Abstract
mTOR-complex 2 (mTORC2) contains the mammalian target of rapamycin (mTOR) kinase and the rictor regulatory protein, and phosphorylates Akt. Whether this function of mTORC2 is critical for cancer progression is unknown. Here, we show that transformed human prostate epithelial cells lacking PTEN require mTORC2 to form tumors when injected into nude mice. Furthermore, we find that Rictor is a haploinsufficient gene and deleting one copy protects Pten heterozygous mice from prostate cancer. Finally, we show that the development of prostate cancer caused by Pten deletion specifically in the prostate epithelium requires mTORC2, but that for normal prostate epithelial cells mTORC2 activity is nonessential. The selective requirement for mTORC2 in tumor development suggests that mTORC2 inhibitors may be of substantial clinical utility.
Significance
Small molecule inhibitors that compromise cancer but not normal cell functions would be valuable anti-cancer therapeutics. Identifying intracellular targets for this type of inhibitor is challenging. We present genetic evidence that mTOR complex 2 (mTORC2) is a candidate target for such an inhibitor as the development of invasive prostate cancer induced by Pten loss in mice requires mTORC2 activity. However, mTORC2 activity is dispensable for the development of a normal prostate epithelium in mice and for the proliferation and survival of primary mouse fibroblasts in culture. PTEN loss activates the PI3K signaling pathway, which is inappropriately activated in many human cancers. Our findings suggest that mTORC2 inhibitors could have broad clinical applications.
Keywords: mTOR, mTORC1, mTORC2, PTEN, Akt, PKB, prostate cancer, rapamycin
Introduction
The PI3K signaling pathway is aberrantly active in many human cancers. The best-characterized downstream target of PI3K activation is the Akt kinase, which influences cancer cell metabolism, survival, growth, proliferation, angiogenesis, and migration by phosphorylating a diverse array of substrates [Reviewed in (Manning and Cantley, 2007)]. Two common causes of aberrant PI3K activation include loss of the PTEN tumor suppressor and activating mutations in PI3K. Either event results in PtdIns (3,4,5)P3 accumulation at cell membranes, which serves as a docking site for Akt localization and activation. Akt requires phosphorylation on two sites for full activation [Reviewed in (Guertin and Sabatini, 2007; Hanada et al., 2004; Manning and Cantley, 2007)]. Following membrane recruitment, PDK1 phosphorylates Akt at one site (T308 in Akt1) in the kinase domain while mTOR phosphorylates Akt at another site (S473 in Akt1) in a C-terminal hydrophobic motif [Reviewed in (Guertin and Sabatini, 2007; Hanada et al., 2004; Manning and Cantley, 2007).
The mTOR kinase, which is the target of the drug rapamycin, assembles into at least two distinct complexes called mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), each having unique substrates [Reviewed in (Guertin and Sabatini, 2007)]. In addition to mTOR, mTORC1 contains Raptor, PRAS40, and mLST8/GβL and regulates cell growth by controlling the activity of the S6 kinases and the 4E-BP proteins. mTORC2 contains mLST8/GβL and two unique regulatory proteins named Rictor and SIN1, and a protein of unknown function called PROTOR/PRR5. When assembled into mTORC2, mTOR phosphorylates Akt (Guertin et al., 2006; Jacinto et al., 2006; Sarbassov et al., 2005; Shiota et al., 2006). Rapamycin, despite being a potent inhibitor of mTORC1, does not appear to be a general inhibitor of mTORC2. However, in a subset of human cancer cells, rapamycin inhibits mTORC2 by preventing its assembly but the determinants of this phenomenon are unknown (Sarbassov et al., 2006).
Efforts to develop PI3K inhibitors or inhibitors of downstream effectors like Akt are intense. One Akt-regulated pathway receiving considerable interest from drug development enterprises is the mTORC1 growth pathway [Reviewed in (Abraham and Eng, 2008)]. Akt activates mTORC1 by phosphorylating and inhibiting TSC2, a GTPase activating protein that negatively regulates mTORC1 activity [Reviewed in (Manning and Cantley, 2007)]. Akt also activates mTORC1 directly by phosphorylating the PRAS40 subunit (Haar et al., 2007; Huang and Porter, 2005; Kovacina et al., 2003; Sancak et al., 2007). Other well-known targets of Akt include the FoxO transcription factors and the GSK3 kinase [Reviewed in (Manning and Cantley, 2007)].
Genetic studies in mice have been crucial to understanding the role of PI3K activation in tumorigenesis (Salmena et al., 2008). Pten heterozygous mice spontaneously develop neoplasms in multiple organs, resulting in a shortened lifespan (Di Cristofano et al., 1998; Freeman et al., 2006; Podsypanina et al., 1999; Suzuki et al., 1998a). Reports demonstrating that mice expressing a hypomorphic allele of Pdk1 or lacking Akt1 (one of three Akt genes in mammals) are protected from tumor development induced by Pten loss highlight the importance of Akt activity (Bayascas et al., 2005; Chen et al., 2006).
Mice expressing conditional alleles of Pten have also been developed to study the role of PTEN in tissue specific cancers (Backman et al., 2001; Trotman et al., 2003; Wang et al., 2003). Human prostate cancer in particular shows strong association with PTEN loss (Dahia, 2000; Sellers and Sawyers, 2002; Suzuki et al., 1998b). Expressing Cre recombinase in prostate epithelial cells of PtenloxP/loxP mice results in Pten deletion and Akt hyper-phosphorylation in the prostate epithelium (Wang et al., 2003). These mice develop epithelial hyperplasia, which progresses to prostatic intraepithelial neoplasia (PIN) within 6 weeks of age, and to invasive adenocarcinoma and metastasis by 9-12 weeks in manner that recapitulates the disease progression in humans. Similar to human prostate cancer, tumors in these mice derive from the prostate epithelium, respond to androgen ablation, and exhibit similar gene expression changes, making them a useful model to study the disease.
An ideal target for an anti-cancer drug is one that when inhibited has no effect on normal cells but compromises the proliferation and/or survival of cancer cells. We hypothesize that mTORC2 may be such a target in cancers driven by PI3K-activation because previous studies show it is not required for survival of mouse embryo fibroblasts or for the development of Drosophila embryos (Guertin et al., 2006; Hietakangas and Cohen, 2007; Jacinto et al., 2006; Shiota et al., 2006). The role of mTORC2 in tumorigenesis cannot be tested pharmacologically because specific inhibitors of mTORC2 are currently unavailable. Therefore, to test our hypothesis we investigated the role of mTORC2 activity in (1) a human prostate cancer epithelial cell line which lacks PTEN expression; (2) in a spontaneous mouse model of cancer dependent upon Pten loss; and (3) in a mouse model of prostate cancer initiated by Pten deletion specifically in prostate epithelial cells.
Results
Rictor is required for PC-3 cells to form tumor xenografts
Human prostate cancer is associated with loss of the PTEN tumor suppressor (Dahia, 2000; Sellers and Sawyers, 2002; Suzuki et al., 1998b). To test if PTEN-deficient human prostate cancer cells require mTORC2 activity to form tumors, we asked if knocking down Rictor in the PC-3 cell line (a human prostate cancer cell line null for PTEN) impairs their ability to form tumors when injected into nude mice. Two independent shRNA hairpins targeting Rictor (shRictor1 and shRictor2) or a control shRNA targeting luciferase (shLuc) were delivered by lentivirus and stably expressed in PC-3 cells. We selected hairpins that differentially decrease Rictor expression and show that in vitro, shRictor1 robustly decreases total Rictor protein while shRictor2 reduces Rictor to an intermediate degree [Figure 1A]. Both Rictor hairpins reduce AktS473 phosphorylation to levels consistent with the level of Rictor knockdown [Figure 1A]. In vitro, both shRictor1 and shRictor2 knockdown cells have a proliferation defect, the severity of which also correlates with the level of Rictor knockdown [Figure 1B].
Figure 1. PC-3 prostate cancer cells require Rictor to form tumors as xenografts.
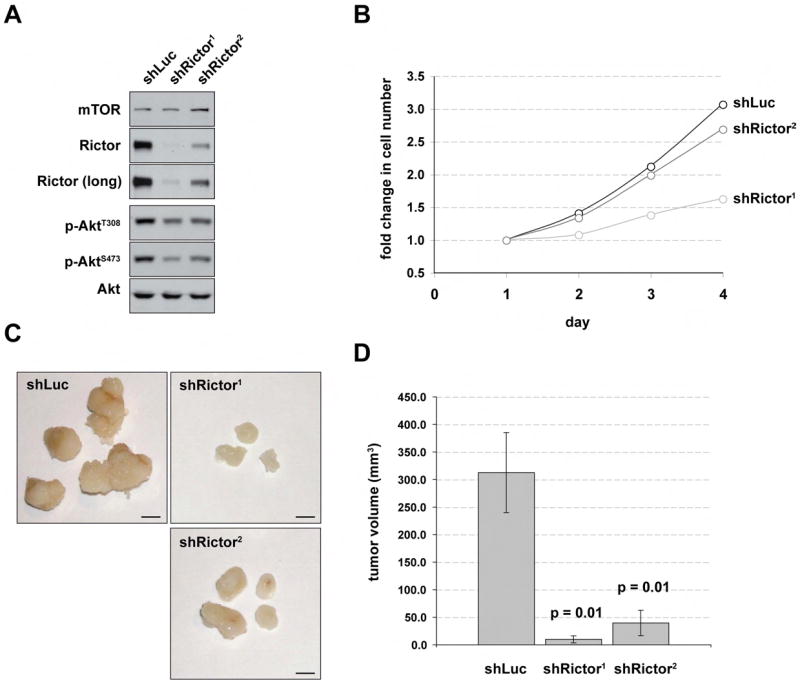
(A) Generation of PC-3 cells with stable knockdown of Rictor. Using a lentiviral delivery system, two independent shRNAs (shRictor1 or shRictor2) that silence Rictor expression or a control shRNA targeting luciferase (shLuc) were stably expressed in PC-3 cells. Protein lysates prepared 6 days post-infection were probed with the indicated antibodies. (B) Rictor knockdown impairs cell proliferation in vitro. PC-3 cells stably expressing shLuc, shRictor1 or shRictor2 were seeded in triplicate at equal density and cell number was counted on four consecutive days. Fold change in cell number is shown. (C) PC-3 cells require mTORC2 to form tumors as xenogfrafts. PC-3 cells stably expressing shLuc, shRictor1 or shRictor2 were injected subcutaneously into nude mice. Tumors were dissected and photographed 28 days post injection. Scale bar = 2mm (D) Average tumor volume (mm3) and SEM is plotted.
To determine if Rictor depletion affects the ability of PC-3 cells to form solid tumors in vivo, we injected the PC-3 cells expressing shLuc, shRictor1, or shRictor2 subcutaneously into nude mice and monitored tumor formation for 28 days, at which point tumors were removed and measured. PC-3 cells expressing shLuc form tumors that are visibly larger than tumors formed by PC-3 cells expressing either shRictor1 or shRictor2 [Figure 1C]. The shLuc-expressing cells grow tumors to an average volume of 312 mm3 [Figure 1D]. In contrast, shRictor1- and shRictor2-expressing cells grow tumors to 10.1 mm3 (p = 0.01) and 39.6 mm3 (p = 0.01) respectively with the difference in tumor volume again corresponding to Rictor knockdown levels [Figure 1D]. Our findings indicate that PC-3 cells require mTORC2 activity to form tumors in vivo.
Partial loss of mTORC2 activity extends the lifespan of Pten+/- mice and can protect mice against prostate cancer
Our xenograft study suggests that mTORC2 activity may be important in prostate cancer development when the PTEN tumor suppressor is lost. We next asked if reducing mTORC1 and/or mTORC2 activity in a genetic model of cancer dependent upon Pten loss could extend lifespan. Pten+/- mice spontaneously develop tumors in multiple organs resulting in a shortened lifespan (Di Cristofano et al., 1998; Freeman et al., 2006; Podsypanina et al., 1999; Suzuki et al., 1998a). We crossed Pten+/- mice with mice heterozygous for the mtor, Raptor, mlst8, or Rictor genes and the offspring were monitored for 52 weeks. Interestingly, Pten+/-mtor+/- (p = 0.023) and Pten+/-mlst8+/- (p = 0.044) mice live longer than strain-matched Pten+/- controls [Figure 2A]. In contrast, Pten+/-Raptor+/- mice show no difference in lifespan compared to Pten+/- controls. Pten+/-Rictor+/- mice also tend to live longer than Pten+/- mice but the effect is less pronounced due to a difference in the Rictor+/- mouse strain composition (see Methods and (Freeman et al., 2006). In developing mouse embryos, only mTORC2 requires mlst8 and Rictor, while mTORC1 requires Raptor (Guertin et al., 2006). Thus lifespan extension of Pten+/-mice doubly heterozygous for mtor, mlst8, or Rictor likely reflects a reduced capacity for mTORC2 signaling, although this does not rule out the possibility that reducing mTORC1 activity may also contribute to extending lifespan.
Figure 2. Phenotypes associated with Pten heterozygosity require mTORC2.
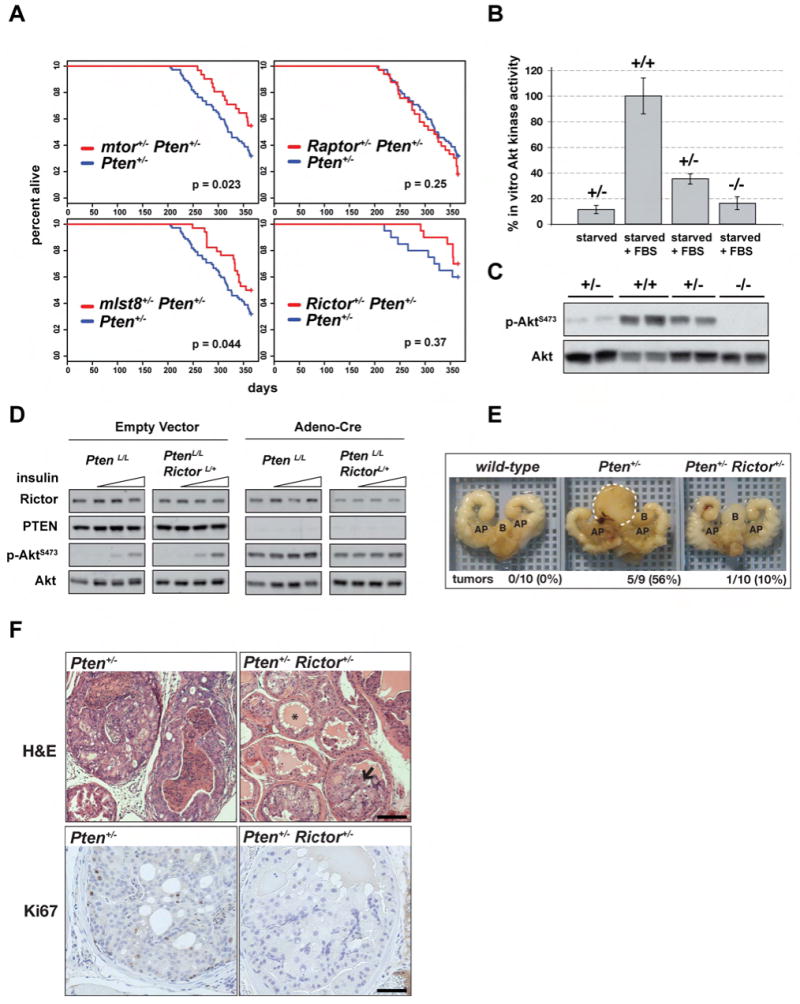
(A) Pten+/- mice lacking one mtor, mlst8, or Rictor gene tend to live longer. Survivability was monitored for 1 year and displayed using Kaplan-Meyer plots. (B-C) Rictor+/- MEFs have reduced Akt activity. (B) Akt was isolated from serum deprived (starved) or stimulated (+FBS) MEFs that were wild-type or deleted for one or both Rictor genes. The ability of immunopurified Akt to incorporate radiolabeled phosphate into a synthetic substrate was measured. Bars indicate standard error. (C) Immunoblot corresponding to (b) showing phospho-AktS473 and total Akt levels. (D) Adeno-Cre was added to MEFs harboring conditional alleles of Pten and Rictor (PtenL/L and PtenL/LRictorL/+) to generate Pten-null MEFs lacking only one Rictor gene. Empty vector was added to generate control cells. Lysates were prepared from serum-deprived cells that were stimulated with 0, 1, 10, or 100nm insulin for 10 minutes and probed with the indicated antibodies. (E-F) Pten+/-Rictor+/- mice are protected against prostate tumor development. (E) GU tracts of wild-type, Pten+/- and Pten+/-Rictor+/- mice that survived to 1 year were dissected and photographed. Bladder (B) and Anterior Prostate (AP) are indicated for orientation. An example of a large tumor associated with a Pten+/- prostate is circled with a white dotted line. (F) Pten+/- and Pten+/-Rictor+/- prostate tissue was sectioned and stained by Hematoxylin & Eosin (top) or labeled with Ki-67 antibody (bottom). Representative images are shown. The star (*) indicates a normal prostate ductule and the arrow points to a ductule containing large hyperplastic cells. Scale bar = 50μm (top) and 25μm (bottom).
To confirm that deleting one Rictor gene reduces mTORC2 signaling, we examined Akt activity in Rictor heterozygous MEFS. We find that deleting one Rictor gene reduces the serum-stimulated in vitro kinase activity of Akt [Figure 2B] and that this correlates with reduced AktS473 phosphorylation [Figure 2C]. To generate MEFs that are null for Pten and are also missing only one Rictor gene, we infected PtenloxP/loxP and PtenloxP/loxPRictorLoxP/+ MEFs with a control Adenovirus or Adenovirus expressing the Cre recombinase (Adeno-CRE) and asked whether partial loss of Rictor impairs Pten-deletion induced AktS473 phosphorylation. We find that deleting one Rictor gene slightly reduces the increase in AktS473 phosphorylation caused by Pten-deletion and prevents insulin from further increasing the phospho-AktS473 signal [Figure 2D]. Moreover, loss of one Rictor gene reduces the expected Mendelian ratio at birth (Guertin et al., 2006) and decreases AktS473 phosphorylation in liver tissue (Yang et al., 2006). Thus, Rictor is a haploinsufficient gene and deleting one copy diminishes mTORC2 activity.
Because we are interested in the role of mTORC2 in prostate cancer, we examined the prostate tissue of offspring born from Pten+/- and Rictor+/- crosses that had survived for one year. Five out of nine Pten+/- male mice had visible tumors, while only one of ten surviving Pten+/-Rictor+/- mice had a similar phenotype [Figure 2E]. No tumors were detectable in any of the wild-type controls (n = 10). A comparison of hematoxylin & eosin (H&E) stained sections generated from these samples indicates that Pten+/- mice develop severe neoplasia [Figure 2F]. Prostates from Pten+/-Rictor+/- mice exhibit signs of hyperplasia [Figure 2F]. However, in contrast to the lesions observed in Pten+/- mice, the lesions in the double heterozygous mice appear less severe and contain larger cells. There are also fewer proliferating cells in the Pten+/-Rictor+/- samples as only 2.3% (19/836) of the cells in three representative images are positive for the proliferation marker Ki67 compared with 10.7% (203/1902) of the cells from Pten+/-samples (p < 0.001) [Figure 2F]. Thus, the haploinsufficiency associated with losing one Rictor gene protects Pten+/- mice from prostate cancer. Because of a difference in genetic background between the Rictor+/- mice and the mtor, Raptor, and mlst8 heterozygous mice, Pten+/- controls born from mtor+/-, Raptor+/-, or mlst8+/- and Pten+/- parents are less susceptible to prostate cancer and this precludes an analysis of prostate cancer in these cohorts (Freeman et al., 2006; Guertin et al., 2006).
Rictor is required for Pten-deletion-induced Akt phosphorylation and transformation of prostate epithelial cells in vivo
The observation that partial loss of Rictor protects Pten+/- mice from prostate cancer prompted us to examine this role of mTORC2 more rigorously. Conditional deletion of Pten specifically in the prostate epithelium leads to prostate cancer with short latency and a histological pattern of disease progression modeling that of human patients with prostate cancer (Wang et al., 2003). In this model, expression of Cre recombinase from a modified rat probasin promoter (Wu et al., 2001) in conditional Pten mice (PtenloxP/loxPPB-Cre+) induces post-natal deletion of Pten in prostate epithelial cells (Wang et al., 2003; Wu et al., 2001). PtenloxP/loxPPB-Cre+ mice develop mPIN by 6 weeks of age, which progresses to invasive adenocarcinoma by 9 weeks, and then to metastatic cancer by 12 weeks (Wang et al., 2003). To determine the role of mTORC2 in the progression of cancer in this model, we used conditional alleles to delete Rictor in combination with Pten.
To test the efficiency of the double knockout strategy, primary MEFs derived from PtenloxP/loxP or PtenloxP/loxPRictorloxP/loxP embryos were infected with Cre-expressing adenoviruses (Adeno-Cre) and cultured for five days. PTEN and Rictor protein and Akt phosphorylation levels were then measured by Western analysis. As expected, infection of PtenloxP/loxP MEFs with Adeno-Cre reduces PTEN expression, and elevates both AktS473 and AktT308 phosphorylation in serum-deprived cells [Figure 3A, lanes 1 & 3] and in insulin stimulated cells [Figure 3A, lanes 2 & 4]. In contrast, PtenloxP/loxPRictorloxP/loxP MEFs infected with Adeno-CRE show no increase in AktS473 phosphorylation despite reduced Pten expression [Figure 3A, lanes 5 & 7]. Furthermore, these MEFs exhibit only a small increase in S473 phosphorylation following insulin stimulation [Figure 3A, lanes 6 & 8]. This slight increase in S473 phosphorylation in insulin-stimulated PtenloxP/loxPRictorloxP/loxP MEFs likely results from a subpopulation of cells that Adeno-Cre failed to infect as an immunoblot analysis clearly shows that not all Rictor is lost. As expected in MEFs, insulin regulation of AktT308 phosphorylation and S6KT389 phosphorylation (a reporter for mTORC1 activity) are unaffected by Rictor deletion [Figure 3 and (Guertin et al., 2006)].
Figure 3. Pten-deletion-induced phosphorylation of AktS473 in MEFs and in prostate epithelial cells requires Rictor.
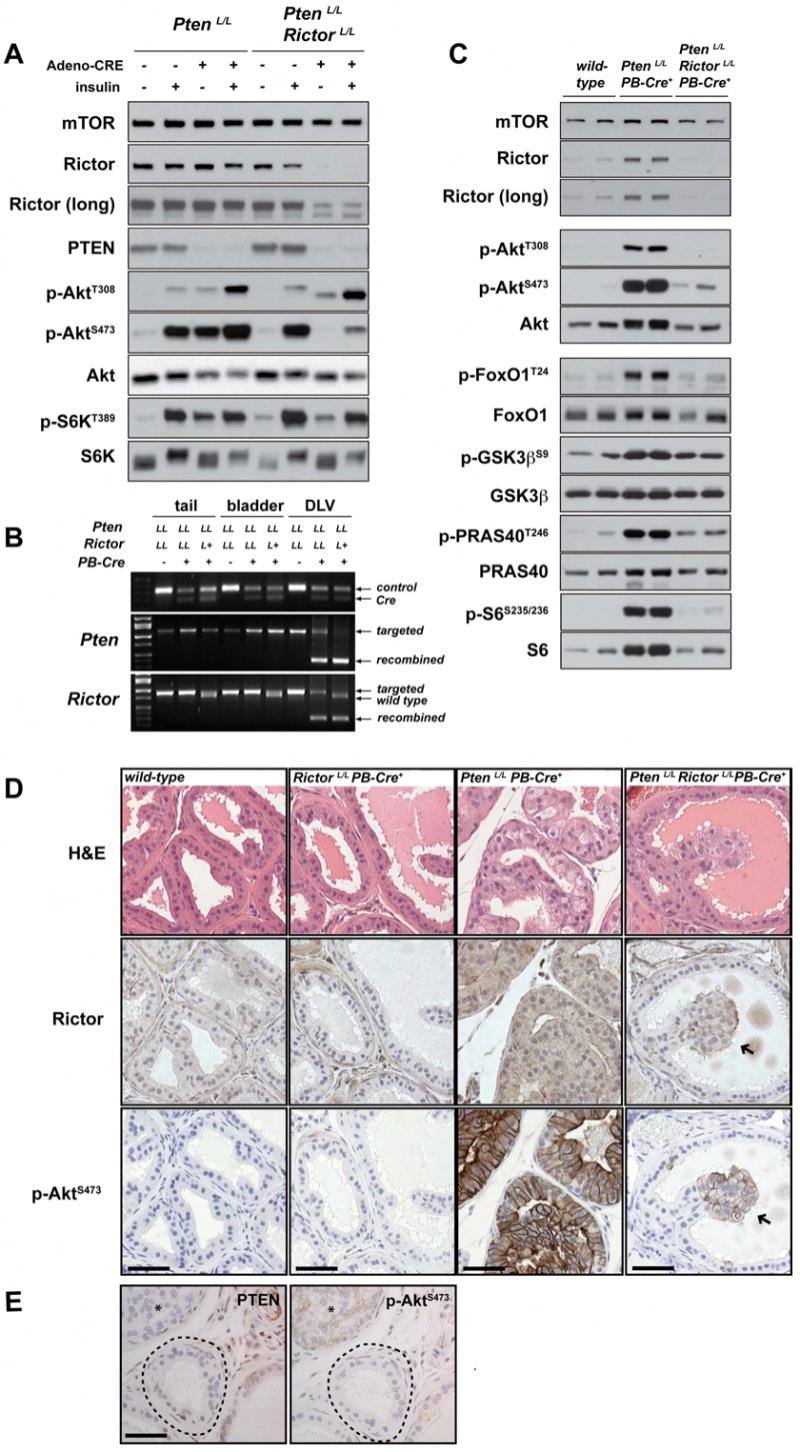
(A) Deleting Rictor in combination with Pten blocks hyper-phosphoyrlation of AktS473 in MEFs. PtenLoxP/LoxP or PtenLoxP/LoxP RictorLoxP/LoxP MEFs were infected with Adeno-Cre virus. After 5-days, cells were starved and stimulated with 300nm insulin for 15 minutes. Protein lysates were prepared and probed with the indicated antibodies. For the Rictor immunoblot, ‘”long” indicates longer exposure. (B) The PB-Cre4 transgene induces recombination of Pten and Rictor conditional alleles only in prostate tissue and not in bladder or tail tissue. A sample set of PCR reactions is shown. DLV indicates pooled tissue from dorsal, lateral, and ventral prostate. (C) Deleting Rictor in combination with Pten inhibits AktS473 phosphorylation and activity towards downstream substrates in vivo. Protein lysates were prepared from dorsolateral prostates dissected from wild-type, PtenLoxP/LoxP PB-Cre+ or PtenLoxP/LoxP RictorLoxP/LoxP PB-Cre+ mice and probed with the indicated antibodies. (D-E) Rictor deletion does not affect normal prostate architecture but is required for AktS473 phosphorylation induced by Pten deletion. (D) Serial sections of 7-week old prostate tissue stained by H&E, or labeled with antibodies to Rictor or phospho-AktS473 are shown. Arrows point to a patch of cells in which inefficient Rictor deletion results in abnormal AktS473 positive cells. Scale bar = 50μM. (E) Cells lacking PTEN and phospho-AktS473 appear normal (circled area), consistent with loss of mTORC2 activity blocking transformation. For comparison, this example includes a nearby section of hyperplastic cells (indicated by the star) that stain negative for PTEN and positive for AktS473, which is indicative of inefficient Rictor deletion. Scale bar = 50μm.
We then asked whether conditional deletion of Rictor inhibits the increase in AktS473 phosphorylation induced by Pten-deletion in the prostate. PCR analysis of prostate tissue samples from Pten conditional mice that harbor either one or two conditional alleles of Rictor and the PB-Cre+ transgene indicates that recombination occurs simultaneously in the prostate epithelium, but not in tail or bladder tissue prepared from the same animals [Figure 3B]. Next, we generated protein lysates from dosolateral prostate tissue of wild-type, PtenloxP/loxP PB-Cre+, and PtenloxP/loxPRictorloxP/loxP PB-Cre+ animals. By immunoblot analysis, we find that mTOR protein levels remain unchanged in all three genotypes [Figure 3C]. In contrast, total Rictor protein increases in PtenloxP/loxP PB-Cre+ prostate tissue, but is mostly absent in PtenloxP/loxPRictorloxP/loxP PB-Cre+ tissue [Figure 3C]. As expected, Pten-loss substantially elevates both AktT308 and AktS473 phosphorylation. Importantly, the increase in phospho-AktS473 is suppressed in PtenloxP/loxPRictorloxP/loxP PB-Cre+ tissue indicating that AktS473 phosphorylation caused by Pten deletion requires mTORC2. Interestingly, we find that the increase in T308 phosphorylation induced by Pten loss also requires mTORC2. This is different to what we observe in MEFs [Figure 3A and (Guertin et al., 2006)] but is more similar to the situation in human cancer cells in which phosphorylation at T308 requires mTORC2 activity [Figure 1A and (Hresko and Mueckler, 2005; Sarbassov et al., 2005)].
Genitourinary tracts were isolated from 7-week-old wild-type, Rictor conditional, Pten conditional, or doubly conditional PB-Cre+ mice. H&E staining and immunohistochemisty with a Rictor antibody performed on dorsolateral prostate tissue indicates that compared to wild-type epithelial cells, RictorloxP/loxPPB-Cre+ cells are slightly smaller, but the overall architecture of the prostate epithelial cell layer is normal [Figure 3D and S1]. Consistent with this observation, immortalized Rictor-null MEFs are smaller than wild-type MEFs in volume by ∼10% while they proliferate at the same rate [Supplemental Figure S1]. We have examined the histology of prostate tissue from RictorloxP/loxPPB-Cre+ mice as old as 16 weeks and find no obvious differences when compared to age matched wild-type tissue [data not shown]. In both wild-type and RictorloxP/loxPPB-Cre+ tissue, AktS473 phosphorylation is undetectable [Figure 3D]. Thus, Rictor, and by extension, mTORC2, is not required to maintain the integrity of a normal prostate epithelium.
Consistent with previous studies, PtenloxP/loxP PB-Cre+ mice develop mPIN by 7 weeks of age, which is characterized by extensive epithelial cell hyperplasia within pre-existing ductules [Figure 3D]. In general, the individual prostate ductules are enlarged, but remain intact with no signs of a desmoplastic response in the surrounding stromal tissue. The individual atypical epithelial cells are clearly larger than wild-type cells and are positive for AktS473 phosphorylation, with the most intense signal at the cell membrane. Pten deletion also appears to increase total Rictor protein level, which is consistent with the immunoblot data in Figure 3C, and possibly suggests feedback activation of Rictor gene expression.
In contrast, prostatic ductules from PtenloxP/loxPRictorloxP/loxPPB-Cre+ exhibit a mixed phenotype containing mostly normal, organized epithelial cells and patches of large hyperplastic disorganized cells [Figure 3D]. We suspected that Rictor, which is required for AktS473 phosphorylation, might be inefficiently deleted in these atypical cells. Indeed, comparison of Rictor protein levels and the phospho-AktS473 signal indicate that normal cells deficient in Rictor protein expression are negative for AktS473 phosphorylation, while atypical cells maintain Rictor protein expression and stain positively for phosphorylated S473 [Figure 3D & S2]. This is consistent with the immunoblot data showing that AktS473 phosphorylation is not completely lost in PtenloxP/loxPRictorloxP/loxPPB-Cre+ tissue [Figure 3C]. Also consistent with inefficient Rictor deletion, we clearly see some PTEN-negative cells that are positive for phospho-AktS473 and others that are negative for phospho-AktS473 signal [Figure 3E]. Importantly, PTEN deficient phospho-AktS473 negative cells appear normal. A recent study suggests that Pten loss down-regulates the probasin promoter, which could explain the recombination inefficiency (Jiao et al., 2007; Lei et al., 2006).
By 9-10 weeks of age, prostate adenocarcinoma is detectable in PtenloxP/loxP PB-Cre+ mice. Nearly all of the dorsolateral lobes at this age contain extensive malignant cells characterized by AktS473 phosphorylation, especially at the membrane [Figure 4A]. Compared to wild-type tissue, the diseased glands are enlarged and the ductule boundaries are disorganized. Invasive AktS473 positive cells are also detectable in the surrounding stroma, which is characterized by hypercellularity [Figure 4B]. Ki-67 staining indicates that the malignant cells are highly proliferative, with proliferating cells concentrating at the stromal interface and invading into the stromal region [Figure 4C]. Invasive adenocarcinoma induces a phenomenon called the desmoplastic response, in which collagenous material is deposited in the surrounding stromal tissue. The trichrome stain, which marks collagen blue and fibrin pink, reveals a recruitment of connective tissues to the diseased areas [Figure 4C]. Rictor protein is clearly detectable in malignant cells, and notably, some cells at the stromal interface express much higher levels of Rictor protein [Figure 4C].
Figure 4. Pten-deletion-induced invasive adenocarcinoma requires Rictor.
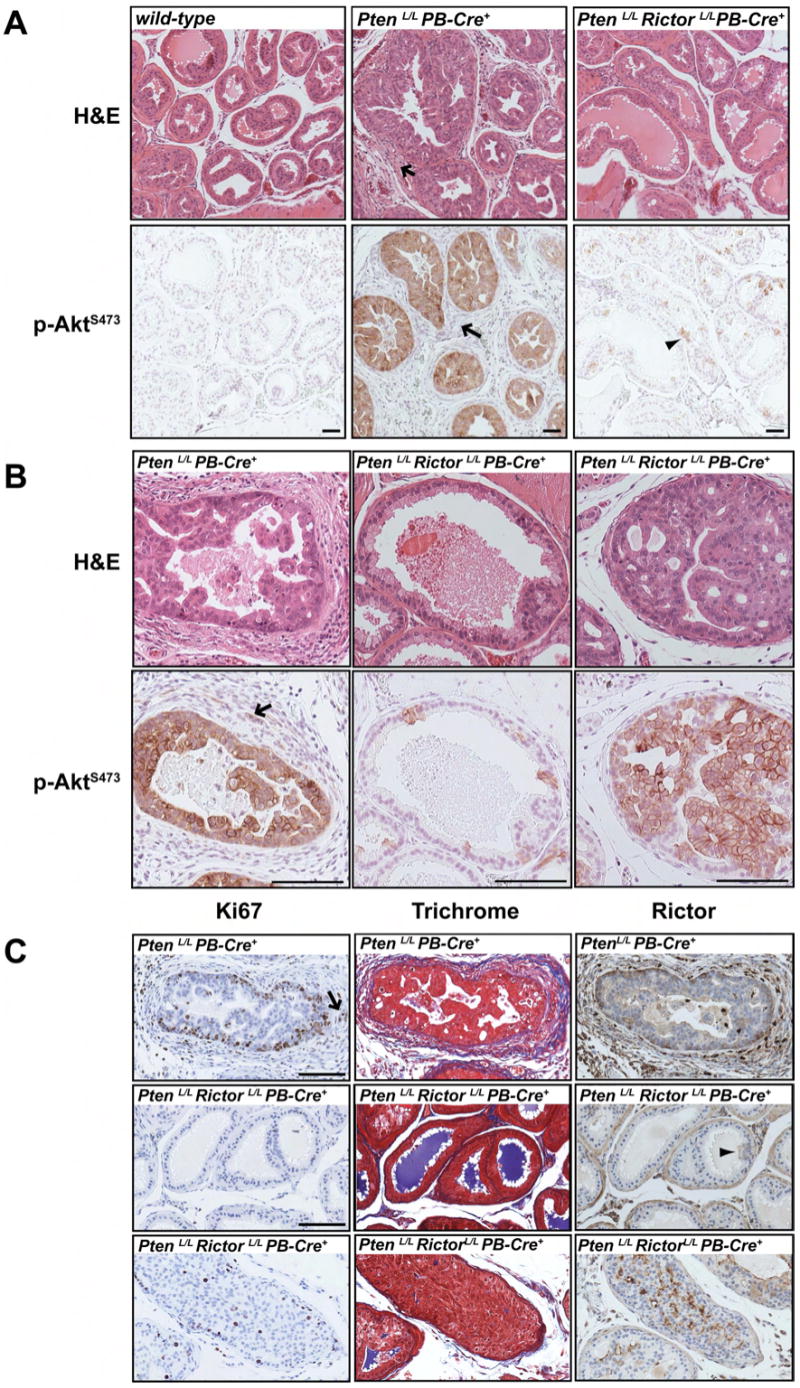
(A) Prostate tissue serial sections from 9-week old mice were stained by H&E or labeled with a phospho-AktS473 antibody and imaged at 10X. The arrows point to changes in the stroma. The arrowhead indicates a patch of phospho-AktS473 positive cells. Scale bar = 50μm. (B) Higher magnification images (20X) of serial sections stained by H&E or labeled with a phospho-AktS473 antibody. Invasive phospho-AktS473 positive cells are indicated with an arrow. Scale bar = 50μM. (C) Labeling with Ki-67 antibody (left), trichrome stain (middle) and Rictor antibody (right) are shown. The arrow indicates proliferating (Ki-67 positive) cells in the stroma. The arrowhead points to a small patch of cells that have not lost Rictor expression. Scale bar = 50μm.
In contrast to PtenloxP/loxP PB-Cre+ mice, prostate epithelial cells from 9-10 week old PtenloxP/loxPRictorloxP/loxP PB-Cre+ mice are protected from transformation [Figure 4A & B]. Individual ductules in many cases are more similar in appearance to wild-type prostate tissue, with little evidence of hyperplasia. Nearly all of these cells are non-proliferating as determined by Ki-67 staining, and there is no desmoplastic response [Figure 4C]. Consistent with the inefficient Rictor deletion noted above, we again observe patches of AktS473 positive epithelial cells [Figure 4A & B]. This often results in AktS473 positive epithelial cells mixed among many cells that do not stain for AktS473 [Figure 4A & B]. In some instances we observe more extensive hyperplasia of large AktS473 positive cells [Figure 4B]; however, the phenotype is less severe as the epithelial-stromal boundaries are intact and invasive cells are not detectable. A proliferation increase is associated with the patches of hyperplastic cells in the double deletion, but it is not to the extent as that seen in PtenloxP/loxP PB-Cre+ tissue [Figure 4C]. Furthermore, connective tissue is not recruited to the surrounding stroma [Figure 4C]. As expected, the AktS473 positive cells in the double deletion tissue co-stain for Rictor protein although the signal is less pronounced compared to PtenloxP/loxP PB-Cre+ tissue [Figure 4C]. Perhaps in these cases, only one allele of Rictor is deleted.
A close inspection of Rictor protein expression in the Pten-deficient prostate epithelium indicates that Rictor is often localized to cell membranes, particularly in the luminal epithelial cells [Figure 4C and S2]. This suggests that a fraction of active mTORC2 co-localizes with Akt at cell membranes. Collectively, these results argue that loss of Rictor expression does not impair development of the prostate epithelium but protects cells from transformation caused by Pten-deletion.
Rictor is required for Akt signaling
Deletion of Rictor in MEFs ablates AktS473 phosphorylation, and this partly impairs the ability of Akt to phosphorylate FoxO, but not TSC2 or GSK3β suggesting that in these cells Akt retains activity (Guertin et al., 2006; Jacinto et al., 2006). Therefore, we asked whether Akt is still capable of phosphorylating downstream substrates in PtenloxP/loxPRictorloxP/loxP PB-Cre+ tissue. Akt phosphorylates the FoxO transcription factors and this inhibits their activity by excluding them from the nucleus. Immunoblot analysis indicates that FoxO1T24 phosphorylation increases in tissue lysates prepared from PtenloxP/loxP PB-Cre+ but not from PtenloxP/loxPRictorloxP/loxP PB-Cre+ mice [Figure 3C]. We also examined FoxO1 localization. In wild-type prostatic epithelial cells, AktS473 phosphorylation is undetectable, and a very faint FoxO1 signal is present both in the nucleus and cytoplasm of these cells [Figure 5A, left]. In PtenloxP/loxP PB-Cre+ prostates, FoxO1 is excluded from the nucleus and concentrates in the cytoplasm [Figure 5A, middle]. In contrast to both wild-type and PtenloxP/loxP PB-Cre+ prostate epithelial cells, we detect strong nuclear localization of FoxO1 in AktS473 negative cells in PtenloxP/loxPRictorloxP/loxPPB-Cre+ tissue [Figure 5A, right]. Importantly, FoxO1 is excluded from the nucleus in neighboring AktS473 positive cells in the same tissue. Thus, the absence of Akt phosphorylation in PtenloxP/loxPRictorloxP/loxP PB-Cre+ cells reduces FoxO1 phosphorylation and promotes FoxO1 accumulation in the nucleus.
Figure 5. Akt activity towards downstream substrates in Pten-deficient prostate epithelial cells requires Rictor.
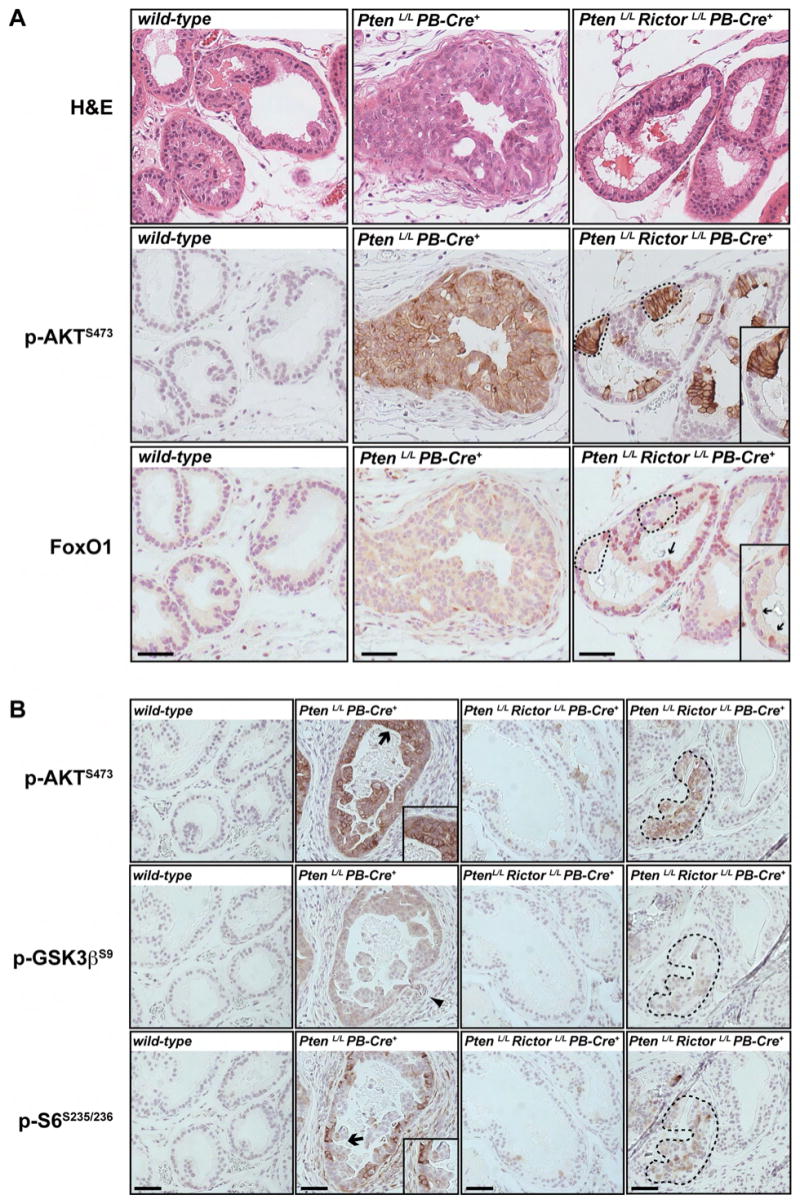
(A) Serial sections from wild-type, PtenLoxP/LoxP PB-Cre+ or PtenLoxP/LoxP RictorLoxP/LoxP PB-Cre+ tissue stained by H&E, or labeled with antibodies to phospho-AktS473 or to FoxO1 are shown. The dotted circles indicate phospho-AktS473 positive cells in which Foxo1 is excluded from the nucleus. Arrows point to phospho-AktS473 negative cells in which Foxo1 concentrates in the nucleus. An enlarged section is show in the boxed insert. Scale bar = 25μm. (B) Serial sections from wild-type, PtenLoxP/LoxP PB-Cre+ or PtenLoxP/LoxP RictorLoxP/LoxP PB-Cre+ tissue labeled with antibodies to phospho-AktS473, phospho-GSK3βS9, or phospho-S6S235/236 are shown. Arrows indicate regions highlighted in the boxed inserts. The arrowhead points to invasive cells. The encircled area in the right panels indicates a patch of phospho-AktS473 positive cells. Scale bar = 25μm.
We next examined phosphorylation of the Akt substrates GSK3β and PRAS40 using antibodies to the Akt-dependent Ser9 or T246 phosphorylation sites, respectively. In prostate tissue lysates from wild-type, PtenloxP/loxP PB-Cre+, or PtenloxP/loxPRictorloxP/loxP PB-Cre+ mice, total GSK3β and PRAS40 protein levels are unchanged [Figure 3C]. In PtenloxP/loxP PB-Cre+ samples, Pten loss elevates GSK3βS9 and PRAS40T246 phosphorylation. However, the increases in GSK3βS9 and PRAS40T246 phosphorylation are greatly diminished in PtenloxP/loxPRictorloxP/loxP PB-Cre+ tissue samples [Figure 3C]. By immunohistochemcial analysis, GSK3βS9 phosphorylation is below the level of detection in wild-type sections [Figure 5B]. Consistent with the immunoblot results, GSK3βS9 phoshporylation, like AktS473 phosphorylation, uniformly increases in all PtenloxP/loxP PB-Cre+ prostate epithelial cells. However, in PtenloxP/loxPRictorloxP/loxP PB-Cre+ prostate tissue GSK3βS9 phosphorylation is only detectable in the subpopulation of AktS473 positive cells. We were unable to achieve reliable detection of PRAS40T246 phosphorylation by IHC.
Finally we examined the activity of the mTORC1 pathway. TSC2, which Akt phosphorylates and inhibits, suppresses mTORC1 activity. Phosphorylation of S6S235/236, a target of the mTORC1 substrate S6K1 is commonly used as a reporter for activation of the mTORC1 pathway by IHC. In lysates from wild-type prostate tissue, S6S235/236 phosphorylation is undetectable, while in PtenloxP/loxP PB-Cre+ prostate tissue phosphorylation increases [Figure 3C]. However, total S6 levels also increase and therefore it is unclear to what extent Pten loss affects the catalytic activity of mTORC1. Both S6S235/236 phosphorylation and total S6 levels in the PtenloxP/loxPRictorloxP/loxP PB-Cre+ prostate tissue lysates are comparable to what is detected in wild-type lysates. Thus, whatever mechanism is responsible for increasing total S6 protein requires mTORC2. By IHC, S6 phosphorylation is undetectable in wild-type prostate epithelial cells [Figure 5B]. Similar to the phospho-AktS473 signal, all cells in the PtenloxP/loxP PB-Cre+ tissue samples display and increase in the phospho-S6S235/236 signal, however, a subset of cells--particularly those near the stromal interface--exhibit a greater increase in phospho-S6 intensity. This variability may reflect the fact that mTORC1 activity is additionally sensitive to nutrient and oxygen availability. Again, consistent with the immunoblots, S6S235/236 phosphorylation levels are comparable to those of wild-type cells in prostate tissue from PtenloxP/loxPRictorloxP/loxPPB-Cre+ mice, and only in patches of AktS473 positive cells can we detect phospho-S6 signal [Figure 5B]. These findings support a model in which Akt requires mTORC2-dependent phosphorylation of AktS473 in order to phosphorylate downstream substrates when Pten is deleted in prostate epithelial cells.
Discussion
In this report we investigate the role of mTORC2 in prostate cancer caused by Pten loss. First, we show that the PTEN-deficient PC-3 cell line requires Rictor, which an essential component of mTORC2, to form tumors in nude mice. Second, we find that the Rictor gene is haploinsufficient in mice, and that Pten heterozygous mice prone to develop prostate cancer are more resistant to the disease when expressing only one copy of the Rictor gene. Finally, we show that in vivo prostate epithelial cells require Rictor to be transformation by Pten deletion, but Rictor has no significant role by itself in maintaining the integrity of a normal prostate epithelium. We propose that cancers driven by PTEN loss or aberrant PI3K activation require mTORC2 signaling and that targeting mTORC2 in these cancers could be a promising therapeutic strategy.
In cultured cells, mTOR, when assembled into mTORC2, is an AktS473 kinase (Sarbassov et al., 2005). Genetic studies in mice confirm that in the developing embryo mTORC2 is the critical AktS473 kinase complex (Guertin et al., 2006; Jacinto et al., 2006; Shiota et al., 2006). Other Akt S473 kinases have been described [Reviewed in (Bhaskar and Hay, 2007)](Bozulic et al., 2008) leaving open the possibility that when cells become transformed, kinases other than mTOR could phosphorylate Akt at S473. Our results argue that mTORC2 is the primary kinase that phosphorylates AktS473 in a Pten-deletion-dependent model of prostate cancer.
The function of TORC2 as an AktS473 kinase is conserved in Drosophila and Dictyostelium (Hietakangas and Cohen, 2007; Lee et al., 2005; Sarbassov et al., 2005). Unlike developing mouse embryos, Drosophila embryos lacking mTORC2 activity are viable and display only minor growth defects (Guertin et al., 2006; Hietakangas and Cohen, 2007; Shiota et al., 2006). However, tissue overgrowth in the eye caused by PTEN loss requires dTORC2 (Hietakangas and Cohen, 2007). Thus the genetic requirement for TORC2 under conditions of high PI3K activity, but not necessarily under normal conditions, is conserved.
Inhibition of mTOR in cancer therapy
The most developed mTOR inhibitor for use in oncology is rapamycin. Initial studies suggest that rapamycin is effective against tumors with PTEN loss (Bjornsti and Houghton, 2004; Rowinsky, 2004; Vignot et al., 2005). However, clinical studies show variable and unpredictable successes with rapamycin as an anti-cancer agent (Chiang and Abraham, 2007). In cells, rapamycin binds to its intracellular receptor FKBP12, and together the rapamycin-FKBP12 complex binds to mTOR adjacent to the kinase domain, inhibiting its in vivo activity towards S6K1 at nanomolar concentrations. Numerous studies show that S6K1T389 phosphorylation but not AktS473 phosphorylation is potently inhibited by rapamycin in most cell types suggesting that rapamycin is an mTORC1-specific inhibitor.
It is now appreciated that prolonged exposure to rapamycin inhibits both S6K1T389 and AktS473 phosphorylation in a subset of cell types (Phung et al., 2006; Sarbassov et al., 2006; Zeng et al., 2006). The mechanism of mTORC2 inhibition by rapamycin is under investigation as rapamycin-FKBP12 binds mTORC1 but not mTORC2 (Sarbassov et al., 2004). However, rapamycin-FKBP12 also binds newly synthesized mTOR and in some cells this appears to block mTORC2 activity by preventing the assembly of new mTORC2 complexes (Sarbassov et al., 2006). Why this phenomenon is not more ubiquitous is a mystery. Part of the reason could be that in many cells, a negative feedback loop masks this effect. Studies show that inhibiting mTORC1 releases PI3K signaling from negative feedback inhibition resulting in strong Akt activation (Chiang and Abraham, 2007; Manning, 2004). According to this model, rapamycin would inhibit both mTORC1 and mTORC2, but push upstream Akt signaling in two directions; inhibiting Akt by blocking mTORC2 assembly; and activating Akt by releasing PI3K from mTORC1 negative feedback inhibition. This would generate counteractive signals and may result in no net observable difference in Akt phosphorylation levels. There are currently no cancer cell biomarkers predictive of rapamycin sensitivity. Interestingly, rapamycin inhibits mTOR at micromolar concentrations independently of FKBP12 (Shor et al., 2008). This phenomenon termed the “high dose effect” broadens the effectiveness of rapamycin and correlates with inhibition of both mTORC1 and mTORC2.
Next generation mTOR inhibitors will likely target the catalytic domain of mTOR and thus achieve universal inhibition of both mTOR complexes. It has been suggested that Akt-driven transformation of some cell types preferentially requires mTORC1 activity (Skeen et al., 2006). An mTOR kinase inhibitor would therefore target both an upstream activator and downstream target of Akt. The major caveat being that such a drug could exhibit considerable toxicity in vivo. In theory, inhibiting the catalytic activity of both complexes may circumvent issues with feedback activation of Akt following mTORC1 inhibition since mTORC2 would also be inactive. However, if mTOR activity is not completely inhibited for technical reasons (i.e. a resistant pool of mTORCs) or it cannot be inhibited for dose-dependent toxicity reasons, then a negative feedback loop in some cells could still be problematic. Until such a class of drugs is available, we can only speculate on their effectiveness.
Potential utility of an mTORC2-specific inhibitor
In this report, we present evidence that an mTORC2-specific inhibitor might be a promising anti-cancer therapeutic. We believe that mTORC2 is an attractive target for drug development for two reasons: (1) By targeting only mTORC2, there may not be feedback activation of Akt signaling since there would be no direct inhibition of mTORC1; (2) an mTORC2 inhibitor may be well tolerated since its activity is not required in normal prostate epithelial cells (or in cultured MEFs), but rather it is required only for prostate epithelial cell transformation when Pten is deleted. This may be evidence for a therapeutic window in which inhibition of mTORC2 could be more deleterious to cancer cells than to normal cells. Because little is known about the physiological roles of mTORC2, it is difficult to predict what effects a theoretical mTORC2 inhibitor could have on other processes. Interestingly, a recent report indicates muscle-specific deletion of Rictor in mice, while sufficient to block insulin stimulated AktS473 phosphorylation, only results in a mild impairment in glucose tolerance (Kumar et al., 2008).
The extent to which other cancers require mTORC2 is unclear although we expect that cancers driven by mutations directly promoting Akt signaling, such as activating mutations in PI3K, may also require mTORC2 activity. A recent study finds that in some glioma cell lines, Rictor expression is increased and this elevates mTORC2 activity and promotes anchorage independent growth and tumor formation in xenografts (Masri et al., 2007). The increase in mTORC2 activity does not correlate with PTEN status suggesting that aberrant up-regulation of mTORC2 activity might occur independently of PI3K activity. This might define another class of cancers preferentially susceptible to an mTORC2 inhibitor. However, it was not determined whether other mutations activate PI3K activity in these cells.
It is currently unclear if Akt is the only relevant mTORC2 target in cancer. The three mammalian Akt proteins (Akt1, 2, and 3) belong to a family of structurally related kinases called the AGC family, which in addition to Akt includes the S6K, PKC, SGK, and RSK kinases (Hanada et al., 2004). AGC kinases are co-regulated by PDK1 phosphorylation and phosphorylation by a hyrdrophobic motif kinase. Because mTOR phosphorylates the hydrophobic motif sites of Akt (S473) and S6K (T389) when assembled into mTOR Complex 2 or 1 respectively, it seems likely other AGC kinases could be targeted by mTOR. The SGKs are good candidates for mTORC2 regulation in cancer because they are structurally very similar to Akt, are regulated by PI3K signaling, and have cell functions that both overlap with and are distinct from Akt (Tessier and Woodgett, 2006). It was recently shown that mTORC2 controls hydrophobic motif phosphorylation of SGK1 suggesting that tumors with SGK activation might also be good candidates for treatment with mTORC2 inhibitors (Garcia-Martinez and Alessi, 2008). mTORC2 also regulates phosphorylation of the hydrophobic motif site in PKCα (S657) but it is not know if this is a direct effect (Guertin et al., 2006; Sarbassov et al., 2004).
In addition to directly phosphorylating the hydrophobic motif of Akt, mTORC2 is required for the growth-factor independent phosphorylation of Akt and PKC on the turn motif site, although the exact role of mTORC2 in this event is unclear (Facchinetti et al., 2008; Ikenoue et al., 2008). This constitutive phosphorylation event occurs during or shortly after translation and is important for maintaining protein stability. Interestingly, Hsp90 chaperones prevent Akt from degrading in mTORC2-deficient cells, in which Akt lacks both hydrophobic and turn motif phosphorylation (Facchinetti et al., 2008). These findings suggest mTORC2 may have a broader role in regulating AGC kinases beyond hydrophobic motif phosphoryaltion and combining mTORC2 inhibition with chaperone inhibitors could be an effective therapeutic strategy.
To develop mTORC2 inhibitors, we need detailed knowledge about the structure and assembly of the mTOR complexes. One possible strategy is to disrupt protein-protein interactions required for mTORC2 activity. For instance, dissociation of Rictor, mSIN, or mLST8 from mTOR results in disassembly and inactivation of the complex (Frias et al., 2006; Guertin et al., 2006; Jacinto et al., 2006; Sarbassov et al., 2005). Another possible strategy is to inhibit kinase-substrate interactions, for example by disrupting the intracellular localization of the complex. We observe that Rictor concentrates at cell membranes in Pten-deficient prostate epithelial cells. Perhaps disrupting mTORC2 localization might prevent it from phosphorylating Akt.
Co-regulation of Akt by PDK1 and mTORC2
One noteworthy finding from the genetic studies of mTORC2 is that Akt has different sensitivities to mTORC2 inhibition in normal cells when compared to cancer cells. For example, Akt retains activity in Rictor-null MEFs despite the absence of hydrophobic motif phosphorylation on S473 [this study & (Guertin et al., 2006; Jacinto et al., 2006; Shiota et al., 2006)]. Hydrophobic motif phosphorylation is a prerequisite for PDK1 to phosphorylate S6K, SGK, and RSK, but this may not be the case for Akt even though full Akt activity in vitro requires phosphorylation at both sites (Alessi et al., 1996; Biondi et al., 2001; Collins et al., 2003). In MEFs, PDK1-dependent phosphorylation of AktT308 is unaffected by Rictor deletion supporting the idea that loss of S473 phosphorylation is not sufficient to block Akt activity in some cells [this study & (Guertin et al., 2006; Jacinto et al., 2006; Shiota et al., 2006)]. Interestingly, we find that downstream targets of Akt require mTORC2 activity to be phosphorylated in Pten-deficient prostate epithelial cells. We also show that unlike the case in MEFs, mTORC2 is required for both AktS473 and AktT308 phosphorylation when Pten is deleted. This is similar to what is observed in human cancer cells in which knocking down Rictor decreases both S473 and T308 phosphorylation (Hresko and Mueckler, 2005; Sarbassov et al., 2005). We are developing AktT308 antibodies for IHC and our preliminary results are consistent we this finding [see Supplemental Figure 3]. This intriguing observation suggests that co-regulation of Akt by mTORC2 and PDK1 is tethered in cancer cells, while in otherwise “normal” cells, these two inputs are uncoupled. This could explain why inhibiting mTORC2 in cancer cells is more deleterious to Akt activity.
Methods
RNAi
PC-3 cells stably expressing the control or Rictor shRNAs were generated as described previously using a lentiviral system (Sarbassov et al., 2005). PC-3 cell lysates for immunoblot analysis were prepared 6 days post infection. For proliferation analysis, cells were seeded at equal density onto 12-well cell culture dishes immediately following puromycin selection and counted each day with a Coulter Counter. For xenografts, 1M PC-3 cells were injected on the back of pre-irradiated (4Gray) ncr-nu/nu male mice and tumors were monitored for 28 days.
MEFs
Rictor deficient MEFs were described previously (Guertin et al., 2006). Akt activity was measured in MEFs using the Akt1/PKBα Immunoprecipitation Kinase Assay Kit (Upstate). To generate Pten null Rictor heterozygous MEFs, we crossed PtenloxP/loxPRictorLoxP/+ males with PtenLoxP/LoxPRictorLoxP/LoxP females and prepared MEFs from d14 embryos. Adeno-Cre (U of Iowa) was added directly to the medium on two consecutive days. Five days after the initial infection, cells were starved 6 hours in serum free medium, stimulated with insulin for 10 minutes, then lysed.
Mice
Pten+/- mice (Podsypanina et al., 1999) that are 129/B6 were crossed with mtor+/-, Raptor+/-, or mlst8+/- mice of the same strain background or Rictor+/- mice that are of a mixed 129/B6/BalbC background (Guertin et al., 2006). The severity and latency of tumor development in Pten+/- mice varies depending on the strain composition (Freeman et al., 2006). To generate mice with conditional alleles, PtenloxP/+RictorloxP/+PB-Cre4+ mice were crossed with PtenloxP/+RictorloxP/+ or PtenloxP/loxPRictorloxP/loxP mice (all on a mixed background) (Shiota et al., 2006; Wang et al., 2003; Wu et al., 2001). Cre is expressed in all four lobes of the prostate with Cre expression highest in the dorsolateral (DL) and ventral (V) lobes. All procedures are approved by the Massachusetts Institute of Technology Division of Comparative Medicine's Committee on Animal Care and conform to the legal mandates and federal guidelines for the care and maintenance of laboratory animals.
Protein biochemistry
Cell lysates were prepared using conditions described in (Kim et al., 2002). For tissue lysates, frozen samples were homogenized for 8 seconds in detergent free buffer using a Brinkmann Homogenizer and immediately after, detergent was added to the final concentrations of 0.1%SDS/1.0% Sodium Deoxycholate/1.0% Triton. Primary antibodies are as follows: From CST, mTOR (#2893); Rictor (#2114); AktT308 (#2965); AktS473 (#4060); Akt (#4685); PTEN (#9559); S6KT389 (#9206); S6K (#9202); FoxO1 (#2880); GSK3βS9 (#9336); GSK3 (#9315); S6S235/236 (#4856); S6 (#2217); and Foxo1T24 (Upstate # 06-952); PRAS40T246 (BioSource #44-1100G); and PRAS40 (Upstate #05-988).
Immunohistochemistry
Slides were deparaffinized in three changes of xylene and rehydrated through graded ethanols. Antigen retrieval was performed using 10mM citrate buffer, pH 6.0. Slides were quenched in 3% hydrogen peroxide and blocked with TBST/5% normal goat serum. Primary antibodies were diluted in TBST/5% normal goat serum (CST# 4858) or SignalStain (R) Antbody Diluent (CST #8112) and incubated overnight at 4 degrees (CST# 4060, 2880, 9323). Detection was performed using Vector ABC Elite (Vector Laboratories) and NovaRed (Vector Laboratories). Rictor antibody (Santa Cruz #50678) was detected using a goat secondary probe/goat polymer system (Biocare Meidcal #ghp516) followed by DAB. Ki-67 (BD Pharmingen #550609) IHC was done using the DakoCytomation ARK kit (#K3954).
Supplementary Material
Acknowledgments
We thank Kathleen Cormier (MIT DCM) and Mike Brown (MIT Koch Center) for histological support, and Randy Wetzel, Wan Cheung Cheung, and Krystyna Zuberek (Cell Signaling Technology) for reagents. This work is supported by grants from the National Institutes of Health (R01 CA103866 and R01 AI04389) to D.M.S and R01 CA107166 to H.W.; An award from the Keck Foundation to D.M.S; A fellowship from the Damon Runyon Cancer Research Foundation and a Career Development Award from the Leukemia & Lymphoma Society to D.A.G.; and a grant form the National Institutes of Health (K99 CA1296613-01A1) to D.A.G. D.M.S. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets. 2008;12:209–222. doi: 10.1517/14728222.12.2.209. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/-) mice. Curr Biol. 2005;15:1839–1846. doi: 10.1016/j.cub.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Biondi RM, Kieloch A, Currie RA, Deak M, Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. Embo J. 2001;20:4380–4390. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Chen ML, Xu PZ, Peng XD, Chen WS, Guzman G, Yang X, Di Cristofano A, Pandolfi PP, Hay N. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/- mice. Genes Dev. 2006;20:1569–1574. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13:433–442. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Collins BJ, Deak M, Arthur JS, Armit LJ, Alessi DR. In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. Embo J. 2003;22:4202–4211. doi: 10.1093/emboj/cdg407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahia PL. PTEN, a unique tumor suppressor gene. Endocr Relat Cancer. 2000;7:115–129. doi: 10.1677/erc.0.0070115. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. Embo J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Lesche R, Kertesz N, Wang S, Li G, Gao J, Groszer M, Martinez-Diaz H, Rozengurt N, Thomas G, et al. Genetic background controls tumor development in PTEN-deficient mice. Cancer Res. 2006;66:6492–6496. doi: 10.1158/0008-5472.CAN-05-4143. [DOI] [PubMed] [Google Scholar]
- Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 Is Necessary for Akt/PKB Phosphorylation, and Its Isoforms Define Three Distinct mTORC2s. Curr Biol. 2006 doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez JM, Alessi DR. mTOR complex-2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum and glucocorticoid induced protein kinase-1 (SGK1) Biochem J. 2008 doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the Role of mTOR in Cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in Mice of the mTORC Components raptor, rictor, or mLST8 Reveals that mTORC2 Is Required for Signaling to Akt-FOXO and PKCalpha, but Not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Haar EV, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hietakangas V, Cohen SM. Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 2007;21:632–637. doi: 10.1101/gad.416307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko RC, Mueckler M. mTOR/RICTOR is the Ser473 kinase for Akt/PKB in 3T3-L1 adipocytes. J Biol Chem. 2005 doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- Huang B, Porter G. Expression of proline-rich Akt-substrate PRAS40 in cell survival pathway and carcinogenesis. Acta Pharmacol Sin. 2005;26:1253–1258. doi: 10.1111/j.1745-7254.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. Embo J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 Maintains rictor-mTOR Complex Integrity and Regulates Akt Phosphorylation and Substrate Specificity. Cell. 2006 doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, Wu H. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–6091. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR Interacts with Raptor to Form a Nutrient-Sensitive Complex that Signals to the Cell Growth Machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278:10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC., Jr Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol. 2008;28:61–70. doi: 10.1128/MCB.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Comer FI, Sasaki A, McLeod IX, Duong Y, Okumura K, Yates JR, 3rd, Parent CA, Firtel RA. TOR Complex 2 Integrates Cell Movement during Chemotaxis and Signal Relay in Dictyostelium. Mol Biol Cell. 2005;16:4572–4583. doi: 10.1091/mbc.E05-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Jiao J, Xin L, Chang CJ, Wang S, Gao J, Gleave ME, Witte ON, Liu X, Wu H. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell. 2006;9:367–378. doi: 10.1016/j.ccr.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri J, Bernath A, Martin J, Jo OD, Vartanian R, Funk A, Gera J. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Res. 2007;67:11712–11720. doi: 10.1158/0008-5472.CAN-07-2223. [DOI] [PubMed] [Google Scholar]
- Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky EK. Targeting the molecular target of rapamycin (mTOR) Curr Opin Oncol. 2004;16:564–575. doi: 10.1097/01.cco.0000143964.74936.d1. [DOI] [PubMed] [Google Scholar]
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 Is an Insulin-Regulated Inhibitor of the mTORC1 Protein Kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sarbassov D, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sellers WR, Sawyers CL. Somatic Genetics of Prostate Cancer: Oncogenes and Tumor Suppressors. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic Disruption of the rictor Gene in Mice Reveals that mTOR Complex 2 Is Essential for Fetal Growth and Viability. Dev Cell. 2006 doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Shor B, Zhang WG, Toral-Barza L, Lucas J, Abraham RT, Gibbons JJ, Yu K. A new pharmacologic action of CCI-779 involves FKBP12-independent inhibition of mTOR kinase activity and profound repression of global protein synthesis. Cancer Res. 2008;68:2934–2943. doi: 10.1158/0008-5472.CAN-07-6487. [DOI] [PubMed] [Google Scholar]
- Skeen JE, Bhaskar PT, Chen CC, Chen WS, Peng XD, Nogueira V, Hahn-Windgassen A, Kiyokawa H, Hay N. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–280. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998a;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998b;58:204–209. [PubMed] [Google Scholar]
- Tessier M, Woodgett JR. Serum and glucocorticoid-regulated protein kinases: variations on a theme. J Cell Biochem. 2006;98:1391–1407. doi: 10.1002/jcb.20894. [DOI] [PubMed] [Google Scholar]
- Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005 doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Sarbassov DD, Samudio IJ, Yee KW, Munsell MF, Jackson CE, Giles FJ, Sabatini DM, Andreeff M, Konopleva M. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2006 doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


