Abstract
Background
Efforts to characterize changes in social functioning in frontotemporal dementia (FTD) have failed to elicit clear dissociation between frontal and temporal variants of the disease based on behavioral measures.
Methods
This study obtained premorbid and current first-degree relative ratings using an established measure of interpersonal functioning, the Interpersonal Adjectives Scales, to measure personality change in 16 patients with frontal variant (FLV) and 13 with temporal variant (TLV) FTD, and in a control group of 16 patients with AD.
Results
All three groups showed significant change over time in multiple domains, including increased introversion (FG) and submissiveness (HI). However, patients with both FTD subtypes evidenced significantly greater increases in overall interpersonal pathology vector length [VL] than did patients with AD, who remained within the normal range on all scores. Patients with FLV showed a 2 SD increase in submissiveness (HI), but their cold-heartedness (DE) change scores were not significantly different from those of patients with AD. Conversely, the TLV cold-heartedness (DE) score increased 2 SD compared to minimal change for the AD and FLV groups, yet change in submissiveness (HI) did not differentiate between AD and TLV groups.
Conclusions
The Interpersonal Adjectives Scales differentiated both FTD groups from patients with AD on the basis of both degree and direction of personality change. Also, the two subtypes of FTD showed distinctly different patterns of change in social functioning: patients with temporal variant shifted toward severe interpersonal coldness with mild loss of dominance, whereas patients with frontal variant showed the opposite pattern.
Diagnostic criteria for frontotemporal dementia (FTD) agree that alterations in personality and social conduct are a central clinical feature of the disease.1,2 However, quantitative instruments have not been used to systematically measure these changes. Similarly, specific social deficits in the FTD syndrome have not been isolated to either the frontal or temporal variants of the disease,3 nor has the neuroanatomy of these deficits been satisfactorily elucidated.
One source of confusion may be that to date, clinicians and researchers have focused on discrete behavioral changes,3–5 but have not had the tools to observe patients’ social processing on a higher level. Personality becomes fixed by early adulthood and remains constant throughout old age with only gradual fluctuation during the course of maturation.6–8 Thus, significant changes in personality during adulthood typically have a neuropathologic etiology.9 Because the primary brain areas affected in FTD are the frontal lobes, the anterior temporal lobes, and the amygdala, the observed personality changes ostensibly arise from a disruption of these structures’ particular contribution to higher social functioning. Early in the disease, social functioning remains preserved in AD, most likely because of the relative sparing of anterior structures.10–12 Correlation of personality change with areas of atrophy should elucidate important brain–behavior relationships.
The primary goal of this study was to objectively measure and compare the personality changes seen in patients with frontal lobe variant (FLV) FTD and temporal lobe variant (TLV) FTD with those in a control group of patients with AD. We hypothesized that patients with FTD would show profound changes in personality, whereas those with AD would show only minor changes. We also predicted that the three groups would also show divergent patterns of change. Specifically, we expected that patients with FLV would show greater loss of social dominance than patients with TLV owing to the greater apathy in patients with FLV, whereas patients with TLV would show greater coldness and lack of empathy because of greater amygdala damage.13
Methods
Subjects
Forty-five patients were recruited through the Memory and Aging Center at the University of California San Francisco. Patients seen at this dementia clinic represented a broad sample of the population in terms of ethnicity, sex, education level, and socioeconomic status, and an attempt was made to recruit all available patients for this study. Patient diagnosis was derived by a multidisciplinary team of neurologists, neuropsychologists, psychiatrists, and nurses, who performed extensive behavioral, neuropsychological, and neuroimaging assessments of these patients. Twenty-nine of the patients were diagnosed with FTD according to the Lund-Manchester criteria.1 All patients with FTD were subtyped into FLV and TLV groups by consensus review of patient MRI scans to determine whether the patient had predominantly frontal or anterior temporal pathology. Probable AD was diagnosed in 16 patients using the National Institute of Neurologic and Communicative Diseases and Stroke—Alzheimer’s Disease and Related Disorders Association criteria.14 MRI scans of patients with FTD and AD were done to rule out dementia due to cerebrovascular disease.
Personality data were obtained on 16 patients diagnosed with frontal variant FTD, 13 patients diagnosed with temporal variant FTD, and 16 patients with AD (table). The FTD groups were significantly younger than the AD group. Groups did not differ significantly on educational level, ethnicity, or informant relationship. Because the groups were also unbalanced by patient sex, all between-group analyses for this study were performed statistically controlling for the effects of sex and age.
Table.
Within-group change in average IAS T-score* over time
| AD |
Frontal variant |
Temporal variant |
||||
|---|---|---|---|---|---|---|
| IAS facet score | Premorbid | Current | Premorbid | Current | Premorbid | Current |
| PA—Assured/dominant | 51.87 | 38.19‡ | 50.94 | 31.25‡ | 51.77 | 45.85 |
| BC—Arrogant/calculating | 42.75 | 40.75 | 47.31 | 42.00 | 43.31 | 45.38 |
| DE—Cold-hearted | 49.19 | 51.25 | 52.25 | 62.13 | 50.85 | 68.77‡ |
| FG—Aloof/introverted | 46.06 | 53.81‡ | 45.00 | 67.38§ | 45.92 | 64.69‡ |
| HI—Unassured/submissive | 42.94 | 53.50‡ | 40.19 | 61.50§ | 44.62 | 55.23† |
| JK—Unassuming/ingenuous | 57.56 | 59.69 | 54.31 | 59.81 | 58.62 | 51.69† |
| LM—Warm/agreeable | 48.44 | 44.50 | 43.44 | 32.81 | 50.92 | 30.92§ |
| NO—Gregarious/extraverted | 54.94 | 46.06‡ | 56.31 | 32.00§ | 54.62 | 29.23§ |
| Vector length (intensity) | 57.75 | 59.81 | 53.44 | 74.06§ | 61.15 | 80.38† |
| Age, y, mean ± SD | 77.63 ± 9.60 | 63.13 ± 14.63 | 65.50 ± 16.54 | |||
| Female/male | 10/6 | 4/12 | 3/10 | |||
| Education, y, mean ± SD | 14.33 ± 4.35 | 14.63 ± 2.60 | 16.54 ± 3.73 | |||
Bold type signifies score > 1.5 SD from the mean.
Mean = 50, SD = 10.
Significant change at p < 0.05,
p < 0.01,
p < 0.001.
Procedures
Patients were identified from the clinic subject pool by diagnosis and then were recruited as potential study participants. These subjects and their caregivers signed a institutional review board–approved research consent form including an agreement to fill out questionnaires for research purposes.
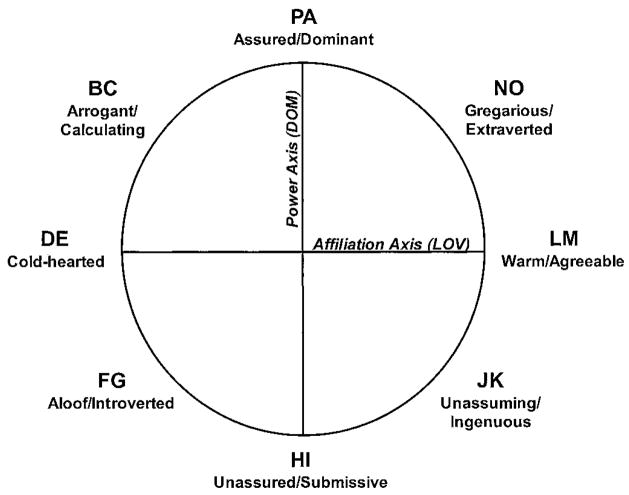
The Interpersonal Adjectives Scale (IAS)15 is a self- or other-report questionnaire based on the circumplex model of personality, a personality theory based on interpersonal constructs16 (figure 1). This theory posits that all social interaction falls along two orthogonal axes: power, or dominance (DOM), and love, or affiliation (LOV). Social interactions, and by extension, individual personality styles, can be mapped onto the grid along these two axes. The IAS yields an efficient, valid, and theoretically sound evaluation of two orthogonal personality constructs (dominance and affiliation) and provides information about how these two primary axes work together to create an overall personality. Summary scores are generated to represent the average vector of the overall profile (angular location [AL] as well as the intensity with which the overall profile is expressed (vector length [VL]). Also, the IAS provides information about changes in the profile compared to normative samples. This scale was constructed to be sensitive to how small shifts in the underlying constructs impact the patient’s overall social environment.
Figure 1.
The Interpersonal Adjectives Scales Interpersonal Circumplex model15 depicts interpersonal functioning as eight facet scores derived from two main axes: power and affiliation.
Caregivers were asked to fill out each personality questionnaire twice, first describing the patient’s current characteristics, and then describing how he or she was before the onset of the disease. Raters were selected on a case-by-case basis with consideration given to the informant’s frequency of contact with the patient, their described level of closeness, the rater’s own cognitive capacity (e.g., in the case of an aging spouse), and willingness to participate. Spouses were used whenever possible (73%), an adult son or daughter if no spouse was available (24%), and in one case, a sibling caregiver was used as an informant. Personality assessment by first-degree relatives of patients with dementia has been demonstrated to have very good inter-rater reliability,10,17 and informant ratings using the IAS in particular have excellent internal and temporal reliability.18 Questionnaires were scored using the IAS computer scoring program,19 which generates T-scores by comparing patient scores to the normative sample data set collected by the IAS developers.15 Change scores were calculated by comparing current and retrospective caregiver ratings.
Results
Change over time
In order to test the hypothesis that each group would undergo significant change in personality after diagnosis of dementia, the “before” and “current” scores were analyzed for each group separately. Repeated-measures analyses of variance were used to determine if significant change over time had occurred on any of the eight facet scores or on the four summary scores (DOM, LOV, VL, AL). Both raw scores and T-scores were analyzed, and no covariates were used because comparisons were within group.
All three groups showed significant changes in many of the eight facet scores and the four summary scores (see the table). All patients showed significant increases in introversion (p < 0.01) and decreases in extraversion (p < 0.01). Patients with TLV had significantly increased cold-heartedness (DE) (p < 0.01), decreased warmth (LM) (p < 0.001), and decreased ingenuousness (p < 0.05). Both the FLV and AD groups showed significant decreases in assuredness (PA) (p < 0.01) not seen in the patients with TLV, although both FTD subtypes showed increased submissive behaviors (HI) (p < 0.01).
Analyses of covariance (ANCOVA) (controlling for sex and age) were then performed to compare overall degree of change for pairs of groups. Both FTD subtypes showed significantly greater increases in overall personality pathology (VL) than the AD group (change in T-scores: FLV +20.63 ± 18.76, TLV +19.23 ± 28.93, AD +2.06 ± 12.88; p < 0.05). The FTD subtypes’ current VL T-scores were in the pathologic range (FLV 74.06, TLV 80.38), whereas the AD overall pathology scores (59.81) were less than 1.5 SD from the mean, suggesting they were in the normal range. Although patients with AD showed significant change in some of the same areas as the patients with FTD, their current scores were all within 1.5 SD from the mean (current T-scores ranged from 38.19 to 59.81), whereas the FTD groups’ current scores were up to 3 SD from normal (T-scores ranged from 29.23 to 80.38). Thus, in accordance with hypothesis 1, patients with FTD showed a much greater degree of change than patients with AD (figure 2).
Figure 2.
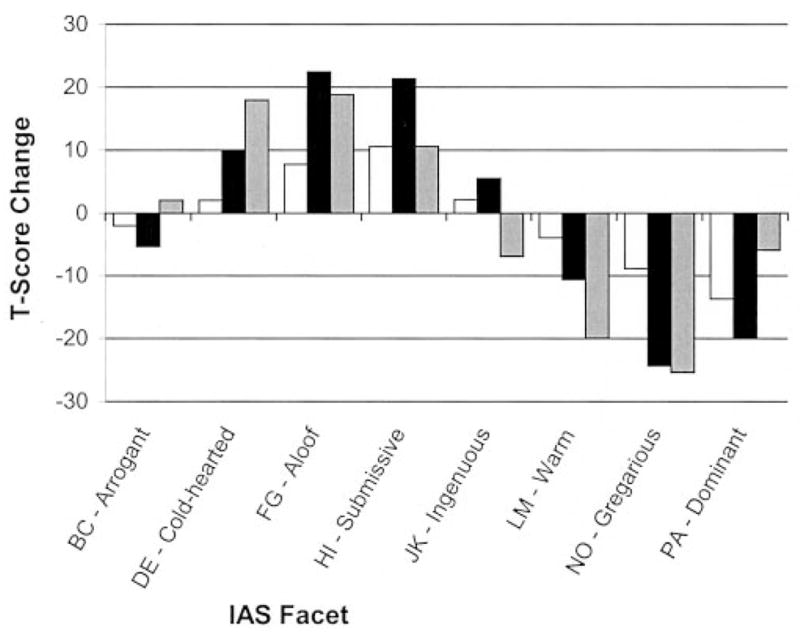
Divergent patterns were found among frontal variant frontotemporal dementia (FLV) (black bars), temporal variant frontotemporal dementia (TLV) (gray bars), and AD (white bars) groups on Interpersonal Adjectives Scales (IAS) facet T-scores measuring change from pre-morbid to current interpersonal style.
Different patterns of change among the three groups
No premorbid group differences were found on any of the personality subscales, suggesting that the retrospective ratings were not significantly biased by the patients’ current diagnosis, and the baselines were equivalent among groups. Only change scores were analyzed because change in personality (rather than absolute current personality) is the variable that theoretically represents pathology in this population. T-scores were analyzed instead of raw scores because they contained additional information based on the way the patients compared to a normative sample. Because the IAS generates a large number of intercorrelated variables, group differences were explored utilizing multivariate statistics. The dataset was reduced by running univariate ANCOVA (controlling for sex and age) to determine which variables significantly predicted group. Then, discriminant function analyses were performed using these significant variables to determine which variables were best able to predict disease group. Although discriminant function analysis works under the assumption that no variable may be a linear combination of the other discriminating variables, summary scores were included because they were analyzed in conjunction with only a subset of the facet scores. Thus, summary scores also contained additional unique information derived from variables that were not included in the discriminant function. Because a Box M test for the equality of population covariances was significant, individual group covariance matrices were used instead of the pooled variances to compute the probability of group membership for all discriminant functions. Violation of this assumption can decrease a discriminant function’s capacity to provide maximum separation among the groups; thus, it is likely that the percentage of correct classifications is actually an underestimate of the true discriminating power of the variables in these analyses.
All three groups were analyzed together in one discriminant function analysis. The best discriminability was obtained using a function of five variables that had been found to significantly predict group via omnibus ANCOVA analysis. The standardized canonical function coefficients for the first function (Wilks lambda = 0.556, p < 0.01) were, in order of influence, gregarious/extraverted (NO) (1.414), DOM (−1.200), AL (0.628), HI (−0.318), and PA (0.122), and the second function’s coefficients were DOM (−1.467), PA (1.097), AL (0.411), NO (0.079), and HI (−0.009) (Wilks lambda = 0.771, p < 0.05). Together, these two functions correctly categorized 72.9% of the patients into their diagnostic groups, showing a clear improvement over the 33% that would be expected at chance levels.
To better characterize the results of this omnibus discriminant function, additional discriminant functions were then derived to compare each pair of groups. When patients with TLV were compared to the AD controls, 93.8% of the 29 patients were correctly classified by a single function (Wilks lambda = 0.577, p < 0.01) made up of the following variables: LOV (3.983), LM (−1.926), NO (−1.869), DE (1.736), and unassuming/ingenuous (JK) (−0.800). The TLV level of warmth/agreeableness (LM) decreased 2 SD (20.00 ± 14.73) compared to a very small (3.89 ± 12.06) change in the patients with AD. Similarly, the TLV cold-heartedness (DE) score increased 2 SD (T-score change: +17.92 ± 20.52) compared to a much smaller change for patients with AD (+2.05 ± 12.90). Degree of change in submissiveness (HI) did not contribute significantly to discriminating TLV from AD. Conversely, the FLV group could be discriminated from AD controls through a function that correctly categorized 80.0% of the 32 patients (Wilks lambda = 0.584, p < 0.01; standardized canonical coefficients: HI [1.143], DOM [0.755], LOV [−0.641], VLT [−0.582], and arrogant/calculating [BC] [−0.571]), but in this case, cold-heartedness (DE) change scores did not contribute significantly to group discrimination. Unlike TLV, the FLV group showed a 2 SD increase in submissiveness (HI) (T-score change: +21.31 ± 12.59) compared to a much smaller change in the patients with AD (+9.58 ± 11.62). When patients with TLV were directly compared to patients with FLV, 79.3% of the patients could be correctly categorized with a single discriminant function (Wilks lambda = 0.667, p < 0.05) made up of four variables: JK (0.996), AL (0.872), DOM (0.797), and HI (0.361). The strongest discriminating variable was the unassuming/ingenuous facet, which showed an increase in the patients with FLV (5.50 ± 14.44) but decreased in the patients with TLV (−6.92 ± 10.38). Patients with TLV also showed half of the decrease in submissiveness (HI) that was seen in patients with FLV.
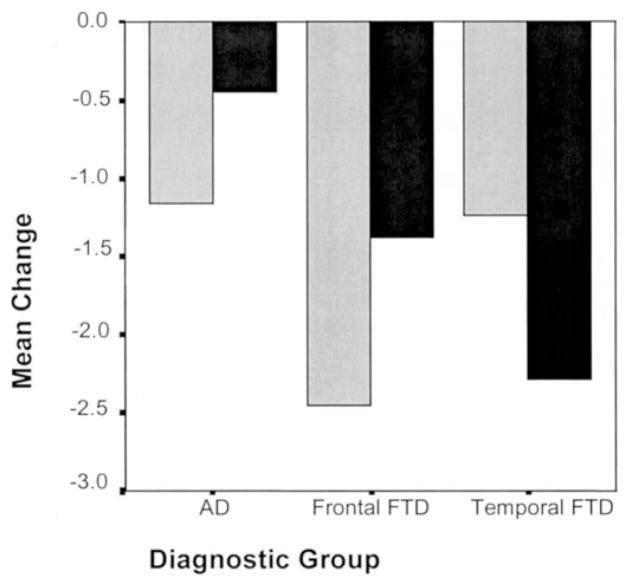
To further investigate the nature of these significant group differences, the change scores for the summary variables DOM and LOV for all three groups were used in a multivariate ANCOVA (controlling for sex and age) to calculate each group’s change in overall interpersonal style. A group by style interaction was found (F[2,43] = 3.69, p < 0.05) (figure 3). The FLV group had almost double the drop in DOM score (−2.45 ± 1.8) than was seen in either patients with AD (−1.16 ± 1.34) or patients with TLV (−1.23 ± 1.09). Conversely, whereas patients with FLV showed almost three times as large a drop in LOV score (−1.38 ± 2.03) as patients with AD (−0.44 ± 1.16), TLV patients’ LOV scores (−2.28 ± 1.89) dropped five times lower than the scores of the patients with AD. Thus, patients with TLV and FLV showed opposite patterns, with patients with FLV losing more overall dominance than affiliation, but patients with TLV losing more affiliation than dominance.
Figure 3.
When Interpersonal Adjectives Scales (IAS) summary scores were used to examine overall personality style, patients with AD, frontal variant frontotemporal dementia (FLV), and temporal variant frontotemporal dementia (TLV) evidenced a significant interaction (p < 0.05). Patients with TLV showed a greater drop in affiliation (black bars) than in social dominance (gray bars), whereas patients with FLV showed the opposite pattern. Both frontotemporal dementia subtypes showed a greater overall degree of change than did AD.
Discussion
The important findings from this study were that 1) the IAS differentiated both FTD groups from patients with AD on the basis of both degree and direction of personality change, and 2) the two subtypes of FTD showed distinctly different patterns of personality change, with patients with TLV evidencing a dramatic shift toward interpersonal coldness with only a small loss of dominance, whereas patients with FLV showed the opposite pattern.
The IAS allows derivation of T-scores based on a large sample of normative data collected by the instrument’s developers, which provided information about the average degree of pathology for each group. The current scores of both FTD subtypes were in the pathologic range, with overall personality pathology scores (VL) 2.5 to 3 SD higher than average, whereas the level of overall interpersonal pathology for patients with AD was within normal limits. This VL (vector length) score on the IAS was designed to represent the degree to which any personality style is rigidly, dysfunctionally maintained. Thus, regardless of personality traits, patients with FTD approach interpersonal transactions in an exaggerated, inflexible manner, whereas patients with AD maintain a slightly decreased but still normal capacity to adapt flexibly to interpersonal demands. The FLV subtype could be differentiated from the AD group on the basis of degree of change, because both groups showed significantly increased introversion, as well as decreased extraversion and assuredness. The TLV group could be differentiated from the AD group not only by degree of change (patients with TLV became much more introverted), but also by the direction of their personality change. Patients with TLV did not show the significantly decreased assuredness seen in patients with AD, but had increased “cold-hearted” behaviors as well as decreased warmth, agreeableness, and ingenuousness.
In addition to distinguishing FTD from AD, the IAS elicited a double dissociation between the patterns of dominance and affiliation seen in FLV and TLV. Patients with FLV showed extreme loss of social dominance but only mild to moderate loss of nurturance and affiliation compared to controls. Patients with TLV showed the reverse pattern. The mean rating on the cold-heartedness (DE) subscale for the patients with TLV was higher than that of 99% of patients in the normative sample, whereas the patients with FLV did not show a significant change from premorbid rating for either cold-heartedness or warmth/agreeableness. Conversely, the FLV group’s mean scores dropped below the second percentile on the assured-dominant subscale, whereas the TLV group did not show a significant drop. The single variable that most effectively discriminated the two subtypes was the unassuming/ingenuous (JK) facet, because the frontal variant patients tended to become more docile and compliant, whereas the temporal patients became less so.
This clear dissociation has several implications. First, direction of personality change may have differential diagnostic utility toward distinguishing subtypes of FTD, particularly if used in conjunction with standard cognitive and neuroimaging assessment. Second, it provides insight into the distinct contributions of the frontal and anterior temporal lobes to social functioning. Social dominance appears to be mediated by frontal areas typically affected in the frontal variant of FTD, but can remain intact with damage to anterior temporal lobe structures, including the amygdala. This loss of social dominance may be partly related to apathy, a behavioral characteristic linked to the anterior cingulate, a structure that shows greater atrophy in FLV than in TLV.13 Also, our data suggest that anterior temporal lobe structures contribute uniquely to the capacity for social affiliation, nurturance, and empathy. Given that medial orbitofrontal cortex (OFC) damage occurs early in both the frontal and temporal variants of FTD,3,13 this double dissociation between dominance and affiliation suggests that both of these social functions are at least partly mediated by brain structures other than the OFC.
The loss of social affiliation and nurturance seen in TLV may be partly explained by the extensive amygdala damage that occurs early in the course of temporal-variant FTD.13 Recent work with patients with TLV suggests that they have significant emotional processing deficits, particularly with amygdala damage on the non-language-dominant side of the brain.20 Beyond recognizing emotions in others, the amygdala may also help one to recognize the emotional significance of another person’s actions.21–23 Also, PET studies have found activations in the anterior temporal lobes for tasks requiring subjects to invest sensory information with emotional significance.24
Both FTD groups had dramatic increases in introversion compared to patients with AD. Loss of social dominance seemed to intensify introversion in the FLV group (i.e., patients are highly submissive and passive, and thus do not initiate social interaction), whereas the introversion of the patients with TLV seemed to be driven by the loss of interpersonal affiliation and attachment (i.e., they no longer derive positive reinforcement from human contact, and thus may approach social interactions in a neutral or negative stance, or may avoid them altogether).
The particular pattern observed with these patients with FTD echoes what was seen in an early, seminal experiment in which monkeys’ social behaviors were observed after their frontal or anterior temporal cortex was ablated.25 Although both groups of monkeys evidenced profound introversion, monkeys with frontal cortex ablations would not initiate social contact but would passively allow their infants to nurse, whereas monkeys with anterior temporal lobe (AT) ablations forcibly rejected their infants. In addition, some AT group monkeys were capable of spontaneously and appropriately asserting their social position with adult monkeys, even to the extent of utilizing aggressive behaviors, whereas the frontal group monkeys were profoundly submissive and never showed this capacity for social dominance after their surgery.
Owing to the small sample size and the fact that only a subset of the patients with FTD had an entirely unilateral presentation, this study grouped all patients with FLV and all patients with TLV together, regardless of whether the disease was more severe on the right or left hemisphere. The preponderance of data suggests that many higher social functions, including sense of self,26 humor,27 accurate perception of emotional material,28 and the capacity for avoiding socially inappropriate behavior,29,30 are mediated by the right hemisphere. Studies comparing right vs left temporal variants of FTD also suggest that within the subtypes, social and emotional deficits are significantly more severe with predominantly right-sided disease.3,20 If patients with primarily left-sided disease do show less behavioral pathology, then our choice to mix left and right hemisphere patients together in the FLV and TLV groups probably increased the within-group variability in this study. This in turn may have diminished group cohesion at the centroids of the discriminant function, which would explain why the population covariances were unequal. The effect on the univariate analyses would be that the left-hemisphere patients balanced out the more extreme change in the right-sided patients, thereby decreasing the groups’ mean personality change. The fact that our hypotheses were confirmed and significant predictions of group membership could still be derived via discriminant function analysis attests to how robust these effects actually are. Thus, the next logical step in characterizing interpersonal changes in FTD would be to quantify how each variant differs by hemisphere.
Because normal personality is inherently stable but diverse, comparison to an objective baseline measurement is necessary for accurate detection of pathology. The most informative data will be derived from observing change from premorbid personality, not merely by examining current scores. With greater numbers, it will become possible to further characterize patients’ personality change as a function of the timing of their disease progression. Future research in this area should also include investigation of how particular social deficits correlate with neuropsychological functioning and areas of pathology on brain scans.
References
- 1.Brun A, Englund B, Gustafson L, et al. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 3.Edwards-Lee T, Miller BL, Benson DF, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120:1027–1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- 4.Gregory CA, Hodges JR. Dementia of frontal type and the focal lobar atrophies. Int Rev Psychiatry. 1993;5:397–406. [Google Scholar]
- 5.Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2000;69:178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa PT, Jr, McCrae RR. Personality in adulthood: a six-year longitudinal study of self-reports and spouse ratings on the NEO Personality Inventory. J Pers Soc Psychol. 1988;54:853–863. doi: 10.1037//0022-3514.54.5.853. [DOI] [PubMed] [Google Scholar]
- 7.Costa PT, McCrae RR. Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychological Assessment. 1992;4:39–53. [Google Scholar]
- 8.Digman JM. Personality structure: emergence of the five-factor model. Annu Rev Psychol. 1990;41:417–440. [Google Scholar]
- 9.Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1986. [Google Scholar]
- 10.Strauss ME, Pasupathi M, Chatterjee A. Concordance between observers in descriptions of personality change in Alzheimer’s disease. Psychol Aging. 1993;8:475–480. doi: 10.1037//0882-7974.8.4.475. [DOI] [PubMed] [Google Scholar]
- 11.Siegler IC, Dawson DV, Welsh KA. Caregiver ratings of personality change in Alzheimer’s disease patients: a replication. Psychol Aging. 1994;9:464–466. doi: 10.1037//0882-7974.9.3.464. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee A, Strauss M, Smyth KA, Whitehouse PJ. Personality changes in Alzheimer’s disease. Arch Neurol. 1992;49:486–491. doi: 10.1001/archneur.1992.00530290070014. [DOI] [PubMed] [Google Scholar]
- 13.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Wiggins JS. Interpersonal Adjectives Scale. Professional manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 16.Wiggins JS, Trapnell P, Phillips N. Psychometric and geometric characteristics of the Revised Interpersonal Adjective Scales (IAS–R) Multivariate Behavioral Research. 1988;23:1–59. doi: 10.1207/s15327906mbr2304_8. [DOI] [PubMed] [Google Scholar]
- 17.Heinik J, Keren P, Vainer-Benaiah Z, Lahav D, Bleich A. Agreement between spouses and children in descriptions of personality change in Alzheimer’s disease. Isr J Psychiatry Relat Sci. 1999;36:88–94. [PubMed] [Google Scholar]
- 18.Kurtz JE, Lee PA, Sherker JL. Internal and temporal reliability estimates for informant ratings of personality using the NEO-PI-R and IAS. Assessment. 1999;6:103–113. doi: 10.1177/107319119900600201. [DOI] [PubMed] [Google Scholar]
- 19.Wiggins JS, Coutu J. Interpersonal Adjective Scales (IAS) scoring program [computer software] Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 20.Perry RJ, Rosen HR, Kramer JH, et al. Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7:145–160. doi: 10.1093/neucas/7.2.145. [DOI] [PubMed] [Google Scholar]
- 21.Damasio AR, Tranel D, Damasio H. Face agnosia and the neural substrates of memory. Annu Rev Neurosci. 1990;13:89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- 22.Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- 23.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 24.Reiman EM. The application of positron emission tomography to the study of normal and pathologic emotions. J Clin Psychiatry. 1997;58(suppl 16):4–12. [PubMed] [Google Scholar]
- 25.Franzen EA, Myers RE. Neural control of social behavior: prefrontal and anterior temporal cortex. Neuropsychologia. 1973;11:141–157. doi: 10.1016/0028-3932(73)90002-x. [DOI] [PubMed] [Google Scholar]
- 26.Miller BL, Seeley WW, Mychack P, et al. Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology. 2001;57:817–821. doi: 10.1212/wnl.57.5.817. [DOI] [PubMed] [Google Scholar]
- 27.Shammi P, Stuss DT. Humour appreciation: a role of the right frontal lobe. Brain. 1999;122:657–666. doi: 10.1093/brain/122.4.657. [DOI] [PubMed] [Google Scholar]
- 28.Borod JC, Cicero BA, Obler LK, et al. Right hemisphere emotional perception: evidence across multiple channels. Neuropsychology. 1998;12:446–458. doi: 10.1037//0894-4105.12.3.446. [DOI] [PubMed] [Google Scholar]
- 29.Blair RJR, Cipolotti L. Impaired social response reversal: a case of ‘acquired sociopathy’. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 30.Miller BL, Chang L, Mena I, Boone K, Lesser IM. Progressive right frontotemporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia. 1993;4:204–213. doi: 10.1159/000107324. [DOI] [PubMed] [Google Scholar]