The introduction of diethyl ether, chloroform, and nitrous oxide into clinical practice over 150 years ago revolutionized medicine and surgery. However, despite a long period of clinical use, it has been difficult to elucidate the molecular basis for general anesthesia. Even today this question continues to be a source of active inquiry and debate.
Although investigators in the last half of the nineteenth century proposed various theories for the actions of anesthetics, the first ‘modern’ theory for general anesthetic action came from the independent works of Meyer and Overton. The observation by Meyer and Overton, supported by extensive experimental investigation, was that the anesthetic potency of certain compounds (‘narcotics’ in the older terminology) varied in direct proportion to the partition coefficient in a lipid solvent such as olive oil. While these results did not prove any particular molecular target, the results were often interpreted to indicate the lipid membrane as the site of anesthetic action (the Meyer-Overton ‘lipoid’ hypothesis), described as follows by Meyer:
The narcotizing substance enters in a loose physico-chemical combination with the vitally important lipoids of the cell, perhaps with the lecithin, and in so doing changes their normal relationship to the other cell constituents, through which an inhibition of the entire cell chemism results.1
Although Meyer and Overton recognized limitations to their experimental observations, their work was taken as the basis for a unified ‘lipoid’ theory of anesthesia. The Meyer-Overton theory is not the only unitary theory of anesthesia action. At least seventeen distinct unified theories have been proposed between 1847 and 1997 (see Table 1). It is nearly impossible to find a review article or book chapter on the mechanism of anesthetic action that does not in some way mention the Meyer-Overton hypothesis or a related unified theory of anesthetic action.
Table 1.
Major Unitary Theories of General Anesthesia Formulated Between 1847 and 1997
| Theory | Authors |
|---|---|
| Dissolution and removal of fat-like substances in the brain (1847) | E. von Bibra & E. Harless57 |
| Colloid theory (1875) | C. Bernard58 |
| Meyer-Overton ‘lipoid’ hypothesis (1899–1901) | H.H. Meyer1,11 and C.E. Overton14 |
| Alteration of surface tension (1904) | J. Traube59 |
| Action by achieving certain molar concentrations in lipids (1937) | K.H. Meyer60 |
| Thermodynamic activity (1939) | J. Ferguson61 |
| Membrane volume occupation (1954) | L.J. Mullins62 |
| Hydrate microcrystal theory (1961) | L. Pauling63,64 and S.L. Miller65 |
| Membrane disordering (1968) | J.C. Metcalfe et al.66 |
| Membrane expansion (1972) | P. Seeman67 |
| Critical volume hypothesis (1973) | K.W. Miller et al.68 |
| Alteration of lipid phase transition (1976–1977) | A.G. Lee69 and J.R. Trudell70 |
| Multi-site expansion hypothesis (1978) | M.J. Halsey et al.71 |
| Proton-pump leak hypothesis (1980) | A.D. Bangham and W.T. Mason72 |
| Disruption of hydrogen bonds (1990) | H. Brockerhoff et al.73 J-S. Chiou et al.74 |
| Alteration of membrane curvature (1991) | S.M. Gruner and E. Shyamsunder75 |
| Alteration of membrane dipole potential (1995) | Z. Qin et al.76 |
| Alteration of lateral membrane pressures (1997) | R.S. Cantor77 |
While the legacy of Meyer and Overton has dominated investigations of general anesthetics, support for their hypothesis was hardly monolithic. In the last several decades in particular, unified theories of anesthesia have come under increasingly severe attacks and the general,2,3 although by no means unaminous,4–6 consensus is that anesthetics, as with most drugs in current medical use, act at protein receptor targets. A clear symbol of the ‘changing of the guard’ is illustrated by comparing two quotations from the 1990 and 1996 editions of the pharmacology textbook The Pharmacological Basis of Therapeutics. The 1990 edition reads:
Most theories of anesthetic action are based on the physicochemical characteristics of the anesthetic drugs. These proposals relate closely to the correlation between the potency of an anesthetic agent and the solubility of the drug in oil…Interpretation of this fundamental result is thought to be crucial to the understanding of the action of anesthetics.7
In the 1996 edition of this text, the above passage was repeated but with the last sentence amended as follows:
Interpretation of this fundamental results, once thought to be crucial to the understanding of the action of anesthetics, may be only an incidental finding.8 (italics added)
The following sections will focus on three compounds whose actions are inconsistent with the Meyer-Overton hypothesis. Two of the compounds, α-chloralose and chloral hydrate, were anesthetics known to Meyer and Overton, and recognized at least by Overton as having properties which were difficult to explain by his hypothesis. The third compound, flurothyl (hexafluorodiethyl ether) is a volatile, fluorinated ether with convulsant properties which was discovered in 1957. While these three compounds posed a challenge for the Meyer-Overton hypothesis, their properties were mostly ignored by those favoring a unitary theory of anesthesia. The end of this manuscript will speculate on reasons for this.
The Chemical Diversity of General Anesthetics
The chemical diversity of compounds that can produce general anesthesia is impressive and encompasses ethers (diethyl ether, isoflurane), alkanes (chloroform, halothane), alcohols (ethanol, chloral hydrate), chloralose, barbiturates, phenols (propofol), etomidate, steroidal compounds (alphaxalone), and inorganic gases (nitrous oxide, xenon). Many of these chemical classes were known to Meyer and Overton. Indeed, Overton analyzed an extensive breadth of compounds including diethyl ether, chloroform and other halogenated alkanes, chloralose, nitriles, aldehydes, ketones, esters, amides, aromatic hydrocarbons, phenols, carbon dioxide, carbon disulfide, nitrous oxide, and opioids.
To support unified theories of anesthesic actions, the chemical diversity of anesthetic molecules is often emphasized. The following statement from a recent edition of a medical pharmacology textbook is typical:
The shape and electronic configuration of the molecule is evidently unimportant, and the pharmacological action requires only that the molecule has certain physicochemical properties. The lack of chemical specificity argues against there being any distinctive ‘receptor’ for anesthetics.9
Despite the apparent non-specificity of a chemical structure required for anesthetic action, some scientists expressed doubts about the validity of the lipoid hypothesis. For example, R.S. Lillie pointed out in 1916:
First it should be noted that substances belonging to the most various classes may have anæsthetic actions. This fact is overlooked in theories like those of Overton and Meyer, Traube, and others, which refer anasthesia to the special properties of lipoid-solvent substances[;] … however, lipoid-solubility or surface activity is not essential to narcotic action; magnesium sulphate has long been used by naturalists to narcotize marine animals … [Thus,] the parallelism between lipoid-solubility and narcotic action is not an exact one, and many exceptions to the rule are known.10
Lillie touches on a fundamental problem with the Meyer-Overton hypothesis: the correlation between anesthetic potency and lipid solubility is impressive for a certain group of compounds, but exceptions to the rule exist. Meyer and Overton discuss observations that are difficult to explain based on a simple, unified theory. For instance, Meyer recognized that magnesium salts and opioids probably produced their anesthetic effects by a mechanism different than that of the alcohol ‘lipoids’, a finding that was definitively demonstrated by later research.11 Meyer also proposed that the convulsions elicited by some compounds could result from affinity to ‘cell constituents’ other than the ‘cell lipoids’.11
Exceptions to the Meyer-Overton hypothesis can be classified into three general categories.
Highly lipid-soluble molecules which are not anesthetic or produce other physiological effects such as convulsions (e.g., volatile ‘non-immobilizers’, flurothyl).
Molecules which possess low lipid solubility but yet are potent anesthetics (e.g., α-chloralose, chloral hydrate).
Compounds which possess equal lipid solubility but unequal anesthetic potencies (e.g., anesthetic optical isomers).
Closer investigation of chloralose, chloral hydrate, and flurothyl will illustrate these categories.
α-Chloralose
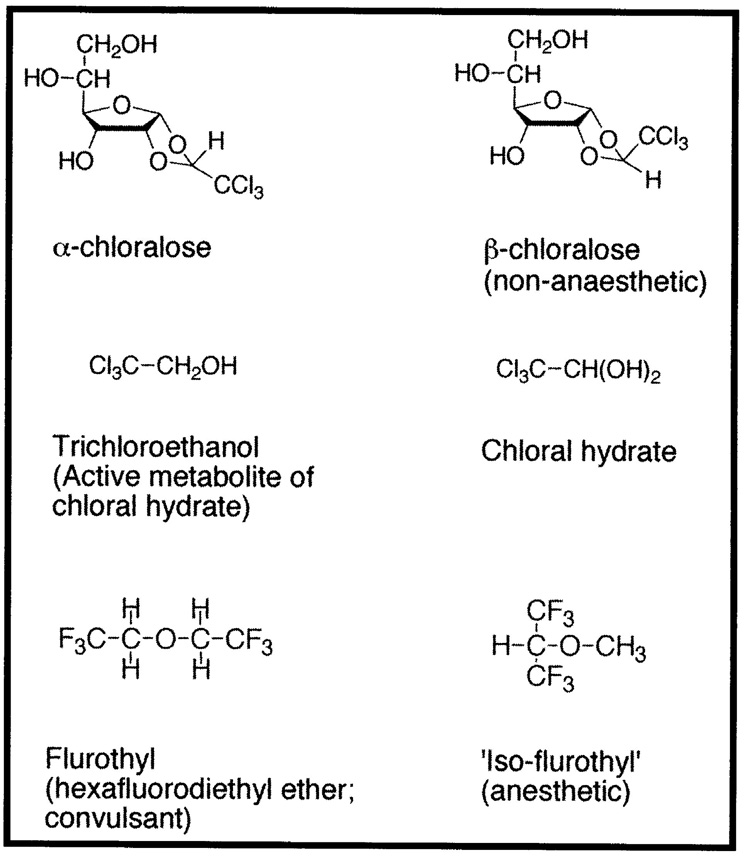
The chloralose molecules are an interesting class of anesthetic compounds, being essentially a hybrid of chloral hydrate and a sugar, with α-chloralose resulting from the condensation of chloral (trichloroacetaldehyde) and glucose (Figure 1). (Chloral hydrate is formed from the reaction of chloral with water). Although evaluated only briefly in the early part of the twentieth century as an anesthetic agent for humans, α-chloralose has found extensive use in veterinary anesthesia12 and as a rodenticide.13 In his 1901 publication Studies of Narcosis (Studien über die Narkose),14 Overton found that anesthetic differences between α-chloralose and its structural isomer β-chloralose were hard to explain. Overton summarized his experiments with the chloralose isomers as follows:
The phenomena of narcosis with α-chloralose are not very easy to interpret … Parachloralose [β-chloralose], which is only very slightly soluble in water in most solutions, has absolutely no narcotic effect. Tadpoles can live for weeks in saturate aqueous solution.14
Figure 1.
A number of investigators returned periodically to the problems of the α- and β-chloralose isomers and verified Overton’s initial observations although problems with chemical purity and uncertainty about molecular composition plagued research with chloralose isomers until the mid-twentieth century.15 Finally in 1940, T.C. Butler and colleagues utilized high-purity α- and β-arabinose optical isomers and synthesized two pairs of arabinochloraloses (i.e., α-d- and α-l-arabinochloraloses; β-d- and β-l-arabinochloralose) that showed anesthetic potency differences not only between the α- and β- structural isomers but also between the respective optical isomer pairs.16 Thus, the anesthetic properties of the chloralose molecules are inconsistent with the Meyer-Overton rule in several ways: (1) α-chloralose is a more potent anesthetic than predicted by the Meyer-Overton rule, (2) optical isomers of α-chloralose have different anesthetic potencies but by definition equal physicochemical properties, and (3) the poorly water-soluble β-chloralose is inactive as an anesthetic. These results prompted Butler to point out:
Those theories which still enjoy any favor are either being hedged about with so many protective reservations or are stated in such general terms that it is difficult to conceive of any feasible experiment which could give unequivocal support to any of them. None of the well-known theories gives special consideration to the role of asymmetry in the mechanism of narcosis … Theories (such as that of Meyer and Overton) which seek to correlate narcotic activity with physical properties are of course inadequate to explain unequal activity of antipodes [i.e., optical isomers].16
Since Overton’s theory was proposed, chloralose was ignored by those supporting a unitary theory of anesthesia. In contrast, Eccles and colleagues utilized the tools of electrophysiology to investigate the actions of α-chloralose on synaptic transmission in the spinal cord beginning in the 1960s.17 These studies and others revealed that α-chloralose exerted barbiturate-like actions on synaptic transmission, including potent effects at inhibitory γ-aminobutyric acid type A (GABAA) receptors.18,19 More recent research using recombinant GABAA receptors demonstrated that while α-chloralose enhances GABA-mediated currents at concentrations equivalent to those that produce anesthesia in animals, β-chloralose is completely inactive at GABAA receptors.20,21 Further, β-chloralose does not antagonize the actions of α-chloralose at GABAA receptors, showing that the binding site of the receptor discriminates between these two isomers.20,21 These results finally provide a coherent pharmacodynamic explanation for the striking pharmacological differences between the chloralose structural isomers nearly one hundred years after Overton’s seminal experiments.
Chloral hydrate
Overton also had difficulty explaining the anesthetic actions of the highly water-soluble but poorly lipid-soluble compound chloral hydrate, stating, “[I]t does not seem improbable to me that the mechanism of action of chloral hydrate is more complex than is the case of most other non-specific narcotics.”14 Much of the confusion surrounding the anesthetic properties of chloral hydrate was resolved in 1948 when T.C. Butler determined that chloral hydrate was metabolized to 2,2,2-trichloroethanol22,23 (not chloroform as previously suggested24). Furthermore, trichloroethanol, and not the parent compound, accounted for all or nearly all of the anesthetic effects that resulted from administration of chloral hydrate to mammals.22,25 Chloral hydrate was thus an example of a general anesthetic that was less potent than predicted by the Meyer-Overton hypothesis, because metabolism to another compound was required for its potent anesthetic actions. Butler summarized his findings as follows:
2,2,2-Trichloroethanol is a substance which, on intravenous injection, appears to be a more active narcotic than chloral hydrate. It is a substance the physical properties of which are more consistent with those of other typical narcotics of comparable activity than are those of chloral hydrate.22
This clashed with the view of general anesthetics as chemically inert molecules which non-specifically alter membrane lipids.
Following the work of Overton, chloral hydrate and trichloroethanol were scarcely discussed during investigations supporting unitary theories of anesthesia, even though the physical properties of trichloroethanol are better compatible with the Meyer-Overton correlation than those of chloral hydrate. A similar lack of attention was paid to the homologous anesthetic 2,2,2-tribromoethanol (Avertin), even though this compound was widely used in humans in the first half of the twentieth century.26 Only in the 1990s did electrophysiology studies reveal that these two halogenated alcohol anesthetics potently modulate GABAA receptor function,21,27 including prolongation of GABAA receptor-mediated inhibitory synaptic transmission of hippocampal neurons.28 Chloral hydrate itself also modulates GABAA receptor function, albeit with a lower potency than trichloroethanol, but at concentrations similar to those which produce loss of righting reflex in tadpoles.21 The research on chloral hydrate also resolved confusion about the homologous compound bromal hydrate, which is analogously metabolized to tribromoethanol.26,29 It should be noted that chloral (or bromal) hydrate probably accounts for some of the anesthetic effect in tadpoles, who inefficient drug metabolism would struggle to hydrolyze the hydrate present in the relatively large solution volume in which they are immersed for the loss of righting reflex assay.30
Flurothyl
The properties of flurothyl (hexafluorodiethyl ether; Figure 1) presented a very different challenge for unitary theories of anesthesia than chloralose or chloral hydrate. Flurothyl was discovered during the active medicinal chemistry efforts of the mid-twentieth century to develop novel inhaled general anesthetics. To improve upon the shortcomings of diethyl ether and chloroform, special attention was paid to compounds which were non-explosive and less toxic. Advances in fluorine chemistry, stimulated by research during the Second World War yielded the modern generation of alkane and ether anesthetics including halothane, enflurane, isoflurane, and methoxyflurane.7,8
These efforts also resulted in the analysis of hundreds of other halogenated ethers and alkanes.31–34 Many of these compounds were rejected because of chemical instability, unpleasant odor, and/or high toxicity and received no further attention. However, some compounds were found to be potent convulsants despite chemical structures similar to compounds which were anesthetics. Flurothyl was one of the compounds with convulsant activity and no apparent anesthetic activity.35–38 As described by Krantz and colleagues in 1957:
White rats exposed to the vapor hexafluorodiethyl ether [i.e., flurothyl] in concentrations as low as 30 ppm … convulsed violently within 30 seconds. There were marked clonic and tonic seizures, and there was some degree of emprosthotonus. The convulsions stopped promptly when the agent was removed from the inspired air. Repeated exposure … did not appear to produce injury to the animals.38
These properties were more striking with the characterization of ‘iso-flurothyl’ (1,1,1,3,3,3-hexafluoro-2-methoxy-propane), a structure isomer of flurothyl which was a potent anesthetic without convulsive properties (Figure 1).39,40 Flurothyl thus provided one of the first well-characterized examples of a highly lipid-soluble general anesthetic analog that lacked anesthetic properties. Despite intense investigation, research failed to provide an explanation consistent with unitary anesthesia theory for the pharmacologic properties of flurothyl, or of the differences between flurothyl and iso-flurothyl, although Koblin, Eger, and colleagues suggested that flurothyl may possess low potency anesthetic properties which are masked by its potent convulsive properties.39 Following extensive investigation into the properties of flurothyl, Krantz and colleagues remarked in 1967, “Not any of the physicochemical properties of measurements conducted on the isomers, flurothyl and ISO[-flurothyl], revealed a marked difference which might be considered responsible for their respective pharmacologic responses.”40 As a historical sidenote, flurothyl ended up having an interesting but brief clinical history in humans when it was marketed under the trade name Indoklon as an inhaled chemical replacement for electroconvulsive therapy in psychiatry.35,36,38
As with chloral hydrate, a plausible molecular mechanism of action for flurothy had to wait until the 1990s. A study published by Wakamori and colleagues in 1991 showed that flurothyl acted as a potent, non-competitive antagonist at GABAA receptors in brainstem neurons, in contrast to halothane and enflurane which were positive allosteric modulators.41 In retrospect, this explained the initial observation by Krantz et al.37,38 that flurothyl convulsions resembled those produced by pentylenetetrazol and picrotoxin.42,43 two compounds later shown to produce convulsions by acting as non-competitive antagonists.44 The differing properties of flurothyl and iso-flurothyl were finally explained in 2000 by the observation that flurothyl antagonized the function of recombinant GABAA receptors while iso-flurothyl enhanced GABAA receptor activity in a manner similar to that produced by isoflurane and sevoflurane.21,45
In addition to α-chloralose, chloral hydrate, and flurothyl, more nettlesome problems for unitary anesthesia theories arose with other anesthetic compounds such as the barbiturates, etomidate, and steroid anesthetics. Examples mounted of isomers or close congeners of anesthetics which were inactive as anesthetics, convulsant, or even able to antagonize the actions of their anesthetic cousins. Further, like the arabinochloraloses, some classes of compounds such as the barbiturates, steroid anesthetics, etomidate, and halogenated ethers shows stereoselectivity in their anesthetics effects (Table 2).
Table 2.
Experimental Observations in Conflict with Unitary Theories of Anesthesia
| Experimental Observation | First year described |
|---|---|
| Barbiturates | |
| Stereoisomers have different anesthetic potencies78 | 1934 |
| Convulsant barbiturates described79 | 1940 |
| Chloral hydrate | |
| Anesthetic properties inconsistent with Meyer-Overton hypothesis14 | 1901 |
| Metabolized to trichloroethanol, which is the active anesthetic compound in mammals22,25 | 1948 |
| Chloraloses | |
| α-Chloralose lack anesthetic activity14 | 1901 |
| Arabinochloraloses show anesthetic potency differences between both optical and structural isomers16 | 1940 |
| Etomidate | |
| Stereoisomers have different anesthetic potencies80,81 | 1975 |
| Stereoisomers equally soluble in lipid bilayers81 | 1998 |
| Flurothyl | |
| Convulsant properties described37,38 | 1957 |
| Isomer ‘iso-flurothyl’ is anesthetic39,40 | 1967 |
| Isoflurane | |
| Stereoisomers have different anesthetic potencies82–84 | 1992 |
| Stereoisomers equally soluble in lipid bilayers85 | 1991 |
| Propofol (2,6-diisopropylphenol) | |
| Alkylphenol analogs such as 2,6-di-tert-butylphenol inactive86–89 | 1980 |
| Steroid anesthetics | |
| Inactive congeners of anesthetic steroids described90,91 | 1965 |
| Stereoselectivity of steroid anesthetics shown92 | 1996 |
| Volatile non-immobilizers | |
| Highly lipid-soluble volatile alkanes and ethers found which lack anesthetic effects93–95 | 1994 |
The Allure of Unitary Theories
Despite some shortcomings, unitary theories have held strong throughout most of the twentieth century. In the last two decades, however, unitary theories have been subjected to a sustained assault by a number of researchers, most prominently Franks and Lieb. Utilizing a combination of physical chemistry and electrophysiology, Franks and Lieb directly challenged the validity of lipid-based theories of anesthesia.2,46–49
Why have unitary theories of anesthetic action proven so compelling? There are several reasons. One, the desire to provide a straightforward answer to an important scientific question is very strong. T.C. Butler speculated on this issue in a 1950 review article:
The metaphysical desire for unification on the part of Claude Bernard and his intellectual heirs among the general physiologists may well have delayed arrival at a more satisfactory solution … Many workers of a later era, likewise impatient to find unifying principles of general physiology, have been led into a disregard of the logical steps needed to establish the relationship of various phenomenon … The concept of one all inclusive phenomenon of ‘narcosis’ does not rest on a very satisfactory basis.50
The Meyer-Overton hypothesis is elegant, compelling, and easily remembered by generations of physicians and scientists. Two, the practice of anesthesiology currently does not require a knowledge of general anesthetic pharmacodynamics. Whether the anesthetics produce their effects by disordering lipids or modulating GABAA receptors does not affect clinical decisions, at least not for the foreseeable future. Three, general anesthetic drugs were in clinical use well before the concept of specific receptors was developed by pioneers such as Clark, Ehrlich, and Langley.51–53 Although some of the initial research into specific protein targets began in the late nineteenth century, these theories were not widely disseminated until after the work of Meyer and Overton was entrenched. Four, unitary theories of anesthesia are versatile, as indicated by the large number of existing theories (Table 1), and difficult to disprove directly due to the technical problems in studying lipids. With a few exceptions such as Franks and Lieb, most researchers who oppose unitary theories do not have the experimental arsenal to challenge the theories directly.
These factors have led to a remarkable resilience of the legacy of Meyer and Overton. Borrowing the terminology of Kuhn from The Structure of Scientific Revolutions, the ‘paradigm shift’ from non-specific to specific mechanisms of anesthesia has progressed slowly.54,55 Perhaps, for the unitary theories, the future will be as Max Planck wrote, “A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents die, and a new generation grows up that is familiar with it.”56
Acknowledgements
The author is supported by NIH grant F30 MH011504.
References
- 1.Meyer H. Welche eigenschaft der Anasthetica bedingt ihre Narkotische wirkung? Naunyn-Schmied Arch Exp Path Pharakol. 1899;42:109–118. [Google Scholar]
- 2.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 3.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckenhoff RG. Do specific or nonspecific interactions with proteins underlie inhalational anesthetic action? Mol Pharmacol. 1998;54:610–615. [PubMed] [Google Scholar]
- 5.Elliott JR, Urban BW. Integrative effects of general anaesthetics: why nerve axons should not be ignored. Eur J Anaesthesiol. 1995;12:41–50. [PubMed] [Google Scholar]
- 6.Halsey MJ. Molecular interactions of anaesthetics with biological membranes. Gen Pharmacol. 1992;23:1013–1016. doi: 10.1016/0306-3623(92)90279-s. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy SK, Longnecker DE. History and principles of anesthesiology. In: Gilman AG, Rall TW, Nies AS, editors. The Pharmacological Basis of Therapeutics. New York: McGraw Hill, Inc.; 1990. pp. 281–282. [Google Scholar]
- 8.Kennedy SK, Longnecker DE. History and principles of anesthesiology. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. pp. 304–305. [Google Scholar]
- 9.Rang HP, Dale MM, Ritter JM, Gardner P. Pharmacology. New York: Churchhill Livingstone; 1995. General anesthetic drugs; p. 533. [Google Scholar]
- 10.Lillie RS. The theory of anæsthesia. Biol Bull. 1916;30:311–366. [Google Scholar]
- 11.Meyer H. Zur Theorie der Alkolnarkose: der Einfuss wehselnder Temperatur auf Wirkungsstarke und Theilungscoefficient der Narcotica. Naunyn-Schmied Arch Exp Path Pharakol. 1901;46:338–346. [Google Scholar]
- 12.Silverman J, Muir WW., 3rd A review of laboratory animal anesthesia with chloral hydrate and chloralose. Lab Anim Sci. 1993;43:210–216. [PubMed] [Google Scholar]
- 13.Gratz NG. A critical review of currently used single-dosed rodenticides. Bull World Health Organiz. 1973;48:469–477. [PMC free article] [PubMed] [Google Scholar]
- 14.Overton E. Studien uber die Narkose, zugleich ein Beitrag zur allgemeiner Pharmakologie. Jena, Switzerland: Gustav Fischer; 1901. [Google Scholar]
- 15.Balis GU, Monroe RR. The pharmacology of chloralose. A review. Psychopharmacologia. 1964;6:1–30. doi: 10.1007/BF00710911. [DOI] [PubMed] [Google Scholar]
- 16.Butler TC. The anesthetic activity of optical antipodes. II. Arabinochloraloses. J Pharmacol Exp Ther. 1940;69:229–235. [Google Scholar]
- 17.Eccles JC, Schmidt R, Willis WD. Pharmacological Studies on Presynaptic Inhibition. J Physiol. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizuka S, Sikdar SK, Yasui S, Oyama Y, Akaike N. α-Chloralose opens the chloride channel of frog isolated sensory neurons. Brain Res. 1989;498:181–184. doi: 10.1016/0006-8993(89)90418-6. [DOI] [PubMed] [Google Scholar]
- 19.Nicoll RA, Wojtowicz JM. The effects of pentobarbital and related compounds on frog motoneurons. Brain Res. 1980;191:225–237. doi: 10.1016/0006-8993(80)90325-x. [DOI] [PubMed] [Google Scholar]
- 20.Garrett KM, Gan J. Enhancement of γ-aminobutyric acidA receptor activity by a-chloralose. J Pharmacol Exp Ther. 1998;285:680–686. [PubMed] [Google Scholar]
- 21.Krasowski MD, Harrison NL. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br J Pharmacol. 2000;129:731–743. doi: 10.1038/sj.bjp.0703087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler TC. The metabolic fate of chloral hydrate. J Pharmacol Exp Ther. 1948;92:49–58. [PubMed] [Google Scholar]
- 23.Butler TC. The introduction of chloral hydrate into medical practice. Bull Hist Med. 1970;44:168–172. [PubMed] [Google Scholar]
- 24.Buchheim R. Die Heilmittellehre und die organische Chemie. Arch Pathol Anat Physiol Klin Med. 1872;56:1–14. [Google Scholar]
- 25.Butler TC. Reduction and oxidation of chloral hydrate by isolated tissues in vitro. J Pharmacol Exp Ther. 1949;95:360–362. [PubMed] [Google Scholar]
- 26.Edwards G. Tribromethyl alcohol (avertin, bromethol), 1928–1945. Proc R Soc Med. 1945;39:71–76. doi: 10.1177/003591574503900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krasowski MD, Finn SE, Ye Q, Harrison NL. Trichloroethanol modulation of recombinant GABAA, glycine and GABA r1 receptors. J Pharmacol Exp Ther. 1998;284:934–942. [PubMed] [Google Scholar]
- 28.Lovinger DM, Zimmerman SA, Levitin M, Jones MV, Harrison NL. Trichloroethanol potentiates synaptic transmission mediated by γ-aminobutyric acidA receptors in hippocampal neurons. J Pharmacol Exp Ther. 1993;264:1097–1103. [PubMed] [Google Scholar]
- 29.Lehman G, Knoefel PK. Trichloroethanol, tribromoethanol, chloral hydrate, and bromal hydrate. J Pharmacol Exp Ther. 1938;63:453–465. [Google Scholar]
- 30.Brodie BB, Maickel RP. Comparative biochemistry of drug metabolism. In: Brodie BB, Erdos EG, editors. Proceedings of the First International Pharmacological Meeting; New York. MacMillan Co.; 1962. pp. 299–324. [Google Scholar]
- 31.Speers L, Szur AJ, Terrell RC. General anesthetics. 4. Methyl pentahaloethyl and methyl heptahaloisopropyl ethers as anesthetic agents. J Med Chem. 1972;15:606–608. doi: 10.1021/jm00276a009. [DOI] [PubMed] [Google Scholar]
- 32.Speers L, Szur AJ, Terrell RC, Treadwell J, Ucciardi TU. General anesthetics. 2. Halogenated methyl isopropyl ethers. J Med Chem. 1971;14:593–595. doi: 10.1021/jm00289a009. [DOI] [PubMed] [Google Scholar]
- 33.Terrell RC, Speers L, Szur AJ, Treadwell J, Ucciardi TR. General anesthetics. 1. Halogenated methyl ethyl ethers as anesthetic agents. J Med Chem. 1971;14:517–519. doi: 10.1021/jm00288a014. [DOI] [PubMed] [Google Scholar]
- 34.Terrell RC, Speers L, Szur AJ, Ucciardi T, Vitcha JF. General anesthetics. 3. Fluorinated methyl ethyl ethers as anesthetic agents. J Med Chem. 1972;15:604–606. doi: 10.1021/jm00276a008. [DOI] [PubMed] [Google Scholar]
- 35.Gander DR, Bennett PJ, Kelly DH. Hexafluorodiethyl ether (indoklon) convulsive therapy: a pilot study. Br J Psychiatry. 1967;113:1413–1418. doi: 10.1192/bjp.113.505.1413. [DOI] [PubMed] [Google Scholar]
- 36.Krantz JC, Jr, Esquibel A, Truitt EB, Jr, Ling AS, Kurland AA. Hexafluorodiethyl ether (indoklon); an inhalant convulsant; its use in psychiatric treatment. J Am Med Assoc. 1958;166:1555–1562. doi: 10.1001/jama.1958.02990130015004. [DOI] [PubMed] [Google Scholar]
- 37.Krantz JC, Jr, Truitt EB, Jr, Ling AS, Speers L, Anesthesia LV. The pharmacologic response to hexafluorodiethyl ether. J Pharmacol Exp Ther. 1957;121:362–368. [PubMed] [Google Scholar]
- 38.Krantz JC, Jr, Truitt EB, Jr, Speers L, Ling AS. New pharmacoconvulsive agent. Science. 1957;126:353–354. doi: 10.1126/science.126.3269.353. [DOI] [PubMed] [Google Scholar]
- 39.Koblin DD, Eger EI, 2nd, Johnson BH, Collins P, Terrell RC, Speers L. Are convulsant gases also anesthetics? Anesth Analg. 1981;60:464–470. [PubMed] [Google Scholar]
- 40.Krantz JC, Jr, Rudo FG, Loecher CK. Anesthesia LXXI. Pharmacologic and physicochemical comparison of flurothyl and its isomer. Proc Soc Exp Biol Med. 1967;124:820–822. doi: 10.3181/00379727-124-31861. [DOI] [PubMed] [Google Scholar]
- 41.Wakamori M, Ikemoto Y, Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J Neurophysiol. 1991;66:2014–2021. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- 42.Bleckwenn WJ, Hodgson ER, Herwick RP. A clinical comparison of picrotoxin, metrazol, and coriamyrtin as analeptics and convulsants. J Pharmacol Exp Ther. 1940;69:81–88. [Google Scholar]
- 43.Browne JC. On the actions of picrotoxine, and the antagonism between picotoxine and chloral hydrate. Br Med J. 1875;1:409–411. doi: 10.1136/bmj.1.743.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker JL, Nicoll RA, Padjen A. Studies on convulsants in the isolated frog spinal cord. I. Antagonism of amino acid responses. J Physiol. 1975;245:521–536. doi: 10.1113/jphysiol.1975.sp010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krasowski MD. Differential modulatory actions of the volatile convulsant flurothyl and its anesthetic isomer at inhibitory ligand-gated ion channels. Neuropharmacology. 2000;39:1168–1183. doi: 10.1016/s0028-3908(99)00221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franks NP, Lieb WR. Where do general anaesthetics act? Nature. 1978;274:339–342. doi: 10.1038/274339a0. [DOI] [PubMed] [Google Scholar]
- 47.Franks NP, Lieb WR. Is membrane expansion relevant to anaesthesia? Nature. 1981;292:248–251. doi: 10.1038/292248a0. [DOI] [PubMed] [Google Scholar]
- 48.Franks NP, Lieb WR. Molecular mechanisms of general anaesthesia. Nature. 1982;300:487–493. doi: 10.1038/300487a0. [DOI] [PubMed] [Google Scholar]
- 49.Franks NP, Lieb WR. Partitioning of long-chain alcohols into lipid bilayers: implications for mechanisms of general anesthesia. Proc Natl Acad Sci U S A. 1986;83:5116–5120. doi: 10.1073/pnas.83.14.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler TC. Theories of general anesthesia. J Pharmacol Exp Ther. 1950;98:121–160. [PubMed] [Google Scholar]
- 51.Clark AJ. The Mode of Action of Drugs on Cells. London: Edward Arnold & Co.; 1933. [Google Scholar]
- 52.Ehrlich P. Über den jetzigen stand der Chemotherapie. Berl Deutsch Chem Ges. 1909;42:17–47. [Google Scholar]
- 53.Langley JN. On the physiology of salivary secretions. Part II. On the mutual antagonism of atropin and pilocarpin, having especial reference to their relations to in Sub-maxillary Gland of the Cat. J Physiol. 1878;1:339–369. doi: 10.1113/jphysiol.1878.sp000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuhn TS. The Copernican Revolution: Planetary Astronomy in the Development of Western Thought. New York: Vintage Press; 1959. [Google Scholar]
- 55.Kuhn TS. The Structure of Scientific Revolution. Chicago: University of Chicago Press; 1970. [Google Scholar]
- 56.Planck M. Scientific Autobiography. New York: Philosophical Library; 1949. [Google Scholar]
- 57.von Bibra E, Harless E. Die Wirkung des Schwefeläthers in chemischen and physiologischen bezigkung. Germany: Erlangen; 1847. [Google Scholar]
- 58.Bernard C. Leçons sur les anesthesique et sur l'asphyxie. Paris: J.B. Balliere et Fils; 1875. [Google Scholar]
- 59.Traube J. Theorie der Osmose and Narkose. Pflüg Arch Physiol. 1904;105:541–558. [Google Scholar]
- 60.Meyer KH. Contribution to the theory of narcosis. Trans Faraday Soc. 1937;33:1062–1068. [Google Scholar]
- 61.Ferguson J. The use of chemical potentials as indices of toxicity. Proc Roy Soc Lond - Ser B: Biol Sci. 1939;197:387–404. [Google Scholar]
- 62.Mullins LJ. Some physical mechanisms in narcosis. Chem Rev. 1954;54:289–323. [Google Scholar]
- 63.Pauling L. A molecular theory of general anesthesia. Science. 1961;134:15–21. doi: 10.1126/science.134.3471.15. [DOI] [PubMed] [Google Scholar]
- 64.Pauling L. The Hydrate Microcrystal Theory of General Anesthesia. Anesth Analg. 1964;43:1–10. [PubMed] [Google Scholar]
- 65.Miller SL. A theory of gaseous anesthetics. Proc Natl Acad Sci U S A. 1961;47:1515–1524. doi: 10.1073/pnas.47.9.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Metcalfe JC, Seeman P, Burgen AS. The proton relaxation of benzyl alcohol in erythrocyte membranes. Mol Pharmacol. 1968;4:87–95. [PubMed] [Google Scholar]
- 67.Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972;24:583–655. [PubMed] [Google Scholar]
- 68.Miller KW, Paton WD, Smith RA, Smith EB. The pressure reversal of general anesthesia and the critical volume hypothesis. Mol Pharmacol. 1973;9:131–143. [PubMed] [Google Scholar]
- 69.Lee AG. Interactions between anesthetics and lipid mixtures. Normal alcohols. Biochemistry. 1976;15:2448–2454. doi: 10.1021/bi00656a031. [DOI] [PubMed] [Google Scholar]
- 70.Trudell JR. A unitary theory of anesthesia based on lateral phase separations in nerve membranes [proceedings] Biophys J. 1977;18:358–359. doi: 10.1016/S0006-3495(77)85622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halsey MJ, Wardley-Smith B, Green CJ. Pressure reversal of general anaesthesia--a multi-site expansion hypothesis. Br J Anaesth. 1978;50:1091–1097. doi: 10.1093/bja/50.11.1091. [DOI] [PubMed] [Google Scholar]
- 72.Bangham AD, Mason WT. Anesthetics may act by collapsing pH gradients. Anesthesiology. 1980;53:135–141. doi: 10.1097/00000542-198008000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Brockerhoff H, Zingoni J, Brockerhoff S. Mechanism of anesthesia: anesthetics may restructure the hydrogen belts of membranes. Neurochem Intl. 1990;17:15–19. doi: 10.1016/0197-0186(90)90062-x. [DOI] [PubMed] [Google Scholar]
- 74.Chiou JS, Ma SM, Kamaya H, Ueda I. Anesthesia cutoff phenomenon: interfacial hydrogen bonding. Science. 1990;248:583–585. doi: 10.1126/science.2159183. [DOI] [PubMed] [Google Scholar]
- 75.Gruner SM, Shyamsunder E. Is the mechanism of general anesthesia related to lipid membrane spontaneous curvature? Ann N Y Acad Sci. 1991;625:685–697. doi: 10.1111/j.1749-6632.1991.tb33902.x. [DOI] [PubMed] [Google Scholar]
- 76.Qin Z, Szabo G, Cafiso DS. Anesthetics reduce the magnitude of the membrane dipole potential. Measurements in lipid vesicles using voltage-sensitive spin probes. Biochemistry. 1995;34:5536–5543. doi: 10.1021/bi00016a027. [DOI] [PubMed] [Google Scholar]
- 77.Cantor RS. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 1997;36:2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 78.Kleiderer EC, Schonle HA. Studies on some optically active barbituric acids. J Am Chem Soc. 1934;56:1772–1774. [Google Scholar]
- 79.Adams RC. Intravenous anesthesia. New York: Paul B. Hoeber, Inc.; 1944. [Google Scholar]
- 80.Heykants JJ, Meuldermans WE, Michiels LJ, Lewi PJ, Janssen PA. Distribution, metabolism and excretion of etomidate, a short-acting hypnotic drug, in the rat. Comparative study of (R)-(+)-(--)-Etomidate. Arch Int Pharmacodyn Ther. 1975;216:113–129. [PubMed] [Google Scholar]
- 81.Tomlin SL, Jenkins A, Lieb WR, Franks NP. Stereoselective effects of etomidate optical isomers on γ-aminobutyric acid type A receptors and animals. Anesthesiology. 1998;88:708–717. doi: 10.1097/00000542-199803000-00022. [DOI] [PubMed] [Google Scholar]
- 82.Dickinson R, White I, Lieb WR, Franks NP. Stereoselective loss of righting reflex in rats by isoflurane. Anesthesiology. 2000;93:837–843. doi: 10.1097/00000542-200009000-00035. [DOI] [PubMed] [Google Scholar]
- 83.Harris B, Moody E, Skolnick P. Isoflurane anesthesia is stereoselective. Eur J Pharmacol. 1992;217:215–216. doi: 10.1016/0014-2999(92)90875-5. [DOI] [PubMed] [Google Scholar]
- 84.Lysko GS, Robinson JL, Casto R, Ferrone RA. The stereospecific effects of isoflurane isomers in vivo. Eur J Pharmacol. 1994;263:25–29. doi: 10.1016/0014-2999(94)90519-3. [DOI] [PubMed] [Google Scholar]
- 85.Franks NP, Lieb WR. Stereospecific effects of inhalational general anesthetic optical isomers on nerve ion channels. Science. 1991;254:427–430. doi: 10.1126/science.1925602. [DOI] [PubMed] [Google Scholar]
- 86.James R, Glen JB. Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents. J Med Chem. 1980;23:1350–1357. doi: 10.1021/jm00186a013. [DOI] [PubMed] [Google Scholar]
- 87.Krasowski MD, Hong X, Hopfinger AJ, Harrison NL. 4D-QSAR analysis of a set of propofol analogues: mapping binding sites for an anesthetic phenol on the GABAA receptor. J Med Chem. 2002;45:3210–3221. doi: 10.1021/jm010461a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of γ-aminobutyric acid (GABA) current at the GABAA receptor but not with lipid solubility. J Pharmacol Exp Ther. 2001;297:338–351. [PubMed] [Google Scholar]
- 89.Krasowski MD, Nishikawa K, Nikolaeva N, Lin A, Harrison NL. Methionine 286 in transmembrane domain 3 of the GABAA receptor β subunit controls a binding cavity for propofol and other alkylphenol general anesthetics. Neuropharmacology. 2001;41:952–964. doi: 10.1016/s0028-3908(01)00141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atkinson RM, Davis B, Pratt MA, Sharpe HM, Tomich EG. Action of some steroids on the centtral nervous system of the mouse. II. Pharmacology. J Med Chem. 1965;8:426–432. doi: 10.1021/jm00328a004. [DOI] [PubMed] [Google Scholar]
- 91.Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- 92.Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced γ-aminobutyric acidA receptor modulation and anesthesia. Mol Pharmacol. 1996;50:1581–1586. [PubMed] [Google Scholar]
- 93.Eger EI, 2nd, Koblin DD, Sonner J, Gong D, Laster MJ, Ionescu P, Halsey MJ, Hudlicky T. Nonimmobilizers and transitional compounds may produce convulsions by two mechanisms. Anesth Analg. 1999;88:884–892. doi: 10.1097/00000539-199904000-00037. [DOI] [PubMed] [Google Scholar]
- 94.Koblin DD, Chortkoff BS, Laster MJ, Eger EI, 2nd, Halsey MJ, Ionescu P. Polyhalogenated and perfluorinated compounds that disobey the Meyer-Overton hypothesis. Anesth Analg. 1994;79:1043–1048. doi: 10.1213/00000539-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Mihic SJ, McQuilkin SJ, Eger EI, 2nd, Ionescu P, Harris RA. Potentiation of γ-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol Pharmacol. 1994;46:851–857. [PubMed] [Google Scholar]