Abstract
Calbindin D9k (CaBP) is critical for intestinal calcium absorption; its in vivo expression is restricted to differentiated enterocytes of the small intestine. Our goal was to identify factors controlling the transcriptional regulation of this gene in the human intestine. Both the natural gene and a 4600-bp promoter construct were strongly regulated by differentiation (>100-fold) but not by treatment with 1,25(OH)2 vitamin D (<2-fold) in the Caco-2 clone TC7. Deletion-mutation studies revealed that conserved promoter sequences for cdx-2 (at −3158 bp) and hepatocyte nuclear factor (HNF)-1 (at −3131 and at −98 bp) combined to control CaBP expression during differentiation. Other putative response elements were not important for CaBP regulation in TC7 cells (CCAAT enhancer binding protein, pancreatic duodenal homebox-1 (pdx-1), a proximal cdx-2 element). Mutation of the distal HNF-1 site had the greatest impact on CaBP gene expression through disruption of HNF-1α binding; both basal and differentiation-mediated CaBP expression was reduced by 80%. In contrast, mutation of the distal cdx-2 element reduced only basal CaBP expression. Whereas a 60% reduction of CaBP mRNA in the duodenum of HNF-1α null mice confirmed the physiological importance of HNF-1α for CaBP gene regulation, additional studies showed that maximal CaBP expression requires the presence of both HNF-1α and cdx-2. Our data suggest that cdx-2 is a permissive factor that influences basal CaBP expression in enterocytes and that HNF-1α modulates CaBP gene expression during cellular differentiation.
Keywords: intestine; enterocyte; 1,25 dihydroxyvitamin D
The Normal Function of Intestinal mucosa relies on the appropriate renewal and differentiation of cells along the cryptvillus axis (25, 29). Developing therapies that promote recovery of a damaged epithelium or prevent malignancy (50) require understanding of the molecular pathways programming enterocyte differentiation. A classical approach to studying the program dictating this process has been to examine the regulation of intestine-specific genes, such as sucrase-isomaltase and lactase-phlorizin hydrolase. Extensive studies (3, 4, 17, 19, 33, 40) on their promoters have identified several transcription factors important for intestinal expression of these genes [cdx-2, hepatocyte nuclear factor (HNF)-1, and GATA family members]. However, intestinal expression of villin and adenosine deaminase depends on enhancer elements in introns 1 and 2, respectively (16, 37). Thus the study of other differentiation-associated genes in intestine will provide new insight into the programming essential for development of a functional absorptive surface.
One such gene encodes calbindin D9k, an EF hand calcium-binding protein critical for calcium absorption that is expressed in the duodenum and cecum of rodents (5, 7). The expression of calbindin D9k is regulated by the hormonally active form of vitamin D, 1,25 dihydroxyvitamin D [1,25(OH)2 D] (7, 22, 47). Whereas previous reports have identified putative vitamin D response elements in the calbindin D9k promoter from various species (12, 28, 45), other data suggest that posttranscriptional regulation may be a more important mode of calbindin D9k regulation by 1,25(OH)2 D (14, 15, 23, 46). In addition to vitamin D regulation, calbindin D9k expression is restricted to the differentiated villus compartment (24, 38, 49, 53), indicating that it is under the control of factors programming intestinal cell differentiation; mutation of a cdx-2 response element at −3.5 kb decreased the intestinal expression of a calbindin D9k reporter gene construct by 99% in transgenic mice (9). Several other potential regulatory sites in the proximal rat calbindin D9k promoter have been reported on the basis of footprinting and gel-shift assays (1, 34), i.e., a proximal cdx-2 element and sites for HNF-1, HNF-4, and CCAAT enhancer binding protein (C/EBP). None of those sites has been assigned a functional role in the regulation of calbindin D9k promoter activity.
Here we characterized 1,25(OH)2 D- and differentiation-mediated regulation of the human calbindin D9k gene promoter in Caco-2 cells, an in vitro model for small intestinal differentiation (2, 13, 18, 51). Our findings demonstrate that the calbindin D9k gene is poorly regulated at the transcriptional level by 1,25(OH)2 D but strongly upregulated during spontaneous differentiation to an enterocyte-like phenotype. Two HNF-1 binding sites, one at −98 and a novel, distal HNF-1 binding site at −3131 are essential for the differentiation-dependent expression of calbindin D9k. In addition, our findings suggest that cdx-2 is a permissive factor that influences the basal expression level and that HNF-1-mediated transcriptional regulation of the calbindin D9k promoter requires a complex interaction between the proximal and the distal HNF-1 response element.
MATERIALS AND METHODS
Cell Culture
Unless otherwise noted, all chemicals were obtained from Fisher Scientific (Fair Lawn, NJ), the media and supplements were purchased from Sigma (St. Louis, MO), and tissue culture plates were purchased from Corning Costar (Cambridge, MA).
The parental strain of Caco-2 cell (HTB-37) and the BBe clone (CRL-2102) were obtained from American Type Culture Collection (Rockville, MD). TC7 cells were first described by Chantret et al. (6). These were provided to us at passage 69 by Dr. Mark Failla (Ohio State University). Cells were cultured as previously described (20). The BBe and TC7 clones were used because they have previously been demonstrated to develop a more differentiated phenotype (e.g., higher sucrase activity) compared with the parental line (6, 42). To ensure that the cell lines would reach confluence concurrently (4 days in culture), different seeding densities were used for each cell line, i.e., parental Caco-2, BBe, and TC7 cells were seeded onto six-well dishes at a density of 128,000, 256,000, and 64,000 cells/well, respectively. For transfection experiments, TC7 cells were seeded onto 24-well dishes at a density of 15,000 cells/well.
Regulation of Calbindin D9k Expression by 1,25(OH)2 D and Spontaneous Differentiation
Spontaneous differentiation
Parental Caco-2, BBe, and TC7 cells were grown in culture until 50% confluent (2 days in culture), confluent (4 days in culture), 4 days postconfluent (8 days in culture), and 11 days postconfluent (15 days in culture). At each time point, cells (n = 3 wells per cell line) were harvested into 1 ml TriReagent (Molecular Research Center, Cincinnati, Ohio), total RNA was isolated following the manufacturer’s directions, and specific messages were assessed by RT-PCR. The experiment was repeated twice (n = 6 per time point per cell line).
To evaluate whether the expression of calbindin D9k mRNA was transcriptionally activated during spontaneous differentiation, expression of a full-length calbindin D9k promoter reporter gene construct (CaBP4600) was examined under two protocols. In one study, proliferating cultures of TC7 cells were transfected and then harvested at 50% confluent, 5 days in culture (1 days postconfluent), 9 days in culture (5 days postconfluent), and 13 days in culture (9 days postconfluent). In the second study, 2- or 9-day cultures of BBe and TC7 cells were transiently transfected and studied 24 h later. This second protocol was utilized for all subsequent experiments. Cells were either seeded onto six-well dishes (for the study of proliferating cells) or 24-well dishes (for the study of postproliferative cultures) and transfected with the Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA). Briefly, 2 µg of the specific experimental vector was cotransfected along with a Renilla-expressing control vector, pRL-CMV (Promega, Madison, WI; molar ratio between the experimental and control vector = 250:1). In proliferating cells, transfection medium was replaced with 10% FBS DMEM 2 h after transfection. For differentiated cells, an equal volume of 20% FBS DMEM was added to each well 8 h after transfection, and new medium was added at 24 h. The prolonged exposure of differentiated cells to plasmid was necessary to overcome the low transfection efficiency we observed in these tight monolayers of nonproliferating cells. Cells were harvested, and luciferase activity was measured with the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) in a luminometer (model TD-20/20; Twin Designs, Sunnyvale, CA). The firefly luciferase activity (experimental plasmid) was normalized to Renilla luciferase activity (control plasmid) to control for variability in transfection efficiency. All treatments were examined in triplicate, and each experiment was conducted at least twice.
1,25(OH)2 D treatment
TC7 cells were seeded into six-well dishes and cultured for 2, 8, or 15 days. Cells were treated with either 100 nM 1,25(OH)2 D or ethanol vehicle (0.1% final concentration) for 48 h and then harvested for the examination of gene expression by RT-PCR.
Transcriptional regulation of the CaBP4600 construct was examined in 13-day cultures of TC7 cells. Cells were transiently transfected with either the CaBP4600 construct or a rat 24-hydroxylase promoter reporter construct provided to us by Dr. John Omdahl (30). Twenty-four hours after the transfection, cells were treated with 100 nM 1,25(OH)2 D for 24 h, and promoter activity was assessed after an additional 24 h as described above. For both natural and reporter-gene studies, the treatments were examined in triplicate and each experiment was conducted twice.
RT-PCR analysis
Levels of specific messages were assessed by RT-PCR as described previously (21, 22). PCR primers used were sucrase (GenBank accession no. X63597): forward, 5′-GGTGGTCACATCCTACCATGTCAAG-3′, reverse, 5′-CTGGGATATCTTTTTACTAA-3′ (annealing temperature = 55°C); calbindin D9k (GenBank accession no. X65869): forward, 5′-ATGAGTACTAAAAAGTCTCCT-3′, reverse, 5′-CTGGGATATCTTTTTTACTAA-3′ (annealing temperature = 55°C); cdx-2 (GenBank accession no. U51096): forward, 5′-AGCCAAGTGAAAACCAGGAC-3′, reverse, 5′-CAGGGACAGAGCCAGACAC-3′ (annealing temperature = 55°C); GAPDH (GenBank accession no. X02231): forward, 5′-CCATACAGGCAGCTTCGG-3′, reverse, 5′-AGTCATCCACGAGCGATTTG-3′ (annealing temperature = 55°C); Pdx-1 (GenBank accession no. U35632): forward, 5′-GCCGCATGAAGTGGAAAAAGG-3′, reverse, 5′-TGTGGCGACGCGCTTAAGG-3′ (annealing temperature = 55°C); HNF-1α (GenBank accession no. NM_000545): forward, 5′-TAGTGGAGGAGTGCAACAGGGC-3′, reverse, 5′-TGGGAGAACTGGACGGGCTG-3′, (annealing temperature = 60°C); HNF-1β (GenBank accession no. NM_000458): forward, 5′-GATGCCCACACACCACTTAC-3′, reverse, 5′-TACGGCTTTCTTGCTTCCTC-3′ (annealing temperature = 57°C); C/EBPα (GenBank accession no. XM009180): forward, 5′-CCCGAGTCACACCAGAAAG-3′, reverse, 5′-CCGAGCAAAACCAAAACAA-3′ (annealing temperature = 57°C). All PCR reactions used the cycling conditions of 94°C for 15 s and primer annealing temperature for 1 min 30 s, and 72°C for 45 s. PCR cycle numbers used were sucrase-isomaltase (27 cycles), cdx-2 (27 cycles), GAPDH (17 cycles), calbindin D9k (2 and 4 days = 35 cycles; 8 days = 27 cycles for BBe and P, 23 cycles for TC7, 15 days = 27 cycles for BBe and P; 21 cycles for TC7), HNF-1α (28 cycles), HNF-1β (24 cycles), C/EBPα (27 cycles), and Pdx-1 (35 cycles). These cycles were chosen so that the amplification was conducted in the linear range of amplification efficiency for each of the primer sets (data not shown). The resulting PCR products were subjected to electrophoresis on 2.5% agarose gels containing ethidium bromide, and bands were visualized under UV light. Gel data were recorded by using the Bio-Rad FluorS Imaging System, and relative densities of the bands were determined by using Quantity One software (Bio-Rad laboratories, Hercules, CA). Data were normalized for the expression of GAPDH within the sample. For calbindin D9k, we adjusted the data to account for differences in cycle numbers using the equation An = Ao(1 + R)n to determine a correction factor [where An = the amount of product produced at n cycles, Ao = the starting amount of cDNA, R = the amplification efficiency (assumed to be 0.8), and n = the cycle number]. Use of this correction factor permitted us to compare the transcript levels for calbindin D9k across time and between cell lines.
In Silico Analysis of the Calbindin D9k Gene Promoter
A computer-based comparison of the calbindin D9k gene promoter from mouse, rat, and human was conducted by using sequences available in GenBank (rat = GenBank accession no. X16635; mouse = GenBank accession no. AY034822 submitted from our laboratory; human = GenBank accession nos. X13042 and AL445467). The Compare (16 bp/21 bp window homology match) and DotPlot programs (GCG, Madison, WI) were used to identify promoter regions (clusters) with high cross-species sequence homology. These conserved clusters were examined for potential response elements using the computer program SIGSCAN (Advanced Bio-science Computer Center of The National Institutes of Health, http://bimas.dcrt.nih.gov/molbio/signal/) and the mammalian response element database TRANSFAC (54). When a putative response element was identified in the human calbindin D9k promoter, we examined the rat and mouse promoters to determine whether the element was conserved in these species.
Deletion-Mutation and Transcription Factor Activation Studies of the Human Calbindin D9k Gene Promoter
TC7 cells were either seeded onto six-well dishes (for the study of proliferating cells) or 24-well dishes (for the study of postproliferative cultures) and transfected with various constructs containing deletions of large promoter regions or mutations of specific response elements. Analysis of promoter activity was conducted as described earlier in Spontaneous differentiation. All treatments were examined in triplicate, and each experiment was conducted at least twice. In other experiments, proliferating cultures of TC7 or HeLa cells were transiently transfected with the CaBP4600 construct in the presence or absence of expression vectors for mouse cdx-2, HNF-1α, or HNF-1β either alone or in combination. Promoter activity was examined 24 h after transfection. Expression vectors for HNF-1α (pBJ5-HNF-1α) and HNF-1β (pBJ5-HNF-1β) were gifts from Dr. G. R. Crabtree, Stanford University. The expression vector for mouse cdx-2 (pCB-mcdx-2) and its control empty vector (pCB6) were previously described (36). The sucrase-isomaltase promoter-luciferase reporter gene vector (pGL3-SI256) was provided by Dr. J. T. Troelsen (University of Copenhagen, Copenhagen, Denmark).
Reporter gene plasmid construction
A 5.4-kb fragment of the human calbindin D9k promoter (−4478 to +889 bp) was obtained by PCR using human genomic DNA as template and the PCR primers: forward, 5′-GCTGCTATGGTTTGAATGCATTCC-3′; reverse, 5′-TCCTCTTCAGTTCCTCAGGAGAC-3′. A gel-purified PCR fragment was subcloned into the pCR4-Blunt vector (Invitrogen, Carlsbad, CA; pCR4-CaBP5400) and resequenced (Iowa State University DNA Sequencing Facility, Ames, IA). A full-length calbindin D9k promoter-luciferase reporter gene construct was assembled in three steps. First, a 3400-bp SacI digestion fragment of pCR4-CaBP5400 (−3342 to +74) was subcloned into the SacI site of pGL3-basic (Promega, Madison, WI; pGL3-CaBP3400). Second, a 3.1-kb EcoRI fragment of pCR4-CaBP5400 (−4580 to −1500) was subcloned into pCR2.1 (Invitrogen, Carlsbad, CA; pCR2.1-CaBP3100). Finally, the pGL3-CaBP3400 and pCR2.1-CaBP3100 vectors were digested with KpnI and ApaI, and the fragment from pCR2.1-CaBP3100 (−4478 to −2898) was ligated into the backbone from pGL3-CaBP3400 to yield pGL3-CaBP4600 (−4478 to +74 bp).
A construct lacking conserved cluster I was prepared by digesting pGL3-CaBP3400 with KpnI and NsiI. The sticky ends were blunted by using Klenow Large Fragment (New England Biolabs, Beverly, MA) and then religated to create pGL3-CaBP1700 (−1558 to +74 bp). A construct lacking conserved clusters I and II was prepared by digesting pGL3-CaBP3400 with KpnI and BstXI followed by bluntend ligation of the backbone to form pGL3-CaBP500 (−408 to +74 bp). A construct lacking conserved cluster II (pGL3-CaBP1&3) was prepared by deleting the 1603-bp BbvCI fragment (−2153 to −550) from pGL3-CaBP4600, whereas a construct lacking three-fourths of cluster III (pGL3-CaBP1 and 2) was prepared by deleting the 631-bp MscI fragment (−710 to −79) from pGL3-CaBP4600.
Point mutations in specific response elements were introduced onto the wild-type calbindin D9k promoter-luciferase reporter gene plasmid (pGL3-CaBP4600) using the QuikChanger XLSite-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) following the manufacturer’s directions. The oligonucleotides used to introduce mutations were: distal cdx-2, 5′-CTCTTGTGAACACACCCTGTAA(TT/GG)ATCACTTGTCTCC-3′; distal HNF-1, 5′-CACTTGTCTCCATATATTTTTAGT(TA/GG)ATGTTTCACAAGAGTATCTATTAG-3′; proximal C/EBP, 5′-CGACTCTGAACCATGAAGC(AA/GG)CTGTCGAGATGGACC-3′; proximal cdx-2, 5′-GGTGGCGTGCCCGT(AA/GG)AGACTATAAAAGTGTCATG-3′; and proximal HNF-1, 5′-GCCTGAGTTTCAAAAACCATT(AA/GG)TAATTACCCTTAAATGGCCAAC-3′, where the underlined section is the response element and the bracketed bases represent the wild-type sequence followed by bolded nucleotides that replaced them in the mutated version. Plasmids with mutated response elements were isolated by using the Plasmid Mini Kit (Qiagen, Valencia, CA). All plasmids were sequenced to confirm the presence of the mutation (Iowa State University DNA Sequencing Facility, Ames, IA).
EMSA Analysis of Novel HNF-1 Response Element
Single-stranded oligonucleotides were synthesized and purified by Integrated DNA Technologies (Coralville, IA). The sequences used were lactase promoter HNF-1 site, 5′-AACCCAGTTAAATATTAAGTC-3′, −125 to −105 (33); calbindin D9k distal HNF-1 site, 5′-TTTAGTTAATGTTTCACAAGAGTAT-3′, −3151 to −3127; and mutated calbindin D9k distal HNF-1 site, 5′-TTTAGTGGATGGTTTCACAAGAGTAT-3′, −3151 to −3127 (underlined portions refer to the HNF-1 site; the bold GG in the mutated version is the site of mutation). Double-stranded probes were made by annealing equal molar amounts of two complementary oligonucleotides in annealing buffer (10 mM Tris·HCl, pH = 7.5, 1 mM EDTA, 100 mM NaCl). The annealing reaction was conducted by heating samples at 90°C for 10 min with gentle agitation and then slowly cooling to room temperature for 1 h. The double-stranded probe was labeled with 32P by end-labeling. Briefly, 3.5 pmol of the probe was incubated with 1 µl of T4 polynucleotide kinase 10× buffer (700 mM Tris·HCl, pH 7.6, 100 mM MgCl2, 50 mM DTT), 1 µl of [γ-32P]ATP (3,000 Ci/mmol at 10 mCi/ml, Amersham Biosciences, Piscataway, NJ), and 1 µl of T4 polynucleotide kinase (5–10 U/µl) in a 10 µl reaction at 37°C for 10 min. The reaction was stopped by adding 1 µl of 0.5 M EDTA. The labeled probe was diluted 10-fold with Tris-EDTA buffer (pH = 7.4) and stored at −20°C for ≤2 wk. Only probes with >40% labeling efficiency were used.
Nuclear extracts were isolated from 50% confluent and 5-day postconfluent TC7 cell cultures using the Nuclear Extract Kit (Active Motif, Carlsbad, CA). Protein yield was determined by the Bio-Rad protein assay. Ten micrograms of nuclear extract were preincubated for 10 min at room temperature in binding buffer (in mM): 10 Tris·HCl, pH 7.5, 5 MgCl2, 50 NaCl, 0.5 DTT, 0.5 EDTA, and 4% glycerol plus 0.025 µg/ml poly(dIdC) with or without unlabeled competitor probes or antibodies (anti-HNF-1α antibody or goat IgG, 2 mg/ml, Santa Cruz Biotechnology, Santa Cruz, CA) in 9-µl total volume. 32P-labeled probe (0.035 pmol) was then added, and the reaction was incubated at room temperature for another 20 min, at which time 1 µl of 10× gel loading buffer was added (250 mM Tris·HCl, pH 7.5, 0.2% bromophenol blue, 40% glycerol). The whole reaction was loaded onto a 6% DNA retardation gel (Invitrogen, Carlsbad, CA), and electrophoresis was conducted by using 0.5× TBE running buffer (44.5 mM Tris base, pH 8.3, 44.5 mM boric acid, 22.1 mM EDTA) at 250 V for 15 min. The gel was dried by using a GelAir gel drying system (Bio-Rad) for 1 h, and specific bands were detected by autoradiography using Kodak XOMAT film.
Analysis of Calbindin D9k Expression in HNF-1α Null Mice
HNF-1α null mice were generated by using Cre-loxP-mediated deletion of exon 1 of the HNF-1α gene. The description and characterization of these mice has been presented elsewhere (35). Eight to twelve-wk-old HNF-1α heterozygous (+/−) and age-matched HNF-1α homozygous null (−/−) mice were used in this study (n = 6 per genotype). Mice were anesthetized with avertin (240 mg/kg), and the proximal 1 cm of small intestine adjacent to the pylorus was removed and immediately frozen on dry ice. All dissections were conducted between 1:00 and 4:00 PM to minimize variability due to circadian expression of HNF-1α (43).
The abundance of calbindin D9k mRNA in HNF-1α +/− and HNF-1α −/− mice was determined by RNase protection assays (RPA) using previously established methods (31). Total RNA was isolated from mouse tissues using the RNeas kit (Qiagen), quantified by optical density at A260 nm, and checked on an agarose gel. Antisense RNA probes for mouse calbindin D9k (a cDNA for the coding region of the mouse gene in pCR2.1) and mouse β-actin (32) were linearized, transcribed with T7 RNA polymerase, labeled by using [32P]ATP (Perkin-Elmer Life Sciences) and hybridized to RNA at 68°C in 50% formamide overnight, and digested with RNase T1 (1 µg/µl), and the protected fragments were separated on 6% denaturing polyacrylamide gels and then detected by autoradiography. The bands were quantified by using a Gel Doc system for image acquisition and Quantity One (Bio-Rad) software for analysis of band density. Calbindin D9k mRNA levels were corrected for the expression of β-actin mRNA levels before analysis. These studies were conducted under protocols approved by the Animal Research at Children’s Hospital Animal Care and Use Committee.
Statistical Analysis
Data are reported as the means ± SE. Studies were examined by ANOVA or t-test (HNF-1α null mouse only) using the SYSTAT statistical software package (SAS Institute, Cary, NC). Comparisons of multiple group means were performed by using Fishers protected least significant difference. Comparisons with P < 0.05 were considered significantly different.
RESULTS
Regulation of Calbindin D9k Gene Expression by Differentiation
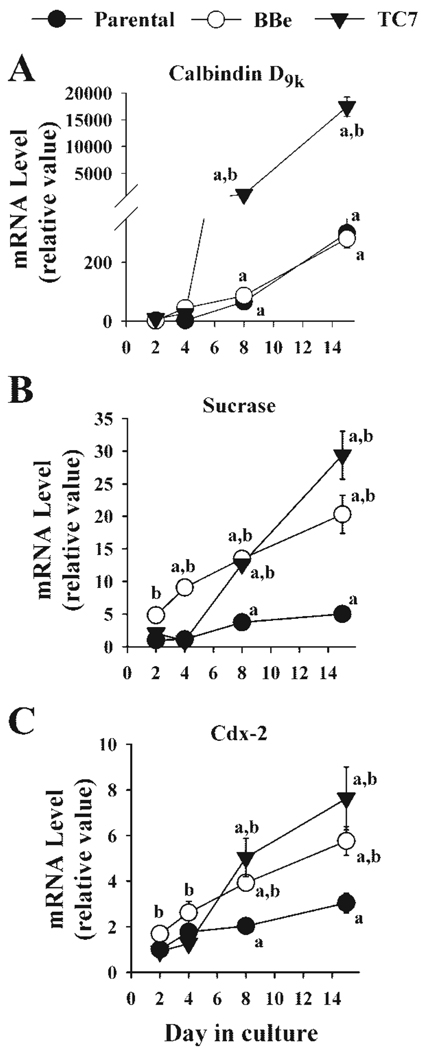
In the parental Caco-2 line and the two well-differentiating clones, BBe and TC7, sucrase-isomaltase and calbindin D9k mRNA levels significantly increased as the cells differentiated (Fig. 1), and the two clonal lines expressed sucrase-isomaltase mRNA at a significantly higher level at every point after confluence (Fig. 1B). None of the three cell lines expressed high levels of calbindin D9k mRNA at 2 or 4 days in culture. However, by 8 days in culture, the calbindin D9k message was higher in TC7 (1036.40 ± 43.42 arbitrary units) than either parental or BBe cells (66.43 ± 3.97 and 86.85 ± 18.60 arbitrary units, P < 0.001, Fig. 1A). This pattern was also seen at 15 days in culture (TC7 60-fold > BBe = parental). Figure 1C shows that the level of cdx-2 mRNA was similar in proliferating cells (parental = 0.29 ± 0.04, BBe = 0.49 ± 0.01, TC7 = 0.27 ± 0.02) and increased significantly in all three Caco-2 lines from 2 to 15 days in culture (parental = 0.89 ± 0.12, BBe = 1.68 ± 0.18, TC7 = 2.23 ± 0.40).
Fig. 1.
Comparison of calbindin D9k, sucrase-isomaltase, and cdx-2 mRNA level among three Caco-2 cell lines. Parental Caco-2 (P) and two well differentiating clones of Caco-2 (BBe and TC7) were harvested at 2 days (50% confluent, proliferating cultures), 4 days (confluence), 8 days (4 days postconfluent, postproliferative), and 15 days (11 days postconfluent, well-differentiated) after seeding. Calbindin D9k, sucrase-isomaltase, and cdx-2 mRNA levels were analyzed by semiquantitative RT-PCR. Each value represents the means ± SE (n = 6) for calbindin D9k (A), sucrase-isomaltase (B), and cdx-2 (C). a, Significantly different from 2-day expression; b, significantly different from parental expression (Fishers least significant difference test, P < 0.05).
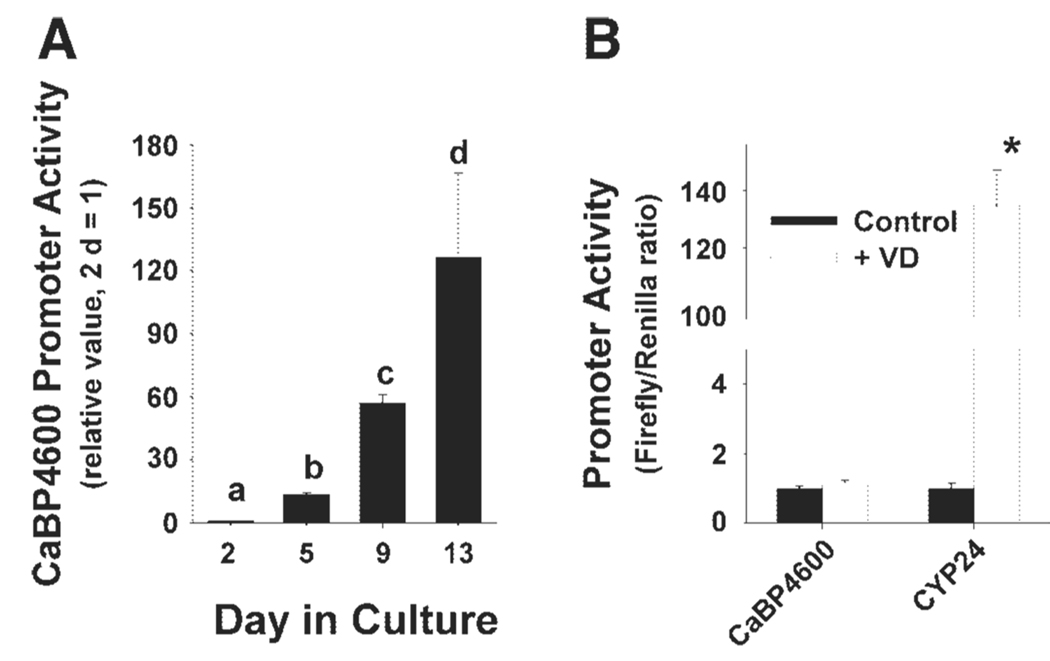
We confirmed that the full-length calbindin D9k promoter-reporter gene construct recapitulated the natural calbindin D9k. In TC7 cells transfected with the CaBP4600 promoter construct while proliferating and then studied at 1, 5, and 9 days postconfluence (5, 9, and 13 days in culture), calbindin D9k promoter activity increased dramatically relative to the activity measured in proliferating TC7 cells (Fig. 2A). A 30-fold increase in calbindin D9k promoter activity from 50% confluence (2 days in culture) to 5 days postconfluence (9 days in culture) was also observed by using the protocol in which TC7 cells were transiently transfected during proliferation or at 4 days postconfluence and studied 24 h later (data not shown). With the use of the later protocol, we found that the increase in promoter activity between 2 and 9 days in BBe cells was less than that observed in TC7 cells (BBe ratio = 10% TC7 response, data not shown).
Fig. 2.
Regulation of calbindin D9k mRNA levels and promoter activity by differentiation. A: regulation of calbindin D9k promoter activity during TC7 cell differentiation using a full-length calbindin D9k promoter-reporter gene construct (CaBP4600). TC7 cells were transfected with a CaBP4600 at 1 day in culture, and reporter activity was examined at 2, 5, 9, and 13 days in culture by measuring luciferase activity. B: vitamin D (VD) regulated promoter activity in 15-day cultures of TC7 cells. Cells were transfected with either CaBP4600 or a 24-hydroxylase promoter construct (CYP24), treated for 24 h with 100 nM 1,25(OH)2 vitamin D, and promoter activity was assessed by measuring luciferase activity. Bars represent the means ± SE. Bars with different letters (in A) are significantly different (P < 0.05). In B, the bar with an asterisk (*) indicates significant difference from vehicle treatment (P < 0.05).
Several transcription factors have been implicated in the regulation of calbindin D9k in the enterocyte. A brief survey of their expression revealed that Pdx-1 mRNA was not expressed in either proliferating or differentiated TC7 or BBe cells. In contrast, cdx-2, HNF-1α, HNF-1β, and C/EBPα mRNA were all expressed in proliferating cells, and their mRNA levels were higher in differentiated cells (data not shown).
Regulation of Calbindin D9k Expression in TC7 Cells by 1,25(OH)2 D
1,25(OH)2 D treatment caused a small (2-fold) increase in the expression of calbindin D9k mRNA in proliferating, postproliferative, and differentiated TC7 cells (data not shown). In contrast, calbindin D9k promoter activity was not influenced by 100 nM 1,25(OH)2 D treatment, although CYP24 promoter activity was increased > 100-fold under the same conditions (Fig. 2B).
Identification of Conserved Sequence Clusters and Response Elements in the Human Calbindin D9k Gene Promoter
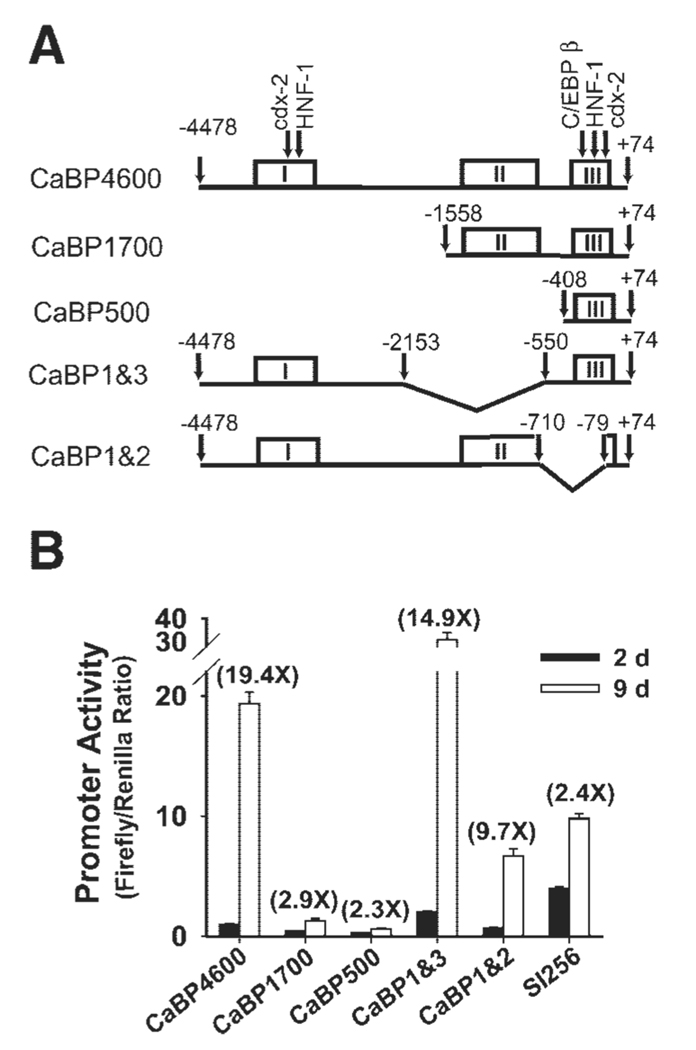
Conserved promoter regions are more likely to contain regulatory elements controlling the expression of a gene (26). In silico analysis of the human, rat, and mouse calbindin D9k promoter sequences revealed three clusters with a high degree of homology across species. In the human promoter, these were located at −3512 to −2932 (cluster I), −1452 to −682 (cluster II) and −272 to −1 (cluster III, Fig. 3A). DNase I hypersensitive sites previously identified in the rat promoter (41) occur within cluster I (HS1), between clusters II and III (HS2 and 3), and in cluster III (HS4 and 5). Table 1 lists all of the previously reported transcription factor binding sites and relates their location to the conserved clusters we identified. Only the proximal cdx-2 response element (including the TATA box), a putative Pdx-1 response element, and the C/EBP response element at −61 bp were 100% conserved across all three species. HS2, HS3 (neither of which are intestine specific) are within a segment that was deleted in mice and humans. Of the previously identified vitamin D response elements (VDREs), none were well conserved between species (e.g., VDRE3 had missing segments in mouse and rat; VDRE1 is in a nonconserved portion of the promoter and is absent in human). SIGSCAN analysis of the three clusters identified 13 putative sites that were completely conserved among all three species, including a HNF-1 site [−3131 to −3120 bp, (−) strand, 5′-GGTTAATNATTATCA-3′, other data not shown]. We also identified two 100% conserved stretches among the species in cluster II: a 19-bp sequence [on the (+) strand starting at −807 bp 5′-TTAAACCTGCTTCTGAAGC-3′] and a 16-bp sequence [on the (−) strand starting at −932 bp 5′-TTTTATTTCTCATTAT-3′]. The 16-bp stretch (5′-TTTTATTTC-3′) is similar to a cdx-2 consensus sequence (5′-TTTTTATA/GGC-3′), but no known response element consensus sequence was found in the 19-bp stretch.
Fig. 3.
Deletion analysis of conserved clusters in the human calbindin D9k gene reveals important regulatory regions. A: schematic representation of the promoter constructs [CaBP4600 or truncated calbindin D9k promoters (CaBP1700, CaBP1&2, CaBP1&3, and CaBP500)] used in experiments. The sequence containing all 3 of the conserved sequence clusters is CaBP4600. Versions of the promoter lacking one or more conserved clusters are shown under the full-length promoter construct. In CaBP4600, arrows point to the sites of putative response elements that were functionally evaluated by mutation analysis. B: reporter constructs containing the sucrase-isomaltase promoter (SI256), CaBP4600, or CaBP1700, CaBP1&2, CaBP1&3, and CaBP500 were transiently transfected into 50% confluent (day 2) and 5-day postconfluent (day 9) TC7 cells. After 24 h, promoter activity was assessed by measuring luciferase activity. All data are expressed relative to the expression observed for CaBP4600 at 2 days (relative value = 1). Values in parentheses are the ratio of promoter activity at 9 days to that measured at 2 days. Each experiment was repeated at least 3 times with triplicate observations within each experiment. Data from 1 representative experiment is shown as means ± SE.
Table 1.
Response elements in the calbindin D9k promoter
| Name | Species | Position | Sequence* | Ref. No. |
|---|---|---|---|---|
| Cdx-2 (P) | H | −40/−25 | GTAAAG ACT ATAAAAG | 34 |
| R | −38/−23 | GTAAAG ACT ATAAAAG | ||
| M | −43/−28 | GTAAAG ACT ATAAAAG | ||
| Cdx-2 (D) | H | −3158/−3152 | TGtAaTTATcaCt | 9 |
| R | −3439/−3433 | TGCATTTATGGCA | ||
| M | −3044/−3038 | TGCcTTTATGGCA | ||
| ERE | H | +51/+63 | GGTtA GTG TGATt | 11 |
| R | +50/+62 | GGTCA GGG TGATC | ||
| M | +50/+62 | GGTCA GTG TGgTC | ||
| PDX-1 | H | −145/−141 | CCTTAATTATG | 1 |
| R | −131/−127 | CCTTAATTgaG | ||
| M | −136/−132 | CCTTAATTgaG | ||
| C/EBP | H | −61/−53 | TTTCATAAT | 34 |
| R | −62/−54 | TTTCATAAT | ||
| M | −67/−59 | TTTCATAAT | ||
| HNF-1 | H | −98/−86 | ATTAATaATTACC | 34 |
| R | −98/−86 | ATTAATCATTACC | ||
| M | −103/−91 | ATTAATCATTACC | ||
| C/EBP | H | −183/−174 | TgAAGCAAcT | 34 |
| R | −172/−164 | TTAAGCAAT | ||
| M | −177/−169 | TTAAGCAAT | ||
| HNF-4 | H | −260/−251 | TGaGCAAa-T-A | 34 |
| R | −256/−245 | TGCGCAAGGTCA | ||
| M | −261/−250 | TGCGCAAGGTCA | ||
| VDRE1 | H | n/a | Not present | 11 |
| R | −489/−475 | GGGTGT CGG AAGCCC | ||
| M | −508/−493 | GGGTGT CAG AAaCCC | ||
| VDRE2 | H | −1247/−1233 | AGGTGA TAT AAGGCA | 28 |
| R | −1648/−1634 | AGGgaA TAT ActGtA | ||
| M | −1260/−1246 | AGGTaA TAT ActGtA | ||
| VDRE3 | H | −151/−130 | TGCCCT TAATTATGG GGTTCATGCAGT | 45 |
| R | −137/−122 | TGaCCT TAATTGAG - - - - - - - - - - AGT | ||
| M | −142/−127 | TGCCCT TAATTGAG - - - - - - - - - - AGT |
Underlined is the consensus sequence reported in the reference; bases different from the reported sequence are written in lower case. All sequences are from the (+) strand and are written 5′- to -3′. P, proximal; D, distal; ERE, estrogen response element; PDX-1, pancreatic duodenal homebox-1; C/EBP, CCAAT enhancer binding protein; HNF-1, hepatocyte nuclear factor 1; VDRE, vitamin D response element; H, human; R, rat; M, mouse.
Role of Conserved Promoter Sequence Clusters and Potential cis Elements in Calbindin D9k Gene Expression
To investigate the contribution of each conserved promoter cluster to the control of calbindin D9k gene expression, we made a series of deletion constructs as illustrated in Fig. 3A. The result of studies using these deletion constructs are shown in Fig. 3B. Removal of cluster I reduced basal promoter activity (expression in 2-day cultures) by 80% and the fold change associated with differentiation (9:2 days expression ratio) by 90% (CaBP1700). Removal of cluster II significantly increased promoter activity in both 2- and 9-day cultures by 60% (CaBP1 and 3), whereas removal of three-fourths of cluster III significantly blocked basal promoter activities as well as the fold change associated with differentiation by 50% (CaBP1 and 2).
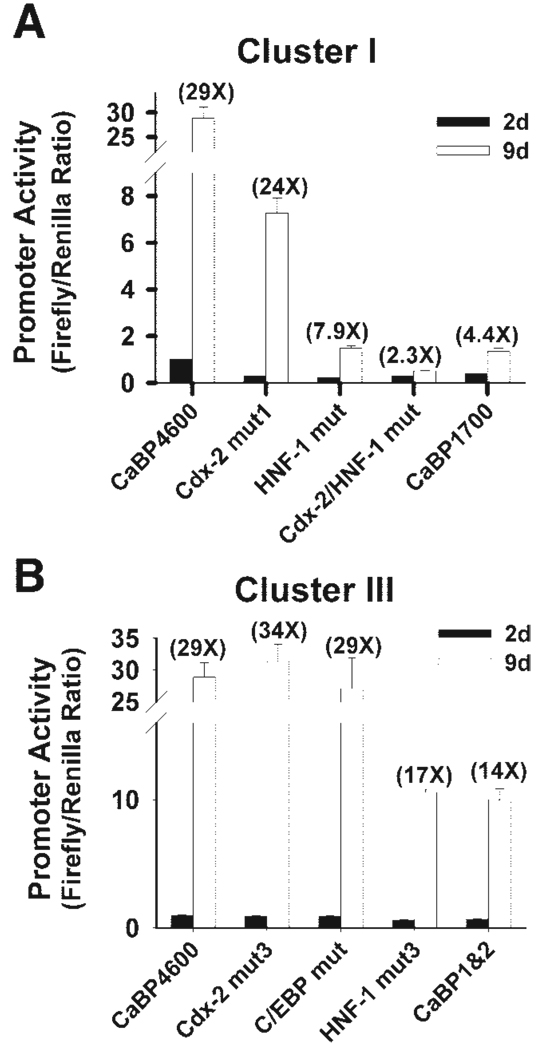
Five cis elements were examined in this study. Within cluster I, mutation of the cdx-2 site (−3158 to −3152 bp, cdx-2 mut1), the HNF-1 site (−3131 to −3120 bp, HNF-1 mut1), or both of the sites (Cdx-2 mut + HNF-1 mut) reduced the basal promoter activity by 75, 80, and 75%, respectively (P < 0.01), and the fold induction due to differentiation by 12, 75, and 90% (P < 0.01), respectively (Fig. 4A). In cluster III, mutation of either the proximal cdx-2 site (−40 to −25 bp, Cdx-2 mut3) or the C/EBP site (−183 to −174 bp) had no detectable effect on calbindin D9k promoter activity at either stage (Fig. 4B). Similar to the removal of three-fourths of cluster III (CaBP1 and 2), mutation of the proximal HNF-1 site in cluster III (−98 to −86 bp, HNF-1 mut3) decreased basal promoter activity by 36% (P < 0.01), and suppressed the differentiation-associated increase by 40% (P < 0.01, Fig. 4B).
Fig. 4.
The role of cis elements in conserved clusters I and III in differentiation-induced regulation of calbindin D9k promoter activity. Luciferase reporter constructs containing CaBP4600 or various truncated or mutated promoter constructs were transiently transfected into 50% confluent (day 2) and 5-day postconfluent TC7 cells (day 9). After 24 h, promoter activity was assessed by measuring luciferase activity. All data are expressed relative to the expression observed for CaBP4600 at 2 days (relative value = 1). Values in parentheses are the ratio of promoter activity at 9 days to that measured at 2 days. Each experiment was repeated at least 3 times with triplicate observations within each experiment. Data from 1 representative experiment are shown as means ± SE. A: cluster I mutants. A truncated calbindin D9k promoter without cluster I (CaBP1700), or promoters with mutations within the distal cdx-2 site (Cdx-2 mut1), the distal HNF-1 site (HNF-1 mut1), or both sites (Cdx-2 mut + HNF-1 mut) were examined. B: cluster III mutants. A truncated calbindin D9k promoter without ¾ of cluster III (CaBP1&2) or promoters with mutations at the proximal cdx-2 site (Cdx-2 mut3), the C/EBP site (C/EBP), or the proximal HNF-1 site (HNF-1 mut3) were examined.
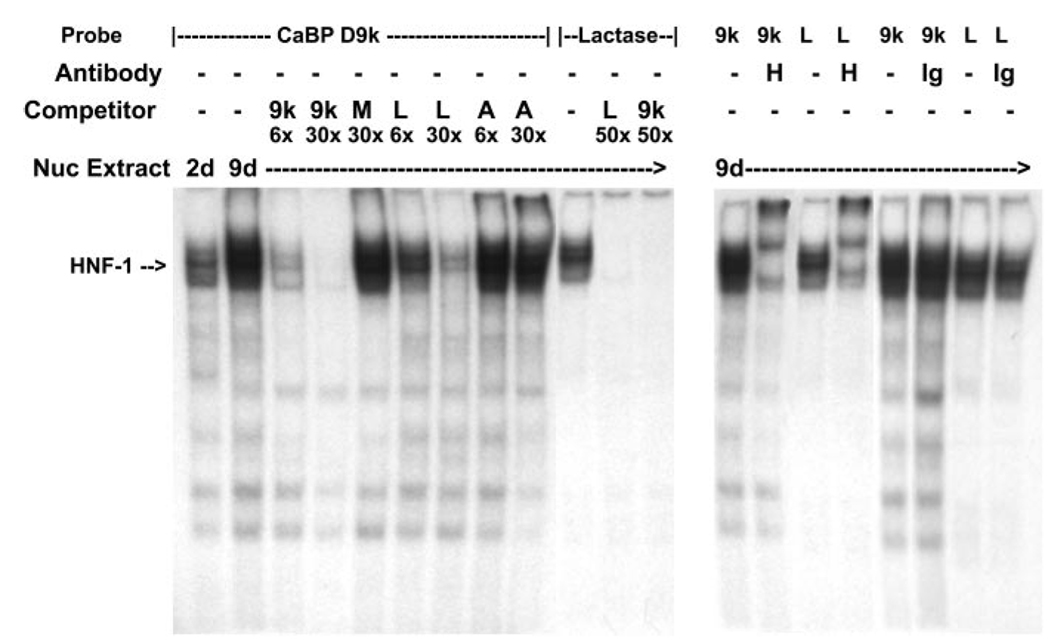
Characterization of the Distal HNF-1 Site by Competitive EMSA
To further characterize the novel distal HNF-1 site in cluster I, a competitive EMSA was conducted. A major complex was formed by incubating the [32P]calbindin D9k-HNF-1 probe with Caco-2 nuclear extract from either preconfluent or postconfluent cultures. Binding in 9-day extracts was greater than that seen in 2-day extracts (Fig. 5). This complex disappeared in the presence of a 30-fold molar excess of unlabeled calbindin D9k-HNF-1 probe as well as a probe for the HNF-1 site previously characterized in the lactase gene promoter (33). A 30-fold molar excess of unlabeled mutated calbindin D9k-HNF-1 probe or a nonspecific AP2 probe did not compete with the Caco-2 nuclear extract binding to the labeled calbindin D9k-HNF-1 probe (Fig. 5). The major complexes formed with the calbindin D9k-HNF-1 probe or the lactase-HNF-1 probe could be supershifted by the addition of an anti-HNF-1α antibody, but not IgG, to the binding reaction (Fig. 5).
Fig. 5.
EMSA analysis of HNF-1 binding to the distal HNF-1 response element in conserved cluster I of the calbindin D9k promoter. Oligonucleotides containing the putative HNF-1 site from cluster I in the human calbindin D9k promoter (CaBP D9k) or a well-characterized HNF-1 site from the lactase promoter (Lactase) were labeled with [γ-32P]ATP by T4 polynucleotide kinase. Left: competitive gel-shift assay. Nuclear extracts (Nuc Extract; 10 µg) from preconfluent (2 days) or 5-day postconfluent (9 days) TC7 cells were used for each binding reaction. The specific HNF-1α-containing complex is marked with an arrow. Specificity of complex formation was confirmed by competition with a 6-fold (6×) or 30-fold (30×) molar excess of unlabeled probe (9k, CaBP-HNF-1; M, mutated CaBP-HNF-1; L, lactase HNF-1; A, AP2). Specificity of complex formation on the labeled lactase HNF-1 probe was confirmed by competition with a 50-fold molar excess of unlabeled calbindin D9k HNF-1 probe or lactase HNF-1 probe. Right, HNF-1α supershift assay. Nuclear extracts (10 µg) from 9-day cultures of TC7 cells were preincubated with HNF-1α antibody (H) or a goat IgG (Ig) before incubation with 32P-labeled calbindin D9k HNF-1 probe or lactase HNF-1 probe.
Effect of HNF-1α Overexpression on Human Calbindin D9k Promoter Activity
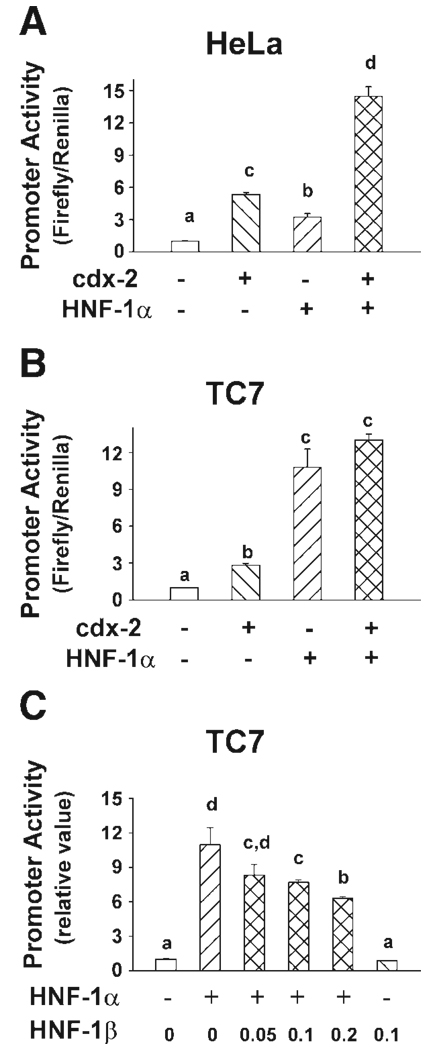
In proliferating TC7 cells, which naturally express both cdx-2 and HNF-1α, overexpression of cdx-2 stimulated calbindin D9k promoter activity by only 2.5-fold, whereas overexpression of HNF-1α increased calbindin D9k promoter activity by 10-fold (P < 0.001, Fig. 6B). The overexpression of both cdx-2 and HNF-1α had an additive effect on promoter activity (12-fold, P < 0.001). In HeLa cells, which lack cdx-2 and HNF-1α, overexpression of either cdx-2 or HNF-1α stimulated calbindin D9k promoter activity by 5.3-fold and 3.2-fold, respectively, and the combined overexpression of cdx-2 and HNF-1α in HeLa cells had a synergetic effect on calbindin D9k promoter activity (Fig. 6A, 14.5-fold, P < 0.001 vs. control; P = 0.012 for cdx-2 and HNF-1α interaction).
Fig. 6.
Effect of HNF-1α, HNF-1β, and cdx-2 expression on calbindin D9k promoter activity in intestinal (TC7) and nonintestinal (HeLa) cells. Luciferase reporter constructs containing CaBP4600 were cotransfected into 50% confluent HeLa (A) or TC7 (B) cells with control vector (250 ng of pCB6), mouse cdx-2 expression vector (250 ng of pCB6-mcdx-2), mouse HNF-1α expression vector (250 ng of pBJ5-HNF-1α), or both cdx-2 and HNF-1α expression vector (250 ng of each). C: 50% confluent cultures of TC7 cells were transfected with CaBP4600 along with control vector, HNF-1α expression vector (0.05 µg), HNF-1β expression vector (0.1 µg), or HNF-1α expression vector mixed with increasing amounts of HNF-1β expression vector (0.05, 0.1, or 0.2 µg). Promoter activities were assessed 24 h after transfection and are expressed relative to the induction seen in the control vector transfected cells (relative value = 1). In A and B, each bar represents the means ± SE (n = 6). In C, each bar represents the means ± SE (n = 3). Bars with different letters are significantly different from one another (Fishers least significant difference test, P < 0.05).
HNF-1β significantly suppressed the induction of calbindin D9k promoter activity by HNF-1α in proliferating TC7 cells (Fig. 6C). HNF-1α-induced calbindin D9k promoter activity was suppressed by 24, 30, and 43% in the presence of 0.05, 0.1, and 0.2 µg of the HNF-1β-expressing vector, respectively.
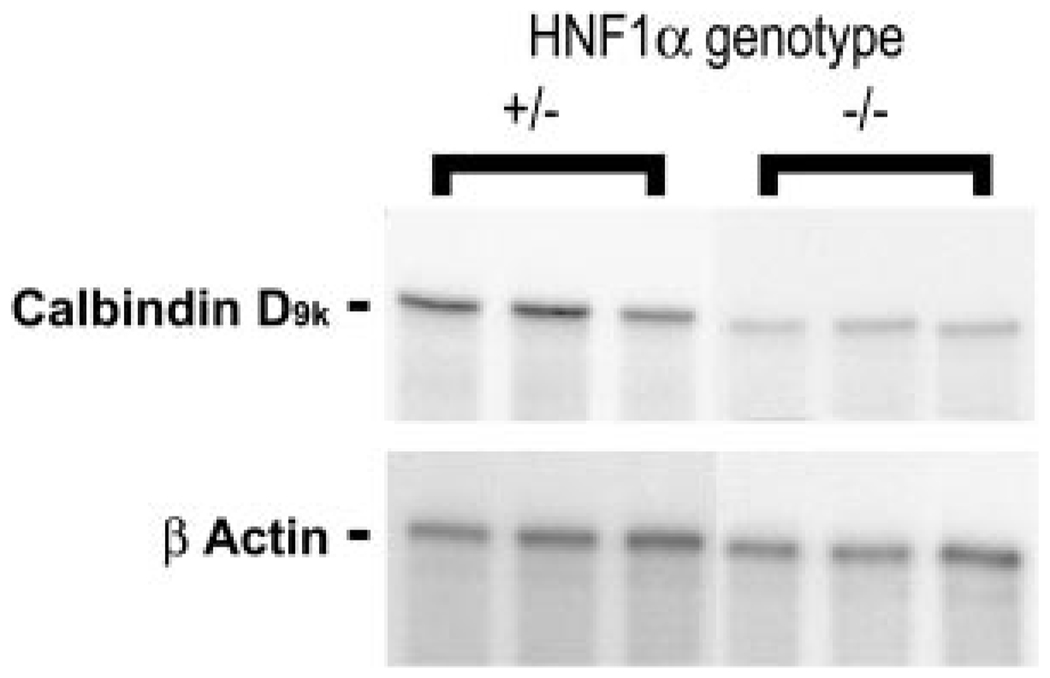
Calbindin D9k Expression in HNF-1α Null Mice
To further define the importance of our findings regarding HNF-1α-mediated calbindin D9k gene expression during enterocyte differentiation, we sought to determine whether these results could be extended to an in vivo setting. Duodenal RNA from HNF-1α knockout mice were examined for calbindin D9k expression by RPA, and this was compared with the expression seen in HNF-1α +/− mice. HNF-1α +/− mice are phenotypically normal and comparable to their wild-type littermates (35). As shown for the representative samples in Fig. 7, actin-corrected calbindin D9k mRNA levels in HNF-1α −/− mice (0.16 ± 0.03 arbitrary units) were 60% lower than those of the age-matched HNF-1α +/− mice (0.41 ± 0.07 arbitrary units, P < 0.05).
Fig. 7.
HNF-1α is an activator of calbindin D9k gene expression in vivo. RNase protection assays were carried out on RNA isolated from the duodenum of mice heterozygous (+/−) and homozygous (−/−) for the HNF-1α null allele using labeled antisense RNA probes for mouse calbindin D9k and β-actin. Two micrograms of RNA were used for calbindin D9k analysis, and 1 µg of RNA was used for β-actin analysis. The analysis from 3 representative mice is shown.
DISCUSSION
Due to its proposed role in the control of intestinal calcium absorption (5), most of the research on calbindin D9k gene expression has focused on its regulation by 1,25(OH)2 D. Our findings indicate that in TC7 cells, calbindin D9k mRNA levels are not transcriptionally regulated by 1,25(OH)2 D. This is consistent with our previous work in the parental Caco-2 cell line and in mice, suggesting a posttranscriptional regulation mechanism, i.e., in Caco-2 cells. Induction of calbindin D9k mRNA requires high doses, occurs gradually (over 48 h), and is inhibited by cycloheximide (22, 23), whereas in mice, calbindin D9k mRNA slowly rises in response to a large dose of 1,25(OH)2 D (47) and significant calbindin D9k expression is seen in the duodenum of vitamin D receptor null mice (46). Dupret et al. (14, 15) previously showed that induction of calbindin D9k mRNA was not inhibited by inhibitors of transcription and that posttranscriptional events account for the accumulation of calbindin D9k mRNA in rat duodenum. The lack of a transcriptional response is also consistent with the observation that the calbindin D9k VDREs do not have a high degree of similarity to the consensus VDRE (5′-GGGTCA NNR RGTTCA-3′, where N = any base and R = G or A) and that these VDREs are not well conserved across species. For example, the direct repeat with 3-bp spacing (DR3)-type VDRE identified in the rat promoter by Darwish et al. (10) and studied by Colnot et al. (8) is not present in the human promoter. In addition, the inverted palindromes spaced by nine nucleotides VDRE identified in the human promoter by Schrader et al. (45) is not intact in the rat or mouse promoter and has not been demonstrated to be active in a natural promoter context.
In contrast to the lack of vitamin D-mediated gene regulation, the calbindin D9k promoter was strongly activated during TC7 cell differentiation to an enterocyte-like phenotype. This is consistent with earlier in vivo data (24, 38, 49, 53). Previous research implicated two cdx-2 response elements as the important determinants for intestinal expression of the calbindin D9k gene (9, 34). Both elements bind to cdx-2 by ESMA (1, 9, 34), and mutation of the distal element at −3500 bp almost completely eliminated expression of a 4.6-kb rat calbindin D9k promoter-CAT reporter gene construct in transgenic mice (9). In our studies, mutation of the distal cdx-2 response element reduced basal promoter activity in TC7 cells but did not have a strong effect on differentiation-associated promoter activity. This indicates that other factors are important for increased calbindin D9k gene expression during enterocyte differentiation. We took several steps to identify these factors.
First, we showed that whereas GATA 4–5 and Pdx-1 have been implicated in the control of other intestinal genes, they may not be critical for calbindin D9k gene regulation during enterocyte differentiation. For example, whereas a previously identified Pdx-1 site (1) was reasonably conserved across species, Pdx-1 mRNA was not detectable in either proliferating or differentiated TC7 cells (data not shown) and therefore cannot account for the dramatic upregulation of calbindin D9k mRNA levels observed during TC7 cell differentiation. However, the participation of Pdx-1 in the regulation of the calbindin D9k gene in vivo, specifically in the duodenum in which Pdx-1 is expressed, cannot be ruled out based on our experiments. We were also unable to find phylogenically conserved GATA sites in the conserved clusters of the calbindin D9k promoter using the variants of the GATA 4/5/6 consensus sequence reported by Krasinski et al. (33). Finally, we identified a HNF-1 site that was within 25 bp of the cdx-2 response element in cluster I. Our subsequent analysis focused on the distal HNF-1 and cdx-2 sites as well as a number of phylogenically conserved sites that had been previously identified within cluster III, i.e., cdx-2 (the 5′ half site), C/EBP (−53 to −61 bp), and HNF-1 (−86 to −98 bp) response elements (34).
The major finding of our analysis is that the newly identified distal HNF-1 site is critical for the control of both basal and differentiation-associated calbindin D9k promoter activity. Whereas mutation of the distal cdx-2 site lowered only the basal promoter activity (by 80%), mutation of the distal HNF-1 site reduced differentiation-associated calbindin D9k promoter activity by 75% and accounted for >90% of the influence of cluster I on calbindin D9k gene expression. We subsequently showed that the putative HNF-1 response element binds avidly to a protein from 9-day TC7 cell nuclear extracts, that the binding is reduced in the presence of a the HNF-1 response element from the lactase gene (33), and that the specific binding to this sequence can be supershifted by an antibody against HNF-1α. In addition, a proximal HNF-1 site in cluster III is also required for basal and differentiation-associated calbindin D9k gene expression in enterocytes; mutation of this site reduced promoter activity by 40% in 9-day cultures. The impact of HNF-1 site mutations we observed in TC7 cells was similar to the reduction in calbindin D9k mRNA level we observed in HNF-1α null mice (60% reduction). This confirms that HNF-1α is important for intestinal expression of calbindin D9k and demonstrates that regulation of promoter activity through the two HNF-1α sites is physiologically relevant.
Our data identifies at least two important determinants that modulate HNF-1α-mediated regulation of calbindin D9k gene expression. First is the presence of cdx-2. We found that in HeLa cells with low natural expression of cdx-2 and HNF-1α, coexpression of the two transcription factors was required for maximal induction of calbindin D9k promoter activity. When our data are considered in combination with the earlier studies by Colnot et al. (9), our studies support a model whereby cdx-2 is a permissive factor necessary for basal expression of the calbindin D9k gene in enterocytes and in which HNF-1α accounts for the increased expression of this gene along the crypt-villus axis of the small intestine. The close proximity of the distal HNF-1 and cdx-2 sites (within 25 bases) suggests that the two transcription factors may directly interact to modulate calbindin D9k gene expression; [i.e., as for the lactase gene (40)]. Additional studies will be necessary to confirm these interactions as well as to assess the potential for direct communication between the proximal and distal HNF-1 response elements.
In addition to the role that cdx-2 plays, we found that HNF-1β antagonizes HNF-1α action similar to what Boudreau et al. (4) reported for sucrase-isomaltase gene regulation. Although Boudreau et al. (4) showed that the β- to α-protein ratio falls with Caco-2 cell differentiation (from ~0.8 to 0.3 due to a decline in HNF-1β levels), we found that β- to α-transfection ratios >4:1 did not completely suppress calbindin D9k promoter activity (43% reduction). This suggests that the release of HNF-1β-mediated suppression alone does not account for the dramatic increase in calbindin D9k gene expression we see during TC7 cell differentiation. Because HNF-1 and cdx-2 are both abundant in proliferating Caco-2 cells (4), a more likely mechanism for their role in calbindin D9k gene regulation is that these transcription factors are activated during enterocyte differentiation. It has been well documented that posttranscriptional modification (e.g., phosphorylation) can alter the DNA binding activity of transcription factors including cdx-2 (27, 44). Hence, it is possible that differentiation-associated changes in the phosphorylation status of HNF-1α or cdx-2 induces direct and indirect interactions between the transcription factors at critical DNA response elements or with essential coactivators (e.g., dimerization co-factor of HNF-1, cAMP response element binding protein, and CREB-binding protein-associated factors) (3, 39, 40, 48, 52). Future studies are necessary to define whether posttranslational modifications of HNF-1α or cdx-2 are necessary for differentiation-induced calbindin D9k gene activation.
ACKNOWLEDGMENTS
We are indebted to Dr. Frank J. Gonzalez, Laboratory of Metabolism, National Institutes of Health (Bethesda, MD) for the HNF-1α null mice.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-54111 (to J. C. Fleet).
Footnotes
These data were presented, in part, at the Digestive Disease Week 2002 meeting of the American Gastroenterological Association in San Francisco, CA, May 19–22, 2002.
REFERENCES
- 1.Barley NF, Prathalingam SR, Zhi P, Legon S, Howard A, Walters JRF. Factors involved in the duodenal expression of the human calbindin-D9k gene. Biochem J. 1999;341:491–500. [PMC free article] [PubMed] [Google Scholar]
- 2.Basson MD, Turowski G, Emenaker NJ. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp Cell Res. 1996;225:301–305. doi: 10.1006/excr.1996.0180. [DOI] [PubMed] [Google Scholar]
- 3.Boudreau F, Rings EH, Van Wering HM, Kim RK, Swain GP, Krasinski SD, Moffett J, Grand RJ, Suh ER, Traber PG. Hepatocyte nuclear factor-1α, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem. 2002;277:31909–31917. doi: 10.1074/jbc.M204622200. [DOI] [PubMed] [Google Scholar]
- 4.Boudreau F, Zhu Y, Traber PG. Sucrase-isomaltase gene transcription requires the hepatocyte nuclear factor-1 (HNF-1) regulatory element and is regulated by the ratio of HNF-1α to HNF-1β. J Biol Chem. 2001;276:32122–32128. doi: 10.1074/jbc.M102002200. [DOI] [PubMed] [Google Scholar]
- 5.Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol Gastrointest Liver Physiol. 1986;250:G561–G569. doi: 10.1152/ajpgi.1986.250.5.G561. [DOI] [PubMed] [Google Scholar]
- 6.Chantret I, Rodolosse A, Barbat A, Dussaulx E, Brot-Laroche E, Zweibaum A, Rousset M. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107:213–225. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- 7.Christakos S, Gabrielides C, Rhoten WB. Vitamin D-dependent calcium binding proteins: chemistry distribution, functional considerations, and molecular biology. Endocr Rev. 1989;10:3–26. doi: 10.1210/edrv-10-1-3. [DOI] [PubMed] [Google Scholar]
- 8.Colnot S, Ovejero C, Romagnolo B, Porteu A, Lacourte P, Thomasset M, Perret C. Transgenic analysis of the response of the rat calbindin-D9k gene to vitamin D. Endocrinology. 2000;141:2301–2308. doi: 10.1210/endo.141.7.7557. [DOI] [PubMed] [Google Scholar]
- 9.Colnot S, Romagnolo B, Lambert M, Cluzeaud F, Porteu A, Vandewalle A, Thomasset M, Kahn A, Perret C. Intestinal expression of the calbindin-D9K gene in transgenic mice. Requirement for a Cdx2-binding site in a distal activator region. J Biol Chem. 1998;273:31939–31946. doi: 10.1074/jbc.273.48.31939. [DOI] [PubMed] [Google Scholar]
- 10.Darwish HM, DeLuca HF. Identification of a 1,25-dihydroxyvitamin D3-response element in the 5′-flanking region of the rat calbindin D-9k gene. Proc Natl Acad Sci USA. 1992;89:603–607. doi: 10.1073/pnas.89.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darwish HM, DeLuca HF. Analysis of binding of the 1,25-dihydroxyvitamin D3 receptor to positive and negative vitamin D response elements. Arch Biochem Biophys. 1996;334:223–234. doi: 10.1006/abbi.1996.0450. [DOI] [PubMed] [Google Scholar]
- 12.Darwish HM, Krisinger J, Strom M, DeLuca HF. Molecular cloning of the cDNA and chromosomal gene for vitamin D-dependent calcium-binding protein of rat intestine. Proc Natl Acad Sci USA. 1987;84:6108–6111. doi: 10.1073/pnas.84.17.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding QM, Ko TC, Evers BM. Caco-2 intestinal cell differentiation is associated with G1 arrest and suppression of CDK2 and CDK4. Am J Physiol Gastrointest Liver Physiol. 1998;275:G1193–G1200. doi: 10.1152/ajpcell.1998.275.5.C1193. [DOI] [PubMed] [Google Scholar]
- 14.Dupret JM, Brun P, Perret C, Lomri N, Thomasset M, Cuisinier-Gleizes P. Transcriptional and post-transcriptional regulation of vitamin D-dependent calcium-binding protein gene expression in the rat duodenum by 1,25-dihydroxycholecalciferol. J Biol Chem. 1987;262:16553–16557. [PubMed] [Google Scholar]
- 15.Dupret JM, Brun P, Thomasset M. In vivo effects of transcriptional and translational inhibitors on duodenal vitamin D-dependent calcium-binding protein messenger ribonucleic acid stimulation by 1,25-dihydroxycholecalciferol. Endocrinology. 1986;119:2476–2483. doi: 10.1210/endo-119-6-2476. [DOI] [PubMed] [Google Scholar]
- 16.Dusing MR, Brickner AG, Lowe SY, Cohen MB, Wiginton DA. A duodenum-specific enhancer regulates expression along three axes in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1080–G1093. doi: 10.1152/ajpgi.2000.279.5.G1080. [DOI] [PubMed] [Google Scholar]
- 17.Dusing MR, Florence EA, Wiginton DA. High-level activation by a duodenum-specific enhancer requires functional GATA binding sites. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1053–G1065. doi: 10.1152/ajpgi.00483.2002. [DOI] [PubMed] [Google Scholar]
- 18.Evers BM, Ko TC, Li J, Thompson A. Cell cycle protein suppression and p21 induction in differentiating Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 1996;271:G722–G727. doi: 10.1152/ajpgi.1996.271.4.G722. [DOI] [PubMed] [Google Scholar]
- 19.Fang R, Olds LC, Santiago NA, Sibley E. GATA family transcription factors activate lactase gene promoter in intestinal Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G58–G67. doi: 10.1152/ajpgi.2001.280.1.G58. [DOI] [PubMed] [Google Scholar]
- 20.Fleet JC, Eksir F, Hance KW, Wood RJ. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol Gastrointest Liver Physiol. 2002;283:G618–G625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- 21.Fleet JC, Turnbull AJ, Bourcier M, Wood RJ. Vitamin D-sensitive and quinacrine-sensitive zinc transport in human intestinal cell line Caco-2. Am J Physiol Gastrointest Liver Physiol. 1993;264:G1037–G1045. doi: 10.1152/ajpgi.1993.264.6.G1037. [DOI] [PubMed] [Google Scholar]
- 22.Fleet JC, Wood RJ. Identification of calbindin D-9k mRNA and its regulation by 1,25-dihydroxyvitamin D3 in Caco-2 cells. Arch Biochem Biophys. 1994;308:171–174. doi: 10.1006/abbi.1994.1024. [DOI] [PubMed] [Google Scholar]
- 23.Fleet JC, Wood RJ. Specific 1,25(OH)2 D3-mediated regulation of transcellular calcium transport in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 1999;276:G958–G964. doi: 10.1152/ajpgi.1999.276.4.G958. [DOI] [PubMed] [Google Scholar]
- 24.Freeman TC. Parallel patterns of cell-specific gene expression during enterocyte differentiation and maturation in the small intestine of the rabbit. Differentiation. 1995;59:179–192. doi: 10.1046/j.1432-0436.1995.5930179.x. [DOI] [PubMed] [Google Scholar]
- 25.Gordon JI, Hermiston ML. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994;6:795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 26.Hardison R. Conserved noncoding sequences are reliable guides to regulatory elements. Trends Genet. 2000;16:369–372. doi: 10.1016/s0168-9525(00)02081-3. [DOI] [PubMed] [Google Scholar]
- 27.Houde M, Laprise P, Jean D, Blais M, Asselin C, Rivard N. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. J Biol Chem. 2001;276:21885–21894. doi: 10.1074/jbc.M100236200. [DOI] [PubMed] [Google Scholar]
- 28.Jeung EB, Leung PC, Krisinger J. The human calbindin-D9k gene. Complete structure and implications on steroid hormone regulation. J Mol Biol. 1994;235:1231–1238. doi: 10.1006/jmbi.1994.1076. [DOI] [PubMed] [Google Scholar]
- 29.Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–D298. doi: 10.2741/karam. [DOI] [PubMed] [Google Scholar]
- 30.Kerry DM, Dwivedi PP, Hahn CN, Morris HA, Omdahl JL, May BK. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;271:29715-–29721. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- 31.Krasinski SD, Estrada G, Yeh KY, Yeh M, Traber PG, Rings EH, Buller HA, Verhave M, Montgomery RK, Grand RJ. Transcriptional regulation of intestinal hydrolase biosynthesis during postnatal development in rats. Am J Physiol Gastrointest Liver Physiol. 1994;267:G584–G594. doi: 10.1152/ajpgi.1994.267.4.G584. [DOI] [PubMed] [Google Scholar]
- 32.Krasinski SD, Upchurch BH, Irons SJ, June RM, Mishra K, Grand RJ, Verhave M. Rat lactase-phlorizin hydrolase/human growth hormone transgene is expressed on small intestinal villi in transgenic mice. Gastroenterology. 1997;113:844–855. doi: 10.1016/s0016-5085(97)70179-3. [DOI] [PubMed] [Google Scholar]
- 33.Krasinski SD, Van Wering HM, Tannemaat MR, Grand RJ. Differential activation of intestinal gene promoters: functional interactions between GATA-5 and HNF-1 alpha. Am J Physiol Gastrointest Liver Physiol. 2001;281:G69–G84. doi: 10.1152/ajpgi.2001.281.1.G69. [DOI] [PubMed] [Google Scholar]
- 34.Lambert M, Colnot S, Suh E, L'Horset F, Blin C, Calliot ME, Raymondjean M, Thomasset M, Traber PG, Perret C. cis-Acting elements and transcription factors involved in the intestinal specific expression of the rat calbindin-D9k gene. Binding of the intestine-specific transcription factor Cdx-2 to the TATA box. Eur J Biochem. 1996;236:778–788. doi: 10.1111/j.1432-1033.1996.00778.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee YH, Sauer B, Gonzalez FJ. Laron dwarfism and non-insulin-dependent diabetes mellitus in the Hnf-1α knockout mouse. Mol Cell Biol. 1998;18:3059–3068. doi: 10.1128/mcb.18.5.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorentz O, Duluc I, De Arcangelis A, Simon-Assmann P, Kedinger M, Freund JN. Key role of the cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J Cell Biol. 1997;139:1553–1565. doi: 10.1083/jcb.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 38.Marche P, Cassier P, Mathieu H. Intestinal calcium-binding protein. A protein indicator of enterocyte maturation associated with the terminal web. Cell Tissue Res. 1980;212:63–72. doi: 10.1007/BF00234033. [DOI] [PubMed] [Google Scholar]
- 39.Mendel DB, Hansen LP, Graves MK, Conley PB, Crabtree GR. Hnf-1-α and Hnf-1-β (Vhnf-1) Share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev. 1991;5:1042–1056. doi: 10.1101/gad.5.6.1042. [DOI] [PubMed] [Google Scholar]
- 40.Mitchelmore C, Troelsen J, Spodsberg N, Sjostrom H, Noren O. Interaction between the homeodomain proteins cdx2 and HNF1α mediates expression of the lactase-phlorizin hydrolase gene. Biochem J. 2000;346:529–535. [PMC free article] [PubMed] [Google Scholar]
- 41.Perret C, L’Horset F, Thomasset M. DNase I-hypersensitive sites are associated, in a tissue-specific manner, with expression of the calbindin-D9k-encoding gene. Gene. 1991;108:227–235. doi: 10.1016/0378-1119(91)90438-h. [DOI] [PubMed] [Google Scholar]
- 42.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci. 1992;102:581–600. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- 43.Rhoads DB, Rosenbaum DH, Unsal H, Isselbacher KJ, Levitsky LL. Circadian periodicity of intestinal Na+/glucose cotransporter 1 mRNA levels is transcriptionally regulated. J Biol Chem. 1998;273:9510–9516. doi: 10.1074/jbc.273.16.9510. [DOI] [PubMed] [Google Scholar]
- 44.Rings EHHM, Boudreau F, Taylor JK, Moffett J, Suh ER, Traber PG. Phosphorylation of the serine 60 residue within the Cdx2 activation domain mediates its transactivation capacity. Gastroenterology. 2001;121:1437–1450. doi: 10.1053/gast.2001.29618. [DOI] [PubMed] [Google Scholar]
- 45.Schrader M, Nayeri S, Kahlen JP, Muller KM, Carlberg C. Natural vitamin D3 response elements formed by inverted palindromes: polarity-directed ligand sensitivity of vitamin D3 receptor-retinoid X receptor heterodimer-mediated transactivation. Mol Cell Biol. 1995;15:1154–1161. doi: 10.1128/mcb.15.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song Y, Kato S, Fleet JC. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr. 2003;133:374–380. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- 47.Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 48.Soutoglou E, Papafotiou G, Katrakili N, Talianidis I. Transcriptional activation by hepatocyte nuclear factor-1 requires synergism between multiple coactivator proteins. J Biol Chem. 2000;275:12515–12520. doi: 10.1074/jbc.275.17.12515. [DOI] [PubMed] [Google Scholar]
- 49.Taylor AN, Gleason WA, Lankford GL. Immunocytochemical localization of rat intestinal vitamin D-dependent calcium-binding protein. J Histochem Cytochem. 1984;32:153–158. doi: 10.1177/32.2.6363517. [DOI] [PubMed] [Google Scholar]
- 50.Thomson AB, Keelan M, Thiesen A, Clandinin MT, Ropeleski M, Wild GE. Small bowel review: diseases of the small intestine. Dig Dis Sci. 2001;46:2555–2566. doi: 10.1023/a:1012782321827. [DOI] [PubMed] [Google Scholar]
- 51.Troelsen JT, Mitchelmore C, Spodsberg N, Jensen AM, Noren O, Sjostrom H. Regulation of lactase-phlorizin hydrolase gene expression by the caudal-related homoeodomain protein Cdx-2. Biochem J. 1997;322:833–838. doi: 10.1042/bj3220833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Wering HM, Huibregtse IL, van der Zwan SM, de Bie MS, Dowling LN, Boudreau F, Rings EHHM, Grand RJ, Krasinski SD. Physical interaction between GATA-5 and hepatocyte nuclear factor-1α results in synergistic activation of the human lactase-phlorizin hydrolase promoter. J Biol Chem. 2002;277:27659–27667. doi: 10.1074/jbc.M203645200. [DOI] [PubMed] [Google Scholar]
- 53.Warembourg M, Tranchant O, Perret C, Desplan C, Thomasset M. In situ detection of vitamin D-induced calcium-binding protein (9 kDa CaBP) messenger RNA in rat duodenum. J Histochem Cytochem. 1986;34:277–280. doi: 10.1177/34.2.3753716. [DOI] [PubMed] [Google Scholar]
- 54.Wingender E, Chen X, Fricke E, Geffers R, Hehl R, Liebich I, Krull M, Matys V, Michael H, Ohnhauser R, Pruss M, Schacherer F, Thiele S, Urbach S. The TRANSFAC system on gene expression regulation. Nucleic Acids Res. 2001;29:281–283. doi: 10.1093/nar/29.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]