Abstract
This paper uses data from the National Longitudinal Study of Adolescent Health to examine the extent to which school-level social and institutional factors moderate genetic tendencies to smoke cigarettes. Our analysis relies on a sub-sample of 1,198 sibling and twin pairs nested within 84 schools. We develop a multilevel modeling extension of regression-based quantitative genetic techniques to calculate school-specific heritability estimates. We show that smoking onset (h2 = .51) and daily smoking (h2 = .58) are both genetically influenced. Whereas the genetic influence on smoking onset is consistent across schools, we show that schools moderate the heritability of daily smoking. The heritability of daily smoking is the highest within schools in which the most popular students are also smokers and reduced within schools in which the majority of the students are non-Hispanic and white. These findings make important contributions to the literature on gene-environment interactions.
Keywords: Smoking, Twins, Schools, Gene-environment interaction
Introduction
Researchers tend to attribute the cause of cigarette smoking to either social or genetic factors. Although there have been calls to integrate environmental and biological explanations for complex health behaviors such as cigarette use (Hernandez and Blazer 2006; Swan et al. 2003; Finch et al. 2001), only a limited number of studies have successfully done so (Kendler et al. 2005; Timberlake et al. 2006). We address this important topic by highlighting normative and institutional aspects of adolescents’ schools that are hypothesized to restrict or enable genetic tendencies to smoke cigarettes. We focus on schools as the environmental background for our study because they denote well-defined social spaces and they serve as critical contexts in which adolescents are socialized regarding smoking (Ennett et al. 1997; Ellickson et al. 2003; Eitle and Eitle 2004). Further, larger social environments such as schools speak more directly to this literature and may be the most appropriate context in which to examine gene-environment interactions among adolescents (Perrin and Lee 2007; Shanahan and Hofer 2005).
The goals of this paper are two-fold. First, we aim to document the extent to which the genetic determinants of smoking vary in magnitude across schools. Second, we attempt to account for the variation in heritability estimates using theoretically informed factors that are hypothesized to influence the heritability of smoking. In doing so, we contextualize the risk factor (Link and Phelan 1995) of genetic vulnerability to smoking and highlight the environment as a fundamental cause of the genetic causes of smoking thereby integrating environmental and biological explanations for cigarette use. Our paper addresses several theoretical and empirical limitations in the existing gene-environment interaction literature that have been noted elsewhere (Perrin and Lee 2007).
Heritability of smoking
It is well known that genetic factors play an important role in the use of tobacco. In a series of highly publicized letters, Fisher (1958a, b) points to the genetic basis of regular cigarette use by comparing the concordance of smoking among identical and fraternal twin pairs. In two different studies, he shows that roughly 75% of identical twin pairs have the same smoking status compared to only 50% among fraternal twin pairs. Based on this information, Fisher states that:
There can be little doubt that the genotype exercises a considerable influence on smoking and on the particular habit of smoking adopted, and that a study of twins on a comparatively small scale is competent to demonstrate the rather considerable differences which must exist between the different groups who classify themselves as a non-smokers, or the different classes of smokers. (1958a, p. 12).
More recently, Slomkowski et al. (2005) estimate heritability of .44 for the frequency of smoking (days in the past month) among adolescents and Maes et al. (1999) use a relatively younger sample (ages 13–16) from the Virginia twin study of Adolescent Behavioral Development and estimate that heritability accounts for 60 and 65% of the variation in current and lifetime tobacco use, respectively. Similar results are reported using data from adults. Compared to fraternal adult male twin pairs, Carmelli et al. (1992) find a greater concordance in experimentation, current smoking, and cessation among identical twin pairs. Even more convincing are heritability estimates for regular tobacco use obtained from adult twins raised in the same home compared to cohort of adult twins raised apart (Kendler et al. 2000). These estimates (h2men = .51; h2women = .64) closely resemble the estimates obtained from meta-analytic reviews for the heritability of cigarette use (Li et al. 2003; Sullivan and Kendler 1999; Hall et al. 2002).
Researchers tend to calculate heritably estimates for total samples and few studies explicitly examine systematic variation across sub-populations. However, there are theoretical reasons to anticipate an interaction between genes and the environment which would lead to significant variation in heritability estimates across groups that have different norms about smoking and differential access to social and material resources (Shanahan and Hofer 2005). At least two studies have tested for gene-environment interactions related to cigarette consumption by comparing heritability estimates across discrete social groups. In the first study, Kendler et al. (2000) compare self-reports of lifetime regular tobacco use and calculate cohort-specific heritabilities for men and women separately among pairs born during 1910–1924, 1925–1939, or 1940–1958. They show that the estimate for men is consistent across birth cohorts (h2 ~ .55) but the estimate for women increased significantly across the periods. The heritability for the first cohort was zero but increased to 21%, and peaked at 64% in the third cohort. Thus by the third cohort, there were no gender differences in the heritability of regular tobacco use. The authors argue that the social restrictions on women’s tobacco use have relaxed significantly across these three cohorts thus enabling genetic tendencies to use tobacco to manifest.
Timberlake and colleagues (2006) use Add Health data to model the heritability of smoking by wave III of the study as a function of adolescents’ self-reported religiosity. Building on work that demonstrates virtually no heritability for alcohol use among those who are raised with a strong religious upbringing (Koopmans et al. 1999), they hypothesize that social pressures to avoid smoking among highly religious families will reduce the extent to which genes will influence smoking initiation. According to their results, the heritability of smoking initiation was roughly 80% among those with low levels of self-reported religiosity but less than 10% among those who self-identified as a religious person and reported that their religious faith was very important to them.
In these examples the normative environment is posited as a restrictive mechanism that places limits upon genetically oriented tendencies to smoke cigarettes. Within the language of gene-environment interactions, this pattern is predicted by the social control model (Shanahan and Hofer 2005) which hypothesizes that social norms constrain the choices available to individuals within a particular social context and thus prevent genetic expression. As further explained:
Thus, social control mechanisms reflect norms and other social forces that “canalize” (i.e., restrict variability in the phenotype of) genetically diverse people. As these canalization forces increase (i.e., norms are more effective and choices are minimal), genetic differences are of diminishing consequence. (p. 69).
The social control model does not differentiate between various levels at which social forces are hypothesized to operate. That is, the social forces may be the norms developed by individuals and groups (e.g., the students) but they may also be the rules, regulations, or laws created and enforced by the institutions in which these individuals interact with one another (e.g., the schools).
While this model anticipates a reduction in heritability due to normative pressures restricting the activities of individuals, it is also possible that normative pressures work to encourage behaviors that may not manifest otherwise (Perrin and Lee 2007). The likelihood that a particular individual will respond to normative pressures to engage in an unhealthy activity, rather than avoiding it, may also depend on their particular genetic makeup. Shanahan and Hofer (2005) refer to this model as a contextual trigger model because the social context is believed to be the underlying mechanism that gives rise to a genetic diathesis.
The most widely cited evidence for this perspective is the work of Caspi et al. (2003) who show that individuals with the short allele of the 5-HTT gene-linked polymorphic region were more likely to suffer from major depression compared to those that were heterozygous at this location or those with two long alleles. However, these effects were limited to those who had experienced a large number of stressful life events. That is, among those that had experienced four or more stressful life events, the homozygous risk allele carriers (SS) had an estimated probability of major depression of nearly .40 compared to only .15 among those with two long alleles at this location. However there was no difference by genotype among those who had not experienced a stressful life event. In this case they observed a non-significant main effect of genotype (b = −.15, P = .29), a significant main effect of stressful life events (b = .37, P < .001), and a marginally significant interaction between the two (b = −.19, P = .056). In this case, the stressful life events are posited as the trigger for genetic expression.
Schools and smoking
Given the critical timing of cigarette initiation during late adolescence (Breslau et al. 2001; Hu et al. 2006) in conjunction with findings suggesting that one-fifth of the variation in smoking among adolescents is between schools (Ennett et al. 1997), schools make a particularly useful level at which to examine gene-environment interactions for smoking behaviors. In this paper we review the literature that links school characteristics to the likelihood that a student will begin to smoke and we focus on four distinct aspects of schools: (a) smoking prevalence; (b) student norms; (c) school policies; and (d) social demographic composition.
Smoking prevalence
Ellickson et al. (2003) demonstrate how the proportion of students in an adolescent’s school who are current smokers uniquely contributes to their risk of smoking, even one year after school attendance. Their findings make a particularly strong case for the role of schools as shaping patterns of cigarette use because they adjust their estimates for the smoking prevalence among respondents’ friends. Eitle and Eitle (2004) show that school effects related to substance use also operate independently of larger levels of aggregation including the county-level social and demographic composition. By adjusting for proximate (peer group smoking) and distal (county-level composition) factors that may potentially confound the effects of schools, these two studies provide convincing evidence that schools have independent effects on the smoking behaviors of the students and that schools are not simply proxies for other social processes.
Both papers account for the school-effects using the epidemiological language of contagion. These models identify direct modeling of behaviors (imitation) and the reward systems that reinforce these behaviors as integral components of the social learning process (Jessor and Jessor 1997) and they posit that the prevalence of smoking within the school will not have a measurable impact on an individual’s risk of smoking until the level of smoking crosses some epidemic threshold; after which the effects will increase monotonically. That is, this model of behavioral imitation anticipates a quadratic relationship between smoking prevalence and the heritability of smoking. In this manner, high rates of smoking may serve as an important trigger for genetic expression.
Smoking norms
The use of smoking prevalence to measure the process of social learning is limited for at least two reasons. First, this explanation tends to focus on the transition from non-use to use rather than explicitly focusing on persistent non-use, per se. While some have used similar language to talk about non-smoking norms (Hill 1971) the direction of the prevalence model does not anticipate non-use as an expected behavior that is equally enforced or rewarded. Second, this approach tends to use the same language to talk about smoking prevalence and smoking norms but it is not clear that these are interchangeable (Alexander et al. 2001).
One way to assess student norms regarding smoking is to measure the popularity and smoking status of all students within a school (Alexander et al. 2001; Ennett and Bauman. 1993; Valente et al. 2005). This work shows that a student’s social connections and social positions within their school can influence their smoking behaviors and the behaviors of those around them. With respect to the spread of smoking within schools, it also suggests that the observed smoking prevalence may be less important than the status of those who are currently smoking. Peer influence is regularly cited as one of the most important factors responsible for the initiation of substance use (Engels et al. 2004) and more popular students, simply because they may have more social connections and thus social influence, may potentially shape both pro and anti-smoking norms for the larger school-community (Ennett and Bauman 1993).
Not only do more popular students have more influence upon their peers, but the attitudes, beliefs, and behaviors of the most popular students often reflect the norms of the existing community (Becker 1970). Thus, by assessing the smoking status of the most popular students, or by describing the association between popularity and smoking status within a school, it is possible to describe smoking norms as well as the extent to which smoking norms are enforced. Importantly, this orientation makes it possible to assess the entire range of smoking norms which includes pro-smoking environments, anti-smoking environments (Pokomy et al. 2004), as well as the majority of environments in which there might not be clear norms regarding smoking behaviors.
In this manner it is also possible to test different models related to the normative environment. That is, social norms may serve as a trigger (in cases in which there are clear pro-smoking norms), a control (in cases in which there are clear anti-smoking norms), or both (in which there are only normative pressures felt at both extremes). To date, little research has addressed the issues related to the functional form of normative environmental models of genetic moderation.
School policies
The explanations above focus on the smoking-related norms that are proscribed and enforced by the students within the school which may or may not be in line with the smoking norms of the larger community. That is, there are clear differences in the ways in which schools monitor and enforce policies regarding cigarette smoking on school grounds. Two studies show somewhat mixed results about the ability of school policies to affect smoking behaviors. Leatherdale et al. (2005) show that school rules allowing students to smoke on the periphery of the school grounds increases the risk of smoking initiation but only among those with friends who would disapprove of smoking. In other words, among those within networks who already approve of and reinforce smoking, the rules of the school seem to have little or no effect. Similarly, Kandel et al. (2004) use data from the Add Health study and predict the likelihood of smoking onset and daily smoking by wave II as a function of several school level factors. They show that smoking prevalence at the school-level strongly predicts the likelihood that a formerly non-smoking student will begin smoking by wave II but the school-level policies do not appear to have a significant impact on the risk of smoking.
It is possible that these rules may not have any average impact on smoking, but they may have a pronounced impact on those students with a genetic vulnerability to tobacco use. Therefore, we anticipate that school-rules regarding smoking are best characterized as a mechanism of social control and clearly enforced rules about non-smoking and the punishment of students for smoking will significantly reduce the genetic influence on smoking.
Social and demographic composition
Finally, there is a well-established literature that links the social and demographic characteristics of schools to the patterns of smoking within schools. For example, Johnson and Hoffmann (2000) show that, among black and Hispanic students, the risk of initiating regular cigarette use decreases as the proportion of racial and ethnic minorities in their schools increase. They argue that smoking differences among black and white students are compounded by the high levels of racial segregation in schools and that this independently contributes to differential norms about smoking. Kandel et al. (2004) also show a significant reduction in the risk of transitioning to daily smoking among those who have experimented with cigarettes as the proportion of minority students within their schools increase. Therefore, it is possible that the heritability of smoking will increase as the proportion of students who are non-Hispanic and white increases. This association, however, should not persist above and beyond school-level controls for smoking prevalence.
It is also possible that the racial composition of schools may capture organizational aspects that differentiate between schools that are not captured with smoking norms or smoking prevalence. That is, schools with a greater proportion of non-Hispanic white students may be more organized and more equipped with resources to control the school environment (Kozol 1991). Similarly, schools with a greater access to economic resources may provide a more controlled social environment and may be better equipped to enforce these rules than schools with less resources. Therefore, once adjusting for the smoking prevalence and norms about smoking, schools with a high proportion of non-Hispanic and white students may have significantly lower levels of heritability simply because these environments may be more highly controlled.
Summary of school-level factors
Taken together, this body of work suggests that school composition and context may influence the genetic etiology of smoking via (a) social triggers or (b) social controls. We expect that social norms that encourage smoking and a high rate of smoking will serve as social triggers. We also expect school policies aimed at the reduction of smoking and peer norms that discourage smoking to serve as social controls. The two models that describe the association between the racial composition of schools and the heritability of smoking are equally plausible.
Data
This study employs the National Longitudinal Study of Adolescent Health data set to examine health and health-related behaviors among a nationally representative sample of adolescents in seventh through twelfth grades (Udry 2003; Harris et al. 2003). In 1994, 90,118 adolescents from 134 different schools completed questionnaires about their daily activities, health-related behaviors, and basic social and demographic characteristics. Following the in-school survey, 20,747 respondents were re-interviewed in their homes (Wave I) between the months of April and December of 1995 and then again one year later (Wave II).
Two aspects of this study are particularly useful for our purposes. First, the Add Health study over sampled twin pairs identified in the in-school survey. During Wave III of the study, respondents who identified that they had a full sibling or a twin during Wave I were asked to provide saliva specimens to be genotyped. Of the 3,139 individuals that were asked, 83% (n = 2,612) agreed to take part in the study. Researchers then used 11 genetic markers to confirm the reported zygosity of the twin and sibling pairs. In total, 34 pairs were reassigned zygosity status as a result of this test. Based on this criterion of zygosity, we use 163 identical twin, 240 fraternal twin, 647 full sibling, and 148 half-sibling pairs for a total of 1,198 unique pairs.1 The pair-design makes it possible to calculate quantitative genetic estimates of genetic and environmental influences (Falconer and Mackay 1996). Second, because nearly all students in the schools responded to the initial survey, it is possible to measure aspects of schools that are otherwise difficult to assess.
The two dependent variables in our analyses were taken from Wave II of the study in which respondents were asked if they had “ever smoked an entire cigarette” and if so, had they ever smoked “at least 1 cigarette a day for 30 days.” We use the same definitions as previous work in this area (Kandel et al. 2004) and refer to these outcomes as smoking onset and daily smoking, respectively. Overall, 44.6% of the respondents reported smoking onset and 21.8% reported daily smoking.
We develop a unique school-level measure that describes the social pressures to smoke or not smoke within a school. This measure characterizes the popularity status of smokers and non-smokers and indicates the extent to which the most popular students either smoke or do not smoke. We believe that this item taps in to school-specific social norms with respect to smoking that are otherwise difficult to measure. All students were asked to write down the names of their five closest female friends and their five closest male friends. Each student in the school was then linked with their respective nominees as well as those that nominated them. Each student received a score that recorded the number of nominations that they received (median = 3; min = 0; max = 36). Students were also asked to report their smoking during the past year. Response options ranged from 0 “never” to 6 “nearly everyday”. We calculated a school-specific correlation between the number of nominations that a respondent received (popularity) and the level of smoking reported by the respondent (mean = −.01; min = −.16; max = .17). Positive values indicate that the most popular students are also those that smoke and negative values indicate that the most popular students are the non-smokers. Because we are concerned with the two ends of this continuum, we use the 10 extreme values of this distribution to characterize pro- and anti-smoking schools.2
In addition to smoking popularity we also consider other aspects of schools that may be associated with the heritability of smoking. Institutional control is measured using two measures from the School Administration component of the Add Health study. First, similar to other work in this area (Kandel et al. 2004) we measure school smoking policies by summing administrator responses regarding the disciplinary action of the school upon the 1st and 2nd incidence of smoking on school-grounds including a) verbal warning; b) minor action; c) in-school suspension; or d) expulsion. This school-level variable ranges from values of 0–7. Second, we also include a dummy variable that assesses whether or not the teachers can smoke on the school grounds. In total 67 of the 84 schools (79.7%) do not allow teachers to smoke.
We also examine the association between the level of smoking in the schools and the corresponding heritability with a measure that captures the prevalence of smoking; the proportion of students who reported that they have ever smoked a cigarette by the date of the in-school survey (min 5.6%; Q1 28.1%; Median 36.5%; Q3 47.6%; max 55.3%). This variable is used to assess the prevalence hypothesis which emphasizes a contagion or epidemic understanding of the “spread” of smoking behaviors. Face-to-face interactions with peers within schools will lead to social imitation that increases as the prevalence of smoking increases. As described earlier we include a linear and quadratic term designed to capture the curvilinear association between smoking prevalence and heritability of smoking anticipated by this model.
Next, given the association between race and smoking (Pampel 2002), we also adjust for the racial composition of the schools with a variable that measures the proportion of students who are non-Hispanic and white. Because of observed differences with smoking and socioeconomic status (Pampel and Rogers 2004), these models also adjust for socioeconomic differences across the schools with a variable that measures the highest level of education attained by students’ mothers. We use this information to record the proportion of students’ mothers who have completed college (mean = 26.8%; min = 5.7%; max = 80.5%).
Methods
We first calculate tetrachoric correlations for identical and fraternal twin pairs and full and half-sibling pairs. These estimates are presented with their respective quantitative genetic estimates describing the relative contribution of heritability, shared environment, and unshared environment to overall phenotypic variation.3 We then propose a unique multi-level application of multivariate regression-based techniques to provide evidence for the heritability of smoking (DeFries and Fulker 1985). This extension provides a parameter estimate that describes the school-level moderation of the heritability of smoking.
The standard model (see equation 1) relies on data obtained from sibling and twin pairs and predicts the outcome of the second sibling of a pair (y2) as a function of the first sibling’s score on the same outcome (y1), a measure of genetic similarity—average proportion of genes identical by descent for each pair type—(g), and an interaction between genetic similarity and the siblings’ score (y1g). Because identical twins share all of their genes, these pairs receive a score of g = 1, fraternal twins and full siblings a score of g = .5, and half-siblings a score of g = .25. If the similarity of the pair is conditional upon their genetic similarity, then a positive and significant value for b3 indicates that the degree of similarity for smoking among sibling pairs is a function of their genetic similarity. If the distribution of the dependent variable is standard-normal, then the parameter estimates for b1 and b3 describe the relative contribution of shared environment (c2) and heritability (h2), respectively, and the remaining proportion is due to nonshared environmental characteristics (e2). This model is quite flexible and it can be extended to include K covariates and it is well-suited to complex sampling designs.
| (1) |
| (2) |
Although the single-level model has undergone considerable modifications (Purcell 2002), it continues to be used to assess the genetic contribution to a trait’s overall variation (Rende et al. 2005; Slomkowski et al. 2005). In equation 2, we show how to extend this model to a generalized linear and mixed modeling framework (Rabe-Hesketh and Skrondal 2005). This parameterization is a particularly useful model because of (a) the ease with which other covariates can be included in the model, (b) the flexibility of the model with respect to the measurement of the dependent variable, and (c) the inclusion of random effects at the school-level allows for a simple test for school moderation of heritabilty without relying on researchers to specify a particular school-level characteristic. Because our dependent variables are binary, we use a logit link to estimate a multi-level logistic model and we adapt this model to the sibling pair approach used elsewhere (DeFries and Fulker 1985). Although the interpretation of the value of the “heritability” estimate is not consistent with the standard normal model, the role of the link function makes it possible to estimate similar models for continuous, count, overdispersed count, and multi-nomial distributions (Rabe-Hesketh and Skrondal 2005).
According to the specification in equation 2, the ith pair is nested within the jth school and this model includes two level-2 residual components (u0 and u1) that describe the random intercept and the random slope, respectively. Variance estimates for these residual terms are described in the final models. If this model is applied to the regression-based approach to quantitative genetics then the score for the first sibling in pair i can be modeled as a function of their sibling’s score (y1ij), the measure of genetic similarity, and the interaction term—or heritability estimate—y1ijgij. Again this model can adjust for additional covariates. The error term at the end (gij) captures an offset to the average heritability estimate for respondents within the jth school and the variance of this estimate (σ2u1) is the primary focus of our analyses. These estimates are presented in Table 2.
Table 2.
Multilevel logistic regression estimates: quantitative genetic estimates based on sibling pairs nested within schools
| Smoking onset |
Daily smoking |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||||||
| p.e. | s.e. | pr. < | p.e. | s.e. | pr. < | p.e. | s.e. | pr. < | p.e. | s.e. | pr. < | |
| Intercept | 0.114 | 1.013 | 0.911 | 0.111 | 1.015 | 0.913 | −3.468 | 0.992 | 0.000 | −3.202 | 0.940 | 0.001 |
| Sociodemographics | ||||||||||||
| Gender [Boy] | ||||||||||||
| Girl | −0.070 | 0.160 | 0.661 | −0.073 | 0.162 | 0.653 | −0.155 | 0.145 | 0.286 | −0.185 | 0.162 | 0.255 |
| Age (years) | −0.084 | 0.055 | 0.124 | −0.084 | 0.055 | 0.127 | 0.009 | 0.062 | 0.880 | −0.005 | 0.060 | 0.928 |
| Race/Ethnicity [Non-White] | ||||||||||||
| Non-Hispanic White | 0.329 | 0.135 | 0.015 | 0.329 | 0.135 | 0.015 | 0.408 | 0.201 | 0.043 | 0.372 | 0.195 | 0.057 |
| Smoking Environment | ||||||||||||
| Friends that are smokers | 1.064 | 0.119 | 0.000 | 1.063 | 0.119 | 0.000 | 2.017 | 0.336 | 0.000 | 2.085 | 0.347 | 0.000 |
| Parents are smokers | 0.061 | 0.156 | 0.696 | 0.057 | 0.154 | 0.710 | 0.422 | 0.176 | 0.017 | 0.416 | 0.174 | 0.017 |
| Proportion of friends in common | 0.719 | 0.493 | 0.145 | 0.719 | 0.495 | 0.146 | 0.721 | 0.546 | 0.186 | 0.542 | 0.604 | 0.370 |
| Quantitative Genetic Estimates | ||||||||||||
| Sibling smoking status | 0.945 | 0.194 | 0.000 | 0.946 | 0.194 | 0.000 | 1.339 | 0.256 | 0.000 | 1.295 | 0.289 | 0.000 |
| Genetic similarity | −3.109 | 0.756 | 0.000 | −3.118 | 0.744 | 0.000 | −2.953 | 0.977 | 0.003 | −2.839 | 0.943 | 0.003 |
| Evidence of heritability | 2.955 | 1.144 | 0.010 | 2.981 | 1.127 | 0.008 | 3.375 | 1.655 | 0.041 | 5.668 | 2.888 | 0.049 |
| School-Level Residual Variance | ||||||||||||
| Intercept | 0.446 | 0.164 | 0.008 | 0.448 | 0.165 | 0.008 | 0.396 | 0.145 | 0.328 | 0.149 | 0.030 | |
| Slope (Evidence of heritability) | 1.161 | 3.752 | 0.758 | 84.190 | 41.564 | 0.046 | ||||||
| Chi-square | 0.124 | 0.725 | 13.004 | 0.000 | ||||||||
Note: Cell entries represent parameter estimates and standard error from two multilevel logistic regression models. Data obtained from the sibling and twin pair sample of the National Longitudinal Study of Adolescent Health (n = 1,198 pairs). Multilevel weights were used in the estimation procedure and standard errors and significance levels reported adjust for the doubling of sibling pairs (Chantala and Tabor 2004). Model fit statistics indicate the χ2 for the inclusion of the random slope estimate
If the presence of school-level moderation is detected with a significant level-2 residual estimate (σ2u1), then we will estimate models that include covariates designed to explain this latent factor. We propose to analyze the empirical Bayes estimates for the random slope (or school-specific heritability estimate) derived from the multilevel regression models. These estimates are then treated as the dependent variable in a series of models designed to account for school-level dispersion for this effect. These estimates are presented in Table 3. All multilevel models are estimated with the GLLAMM procedure in Stata 9.0 (Rabe-Hesketh and Skrondal 2005).4
Table 3.
School-level factors that shape the direction and magnitude of the heritability of daily smoking
| p.e. | beta | s.e. | t | pr < | |
|---|---|---|---|---|---|
| Social and demographic characteristics | |||||
| Proportion of college-educated mothers | −25.23 | −0.357 | 23.78 | −1.061 | 0.292 |
| Proportion non-Hispanic and white | −6.55 | −0.685 | 2.41 | −2.718 | 0.008 |
| Smoking norms | |||||
| Popular students do not smoke | −6.77 | −0.177 | 8.02 | −0.845 | 0.401 |
| Popular students are also smokers | 51.04 | 1.334 | 8.56 | 5.962 | 0.000 |
| Institutional control of smoking | |||||
| Teachers not allowed to smoke on campus | −3.31 | −0.144 | 5.05 | −0.656 | 0.514 |
| School penalties for smoking infractions | 1.72 | 0.264 | 2.34 | 0.736 | 0.464 |
| Smoking prevalence | |||||
| Proportion of students who have smoked | −19.32 | −0.213 | 174.91 | −0.110 | 0.912 |
| Smoking prevalence squared | 31.01 | 0.233 | 236.9 | 0.131 | 0.896 |
Note: Cell entries are parameter estimates the latent school-level heritability factor for daily smoking regressed on various school-level factors. These models were estimated using the GEQS command in the GLLAMM procedure available in STATA 9.2. Data obtained from the sibling and twin pair sample of the National Longitudinal Study of Adolescent Health (n = 1,198 pairs). Parameter estimates were weighted for individual and school-level weights (Chantala and Tabor 2004). The inclusion of these estimates significantly improved overall fit (Chi-square = 16.38, df = 8, P < .037)
Additional considerations: rGE and EEA
In the most basic model, genes and environments are believed to independently and additively affect smoking. As described above, the effects can be multiplicative rather than additive (GE interactions) but there are also reasons to believe that genes and environments are not independent of one another. This is referred to as a gene-environment correlation (rGE)5 To adjust for passive gene-environment correlations, we simply control for maternal smoking behaviors with a dummy variable coded 1 if their mother reported smoking during the Wave I in-home survey (22.9%) and to adjust for active rGE we include a dummy variable coded 1 if any of their friends currently smoke (47.3%) and 0 if otherwise. While this later measure may be subject to projection bias, the direction of this bias will most likely lead to an over-control of active rGE and should be seen as a conservative operationalization of this important concept.
Another important specification is the equal environment assumption (EEA) [Hrubec and Robinette 1984]. This assumption states that identical and fraternal twins share similar environmental exposures through the period of interest in a study. This assumption is critical because it enables researchers to characterize excess similarity among identical twins to genetic similarity.6 We use data from the friend nomination roster to examine the overlap in sibling pairs’ friends. In total, 39% of the identical twins shared at least one friend in common compared to 26% of fraternal twins, 18% of full-siblings, and 6% of half-siblings. Among those that shared at least one friend, identical twins listed the same group of people as their friends 44% of the time compared to 36% of fraternal twins. A measure that taps the overlap in friend nomination networks is used in the models to adjust for these concerns.
Findings
Table 1 presents pairwise correlations for smoking onset and daily smoking for the four types of sibling pairs in our sample. There is a clear gradient between genetic similarity and smoking concordance for both measures of smoking; the sibling correlations are the highest for identical twins and the lowest for half-siblings. Based on the resemblance of sibling pairs as a function of their genetic similarity, we calculate a heritability estimate of .51 for smoking onset and .58 for daily smoking. Thus, as with other research, these estimates provide support for the notion that both smoking onset and daily smoking have an important genetic component.
Table 1.
Sibling and twin correlations and quantitative genetic estimates for smoking onset and daily use
| Sample size | Smoking onset |
Daily smoking |
|||
|---|---|---|---|---|---|
| Tetrachoric correlation | Prevalence | Tetrachoric correlation | Prevalence | ||
| Identical twins | 163 | 0.72 | 0.42 | 0.87 | 0.19 |
| Fraternal twins | 240 | 0.47 | 0.41 | 0.64 | 0.18 |
| Full siblings | 647 | 0.45 | 0.46 | 0.57 | 0.23 |
| Half siblings | 148 | 0.36 | 0.48 | 0.39 | 0.26 |
| Quantitative genetic estimates (twins only) | |||||
| Heritability (h2) | 0.51 | 0.58 | |||
| Shared environment (c2) | 0.20 | 0.29 | |||
| Unshared environment (e2) | 0.29 | 0.13 | |||
Note: Data obtained from the sibling and twin pair sample of the National Longitudinal Study of Adolescent Health (n = 1,198 pairs). Heritability estimates obtained using Mx (Neale et al. 2003)
Onset [χ2 = 140.30, d.f. 11, P < .001]; Daily smoking [χ2= 183.26, d.f. 11, P < .001]
We elaborate on these univariate estimates and take advantage of the complex design of the Add Health study to address the importance of school context on heritability of smoking behaviors. As we described earlier, the dependent variable is simply a dummy variable indicating whether or not a respondent has ever smoked an entire cigarette or if they have smoked daily. These results are presented in Table 2. As expected, having friends that are current smokers strongly predicts current smoking status for both onset and daily smoking. Having a parent that is a smoker increases the risk of daily smoking but not smoking onset.
Evidence for heritability is estimated by including an interaction between the genetic similarity of the pair and the smoking status of the respondent’s sibling. Two models are included for each smoking outcome. The first includes a random intercept only and the second includes the random slope. For smoking onset, we show evidence for genetic influence in both models (b = 2.955, b = 2.981).7 Whereas the random intercept is significant (σ2u0 = .446, P < .008) there is no evidence that the heritability of smoking initiation varies across schools. Specifically, the parameter estimate (σ2u1 = 1.161) statistically non-significant and the inclusion of the random slope parameter estimate does not improve the overall model fit (Δ-2ll = χ2 = .124, n.s.). With respect to daily smoking, these results point to an increased estimate of heritability (b = 5.668, P < .05) and, more importantly, a large and statistically significant estimate for the school-level residual variance increased significantly (σ2u1 = 84.190, P < .05) and improved overall model fit (χ2 = 13.004, P < .001).
The estimates presented in Table 3 are designed to examine the school-level factors that may be responsible for the variation in heritability for daily smoking and they represent the latent heritability factor regressed on the seven school-level factors described above. According to these results, attending a school in which the more popular students are also the most likely to smoke cigarettes significantly increases the heritability of smoking daily (β= 1.334, P < .001). Although the heritability estimate is reduced within schools in which there are normative pressures to avoid smoking (β= −.18, n.s.) this effect is not statistically significant. Importantly, neither the linear nor the quadratic specification of smoking prevalence was significantly associated with the school-level estimate for the heritability of smoking. Similarly, neither form of institutional social control was associated with reduced heritability.
Importantly, we show a significant association between the racial composition of schools and the heritability estimate. Specifically, we show that the heritability of daily smoking is significantly reduced as the proportion of students who are non-Hispanic and white increases (β= −.685, P < .008). Because predominantly white schools have higher rates of smoking compared to other schools (Kandel et al. 2004), it was possible that these schools would also have an increased estimate for heritability. As the results indicate, however, this does not appear to be the case. Indeed, as smoking heritability among adolescents is more strongly affected by smoking norms rather than a simple tabulation of prevalence, the potentially protective factor of smoking rates among students at predominantly minority schools, does not necessarily translate to a controlling mechanism on genetic tendencies to smoke.
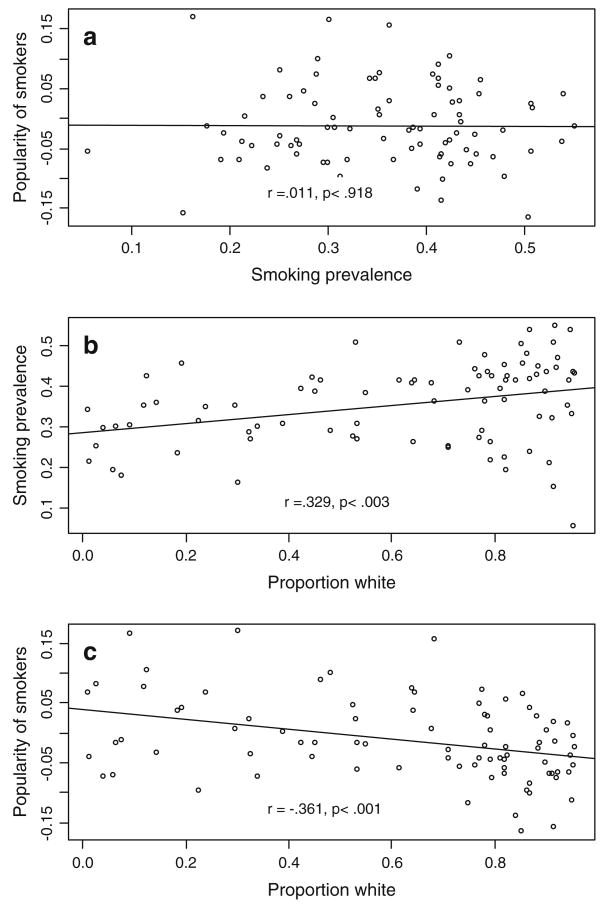
To more fully examine this association, we examined school-level bivariate associations between smoking popularity, smoking prevalence, and racial composition. These associations are presented in Fig. 1. As implied in the result above, Fig. 1a. shows that there is no association between smoking prevalence and the popularity of smokers within schools (r = .011, n.s.). However, as shown in Fig. 1b, predominantly white schools appear to have stronger anti-smoking norms compared to schools with a greater proportion of racial and ethnic minorities. That is, the popularity of non-smoking students increases significantly as the proportion of white students increases (r =−.361, P < .001). But, as described elsewhere (Kandel et al. 2004), this does not correspond with smoking prevalence. Figure 1c shows that predominantly white schools have higher overall smoking rates compared to schools with few white students (r = .329, P < .003). In ancillary analyses (results not shown) we calculate daily smoking rates of 15% for students within predominantly white schools compared to less than 6% in schools with no white students. Students in predominantly white schools smoke more often than do students within other schools, but those that do smoke within predominantly white schools are significantly less popular than their non-smoking counterparts. This same association is not evident within schools with a greater proportion of minority students suggesting that smoking norms may be somewhat less clear within these schools. Further, within predominantly white schools, the popularity-smoking correlations are more tightly bounded compared to schools with a greater number of racial and ethnic minorities. This reduced variability directly addresses the social control model where social forces canalize phenotypic variation and restrict the range of outcomes for diverse populations (Shanahan and Hofer 2005). Taken together with the null effects for the smoking prevalence, this finding suggests that the social mechanisms through which heritability for smoking is moderated may not simply be the exposure to smoking behaviors and subsequent modeling, but rather more direct social pressures.
Fig. 1.
School-level bivariate associations between racial composition, smoking norms, and smoking prevalence
Discussion
In this paper, we estimate that a substantial portion of the variance in smoking may be due to genetic factors. We demonstrate that the relative contribution of genetics to the daily use of cigarettes is conditional upon student norms related to cigarette use. These findings make substantive contributions to the fields of sociology and behavioral genetics and they help to move the discussion away from characterizing phenomena as socially or genetically oriented. Indeed, it would very difficult to describe the likelihood that two siblings will be concordant for smoking without knowing the degree to which they are genetically similar as well as a detailed account of their friends and social environments.
Importantly, we do not find evidence for the strongest version of the contextual trigger argument. That is, even after accounting for school-level differences in smoking and school-level differences in the heritability of smoking, we still show evidence that genetic factors matter for regular smoking. In other words, we argued that the driving factor responsible for genetic expression was the social environment and we expected to find that, without a normative environment in which cigarette use is supported, the genetic determinants of smoking would be non-existent; but this is not the case with our findings.
However, our findings are important because they speak to the continuing discussion regarding the relative contribution of biological as opposed to social factors as the primary determinants of complex behaviors. There are strong theoretical reasons (Shannahan and Hofer 2005; Deater-Deckard and Mayr 2005) and corresponding empirical evidence (Turkheimer et al. 2003; Purcell 2002; Purcell and Sham 2003) to suggest that a full understanding of health-related behaviors such as smoking requires information on processes both inside and outside the body (Duster 2006). That is, smoking-related behaviors are in part genetically caused, but the expression of a gene that may affect tobacco use is best understood only when situated in a particular social or environmental context. If the degree to which behaviors such as smoking are heritable is conditional upon social contexts, then it is important to develop theories, hypotheses, and methods specifically designed to address the interaction between genes and environments. This point is summarized nicely by Hewitt and Turner (1995) in the introduction to Behavior Genetic Approaches in Behavioral Medicine:
Behavior genetics is the study of genetic and environmental determination of individual differences in characteristics with a behavioral component. Traditional genetics has treated environmental variation largely as “noise.” Traditional epidemiology and many of the social sciences have either ignored genetic variation or wished it would go away. In some cases, the emphasis of these traditional disciplines on a particular component, either genetic or environmental, can serve a useful scientific purpose. In other cases, it may be misleading and an obstacle to understanding. (p. 3).
Our findings are also important because they are in line with emerging theories regarding the most appropriate way to conceptualize the environment within gene-environment interaction studies. Perrin and Lee (2007) argue that gene-environment interaction studies need to understand that “interconnections between individuals and social structures can predict important variations in individual behavior without recourse to individual traits” and thus “environments and genetic potentials must be understood as nested and cross-cutting in potentially complex ways” (311). Nevertheless, the bulk of gene-environment studies continue to focus on the social and demographic characteristics of individuals or families rather than on the composition of larger social contexts such as neighborhoods or schools (Swan et al. 2003) and yet studies at this higher level of aggregation speak more directly to theories of gene-environment interactions (Johnston and Edwards 2002; Shanahan and Hofer 2005; Perrin and Lee 2007).
This perspective is particularly relevant when one considers the social pressures responsible for school and neighborhood segregation by race (Massey and Denton 1993). That is, social scientists are somewhat reticent to engage in behavioral genetic inquiry, especially if the part of the explanation emphasizes the role of race as an enabling factor (Frank 2001; Zuberi 2001a, b; Duster 2006). However, the material resources available to communities and schools are highly correlated with the racial composition of the area and there are, at times, equally clear differences with respect to behavioral norms—including cigarette smoking. Therefore, as evident in the results presented above, the concept of race should be understood as a critical component of investigations into the genetic determinants of behavior precisely because of environmental experiences that differ among black, white, Latino, and Asian-Americans.
Perrin and Lee (2007) also make the point that the social environment should be understood as an enabling factor and not simply as a controlling mechanism as with most work in this area. They argue that it is not possible to examine one of the important counterfactuals established by most gene-environment interaction studies. Namely, the notion of “genetic potential” is taken as the starting point and the environment simply constrains the capacity of factors that are genetically determined. However, as our results show, it is also possible that genetic orientations may not emerge unless the environment actively engages individuals in behaviors and reinforces these behaviors. This point builds on the “fundamental causes” explanation for health and health-related behaviors (Link and Phelan 1995). According to Link and Phelan (1995), epidemiologic inquiry into the determinants of disease has focused almost exclusively upon proximate causes rather than the social antecedents to these factors. While the proximate determinants of disease risk may change, the social environment remains a fundamental cause of disease and these risk factors need to be contextualized. Contextualizing risk factors means that researchers should consistently examine the “conditions under which individual risk factors are related to disease” (Link and Phelan 1995, pp. 84–85). That is, not only does the environment shape exposure to risk factors, but environmental aspects fundamentally shape the etiologic trajectories of known risk factors. When genetic vulnerability is characterized as a risk factor, this theoretical perspective is highly relevant to cigarette use because genetic factors may be particularly strong risk factors within certain environments (Shanahan and Hofer 2005).
This same perspective may also help to account for the notable variability in the smoking heritability estimates for adolescents that have been presented elsewhere. For example, our calculation of smoking heritability (roughly 51–58%) is within the range of estimates obtained from other studies but this range is as low as 44% (Slomkowski et al. 2005) and as high as 65% (Maes et al. 1999). On the one hand, this variation can be considered a stochastic component of the parameter estimate and dispersion about this estimate is akin to standard error. For example, Li et al. (2003) use standard meta-analysis techniques to estimate the true population parameter based on the distribution of heritability estimates from previous research. The authors conclude that the true parameter is somewhere between 57 and 61%. This approach remains an important method to characterize dispersion but it is also possible that the magnitude and sign of these residuals can be anticipated with existing theory and they can be tested empirically (Shannahan and Hofer 2005). In other words, sibling resemblance as a function of zygosity can be modeled as a function of theoretically relevant characteristics that are believed to enhance or suppress heritability. If, as we show, the social environment enables or limits genetic effects then we should not expect heritability estimates to remain constant except in cases in which the environment is held constant. Therefore, efforts should be made to identify social, environmental, spatial, or cultural factors that may account for the differences observed in these studies.
Acknowledgments
This paper is part of a larger study funded by the National Institute of Child Health and Human Development (NIH/NICHD K01 HD 50336). Resources were also provided by the Population Center at the University of Colorado (NIH/NICHD R21 HD 51146). This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contact Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC 27516-2524.
Footnotes
Our data consists of a subset of the original sibling and twin pair data (see Harris et al. 2006 for a detailed description of these data). Our analysis is limited to siblings and twins who attended the same school, those with information on smoking at wave II, sibling pairs whose ages are at most three years apart from one another, schools with network data on friend nominations, schools with at least five pairs of siblings, and schools that contained sampling weights. After these deletions our final sample consisted of 1,198 pairs nested within 84 schools.
Because mechanisms of social control and social expression may be happening simultaneously, it is possible that there is no effect of social norms at or near the average of the distribution and it is only in the extreme ends of this distribution in which social forces manifest. This most closely resembles a cubic distribution with a positive linear, negative quadratic, and positive cubic term, but these models are difficult to estimate with the limited number of schools that we have in the data. Accordingly, we took the extreme schools to capture expression and control, respectively, in which their combined effects are interpreted in relation to the average normative environment.
We use Mx, a structural equation modeling package that contains a number of standard procedures, to calculate quantitative genetic parameter estimates for genetic and environmental components of phenotypic variance. There are a number of Mx scripts developed by the GenomeUTwin group (Posthuma et al. 2003) that are freely available at: http://www.psy.vu.nl/mxbib/. We use the contingency table script ctVCut2c.mx to estimate the ACE parameters presented in Table 1.
The GLLAMM procedure is comprised of a bundle of statistical procedures that are remarkably flexible and well-suited to the complex design of the Add Health study. As an example, all parameter estimates in this paper are calculated using the sampling weights for both individuals (GSWGT2) and schools (SCHWT1) provided by the Add Health study (Chantala and Tabor 2004). Because we estimate random effects at the school level, individual and school weights were calculated using a PWIGLS macro in STATA 9.0 that was designed for survey data obtained from more than one level (see Chantala et al. 2006 for a detailed discussion of this method).
Jaffee and Price (2007) define gene-environment correlations as “genetic differences in exposure to a particular environment” (p. 2) and describe both causal and non-causal variants. We are primarily concerned with two forms of rGE; both of which are causal. The first is the passive form of rGE where children inherit both genes and the environment from their parents. Therefore, if a parent smokes because of genetic reasons, then these genes will be shared with their children but their children will also be raised in an environment in which cigarettes are easier to obtain and smoking behaviors are not negatively sanctioned. For example, Hill et al. (2005) show that, controlling for other important factors, the onset of smoking among adolescents is strongly shaped by both parental smoking and household norms about smoking but these environmental factors may have a genetic component that needs to be modeled explicitly. Active correlation is the second major form of rGE. This model describes a situation in which individuals with genetically oriented tendencies to smoke cigarettes will select in to social environments in which smoking is normative or rewarded. Some of the strongest evidence for active rGE related to adolescent smoking comes from a study by Cleveland et al. (2005) who use traditional behavioral genetic models to estimate the heritability of substance using or non-substance using friendship networks. That is, do genetics shape the composition of adolescents’ friends as related to smoking, drinking, and drug use? According to their estimates, nearly two-thirds of the variance (h2 = .64) of the substance using behaviors of adolescents’ networks has a genetic component. This estimate is derived by comparing the pair-wise correlations across identical twin (r = .61), fraternal twin (r = .27), full-sibling (r = .28), and half-sibling (r = .18) pairs. As Jaffee and Price (2007) highlight, these two forms or rGE are particularly problematic for research on gene-environment interactions. According to these authors “rGE does not have to reach statistical significance to profoundly affect the interpretation of G x E estimates” (6). To deal with the possibility that our environmental measures may have a genetic component, we include statistical controls that reduce the likelihood of rGE confounding our gene-environment interaction results.
Using data from Add Health, Slomkowski et al. (2005) show that the similarity of sibling pairs is strongly conditional upon the social connectedness of the pair. That is, pairs that report spending more time with one another, sharing similar friends, and having affection for one another are much more alike one another than those who do not report this same level of closeness. Importantly, in wave II of the study, identical twins with high levels social connectedness reported a sibling correlation for smoking of .83 compared to a value of .54 for DZ twins with the same level of connection. However, when these sibling correlations were compared for those who reported low social connection, the identical pair correlation was only .63 and the fraternal twin correlation remained roughly the same (r = .52). Although their analysis of the sibling pair data does not show systematic differences in the heritability estimate by social connection status, it is an important caveat to consider. Kendler and Gardner (1998) analyze three aspects of the EEA using retrospective data from an adult sample of twin pairs: a) childhood treatment (i.e., how similarly they two were treated by others), b) co-socialization (i.e., how much the twins tried to act like one another), and c) similitude (i.e., were they treated as individuals or were they always treated as a “pair”). These factors were then used to compare concordance rates for smoking across the groups. While treatment and similitude were not associated with smoking initiation, the sibling correlations were significantly moderated by their responses to the co-socialization measure. High co-socializing identical and fraternal twins had a tetrachoric correlation of .87 and .51, respectively, but their low-socializing counterparts had scores of .74 and .37. Again, these differences may have important implications for the heritability estimate.
Because regression-based techniques are typically used in proband-based designs, the twins and siblings are double entered in the multivariate models to adjust for the lack of an a priori proband in this sample. Standard errors are adjusted by a constant factor (2.5) when calculating test statistics and corresponding statistical significance.
Contributor Information
Jason D. Boardman, Department of Sociology and Population Program, Institute of Behavioral Science, University of Colorado at Boulder, 327 UCB, Boulder, CO 80309-0327, USA, e-mail: boardman@colorado.edu
Jarron M. Saint Onge, Department of Sociology, University of Houston, 489 PGH, Houston, TX 77204, USA, e-mail: jmsaintonge@uh.edu
Brett C. Haberstick, Institute for Behavioral Genetics, University of Colorado at Boulder, UCB 447, Boulder, CO 80309-0447, USA e-mail: Brett.Haberstick@colorado.edu
David S. Timberlake, Health Sciences and Public Health, University of California at Irvine, 120 Theory #100, Irvine, CA 92697-3956, USA, e-mail: dtimberl@uci.edu
John K. Hewitt, Department of Psychology and Institute for Behavioral Genetics, University of Colorado at Boulder, UCB 447, Boulder, CO 80309-0447, USA, e-mail: hewitt@ibg.colorado.edu
References
- Alexander C, Piazza M, Mekos D, Valente T. Peers, schools, and adolescent cigarette smoking. J Adolescent Health. 2001;29:22–30. doi: 10.1016/s1054-139x(01)00210-5. [DOI] [PubMed] [Google Scholar]
- Becker MH. Sociometric location and innovativeness: reformulation and extension of the diffusion model. Am Sociol Rev. 1970;35:267–82. [Google Scholar]
- Breslau N, Johnson EO, Hiripi E, Kessler R. Nicotine dependence in the United States—prevalence, trends, and smoking persistence. Arch Gen Psychiatry. 2001;58:810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking: a study of male twins. N Engl J Med. 1992;327:829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington HL, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chantala K, Tabor J. [Accessed 31 July 2007];Strategies to perform a design-based analysis using the add health. 2004 http://www.cpc.unc.edu/projects/addhealth/files/weight1.pdf.
- Chantala K, Blanchette D, Suchindran C. [Accessed 31 July 2007];Software to compute sampling weights for multilevel analysis. 2006 http://www.cpc.unc.edu/restools/data_analysis/ml_sampling_weights.
- Cleveland HH, Wiebe RP, Rowe DC. Source of exposure to smoking and drinking friends among adolescents: a behavioral-genetic evaluation. J Genet Psychol. 2005;166(2):153–169. [PubMed] [Google Scholar]
- Deater-Deckard K, Mayr U. Cognitive change in aging: identifying gene-environment correlation and nonshared environment mechanisms. J Gerontol B Psychol Sci Soc Sci. 2005;60:24–31. doi: 10.1093/geronb/60.special_issue_1.24. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Fulker DW. Multiple-regression analysis of twin data. Behav Genet. 1985;15:467–473. doi: 10.1007/BF01066239. [DOI] [PubMed] [Google Scholar]
- Duster T. Comparative perspectives and competing explanations: taking on the newly configured reductionist challenge to sociology. Am Sociol Rev. 2006;71:1–15. [Google Scholar]
- Eitle DJ, Eitle TM. School and county characteristics as predictors of school rates of drug, alcohol, and tobacco offenses. J Health Soc Behav. 2004;45:408–421. doi: 10.1177/002214650404500404. [DOI] [PubMed] [Google Scholar]
- Ellickson PL, Bird CE, Orlando M, Klein DJ, McCaffrey DF. Social context and adolescent health behavior: does school-level smoking prevalence affect students’ subsequent smoking behavior? J Health Soc Behav. 2003;44:525–535. [PubMed] [Google Scholar]
- Engels RCME, Vitarob F, Bloklandc EDE, de Kempa R, Scholte RHJ. Influence and selection processes in friendships and adolescent smoking behaviour: the role of parental smoking. J Adolesc. 2004;27(5):531–544. doi: 10.1016/j.adolescence.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Ennett ST, Bauman KE. Peer group structure and adolescent cigarette smoking: a social network analysis. J Health Soc Behav. 1993;34:226–236. [PubMed] [Google Scholar]
- Ennett ST, Flewelling RL, Lindrooth RC, Norton EC. School and neighborhood characteristics associated with school rates of alcohol, cigarette, and marijuana use. J Health Soc Behav. 1997;38:55–71. [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. Pearson-Prentice Hall; New York: 1996. [Google Scholar]
- Finch CE, Vaupel JW, Kinsella K, editors. Cells and surveys: should biological measures be included in social science research? National Academies Press; Washington: 2001. [PubMed] [Google Scholar]
- Fisher RA. Lung cancer and cigarettes? Nature. 1958a;182:108. doi: 10.1038/182108a0. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Cancer and smoking. Nature. 1958b;182:596. doi: 10.1038/182596a0. [DOI] [PubMed] [Google Scholar]
- Frank R. The misuse of biology in demographic research on racial/ethnic differences: a reply to van den Oord and Rowe. Demography. 2001;38:563–567. doi: 10.1353/dem.2001.0034. [DOI] [PubMed] [Google Scholar]
- Hall WPM, Lynskey M. The genetics of tobacco use: methods, findings, and policy implications. Tob Control. 2002;11(2):119–124. doi: 10.1136/tc.11.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Florey F, Tabor J, Bearman PS, Jones J, Udry JR. [Accessed June 25, 2007];The national longitudinal study of adolescent health: research design. 2003 http://www.cpc.unc.edu/projects/addhealth/design.
- Harris KM, Halpern CT, Smolen A, Haberstick BC. The national longitudinal study of adolescent health (add health) twin data. Twin Res Hum Genet. 2006;9(6):988–997. doi: 10.1375/183242706779462787. [DOI] [PubMed] [Google Scholar]
- Hernandez LM, Blazer DG, editors. Genes, behavior, and the social environment: moving beyond the nature/nurture debate. National Academies Press; Washington: 2006. [PubMed] [Google Scholar]
- Hewitt JK, Turner JR. Behavior genetic approaches in behavioral medicine: an introduction. In: Turner JR, Cardon LR, Hewitt JK, editors. Behavior genetic approaches in behavioral medicine. Plenum Press; New York: 1995. pp. 3–13. [Google Scholar]
- Hill D. Peer group conformity in adolescent smoking and its relationship to affiliation and autonomy needs. Aust J Psychol. 1971;23(2):189–199. [Google Scholar]
- Hill KG, Hawkins JD, Catalano RF, Abbott RD, Guo J. Family influences on the risk of daily smoking initiation. J Adolescent Health. 2005;37(3):202–210. doi: 10.1016/j.jadohealth.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Hrubec Z, Robinette CD. The study of human twins in medical research. N Engl J Med. 1984;310:435–441. doi: 10.1056/NEJM198402163100706. [DOI] [PubMed] [Google Scholar]
- Hu M-C, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem behavior and psychosocial development: a longitudinal study of youth. Academic Press; New York: 1997. [Google Scholar]
- Johnson RA, Hoffmann JP. Adolescent cigarette smoking in U.S. racial/ethnic subgroups: findings from the national education longitudinal study. J Health Soc Behav. 2000;41:392–407. [PubMed] [Google Scholar]
- Johnston TD, Edwards L. Genes, interactions, and the development of behavior. Psychol Rev. 2002;109:26–34. doi: 10.1037/0033-295x.109.1.26. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Kiros G-E, Schaffran C, Hu M-C. Racial/ethnic differences in cigarette smoking initiation and progression to daily smoking: a multi-level analysis. Am J Public Health. 2004;94(1):128–135. doi: 10.2105/ajph.94.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO., Jr Twin studies of adult psychiatric and substance dependence disorders: are they biased by differences in the environmental experiences of monozygotic and dizygotic twins in childhood and adolescence? Psychol Med. 1998;28:625–633. doi: 10.1017/s0033291798006643. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Pedersen NL. Tobacco consumption in swedish twins reared apart and reared together. Arch Gen Psychiatry. 2000;57:886–892. doi: 10.1001/archpsyc.57.9.886. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Jacobson CC, Neale MC, Prescott CA. Genetic and environmental influences on illicit drug use and tobacco use across birth cohorts. Psychol Med. 2005;35:1349–1356. doi: 10.1017/S0033291705004964. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, van Baal GCM, Boomsma DI. The influence of religion on alcohol use initiation: evidence for genotype X environment interaction. Behav Genet. 1999;29:573–3297. doi: 10.1023/a:1021679005623. [DOI] [PubMed] [Google Scholar]
- Kozol J. Savage inequalities: children in America’s schools. Crown Publishers, Inc; New York: 1991. [Google Scholar]
- Leatherdale ST, Brown S, Cameron R, McDonald PW. Social modeling in the school environment, student characteristics, and smoking susceptibility: A multi-level analysis. J Adolescent Health. 2005;37(4):330–336. doi: 10.1016/j.jadohealth.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;35:80–94. [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Rutter M, Simonoff E, Pickles A, Carbonneau R, Neale MC, Eaves LJ. Tobacco, alcohol and drug use in eight-to sixteen-year-old twins: the virginia twin study of adolescent behavioral development. J Stud Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Massey DS, Denton N. American apartheid: segregation and the making of the underclass. Harvard University Press; Cambridge: 1993. [Google Scholar]
- Neale MC, Boker SM, Gary X, Maes HH. Mx: statistical modeling. 6. Department of Psychiatry; VCU Box 900126, Richmond, VA 23298: 2003. [Google Scholar]
- Pampel FC. Inequality, diffusion, and the status gradient in smoking. Soc Probl. 2002;49:35–57. [Google Scholar]
- Pampel FC, Rogers RG. Socioeconomic status, smoking, and health: a test of competing theories of cumulative advantage. J Health Soc Behav. 2004;45:306–321. doi: 10.1177/002214650404500305. [DOI] [PubMed] [Google Scholar]
- Perrin AJ, Lee H. The undertheorized environment: sociological theory and the ontology of behavioral genetics. Sociol Perspect. 2007;50(2):303–322. [Google Scholar]
- Pokomy SB, Jason LA, Schoeny ME. Current smoking among young adolescents: assessing school-based contextual norms. Tob Control. 2004;13:301–307. doi: 10.1136/tc.2003.005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthuma D, Leo Beem A, de Geus EJC, van Baal GCM, von Hjelmborg JB, Iachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Res. 2003;6(5):361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Purcell S, Sham PC. A model-fitting implementation of the DeFries-Fulker model for selected twin data. Behav Genet. 2003;33:271–278. doi: 10.1023/a:1023494408079. [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using STATA. Stata Press; College Station: 2005. [Google Scholar]
- Rende R, Slomkowski C, Lloyd-Richardson E, Niaura R. Sibling effects on substance use in adolescence: social contagion and genetic relatedness. J Fam Psychol. 2005;19(4):611–618. doi: 10.1037/0893-3200.19.4.611. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. J Gerontol B Psychol Sci Soc Sci. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Slomkowski C, Rende R, Novak S, Lloyd-Richardson E, Niaura R. Sibling effects on smoking in adolescence: evidence for social influence from a genetically informative design. Addiction. 2005;100(4):430–438. doi: 10.1111/j.1360-0443.2004.00965.x. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1:S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Swan GE, Hudmon KS, Jack LM, Hemberger K, Carmelli D, Khroyan TV, Ring HZ, Hops H, Andrews JA, Tildesley E, McBride D, Benowitz N, Webster C, Wilhelmsen KC, Feiler HS, Koenig B, Caron L, Illes J, Cheng LS-C. Environmental and genetic determinants of tobacco use: methodology for a multidisciplinary, longitudinal family-based investigation. Cancer Epidemiol Biomarkers Prev. 2003;12:994–1005. [PMC free article] [PubMed] [Google Scholar]
- Timberlake DS, Rhee SH, Haberstick BC, Hopfer C, Ehringer M, Lessem JM, Smolen A, Hewitt JK. The moderating effects of religiosity on the genetic and environmental determinants of smoking initiation. Nicotine Tob Res. 2006;8:123–133. doi: 10.1080/14622200500432054. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychol Sci. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Udry JR. The national longitudinal study of adolescent health (add health), waves I & II, 1994–1996; wave III, 2001–2002 [machine-readable data file and documentation] Carolina Population Center, University of North Carolina at Chapel Hill; Chapel Hill: 2003. [Google Scholar]
- Valente TW, Unger JB, Johnson CA. Do popular students smoke? The association between popularity and smoking among middle school students. J Adolescent Health. 2005;37:323–329. doi: 10.1016/j.jadohealth.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Zuberi T. One step back in understanding racial differences in birth weight. Demography. 2001a;38:569–571. doi: 10.1353/dem.2001.0041. [DOI] [PubMed] [Google Scholar]
- Zuberi T. Thicker than blood: an essay on how racial statistics lie. University of Minnesota Press; Minneapolis: 2001b. [Google Scholar]