Abstract
Purpose
To assess the impact of tumor detection method (screening versus symptom-based diagnosis) in predicting breast cancer survival, and investigate how biological features of breast cancer are related to the tumor detection method.
Patients and Methods
The study population consisted of 5481 women diagnosed with primary invasive breast cancer between 1997 and 2005, and received their treatment at M. D. Anderson Cancer Center.
Results
Patients with symptom-detected tumors had an increased risk of recurrence or death (relative risk [RR] = 1.34; P = 0.006) and breast-cancer-specific death (RR = 1.31; P = 0.117) than patients with screen-detected tumors after adjusting for tumor characteristics, and treatments received. This relationship was especially evident among estrogen receptor (ER) negative tumors (RR = 1.60 for breast cancer recurrence for ER-negative tumors and RR = 1.18 for ER-positive tumors). ER status and Ki67 expression were statistically significantly associated with symptom-detection rate after adjusting for patients' age, tumor stage, tumor size, and nuclear grade (odds ratio [OR] of ER-negative versus ER-positive = 1.35, P < 0.001; OR of Ki67 10%-30% versus Ki67 < 10% = 1.40, P = 0.005; OR of Ki67 > 30% versus Ki67 < 10% = 2.11, P < 0.001).
Conclusion
The method of detection was a statistically significant independent predictor of breast cancer recurrence. Information on the method of tumor detection should be collected to improve the prediction of prognosis of breast cancer patients.
Keywords: biomarkers, breast cancer, detection method, recurrence, survival
INTRODUCTION
The marked decrease in the U.S. breast cancer mortality rate since 1990 has been attributed to the use of early detection programs and improved adjuvant chemotherapy and endocrine therapy (1-6). Several studies have indicated that women with tumors detected by screening examinations have more favorable clinical prognoses than women whose tumors are detected symptomatically (7-11). The estimated hazards of death from breast cancer detected symptomatically range from 36% [Shen et al] (7) to 90% [Joensuu et al] (8) greater than that from screen-detected breast cancer, after accounting for characteristics of the disease. Part of this residual screening benefit is due to lead-time and length biases (12-17). Lead time is the period from detection of the disease by screening exam to the time of its usual clinical presentation in the absence of screening. Lead-time bias occurs because breast tumors detected through a screening exam tend to be at an earlier stage of development than those detected symptomatically. Length bias occurs because a screening exam is more likely to detect slower growing tumors, which may be less deadly (15). Length bias may be explained by biological characteristics that determine the tumor growth rate.
In this study, we assessed the impact of the tumor detection method on breast cancer survival after adjusting for important risk factors at the time of diagnosis. We also investigated how the tumor detection method relates to tumor biologic features, including tumor expression of Ki67, a protein associated with cell proliferation, and the tumor status of specific cell receptors: estrogen receptor (ER), progesterone receptor (PR), and HER2/neu, an epidermal growth factor receptor.
PATIENTS AND METHODS
Data Source
We used data on women diagnosed with primary breast cancer between 1997 and 2005 and treated at M. D. Anderson Cancer Center (MDACC). The data were abstracted from medical charts, reviewed and entered into the Breast Cancer Management Systems (BCMS), which is a prospective electronic database initiated since 1997. The data for this study included patient race, age at diagnosis, tumor detection method (screen-detected and symptom-detected), tumor characteristics at diagnosis, treatments received following diagnosis, and times to disease recurrence, death, and last patient follow-up. Tumor characteristics included TNM stage, tumor size, axillary nodal status, nuclear grade, lymphatic/vascular invasion, ER status, PR status, HER2/neu IHC score, and tumor expression of Ki67. All the information had been prospectively collected in the BCMS database by trained personnel. The study was approved by the Institutional Review Board of M. D. Anderson. Tumor staging and size determination were based on pathologic staging and measurement of the tumor size. For patients receiving neoadjuvant therapy, the clinical stage and tumor size measurements prior to the initiation of therapy were used. Tumor HER2/neu score was determined by testing HER2/neu protein expression using immunohistochemistry (IHC).
Patient Inclusion Criteria
We used data from all breast cancer patients (7451) who had been consecutively registered in the M. D. Anderson BCMS database from 1997 to 2005. Then, we excluded patients who were either diagnosed to have DClS and LCIS, were younger than 40 years old at diagnosis, had unknown information on method of detection, or had unknown surgery / treatment information. The resulting study cohort consisted of 5481 patients.
Tumor Detection Method
Physicians in the Breast Center interviewed patients and documented the information of the tumor detection method in the medical charts. Screen-detected breast cancers were defined as those initially detected through an abnormal mammography finding or an abnormal clinical breast exam. Symptom-detected breast cancers were those initially detected through the patient's discovery of disease-related symptoms.
Statistical Analysis
We used the chi-squared test to compare the distributions of tumor characteristics by the detection method. Logistic regression was used to determine whether the probability of a tumor being screen-detected was associated with the observed biologic features, including ER, PR, HER2/neu, and Ki67 status, after controlling for patient age, race, disease stage, tumor size, nodal status, nuclear grade, and lymphatic/vascular invasion. The primary clinical outcomes were recurrence-free survival (RFS) and breast-cancer–specific survival (BCSS). We measured RFS from the time of diagnosis to disease progression (including local recurrence, distant metastasis, or contralateral breast tumor) or death, whichever occurred first. Death was used as the endpoint for any patient who had distant tumor metastasis at diagnosis. We measured BCSS from the time of diagnosis to death due to breast cancer. We estimated Kaplan-Meier curves by tumor detection method and used multivariable Cox models to determine the impact of tumor detection method on survival distributions after adjusting for the potential risk factors (patient age, race, tumor size, nodal status, nuclear grade, lymphatic/vascular invasion, ER status, PR status, HER2/neu IHC score, and Ki67 expression) and treatments received (surgery, radiation, adjuvant chemotherapy, adjuvant endocrine therapy, and neoadjuvant chemotherapy). We obtained the final multivariable model using a backward selection approach, removing the least significant covariate from the full model one at a time, and a P value of less than 0.05 was used as the limit for inclusion. All P values are two-sided. We used SAS 9.1.3 and S-PLUS 7.0 for the analyses.
RESULTS
Tumor Characteristics at Diagnosis by Detection Method
In Table 1, we list patient demographics and tumor characteristics by tumor detection method. Among 5481 patients, 2387 (43.5%) were diagnosed by screening examinations and 3094 (56.5%) were diagnosed as a result of symptoms, which included a breast lump (80.2%), breast pain and discomfort (6.0%), axillary mass (3.5%), breast swelling (2.6%), nipple discharge (2.0%), inverted nipple (2.4%), and other or unknown symptoms (3.3%). In this study cohort, screen-detected tumors were more common in women 60 years or older (41.1%, ages ≥ 60 years; 34.9%, ages at 50-59 and 24.0%, ages < 50 years). Symptom-detected tumors were more common in African American and other minority women than in whites.
Table 1.
Demographic and Tumor Characteristics by Tumor Detection Method
| Screen-detected (n= 2387) |
Symptom-detected (n= 3094) |
Total (n = 5481) |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | No. | (%) | No. | (%) | No. | (%) | P | |
| Age at diagnosis (yrs) | ||||||||
| 40 - 49 | 572 | (24.0) | 1129 | (36.5) | 1701 | (31.0) | ||
| 50 - 59 | 834 | (34.9) | 1067 | (34.5) | 1901 | (34.7) | ||
| ⩾ 60 | 981 | (41.1) | 898 | (29.0) | 1879 | (34.3) | <0.001 | |
| Race | ||||||||
| White | 1903 | (79.7) | 2206 | (71.3) | 4109 | (75.0) | ||
| African American | 199 | (8.3) | 342 | (11.1) | 541 | (9.9) | ||
| Other | 285 | (12.0) | 546 | (17.6) | 831 | (15.1) | <0.001 | |
| Tumor stage | ||||||||
| Stage I | 1432 | (60.0) | 779 | (25.2) | 2211 | (40.3) | ||
| Stage II | 813 | (34.1) | 1541 | (49.8) | 2354 | (43.0) | ||
| Stage III | 125 | (5.2) | 660 | (21.3) | 785 | (14.3) | ||
| Stage IV | 17 | (0.7) | 114 | (3.7) | 131 | (2.4) | <0.001 | |
| Tumor size | ||||||||
| T0 | 16 | (0.7) | 38 | (1.3) | 54 | (1.0) | ||
| T1 | 1817 | (76.3) | 1221 | (39.7) | 3038 | (55.7) | ||
| T2 | 444 | (18.7) | 1192 | (38.8) | 1636 | (30.0) | ||
| T3 or T4 | 103 | (4.3) | 622 | (20.2) | 725 | (13.3) | ||
| Unknown | 7 | 21 | 28 | <0.001 | ||||
| Nodal status | ||||||||
| Negative | 1684 | (70.6) | 1594 | (51.7) | 3278 | (59.9) | ||
| Positive | 700 | (29.4) | 1492 | (48.3) | 2192 | (40.1) | ||
| Unknown | 3 | 8 | 11 | <0.001 | ||||
| Nuclear grade | ||||||||
| Well/moderately | ||||||||
| differentiated | 1453 | (62.1) | 1329 | (44.3) | 2782 | (52.1) | ||
| Poorly differentiated | 885 | (37.9) | 1669 | (55.7) | 2554 | (47.9) | ||
| Unknown | 49 | 96 | 145 | <0.001 | ||||
| Lymphatic/vascular invasion | ||||||||
| No | 1995 | (84.3) | 2261 | (74.9) | 4256 | (79.0) | ||
| Yes | 372 | (15.7) | 758 | (25.1) | 1130 | (21.0) | ||
| Unknown | 20 | 75 | 95 | <0.001 | ||||
| ER status | ||||||||
| Negative | 432 | (19.0) | 975 | (32.7) | 1407 | (26.8) | ||
| Positive | 1839 | (81.0) | 2004 | (67.3) | 3843 | (73.2) | ||
| Unknown | 116 | 115 | 231 | <0.001 | ||||
| PR status | ||||||||
| Negative | 768 | (34.0) | 1267 | (42.8) | 2035 | (39.0) | ||
| Positive | 1492 | (66.0) | 1692 | (57.2) | 3184 | (61.0) | ||
| Unknown | 127 | 135 | 262 | <0.001 | ||||
| HER2/neu IHC score | ||||||||
| 0 or 1+ | 1280 | (75.6) | 1433 | (66.9) | 2713 | (70.7) | ||
| 2+ | 201 | (11.9) | 319 | (14.9) | 520 | (13.6) | ||
| 3+ | 212 | (12.5) | 390 | (18.2) | 602 | (15.7) | ||
| Unknown | 694 | 952 | 1646 | <0.001 | ||||
| Ki67 proliferation | ||||||||
| < 10% | 307 | (30.1) | 216 | (17.2) | 523 | (23.0) | ||
| 10% - 30 % | 518 | (50.7) | 574 | (45.7) | 1092 | (48.0) | ||
| > 30% | 196 | (19.2) | 465 | (37.1) | 661 | (29.0) | ||
| Unknown | 1366 | 1839 | 3205 | <0.001 | ||||
| Surgery and radiation | ||||||||
| BCS alone | 111 | (4.7) | 134 | (4.3) | 245 | (4.5) | ||
| BCS + radiation | 1208 | (50.6) | 1114 | (36.0) | 2322 | (42.4) | ||
| Mastectomy alone | 827 | (34.6) | 935 | (30.2) | 1762 | (32.1) | ||
| Mastectomy + | ||||||||
| radiation | 226 | (9.5) | 841 | (27.2) | 1067 | (19.5) | ||
| None | 15 | (0.6) | 70 | (2.3) | 85 | (1.5) | <0.001 | |
| Adjuvant chemotherapy | ||||||||
| No | 1515 | (63.5) | 1659 | (53.6) | 3174 | (57.9) | ||
| Yes | 872 | (36.5) | 1435 | (46.4) | 2307 | (42.1) | <0.001 | |
| Adjuvant endocrine therapy | ||||||||
| No | 765 | (32.0) | 1260 | (40.7) | 2025 | (36.9) | ||
| Yes | 1622 | (68.0) | 1834 | (59.3) | 3456 | (63.1) | <0.001 | |
| Neo-adjuvant chemotherapy | ||||||||
| No | 2102 | (88.1) | 1896 | (61.3) | 3998 | (72.9) | ||
| Yes | 285 | (11.9) | 1198 | (38.7) | 1483 | (27.1) | <0.001 | |
| Total | 2387 | (43.5) | 3094 | (56.5) | 5481 | (100.0) | ||
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; IHC, immunohistochemistry; BCS, breast-conserving surgery.
As expected, screen-detected tumors tended to be at an earlier stage compared to symptom-detected tumors. Screen-detected tumors were of significantly smaller size and lower nuclear grade, had fewer axillary lymph nodes involved, and were less likely to have invaded intramammary lymphatic or vascular tissue, compared to symptom-detected tumors (all P values < 0.001).
The screen-detected tumors demonstrated favorable biologic features. 81.0% of screen-detected tumors were ER positive, compared with 67.3% of symptom-detected tumors (P < 0.001). We observed the same trend within each age group (respectively, 75.4% screen-detected vs. 64.0% symptom-detected ER-positive for age < 50 years, P < 0.001; 82.7% vs. 69.2% for age ≥ 50 years, P < 0.001). The proportion of low Ki67 expression (Ki67 < 10%) was significantly higher among screen-detected tumors compared to that for symptom-detected tumors (30.1% vs. 17.2%, P < 0.001).
The odds of patients diagnosed with symptoms were statistically higher for ER-negative tumors than for ER-positive tumors (odds ratio [OR] = 1.35; 95% confidence interval [CI] = 1.15 to 1.59) even adjusting for patients' age, tumor stage, tumor size, and nuclear grade (Table 2). Ki67 expression was also significantly associated with detection method after adjusting for the above risk factors. Tumors with a high Ki67 expression (compared to a low expression: Ki67 < 10%) were more likely to be symptomatically detected (OR for Ki67 at 10%-30% = 1.40, 95% CI = 1.11 to 1.77; OR for Ki67 > 30% = 2.11, 95% CI = 1.57 to 2.85). Compared with HER2/neu score 0 or 1+ tumors, tumors with HER2/neu score 2+ were more likely to be symptomatically detected (OR for HER2/neu score 2+ = 1.28, 95% CI = 1.04 – 1.58). PR status was not statistically significantly associated with detection method after adjusting for these risk factors.
Table 2.
Odds Ratios and 95% Confidence Intervals (CIs) for Symptom-Detected vs Screen-Detected Invasive Breast Cancers for the Following Biomarkers
| Univariate models (for single biomarker) |
Multivariable models * (for single biomarker) |
Multivariable model † (for all biomarkers) |
|||||
|---|---|---|---|---|---|---|---|
| Biomarker | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| ER Status | |||||||
| Positive | 1.00 | 1.00 | 1.00 | ||||
| Negative | 2.07 (1.82-2.36) | < 0.001 | 1.35 (1.15-1.59) | < 0.001 | 1.28 (0.93-1.77) | 1.131 | |
| PR Status | |||||||
| Positive | 1.00 | 1.00 | 1.00 | ||||
| Negative | 1.45 (1.30-1.63) | < 0.001 | 1.08 (0.94-1.23) | 0.274 | 0.85 (0.66-1.09) | 0.207 | |
| HER2/neu IHC score | |||||||
| 0 or 1+ | 1.00 | 1.00 | 1.00 | ||||
| 2+ | 1.42 (1.17-1.72) | < 0.001 | 1.28 (1.04-1.58) | 0.022 | 1.13 (0.83 - 1.54) | 0.433 | |
| 3+ | 1.64 (1.37-1.97) | < 0.001 | 1.07 (0.87-1.32) | 0.527 | 0.88 (0.64-1.22) | 0.446 | |
| Ki67 | |||||||
| <10% | 1.00 | 1.00 | 1.00 | ||||
| 10% - 30% | 1.57 (1.28-1.94) | < 0.001 | 1.40 (1.11-1.77) | 0.005 | 1.46 (1.12-1.91) | 0.005 | |
| >30% | 3.37 (2.65-4.29) | < 0.001 | 2.11 (1.57-2.85) | < 0.001 | 2.24 (1.58-3.19) | < 0.001 | |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; IHC, immunohistochemistry
The risk factors - age, stage, tumor size, and nuclear grade were included in the multivariable logistic models for each single biomarker.
The multivariable logistic model for all four biomarkers included ER status, PR status, HER2/neu IHC score, Ki67, and age, stage, tumor size, and nuclear grade.
When including all four biomarkers (ER, PR, HER2/neu and Ki67) in a multivariable model, we found only Ki67 to be a statistically significant predictor of the tumor detection method (OR for Ki67 10% - 30% vs. Ki67 < 10% = 1.46, 95% CI = 1.12 to 1.91; OR for Ki67 > 30% vs. Ki67 < 10% = 2.24, 95% CI = 1.58 to 3.19), while ER, PR, and HER2/neu were not statistically significant in the model.
Disease Prognosis by Tumor Detection Method
Among 5481 patients with invasive tumors, 174 (3.2%) experienced local recurrences, 528 (9.6%) had distant metastases, 78 (1.4%) developed contralateral breast cancers, and 296 (5.4%) died of breast cancer during a median follow-up of 3.2 years (a patient may have experienced more than one of these events). Without adjusting for the risk factors, patients with symptom-detected tumors had a significantly increased risk of recurrence (relative risk [RR] = 2.40; 95 % CI = 2.00 to 2.87; P < 0.001) and breast cancer specific death (RR = 3.09, 95 % CI = 2.33 to 4.10; P < 0.001) compared to patients with screen-detected tumors. The lead-time bias and length bias can contribute to this screening benefit. To minimize the lead-time bias and length bias, we first compared survival distributions by tumor detection method among patients with tumors of the same stage and ER status.
Univariate Analyses
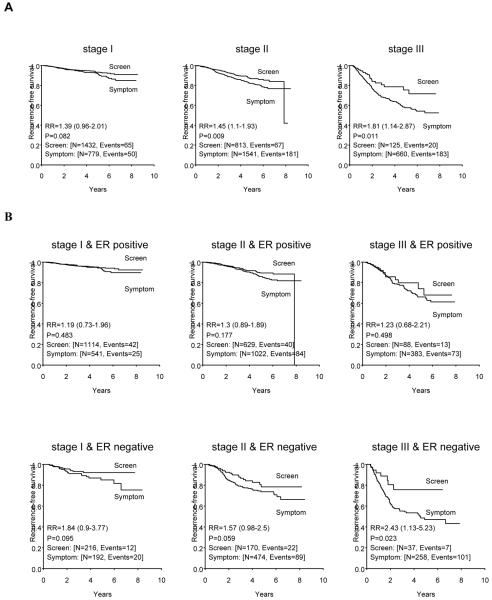
The graphs of Figure 1, panel A show a greater recurrence-free survival (RFS) for patients with screen-detected tumors compared to those with symptom-detected tumors within each stage (relative risk [RR] = 1.39, P = 0.082 for stage I; RR = 1.45, P =0.009 for stage II; RR = 1.81, P = 0.011 for stage III).
Figure 1.
(A) Recurrence-free survival curves for patients with symptom-detected breast cancer compared to those of patients with screen-detected breast cancer, adjusting for the tumor stage at diagnosis. (B) Recurrence-free survival curves for patients with symptom-detected breast cancer compared to those of patients with screen-detected breast cancer, after controlling for tumor stage and estrogen receptor status. RR is relative risk. 95% confidence interval of RR was given in the parenthesis.
Panel B shows a comparison of the RFS distributions by tumor detection method after fixing disease stage and ER status, and indicates that the benefit in RFS due to detection through screening remains. The differences were statistically or marginally significant among patients with ER-negative tumors of stages I-III. The differences were more evident among ER-negative tumors than those among ER-positive tumors.
Multivariable Analyses
Recurrence-free survival (RFS)
In Table 3, we show the results of our multivariable analyses of Cox proportional hazards models for RFS. Race was not a statistically significant predictive factor for RFS in the multivariable models (Model 1). RFS was significantly associated with patient tumor size, nodal status, lymphatic/vascular invasion, and ER status. Patients with symptom-detected tumors had a greater risk of relapse with RR increase of 1.34 (95% CI = 1.09 to 1.66; P = 0.006) compared to patients with screen-detected tumors, after adjusting for the other factors (Model 1).
Table 3.
Cox Proportional Hazards Regression Models of RFS among Patients with Invasive Cancer
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| (All patients) | (Patients with ER- positive tumors) |
(Patients with ER- negative tumors) |
(Adjusted for all biomarkers) |
|
| Variable | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) |
| Unadjusted | ||||
| Method of detection | ||||
| Screen-detected | 1.00 | 1.00 | 1.00 | -- |
| Symptom-detected | 2.40 (2.00-2.87)* | 2.03(1.60-2.59)* | 2.42 (1.77-3.31)* | -- |
| P value | < 0.001 | < 0.001 | <0.001 | -- |
| Number of recurrences or deaths / Total (%) |
626 / 5481 (11.4%) |
304 / 3843 (7.9%) |
281 / 1407 (20.0%) |
-- |
| Adjusted | ||||
| Method of detection | ||||
| Screen-detected | 1.00 | 1.00 | 1.00 | 1.00 |
| Symptom-detected | 1.34 (1.09-1.66)† | 1.18 (0.90-1.56) | 1.60 (1.14-2.25)† | 1.29 (0.89-1.87) |
| P value | 0.006 | 0.233 | 0.007 | 0.181 |
| Age (per 10 years increase) |
‡ | ‡ | ‡ | ‡ |
| Race | ||||
| Non-African American | ‡ | ‡ | ‡ | 1.00 |
| African American | ‡ | ‡ | ‡ | 1.56 (1.02-2.39)† |
| Tumor size | ||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 |
| T2 | 1.68 (1.34-2.10)* | 1.61 (1.19-2.19)† | 1.78 (1.27-2.50)* | 1.76 (1.18-2.63)† |
| T3 | 2.76 (2.02-3.78)* | 2.79 (1.81-4.29)* | 2.77 (1.72-4.46)* | 2.87 (1.72-4.78)* |
| T4 | 3.42 (2.54-4.61)* | 3.43 (2.23-5.28)* | 3.69 (2.41-5.67)* | 3.68 (2.24-6.06)* |
| Nodal status | ||||
| Negative | 1.00 | 1.00 | 1.00 | 1.00 |
| Positive | 1.89 (1.53-2.32)* | 1.75 (1.31-2.33)* | 2.00 (1.49-2.69)* | 1.90(1.33-2.72)* |
| Nuclear grade | ||||
| Well/mod. differentiated | ‡ | ‡ | ‡ | ‡ |
| Poorly differentiated | ‡ | ‡ | ‡ | ‡ |
| Lymphatic/vascular invasion | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.60 (1.33-1.94)* | 1.42 (1.08-1.86)† | 1.90 (1.46-2.48)* | 1.55 (1.10-2.18)† |
| Surgery and radiation | ||||
| BCS alone | 2.37 (1.65-3.42)* | 1.95 (1.20-3.17)† | 3.40 (1.95-5.94)* | ‡ |
| BCS + radiation | 0.81 (0.64-1.03) | 0.76 (0.55-1.06) | 0.85 (0.60-1.21) | ‡ |
| Mastectomy alone | 1.00 | 1.00 | 1.00 | ‡ |
| Mastectomy + radiation | 1.08 (0.84-1.38) | 1.06(0.76-1.48) | 1.03 (0.71-1.49) | ‡ |
| Adjuvant chemotherapy | ||||
| No | 1.00 | ‡ | 1.00 | ‡ |
| Yes | 0.75 (0.63-0.91)† | ‡ | 0.68 (0.52-0.88)† | ‡ |
| Adjuvant endocrine therapy | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.47 (0.37-0.59)* | 0.44 (0.33-0.58)* | 0.48 (0.32-0.73)* | 0.37 (0.24-0.55)* |
| ER status | ||||
| ER positive | 1.00 | ‡ | ‡ | 1.00 |
| ER negative | 1.60 (1.27-2.02)* | ‡ | ‡ | 1.20 (0.75-1.91) |
| PR status | ||||
| PR positive | ‡ | 1.00 | ‡ | 1.00 |
| PR negative | ‡ | 1.37 (1.06-1.77)† | ‡ | 0.95 (0.64-1.39) |
| HER2/neu IHC score | ||||
| 0 or 1+ | ‡ | ‡ | ‡ | 1.00 |
| 2+ | ‡ | ‡ | ‡ | 0.58 (0.33-1.01) |
| 3+ | ‡ | ‡ | ‡ | 0.94(0.64-1.38) |
| Ki67 expression | ||||
| < 10% | § | § | § | 1.00 |
| 10% -30% | § | § | § | 1.29(0.78-2.14) |
| > 30% | § | § | § | 1.86(1.09-3.18)† |
| Number of recurrences or deaths / Total (%) |
536 / 5110 (10.5%) |
280 / 3752 (7.5%) |
254 / 1340 (19.0%) |
166 / 1810 (9.2%) |
Abbreviations: RFS, recurrence-free survival; RR, relative risk; 95% CI, 95 percent confidence interval; Well/mod. differentiated, well/moderately differentiated; BCS, breast-conserving surgery; ER, estrogen receptor; PR, progesterone receptor; IHC, immunohistochemistry.
NOTE:
P < 0.001
P ≥ 0.001 and P < 0.05
The variable was included in the original full model but it was not statistically significant (P ≥ 0.05). Thus, it was not included in the reduced model. A variable representing whether a patient received neoadjuvant chemotherapy was excluded for the same reason. The original full model included method of detection, age, race, tumor size, nodal status, nuclear grade, lymphatic/vascular invasion, surgery and radiation, adjuvant chemotherapy, neoadjuvant chemotherapy, adjuvant endocrine therapy, ER status, PR status, and HER2/neu IHC score.
The variable was not included in the full model.
We performed similar multivariable analyses among subsets of patients, categorized by tumor ER status. Among patients with ER-positive tumors, the adjusted relative risk of recurrence was 1.18 for patients with symptom-detected tumors (95% CI = 0.90 to 1.56; P = 0.233), compared to those with screen-detected tumors (Model 2). Among patients with ER-negative tumors, the relative risk was 1.60 (95% CI = 1.14 to 2.25; P = 0.007) (Model 3).
Breast-cancer–specific survival (BCSS)
Similar to the results for RFS, we found that the patients with symptom-detected tumors had an increased risk of breast cancer death (RR = 1.31; 95% CI = 0.93 to 1.84; P = 0.117), compared to the patients with screen-detected tumors, although the differences were not statistically significant since there were few deaths (Table 4). African American race was associated with a significantly elevated risk of death from breast cancer compared with women of other races (RR = 1.87; 95% CI = 1.36 to 2.58; P < 0.001).
Table 4.
Cox Proportional Hazards Regression Models of BCSS among Patients with Invasive Breast Cancer
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| (All patients) | (Patients with ER- positive tumors) |
(Patients with ER- negative tumors) |
(Adjusted for all biomarkers) |
|
| Variable | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) |
| Unadjusted | ||||
| Method of detection | ||||
| Screen-detected | 1.00 | 1.00 | 1.00 | -- |
| Symptom-detected | 3.09 (2.33-4.10)* | 2.21(1.48-3.30)* | 2.65 (1.73-4.06)* | -- |
| P value | < 0.001 | < 0.001 | <0.001 | -- |
| Number of deaths / Total (%) |
296 / 5481 (5.4%) |
115 / 3843 (3.0%) |
168 / 1407 (11.9%) |
-- |
| Adjusted | ||||
| Method of detection | ||||
| Screen-detected | 1.00 | 1.00 | 1.00 | 1.00 |
| Symptom-detected | 1.31 (0.93-1.84) | 1.13 (0.68-1.86) | 1.46 (0.91-2.35) | 1.19 (0.66-2.15) |
| P value | 0.117 | 0.638 | 0.117 | 0.562 |
| Age (per 10 years increase) |
‡ | 1.46 (1.22-1.76)* | ‡ | ‡ |
| Race | ||||
| Non-African American | 1.00 | 1.00 | ‡ | 1.00 |
| African American | 1.87 (1.36-2.58)* | 2.84 (1.73-4.68)* | ‡ | 2.21 (1.25-3.89)† |
| Tumor size | ||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 |
| T2 | 3.06 (2.08-4.51)* | 1.89 (1.07-3.35)† | 4.86 (2.77-8.55)* | 3.18 (1.58-6.41)† |
| T3 | 4.47 (2.71-7.39)* | 2.28 (1.05-4.99)† | 8.50 (4.19-17.22)* | 4.21 (1.83-9.67)* |
| T4 | 6.47 (4.06-10.32)* | 3.61 (1.78-7.31)* | 11.96 (6.20-23.08)* | 7.19 (3.27-15.81)* |
| Nodal status | ||||
| Negative | 1.00 | 1.00 | 1.00 | 1.00 |
| Positive | 2.22 (1.61-3.07)* | 2.26 (1.31-3.89)† | 2.50 (1.72-3.65)* | 2.50 (1.40-4.46)* |
| Nuclear grade | ||||
| Well/mod. differentiated | 1.00 | 1.00 | ‡ | ‡ |
| Poorly differentiated | 1.62 (1.13-2.33)† | 1.85 (1.20-2.86)† | ‡ | ‡ |
| Lymphatic/vascular invasion | ||||
| No | 1.00 | 1.00 | ‡ | 1.00 |
| Yes | 1.52 (1.15-2.01)† | 2.02 (1.29-3.18)† | ‡ | 2.06 (1.25-3.39)† |
| Surgery and radiation | ||||
| BCS alone | 2.95 (1.73-5.01)* | 2.79 (1.23-6.33)† | 3.92 (2.00-7.70)* | ‡ |
| BCS + radiation | 1.04 (0.70-1.53) | 1.41 (0.74-2.66) | 0.78 (0.48-1.26) | ‡ |
| Mastectomy alone | 1.00 | 1.00 | 1.00 | ‡ |
| Mastectomy + radiation | 1.13 (0.78-1.65) | 1.91 (1.06-3.45)† | 0.83 (0.52-1.32) | ‡ |
| Adjuvant chemotherapy | ||||
| No | 1.00 | ‡ | 1.00 | 1.00 |
| Yes | 0.49 (0.37-0.65)* | ‡ | 0.46 (0.32-0.65)* | 0.42 (0.26-0.69)* |
| Adjuvant endocrine therapy | ||||
| No | 1.00 | 1.00 | ‡ | ‡ |
| Yes | 0.48 (0.34-0.69)* | 0.34 (0.21-0.53)* | ‡ | ‡ |
| ER status | ||||
| ER positive | 1.00 | ‡ | ‡ | 1.00 |
| ER negative | 2.14 (1.49-3.07)* | ‡ | ‡ | 3.74 (2.08-6.72)* |
| PR status | ||||
| PR positive | ‡ | 1.00 | ‡ | 1.00 |
| PR negative | ‡ | 1.86 (1.23-2.83)† | ‡ | 1.41 (0.83-2.38) |
| HER2/neu IHC score | ||||
| 0 or 1+ | ‡ | ‡ | ‡ | 1.00 |
| 2+ | ‡ | ‡ | ‡ | 0.69 (0.32-1.46) |
| 3+ | ‡ | ‡ | ‡ | 0.60 (0.33-1.09) |
| Ki67 expression | ||||
| < 10% | § | § | § | 1.00 |
| 10% -30% | § | § | § | 1.77 (0.72-4.36) |
| > 30% | § | § | § | 2.52 (1.01-6.35)† |
| Number of deaths / Total (%) |
237 / 5024 (4.7%) |
95 / 3693 (2.6%) |
146 / 1355 (10.7%) |
76 / 1810 (4.2%) |
Abbreviations: BCSS, breast-cancer-specific survival; RR, relative risk; 95% CI, 95 percent confidence interval; Well/mod. differentiated, well/moderately differentiated; BCS, breast-conserving surgery; ER, estrogen receptor; PR, progesterone receptor; IHC, immunohistochemistry.
NOTE: The original full model included method of detection, age, race, tumor size, nodal status, nuclear grade, lymphatic/vascular invasion, surgery and radiation, adjuvant chemotherapy, neoadjuvant chemotherapy, adjuvant endocrine therapy, ER status, PR status, and HER2/neu IHC score.
P < 0.001
P ≥ 0.001 and P < 0.05
The variable was included in the original full model but it was not statistically significant (P ≥ 0.05). Thus, it was not included in the reduced model. A variable representing whether a patient received neoadjuvant chemotherapy was excluded for the same reason.
The variable was not included in the full model.
In subset analyses, the adjusted relative risk of breast cancer death was 1.13 (95% CI = 0.68 to 1.86; P = 0.638) among subgroup with ER positive tumors and 1.46 (95% CI = 0.91 to 2.35; P = 0.117) among subgroup with ER negative tumors.
The survival model (Model 4) included tumor characteristics, treatments, and HER2/neu and Ki67. Among 1810 patients with all biomarkers data available, the adjusted relative risk of tumor method of detection was 1.29 for RFS (95% CI = 0.89 to 1.87) and 1.19 for BCSS (95% CI = 0.66 to 2.15), which were similar to the results in Model 1. Because of the smaller number of event, the method of detection was not a statistically significant predictor for RFS and BCSS.
DISCUSSION
In summary, we compared the clinical outcomes of patients whose tumors were detected by screening examinations with those whose tumors were detected symptomatically. After adjusting for the known prognostic factors and major treatments received, we found the tumor detection method to be an independent predictor of disease recurrence. Specifically, patients with symptom-detected breast tumors had a greater risk of recurrence and death (RR for RFS =1.34, 95% CI = 1.09 to 1.66, P = 0.006; RR for BCSS = 1.31, 95% CI = 0.93 to 1.84, P = 0.117) than patients with screen-detected tumors.
The only tumor characteristic significantly associated with method of detection was Ki67, when including all four biomarkers in the regression model (the last column in Table 2). Our observation that screen-detected tumors tended to have lower Ki67 expression and ER-positive status is consistent with earlier observations that screening examinations preferentially identify cancers with these prognostic features (18-20). Because of missing Ki67 and HER2/neu score in the data, we performed exploratory analyses to examine the missing patterns of Ki67 expression and HER2/neu score by patient age, race, and other tumor characteristics. The results indicated that the subgroup of patients who had Ki67 information was sufficiently representative of the general cohort of patients with breast cancer at M. D. Anderson, because the missing Ki67 data were evenly distributed among each of the above factors. The analysis results using Ki67 data should be representative enough in Tables 2-4. In contrast, the missing pattern of HER2/neu IHC score was dependent on the year of diagnosis. Patients diagnosed between 1997 and 1998 were less likely to have HER2/neu data, as expected.
Similar to the findings in many other studies (21-23), we found that African American women had an elevated risk of death from breast cancer (RR = 1.87; 95% CI = 1.36 to 2.58) compared to women of other races, after adjusting for method of detection, tumor characteristics and treatments. While it is critical to promote targeting education and screening services to minority populations, a parallel effort is to better understand biologic difference in tumors among different ethnic groups to improve disease prognosis among minorities.
Our results are also consistent with conclusions from randomized screening trials, including a study of the Health Insurance Plan [HIP] and the Canadian National Breast Screening Studies [CNBSS] (7), and an observational study based on the Finnish Cancer Registry (8). In the former study, patients in the control groups had an increased risk of breast-cancer–specific death (RR = 1.36; 95% CI = 1.10 to 1.68) than patients in the screening groups, after accounting for tumor size, lymph node status, and disease stage. In the latter study, patients in the non-screened group had 1.90 times greater risk of distant recurrence (RR = 1.90; 95% CI = 1.15 to 3.11) and 2.11 times greater risk of breast-cancer–specific death (RR = 2.11; 95% CI = 1.16 to 3.85) after controlling for the number of positive lymph nodes, tumor size, PR status, histological grade, HER2/neu amplification, and patient age. The differences separating the current study from the earlier studies with similar conclusions include the following: 1. this study uses the largest sample size from one of the largest multidisciplinary breast cancer centers in the U.S.; 2. this study focuses on recently diagnosed patients (from 1997-2005) who have received adjuvant chemotherapy and hormonal therapies in the modern era; and 3. patient information in this study includes biomarker data such as Ki67 expression and HER2/neu IHC score that has become available only recently.
There are several limitations of our study. First, it is observational. Unlike randomized controlled screening trials, women who chose to have screening examinations may have very different characteristics from those who did not. However, despite the possibility of such a bias, our conclusions from this observational study are consistent with those of our earlier study of the HIP and CNBSS randomized trials (7). Second, conclusions from this study were based on the data from a single hospital, which may be subject to some selection bias. Compared with the Surveillance, Epidemiology, and End Results [SEER] data collected from 17 geographic areas of the United States for women aged 40 years and older and diagnosed with primary invasive breast cancer between 1997 and 2003, we found that more patients represented in the breast cancer database at M. D. Anderson tended to have later-stage breast cancer (16.7% stage III/IV tumors compared to 10.4% stage III/IV tumors from SEER), and to be of a racial/ethnic minority (9.9% African American and 15.1% Hispanic and other minority women, compared to 8.3% African American and 7.2% other minority women from SEER). Patients represented in the database at M. D. Anderson were also younger than those from the SEER database: 36.4% compared to 18.4% of patients, respectively, were younger than 50 years. Finally, the information on breast density at diagnosis is not available in our database.
Part of the observed residual survival benefit associated with tumor screening may not be completely due to screening itself but is partially due to lead-time bias and length bias. As indicated in our results, women diagnosed by screening examinations often had better tumor characteristics at diagnosis than those otherwise diagnosed, which explained the lead-time bias. The amount of time by which the diagnosis is advanced as a result of early detection by screening is the lead-time. Length-bias is revealed from the fact that tumors detected by screening examination may have biologic profiles (e.g., slower growing or less aggressive) that are different from tumors otherwise diagnosed (7). The finding that patients with screen-detected tumors still have better prognosis than those with symptomatically detected tumors after adjusting for tumor characteristics such as ER/PR status, Ki67 expression and HER2/neu score in the models indicates that the improved prognosis of screen-detected patients is due to some combination of length bias and actual screening benefit. But it is impossible to separate length-bias and the actual screening benefit.
Breast tumors are heterogeneous. It is still impossible now to obtain a complete profile to describe the heterogeneity of the tumors. Including method of detection in the model better enables predicting patients' prognoses. Method of detection is not a prognostic factor in the conventional sense (such as tumor size and nodal status), but it gives important information and partially adjusts for length bias.
This study suggests that the information of the detection method should be routinely collected in the breast cancer database to help clinical trialists and health care providers improve prognosis prediction for patients with breast cancer.
Acknowledgments
Grant support: Supported by Research Grants No. CA-79466 and CA-016672 from National Cancer Institute, and a grant from the National Comprhensive Cancer Network to fund, in part, the Breast Cancer Medical System database of M. D. Anderson Cancer Center.
REFERENCES
- 1.Berry DA, Inoue L, Shen Y, et al. Modeling the impact of treatment and screening on U.S. breast cancer mortality: a Bayesian approach. J Natl Cancer Inst Monogr. 2006;36:30–6. doi: 10.1093/jncimonographs/lgj006. [DOI] [PubMed] [Google Scholar]
- 2.Tabar L, Yen MF, Vitak B, et al. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361:1405–10. doi: 10.1016/S0140-6736(03)13143-1. [DOI] [PubMed] [Google Scholar]
- 3.Otto SJ, Fracheboud J, Looman CW, et al. Initiation of population-based mammography screening in Dutch municipalities and effect on breast-cancer mortality: a systematic review. Lancet. 2003;361:1411–7. doi: 10.1016/S0140-6736(03)13132-7. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:347–60. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 5.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–99. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 6.Howe HL, Wingo PA, Thun MJ, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001;93:824–42. doi: 10.1093/jnci/93.11.824. [DOI] [PubMed] [Google Scholar]
- 7.Shen Y, Yang Y, Inoue LY, et al. Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. J Natl Cancer Inst. 2005;97:1195–203. doi: 10.1093/jnci/dji239. [DOI] [PubMed] [Google Scholar]
- 8.Joensuu H, Lehtimaki T, Holli K, et al. Risk for distant recurrence of breast cancer detected by mammography screening or other methods. Jama. 2004;292:1064–73. doi: 10.1001/jama.292.9.1064. [DOI] [PubMed] [Google Scholar]
- 9.Chu KC, Smart CR, Tarone RE. Analysis of breast cancer mortality and stage distribution by age for the Health Insurance Plan clinical trial. J Natl Cancer Inst. 1988;80:1125–32. doi: 10.1093/jnci/80.14.1125. [DOI] [PubMed] [Google Scholar]
- 10.Miller AB, Baines CJ, To T, et al. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. Cmaj. 1992;147:1477–88. [PMC free article] [PubMed] [Google Scholar]
- 11.McPherson CP, Swenson KK, Jolitz G, et al. Survival of women ages 40-49 years with breast carcinoma according to method of detection. Cancer. 1997;79:1923–32. doi: 10.1002/(sici)1097-0142(19970515)79:10<1923::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro S, Goldberg JD, Hutchison GB. Lead time in breast cancer detection and implications for periodicity of screening. Am J Epidemiol. 1974;100:357–66. doi: 10.1093/oxfordjournals.aje.a112046. [DOI] [PubMed] [Google Scholar]
- 13.Connor RJ, Chu KC, Smart CR. Stage-shift cancer screening model. J Clin Epidemiol. 1989;42:1083–95. doi: 10.1016/0895-4356(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 14.Schootman M, Fuortes L, Aft R. Prognosis of metachronous contralateral breast cancer according to stage at diagnosis: the importance of early detection. Breast Cancer Res Treat. 2006;99:91–5. doi: 10.1007/s10549-006-9185-0. [DOI] [PubMed] [Google Scholar]
- 15.Morrison AS. The effects of early treatment, lead time and length bias on the mortality experienced by cases detected by screening. Int J Epidemiol. 1982;11:261–7. doi: 10.1093/ije/11.3.261. [DOI] [PubMed] [Google Scholar]
- 16.Baum M. Breast cancer screening comes full circle. J Natl Cancer Inst. 2004;96:1490–1. doi: 10.1093/jnci/djh311. [DOI] [PubMed] [Google Scholar]
- 17.Krzyzanowska MK, Tannock IF. Should screen-detected breast cancers be managed differently? J Natl Cancer Inst. 2005;97:1170–1. doi: 10.1093/jnci/dji246. [DOI] [PubMed] [Google Scholar]
- 18.Porter PL, El-Bastawissi AY, Mandelson MT, et al. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91:2020–8. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]
- 19.Gilliland FD, Joste N, Stauber PM, et al. Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer Inst. 2000;92:743–9. doi: 10.1093/jnci/92.9.743. [DOI] [PubMed] [Google Scholar]
- 20.Narod SA, Dube MP. Re: Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer Inst. 2001;93:151–2. doi: 10.1093/jnci/93.2.151. [DOI] [PubMed] [Google Scholar]
- 21.Shen Y, Dong W, Esteva FJ, et al. Are there racial differences in breast cancer treatments and clinical outcomes for women treated at M.D. Anderson Cancer Center? Breast Cancer Res Treat. 2007;102:347–56. doi: 10.1007/s10549-006-9337-2. [DOI] [PubMed] [Google Scholar]
- 22.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97:134–47. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 23.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144:541–53. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]