Abstract
The administration of low dose opioid antagonists has been explored as a potential means of detoxification in opiate dependence. Previous results from our laboratory have shown that concurrent administration of low dose naltrexone in the drinking water of rats implanted with subcutaneous morphine pellets attenuates behavioral and biochemical signs of withdrawal in brainstem noradrenergic nuclei. Noradrenergic projections originating from the nucleus tractus solitarius (NTS) and the locus coeruleus (LC) have previously been shown to be important neural substrates involved in the somatic expression of opiate withdrawal. The hypothesis that low dose naltrexone treatment attenuates noradrenergic hyperactivity typically associated with opiate withdrawal was examined in the present study by assessing norepinephrine tissue content and norepinephrine efflux using in vivo microdialysis coupled to high performance liquid chromatography (HPLC) with electrochemical detection (ED). The frontal cortex (FC), amygdala, bed nucleus of the stria terminalis (BNST) and cerebellum were analyzed for tissue content of norepinephrine following withdrawal in morphine dependent rats. Naltrexone precipitated withdrawal elicited a significant decrease in tissue content of norepinephrine in the BNST and amygdala. This decrease was significantly attenuated in the BNST of rats that received low dose naltrexone pretreatment compared to controls. No significant difference was observed in the other brain regions examined. In a separate group of rats, norepinephrine efflux was assessed with in vivo microdialysis in the BNST or the FC of morphine dependent rats or placebo treated rats subjected to naltrexone-precipitated withdrawal that received either naltrexone in their drinking water (5 mg/L) or unadulterated water. Following baseline dialysate collection, withdrawal was precipitated by injection of naltrexone and sample collection continued for an additional four hours. At the end of the experiment, animals were transcardially perfused and the brains were removed for verification of probe placement. Low dose naltrexone pre-treatment significantly attenuated withdrawal-induced increases of extracellular norepinephrine in the BNST, with a smaller effect in the FC. These findings suggest that alterations in norepinephrine release associated with withdrawal may be attenuated in forebrain targets of noradrenergic brainstem neurons that may underlie reduced behavioral signs of withdrawal following low dose naltrexone administration.
Keywords: HPLC, microdialysis, morphine, naltrexone, norepinephrine, withdrawal
INTRODUCTION
Since their first use in opiate detoxification over 30 years ago, the administration of opiate antagonists (e.g. naloxone and naltrexone) during detoxification has varied either by differences in interval of administration between agonist and antagonist, or by quantity of antagonist administered (Kurland and McCabe, 1976; Mannelli et al., 2004). The closer the antagonist is administered to opiate agonist exposure, the more acute the withdrawal syndrome. Interestingly, the administration of small quantities of naltrexone or naloxone during opiate exposure induces “anti-withdrawal” effects (Shen and Crain, 1997; Mannelli et al., 2004). A better knowledge of the effect of opiate antagonists and their potential role during the detoxification process may help improve the quality of existing treatments.
Our previous studies have demonstrated that concurrent administration of low dose naltrexone in the drinking water of rats implanted with subcutaneous morphine pellets attenuates behavioral signs of withdrawal and decreases the expression of c-Fos (used as an index of cellular activation in autonomic brain areas (Sagar et al., 1988)) in the noradrenergic locus coeruleus (LC) and nucleus of the solitary tract (NTS) (Mannelli et al., 2004). The LC and the NTS, known to be hyperactive following withdrawal from opiates (Hayward et al., 1990; Stornetta et al., 1993; Beckmann et al., 1995; Chieng et al., 1995; Georges et al., 2000; Mannelli et al., 2004), have previously been shown to be important neural substrates involved in the somatic expression of opiate withdrawal (Nestler et al., 1994; Aston-Jones et al., 1999; Mannelli et al., 2004). In addition, immunoblot analysis of intracellular messengers, cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PKA) and cAMP-response element-binding protein (CREB), demonstrated to be dramatically increased in noradrenergic nuclei following opiate withdrawal (Guitart et al., 1992; Lane-Ladd et al., 1997), showed a decrease in expression (primarily in the NTS) in rats that had received low doses of naltrexone in their drinking water and that were subjected to pharmacological precipitation of opiate withdrawal (Mannelli et al., 2004). Potential explanations of the efficacy of the naltrexone treatment include the possibility that low antagonist dosing may act to facilitate enkephalin release by blocking opioid presynaptic receptors (Ueda et al., 1994). Alternatively, low dose naltrexone may antagonize excitatory opiate receptor functions and unmask the inhibitory effects of opioids (Shen and Crain, 1997). We also reported increases in opioid receptor expression (specifically of the mu subtype) in the NTS following antagonist treatment that may contribute to the attenuation of behavioral expression of withdrawal following low dose naltrexone treatment (Van Bockstaele, 2006). Increased expression of mu-opioid receptors may result in increased tonic inhibition of noradrenergic neurons in the NTS that provide noradrenergic innervation to forebrain targets resulting in decreased release of norepinephrine in limbic and cortical targets (Van Bockstaele, 2006).
In the present study, the hypothesis that low dose naltrexone treatment in morphine dependent rats attenuates forebrain noradrenergic hyperactivity usually associated with pharmacological precipitation of withdrawal (Crawley et al., 1979; Swann et al., 1982; Grasing et al., 1997; Fuentealba et al., 2000) was tested. First, we measured (from morphine dependent rats that received low dose naltrexone or unadulterated water) tissue content of norepinephrine from two forebrain regions that are targeted by brainstem noradrenergic nuclei: the bed nucleus of the stria terminalis (BNST) that receives noradrenergic projections primarily from the NTS (Dunn and Williams, 1995; Delfs et al., 2000) and the frontal cortex (FC) that receives afferents primarily from the LC (Robbins, 1984; Aston-Jones, 1985). We also examined tissue samples from the amygdala, a region that receives noradrenergic innervation from both the LC and NTS (Robbins, 1984; Aston-Jones, 1985; Dunn and Williams, 1995; Delfs et al., 2000), and cerebellum, which receives noradrenergic afferents solely from the LC. Subsequently, norepinephrine efflux in the FC and BNST was assessed using high performance liquid chromatography (HPLC) with electrochemical detection (ED).
METHODS
Animals
Male, Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 225–250g at the start of experiments were used for these studies. All procedures were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University and are in compliance with the guidelines of the National Institutes of Health.
Immunoperoxidase labeling of DβH
Tissue sections through the forebrain of one (n=1) rat were processed for the immunocytochemical detection of the noradrenergic synthesizing enzyme, dopamine-beta-hydroxylase (DβH). The rat was deeply anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg) and transcardially perfused with 200 ml of 4% paraformaldehyde in 0.1M phosphate buffer (pH=7.4). The brain was extracted, and thirty-micron-thick coronal sections through the BNST were cut using a Vibratome and collected into chilled 0.1 M phosphate buffer. Tissue sections were incubated in 1% sodium borohydride solution for 30 min and then rinsed in 0.1 M phosphate buffer and 0.1 M Tris–saline buffer (pH 7.6). Sections were blocked in 0.5% bovine serum albumin in 0.1 M Tris-saline buffer for 30 min and rinsed extensively in 0.1 M Tris-saline buffer. Tissue sections were incubated in a mouse anti-DβH (1:1000) primary antibody made in 0.1 M Tris-saline buffer containing 0.1% bovine serum albumin at room temperature overnight on a rotary shaker. Next, tissue sections were rinsed in 0.1 M Tris-saline buffer and then incubated in biotinylated donkey anti-mouse IgG (Vector Laboratories, Burlingame, CA; 1:200) made in 0.1 M Tris-saline buffer containing 0.1% bovine serum albumin for 30 min. Tissue was rinsed in 0.1 M Tris-saline buffer, incubated in an avidin–biotin complex solution (Vector Laboratories) for 30 min and then washed again in 0.1 M Tris-saline buffer. A peroxidase color reaction was obtained by exposing the tissue sections to a solution containing 22 mg of 3,3-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, MO) in a 0.1 M Tris-saline solution containing 0.05% hydrogen peroxide for 6 min. Tissue was rinsed with 0.1 M Tris-saline buffer, followed by 0.1 M phosphate buffer. Sections were mounted onto gelatin-coated microscope slides and the distribution of DβH containing fibers within the BNST was photographed. The distribution of DβH fibers in this region was compared to a rat brain atlas (Paxinos and Watson, 1986) and aided in the appropriate selection of coordinates for microdialysis probe placement (data not shown).
Drug Treatment for Tissue Analysis and High Pressure Liquid Chromatography for Microdialysis
For norepinephrine tissue analysis, fifteen rats were used. Ten rats were implanted subcutaneously with two slow release morphine pellets each (75 mg morphine base; National Institute on Drug Abuse). On Day 3, five of the ten rats received very low dose naltrexone in their drinking water (5 mg/L). Control rats received placebo pellets (n = 5). Rats were euthanized 45 minutes after naltrexone or saline administration on Day 8 and brains were rapidly dissected on ice. Samples from the FC, BNST, amygdala and cerebellum were frozen on dry ice for subsequent analysis. Tissue samples were homogenized in 0.1 N perchloric acid with 100 μM ethylenediamine tetraacetic acid (EDTA) (15 μl/mg tissue) using an ultrasonic processor. Samples were centrifuged at 15,000 rpm (23,143 g) for 15 min. The supernatant was filtered through 0.45 μm nylon acrodisk syringe filters. Tissue samples were analyzed for norepinephrine content using HPLC-ED.
Male rats were implanted subcutaneously with two slow release morphine pellets each (75 mg each morphine base; National Institute on Drug Abuse). This dose induces morphine dependence that is maintained for almost two weeks (Gold et al., 1994). Control rats received placebo pellets. On Day 3, a subset of the morphine implanted rats received very low dose naltrexone in their drinking water (5 mg/L). The concentration of naltrexone in the drinking water is based on an estimated water consumption of 22 ml/rat/day (Mannelli et al., 2004). During the duration of the treatment, rats were monitored daily to verify that the dose of naltrexone ingested did not elicit any overt signs of withdrawal or behavioral disruption. Animals were maintained on a 12-hr light:dark cycle. To precipitate withdrawal, naltrexone (100 mg/kg) was administered systemically. This dose was selected based on our previously published studies (Mannelli et al., 2004).
The HPLC system consisted of an ESA solvent delivery system (ESA Inc., Chelmsford, MA) and an MD 150 reverse phase narrowbore column (150 x 2 mm, 3 μm; ESA Inc., Chelmsford, MA). The mobile phase consisted of 60 mM sodium phosphate buffer (pH = 4.2) with 100 μM EDTA, 1.5 mM sodium octyl-sulfate, and 3.5% (v.v) methanol. The flow rate through the system was 300 μl/min. The detection system consisted of an ESA Coulochem III electrochemical detector with a guard cell and a 5041 enhanced amperometric analytical cell (ESA Inc., Chelmsford, MA) with a glassy carbon in ceramic target electrode in series. The applied potential of the guard cell was −150 mV and the compounds of interest were quantified at the target electrode set at +200 mV. Peak heights were measured and compared to the peak heights of a 10−8 M standard calibrated daily. The detection limit, defined as the sample amount producing a peak height that is twice the height of the background noise, was approximately 0.5 pg of norepinephrine. Absolute values of tissue concentrations of norepinephrine (pg/15 mg tissue) were compared between groups using one-way ANOVA.
Dialysis Probe Construction and Microdialysis
Vertical concentric microdialysis probes were used. A piece of fused silica (Polymicro Technologies, Phoenix, AZ) was inserted through PE 10 tubing and semipermeable membrane made from hollow rayon fibers with a 224 μm o.d. and 35,000 MW cutoff was fixed over the fused silica and into the PE 10 tubing with epoxy. The open end of the dialysis fiber was sealed with a 0.5 mm epoxy plug and 2 mm of the membrane was coated with epoxy leaving an active area of 3 mm (FC) or 2 mm (BNST) for exchange across the membrane. The in vitro recovery rate was determined by placing the probe in a beaker of artificial cerebrospinal fluid (aCSF: 174 mM NaCl, 1.7 mM CaCl2, 0.9 mM Mg Cl2, and 4 mM KCl) containing a known concentration of norepinephrine standard. The concentration of norepinephrine in the dialysate was compared to the amount in the bath. Probes that did not correspond to an acceptable range of recovery (12–24%) were eliminated. Because the diffusion properties of neurochemicals in brain tissue are likely different from in vivo conditions, reported dialysate values were not corrected for recovery of the probe.
On Day 7, rats were anesthetized with isofluorane and placed in a stereotaxic apparatus with the skull flat. A small burr hole was made in the skull centered at 3.2 mm anterior and ± 0.7 mm lateral to Bregma (FC) or −0.3 mm and ± 1.5 mm lateral (for BNST). The dura was removed and the microdialysis probe was slowly lowered 5.0 mm from the brain surface into the infralimbic and prelimbic areas of the FC or 7.2 mm for the BNST and secured with skull screws and dental acrylic. The inlet of the probe was connected to a fluid swivel (Instech Laboratories, Plymouth Meeting, PA) and the rat was placed into a cylindrical plexiglass container covered with bedding and allowed to recover. Food and water were freely accessible. ACSF was continuously perfused through the probe at a rate of 1.5 μl/min by a microliter infusion pump (Harvard Pump ‘11’ VPF Dual Syringe, Harvard Apparatus, Holliston, MA). Rats were allowed to recover overnight. Approximately 18 hours following surgery, dialysate samples were collected every 20 minutes for 6 hours. An injection of naltrexone (100 mg/kg, i.p.) was given after 6 baseline samples were collected. At the conclusion of the experiment, rats were deeply anesthetized and 2% pontamine sky blue dye (Alfa Aesar, Ward Hill, MA) was infused through the probe to mark its location. The rats were transcardially perfused with 10% formalin (Fisher Scientific, Pittsburgh, PA), decapitated and the brains removed for subsequent histological verification of probe placement. The data were not included in the analysis if the placement was outside the infralimbic and prelimbic areas of the FC or in the BNST.
Data Analysis
For analysis of tissue extracts, norepinephrine levels were compared between groups using a one way analysis of variance (ANOVA). For microdialysis experiments, a 2x3x6 repeated measures ANOVA crossing region, study condition, and time (i.e., the 6 pre-withdrawal measures of norepinephrine) was conducted to establish the absence of any systematic pre-intervention differences on basal levels of norepinephrine. The absence of any significant main or interaction effects would allow for the computation of a single pre-intervention norepinephrine level summary statistic (i.e., the mean) for each animal. To test the primary hypothesis that the administration of low dose naltrexone prior to opiate withdrawal induction would attenuate the levels of norepinephrine efflux differentially at specific brain regions (FC, BNST) was tested via a 2x3 analysis of covariance that crossed region and study condition while using the baseline measure as a covariate. The primary dependent measure for this analysis was norepinephrine levels recorded twenty minutes post-withdrawal induction. While a significant interaction term would be suggestive of an effect, planned contrasts were designed to test norepinephrine efflux levels between study conditions at the FC and BNST regions. All statistics were performed using JMP software (SAS Institute, Cary, NC).
RESULTS
Effect of low dose naltrexone on tissue norepinephrine levels following withdrawal
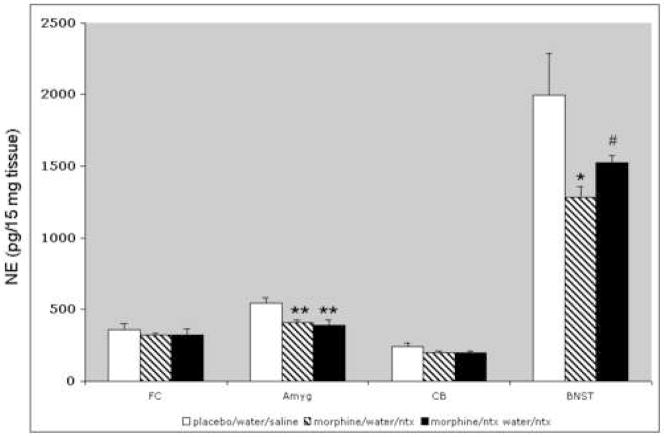
Norepinephrine tissue content was assessed in tissue extracts from four brain regions. These included the amygdala, BNST, frontal cortex, and cerebellum. Withdrawal precipitated by systemic injection of naltrexone (100 mg/kg) was associated with a reduction of tissue norepinephrine in the amygdala (F[2,12] = 8.35; p < .01) and the BNST (F[2,12] = 4.24; p < .05 one way ANOVA) with no significant change in the FC or cerebellum (Fig. 1). Pretreatment with low dose naltrexone in the drinking water significantly attenuated the withdrawal-induced decrease in the BNST but had no effect in the FC, cerebellum or amygdala.
Figure 1.
Tissue levels of norepinephrine were significantly decreased in morphine-dependent rats after naltrexone-precipitated withdrawal in the amygdala and BNST only. (One-way ANOVA, **p < .01, *p < .05; n = 5 for each group). Pretreatment with low dose naltrexone in the drinking water attenuated the decrease only in the BNST (one-way ANOVA, #p < .05, compared to unadulterated water). Abbreviations: FC: frontal cortex; Amyg: amygdala; CB: cerebellum.
Extracellular NE levels in the BNST and FC were monitored using in vivo microdialysis and HPLC-ED
To better define the localization of noradrenergic fibers in the BNST to guide microdialysis probe placement, tissue sections from one rat were processed for the immunocytochemical identification of DβH in this region (data not shown). Immunoperoxidase labeling for DβH revealed a circumscribed region of dense immunoreactivity ventral to the anterior commissure corresponding to the level 0.26 mm posterior to Bregma (Paxinos and Watson, 1986). This is the area of the ventral BNST known to contain the highest concentration of norepinephrine (Kozicz and Arimura, 2000) and corresponds to the area that receives significant input from the NTS (Dunn and Williams, 1995).
Extracellular NE levels in the BNST and FC were monitored using in vivo microdialysis and HPLC-ED. Representative photomicrographs of microdialysis probe placements can be seen for the BNST in Figure 2A and for the FC in Figure 3A. Two hours of baseline sample collection were collected to determine basal extracellular norepinephrine efflux prior to naltrexone injection (Fig. 2B, 3B arrow). Placebo treated rats received unadulterated water followed by a saline injection (Fig. 2B, 3B; straight black line). Withdrawal was induced by a systemic injection of naltrexone in rats that received either unadulterated water (Fig. 2B, 3B; circles) or that received low doses of naltrexone in their drinking water (Fig. 2B, 3B; filled circles).
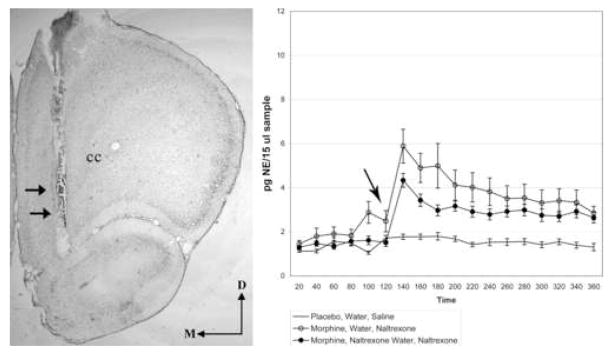
Figure 2.
A. Brightfield photomicrograph of a neutral red labeled tissue section showing a representative trajectory (arrows) of a microdialysis probe placement within the ventral BNST. The region targeted for probe placement corresponds to 0.26 mm posterior to Bregma from the rat brain atlas of Paxinos and Watson (Paxinos and Watson, 1986). B. Absolute values of extracellular norepinephrine (pg/15 μl sample) are reported over a four hour period in the BNST. Arrow indicates timepoint of naltrexone administration. Abbreviations: D = dorsal, M = medial; cc = corpus callosum, LV = lateral ventricle, CPu = caudate putamen.
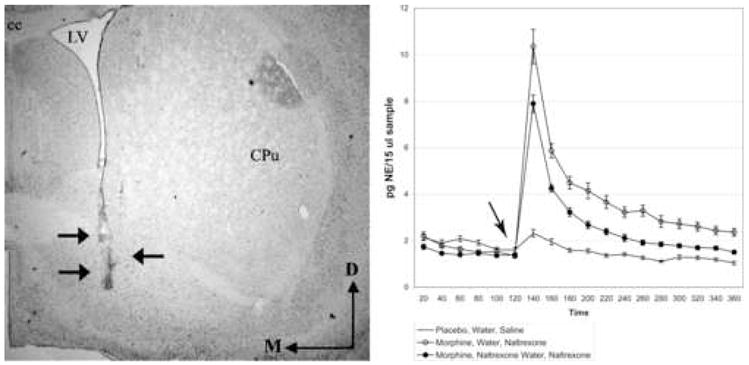
Figure 3.
A. Brightfield photomicrograph of a neutral red labeled tissue section showing the trajectory of the microdialysis probe into the FC. B. Absolute values of extracellular norepinephrine (pg/15 μl sample) are reported over a four hour period in the FC. Arrow indicates timepoint of naltrexone administration. Abbreviations: D = dorsal, M = medial, cc = corpus callosum, LV = lateral ventricle, CPu = caudate putamen.
Pre-Intervention Data
The presence of multiple pre-intervention, baseline data points led us to examine whether any differences existed by treatment condition or region. A 2x3x6 repeated measures analysis of variance crossing site (BNST/FC), intervention (control, morphine alone, morphine and low dose naltrexone treatment) and time was conducted. No significant main or interaction effects were observed. As a result, to ease the interpretability of the results, a single summary measure (i.e., average) of pre-intervention levels of norepinephrine was computed for each study participant.
Effect of Low Dose Naltrexone on Basal and Withdrawal-Induced Norepinephrine Efflux Across Region: Peak Effect
To test the hypothesis that naltrexone induced opiate withdrawal preceded by treatment with very low doses of naltrexone could attenuate norepinephrine efflux, a 2x3 analysis of covariance, crossing treatment condition and site, using the baseline average as a covariate, was conducted. For this analysis, norepinephrine efflux data 20 minutes post withdrawal induction represented the primary outcome measure. While significant main effects of both region, F (1, 31) = 12.40, p < .001 and study condition, F (2, 31) = 31.65, p < .001 were observed, these effects were mediated by a significant site x condition interaction, F (2, 31) = 5.02, p < .01. While the presence of this significant interaction term provides evidence of the hypothesized differences in efflux levels by region and study condition, planned contrasts were used to explicate the nature of the effect. These contrasts indicated a) that the differences in norepinephrine efflux observed at the FC region did not differ significantly between the low dose naltrexone treated and unadulterated water treated opiate addicted groups (95% confidence intervals = −2.71 and 2.07, p = .79), and b) a trend for norepinephrine efflux to be significantly attenuated with low dose naltrexone treatment at the BNST region (95% confidence intervals = −.422 and .31, p = .08).
Effect of Low Dose Naltrexone on Basal and Withdrawal-Induced Norepinephrine Efflux Across Region: Area Under the Curve
To further examine whether naltrexone induced opiate withdrawal preceded by treatment with very low doses of naltrexone could attenuate norepinephrine efflux, two measures of AUC data were analyzed via a 2x3 analysis of variance again crossing treatment condition and site. In the first analysis, AUC data were computed for the 5 assessments most proximal to the administration of naltrexone (Times 140, 160, 180, 200, 220). A similar pattern of main effects as observed for the peak effect data were noted (study condition, p < .01, location, p < .05.) While the interaction failed to reach conventional standards of significance, F (2, 31) = 2.61, p = .09, an examination of the means yielded a similar pattern of results. The exploratory nature of this study led us to further decompose this interaction. Results indicated that norepinephrine efflux in the first 100 minutes post-withdrawal induction was significantly attenuated at the BNST (95% confidence intervals = −864.46 and −133.42, p < .05.) This effect was not noted at the FC site (95 CI = −654.28 and 115.11, p > .05.)
DISCUSSION
The present findings build upon our previously published results (Mannelli et al., 2004; Van Bockstaele, 2006) as well as that of others (Crain and Shen, 1995; Shen and Crain, 1997) that show that morphine dependent rats that receive low doses of naltrexone in their drinking water exhibit attenuated withdrawal behaviors (e.g. decreased wet dog shakes, teeth chatters, digging, lacrimation and diarrhea) following pharmacological precipitation of withdrawal. Here, we provide neurochemical data showing that withdrawal-induced increases in norepinephrine efflux are attenuated in forebrain sites following pre-treatment with low doses of naltrexone. This attenuation of forebrain norepinephrine release may explain, in part, the efficacy of the low dose naltrexone treatment in ameliorating withdrawal symptomatology in opiate dependent rats.
The drug administration used in the present study was designed to maintain the animal’s natural environment and routine habits. Previous studies have shown that oral administration of naltrexone results in more effective analgesia and dependence-reducing effects as compared to the subcutaneous mode of drug administration (Shen and Crain, 1997). We modeled our drug delivery in rats following a clinical report of the accidental ingestion of an opiate antagonist by an active human opioid abuser (Mannelli et al., 1999). In the case of a methadone maintained patient, 50 mg naltrexone induced acute withdrawal requiring hospital admission. After approximately 47 hours of deep sedation, the patient became quite comfortable and exhibited no further signs of spontaneous or repeatedly induced withdrawal, up to 75 hours from the accident (Mannelli et al., 1999). Nevertheless, naltrexone, whose half-life for receptor occupation is about 70 hours, and methadone, as detected by urinalyses, were still present in the patient’s body. The total absence of symptoms upon additional acute administration of opiate antagonists (1.2 mg naloxone tests were repeatedly performed in the presence of the agonist) indicated that the findings could not be readily explained by competitive antagonism of the mu-opioid receptor. As described in our previous studies (Mannelli et al., 2004; Van Bockstaele, 2006), naltrexone administration in the drinking water did not cause any withdrawal symptoms or change in food or water consumption compared to control animals. Furthermore, as described here, no changes in baseline norepinephrine efflux were observed in any brain region analyzed during the naltrexone pre-treatment. Finally, our results are not likely explained on the basis of competitive antagonism of the mu-opioid receptor as withdrawal responses could be elicited every time full blocking quantities of antagonist were administered during low dose naltrexone exposure indicating that dependence had occurred during the pre-treatment period (Zimmerman and Leander, 1990) (Shen and Crain, 1997).
It is widely accepted that noradrenergic neurons of the LC and NTS are involved in mediating somatic signs of the opiate withdrawal syndrome (Gold et al., 1981; Kantak and Miczek, 1988; Hayward et al., 1990; Koob et al., 1992; Stornetta et al., 1993; Nestler et al., 1994; Beckmann et al., 1995; Chieng et al., 1995; Aston-Jones et al., 1999; Georges et al., 2000; Mannelli et al., 2004). Opiate dependent rats show a marked increase in the discharge rates of LC neurons during antagonist-induced withdrawal (Rasmussen et al., 1990) which correlates with the behavioral expression of the opiate withdrawal syndrome (Grant et al., 1988; Akaoka and Aston-Jones, 1991; Guitart et al., 1993; Aghajanian et al., 1994; Kogan and Aghajanian, 1995; Maldonado et al., 1995; Rasmussen, 1995; Dossin et al., 1996; Krystal et al., 1996; Rasmussen et al., 1996; Aston-Jones et al., 1997; Javelle et al., 1997). Biochemical studies have shown increases in numerous intracellular messengers during early stages of withdrawal in both regions (Duman et al., 1988; Nestler et al., 1989; Hayward et al., 1990; Rasmussen et al., 1990; Guitart et al., 1992; Kogan et al., 1992; Guitart and Nestler, 1993; Stornetta et al., 1993; Nestler et al., 1994; Koob and Nestler, 1997; Lane-Ladd et al., 1997). Increased cAMP activity and downstream effectors have been widely related to opiate withdrawal adaptations (for a review see (Nestler, 2001) and a reduction in intracellular messengers that are part of the cAMP pathway was previously shown in morphine dependent rats pretreated with low dose naltrexone in both the LC and NTS (Mannelli et al., 2004). The LC and NTS provide the major source of noradrenergic afferents to the forebrain with the LC using the dorsal noradrenergic bundle to target cortical areas and the NTS using the ventral noradrenergic bundle to target limbic regions (Robbins, 1984; Aston-Jones, 1985; Dunn and Williams, 1995; Aston-Jones et al., 1999; Delfs et al., 2000).
The present results indicate that administration of low dose naltrexone significantly attenuates increases in norepinephrine efflux in the BNST, a region that typically exhibits a dramatic increase in norepinephrine efflux following pharmacological precipitation of opiate withdrawal (Funada et al., 1994; Aston-Jones et al., 1999; Fuentealba et al., 2000). The attenuation of norepinephrine release in the BNST may explain, in part, the efficacy of the low dose naltrexone treatment in ameliorating withdrawal symptomatology in opiate dependent rats as many of the observed withdrawal symptoms are consistent with projections of noradrenergic neurons of the NTS to limbic forebrain areas (Funada et al., 1994; Aston-Jones et al., 1999; Fuentealba et al., 2000). A potential explanation for the attenuation of norepinephrine efflux in the BNST of morphine dependent animals following low dose naltrexone pretreatment is that low antagonist dosing may modulate presynaptic opioid receptors in this region resulting in alterations in norepinephrine release as suggested for spinal circuitry (Ueda et al., 1994). Alternatively, we hypothesized that increases in opioid receptor expression (specifically of the mu subtype) in the NTS could potentially contribute to the attenuation of behavioral expression of withdrawal following low dose naltrexone treatment by providing a greater tonic inhibition of these noradrenergic neurons resulting in decreased norepinephrine release in forebrain targets (Van Bockstaele, 2006). This would be consistent with reported modifications of mu-opioid receptor expression induced by naltrexone in non-dependent animals (Unterwald et al., 1998). Further mechanistic studies are required to address the cellular mechanisms underlying the observed decreases in norepinephrine efflux following low dose naltrexone pretreatment.
Tissue levels of norepinephrine were significantly decreased in morphine dependent rats after naltrexone precipitated withdrawal in the amygdala and BNST but not in the cerebellum or frontal cortex. Tissue analysis is an indirect measure of neurotransmitter release so less norepinephrine in tissue levels correlates with a greater amount of released transmitter. The observed decrease in tissue norepinephrine levels in the BNST and amygdala following pharmacological precipitation of withdrawal is indicative of an increase in norepinephrine release, a result that is consistent with data of others (Funada et al., 1994) and with our microdialysis data. Our data suggest that norepinephrine levels in the amygdala follow a similar pattern as the BNST following morphine treatment and induction of opiate withdrawal although we did not conduct microdialysis experiments there. However, the effect of low-dose naltrexone pre-treatment shows a differential effect in the amygdala vs BNST. Pre-treatment with low dose naltrexone only attenuated the decrease in norepinephrine tissue levels in the BNST. This may be related to the lower levels of norepinephrine present in the amygdala that may compromise adequate detection of small fluctuations in neurotransmitter release. Alternatively, effects of pre-treatment with low doses of naltrexone may be more complex and target noradrenergic circuits to the amygdala differently than pathways projecting to the BNST. This may be due in part to the fact that the amygdala receives noradrenergic afferents from both the LC and the NTS (Robbins, 1984; Aston-Jones, 1985; Dunn and Williams, 1995; Delfs et al., 2000) so this may account for the shared effect on norepinephrine efflux following precipitated withdrawal but also explain the differential response to low dose pre-treatment.
The presence of an attenuation by low dose naltrexone treatment on norepinephrine efflux in the FC in the microdialysis study but no effect in tissue analysis may arise from the fact that levels of norepinephrine are lower in this brain region as compared to the BNST which exhibits one of the highest concentrations or norepinephrine in the forebrain. The lack of a significant reduction in tissue norepinephrine reflects a lack of a major effect in terms of norepinephrine available for release. The tissue analysis data suggest that a similar amount of norepinephrine is available in all treatment conditions at the time of sampling. For the microdialysis experiments, a modest attenuation of norepinephrine efflux in this region following low dose naltrexone pre-treatment could contribute to the reduction of selected withdrawal symptoms (Mannelli et al., 2004). Some behaviors such as locomotor symptoms (rearing, freezing, jumping) are attributed to LC efferent projections to the forebrain (Maldonado and Koob, 1993). Future studies examining activity of the norepinephrine transporter in the frontal cortex under the different experimental conditions may shed light (and possibly provide better resolution) of adaptations in the coeruleo-cortical circuit following low dose naltrexone pre-treatment.
Clinically, the effect of low dose antagonist treatment on noradrenergic forebrain targets may parallel the efficacy of medications that regulate noradrenaline release in controlling opioid withdrawal (e.g. clonidine) without confounding side effects. Thus, chronic low dose naltrexone may help regulate sensitive regions of the opioid dependent brain. How this could influence other aspects of the addictive behavior (drug seeking, relapse, craving) remains to be explored. In summary, the present study has important implications for clinical detoxification approaches and for the understanding of adaptations in neural circuits underlying the actions of opiate antagonists and their interaction with agonist drugs.
TABLE 1. Norepinephrine extracellular levels.
Microdialysis probes were implanted in either the BNST or FC of placebo or morphine treated rats that received either low dose naltrexone in their drinking water or unadulterated water. Adjusted means are presented with standard error of the mean. Control rats received placebo pellets and unadulterated water and showed no significant changes in extracellular norepinephrine efflux following a systemic injection of saline on day 8. A single summary measure (i.e., average) of pre-intervention levels of norepinephrine was computed and these values are reported. Basal norepinephrine efflux data are shown for the time point of 20 minutes following a systemic naltrexone withdrawal (100 mg/kg, i.p., at arrow) injection used as the primary outcome measure. Naltrexone precipitated withdrawal in morphine dependent rats was associated with a significant increase of extracellular norepinephrine in the BNST and FC. Low dose naltrexone administration significantly abrogated withdrawal-induced increases of extracellular norepinephrine in the BNST. Prior pretreatment with low dose naltrexone in the drinking water only slightly attenuated withdrawal-induced increases of norepinephrine in the FC but did not reach statistical significance.
| NE (pg/15μl sample) |
||||||
|---|---|---|---|---|---|---|
| Bed Nucleus of the Stria Terminalis |
Frontal Cortex |
|||||
| Treatment | Basal | Naltrexone | n | Basal | Naltrexone | n |
| Placebo/Water/Saline | 1.84±.60 | 2.33 ± .90 | 8 | 1.44±.55 | 1.77 ± .52 | 5 |
| Morphine/Water/Naltrexone | 1.48±.65 | 10.06 ± 4.43* | 7 | 1.35±.35 | 5.14 ± 1.82 | 5 |
| Morphine/Naltrexone Water/Naltrexone |
1.40±.34 | 7.89 ± 1.85^ | 6 | 1.49±.81 | 5.19 ± 2.88 | 7 |
TABLE 2. Norepinephrine extracellular levels AUC.
Two-way analysis of variance on the complete AUC data revealed a single main effect of study condition, F (2, 31) = 32.97, p < .01. Examination of the means suggested that pre-treatment with low dose naltrexone significantly reduced norepinephrine efflux independent of location. No other effects were noted for this measure most likely the result of the effects’ decay over time and the accumulated variance associated with this decay.
| NE (pg/15 μl sample) |
||||
|---|---|---|---|---|
| Bed Nucleus of the Stria Terminalis |
Frontal Cortex |
|||
| Treatment | Naltrexone | n | Naltrexone | n |
| Placebo/Water/Saline | 531.40±144.21 | 8 | 581.04±160.42 | 5 |
| Morphine/Water/Naltrexone | 1946.85±578.76 | 7 | 1400.83±396.42 | 5 |
| Morphine/Naltrexone Water/Naltrexone |
1447.91±267.38 | 6 | 1131.25±118.76 | 7 |
Acknowledgments
Supported by PHS DA15123 and DA15395 to EVB.
Abbreviations
- ABC
avidin–biotin complex solution
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- AUC
area under the curve
- BNST
bed nucleus of the stria terminalis
- BSA
bovine serum albumin
- cAMP
cyclic adenosine monophosphate
- CREB
cAMP-response element-binding protein
- DAB
3,3-diaminobenzidine tetrahydrochloride
- DβH
dopamine-beta-hydroxylase
- ED
electrochemical detection
- EDTA
ethylenediamine tetraacetic acid
- FC
frontal cortex
- HPLC
high performance liquid chromatography
- LC
locus coeruleus
- NTS
nucleus tractus solitarius
- PB
phosphate buffer
- PKA
protein kinase
- TS
Tris–saline buffer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Kogan JH, Moghaddam B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 1994;636:126–130. doi: 10.1016/0006-8993(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G. Behavioral functions of locus coeruleus derived from cellular attributes. Physiol Psychol. 1985;13:118–126. [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Hirata H, Akaoka H. Local opiate withdrawal in locus coeruleus in vivo. Brain Res. 1997;765:331–336. doi: 10.1016/s0006-8993(97)00682-3. [DOI] [PubMed] [Google Scholar]
- Beckmann AM, Matsumoto I, Wilce PA. Immediate early gene expression during morphine withdrawal. Neuropharmacology. 1995;34:1183–1189. doi: 10.1016/0028-3908(95)00089-o. [DOI] [PubMed] [Google Scholar]
- Chieng B, Keay KA, Christie MJ. Increased fos-like immunoreactivity in the periaqueductal gray of anaesthetised rats during opiate withdrawal. Neurosci Lett. 1995;183:79–82. doi: 10.1016/0304-3940(94)11119-4. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci U S A. 1995;92:10540–10544. doi: 10.1073/pnas.92.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Laverty R, Roth R. Clonidine reversal of increased noradrenaline metabolite levels during morphine withdrawal. European Journal of Pharmacology. 1979;57:247–250. doi: 10.1016/0014-2999(79)90372-8. [DOI] [PubMed] [Google Scholar]
- Delfs J, Zhu Y, Druhan J, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Dossin O, Hanoun N, Zajac JM. Involvement of locus coeruleus projections in opiate withdrawal but not in opiate tolerance in mice. Eur J Pharmacol. 1996;308:271–274. doi: 10.1016/0014-2999(96)00318-4. [DOI] [PubMed] [Google Scholar]
- Duman RS, Tallman JF, Nestler EJ. Acute and chronic opiate-regulation of adenylate cyclase in brain: specific effects in locus coeruleus. J Pharmacol Exp Ther. 1988;246:1033–1039. [PubMed] [Google Scholar]
- Dunn JD, Williams TJ. Cardiovascular responses to electrical stimulation of the bed nucleus of the stria terminalis. J Comp Neurol. 1995;352:227–234. doi: 10.1002/cne.903520206. [DOI] [PubMed] [Google Scholar]
- Fuentealba JA, Forray MI, Gysling K. Chronic morphine treatment and withdrawal increase extracellular levels of norepinephrine in the rat bed nucleus of the stria terminalis. J Neurochem. 2000;75:741–748. doi: 10.1046/j.1471-4159.2000.0750741.x. [DOI] [PubMed] [Google Scholar]
- Funada M, Suzuki T, Sugano Y, Tsubai M, Misawa M, Ueda H, Misu Y. Role of beta-adrenoceptors in the expression of morphine withdrawal signs. Life Sci. 1994;54:L113–118. doi: 10.1016/0024-3205(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Georges F, Stinus L, Le Moine C. Mapping of c-fos gene expression in the brain during morphine dependence and precipitated withdrawal, and phenotypic identification of the striatal neurons involved. Eur J Neurosci. 2000;12:4475–4486. doi: 10.1046/j.0953-816x.2000.01334.x. [DOI] [PubMed] [Google Scholar]
- Gold LH, Stinus L, Inturrisi CE, Koob GF. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. Eur J Pharmacol. 1994;253:45–51. doi: 10.1016/0014-2999(94)90755-2. [DOI] [PubMed] [Google Scholar]
- Gold MS, Pottash AC, Extein IL, Kleber HD. Neuroanatomical sites of action of clonidine in opiate withdrawal: the locus coeruleus connection. Prog Clin Biol Res. 1981;71:285–298. [PubMed] [Google Scholar]
- Grant SJ, Huang YH, Redmond DE., Jr Behavior of monkeys during opiate withdrawal and locus coeruleus stimulation. Pharmacol Biochem Behav. 1988;30:13–19. doi: 10.1016/0091-3057(88)90419-4. [DOI] [PubMed] [Google Scholar]
- Grasing K, Bills D, Ghosh S, Schlussman SD, Patel AH, Woodward JJ. Opiate modulation of striatal dopamine and hippocampal norepinephrine release following morphine withdrawal. Neurochem Res. 1997;22:239–248. doi: 10.1023/a:1022474318541. [DOI] [PubMed] [Google Scholar]
- Guitart X, Kogan JH, Berhow M, Terwilliger RZ, Aghajanian GK, Nestler EJ. Lewis and Fischer rat strains display differences in biochemical, electrophysiological and behavioral parameters: studies in the nucleus accumbens and locus coeruleus of drug naive and morphine-treated animals. Brain Res. 1993;611:7–17. doi: 10.1016/0006-8993(93)91770-s. [DOI] [PubMed] [Google Scholar]
- Guitart X, Nestler EJ. Second messenger and protein phosphorylation mechanisms underlying opiate addiction: studies in the rat locus coeruleus. Neurochem Res. 1993;18:5–13. doi: 10.1007/BF00966918. [DOI] [PubMed] [Google Scholar]
- Guitart X, Thompson MA, Mirante CK, Greenberg ME, Nestler EJ. Regulation of cyclic AMP response element-binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J Neurochem. 1992;58:1168–1171. doi: 10.1111/j.1471-4159.1992.tb09377.x. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Duman RS, Nestler EJ. Induction of the c-fos proto-oncogene during opiate withdrawal in the locus coeruleus and other regions of rat brain. Brain Res. 1990;525:256–266. doi: 10.1016/0006-8993(90)90872-9. [DOI] [PubMed] [Google Scholar]
- Javelle N, Renaud B, Lambas-Senas L. Monoamine metabolism in the locus coeruleus measured concurrently with behavior during opiate withdrawal: an in vivo microdialysis study in freely moving rats. J Neurochem. 1997;68:683–690. doi: 10.1046/j.1471-4159.1997.68020683.x. [DOI] [PubMed] [Google Scholar]
- Kantak K, Miczek K. Social. motor, and autonomic signs of morphine withdrawal: differential sensitivities to catecholaminergic drugs in mice. Psychopharmacology. 1988;96:468–476. doi: 10.1007/BF02180026. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Aghajanian GK. Long-term glutamate desensitization in locus coeruleus neurons and its role in opiate withdrawal. Brain Res. 1995;689:111–121. doi: 10.1016/0006-8993(95)00545-2. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Nestler EJ, Aghajanian GK. Elevated basal firing rates and enhanced responses to 8-Br-cAMP in locus coeruleus neurons in brain slices from opiate-dependent rats. Eur J Pharmacol. 1992;211:47–53. doi: 10.1016/0014-2999(92)90261-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Arimura A. Synaptic interaction between galanin immunoreactive neurons and axon terminals immunopositive for VIP and PACAP in the bed nucleus of the stria terminalis in the rat. Ann N Y Acad Sci. 2000;921:327–332. doi: 10.1111/j.1749-6632.2000.tb06987.x. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Compere S, Nestler EJ, Rasmussen K. Nimodipine reduction of naltrexone-precipitated locus coeruleus activation and abstinence behavior in morphine-dependent rats. Physiol Behav. 1996;59:863–866. doi: 10.1016/0031-9384(95)02206-6. [DOI] [PubMed] [Google Scholar]
- Kurland AA, McCabe L. Rapid detoxification of the narcotic addict with naloxone hydrochloride. A preliminary report. J Clin Pharmacol. 1976;16:66–74. doi: 10.1002/j.1552-4604.1976.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Koob GF. Destruction of the locus coeruleus decreases physical signs of opiate withdrawal. Brain Res. 1993;605:128–138. doi: 10.1016/0006-8993(93)91364-x. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Garbay C, Roques BP. Protein kinases in the locus coeruleus and periaqueductal gray matter are involved in the expression of opiate withdrawal. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:565–575. doi: 10.1007/BF00169392. [DOI] [PubMed] [Google Scholar]
- Mannelli P, De Risio S, Pozzi G, Janiri L, De Giacomo M. Serendipitous rapid detoxification from opiates: the importance of time-dependent processes. Addiction. 1999;94:589–591. doi: 10.1046/j.1360-0443.1999.94458913.x. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Gottheil E, Peoples JF, Oropeza VC, Van Bockstaele EJ. Chronic very low dose naltrexone administration attenuates opioid withdrawal expression. Biol Psychiatry. 2004;56:261–268. doi: 10.1016/j.biopsych.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: studies in the rat locus coeruleus. Brain Res Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Erdos JJ, Terwilliger R, Duman RS, Tallman JF. Regulation of G proteins by chronic morphine in the rat locus coeruleus. Brain Res. 1989;476:230–239. doi: 10.1016/0006-8993(89)91243-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- Rasmussen K. The role of the locus coeruleus and N-methyl-D-aspartic acid (NMDA) and AMPA receptors in opiate withdrawal. Neuropsychopharmacology. 1995;13:295–300. doi: 10.1016/0893-133X(95)00082-O. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10:2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Kendrick WT, Kogan JH, Aghajanian GK. A selective AMPA antagonist, LY293558, suppresses morphine withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropsychopharmacology. 1996;15:497–505. doi: 10.1016/S0893-133X(96)00094-2. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Cortical noradrenaline, attention and arousal. Psychol Med. 1984;14:13–21. doi: 10.1017/s0033291700003032. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Ultra-low doses of naltrexone or etorphine increase morphine’s antinociceptive potency and attenuate tolerance/dependence in mice. Brain Res. 1997;757:176–190. doi: 10.1016/s0006-8993(97)00197-2. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Norton FE, Guyenet PG. Autonomic areas of rat brain exhibit increased Fos-like immunoreactivity during opiate withdrawal in rats. Brain Res. 1993;624:19–28. doi: 10.1016/0006-8993(93)90055-r. [DOI] [PubMed] [Google Scholar]
- Swann AC, Elsworth JD, Charney DS, Jablons DM, Roth RH, Redmond DE, Jr, Maas JW. Brain catecholamine metabolites and behavior in morphine withdrawal. Eur J Pharmacol. 1982;86:167–175. doi: 10.1016/0014-2999(82)90314-4. [DOI] [PubMed] [Google Scholar]
- Ueda H, Miyamae T, Fukushima N, Misu Y. Protein kinase inhibitor potentiates opioid delta-receptor currents in Xenopus oocytes. Neuroreport. 1994;5:1985–1988. doi: 10.1097/00001756-199410000-00037. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Anton B, To T, Lam H, Evans CJ. Quantitative immunolocalization of mu opiod receptors: regulation by naltrexone. Neuroscience. 1998;85:897–905. doi: 10.1016/s0306-4522(97)00659-3. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele RC, Mannelli P, Oropeza V, Qian Y. Elevated mu-opioid receptor expression in the nucleus of the solitary tract accompanies attenuated withdrawal signs after chronic low dose naltrexone in opiate-dependent rats. J Neurosci Res. 2006;83:508–514. doi: 10.1002/jnr.20738. [DOI] [PubMed] [Google Scholar]
- Zimmerman DM, Leander JD. Opioid antagonists: structure activity relationships. NIDA Res Monogr. 1990;96:50–60. [PubMed] [Google Scholar]