Abstract
Previously, utilizing a series of genome-wide association, brain imaging and gene expression studies we implicated the KIBRA gene and the RhoA/ROCK pathway in hippocampal-mediated human memory. Here we show that peripheral administration of the ROCK inhibitor hydroxyfasudil improves spatial learning and working memory in the rodent model. This study supports the action of ROCK on learning and memory, suggests the potential value of ROCK inhibition for the promotion of cognition in humans and highlights the powerful potential of unbiased genome-wide association studies to inform potential novel uses for existing pharmaceuticals.
Keywords: learning, memory, ROCK, fasudil, aging
INTRODUCTION
The molecular processes involved in learning and memory provide promising targets for putative memory-enhancing (i.e. nootropic) pharmaceuticals, which might be helpful in the treatment of Alzheimer's disease, other dementias, mild cognitive impairment and other disorders associated with impairments in learning and memory. If safe and well tolerated, these medications could even have roles in the treatment of the non-disabling learning and memory declines associated with healthy aging as well as in the enhancement of normal learning and memory.
To date, nootropic drug discovery efforts have focused on the enhancement of cholinergic, glutaminergic, and serotonergic neurotransmission and phosphodiesterase inhibition, and have had limited benefits (Sarter, 2006). Examples include the cholinesterase inhibitors and N-methyl-d-aspartate (NMDA) agonists currently approved for the treatment of Alzheimer's disease, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor agonists and nicotinic alpha 7 receptor agonists.
Using a hypothesis-free 500K single nucleotide polymorphism (SNP) genome-wide association study, followed by functional brain imaging and gene expression studies, we recently associated the KIBRA gene with the variation in normal episodic memory performance in both young (median age of 22; ranging from 18-48 years) and late middle aged (median age of 55; ranging from 20-81 years) adults (Papassotiropoulos et al., 2006) Our initial genetic findings have since been replicated by a different research team in a unique cohort of older individuals (mean age of 67) thereby further supporting KIBRA's role in episodic memory, as well as extending this relationship to the aged population (Schaper, Kolsch, Popp, Wagner, & Jessen, 2007). Additionally, two other groups have published studies using independent cohorts that further support a genetic link between KIBRA and memory variation in healthy individuals (Almeida et al., 2008; Nacmias et al., 2008). The genetic link between KIBRA and human memory disorders has also been investigated. One group recently reported no effect on risk for development of Mild Cognitive Impairment (Almeida et al., 2008), however, a manuscript published in 2007 as well as a recently published manuscript by members of our group support a link between KIBRA genetic variation and Alzheimer's disease in (Rodriguez-Rodriguez et al., 2007) and (Corneveaux et al., 2008 [in press]). There has also been a single recent report suggesting no association between KIBRA and multiple verbal memory tasks (Need et al., 2008).
Based on this finding and a pathway analysis approach, we hypothesized that KIBRA activity would be altered via the RhoA/ROCK/Rac pathway through the putative modulation of PKC-ζ (Van Kolen & Slegers, 2006). KIBRA is a demonstrated substrate for PKC-ζ (Buther, Plaas, Barnekow, & Kremerskothen, 2004) and has been shown to interact with Dendrin (Kremerskothen et al., 2003), a postsynaptic cytoskeleton modulatory molecule. Recently, KIBRA has been also shown to co-localize with both a postsynaptic marker protein (ProSAP2/Shank3) and in close contact with a presynaptic marker (bassoon) in primary rat hippocampal neurons (Johannsen, Duning, Pavenstadt, Kremerskothen, & Boeckers, 2008). In multiple cell types the RhoA/ROCK/Rac pathway has been demonstrated to be upstream of PKC-ζ (Kampfer et al., 2001; Scott, Arioka, & Jacobs, 2007; Uberall et al., 1999; Van Kolen & Slegers, 2006). Additionally, since the RhoA/ROCK/Rac pathway has been implicated in key neurobiological processes that underlie cognitive function, such as neurite outgrowth and growth cone modulation (Gopalakrishnan et al., 2008; Lingor et al., 2007; Loudon, Silver, Yee, & Gallo, 2006; Woo & Gomez, 2006), we postulated that an inhibitor of this pathway might be useful as a treatment for the enhancement of learning and memory.
Several existing compounds have the capability to modulate the RhoA/ROCK pathway. While a recently developed inhibitor of ROCK, fasudil, has been investigated in patients as a potential treatment for vasospasm and angina, fasudil or its active metabolite hydroxyfasudil has not been evaluated in laboratory animals or human subjects for effects on learning and memory (Hirooka & Shimokawa, 2005).
METHODS
Subjects and Treatment Procedures
Subjects were 27 seventeen month old Fischer-344 male rats (eighteen months old at the time of behavioral testing) born and raised at the aging colony of the National Institute on Aging at Harlan Laboratories (Indianapolis, IN). After arrival at Arizona State University, animals were pair housed with a same-age cage mate, had exposure to food and water ad-lib, and were maintained on a 12-h light/dark cycle. All procedures were approved by the local IACUC committee and adhered to NIH standards. The experimenters who performed the behavioral testing and brain dissections were blind to treatment group.
One daily injection of the assigned substrate began four days prior to behavioral testing and continued throughout testing. The half-life of hydroxyfasudil in humans has been estimated at between 5-7 hours (Hinderling et al., 2007) therefore we administered the drug dose each morning prior to behavioral testing. The initial four day period before testing was incorporated to habituate the animals to daily drug or vehicle delivery. Injections were given subcutaneously into the scruff of the neck. There were three experimental groups: nine animals received saline vehicle (“Aged Vehicle”), nine animals received hydroxyfasudil (Sigma-Aldrich, St. Louis, MO) at a dose of 0.1875 mg (“Aged Low Dose”), and nine animals received hydroxyfasudil at a dose of 0.3750 mg (“Aged High Dose”).
Water Radial-arm Maze
A schematic of the water radial-arm maze is shown in Supplementary Figure 1A. This win-shift radial-arm maze tests working and reference memory, and utilizes water escape onto hidden platforms as the reinforcer (Bimonte & Denenberg, 1999, 2000; Bimonte, Granholm, Seo, & Isacson, 2002; Bimonte, Hyde, Hoplight, & Denenberg, 2000; Hyde, Hoplight, & Denenberg, 1998; Hyde, Sherman, & Denenberg, 2000). The 8-arm maze had submerged escape platforms placed in the ends of 4 of arms. Each subject had different platform locations that remained fixed throughout the experiment. A subject was released from the start arm, which remained constant throughout testing and for all subjects, and had 3 minutes to locate a platform. Once a platform was found, the animal remained on it for 15 s, and was then returned to its heated home cage for 30 s until its next trial. During the inter-trial-interval, the just-chosen platform was removed from the maze. The animal was placed again into the start alley and allowed to locate another platform. The same sequence of events was repeated daily until all four platforms were located. Consequently, for each animal a daily session consisted of four trials per session, with the number of platformed arms reduced by one on each subsequent trial resulting in one more item of information needing to be remembered after every trial. Hence, the working memory system was increasingly taxed as trials progressed. Animals performed for 4 trials each day for 12 days.
Entry into an arm was counted when the tip of a rat's snout reached a mark delineated on the outside of the arm (11 cm into the arm). Errors were quantified for each daily session using the orthogonal measures of working and reference memory errors (Jarrard, Okaichi, Steward, & Goldschmidt, 1984), and blocked into the initial and latter test phases based on previous studies using the water escape radial-arm maze (Bimonte-Nelson, Francis, Umphlet, & Granholm, 2006; Bimonte-Nelson, Hunter, Nelson, & Granholm, 2003; Bimonte-Nelson, Singleton, Williams, & Granholm, 2004; Bimonte & Denenberg, 1999, 2000; Bimonte et al., 2002; Bimonte et al., 2000; Bimonte, Nelson, & Granholm, 2003; Hyde et al., 1998; Hyde et al., 2000). Day 1 was considered a training session because the animal had no previous experience in the maze. Days 2-12 were testing sessions. The 11 testing days were blocked into two phases: the initial phase consisting of testing days 2-7 which is considered an acquisition measure as used in the Learning Index measure described below, and the latter phase consisting of days 8-12 which is considered a more steady-state measure of memory after the rules of the task have been learned. Working Memory Correct errors were the number of first and repeat entries into any arm from which a platform had been removed during that session. Reference Memory errors were the number of first entries into any arm that never contained a platform. Working Memory Incorrect errors were the number of repeat entries into an arm that never contained a platform in the past. A total error score was also obtained. The ability to handle an increasing working memory load was tested by evaluating performance as trials increased during the latter testing phase for the Working Memory Correct and Incorrect variables. We have shown that young rats can learn to handle high working memory load as testing days progress on this task, while experimentally unaltered aged rats cannot (Bimonte et al., 2003). Thus, to evaluate learning of the water radial-arm maze task at the highest memory load, we used a Learning Index. Total, Working Memory Correct, and Working Memory Incorrect errors on trial 4 were used to determine the Learning Index for each day of testing following the first day. This measure is based on similar indices of learning using a difference score, and corrects for potential differences in baseline variances across animals . In addition to learning, we also measured memory competence by evaluating error scores on the latter testing phase via a repeated measures ANOVA, with Treatment as the between-subjects factor and Days and Trials as the repeated measures. Each drug-treated group was compared to the saline group, set a priori.
Spatial Reference Memory Morris maze
A schematic of the Morris water maze is shown in Supplementary Figure 1B. The Morris maze (Morris, 1984) consisted of a round tub (188 cm in diameter) filled with room temperature (19-22°C) water made opaque with black non-toxic paint. A black platform (10 cm) was submerged just below the water surface. The rat was placed in the maze, facing the tub wall, from any of four locations (North, South, East, or West) and had 60 s to locate the hidden platform which remained in a fixed location throughout testing. After 15 s on the platform, the rat was removed from the maze and placed into its heated cage until the next trial. The inter-trial-interval was approximately 5-7 minutes. The rats were given 5 trials a day for 4 days, plus a probe trial on trial 6, day 5 where the platform was removed to test platform spatial localization. A video camera suspended on the ceiling above the maze tracked the rat's path and a tracking system (Ethovision system, Noldus Instruments) was used to analyze each rat's tracing. Performance was assessed by swim path distance to the platform. Distance scores were analyzed using repeated measures ANOVA with Treatment as the between-subjects factor and Days and Trials as the repeated measures for the test trials.
RESULTS AND DISCUSSION
Based on results from our prior whole-genome association study, we evaluated the effects of two doses of the ROCK inhibitor hydroxyfasudil on spatial learning and memory in aged rats. For the water radial-arm maze, the Aged High Dose group showed superior learning for all three measures evaluated (Figure 1; a-c). For the measure of Total Errors (Figure 1a), the Aged High Dose group showed the best learning, with a significantly higher Learning Index compared to the Aged Vehicle group [t(16)=2.42; p=0.028]. The Aged High Dose group also showed better learning of both orthogonal working memory measures at the most demanding memory load of the radial arm maze, on trial 4. Indeed, the Aged High Dose group had a higher Learning Index than the Aged Vehicle group for Working Memory Correct [t(16)=2.70; p=0.016; Figure 1b] and Working Memory Incorrect [t(16)=2.19; p=0.043; Figure 1c]. The Aged Low Dose group had only a marginally higher Learning Index for Working Memory Incorrect errors compared to the Aged Vehicle group [t(16)=1.79; p=0.09; Figure 1c]. This finding may be due to a slight under-powering to detect this effect in the Aged Low Dose animals. However, for every Learning Index measure, values for the Aged Low Dose group were intermediate between the Aged Vehicle and Aged High Dose groups thereby suggesting a dose-dependent effect on learning the working memory task. Regression analyses confirmed this relationship between treatment dose and performance, with significant linear trends across groups for the Learning Index for Total [ANOVA for linear trend: F(1,25)=7.24; p=0.013], Working Memory Correct [ANOVA for linear trend: F(1,25)=6.10; p=0.021] and Working Memory Incorrect [ANOVA for linear trend: F(1,25)= 6.24; p=0.019] errors.
Figure 1. Hydroxyfasudil treatment is associated with a dose-related increase in learning proficiency in aged rats.
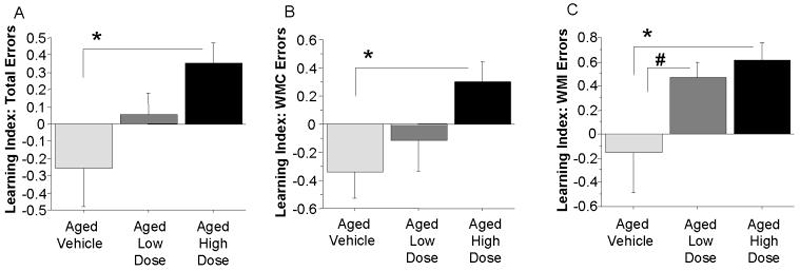
Shown are the mean ± S.E. Learning Index scores for A) Total Errors, B) Working Memory Correct (WMC) Errors and C) Working Memory Incorrect (WMI) Errors for each group for the trial with the highest memory demand. A higher Learning Index is indicative of better learning. Aged rats given high dose hydroxyfasudil showed better learning on all three measures, and linear trends showed that drug dose was correlated with a higher Learning Index for each of the three variables. *P < 0.05, #P = 0.09
In addition to demonstrating better learning of the working memory task, the Aged High Dose group also outperformed the Aged Vehicle group on working memory on the latter phase of testing, with high dose hydroxyfasudil improving the ability to handle the maximally increased memory load [Drug Treatment x Trial significant interaction for Working Memory Incorrect: F(3,48)=2.84; p=0.048, Figure 2a; Drug Treatment x Trial marginal interaction for Working Memory Correct: F(2,32)=3.14; p=0.057, Figure 2b]. Low-dose treatment did not significantly enhance working memory performance for any variable. Finally, neither dose of hydroxyfasudil was associated with significantly altered spatial reference memory performance on the water radial-arm maze or Morris water maze. Indeed, there were no Drug Treatment main effects or interactions for Reference Memory errors on the water radial-arm maze, nor for Distance scores on the Morris maze.
Figure 2. Hydroxyfasudil treatment improves working memory in aged rats.
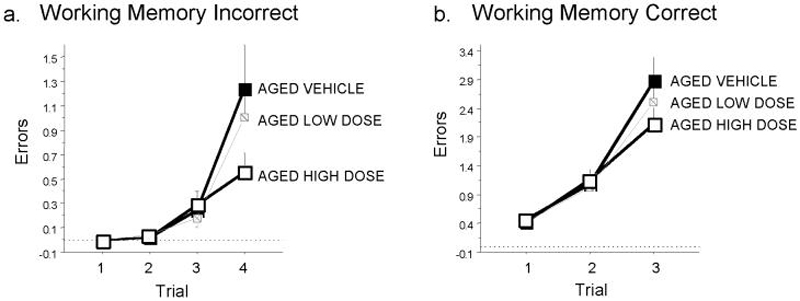
Shown are the mean ± S.E. Working Memory Incorrect errors across trials for the latter testing phase of the water-escape radial arm maze. Aged animals given high dose hydroxyfasudil treatment were better able to handle an increased memory load as compared to aged animals given vehicle. P = 0.048
The data presented here implicate ROCK activity in the processes of both learning and the ability to handle a high working memory load. Interestingly, there were no effects of hydroxyfasudil on reference memory. Thus, the effects of hydroxyfasudil were working memory specific, which may implicate effects, in part, on the frontal cortex. Indeed, the frontal cortex is intimately involved with working memory in both humans and rodents (Miotto, Bullock, Polkey, & Morris, 1996; Poucet, 1990; Poucet & Herrmann, 1990). While there has been some research evaluating the effects of ROCK inhibition on variables such as pain perception and anxiety, and one study evaluating memory retention after hippocampal infusion of a ROCK inhibitor in the young rodent, aside from the current report there has been no study evaluating the effects of peripheral administration of a ROCK inhibitor on spatial cognition (Buyukafsar et al., 2006; Dash, Orsi, Moody, & Moore, 2004; Lamprecht, Farb, & LeDoux, 2002; Saitoh et al., 2006). Thus, the current study is the first to examine the effect of peripheral delivery of hydroxyfasudil on spatial cognition as well as the first to test the mnemonic effects of ROCK inhibition in an older population of animals.
These findings underscore the potential of the promises of genome-wide association studies. Our interest in ROCK as a potential target for cognitive enhancement arose from the elucidation of the genetic involvement of KIBRA in human episodic memory performance in our previously published association study (Papassotiropoulos et al., 2006). The evidence detailed in this manuscript illustrates that it is possible to move from a validated human genetic association to an informed pharmaceutical decision. In our approach we first searched for targets with existing pharmaceutical agents that were at an advanced clinical stage. The optimal study design might be envisioned to directly pharmaceutically target KIBRA, and the investigation of agents that may be capable of disrupting key KIBRA interactions are currently ongoing. In lieu of directly altering the activity of the genetically associated gene product, the approach used in the present study was to influence the biomolecules that interact with it, in turn, resulting in the functional consequences of cognitive change This approach may be widely applicable to many of the associated genes currently being reported in the literature.
Our current working hypothesis is that PKC-ζ activity modulation alters learning and memory through KIBRA. It has been shown in astrocytoma cells that ROCK inhibition leads to a subsequent increase in Rac1 activity (Salhia et al., 2005). Rac1 activation has also been demonstrated to modulate the activity of PKC-ζ (Liu et al., 2006; Scott et al., 2007; Uberall et al., 1999). Additionally, pioneering work has shown that a unique form of PKC-ζ, termed PKM-ζ, is essential for long term potentiation and plays a significant role in the process of differing forms of memory as well (Ling et al., 2002; Pastalkova et al., 2006; Shema, Sacktor, & Dudai, 2007). Based on these reports, we hypothesized that inhibition of ROCK leading to activation of Rac1 and PKC-ζ would in turn phosphorylate KIBRA and alter its activity. However, as of yet the mechanism related to the nootropic benefits we observed with hydroxyfasudil are unknown. Fasudil, the parent compound, has been shown to exhibit direct neuroprotective effects against ischemia (Satoh, Toshima, Ikegaki, Iwasaki, & Asano, 2007; Yamashita et al., 2007) as well as influence vasodilation, which could then in turn influence cognitive function (Jiang et al., 2007; Nagata et al., 1993). While further experiments are necessary to confirm the mechanism/s underlying hydroxyfasudil-induced functional improvements on learning and memory, the present results can be considered an initial step in that direction providing support for the hypothesis that ROCK inhibition initiates a cascade of events that can influence cognition.
Our findings suggest that peripheral administration of the ROCK inhibitor hydroxyfasudil improves spatial learning and memory, a finding that may have clinical relevance considering that the parent drug (Fasudil) was found to be safe and extremely well tolerated when used in the human clinic in multiple dosage forms. The collected findings and the relative safety of fasudil support the potential of this ROCK inhibitor as a cognitive enhancer in humans that have age- or neurodegenerative- related memory dysfunction.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to acknowledge financial support from the Evelyn F. McKnight Brain Research Foundation, the NIH National Institute on Aging (grant #AG19610), the NIH National Institute of Neurological Disorders and Stroke (grant #NS059873), and the State of Arizona. We thank Ms. Cynthia Zay and Ms. Britny Sundin for their excellent technical support.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/bne/
REFERENCES
- Almeida OP, Schwab SG, Lautenschlager NT, Morar B, Greenop KR, Flicker L, Wildenauer D. KIBRA Genetic Polymorphism Influences Episodic Memory in Later Life, but Does Not Increase the Risk of Mild Cognitive Impairment. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24(1):229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139(12):47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118(4):707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24(2):161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Sex differences in vicarious trial-and-error behavior during radial arm maze learning. Physiol Behav. 2000;68(4):495–499. doi: 10.1016/s0031-9384(99)00201-2. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Granholm AC, Seo H, Isacson O. Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not aged, female rats. Neurosci Lett. 2002;328(1):50–54. doi: 10.1016/s0304-3940(02)00442-1. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70(34):311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24(1):37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Buther K, Plaas C, Barnekow A, Kremerskothen J. KIBRA is a novel substrate for protein kinase Czeta. Biochem Biophys Res Commun. 2004;317(3):703–707. doi: 10.1016/j.bbrc.2004.03.107. [DOI] [PubMed] [Google Scholar]
- Buyukafsar K, Yalcin I, Kurt AH, Tiftik RN, Sahan-Firat S, Aksu F. Rho-kinase inhibitor, Y-27632, has an antinociceptive effect in mice. Eur J Pharmacol. 2006;541(12):49–52. doi: 10.1016/j.ejphar.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moody M, Moore AN. A role for hippocampal Rho-ROCK pathway in long-term spatial memory. Biochem Biophys Res Commun. 2004;322(3):893–898. doi: 10.1016/j.bbrc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan SM, Teusch N, Imhof C, Bakker MH, Schurdak M, Burns DJ, Warrior U. Role of Rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells. J Neurosci Res. 2008;86(10):2214–2226. doi: 10.1002/jnr.21671. [DOI] [PubMed] [Google Scholar]
- Hinderling PH, Karara AH, Tao B, Pawula M, Wilding I, Lu M. Systemic availability of the active metabolite hydroxy-fasudil after administration of fasudil to different sites of the human gastrointestinal tract. J Clin Pharmacol. 2007;47(1):19–25. doi: 10.1177/0091270006293767. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Shimokawa H. Therapeutic potential of rho-kinase inhibitors in cardiovascular diseases. Am J Cardiovasc Drugs. 2005;5(1):31–39. doi: 10.2165/00129784-200505010-00005. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785(2):236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Sherman GF, Denenberg VH. Non-spatial water radial-arm maze learning in mice. Brain Res. 2000;863(12):151–159. doi: 10.1016/s0006-8993(00)02113-2. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav Neurosci. 1984;98(6):946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Tawara S, Abe K, Takaki A, Fukumoto Y, Shimokawa H. Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;49(2):85–89. doi: 10.1097/FJC.0b013e31802df112. [DOI] [PubMed] [Google Scholar]
- Johannsen S, Duning K, Pavenstadt H, Kremerskothen J, Boeckers TM. Temporal-spatial expression and novel biochemical properties of the memory-related protein KIBRA. Neuroscience. 2008;155(4):1165–1173. doi: 10.1016/j.neuroscience.2008.06.054. [DOI] [PubMed] [Google Scholar]
- Kampfer S, Windegger M, Hochholdinger F, Schwaiger W, Pestell RG, Baier G, Grunicke HH, Uberall F. Protein kinase C isoforms involved in the transcriptional activation of cyclin D1 by transforming Ha-Ras. J Biol Chem. 2001;276(46):42834–42842. doi: 10.1074/jbc.M102047200. [DOI] [PubMed] [Google Scholar]
- Kremerskothen J, Plaas C, Buther K, Finger I, Veltel S, Matanis T, Liedtke T, Barnekow A. Characterization of KIBRA, a novel WW domain-containing protein. Biochem Biophys Res Commun. 2003;300(4):862–867. doi: 10.1016/s0006-291x(02)02945-5. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Farb CR, LeDoux JE. Fear memory formation involves p190 RhoGAP and ROCK proteins through a GRB2-mediated complex. Neuron. 2002;36(4):727–738. doi: 10.1016/s0896-6273(02)01047-4. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5(4):295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Lingor P, Teusch N, Schwarz K, Mueller R, Mack H, Bahr M, Mueller BK. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J Neurochem. 2007;103(1):181–189. doi: 10.1111/j.1471-4159.2007.04756.x. [DOI] [PubMed] [Google Scholar]
- Liu LZ, Zhao HL, Zuo J, Ho SK, Chan JC, Meng Y, Fang FD, Tong PC. Protein kinase Czeta mediates insulin-induced glucose transport through actin remodeling in L6 muscle cells. Mol Biol Cell. 2006;17(5):2322–2330. doi: 10.1091/mbc.E05-10-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF, Jr., Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 2006;66(8):847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto EC, Bullock P, Polkey CE, Morris RG. Spatial working memory and strategy formation in patients with frontal lobe excisions. Cortex. 1996;32(4):613–630. doi: 10.1016/s0010-9452(96)80034-7. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nacmias B, Bessi V, Bagnoli S, Tedde A, Cellini E, Piccini C, Sorbi S, Bracco L. KIBRA gene variants are associated with episodic memory performance in subjective memory complaints. Neurosci Lett. 2008;436(2):145–147. doi: 10.1016/j.neulet.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Nagata K, Kondoh Y, Satoh Y, Watahiki Y, Yokoyama E, Yuya H, Hirata Y, Shishido F, Hatazawa J, Kanno I, et al. Effects of fasudil hydrochloride on cerebral blood flow in patients with chronic cerebral infarction. Clin Neuropharmacol. 1993;16(6):501–510. doi: 10.1097/00002826-199312000-00003. [DOI] [PubMed] [Google Scholar]
- Need AC, Attix DK, McEvoy JM, Cirulli ET, Linney KN, Wagoner AP, Gumbs CE, Giegling I, Moller HJ, Francks C, Muglia P, Roses A, Gibson G, Weale ME, Rujescu D, Goldstein DB. Failure to replicate effect of Kibra on human memory in two large cohorts of European origin. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(5):667–668. doi: 10.1002/ajmg.b.30658. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, Huynh KD, Brunner F, Corneveaux J, Osborne D, Wollmer MA, Aerni A, Coluccia D, Hanggi J, Mondadori CR, Buchmann A, Reiman EM, Caselli RJ, Henke K, de Quervain DJ. Common Kibra alleles are associated with human memory performance. Science. 2006;314(5798):475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313(5790):1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Poucet B. A further characterization of the spatial problem-solving deficit induced by lesions of the medial frontal cortex in the rat. Behav Brain Res. 1990;41(3):229–237. doi: 10.1016/0166-4328(90)90110-z. [DOI] [PubMed] [Google Scholar]
- Poucet B, Herrmann T. Septum and medial frontal cortex contribution to spatial problem-solving. Behav Brain Res. 1990;37(3):269–280. doi: 10.1016/0166-4328(90)90139-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez E, Infante J, Llorca J, Mateo I, Sanchez-Quintana C, Garcia-Gorostiaga I, Sanchez-Juan P, Berciano J, Combarros O. Age-dependent association of KIBRA genetic variation and Alzheimer's disease risk. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yamada M, Yamada M, Kobayashi S, Hirose N, Honda K, Kamei J. ROCK inhibition produces anxiety-related behaviors in mice. Psychopharmacology (Berl) 2006;188(1):1–11. doi: 10.1007/s00213-006-0466-4. [DOI] [PubMed] [Google Scholar]
- Salhia B, Rutten F, Nakada M, Beaudry C, Berens M, Kwan A, Rutka JT. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res. 2005;65(19):8792–8800. doi: 10.1158/0008-5472.CAN-05-0160. [DOI] [PubMed] [Google Scholar]
- Sarter M. Preclinical research into cognition enhancers. Trends Pharmacol Sci. 2006;27(11):602–608. doi: 10.1016/j.tips.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Satoh S, Toshima Y, Ikegaki I, Iwasaki M, Asano T. Wide therapeutic time window for fasudil neuroprotection against ischemia-induced delayed neuronal death in gerbils. Brain Res. 2007;1128(1):175–180. doi: 10.1016/j.brainres.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Schaper K, Kolsch H, Popp J, Wagner M, Jessen F. KIBRA gene variants are associated with episodic memory in healthy elderly. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Scott GA, Arioka M, Jacobs SE. Lysophosphatidylcholine mediates melanocyte dendricity through PKCzeta activation. J Invest Dermatol. 2007;127(3):668–675. doi: 10.1038/sj.jid.5700567. [DOI] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317(5840):951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Uberall F, Hellbert K, Kampfer S, Maly K, Villunger A, Spitaler M, Mwanjewe J, Baier-Bitterlich G, Baier G, Grunicke HH. Evidence that atypical protein kinase C-lambda and atypical protein kinase C-zeta participate in Ras-mediated reorganization of the F-actin cytoskeleton. J Cell Biol. 1999;144(3):413–425. doi: 10.1083/jcb.144.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kolen K, Slegers H. Atypical PKCzeta is involved in RhoA-dependent mitogenic signaling by the P2Y(12) receptor in C6 cells. Febs J. 2006;273(8):1843–1854. doi: 10.1111/j.1742-4658.2006.05205.x. [DOI] [PubMed] [Google Scholar]
- Woo S, Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J Neurosci. 2006;26(5):1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Kotani Y, Nakajima Y, Shimazawa M, Yoshimura S, Nakashima S, Iwama T, Hara H. Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Res. 2007;1154:215–224. doi: 10.1016/j.brainres.2007.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


