larger region than is required for cell-specific expression (Hunt-Newbury et al., 2007). However, a recent analysis of all the promoter-driven GFP constructs generated by the C. elegans Gene Expression Consortium revealed that virtually all 5’ region promoter sequences drive GFP expression in multiple tissues and in multiple stages of development (Hunt-Newbury et al., 2007). Other recent analyses suggest that at least 25% of the genome can be expressed in any particular cell type or tissue (McKay et al., 2004). It is also not clear from an analysis of this sort if functional protein is produced in all the cells in which mRNA is produced. Finally, it is possible that different alternately-spliced forms of PTL-1 are expressed in different tissues or cell types. Our current work is addressing these questions.
The ptl-1 deficient worms exhibit a moderate, and significant, reduction in touch responsiveness. Further, when crossed into mec-7 or mec-12 mutant backgrounds, the touch insensitivity of these genetic combinations is significantly enhanced. These findings indicate that PTL-1 plays a role in mechanosensation. While PTL-1 is not essential for touch sensitivity or microtubule structure, it influences the responsiveness or sensitivity of the worms to multiple touches. This finding suggests that PTL-1 is not required for the establishment or maintenance of the touch response proteins. In particular, PTL-1 is not required for the formation or localization of the specialized microtubules found in adult touch neurons, as evidenced by the similar overall immunoreactivity of tubulin and the specialized acetylated α-tubulin (MEC-12) found in the touch neurons. We speculate that PTL-1, by influencing the stiffness of the 15 protofilament microtubules, might alter the sensitivity of the mechanosensory neurons to a gentle touch stimulus thereby influencing the responsiveness of the worm to the touch. If, in the absence of PTL-1, the microtubules are less stiff, then the worm would exhibit a reduced responsiveness, particularly with multiple touches. It is also possible that PTL-1 plays a role in the attachment of the 15 protofilament microtubules to the plasma membrane (Chalfie and Thomson, 1982) or may regulate the spacing of microtubules (Cueva et al., 2007) by virtue of its N-terminal projection domain. The attachments of the microtubule bundles would serve to provide stiffness or increased rigidity to the mechanosensory neuron membrane in the regions between the ion channel clusters, where the microtubule bundles appear to contact the plasma membrane (Cueva et al., 2007). This cell architecture could transmit the mechanical energy to the ion channel clusters, thereby increasing the “gain” or sensitivity to a touch stimulus. This arrangement might also serve to localize the touch stimulus to a particular spatial location along the nerve process. A reduction in the attachments would reduce these capabilities, and thus reduce the sensitivity or behavioral responsiveness to touch without interfering with the actual transduction mechanism. These possible functions of PTL-1 have not yet been tested.
The structural MAP family interacts with a number of proteins in addition to tubulin, including actin and a wide range of other proteins (Dehmelt and Halpain, 2004). MAP2 binds tightly and specifically to components of the NMDA receptor in adult rat hippocampal neurons (Buddle et al., 2003) and might play a role in the spatial localization of receptor complexes. Recent work on human tau has shown that, depending on how tau interacts with microtubules, it can influence the function of motor proteins and so alter intracellular protein and organelle trafficking (Dehmelt and Halpain, 2004). These more recent studies depict a role for structural MAPs in protein trafficking or subcellular localization at the membrane. It is possible that PTL-1, through these kinds of interactions, might influence the delivery, placement or stability of ion channel transduction complexes, thus altering the sensitivity or responsiveness to gentle touch.
Acknowledgments
This work was supported by internal grant awards from the Dean of the Faculty Research Fund, the Abbey Fund and an NIH- AREA award 1R15NS057808-01(to K.M.R.-S.). We would like to thank Cristian Opazo, Richard Wing, Amory Meltzer, Tasnim Rahman and Charlene Scotland for helpful assistance and Jerry Calvin for assistance with the confocal microscope. We would also like to thank Martin Chalfie for helpful discussions. This paper is dedicated to the memory of Michele Buddle (1962–2004).
References
- Altun ZF, Hall DH. [Cited 5 May 2008];WormAtlas. 2002–2006 http://www.wormatlas.org/
- Buddle M, Eberhardt E, Ciminello LH, Levin T, Wing R, DiPasquale K, Raley-Susman KM. Microtubule-associated protein 2 (MAP2) associates with the NMDA receptor and is spatially redistributed within rat hippocampal neurons after oxygen-glucose deprivation. Brain Res. 2003;978:38–50. doi: 10.1016/s0006-8993(03)02758-6. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston J, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in C. elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Thomson JN. Structural and functional diversity in the neuronal microtubules of Caenorhabditis elegans. J Cell Biol. 1982;93:15–23. doi: 10.1083/jcb.93.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- Cueva JG, Mulholland A, Goodman MB. Nanoscale organization of the MEC-4/DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J Neurosci. 2007;27:14089–14098. doi: 10.1523/JNEUROSCI.4179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Ann Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. Actin and microtubules in neurite inititation: Are MAPs the missing link? J Neurobio. 2004;58:18–33. doi: 10.1002/neu.10284. [DOI] [PubMed] [Google Scholar]
- Doerflinger H, Benton R, Shulman JM, St. Johnson D. The role of PAR-1 in regulating the polarized microtubule cytoskeleton in the Drosophila follicular epithelium. Dev. 2003;130:3967–3975. doi: 10.1242/dev.00616. [DOI] [PubMed] [Google Scholar]
- Duerr JS. Immunohistochemistry. In: WormBook, editor. The C. elegans Research Community. 2006. http://www.wormbook.org. [Google Scholar]
- Ellgaard L, Riek R, Braun D, Herrmann T, Helenius A, Wuthrich K. Three-dimensional structure topology of the calreticulin P-domain based on NMR assignment. FEBS Letters. 2001;488:69–73. doi: 10.1016/s0014-5793(00)02382-6. [DOI] [PubMed] [Google Scholar]
- Ernstrom GG, Chalfie M. Genetics of sensory mechanotransduction. Ann Rev Genetics. 2002;36:411–453. doi: 10.1146/annurev.genet.36.061802.101708. [DOI] [PubMed] [Google Scholar]
- Fukushige T, Siddiqui ZK, Chou M, Culotti JG, Gogonea CB, Siddiqui SS, Hamelin M. MEC-12, an α-tubulin required for touch sensitivity in C.elegans. J Cell Sci. 1999;112:395–403. doi: 10.1242/jcs.112.3.395. [DOI] [PubMed] [Google Scholar]
- Goedert M. Tau gene mutations and their effects. Movement Disorders. 2005;20:S45–S52. doi: 10.1002/mds.20539. [DOI] [PubMed] [Google Scholar]
- Goedert M, Baur CP, Ahringer J, Jakes R, Hasegawa M, Spillantini MG, Smith MJ, Hill F. PTL-1, a microtubule-associated protein with tau-like repeats from the nematode Caenorhabditis elegans. J Cell Sci. 1996;109:2661–2672. doi: 10.1242/jcs.109.11.2661. [DOI] [PubMed] [Google Scholar]
- Gu G, Caldwell GA, Chalfie M. Genetic interactions affecting touch sensitivity in Caenorhabditis elegans. Proc Nat Acad Sci. 1996;93:6577–6582. doi: 10.1073/pnas.93.13.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M, Borisova S, Schrag JD, Tessier DC, Zapun A, Tom R, Kamen AA, Bergeron JJ, Thomas DY, Cygler M. Identification and crystallization of a protease-resistant core of calnexin that retains biological activity. J Struct Biol. 1998;123:260–264. doi: 10.1006/jsbi.1998.4032. [DOI] [PubMed] [Google Scholar]
- Harris TW, Chen N, Cunningham F, Tello-Ruiz M, Antoshechkin I, Bastiani C, Bieri T, Blasiar D, Bradnam K, Chan J, Chen C, Chen WJ, Davis P, Kenny E, Kishore R, Lawson D, Lee R, Muller H, Nakamura C, Ozersky P, Petcherski A, Rogers A, Sabo A, Schwarz EM, Van Auken K, Wang Q, Durbin R, Spieth J, Sternberg PW, Stein LD. WormBase: a multi-species resource for nematode biology and genomics. Nucleic Acids Res. 2004;32:D411–D417. doi: 10.1093/nar/gkh066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Smith MR, Goedert M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Letters. 1998;437:207–210. doi: 10.1016/s0014-5793(98)01217-4. [DOI] [PubMed] [Google Scholar]
- Heidary G, Fortini ME. Identification and characterization of the Drosophila tau homolog. Mech Dev. 2001;108:171–178. doi: 10.1016/s0925-4773(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Hunt-Newbury R, Viveiros R, Johnsen R, Mah A, Anastas D, Fang L, Halfnight E, Lee D, Lin J, Lorch A, McKay S, Okada HM. High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLOS Biol. 2007;5:1981–1997. doi: 10.1371/journal.pbio.0050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JB, Aamodt S, Aamodt E. ptl-1, a Caenorhabditis elegans gene whose products are homologous to the tau microtubule-associated proteins. Biochem. 1996;35:9415–9423. doi: 10.1021/bi952646n. [DOI] [PubMed] [Google Scholar]
- McKay SJ, Johnsen R, Khattra J, Asano J, Baillie DL, Chan S, Dube N, Fang L, Goszczynski B, Ha E, Halfnight E. Gene expression profiling of cells, tissues, and developmental stages of the nematode C. elegans. Cold Spring Harbor Symp Quant Biol. 2004;68:159–169. doi: 10.1101/sqb.2003.68.159. [DOI] [PubMed] [Google Scholar]
- Mukrasch MD, von Bergen M, Biernat J, Fishcher D, Griesinger C, Mandelkow E, Zweckstetter M. The "Jaws" of the Tau-Microtubule interaction. J Biol Chem. 2007;282:12230–12239. doi: 10.1074/jbc.M607159200. [DOI] [PubMed] [Google Scholar]
- O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- Permana S, Hisanage S, Nagatomo Y, Iida J, Hotani H, Itoh TJ. Truncation of the projection domain of MAP4 (Microtubule-Associated Protein) leads to attenuation of microtubule dynamic instability. Cell Struct Function. 2005;29:147–157. doi: 10.1247/csf.29.147. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- Rolls MM, Satoh D, Clyne PJ, Henner AL, Uemura T, Doe CQ. Polarity and intracellular compartmentalization of Drosophila neurons. Neural Dev. 2007;2:2007. doi: 10.1186/1749-8104-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The Neighbor-joining Method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobio. 2000;61:133–168. doi: 10.1016/s0301-0082(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Savage C, Hamelin M, Culotti JG, Coulson A, Albertson DG, Chalfie M. mec-7 is a β-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev. 1989;3:870–881. doi: 10.1101/gad.3.6.870. [DOI] [PubMed] [Google Scholar]
- Savage C, Xue Y, Mitani S, Hall D, Zakhay R, Chalfie M. Mutations in the Caenorhabditis elegans β-tubulin gene mec-7: effects on microtubule assembly and stability and on tubulin autoregulation. J Cell Sci. 1994;107:2165–2175. doi: 10.1242/jcs.107.8.2165. [DOI] [PubMed] [Google Scholar]
- Siddiqui SS, Aamodt E, Rastinejad F, Culotti JG. Anti-tubulin antibodies that bind to specific neurons in Caenorhabditis elegans. J Neurosci. 1989;9:2963–2972. doi: 10.1523/JNEUROSCI.09-08-02963.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Bio. 2006;7:S12. doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bergen M, Barghorn S, Li L, Marx A, Biernat J, Mandelkow EM, Mandelkow E. Mutations of tau protein in fronto-temporal dementia promote aggregation of paired helical filaments by enhancing local β-structure. J Biol Chem. 2001;276:48165–48174. doi: 10.1074/jbc.M105196200. [DOI] [PubMed] [Google Scholar]