Abstract
Background
Classical Kaposi sarcoma (cKS) is a rare complication of Kaposi sarcoma-associated herpes virus (KSHV) infection. We conducted a population-based, frequency-matched case-control study in Sicily to further investigate the reported inverse relationship between smoking and cKS and to identify other factors associated with altered risk.
Methods
All incident, histologically confirmed, cKS cases in Sicily were eligible. A two-stage cluster sample design was applied to select population controls. KSHV seropositivity was determined using 4 antibody assays (K8.1 and orf73 enzyme immunoassays and 2 immunofluroescence assays). Using SAS-callable SUDAAN we compared the characteristics of cKS cases and KSHV seropositive controls. Odds ratios (ORs) and 95% confidence intervals (CIs) are presented.
Results
In total, 142 cKS cases and 123 KSHV seropositive controls were recruited. Current cigarette smoking was associated with reduced risk of cKS (OR 0.20, 95% CI 0.06-0.67). Edema was associated with cKS, but only when it presented on the lower extremities (OR 3.65, 95% CI 1.62-8.23). Irrespective of presentation site, diabetes and oral corticosteroid medications were associated with increased risk (ORs, 95% CIs: 4.73, 2.02-11.1 and 2.34, 1.23-4.45, respectively). Never smoking, diabetes and oral corticosteroid medication use were all independently associated with cKS risk.
Discussion
We confirmed previous reports that cigarette smoking was associated with a reduced risk of cKS, and we found that risk was lowest among current smokers. We also found that cKS risk was strongly and independently associated with oral corticosteroid use and diabetes. Corroboration of these observations and investigation of possible underlying mechanisms are warranted.
Introduction
Kaposi sarcoma-associated herpes virus (KSHV) is the primary cause of all forms of Kaposi sarcoma (KS)[Moore and Chang, 1998], including classical KS (cKS)[Rady, Yen, Martin, III, Nedelcu, Hughes, and Tyring, 1995]. Unlike iatrogenic or acquired immunodeficiency syndrome (AIDS) KS, cKS predominately occurs in elderly men of Mediterranean or Jewish decent, with no apparent immunosuppresion[Touloumi, Hatzakis, Potouridou, Milona, Strarigos, Katsambas, Giraldo, Beth-Giraldo, Biggar, Mueller, and Trichopoulos, 1999;Brown, Fallin, Ruczinski, Hutchinson, Staats, Vitale, Lauria, Serraino, Rezza, Mbisa, Whitby, Messina, Goedert, and Chanock, 2006]. In the Mediterranean area, KSHV is thought to be mainly transmitted through early life exposure, probably to infectious saliva, with little evidence of sexual transmission[Whitby, Luppi, Sabin, Barozzi, Di Biase, Balli, Cucci, Weiss, Boshoff, and Torelli, 2000]. Once infected, KSHV generally remains latent. Factors associated with dissemination of the virus in vivo and via shedding in saliva to susceptible individuals have not been well characterized. Patients with cKS, as well as those with other types of KS, have much higher antibody titers and viral load in peripheral blood cells compared to KSHV seropositive, disease free individuals[Brown, Fallin, Ruczinski, Hutchinson, Staats, Vitale, Lauria, Serraino, Rezza, Mbisa, Whitby, Messina, Goedert, and Chanock, 2006]. Identification of other differences between cKS patients and KSHV seropositive controls may help to explain why only a few infected adults (approximately 1 in 3,000 men and 1 in 8,000 women) progress to cKS annually[Vitale, Briffa, Whitby, Maida, Grochowska, Levin, Romano, and Goedert, 2001].
Our group reported data from a case-control study of cKS patients in Sicily, Naples and Rome (1998-2001)[Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002]. Asthma, allergies in males, topical corticosteriod use and infrequent bathing were associated with increased risk of cKS. Interestingly, cigarette smoking was associated with a reduced risk of cKS[Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002]. This was most prominent in males and those who had the greatest cumulative exposure to cigarette smoking. Cigarette smoking has also been associated with a reduced risk of AIDS KS[Nawar, Mbulaiteye, Gallant, Wohl, Ardini, Hendershot, Goedert, and Rabkin, 2005;Hoover, Black, Jacobson, Martinez-Maza, Seminara, Saah, Von, Anderson, and Armenian, 1993], and this inverse association with smoking is supported by the reduced risk of lung cancer among patients with KS[Safai, Mike, Giraldo, Beth, and Good, 1980;Dictor and Attewell, 1988;Iscovich, Boffetta, Winkelmann, and Brennan, 1999]. Despite these observations, the association has not been characterized in depth, and potential mechanisms of action have not been elucidated. We conducted a case-control study in Sicily to further investigate the inverse relationship between smoking and cKS and to identify other factors associated with the development of cKS.
Methods
Incident, histopathologically confirmed, first primary cKS in native born Italians, diagnosed from July 2002 through June 2006, were identified from all histopathology laboratories on the island of Sicily. Patients diagnosed with human immunodeficiency virus (HIV) were excluded from the study. In agreement with data from the population-based registries in Ragusa, Siracusa and Trapani that monitor cancer incidence in one-fifth of the Sicilian population, a total of 177 incident cases of cKS were identified. Of these, 35 patients did not participate for the following reasons: refusal (n=20), deceased (n=12), unable to locate (n=3). The overall participation rate of cKS patients was 80.2%.
All residents of Italy are assigned to a primary care physician, with rosters maintained by the regional health authority. As illustrated in Figure 1, a stratified two-stage cluster sample design was applied to select controls from the population of Sicily listed on the 4,044 primary care physicians' rosters (minimum roster size of 200 patients) with known sex and age 31-92 (n=3,141,224). Physician practices were stratified by community size (<10,000, 10,000-100,000, >100,000 residents); then 450 practices were randomly sampled, with the probability of selection proportional to the size of the roster. The 450 practices were randomly assigned to one of three Waves, with on average 79 and 68 practices in eastern and western Sicily, respectively. Controls were randomly selected with different probabilities of selection from each roster in order to frequency match them to the distribution by sex (73.6% male) and age (31-50, 51-60, 61-70, 71-80, 81-92) of the anticipated cKS cases. In Wave 1, eight patients were selected from each of the 150 practices (n=1,200). In Waves 2-3, we used an updated file to exclude deceased physicians (n=13, including 4 in Wave 1) and selected 12 patients per practice (n=1,752 and 1,740 in Waves 2 and 3, respectively). Of the 4,692 selected controls, 1,268 were not approached when Wave 3 was terminated for lack of funds, 404 were deceased and 14 were otherwise out-of-scope (non-Italian, insufficient blood for KSHV testing, or had cKS). Of the 3,006 eligible patients, 1,774 were non-respondents, including 590 who could not be approached because the physician refused, had retired, or had died. The final population of controls was n=1,232 (41% of the eligible participants).
Figure 1.
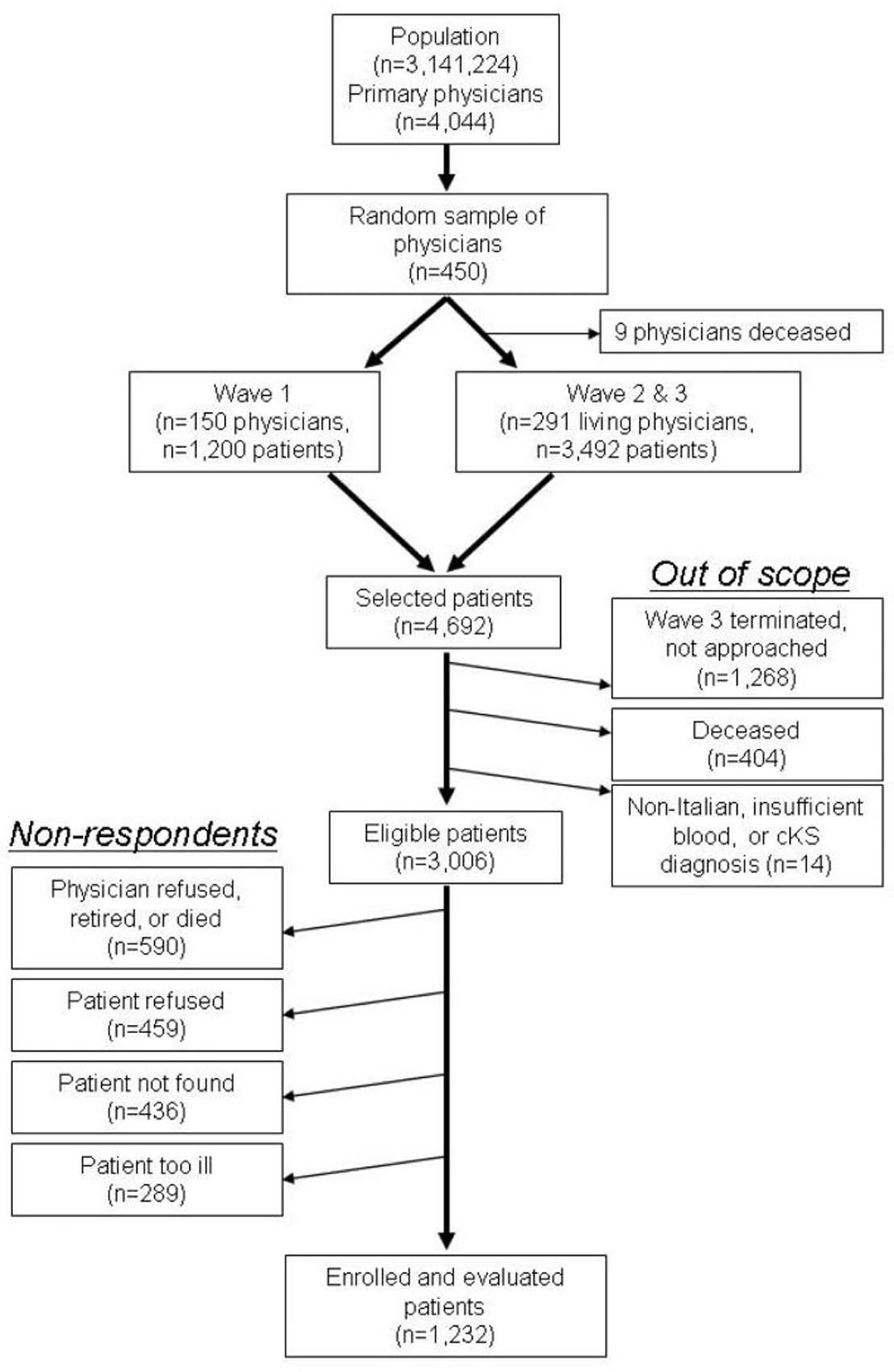
Two-stage sampling procedures for population-controls
All subjects provided signed informed consent, and ethical approval was obtained from the U.S. National Cancer Institute, local institutions in eastern (Ragusa) and western (Palermo) Sicily, and the coordinating center (RTI International).
Exposure assessment
Subjects were interviewed using a standardized questionnaire about demographic characteristics, socio-economic variables, medical history and smoking. Participants were defined as never smokers if they had smoked less than one cigarette per week in their lifetime. Current smokers were defined as those who still smoked at least one cigarette per week, and former smokers as those who had stopped smoking at least one cigarette per week. A 23-25 ml peripheral blood sample was obtained from all cases and controls in EDTA anticoagulated blood vacutainer tubes. Aliquots of plasma were stored at -80°C until testing. One plasma aliquot was tested for HIV-1 antibodies with a licensed immunoassay; all were negative.
Kaposi Sarcoma-associated Herpes Virus antibody testing
KSHV antibody testing was performed as reported previously[Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002]. Briefly, using a 1:120 dilution of plasma and the BCBL-1 cell line with and without induction by tetradecanoyl phorbol-ester acetate (TPA), antibodies against KSHV lytic and latent nuclear antigens, respectively, were detected using immunofluroescence assays (IFA)[Perna, Bonura, Vitale, Viviano, Di Benedetto, Ajello, Villafrate, Prestileo, Mancuso, Goedert, and Romano, 2000]. Enzyme immunoassays (EIAs) with recombinant proteins were used to detect KSHV antibodies against the K8.1 structural glycoprotein and open reading frame (orf) 73 at plasma dilutions of 1:20 or 1:100, respectively[Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002].
Subjects were considered KSHV seropositive if the latent IFA was positive or the K8.1 optical density (OD) was >1.2. KSHV seronegative was defined as latent IFA negative and K8.1 OD≤0.8 and orf73 OD≤0.8. IFA latent and lytic results were unavailable for 4 controls, all of whom were below the K8.1 and orf73 OD cutoffs and negative in investigational KSHV antibody assays (data not presented). These 4 subjects were categorized as KSHV seronegative. All other subjects were categorized as having indeterminant results.
Weighting procedures
To take into account the complex sampling used for the selection of controls, controls were weighted using the product of the reciprocal of the selection probabilities at each stage of sampling. These weights are referred to as the base weights which were adjusted for non-response by first forming non-response categories that were a cross-classification of age (31-50 and 81-90, 51-60, 61-80 years), region (eastern, western Sicily), and gender. The base weights for enrolled controls within each non-response category were then multiplied by the inverse of the (base) weighted proportion of the enrolled controls to the eligible sampled controls. Using a two-dimensional raking method[Little and Rubin, 1987], these non-response-adjusted weights were further adjusted by using post-stratification to constrain the weights to reflect the population totals by age, gender and zone (six zones are made up of three community sizes (<10,000, 10,000-100,000 and >100,000) by two regions (eastern, western Sicily)).
To approximate the frequency matching of the control sample to the age, gender and community size distribution of the cases, the non-response/post-stratification-adjusted weights for the controls were adjusted by applying two-dimensional raking to these weights so that they matched the distribution of all the cases (including the non-responding cases). To reflect the sample size of the enrolled controls, these ratio-adjusted weights were further scale-adjusted so that these weights summed to the sample size of the enrolled controls.
Weights were also constructed for the cases. Since cases were not sampled, the base weights were set equal to one. These base weights were then adjusted for non-response (n=35) in a similar manner to the controls, but the response categories were the cross-classified categories of age (≤80, >80 years) and gender. The non-response-adjusted case weights were further scale-adjusted so that the sum of the case weights was the number of enrolled cases (n=142).
Statistical analysis
Classical chi-square and t-tests were used for descriptive, unweighted, unadjusted demographic comparisons. SAS-callable SUDAAN was used to conduct logistic regression analyses that accounted for the sample weighting and the multistage stratified cluster sampling of the controls. These logistic regression analyses compared potential risk factors between patients with cKS and the KSHV seropositive controls. Categories for continuous measured covariates were defined a priori, using median or tertile values, among KSHV seropositive controls. All analyses were adjusted for age in quartiles (<68, 68-74, 75-80, >80 years), education (1st-4th grade, 5th grade and higher) and gender (or restricted to males).
Results
Of the 142 cKS cases recruited, 135 (95.1%) were KSHV seropositive, 4 (2.8%) were KSHV seronegative, and 3 (2.1%) had indeterminate KSHV status. Of the 1,232 controls, 123 (10.0%) were KSHV seropositive, 1,031 (83.7%) were KSHV seronegative and 78 (6.3%) had indeterminate KSHV status. KSHV seropositive controls were slightly older than KSHV seronegative/indeterminate controls (mean 73.6 vs. 71.1 years, p=0.05), but they did not differ with respect to gender (70% vs. 74% male, p=0.33). KSHV seropositive controls did not differ from cKS cases in either age (mean 73.6 vs. 74.6 years, p=0.45) or gender (70% vs. 63% male, p=0.22). Feet or legs were the site of initial cKS lesions in 34 (23.9%) of the patients with cKS.
Tables 1-3 present odds ratios (OR) and 95% confidence intervals (CI) associated with postulated risk factors for cKS compared to KSHV seropositive controls that take account of the complex sample design, with adjustment for age, gender and where appropriate education. cKS patients were more likely than KSHV seropositive controls to have been educated beyond the 4th grade (OR 2.63, 95% CI 1.48-4.68). cKS patients tended to come from childhood households that had at least 6 residents, but the association did not reach statistical significance. Household crowding, as reflected by the number of people per bedroom in the childhood home, was not associated with risk of cKS. In addition, the number of older and younger siblings did not differ significantly between cKS patients and KSHV seropositive controls.
Table 1.
Sociodemographic characteristics of classic Kaposi sarcoma (cKS) cases and Kaposi sarcoma-associated herpes virus (KSHV) seropositive controls.
| No. of cKS patients (n=142) | No. of KSHV seropositive controls (n=123) | OR (95% CI)* | |
|---|---|---|---|
| Commune size (current) | |||
| <10,000 | 21 | 26 | 1.00 |
| ≥10,000 | 111 | 97 | 1.09 (0.65-1.82) |
| Commune size (most of adult life) | |||
| <10,000 | 48 | 47 | 1.00 |
| ≥10,000 | 89 | 76 | 1.13 (0.63-2.03) |
| Commune size (birth) | |||
| <10,000 | 50 | 37 | 1.00 |
| ≥10,000 | 91 | 86 | 0.81 (0.45-1.45) |
| Education† | |||
| 1st - 4th grade | 44 | 62 | 1.00 |
| 5th grade or higher | 97 | 60 | 2.63(1.48-4.68) |
| No. residents in childhood home | |||
| <6 | 59 | 65 | 1.00 |
| ≥6 | 83 | 58 | 1.65 (0.94-2.92) |
| No. people per bedroom | |||
| <3 | 80 | 66 | 1.00 |
| ≥3 | 62 | 57 | 1.10 (0.62-1.96) |
| No. older siblings | |||
| <2 | 64 | 66 | 1.00 |
| ≥2 | 78 | 57 | 1.36 (0.76-2.46) |
| No. younger siblings | |||
| <3 | 82 | 81 | 1.00 |
| ≥3 | 59 | 41 | 1.54 (0.88-2.80) |
Adjusted for gender, age group (<68, 68-74, 75-80, >80 years) and education (1st-4th grade, 5th grade and higher).
Adjusted for gender and age group (<68, 68-74, 75-80, >80 years) only.
Table 3.
Comparison of select medical conditions and medication usage in classic Kaposi sarcoma (cKS) patients and Kaposi sarcoma-associated herpes virus (KSHV) seropositive controls.
| No. of cKS patients (n=142) | No. of KSHV seropositive controls (n=123) | OR (95% CI)* | |
|---|---|---|---|
| Medical Conditions | |||
| Allergies | 26 | 24 | 1.01 (0.50-2.07) |
| Angina | 9 | 7 | 0.76 (0.24-2.37) |
| Arthritis | 4 | 6 | 0.55 (0.13-2.39) |
| Asthma | 15 | 13 | 1.42 (0.61-3.31) |
| Bronchitis | 41 | 38 | 0.99 (0.54-1.83) |
| Chilblains | 38 | 28 | 1.11 (0.58-2.13) |
| Cirrhosis/Liver failure | 15 | 9 | 2.17 (0.73-6.41) |
| Diabetes | 45 | 15 | 4.73 (2.02-11.1) |
| Edema | 68 | 31 | 3.11 (1.59-6.05) |
| >5 years prior to interview | 34 | 18 | 1.63 (0.74-3.59) |
| Gout | 14 | 8 | 1.28 (0.45-3.62) |
| Heart disease/stroke | 43 | 31 | 0.93 (0.49-1.77) |
| Hypertension | 87 | 70 | 0.87 (0.48-1.56) |
| Kidney failure/Dialysis | 19 | 4 | 3.86 (1.22-12.3) |
| Malaria | 32 | 35 | 0.84 (0.45-1.58) |
| Sexually transmitted disease | 9 | 11 | 0.72 (0.26-1.93) |
| Thalassemia | 3 | 3 | 1.18 (0.18-7.55) |
| Medications† | |||
| Oral Cortisone | 53 | 26 | 2.34 (1.23-4.45) |
| Cortisone cream | 53 | 36 | 1.45 (0.79-2.66) |
| Other medicated non-steroid cream | 62 | 57 | 0.81 (0.45-1.46) |
| Herbal treatments | 26 | 16 | 1.58 (0.70-3.55) |
Logistic regression analyses adjusted for gender, age (<68, 68-74, 75-80, >80 years) and education (1st-4th grade, 5th grade and higher) that account of the sample weighting and the multistage stratified cluster sampling of the controls
Used during the past 10 years.
Current cigarette smoking, compared to never smoking, was strongly associated with a reduced risk of cKS (OR 0.22, 95% CI 0.07-0.69), particularly in males (OR 0.20, 95% CI 0.06-0.67, Table 2). Because there were few current (cases: n=3, controls: n=2) or former (cases: n=2, controls: n=2) female smokers, Table 2 presents the findings for male smokers only. The reduced risk for current smokers was stronger before age 75 years (OR 0.07, 95% CI 0.01-0.43) than at older ages (OR 0.66, 95% CI 0.11-3.87).
Table 2.
Comparison of smoking habits of male patients with classic Kaposi sarcoma (cKS) and Kaposi sarcoma-associated herpes virus (KSHV) seropositive controls.
| No. of male cKS patients | No. of male KSHV seropositive controls | OR (95% CI)* | |
|---|---|---|---|
| Cigarette smoking | |||
| Never | 23 | 12 | 1.00 |
| Former | 59 | 55 | 0.50 (0.19-1.32) |
| Current | 7 | 19 | 0.20 (0.06-0.67) |
| Cigarette smoking in men aged <75 years | |||
| Never | 16 | 7 | 1.00 |
| Former | 23 | 26 | 0.30 (0.09-1.07) |
| Current | 2 | 11 | 0.07 (0.01-0.43) |
| Cigarette smoking in men aged ≥75 years | |||
| Never | 7 | 5 | 1.00 |
| Former | 36 | 29 | 0.79 (0.19-3.36) |
| Current | 5 | 8 | 0.66 (0.11-3.87) |
| Years since quitting smoking | |||
| Never smoker | 23 | 12 | 1.00 |
| ≥ 26 | 23 | 18 | 0.62 (0.20-1.96) |
| 15-25 | 17 | 19 | 0.42 (0.12-1.41) |
| <15 | 18 | 17 | 0.46 (0.15-1.39) |
| Current smoker | 7 | 19 | 0.18 (0.05-0.64) |
| Smoking duration (years) | |||
| Never smoked | 23 | 12 | 1.00 |
| <34 | 24 | 22 | 0.56 (0.19-1.64) |
| 34-48 | 22 | 27 | 0.38 (0.13-1.09) |
| ≥49 | 20 | 25 | 0.35 (0.11-1.07) |
| No. cigarettes per day | |||
| Never smoked | 23 | 12 | 1.00 |
| <10 | 19 | 22 | 0.48 (0.16-1.41) |
| 10-19 | 13 | 18 | 0.33 (0.10-1.10) |
| ≥20 | 34 | 34 | 0.45 (0.16-1.22) |
| Pack years of smoking | |||
| Never smoked | 23 | 12 | 1.00 |
| <14.7 | 21 | 23 | 0.51 (0.18-1.46) |
| 14.7-47 | 19 | 27 | 0.30 (0.10-0.90) |
| ≥48 | 26 | 24 | 0.51 (0.18-1.46) |
| Cigarette type | |||
| Never smoker | 23 | 12 | 1.00 |
| Filtered usually | 29 | 44 | 0.28 (0.10-0.81) |
| Unfiltered usually | 19 | 16 | 0.61 (0.20-1.89) |
| Filtered and unfiltered equally | 18 | 14 | 0.63 (0.20-2.02) |
| Pipe | |||
| No | 79 | 77 | 1.00 |
| Yes | 10 | 9 | 0.98 (0.31-3.10) |
| Cigar | |||
| No | 75 | 75 | 1.00 |
| Yes | 11 | 14 | 1.18 (0.42-3.32) |
| Lived with ≥1 person who smoked daily† | |||
| No | 17 | 24 | 1.00 |
| Yes | 72 | 62 | 0.77 (0.38-1.55) |
| Worked in smoky indoor place† | |||
| No | 68 | 71 | 1.00 |
| Yes | 21 | 15 | 1.29 (0.50-3.38) |
Logistic regression analyses adjusted for gender, age (<68, 68-74, 75-80, >80 years) and education (1st-4th grade, 5th grade and higher) that account of the sample weighting and the multistage stratified cluster sampling of the controls
Within the past 10 years.
Quitting smoking more recently was associated with a reduced risk of cKS (p trend 0.021). In addition, there appeared to be a trend between increasing duration of smoking and reduced risk of cKS (p trend 0.058). There were no clear trends between intensity or pack-years of smoking and risk of cKS (p trend 0.143 and 0.173, respectively). Male cKS patients were less likely than KSHV seropositive controls to usually smoke filtered cigarettes. Cigar and pipe smoking were not associated with cKS risk. Likewise, living or working in an enclosed environment with smokers was not associated with cKS risk in males, females, or never-smokers (data not presented). None of the study participants had ever used nicotine patches or gum.
Among 16 specific medical conditions queried, cKS cases were more likely than KSHV seropositive controls to have been evaluated for, or diagnosed with, kidney failure/dialysis, diabetes, or edema of the legs or feet (Table 3). All three conditions were reported by seven cKS patients (six of whom had taken oral corticosteroids), compared to no controls. For edema of the lower extremities, the association was limited to cKS patients with their first KS lesion on their legs or feet (OR 3.65, 95% CI 1.62-8.23); KS risk was not increased with a first KS lesion at other body sites (OR 0.99, 95% CI 0.25-3.92). In addition, the association between edema and cKS risk was not significant (OR 1.63, 95% CI 0.74-3.59) when the 5-year period before the interview date was excluded to reduce the possibility that the edema was caused by cKS. Increased risk with kidney failure/dialysis was seen for initial KS lesions on the legs/feet (OR 2.91, 95% CI 0.20-41.6) and also at other body sites (OR 3.82, 95% CI 0.19-77.1). Likewise, increased risk with diabetes was independent of the initial site of cKS lesions (legs/feet: OR 3.30, 95% CI 0.99-10.9; other part of body: OR 2.75, 95% CI 0.67-11.3). None of the other medical conditions investigated was significantly associated with cKS. Any oral corticosteroid use during the previous 10 years was associated with a 2.34-fold increased risk of cKS (Table 3). The use of corticosteroid cream, other topical creams and herbal treatments were not significantly associated with cKS risk.
Multiple variable logistic regression models, each adjusted for gender, age category, and education category, were constructed to assess the independence of variables associated with cKS risk. Edema of the legs/feet was not included. Risk of kidney failure/dialysis remained elevated, although not significant, when adjusted for diabetes (OR 3.03, 95% CI 0.25-36.4). In contrast, as shown in Figure 2, cKS risk was independently associated with never smoking, diabetes and oral corticosteroid use (ORs, 95% CIs: 2.66, 1.06-6.64; 4.02, 1.73-9.37 and 2.25, 1.16-4.38, respectively).
Figure 2.
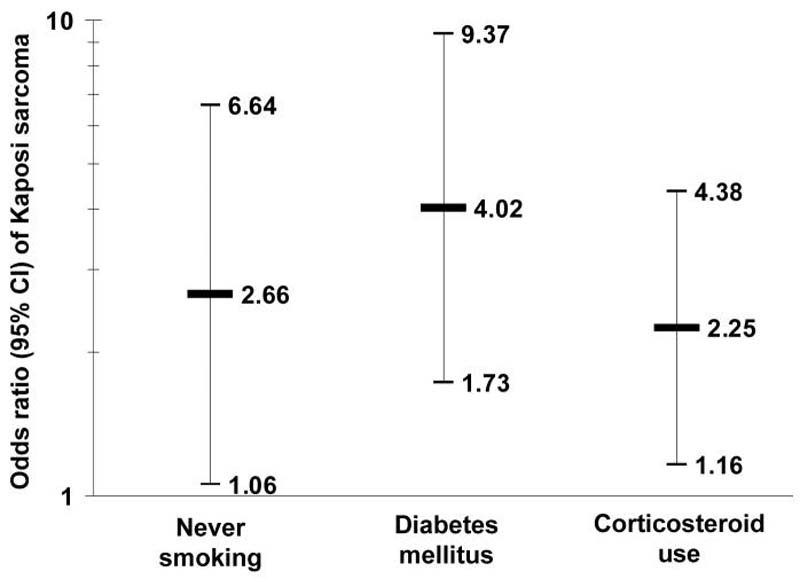
Multiple variable logistic regression model for risk of classic Kaposi sarcoma (cKS), compared to Kaposi sarcoma-associated herpes virus (KSHV) seropositive controls, among men and women in Sicily.
Discussion
In this population-based case-control study, we confirmed the previously reported inverse relationship between cigarette smoking and cKS among men[Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002]. The current study investigated this in more depth, finding that risk of cKS was lowest among current smokers and those who smoked only filtered cigarettes. Independent of smoking, we found that higher education, diabetes and use of oral corticosteroid medications were more common in cKS cases compared to KSHV seropositive controls.
Our findings are consistent with most[Nawar, Mbulaiteye, Gallant, Wohl, Ardini, Hendershot, Goedert, and Rabkin, 2005;Hoover, Black, Jacobson, Martinez-Maza, Seminara, Saah, Von, Anderson, and Armenian, 1993;Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002], but not all[Conley, Bush, Buchbinder, Penley, Judson, and Holmberg, 1996;Guttman-Yassky, Dubnov, Kra-Oz, Friedman-Birnbaum, Silbermann, Barchana, Bergman, and Sarid, 2006], studies of the relation between cigarette smoking and risk of KS. For example, Nawar and colleagues observed a lower relative risk (0.6, 95% CI 0.5-0.9) of AIDS KS among cigarette smokers in a cohort of HIV-positive, KSHV-seropositive homosexual men in the U.S.[Nawar, Mbulaiteye, Gallant, Wohl, Ardini, Hendershot, Goedert, and Rabkin, 2005] In addition, lung cancer is uncommon in patients with KS[Safai, Mike, Giraldo, Beth, and Good, 1980;Dictor and Attewell, 1988;Iscovich, Boffetta, Winkelmann, and Brennan, 1999]. In unpublished data from the Surveillance Epidemiology and End Results program for years 1973 - 2005, risk of a second-primary lung cancer was lower than expected (0.41, 95% CI 0.18-0.80) after a first-primary KS unrelated to AIDS (diagnosed before 1980 or after age 70; Morton, L.M., National Cancer Institute), providing population-based support for the hypothesis that most KS patients were non-smokers.
In Italy[Gallus, Pacifici, Colombo, Scarpino, Zuccaro, Bosetti, Fernandez, Apolone, and La, 2006], as in other developed countries[Franceschi and Naett, 1995;Giovino, Schooley, Zhu, Chrismon, Tomar, Peddicord, Merritt, Husten, and Eriksen, 1994], many of our study participants had discontinued smoking. Remarkably, we found a strong and highly significant trend between smoking within the previous 15 years and reduced risk of cKS. The lower cKS risk was weakly related to duration of smoking, and it was not significantly related to smoking intensity or cumulative exposure, as estimated by cigarettes per day and pack-years, respectively. Never smokers with a high level of education were most at risk of cKS. As was true previously[Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002], cKS risk was unrelated to cigar and pipe smoking.
It has been postulated that immunologic effects of cigarette smoking could reduce the risk of cKS in smokers[Goedert, Scoppio, Pfeiffer, Neve, Federici, Long, Dolan, Brambati, Bellinvia, Lauria, Preiss, Boneschi, Whitby, and Brambilla, 2008;Hoover, Black, Jacobson, Martinez-Maza, Seminara, Saah, Von, Anderson, and Armenian, 1993]. For example, nicotine can affect dendritic cell function[Nouri-Shirazi, Tinajero, and Guinet, 2007;Nouri-Shirazi and Guinet, 2003;Hogg, 2003] and alter cytokine and growth factor production involved in the propagation of KS[Chang, Renne, Dittmer, and Ganem, 2000]. This prompted a recent randomized clinical trial to evaluate whether transdermal nicotine patches, used continuously for 15 weeks, could induce regression of cKS lesions[Goedert, Scoppio, Pfeiffer, Neve, Federici, Long, Dolan, Brambati, Bellinvia, Lauria, Preiss, Boneschi, Whitby, and Brambilla, 2008]. The null results of that trial, with the null association between cKS risk and use of topical creams and ointments in the current study, imply that exposures of the epidermis may have little impact on the pathogenesis of KS. Rather, they suggest that internal factors drive KSHV infection to manifest as KS. Susceptibility of cells to KSHV infection in vitro appears to be suppressed by cigarette smoke extract.[Ford, Hamden, Whitman, Bryan, Chintalgattu, McCubrey, Dyson, and Akula, 2005] Moreover, although not supported by functional data, cigarette smokers have reduced levels of serum neopterin and elevated levels of CD4 lymphocytes and other leukocytes,[Diamondstone, Tollerud, Fuchs, Wachter, Brown, Maloney, Kurman, Nelson, and Blattner, 1994;Tollerud, Clark, Brown, Neuland, Mann, Pankiw-Trost, Blattner, and Hoover, 1989] which is the inverse of the pattern seen among cKS cases [Brown, Whitby, Vitale, Marshall, Mbisa, Gamache, Lauria, Alberg, Serraino, Cordiali-Fei, Messina, and Goedert, 2006;Touloumi, Hatzakis, Potouridou, Milona, Strarigos, Katsambas, Giraldo, Beth-Giraldo, Biggar, Mueller, and Trichopoulos, 1999].
Despite the possibility that constituents of cigarette smoke could modulate cKS risk, other potential explanations should be considered. Cigarette smoking is associated with significant morbidity and mortality. Because cKS is primarily a disease of the elderly, smokers may not survive long enough to develop cKS. If smokers who die prematurely due to smoking related illnesses are more genetically predisposed to cKS than smokers who survive, then the reduced risk of cKS in smokers could be a function of differential survival bias. If true, then risk of cKS related to smoking would be less prominent in younger individuals who have not yet died of a smoking related illness. However, we observed that cKS risk was more markedly reduced in younger male smokers (OR 0.07) than in older male smokers (OR 0.66). In addition, there were no significant decreases in smoking related illnesses, such as bronchitis or heart disease/stroke, in patients with cKS, suggesting that differential survival bias does not fully explain our observations. Given the detrimental health effects of smoking, our findings should not be interpreted to recommend smoking to people infected with KSHV or anyone else.
We observed strong effects of never smoking, use of oral corticosteroids and diabetes. All three factors were independently associated with increased risk of cKS. Our group previously reported that short-term use of oral corticosteroids was associated with a non-significantly elevated risk of cKS (OR 2.17, 95%CI 0.94-5.01)[Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002]. Use of topical corticosteroid creams and ointments in the current study was not significantly related to cKS risk, in contrast to our previous results[Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002]. Unfortunately, the current study lacked detailed information about the duration, frequency or indications for use of oral corticosteroids. Although not observed herein, cKS has previously been reported to be higher in patients with asthma[Goedert, Vitale, Lauria, Serraino, Tamburini, Montella, Messina, Brown, Rezza, Gafa, and Romano, 2002] and rheumatic diseases[Louthrenoo, Kasitanon, Mahanuphab, Bhoopat, and Thongprasert, 2003] that are commonly treated with corticosteroid medications. The immunosuppressive effects of corticosteroid medications could induce KSHV reactivation and dissemination of the virus or the malignant cells. For example, glucocorticoids have been shown to enhance KS cell growth, possibly by blocking TGF-β[Cai, Zheng, Lotz, Zhang, Masood, and Gill, 1997], and remission of KS has been reported with reductions in corticosteroid medication usage[Kroumpouzos, Delaney, and Phillips, 2000;Leung, Fam, and Osoba, 1981]. Together these studies support a role of corticosteroids in the development of cKS.
Diabetes mellitus was also strongly associated with increased risk of cKS in the current study and has been noted among KS patients previously[Weissmann, Linn, Weltfriend, and Friedman-Birnbaum, 2000;Lanternier, Lebbe, Schartz, Farhi, Marcelin, Kerob, Agbalika, Verola, Gorin, Janier, Avril, and Dupin, 2008;Guttman-Yassky, Dubnov, Kra-Oz, Friedman-Birnbaum, Silbermann, Barchana, Bergman, and Sarid, 2006] [add recent Lanternier paper on HIV-neg gay men in AIDS]. Guttman-Yassky et al. suggested a relation between diabetes mellitus and cKS in a case-control study in Israel, although the association was not significant (OR 1.8, 95% CI 0.4-7.4) [Guttman-Yassky, Dubnov, Kra-Oz, Friedman-Birnbaum, Silbermann, Barchana, Bergman, and Sarid, 2006]. It is plausible that ascertainment bias could lead to detection of cKS in diabetic patients since they require careful and frequent clinical inspection of the feet, where initial cKS lesions often arise. However, the increased risk of cKS was not limited to patients who noticed their first KS lesion on their legs or feet. Since subjects were asked if they had ever been `diagnosed with, or investigated for' diabetes, it is possible that the true prevalence of diabetes is lower than reported. However, prevalence of diabetes among our controls is comparable to the prevalence of type 2 diabetes in persons aged >65 years in Italy.[Mazzaglia, Yurgin, Boye, Trifiro, Cottrell, Allen, Filippi, Medea, and Cricelli, 2008] How diabetes might increase the risk of cKS is unknown, but at least two mechanisms are possible. First, impaired microvascular circulation, as can occur in diabetics, could induce tissue hypoxia and KSHV lytic replication through hypoxia-inducible factor-1α (HIF-1α)[Davis, Rinderknecht, Zoeteweij, Aoki, Read-Connole, Tosato, Blauvelt, and Yarchoan, 2001]. If so, this would lead to dissemination of the virus in vivo increasing the total body burden of KSHV and the risk of developing cKS. Second, HIF-1α and 2-α are not only expressed in a KS cell line, but they also are modulated by insulin-like growth factor-I, which is required for the growth of these cells[Catrina, Botusan, Rantanen, Catrina, Pyakurel, Savu, Axelson, Biberfeld, Poellinger, and Brismar, 2006]. In addition, the transformation of dermal microvascular endothelial cells by KSHV is reported to depend on the expression of the insulin receptor[Rose, Carroll, Carroll, DeFilippis, Lagunoff, Moses, Roberts Jr, and Fruh, 2007]. Further work is needed to investigate how cKS risk is altered by the complex insulin-related pathways, taking into consideration the effects of non-smoking and use of corticosteroids.
An elevated risk of cKS was also observed in patients with edema. The association with cKS was limited to patients who developed their first KS lesion on the lower extremities. Lymphedema of the lower extremities can be a consequence of KS. Even though our study was limited to patients with incident cKS, some patients (n=21, 15%) had KS lesions for more than 2 years before seeking medical attention. When we excluded the 5-year period prior to interview, the association between edema and cKS was no longer significant, suggesting that edema did not increase the risk of cKS. Instead, edema was likely to have been a consequence of the disease process.
This is the first population-based field study of cKS. It has several strengths including a broad perspective of potential risk factors, a high response rate amongst cases, restriction to cases who had newly diagnosed KS, inclusion of a representative sample of controls from throughout Sicily, comparison of cKS patients to KSHV seropositive controls to assess risk factors involved in tumor development, and weighted adjustment for non-response amongst the cases and controls.
Some limitations should also be noted. Firstly, participation rates amongst the controls were not optimal which may have biased some of our results. Secondly, since medical chart review was not undertaken, historical recollections by elderly participants may have led to misclassification of exposures. However, this is unlikely to differ greatly between cases and controls. Thirdly, while our data suggest that diabetes may be an independent risk factor for cKS, we cannot exclude the possibility that ketosis-prone diabetes mellitus is a serious complication of KSHV infection[Sobngwi, Choukem, Agbalika, Blondeau, Fetita, Lebbe, Thiam, Cattan, Larghero, Foufelle, Ferre, Vexiau, Calvo, and Gautier, 2008] and thus an intermediate en route to KS. Finally, imprecision of KSHV serology is an important limitation. We used four antibody assays to determine KSHV status and an algorithm combining these results that had good sensitivity (95%) in the cKS cases and probably good specificity, given the conservative seroprevalence (10%) among the controls. Previous estimates of KSHV seroprevalence in Sicily have ranged from 12-38%[Perna, Bonura, Vitale, Viviano, Di Benedetto, Ajello, Villafrate, Prestileo, Mancuso, Goedert, and Romano, 2000;Whitby, Luppi, Barozzi, Boshoff, Weiss, and Torelli, 1998].
In summary, we confirmed previous reports that cigarette smoking was associated with a reduced risk of cKS, and we found that risk was lowest among current smokers. We also found that cKS risk was strongly and independently associated with never smoking, oral corticosteroid use and diabetes. Corroboration of these observations and study of possible underlying mechanisms are needed.
Acknowledgements
We are grateful to G. Arena, G. Antonelli, Colleen Pelser and Dr. Robert Biggar for helpful discussions; to Dr. Lindsay Morten for the analysis of KS risk after lung cancer; to Filippa Bonura, Anna Maria Perna, Fabio Tramuto, Anna Fidilio, Michele Massimino, Stefania Stella, and Georgina Mbisa for specimen processing and KSHV antibody testing; to Enza Viviano, Rosalia Valenti, Elisa Martorana, G. Arena, G. Antonelli, L. Leggio, G. Buscema, V. Paparazzo, M.C. DiPasquale, A. Tortorici, I. Bocchieri, and Laboratorio Brinch-Battaglia for recruitment and collection of data and specimens; to the staff at RTI International, including Mary-Anne Ardini, Dr. Barbara Kroner, Dr. Mansour Fahimi, and Liliana Preiss, for coordination, computation of weights, and analyses; and especially to Dr. Santo LoGalbo of the Assessorato Regionale della Salute for providing the population roster for Sicily.
This study would not have been possible without the KS cases reported by the following pathologists of Sicily: Professors Dr. Batolo and M. Lentini (Messina University); Professors E. Vasquez, G. Micali, S. Lanzafame, and Dr. G. Grasso (Catania University); Dr. S. Castellino (Ragusa); Dr. B. Barino (Vittoria); Dr. C. Castobello (Sircusa); Drs. Tricoli and M. Amore (Caltagirone); Dr. G. Russo (Taormina); Dr. M. Manusia and Dr.ssa E. Salomone (Catania); Dr. Vallone (Enna), Dr.ssa Calcarco and Dr.ssa P. Napoli Nania (Messina); Professors F. Aragona and V. Rodolico (Azienda Ospedaliera Universitaria, Policlinico Paolo Giaccone, Palermo); Dr. L. Marasà (Azienda di Rilievo Nazionale e di Alta Specializzazione, Ospedale Civico e Benfratelli, G. Di Cristina e M.Ascoli, Palermo); Prof. G.Genova and Dr. C. Rimi (Azienda Ospedaliera “Villa Sofia-C.T.O.”, Palermo); Drs. A. Rizzo and T. Mannone (Azienda Ospedaliera “V.Cervello”, Palermo); Drs. G. Pinto and G. Becchina (Azienda USL 6, Presidio Ospedaliero G.F. Ingrassia, Palermo); Dr. D. Ientile (Ospedale “Buccheri- La Ferla” Fatebenefratelli, Palermo); Dr. D. Messina (Azienda. Ospedaliera “S. Antonio Abate”, Trapani); Drs. N.A. Zarcone and Marino (Azienda Unità Sanitaria Locale n.9 Trapani Presidio Ospedaliero “V.Emanuele”, Castelvetrano); Dr. F. Italia (Azienda Ospedale “S. Giovanni di Dio”, Agrigento); and Drs. G. Ulrico and G. Bruno (Azienda Ospedaliera “S.Elia”, Caltanissetta).
This study was funded by the Intramural Research Program of the National Cancer Institute, in part under a contract with RTI International (N02-CP-91027) and in part under contract N01-CO-12400. The Research and Development Office, Northern Ireland, funded Dr. Lesley Anderson to participate in the Cancer Prevention Fellowship Program, Office of Preventive Oncology, National Cancer Institute.
Reference List
- 1.MOORE PS, CHANG Y. Kaposi's sarcoma (KS), KS-associated herpesvirus, and the criteria for causality in the age of molecular biology. Am.J.Epidemiol. 1998;147:217–221. doi: 10.1093/oxfordjournals.aje.a009440. [DOI] [PubMed] [Google Scholar]
- 2.RADY PL, YEN A, MARTIN RW, III, NEDELCU I, HUGHES TK, TYRING SK. Herpesvirus-like DNA sequences in classic Kaposi's sarcomas. J.Med.Virol. 1995;47:179–183. doi: 10.1002/jmv.1890470212. [DOI] [PubMed] [Google Scholar]
- 3.TOULOUMI G, HATZAKIS A, POTOURIDOU I, MILONA I, STRARIGOS J, KATSAMBAS A, GIRALDO G, BETH-GIRALDO E, BIGGAR RJ, MUELLER N, TRICHOPOULOS D. The role of immunosuppression and immune-activation in classic Kaposi's sarcoma. Int.J.Cancer. 1999;82:817–821. doi: 10.1002/(sici)1097-0215(19990909)82:6<817::aid-ijc8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.BROWN EE, FALLIN D, RUCZINSKI I, HUTCHINSON A, STAATS B, VITALE F, LAURIA C, SERRAINO D, REZZA G, MBISA G, WHITBY D, MESSINA A, GOEDERT JJ, CHANOCK SJ. Associations of classic Kaposi sarcoma with common variants in genes that modulate host immunity. Cancer Epidemiol.Biomarkers Prev. 2006;15:926–934. doi: 10.1158/1055-9965.EPI-05-0791. [DOI] [PubMed] [Google Scholar]
- 5.WHITBY D, LUPPI M, SABIN C, BAROZZI P, DI BIASE AR, BALLI F, CUCCI F, WEISS RA, BOSHOFF C, TORELLI G. Detection of antibodies to human herpesvirus 8 in Italian children: evidence for horizontal transmission. Br.J.Cancer. 2000;82:702–704. doi: 10.1054/bjoc.1999.0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BROWN EE, FALLIN D, RUCZINSKI I, HUTCHINSON A, STAATS B, VITALE F, LAURIA C, SERRAINO D, REZZA G, MBISA G, WHITBY D, MESSINA A, GOEDERT JJ, CHANOCK SJ. Associations of classic Kaposi sarcoma with common variants in genes that modulate host immunity. Cancer Epidemiol.Biomarkers Prev. 2006;15:926–934. doi: 10.1158/1055-9965.EPI-05-0791. [DOI] [PubMed] [Google Scholar]
- 7.VITALE F, BRIFFA DV, WHITBY D, MAIDA I, GROCHOWSKA A, LEVIN A, ROMANO N, GOEDERT JJ. Kaposi's sarcoma herpes virus and Kaposi's sarcoma in the elderly populations of 3 Mediterranean islands. Int.J.Cancer. 2001;91:588–591. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1089>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 9.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 10.NAWAR E, MBULAITEYE SM, GALLANT JE, WOHL DA, ARDINI M, HENDERSHOT T, GOEDERT JJ, RABKIN CS. Risk factors for Kaposi's sarcoma among HHV-8 seropositive homosexual men with AIDS. Int.J.Cancer. 2005;115:296–300. doi: 10.1002/ijc.20887. [DOI] [PubMed] [Google Scholar]
- 11.HOOVER DR, BLACK C, JACOBSON LP, MARTINEZ-MAZA O, SEMINARA D, SAAH A, VON RJ, ANDERSON R, ARMENIAN HK. Epidemiologic analysis of Kaposi's sarcoma as an early and later AIDS outcome in homosexual men. Am.J.Epidemiol. 1993;138:266–278. doi: 10.1093/oxfordjournals.aje.a116855. [DOI] [PubMed] [Google Scholar]
- 12.SAFAI B, MIKE V, GIRALDO G, BETH E, GOOD RA. Association of Kaposi's sarcoma with second primary malignancies: possible etiopathogenic implications. Cancer. 1980;45:1472–1479. doi: 10.1002/1097-0142(19800315)45:6<1472::aid-cncr2820450629>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.DICTOR M, ATTEWELL R. Epidemiology of Kaposi's sarcoma in Sweden prior to the acquired immunodeficiency syndrome. Int.J.Cancer. 1988;42:346–351. doi: 10.1002/ijc.2910420307. [DOI] [PubMed] [Google Scholar]
- 14.ISCOVICH J, BOFFETTA P, WINKELMANN R, BRENNAN P. Classic Kaposi's sarcoma as a second primary neoplasm. Int.J.Cancer. 1999;80:178–182. doi: 10.1002/(sici)1097-0215(19990118)80:2<178::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 16.PERNA AM, BONURA F, VITALE F, VIVIANO E, DI BENEDETTO MA, AJELLO F, VILLAFRATE MR, PRESTILEO T, MANCUSO S, GOEDERT JJ, ROMANO N. ntibodies to human herpes virus type 8 (HHV8) in general population and in individuals at risk for sexually transmitted diseases in Western Sicily. Int.J.Epidemiol. 2000;29:175–179. doi: 10.1093/ije/29.1.175. [DOI] [PubMed] [Google Scholar]
- 17.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 18.LITTLE RJA, RUBIN DB. Statistical analysis with missing data. Wiley; New York: 1987. [Google Scholar]
- 19.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 20.NAWAR E, MBULAITEYE SM, GALLANT JE, WOHL DA, ARDINI M, HENDERSHOT T, GOEDERT JJ, RABKIN CS. Risk factors for Kaposi's sarcoma among HHV-8 seropositive homosexual men with AIDS. Int.J.Cancer. 2005;115:296–300. doi: 10.1002/ijc.20887. [DOI] [PubMed] [Google Scholar]
- 21.HOOVER DR, BLACK C, JACOBSON LP, MARTINEZ-MAZA O, SEMINARA D, SAAH A, VON RJ, ANDERSON R, ARMENIAN HK. Epidemiologic analysis of Kaposi's sarcoma as an early and later AIDS outcome in homosexual men. Am.J.Epidemiol. 1993;138:266–278. doi: 10.1093/oxfordjournals.aje.a116855. [DOI] [PubMed] [Google Scholar]
- 22.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 23.CONLEY LJ, BUSH TJ, BUCHBINDER SP, PENLEY KA, JUDSON FN, HOLMBERG SD. The association between cigarette smoking and selected HIV-related medical conditions. AIDS. 1996;10:1121–1126. [PubMed] [Google Scholar]
- 24.GUTTMAN-YASSKY E, DUBNOV J, KRA-OZ Z, FRIEDMAN-BIRNBAUM R, SILBERMANN M, BARCHANA M, BERGMAN R, SARID R. Classic Kaposi sarcoma. Which KSHV-seropositive individuals are at risk? Cancer. 2006;106:413–419. doi: 10.1002/cncr.21614. [DOI] [PubMed] [Google Scholar]
- 25.NAWAR E, MBULAITEYE SM, GALLANT JE, WOHL DA, ARDINI M, HENDERSHOT T, GOEDERT JJ, RABKIN CS. Risk factors for Kaposi's sarcoma among HHV-8 seropositive homosexual men with AIDS. Int.J.Cancer. 2005;115:296–300. doi: 10.1002/ijc.20887. [DOI] [PubMed] [Google Scholar]
- 26.SAFAI B, MIKE V, GIRALDO G, BETH E, GOOD RA. Association of Kaposi's sarcoma with second primary malignancies: possible etiopathogenic implications. Cancer. 1980;45:1472–1479. doi: 10.1002/1097-0142(19800315)45:6<1472::aid-cncr2820450629>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.DICTOR M, ATTEWELL R. Epidemiology of Kaposi's sarcoma in Sweden prior to the acquired immunodeficiency syndrome. Int.J.Cancer. 1988;42:346–351. doi: 10.1002/ijc.2910420307. [DOI] [PubMed] [Google Scholar]
- 28.ISCOVICH J, BOFFETTA P, WINKELMANN R, BRENNAN P. Classic Kaposi's sarcoma as a second primary neoplasm. Int.J.Cancer. 1999;80:178–182. doi: 10.1002/(sici)1097-0215(19990118)80:2<178::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.GALLUS S, PACIFICI R, COLOMBO P, SCARPINO V, ZUCCARO P, BOSETTI C, FERNANDEZ E, APOLONE G, LA VC. Prevalence of smoking and attitude towards smoking regulation in Italy, 2004. Eur.J.Cancer Prev. 2006;15:77–81. doi: 10.1097/01.cej.0000180667.89087.b9. [DOI] [PubMed] [Google Scholar]
- 30.FRANCESCHI S, NAETT C. Trends in smoking in Europe. Eur.J.Cancer Prev. 1995;4:271–284. doi: 10.1097/00008469-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 31.GIOVINO GA, SCHOOLEY MW, ZHU BP, CHRISMON JH, TOMAR SL, PEDDICORD JP, MERRITT RK, HUSTEN CG, ERIKSEN MP. Surveillance for selected tobacco-use behaviors--United States, 1900-1994. MMWR CDC Surveill Summ. 1994;43:1–43. [PubMed] [Google Scholar]
- 32.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 33.GOEDERT JJ, SCOPPIO BM, PFEIFFER R, NEVE L, FEDERICI AB, LONG LR, DOLAN BM, BRAMBATI M, BELLINVIA M, LAURIA C, PREISS L, BONESCHI V, WHITBY D, BRAMBILLA L. Treatment of classic Kaposi sarcoma with a nicotine dermal patch: a phase II clinical trial. J.Eur.Acad.Dermatol.Venereol. 2008 doi: 10.1111/j.1468-3083.2008.02720.x. [DOI] [PubMed] [Google Scholar]
- 34.HOOVER DR, BLACK C, JACOBSON LP, MARTINEZ-MAZA O, SEMINARA D, SAAH A, VON RJ, ANDERSON R, ARMENIAN HK. Epidemiologic analysis of Kaposi's sarcoma as an early and later AIDS outcome in homosexual men. Am.J.Epidemiol. 1993;138:266–278. doi: 10.1093/oxfordjournals.aje.a116855. [DOI] [PubMed] [Google Scholar]
- 35.NOURI-SHIRAZI M, TINAJERO R, GUINET E. Nicotine alters the biological activities of developing mouse bone marrow-derived dendritic cells (DCs) Immunol.Lett. 2007;109:155–164. doi: 10.1016/j.imlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 36.NOURI-SHIRAZI M, GUINET E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology. 2003;109:365–373. doi: 10.1046/j.1365-2567.2003.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.HOGG N. Nicotine has suppressive effects on dendritic cell function. Immunology. 2003;109:329–330. doi: 10.1046/j.1365-2567.2003.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CHANG J, RENNE R, DITTMER D, GANEM D. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology. 2000;266:17–25. doi: 10.1006/viro.1999.0077. [DOI] [PubMed] [Google Scholar]
- 39.GOEDERT JJ, SCOPPIO BM, PFEIFFER R, NEVE L, FEDERICI AB, LONG LR, DOLAN BM, BRAMBATI M, BELLINVIA M, LAURIA C, PREISS L, BONESCHI V, WHITBY D, BRAMBILLA L. Treatment of classic Kaposi sarcoma with a nicotine dermal patch: a phase II clinical trial. J.Eur.Acad.Dermatol.Venereol. 2008 doi: 10.1111/j.1468-3083.2008.02720.x. [DOI] [PubMed] [Google Scholar]
- 40.FORD PW, HAMDEN KE, WHITMAN AG, BRYAN BA, CHINTALGATTU V, MCCUBREY JA, DYSON OF, AKULA SM. Cigarette smoke concentrate inhibits Kaposi's sarcoma-associated herpesvirus infection. Virus Res. 2005;114:172–176. doi: 10.1016/j.virusres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 41.DIAMONDSTONE LS, TOLLERUD DJ, FUCHS D, WACHTER H, BROWN LM, MALONEY E, KURMAN CC, NELSON DL, BLATTNER WA. Factors influencing serum neopterin and beta 2-microglobulin levels in a healthy diverse population. J.Clin.Immunol. 1994;14(6):368–374. doi: 10.1007/BF01546321. [DOI] [PubMed] [Google Scholar]
- 42.TOLLERUD DJ, CLARK JW, BROWN LM, NEULAND CY, MANN DL, PANKIW-TROST LK, BLATTNER WA, HOOVER RN. The effects of cigarette smoking on T cell subsets. A population-based survey of healthy caucasians. Am Rev Respir Disease. 1989;139(6):1446–1451. doi: 10.1164/ajrccm/139.6.1446. [DOI] [PubMed] [Google Scholar]
- 43.BROWN EE, WHITBY D, VITALE F, MARSHALL V, MBISA G, GAMACHE C, LAURIA C, ALBERG AJ, SERRAINO D, CORDIALI-FEI P, MESSINA A, GOEDERT JJ. Virologic, hematologic, and immunologic risk factors for classic Kaposi sarcoma. Cancer. 2006;107:2282–2290. doi: 10.1002/cncr.22236. [DOI] [PubMed] [Google Scholar]
- 44.TOULOUMI G, HATZAKIS A, POTOURIDOU I, MILONA I, STRARIGOS J, KATSAMBAS A, GIRALDO G, BETH-GIRALDO E, BIGGAR RJ, MUELLER N, TRICHOPOULOS D. The role of immunosuppression and immune-activation in classic Kaposi's sarcoma. Int.J.Cancer. 1999;82:817–821. doi: 10.1002/(sici)1097-0215(19990909)82:6<817::aid-ijc8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 45.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 46.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 47.GOEDERT JJ, VITALE F, LAURIA C, SERRAINO D, TAMBURINI M, MONTELLA M, MESSINA A, BROWN EE, REZZA G, GAFA L, ROMANO N. Risk factors for classical Kaposi's sarcoma. J.Natl.Cancer Inst. 2002;94:1712–1718. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 48.LOUTHRENOO W, KASITANON N, MAHANUPHAB P, BHOOPAT L, THONGPRASERT S. Kaposi's sarcoma in rheumatic diseases. Semin.Arthritis Rheum. 2003;32:326–333. doi: 10.1053/sarh.2002.50000. [DOI] [PubMed] [Google Scholar]
- 49.CAI J, ZHENG T, LOTZ M, ZHANG Y, MASOOD R, GILL P. Glucocorticoids induce Kaposi's sarcoma cell proliferation through the regulation of transforming growth factor-beta. Blood. 1997;89:1491–1500. [PubMed] [Google Scholar]
- 50.KROUMPOUZOS G, DELANEY T, PHILLIPS TJ. Combined classic and iatrogenic Kaposi's sarcoma. Corticosteroid withdrawal can result in remission. Postgrad.Med. 2000;108:103–106. doi: 10.3810/pgm.2000.08.1196. [DOI] [PubMed] [Google Scholar]
- 51.LEUNG F, FAM AG, OSOBA D. Kaposi's sarcoma complicating corticosteroid therapy for temporal arteritis. Am.J.Med. 1981;71:320–322. doi: 10.1016/0002-9343(81)90135-2. [DOI] [PubMed] [Google Scholar]
- 52.WEISSMANN A, LINN S, WELTFRIEND S, FRIEDMAN-BIRNBAUM R. Epidemiological study of classic Kaposi's sarcoma: a retrospective review of 125 cases from Northern Israel. J.Eur.Acad.Dermatol.Venereol. 2000;14:91–95. doi: 10.1046/j.1468-3083.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 53.LANTERNIER F, LEBBE C, SCHARTZ N, FARHI D, MARCELIN AG, KEROB D, AGBALIKA F, VEROLA O, GORIN I, JANIER M, AVRIL MF, DUPIN N. Kaposi's sarcoma in HIV-negative men having sex with men. AIDS. 2008;22(10):1163–1168. doi: 10.1097/QAD.0b013e3283031a8a. [DOI] [PubMed] [Google Scholar]
- 54.GUTTMAN-YASSKY E, DUBNOV J, KRA-OZ Z, FRIEDMAN-BIRNBAUM R, SILBERMANN M, BARCHANA M, BERGMAN R, SARID R. Classic Kaposi sarcoma. Which KSHV-seropositive individuals are at risk? Cancer. 2006;106:413–419. doi: 10.1002/cncr.21614. [DOI] [PubMed] [Google Scholar]
- 55.GUTTMAN-YASSKY E, DUBNOV J, KRA-OZ Z, FRIEDMAN-BIRNBAUM R, SILBERMANN M, BARCHANA M, BERGMAN R, SARID R. Classic Kaposi sarcoma. Which KSHV-seropositive individuals are at risk? Cancer. 2006;106:413–419. doi: 10.1002/cncr.21614. [DOI] [PubMed] [Google Scholar]
- 56.MAZZAGLIA G, YURGIN N, BOYE KS, TRIFIRO G, COTTRELL S, ALLEN E, FILIPPI A, MEDEA G, CRICELLI C. Prevalence and antihyperglycemic prescribing trends for patients with type 2 diabetes in Italy: A 4-year retrospective study from national primary care data. Pharmacological Research. 2008 doi: 10.1016/j.phrs.2008.03.009. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 57.DAVIS DA, RINDERKNECHT AS, ZOETEWEIJ JP, AOKI Y, READ-CONNOLE EL, TOSATO G, BLAUVELT A, YARCHOAN R. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97:3244–3250. doi: 10.1182/blood.v97.10.3244. [DOI] [PubMed] [Google Scholar]
- 58.CATRINA S-B, BOTUSAN IR, RANTANEN A, CATRINA AI, PYAKUREL P, SAVU O, AXELSON M, BIBERFELD P, POELLINGER L, BRISMAR K. Hypoxia-Inducible Factor-1Aand Hypoxia-Inducible Factor-2alpha Are Expressed in Kaposi Sarcoma and Modulated by Insulin-like Growth Factor-I. Clin.Cancer Res. 2006;12(15):4506–4514. doi: 10.1158/1078-0432.CCR-05-2473. [DOI] [PubMed] [Google Scholar]
- 59.ROSE PP, CARROLL JM, CARROLL PA, DEFILIPPIS VR, LAGUNOFF M, MOSES AV, ROBERTS CT, JR, FRUH K. The insulin receptor is essential for virus-induced tumorigenesis of Kaposi's sarcoma. Oncogene. 2007;26:1995–2005. doi: 10.1038/sj.onc.1210006. [DOI] [PubMed] [Google Scholar]
- 60.SOBNGWI E, CHOUKEM SP, AGBALIKA F, BLONDEAU B, FETITA L, LEBBE C, THIAM D, CATTAN P, LARGHERO J, FOUFELLE F, FERRE P, VEXIAU P, CALVO F, GAUTIER J. Ketosis-Prone Type 2 Diabetes Mellitus and Human Herpesvirus 8 Infection in Sub-Saharan Africans. JAMA: The Journal of the American Medical Association. 2008;299(23):2770–2776. doi: 10.1001/jama.299.23.2770. [DOI] [PubMed] [Google Scholar]
- 61.PERNA AM, BONURA F, VITALE F, VIVIANO E, DI BENEDETTO MA, AJELLO F, VILLAFRATE MR, PRESTILEO T, MANCUSO S, GOEDERT JJ, ROMANO N. ntibodies to human herpes virus type 8 (HHV8) in general population and in individuals at risk for sexually transmitted diseases in Western Sicily. Int.J.Epidemiol. 2000;29:175–179. doi: 10.1093/ije/29.1.175. [DOI] [PubMed] [Google Scholar]
- 62.WHITBY D, LUPPI M, BAROZZI P, BOSHOFF C, WEISS RA, TORELLI G. Human herpesvirus 8 seroprevalence in blood donors and lymphoma patients from different regions of Italy. J.Natl.Cancer Inst. 1998;90:395–397. doi: 10.1093/jnci/90.5.395. [DOI] [PubMed] [Google Scholar]


