Abstract
Background & Aims
5-hydroxytryptamine is a neurotransmitter and paracrine signaling molecule in the gut. Paracrine signaling by enterchromaffin cells (EC) which release 5-HT has not been studied in neonatals. Our aim was to compare 5-HT disposition in the intestinal mucosa of neonatal and adult guinea pigs.
Methods
5-HT was locally measured in vitro from intestinal segments using a diamond microelectrode and continuous amperometry. The serotonin transporter (SERT) was measured using immunohistochemical and Western blot techniques. 5-HT intestinal content was measured using immunohistochemistry and HPLC with electrochemical detection.
Results
An oxidation current, reflective of local 5-HT release, was recorded with the microelectrode near the mucosal surface and this current was larger in neonatal than in adult tissues. Mechanically stimulating the mucosa with a fine glass probe evoked an additional current in adult but not neonatal tissues. Oxidation currents were reduced by tetrodotoxin and were blocked in calcium-free solutions. Fluoxetine (1 μM) potentiated oxidation currents in adult but not neonatal tissues. SERT levels were lower in neonatal vs. adult tissues. There was no difference in 5-HT content between neonates and adults but 5-HIAA/5-HT ratios were higher in adults. EC cell counts showed no difference in cell number but EC cells were found in the crypts in neonatal and along the villi in adult tissues.
Conclusions
SERT expression is low in neonates and this is associated with high levels of free mucosal 5-HT and reduced metabolism. Postnatal maturation of 5-HT signaling may important for development of neurohumoral control of intestinal motor reflexes.
5-Hydroxytryptamine (5-HT, serotonin) is a signaling molecule released from enteric neurons1,2 and enterochromaffin (EC) cells in the mucosal layer of the gut.3,4 EC cells release 5-HT in a calcium-dependent manner and they express mechano- and chemosensitive ion channels, ligand-gated ion channels, and G-protein-coupled receptors.3,4 Activation of calcium-permeable channels or G-protein-linked receptors leads to a rise in intracellular calcium and 5-HT secretion.3,4 Regulated secretion of 5-HT by EC cells can be enhanced or inhibited by signaling molecules released from surrounding cells and by nerves supplying the mucosa.3,4 EC cells are sensory transducers that respond to mechanical or chemical stimuli applied to the mucosa causing 5-HT release.5 5-HT released from EC cells initiates motor reflexes by activating 5-HT receptors localized to the primary afferent nerve terminals.6,7,8 5-HT released from EC cells initiates antidromic action potentials in the intestinal primary afferent neurons,6 which then activate interneurons and motoneurons in enteric neural circuits mediating peristalsis.7,8 Clearance of 5-HT is also an important determinant of the strength and duration of excitatory signals transmitted by 5-HT. Clearance of 5-HT is accomplished through the activity of the high-affinity serotonin transporter (SERT) which is expressed by enterocytes.9
The ENS begins to mature during embryonic development when neural precursors migrate from the neural crest into the bowel wall.10 When the neural precursors reach the gut, trophic factors and extracellular matrix proteins stimulate neuronal differentiation and circuit formation.11,12 However, the ENS continues to mature in the postnatal period.13 As discussed above, signaling between EC cells and enteric neurons is important for initiation of motor reflexes but the status of the EC cell-ENS interaction in the early postnatal period is unknown.
In order to compare EC cell function in neonatal and adult intestinal tissues, it is essential to measure 5-HT concentrations very close to release sites in the intestinal mucosa. This has been accomplished using electrochemical techniques with carbon fiber microelectrodes positioned on the mucosa of guinea pig ileum maintained in vitro.14 Using this approach, it has been possible to monitor 5-HT release from a few EC cells in real time. However, electrochemical monitoring of 5-HT in vitro or in vivo is often hindered by the tendency of oxidation products to form an insulating film on the carbon fiber surface causing electrode fouling and signal loss.15,16 We showed recently that diamond microelectrodes are resistant to fouling and can be used for sensitive and stable measurement of 5-HT in the intestinal mucosa in vitro.17 In the present study we used diamond microelectrodes to compare 5-HT disposition in the small intestinal mucosa of neonatal and adult guinea pigs. As there were marked differences in 5-HT handling between these groups of animals, we also used chromatographic, immunohistochemical, and Western blot techniques to probe the mechanisms underlying differences in 5-HT handling by the intestinal mucosa of neonatal and adult guinea pigs.
Methods and Materials
Diamond Microelectrode Preparation
Boron-doped diamond (BDD) thin film was deposited on a sharpened 76 μm diameter Pt wire (99.99%, Aldrich Chemical) by microwave-assisted chemical vapour deposition (CVD) (1.5 kW, 2.54 GHz, ASTeX, Woburn, MA, USA).18,19 The diamond-coated Pt wire was affixed to a copper wire using conductive Ag epoxy and the assembly was insulated with polypropylene from a pipet tip. The insulation was applied by inserting the microelectrode into a pipette tip with about 500 μposed from the end and carefully heating the tapered end using the coil of micropipette puller. This softened the polypropylene and caused it to flow evenly over the diamond surface. The resulting microelectrode was conically shaped with diameter at the narrowest point of ca. 10 μm at the widest point of ca. 80 μm. The length of the exposed electrode was 100–200 μm. This method reproducibly coats diamond with a thin and continuous polymer film.
Intestinal Preparation
All animal use protocols were approved by the Institutional Animal Use and Care Committee at Michigan State University. Neonatal guinea-pigs (≤ 48 hours postnatal, weight 75–90 g) and young adult guinea pigs (3–4 months old, 300–400 g) were obtained from Bioport, Inc. (Lansing MI). At this age and weight range, the animals are sexually mature (http://netvet.wustl.edu/species/guinea/guinpig.txt). Guinea pigs were lightly anesthetized via halothane inhalation, stunned and exsanguinated by severing the major neck blood vessels. A segment of ileum was harvested 15 – 20 cm proximal to the ileocecal junction and placed in an oxygenated (95% O2 and 5% CO2) Krebs’ buffer solution, pH 7.4 (composition: 117 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 25 mM NaHCO3 and 11 mM glucose). A small piece (3 cm) of ileum was placed in a Sylgard®-(Dow Corning, USA) lined Teflon recording chamber (6.5 cm long, 6.5 cm wide, 4 mm deep). The segment was cut open along the mesenteric attachment and was lightly stretched and pinned flat (mucosal surface up) to the chamber bottom using small stainless steel pins (Fine Science Tools, Foster City, CA). The bath was mounted on the stage of an inverted microscope (Model 3030, Accu-Scope, USA) and superfused with warm (37 °C) Krebs’ solution at a flow rate of 2 mL/min. The solution temperature was controlled with an immersion heating circulator (Model 1130A, VWR Scientific, USA) and flow was controlled with a peristaltic pump (Masterflex, Cole Parmer, USA). Tissues were exposed to the flowing solution for 30 minutes prior to commencing a series of measurements.
Amperometric measurements of 5-HT
For continuous amperometric recording of 5-HT overflow, a three electrode system was used. A diamond microelectrode, a Pt wire counter electrode and a commercial “no leak” Ag|AgCl (3 M KCl, model EE009, Cypress Systems Inc., USA) reference electrode were mounted in the bath to complete the electrochemical circuit. Measurements were carried out using a BioStat™ multi-channel potentiostat (ESA Biosciences, Inc, USA). The diamond microelectrode was affixed to a micromanipulator (Model 25033, Fine Scientific Tools, USA). The tissue sample and microelectrode were placed in the center of the chamber. The electrode potential was held at 700 mV vs Ag|AgCl, which was sufficient to oxidize 5-HT at a mass transfer limited rate. The electrode was placed several centimeters away from the mucosa for several seconds before the electrode was carefully positioned about 1 mm over the tissue for 20 s. This established a baseline 5-HT oxidation current. A glass capillary (0.5 mm tip diameter) was used to gently touch the nearby mucosa for another 20 s. Movement of the glass capillary was controlled by a micromanipulator. The microelectrode was then used for another 20 s to record the mechanically-stimulated release of 5-HT as an oxidation current. The glass capillary was then moved away from the mucosa to allow the oxidation current to return to the baseline level. Finally, the diamond microelectrode was retracted from the mucosa and the oxidation current returned to the 0 level. This procedure was repeated 5 or 6 times for each tissue section.
HPLC measurements of tissue levels of 5-HT
Using a sharp scalpel, 1 cm2 segments of the mucosal layer were scrapped from ileal tissues from neonatal and adult guinea pigs. The mucosal tissue was placed in 500 μl of ice cold 0.1 M perchloric acid. This sample was then homogenized and centrifuged at 14,000 g for 10 minutes prior to chromatographic analysis. The mucosa tissue samples were homogenized in 500 μl of ice cold 0.1 M perchloric acid and centrifuged at 14,000 g at 4 °C for 10 minutes and were stored in ice prior to analysis. HPLC analysis of 5-HIAA and 5-HT was the carried out on the centrifugate using a CoulArray coulomertic detector (ESA Biosciences, Boston, MA USA) at 400 mV. An ESA autosampler (model 542) and a 20 μl sample loop were used with a column heater maintained at 25 ± 0.15 °C. An ESA HR-880 ODS analytical column (3 μm particle size, 4.6 mm i.d. × 80 mm length) along with a guard column were used for the separation. For the mobile phase (flow rate = 1.1 ml/min, ESA model 582 pump), a commercial ESA Cat A phase® II was used, which consisted of 100 mM phosphate buffer and an ion pairing agent. This was mixed with 16% methanol and 8% acetonitrile. All data analysis was carried out using CoulArray® for Windows.
Immunohistochemistry and analysis of EC cell distribution
Ileal segments were cut open along the mesenteric border and washed with cold phosphate buffered saline solution (PBS, 0.01 M, pH, 7.2). Segments were stretched and pinned flat in a silcone elastomer-lined Petri dish, which was then filled with Zamboni’s fixative (2% (v/v) formaldehyde and 0.2% (v/v) picric acid in PBS). Tissues were fixed overnight (4 °C) and then washed 3 times with DMSO at 10 min intervals, followed by 3 washings in PBS at 10 min intervals. Tissues were dehydrated in PBS/30% sucrose overnight before freezing in OCT mounting medium. After cryostat sectioning, sections were dried in an air-tight dessicator for 1 h. Sections were then incubated overnight in a humidified chamber in diluted (1:200, in PBS) anti-5-HT antibody (YC5/45, rat monoclonal, Abcam, Cambridge, MA, http://www.abcam.com/index.html). Sections were then washed in PBS and incubated for 1 h at room temperature in diluted (1:40) goat anti-rat IgG conjugated to fluoroscein isothiocyanate (FITC, F6258, Sigma Chemical Co., St Louis, MO http://www.sigmaaldrich.com). Sections were washed 3 times with PBS at 10 min intervals and then mounted in buffered glycerol (pH = 8.6) for fluorescence microscopy. Sections were viewed using a Nikon fluorescence microscope (model TE 2000-U ) and images were acquired and analyzed using MetaMorph software.
The distribution of EC cells was quantified by making measurements on four sections of each sample obtained from 4 adult and 4 neonatal guinea pigs. A line was drawn across the image at the crypt-villus border. The number of cells on each side of the line was counted and cell counts obtained in the 4 sections from each animal were summed to provide a single number for crypt or villus EC cells. The mean ± S.E.M. value for the group of 4 animals was then compared using Student’s t-test. The crypt/villus ratio of EC cell distribution for each animal was also calculated and compared.
Western blot analysis
Western blot analysis was performed on protein extracts obtained from mucosal tissues from neonatal and adult guinea pigs. Ileal segments were placed in cold (4 °C phosphate buffered saline, PBS, pH = 7.2) and were cut open along the mesenteric border. The mucosa was scraped from these segments using a sharp scapel and the mucosal tissues were lyzed on ice in lysis buffer (10 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.2% NP40), containing a commercially available protease inhibitor cocktail (cat #P8340, Sigma-Aldrich Inc, St. Louis, MO). Lysates were centrifuged at 700×g for 10 min at 4°C to pellet nuclear proteins and any insoluble debris. The supernatant obtained from each animal was saved separately and total protein in the supernatant was determined using a protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein from each sample described above were mixed with Laemmli buffer [16% mercaptoethanol (w/v), 6% SDS (w/v), 0.1% bromphenol blue (w/v), 30% glycerol (w/v), 240 mM Tris, pH 6.8], separated on a 10% SDS-PAGE gel and then transferred to a nitrocellulose membrane which was kept at 4 °C overnight. Membranes were blocked with 4% milk in PBS-Tween 20 buffer for three hours and then incubated at 4°C overnight with the primary anti-SERT antibody (1:1500 dilution). The SERT antibody (a generous gift from Dr. Randy Blakely, Vanderbilt University) was raised against the C-terminal sequence (amino acids 596–614, antibody #50) of the rat SERT protein. After overnight incubation, the membrane was washed with PBS and then further incubated for one hour at 4°C with a horseradish peroxidase-conjugated secondary antibody. The immunoreactivity was detected by a chemiluminescence kit (Pierce, Rockford, IL) and images were analyzed using Image J software. All membranes were stained with Coomassie Blue to verify equal protein loading and to quantitate total protein in each lane. The intensity of SERT bands and total protein on membranes was quantitated using Image J software (http://rsb.info.nih.gov/ij/). SERT levels in each lane were normalized to the total protein in that lane.
Chemicals
All chemicals and drugs were obtained from Sigma Aldrich (St. Louis, MO USA) and used as received. All solutions were prepared with ultrapure water from a Barnstead E-Pure system (distilled, deionised and passed over activated carbon).
Results
Detection of mucosally-released 5-HT
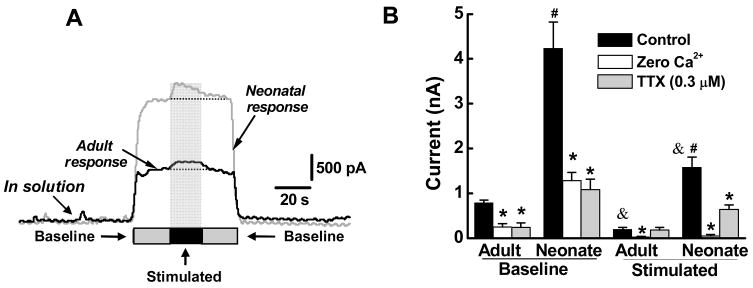
Our previous work17 showed that at an electrode potential of 0.8V, an oxidation current is measured when a diamond microlectrode is positioned on or near the intestinal mucosal due to the overflow of 5-HT. These data are similar to those published previously in studies using carbon fiber electrodes to detect oxidation of 5-HT.14 In the present study, we used a diamond microelectrode because of its enhanced response stability and we compared 5-HT handling by the mucosa of neonatal and adult guinea pigs. We found important differences in the 5-HT signal detected in tissues from neonates and adults. When the tip of the microelectrode was inserted into the solution superfusing the intestinal tissue, no current was detected (Figure 1A). However, when the microelectrode was positioned within 1 mm of the mucosal surface, there was a marked increase in the oxidation current and this baseline response was significantly larger in tissues from neonatal guinea pigs (Figure 1A and 1B). In tissues from both neonates and adults, the baseline 5-HT oxidation current was reduced significantly by the sodium channel blocker, tetrodotoxin (TTX, 0.3 μM)(Figure 1B). Lowering extracellular Ca2+ (from 2.5 to 0.25 mM) produced a further reduction in the baseline 5-HT oxidation current (Figure 1B).
Figure 1.
Increased 5-HT availability in the mucosa of neonatal guinea pigs. (A) Baseline and mechanically-evoked 5-HT oxidation currents in adult and neonatal intestine in vitro. Bars labeled “Baseline” indicate when the electrode was positioned near the mucosa. The hatched area labeled “Stimulated” indicates when a glass probe was pressed against the mucosa near the recording electrode. (B) Analysis of experiments similar to those shown in “A”. The baseline oxidation current in tissues from neonatal guinea pigs (n=6) was larger (#, P <0.001) than that measured in tissues from adult guinea pigs (n=6). The stimulated current was significantly larger in neonatal (#, P < 0.001) vs. adult tissues and stimulation produced a significant increase in the oxidation current over baseline in adult and neonatal tissues (&, P < 0.01). All currents were significantly reduced in low extracellular Ca2+ solutions (0.25 mM)(*, P <0.05 compared to control). Tetrodotoxin (TTX) reduced all currents (*, P <0.05 compared to control) except the stimulated current in adult tissues. Data are mean ± S.E.M.
Mechanical stimulation of the mucosa will cause release of 5-HT from EC cells. We used a fine glass capillary tube (300 μm tip diameter) to mechanically stimulate the mucosal near the end of the diamond microelectrode. Under the control of a micromanipulator, the capillary tube was gently pressed against the mucosa to deform a few villi. Mechanical stimulation evoked a significant increase in the 5-HT oxidation current over baseline levels and the stimulated response was significantly larger in neonatal compared to adult tissues(Figure 1C). The mechanically stimulated response was not altered by TTX in adult tissues but it was reduced by TTX in tissues from neonatal animals (Figure 1B). The mechanically stimulated response was blocked by the low Ca2+ solution in both neonatal and adult tissues (Figure 1B).
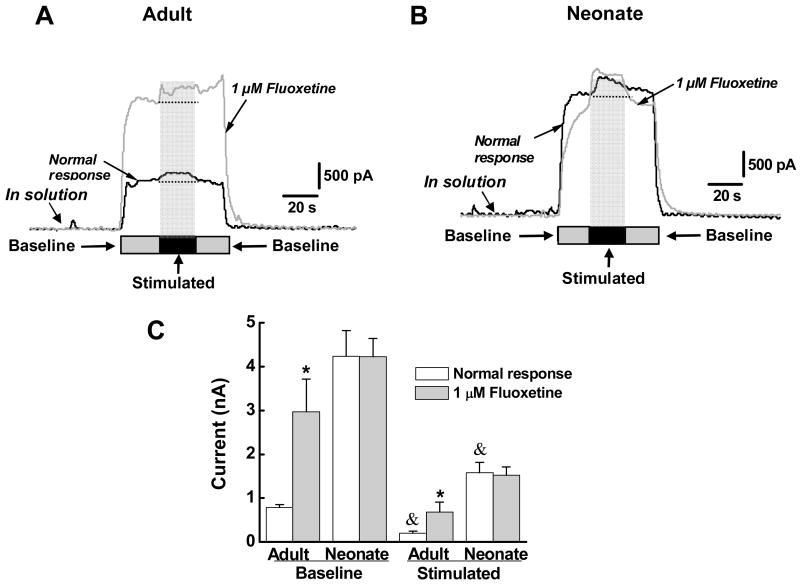
SERT is expressed in high concentrations by enterocytes in the mucosa and is responsible for clearing 5-HT after its release from EC cells.9 It is possible that SERT expression or function is lower in the mucosa of neonates compared to adult guinea pigs. This could account for the elevated basal 5-HT level in the neonatal mucosa. Therefore, the effect of the SERT antagonist, fluoxetine (1 μM), on oxidation currents measured in neonatal and adult tissues was assessed. Fluoxetine increased the baseline response and the mechanically-evoked response in tissues from adult animals (Figure 1A and 1C). However, fluoxetine did not alter responses in neonatal tissues (Figure 2B and 2C).
Figure 2.
Fluoxetine potentiated 5-HT oxidation currents in adult but not neonatal tissues. (A) Baseline and stimulated 5-HT oxidation currents were increased in the presence of the SERT inhibitor fluoxetine. (B) Baseline and stimulated 5-HT oxidation currents were larger in the neonatal tissue compared to the adult tissue (see A) but fluoxetine did not change current amplitude in the neonatal tissue. (C) Data from experiments similar to those shown in “A” and “B”. Baseline currents were larger in neonatal (n=6) vs. adult tissues (n=6) but the current was increased significantly by fluoxetine only in adult tissues (*, P <0.05). Stimulation produced a significant increase in oxidation current over baseline levels in adult and neonatal tissues (&, P <0.01). The stimulated current was also increased significantly by fluoxetine in adult tissues but not in neonatal tissues (*, P < 0.05). Data are mean ± S.E.M.
SERT expression is reduced in neonatal intestine
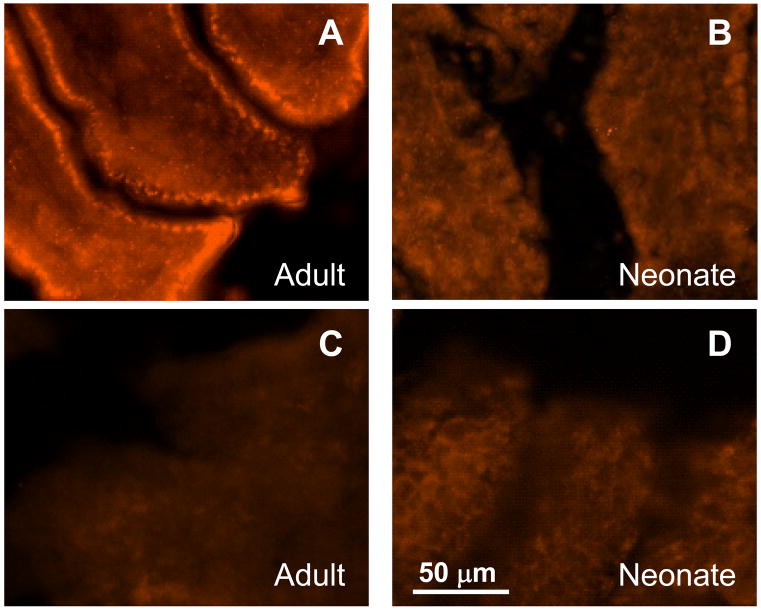
The pharmacological data above suggest that SERT expression is either reduced in neonatal tissues or that SERT activity is lower in neonatal vs. adult intestinal mucosa. In order to distinguish between these possibilities, we localized the SERT protein in frozen sections of neonatal and adult small intestine using immunohistochemical methods. SERT immunoreactivity (ir) was found in enterocytes lining individual mucosal villi in tissues from adult animals (Figure 3A) while little or no SERT-ir was found in sections from neonates. (Figure 3B). Omitting the primary antibody from the immunohistochemical protocol removed localization of SERT in both adult and neonatal tissues (Figure 3C and 3D).
Figure 3.
Immunohistochemical localization of SERT in section neonatal and adult intestine. (A) Section from adult intestine showing SERT-ir in enterocytes lining several villi. (B) A similar section from the neonatal intestine showing absence of SERT-ir. Omitting the primary antibody removes SERT labeling from an adult (C) and neonatal (D) sections.
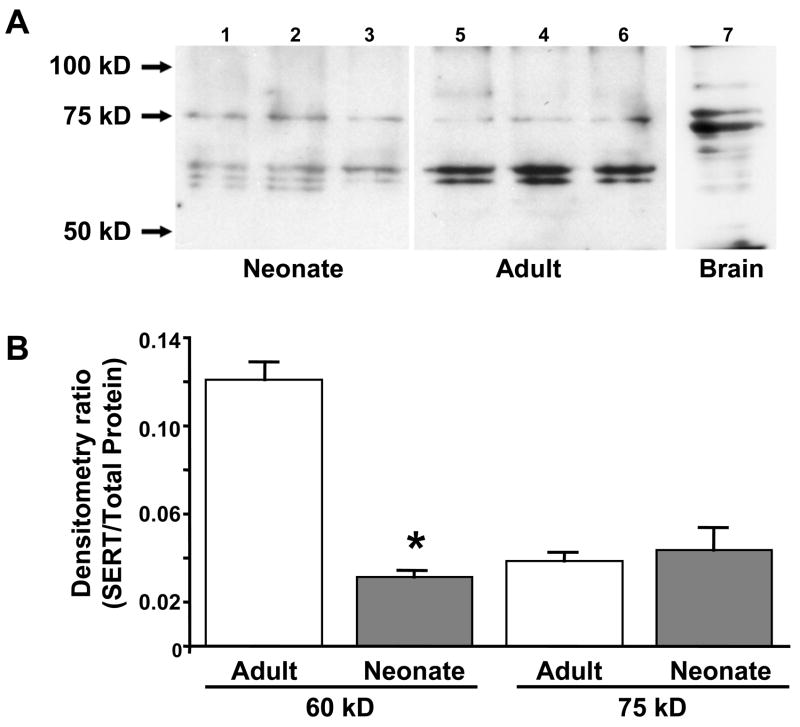
While immunohistochemical techniques provide a qualitative assessment of SERT expression in the mucosa, it is difficult to make quantitative measures using this technique. Therefore, we also used Western blot techniques to compare the relative expression of SERT in the intestine of neonatal (n=3) and adult (n = 3) guinea pigs. Figure 4A shows a Western blot revealing a band near 75 kDa in extracts from the neonatal and adult intestine. This band corresponds to a prominent band at 75 kDa in extracts of adult guinea pig brain (Figure 4A). The intensity of the 75 kD band was similar in adult and neonatal intestinal tissues (Figure 4B). In extracts from adult and neonatal intestine, we also detected a band near 60 kDa. The intensity of the 60 kDa band was much greater in adult samples compared to samples from neonates (Figure 4B). There were also several less intense bands of molecular weights slightly lower than 60 kDa in adult and neonatal tissues.
Figure 4.
Western blot analysis of SERT expression in the neonatal and adult intestine. (A) Western blot showing multiple bands recognized by the SERT antibody in samples from 3 neonatal guinea pigs (lanes 1–3) and 3 adult guinea pigs (lanes 4–6). There is a faint band that corresponds to a prominent band near 75 kD in a protein extract from adult guinea pig brain. A prominent band near 60 kD was detected in adult extracts but this band was present at lower levels in neonatal extracts. (B) Intensity of the 60 and 75 kD bands was normalized to the total protein present in each lane. There was no difference in the intensity of the 75 kD bands between adult and neonatal tissues but the intensity of the 60 kD was significantly greater in adult compared to neonatal tissues (*, P < 0.05). Data are mean ± S.E.M.
5-HT content is similar in neonatal and adult intestine
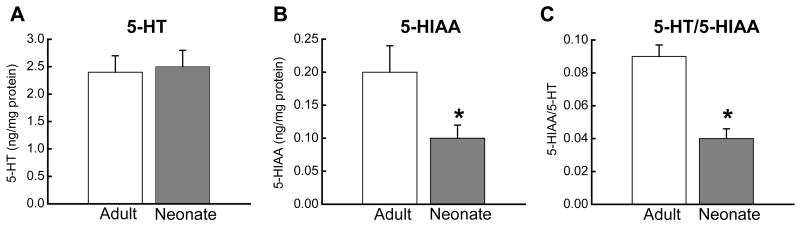
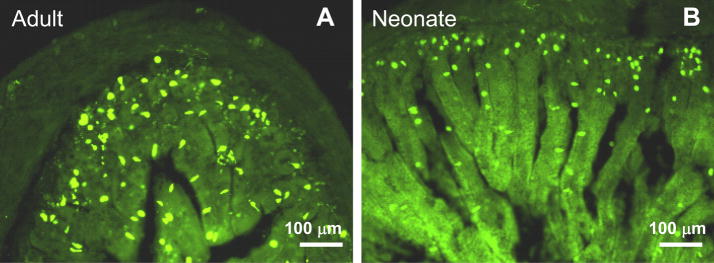
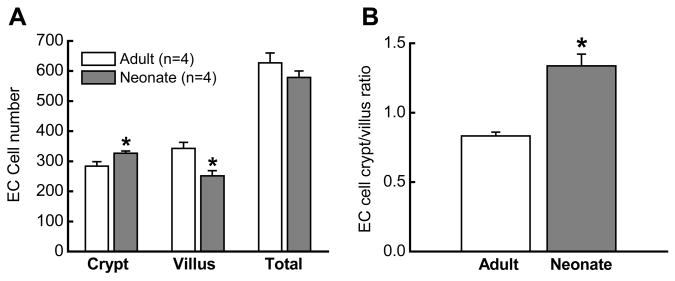
The data presented above indicate that reduced SERT expression could account for the larger 5-HT oxidation currents and reduced fluoxetine-sensitivity in neonatal intestine. It is also possible that the neonatal intestine contains higher levels of 5-HT and/or more EC cells. These issues were addressed in two ways. Firstly, 5-HT content was measured in the intestinal mucosa from neonates and adults. 5-HT levels (Figure 5A) were similar in these two groups but 5-HIAA (the primary metabolite of 5-HT) levels were much higher in adult tissues (Figure 5B). Therefore, the 5-HIAA/5-HT ratio, an index of 5-HT turnover, was significantly higher in adults (Figure 5C). Secondly, we used 5-HT immunohistochemistry to localize 5-HT in the EC cells of the neonatal and adult intestine (Figure 6). EC cells were easily recognized in whole wall intestinal cross sections from adults and neonates. Cell counts revealed that the numbers of EC cells were similar in sections from neonatal and adult intestine but the cell distribution differed (Figure 7). In adult tissues, EC cells were localized predominately along the mucosal villi (Figures 6 and 7A) while in neonatal tissues, EC cells were found predominately in the crypts (Figure 6, 7A). The difference in EC cell distribution was reflected in a difference in the crypt/villus ratio for neonatal and adult tissues (Figure 7B).
Figure 5.
5-HT and 5-HIAA levels in the mucosa of neonatal and adult intestine. (A) There was no difference in 5-HT concentration in the mucosa of neonatal (n=6) vs. adult (n=5) intestine. (B) 5-HIAA levels were lower in neonatal intestine (*, P < 0.05). (C) The 5-HT/5-HIAA ratio was also smaller in neonatal compared to adult intestine (*, P <0.05). Data are mean ± S.E.M.
Figure 6.
Immunohistochemical localization of 5-HT-ir in sections of adult and newborn intestine. (A) A cross section of the adult intestine showing 5-HT-ir EC cells distributed in the crypt region and along the mucosal villi. (B) A cross section of neonatal intestine showing that 5-HT-ir EC cells are localized preferentially to the crypt region.
Figure 7.
Analysis of the distribution of EC cells in the crypts and villi of neonatal and adult guinea pigs. (A) The total number of EC cells in the mucosa did not differ between adult (n=4) and neonatal (n=4) guinea pigs. However, the number of EC cells in the crypt of neonatal intestine was significantly greater (*, P < 0.05) than that in adult intestine. Alternatively, the number of EC cells in adult villi was significantly greater (*, P < 0.05) than that in the neonatal intestine. (B) The EC cell crypt-villus ratio was significantly larger in the neonatal intestine (*, P <0.05). Data are mean ± S.E.M.
Discussion
Amperometric measurement of 5-HT release
5-HT release from intestinal EC cells has been studied previously either by sampling the neurotransmitter content in overflow solutions in vitro or in blood samples obtained in vivo.3,20,21 In order elicit release of detectable amounts of 5-HT, large volume samples and prolonged periods of pharmacological or electrical stimulation were required. While these studies have provided important data about EC cell function, overflow techniques do not allow 5-HT measurements in real time and they have poor spatial resolution as the molecule is released from many hundreds of EC cells from extended lengths of intestine. These issues have been addressed by using amperometric methods with carbon fiber microelectodes, which allow real time measurement of 5-HT from the mucosa of intestinal segments maintained in vitro.14,17 Furthermore, these microelectrode based techniques allow detection of 5-HT release from just a few EC cells near the electrode tip.
While real-time and local measurements of 5-HT are feasible with carbon fibers, the duration of recordings is limited by extensive electrode fouling caused by adsorption of oxidation products and other biological molecules present in the tissue. Electrode fouling reduces electrode sensitivity for detection of 5-HT. Diamond microelectrodes are resistant to fouling enabling these electrodes to be used for extended periods of time for sensitive and reproducible measurement of 5-HT release from the intestine.17
Previous work showed that currents caused by oxidation of 5-HT at the electrode tip can be discriminated from oxidation currents produced by other molecules in the mucosa.14,17 Using diamond microelectrodes, this can be accomplished by setting the voltage at the electrode tip to 0.8 V. When diamond microelectrodes are positioned near the mucosal surface, there is an abrupt increase in the oxidation current. The basal response was partly reduced by the neurotoxin, TTX, which indicates that 5-HT release from EC cells was partly neurogenically driven. EC cells express nicotinic and muscarinic receptors for acetylcholine, which is released from nerves supplying the mucosal layer, and acetylcholine can directly stimulate 5-HT release from EC cells.22,23 In our experiments, it is likely that TTX inhibited this excitatory cholinergic input. However, not all of the 5-HT release required nerve activity as a substantial fraction of the 5-HT oxidation current persisted in the presence of TTX, particularly in adult tissues. The TTX-resistant component was blocked by reducing extracellular Ca2+. This indicates that the release of 5-HT was due to Ca2+-dependent exocytosis. Mechanically stimulating a few villi in adult tissues evoked an additional current that was inhibited by reduced extracellular Ca2+ but not by TTX. This result suggests that the mechanically-evoked response was due either to direct mechanical stimulation of the EC cells or to release of substances from non-neuronal cells which then caused Ca2+-dependent exocytosis from EC cells. An important new finding from the present work is that basal and stimulated 5-HT oxidation currents in neonatal intestine greatly exceeded those measured in adult tissues.
SERT expression is reduced in neonatal intestine
The elevated basal and stimulated 5-HT oxidation currents detected in neonatal compared to adult tissues could be due to one or more factors. The neonatal intestine could contain more EC cells with higher levels of 5-HT or 5-HT could be cleared less efficiently from the extracellular space in the neonatal intestine. As SERT is the primary mechanism for clearing 5-HT from the mucosa,9 the neonatal intestine may express less SERT or SERT may not function as efficiently as it does in the adult intestine. With less efficient recovery, there would be more 5-HT overflow in the neonatal intestine.
Fluoxetine is a SERT inhibitor and if SERT is responsible for clearing 5-HT from the mucosa, it is expected that fluoxetine would enhance the 5-HT current detected near the intestinal mucosa. Consistent with this prediction we found that fluoxetine produced a marked increase in basal and stimulated oxidation currents in adult tissues. However, fluoxetine did not increase basal or stimulated currents in the intestine of neonates. These data suggest that the elevated overflow currents in neonatal tissue are due to either the absence of SERT from the neonatal intestine or that SERT is present but not functional in these tissues.
Immunohistochemical studies using an antibody raised against a portion of the rat SERT sequence revealed that SERT-immunoreactivity (ir) was present in enterocytes lining the mucosal villi in frozen sections of the adult intestine. Similar studies revealed that little or no SERT-ir was detectable in sections from the neonatal intestine. These data are consistent with the functional data discussed above. We sought to confirm the absence of SERT-ir from the neonatal intestine using Western blot techniques and the same rat SERT antibody. These studies revealed a complex expression of SERT-ir in protein extracts obtained from the mucosal of adult and guinea pig intestine. The SERT protein identified in rat and human brain and rat intestine has a molecular weight of approximately 75 kDa.26,27,28 This antibody also revealed a 75 kDa band in protein extracts of whole guinea pig brain and in protein extracts of neonatal and adult guinea pig intestine. There was no difference in the intensity of the 75 kDa band between neonates and adults suggesting that there is no difference in expression of this form of the SERT. However, a new finding presented here is that the antibody also revealed a protein band with a molecular weight of approximately 60 kDa in both the neonatal and adult intestine. This 60 kDa band was absent from the brain but was significantly stronger in protein extracts from the adult intestine. Previous work revealed that there are only modest differences in the nucleotide sequences encoding the rat and human brain and guinea pig intestine SERT protein.28 However, the same study showed that some antibodies recognize the rat brain SERT but do not recognize the SERT protein in the guinea pig intestine. It is possible that the SERT protein in the adult guinea pig intestine undergoes some post-translational modification that shortens the amino acid sequence to yield the mature (functional) form of the transporter. This post-translational processing does not occur in the neonatal intestine and therefore the SERT protein is present but it is expressed in an immature (non-functional) form. The SERT antibody may recognize the denatured 60 kDa protein in Western blots but it may not recognize the native SERT conformation in enterocytes in immunohistochemical studies. It should also be pointed out that our data are the first to describe Western blot analysis of SERT obtained from the guinea pig small intestine. Western blot studies done on protein extracts obtained from guinea pig airway smooth muscle cells maintained in culture revealed a single band with a molecular weight near 115 kDa.29 This band might represent a dimer of individual SERT proteins (each with a MW of approximately 60 kDa). It is also possible that the 60 kDa band represents a degradation product of a larger SERT form expressed in the guinea pig intestine. Our protein isolation protocol included the use of a protease inhibitor cocktail; however, this may have been insufficient to fully block the high levels of protease activity present in the intestinal mucosa.
5-HT content and differential distribution of EC cells in neonatal and adult intestine
There were no differences in mucosal 5-HT content in tissues obtained from neonatal and adult guinea pigs. Therefore, differences in EC cell 5-HT content can not account for the elevated basal levels of extracellular 5-HT detected in neonatal tissues. 5-HIAA (the major degradation metabolite of 5-HT) was higher in adult tissues compared to that in neonatal tissues. The 5-HIAA/5-HT ratio is a measure of 5-HT turnover.24 After 5-HT is released from EC cells it is taken up by SERT expressing enterocytes where it is oxidized by monoamine oxidase to produce 5-HIAA.24 As functional SERT is absent from neonatal enterocytes, 5-HT can not be transported by the enterocytes for metabolism to 5-HIAA.
We found that there were no differences in EC cell numbers in sections of adult and neonatal intestine and this corresponds to the similar 5-HT content measured in adult and neonatal tissues. However, in adult tissues, EC cells were localized along the length of mucosal villi while in sections from neonates, EC cells were found predominately in the crypt region. It is possible that recently differentiated EC cells in the crypt region of neonatal animals have only just begun to migrate towards the villus tip.30 It is also possible that EC cell movement into the villi stimulates SERT expression in enterocytes. Membrane expression and SERT activity are regulated by protein kinase C and p38-MAP kinase-dependent pathways activated by G-protein coupled receptors, including 5-HT receptors.31 Enterocytes in guinea pig mucosal villi and crypts express 5-HT2A receptors32,33 and 5-HT2A receptors can activate MAP-kinase in vascular smooth muscle and renal mesangial cells.34,35 The p38 MAP-kinase dependent pathway is particularly important for maintaining SERT membrane expression and activity. 5-HT released by EC cells as they migrate into the villi could stimulate maturation and membrane expression of SERT in the enterocytes so that as the intestine matures in the postnatal period it is prepared to buffer extracellular 5-HT released by EC cells.
Conclusions
The data presented here indicate that paracrine signaling by EC cells in the intestinal mucosa is not fully developed in the small intestine of neonatal guinea pigs. Specifically, functional SERT expression has not yet developed and therefore, there is no active mechanism for clearance of 5-HT released by EC cells in the neonatal intestine. The absence of functional SERT expression causes enhanced basal and mechanically-stimulated overflow of 5-HT from the mucosa. Pathophysiological changes in SERT function or expression are also associated with increased availability of 5-HT in the intestinal mucosa. In a guinea pig model of colonic inflammation, SERT expression is reduced and 5-HT availability is increased in inflamed tissues under basal and stimulated conditions.36 In addition, fluoxetine increased 5-HT availability in control but not inflamed tissues. It was proposed that the increased free mucosal 5-HT in inflamed tissues might contribute to altered colonic motility known to occur in human and experimental colitis.36 As 5-HT released from EC cells activates motor reflexes elicited by mucosal stimulation, it might be expected that these reflexes would not be fully-developed in the neonatal intestine where SERT expression is low and 5-HT availability is high. Therefore, propulsive motility might be impaired in the neonatal gut as it is in the inflamed gut. Maturation of the 5-HT signaling system in the gut might be required for postnatal development of motility patterns as the neonate transitions to oral intake of nutrients.
Acknowledgments
Grant support: HL084258 (GMS) and DK57039 (JJG). BAP was supported provided by an EPSRC LSI Postdoctoral Fellowship Grant (EP/C532058/1). The authors wish to thank Dr. Veronika Quaiserova-Mocko for preparing the diamond microelectrodes used in this work and Dr. Randy Blakely (Vanderbilt University) for the gift of the SERT antibody.
Abbreviations
- EC
Enterochromaffin
- 5-HIAA
5-hydroxyindole acetic acid
- 5-HT
5-hydroxytryptamine
- HPLC
high performance liquid chromatography
- SERT
serotonin transporter
- TTX
tetrodotoxin
Footnotes
Conflicts of Interest: The authors have no conflicts to disclose.
References
- 1.Gershon MD, Tamir H. Release of endogenous 5-hydroxytryptamine from resting and stimulated enteric neurons. Neuroscience. 1981;6:2277–2286. doi: 10.1016/0306-4522(81)90017-8. [DOI] [PubMed] [Google Scholar]
- 2.Costa M, Furness JB, Cuello AC, Verhofstad AA, Steinbusch HW, Elde RP. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their visualization and reactions to drug treatment. Neuroscience. 1982;7:351–363. doi: 10.1016/0306-4522(82)90272-x. [DOI] [PubMed] [Google Scholar]
- 3.Racke K, Reimann A, Schworer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. 1996;73:83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 4.Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Invest Drugs. 2004;5:55–60. [PubMed] [Google Scholar]
- 5.Bulbring E, Lin R. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis, the local production of 5-hydroxytryptamine and its release in relation to intraluminal pressure and propulsive activity. J Physiol. 1958;140:381–407. [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea pig ileum are excited by 5-hydroxytrypamine acting at 5-hydroxytrypamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 7.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neuroscience. 2000;20:3295–3309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1P receptors on sensory CGRP neurons. Am J Physiol. 1996;270:G778–G782. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- 9.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neuroscience. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young HM, Newgreen D. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. Anat Rec. 2001;262:1–15. doi: 10.1002/1097-0185(20010101)262:1<1::AID-AR1006>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Young HM, Anderson RB, Anderson CR. Guidance cues involved in the development of the peripheral autonomic nervous system. Auton Neurosci. 2004;112:1–14. doi: 10.1016/j.autneu.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Hagl CI, Thil O, Holland-Cunz S, Faissner R, Wandschneider S, Schnolzer M, Lohr M, Schafer KH. Proteome analysis of isolated myenteric plexus reveals significant changes in protein expression during postnatal development. Auton Neurosci. 2005;122:1–8. doi: 10.1016/j.autneu.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Wester T, O’Brian DS, Puri P. Notable postnatal alterations in the myenteric plexus of the normal human bowel. Gut. 1999;44:666–674. doi: 10.1136/gut.44.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand PP. Real-time detection of serotonin release from enterochromaffin cells of the guinea-pig ileum. Neurogastroenterol Motil. 2004;16:511–514. doi: 10.1111/j.1365-2982.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 15.Baur JE, Kristensen EW, May LJ, Wiedemann DJ, Wightman RM. Fast-scan voltammetry of biogenic amines. Anal Chem. 1988;60:1268–1272. doi: 10.1021/ac00164a006. [DOI] [PubMed] [Google Scholar]
- 16.Jackson BP, Dietz SM, Wightman RM. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal Chem. 1995;67:1115–1120. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- 17.Patel B, Bian X, Galligan JJ, Swain GM. In vitro continuous amperometric monitoring of 5-hydroxytrypamine release from enterochromaffin cells of the guinea pig ileum. The Analyst. 2007;132:41–7. doi: 10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- 18.Park J, Quaiserova-Mocko V, Peckova K, Galligan JJ, Fink GD, Swain GM. Fabrication, characterization, and application of a diamond microelectrode for electrochemical measurement of norepinephrine release from the sympathetic nervous system. Diamond Relat Mater. 2006;15:761–772. [Google Scholar]
- 19.Park J, Show Y, Quaiserova V, Galligan JJ, Fink GD, Swain GM. Diamond microelectrodes for use in biological environments. J Electroanal Chem. 2005;583:56–68. [Google Scholar]
- 20.Schworer H, Ramadori G. Autoreceptors can modulate 5-hydroxytryptamine release from porcine and human small intestine in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:548–52. doi: 10.1007/pl00005206. [DOI] [PubMed] [Google Scholar]
- 21.Ginap T, Kilbinger H. NK1- and NK3-receptor mediated inhibition of 5-hydroxytryptamine release from the vascularly perfused small intestine of the guinea-pig. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:689–93. doi: 10.1007/pl00005106. [DOI] [PubMed] [Google Scholar]
- 22.Racke K, Schworer H. Nicotinic and muscarinic modulation of 5-hydroxytryptamine (5-HT) release from porcine and canine small intestine. Clin Invest. 1992;70:190–200. doi: 10.1007/BF00184650. [DOI] [PubMed] [Google Scholar]
- 23.Schworer H, Racke K, Kilbinger H. Cholinergic modulation of the release of 5-hydroxytryptamine from the guinea pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:127–32. doi: 10.1007/BF00165795. [DOI] [PubMed] [Google Scholar]
- 24.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–57. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 25.Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- 26.Austin MC, Bradley CC, Mann JJ, Blakely RD. Expression of serotonin transporter messenger RNA in the human brain. J Neurochemistry. 1994;62:2362–2367. doi: 10.1046/j.1471-4159.1994.62062362.x. [DOI] [PubMed] [Google Scholar]
- 27.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neuroscience. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol. 1998;275:G433–448. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 29.Dodson AM, Anderson GM, Rhoden KJ. Serotonin uptake and metabolism by cultured guinea pig airway smooth muscle cells. Pulm Pharmacol Ther. 2004;17:19–25. doi: 10.1016/j.pupt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Hocker M, Wiedenmann B. Molecular mechanisms of enteroendocrine differentiation. Ann NY Acad Sci. 1998;859:160–74. doi: 10.1111/j.1749-6632.1998.tb11120.x. [DOI] [PubMed] [Google Scholar]
- 31.Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neuroscience. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorica-Howells E, Hen R, Gingrich J, Li Z, Gershon MD. 5-HT2A receptors: location and functional analysis in intestines of wild-type and 5-HT2A knockout mice. Am J Physiol. 2002;282:G877–993. doi: 10.1152/ajpgi.00435.2001. [DOI] [PubMed] [Google Scholar]
- 33.Siriwardena AK, Smith EH, Borum EH, Kellum JM., Jr Identification of a 5-hydroxytryptamine (5-HT2) receptor on guinea pig small intestinal crypt cells. Am J Physiol. 1993;265:G339–446. doi: 10.1152/ajpgi.1993.265.2.G339. [DOI] [PubMed] [Google Scholar]
- 34.Greene EL, Houghton O, Collinsworth G, Garnovskaya MN, Nagai T, Sajjad T, Bheemanathini V, Grewal JS, Paul RV, Raymond JR. 5-HT2A receptors stimulate mitogen-activated protein kinase via H2O2 generation in rat renal mesangial cells. Am J Physiol. 2000;278:F650–658. doi: 10.1152/ajprenal.2000.278.4.F650. [DOI] [PubMed] [Google Scholar]
- 35.Banes A, Florian JA, Watts SW. Mechanisms of 5-hydroxytryptamine2A receptor activation of the mitogen-activated protein kinase pathway in vascular smooth muscle. J Pharmacol Exp Ther. 1999;291:1179–1187. [PubMed] [Google Scholar]
- 36.Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol. 2003;285:G207–216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]