Abstract
Volatile anesthetics protect the heart from ischemia/reperfusion injury but the mechanisms for this protection are poorly understood. Caveolae, sarcolemmal invaginations, and caveolins, scaffolding proteins in caveolae, localize molecules involved in cardiac protection. We tested the hypothesis that caveolae and caveolins are essential for volatile anesthetic-induced cardiac protection using cardiac myocytes (CM) from adult rats and in vivo studies in caveolin-3 knockout mice (Cav-3−/−). We incubated CM with methyl-β-cyclodextrin (MβCD) or colchicine to disrupt caveolae formation, and then exposed the myocytes to the volatile anesthetic isoflurane (30 min, 1.4%), followed by simulated ischemia/reperfusion (SI/R). Isoflurane protected CM from SI/R [23.2±1.6% vs. 71.0±5.8% cell death (assessed by trypan blue exclusion), P<0.001] but this protection was abolished by MβCD or colchicine (84.9±5.5% and 64.5±6.1% cell death, P<0.001). Membrane fractionation by sucrose density gradient centrifugation of CM treated with MβCD or colchicine revealed that buoyant (caveolae-enriched) fractions had decreased phosphocaveolin-1 and caveolin-3 compared to control CM. Cardiac protection in vivo was assessed by measurement of infarct size relative to the area at risk and cardiac troponin levels. Isoflurane-induced a reduction in infarct size and cardiac troponin relative to control (infarct size: 26.5%±2.6% vs. 45.3%±5.4%, P<0.01; troponin: 27.7±4.4 vs. 77.7±11.8 ng/mL, P<0.05). Isoflurane induced cardiac protection was abolished in Cav-3−/− mice (infarct size: 53.4%±6.1% vs. 53.2%±3.5%, P<0.01; troponin: 102.1±22.3 vs. 105.9±8.2 ng/mL, P<0.01). Isoflurane-induced cardiac protection is thus dependent on the presence of caveolae and the expression of caveolin-3. We conclude that caveolae and caveolin-3 are critical for volatile anesthetic-induced protection of the heart from ischemia/reperfusion injury.
Keywords: caveolae, caveolin-3, cardiac protection, volatile anesthetics, ischemia/reperfusion injury
Introduction
Protection of the heart from ischemia/reperfusion injury can be induced by multiple stimuli (e.g., ischemia [1], opioids [2] and volatile anesthetics [3, 4]). Though many volatile anesthetics, including isoflurane [3, 4], sevoflurane [5, 6], and desflurane [6] show cardiac protection in vivo, the precise mechanism for volatile anesthetic-induced cardiac protection has not been elucidated.
Caveolae are small (~100nm diameter), flask-like invaginations [7] of the plasma membrane that are enriched in particular lipids (e.g., cholesterol and glycosphingolipids [8]) and structural proteins, caveolins. There are three known isoforms of caveolin: caveolin-1, caveolin-2 and caveolin-3 [9], each of which has scaffolding domains that interact with signaling molecules [10, 11]. Cardiac myocytes (CM) express the muscle-specific isoform caveolin-3 [12] while other cell types in the heart express caveolin-1 and -2. Recent studies have shown: 1) the presence and interaction of all three caveolin isoforms in adult cardiac myocytes [13, 14], 2) a signaling role for caveolin-1 in cardiac myocytes [15], and 3) that caveolins can scaffold proteins associated with cardiac protection [16, 17].
Caveolae can be disrupted using agents such as methyl-β-cyclodextrin (MβCD), which depletes membrane cholesterol [18, 19], or colchicine, which disrupts microtubules [20]. Colchicine can abolish anesthetic-induced cardiac protection in vivo [21] although the molecular mechanism for this effect is not known. We have recently shown that MβCD can disrupt caveolae and attenuate ischemic- and opioid-induced cardiac myocyte protection [19] but the role of caveolae expression and caveolin-3 in volatile anesthetic-induced cardiac protection is not known. Therefore, in the current study we used both in vitro and in vivo approaches to test the hypothesis that caveolae and the expression of caveolin-3 are essential for volatile anesthetic (e.g., isoflurane)-induced cardiac protection from ischemia/reperfusion injury. Our findings establish that caveolins and caveolae help mediate the action of a wide range of cardiac protective agents.
Materials and Methods
Preparation of CM
CM were isolated from adult Sprague-Dawley rats (Harlan –Indianapolis, IN; 250–300 g, male). All animal use protocols were approved by the VA San Diego Institutional Animal Care and Use Committee. These investigations conform with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Animals were heparinized (1,000U, IP) 5 min before being anesthetized with pentobarbital (80 mg/kg IP). The hearts were removed and placed in ice-cold cardioplegic (20 mM KCl) heart media solution (HM, in mmol/l: 112 NaCl, 5.4 KCl, 1 MgCl2, 9 NaH2PO4, and 11.1 D-glucose; supplemented with 10 HEPES, 30 taurine, 2 DL-carnitine, and 2 creatine, pH 7.4) and then retrograde-perfused on a Langendorff apparatus with Ca2+-free HM for 5 min at 5 ml/min at 37°C, followed by perfusion with Ca2+-free HM containing collagenase II (210 U/mg; Worthington –Lakewood, NJ) for 20 min. After perfusion, both ventricles were removed and minced in collagenase II-containing HM for 10–15 min. The cell solution was then washed several times to remove collagenase II and re-exposed to 1.2 mM Ca2+ over 25 min to produce Ca2+-tolerant CM. Myocytes were then plated in 4% FBS on laminin (2 μg/cm2)-coated plates for 1 hr. Plating/maintenance media was changed to serum-free medium [1% bovine serum albumin (BSA)+0.1% penicillin/streptomycin in 199 medium (Invitrogen – Carlsbad, CA)] to remove all non-myocytes, and CM were incubated at 37°C in 5% CO2 for 24 hr.
Immunoblot analysis
Proteins in individual fractions, whole cell lysates, and whole tissue lysates were separated by SDS-PAGE using 10% polyacrylamide precast gels (Invitrogen – Carlsbad, CA) and transferred to a polyvinylidene difluoride (Millipore – Billerica, MA) membrane by electroelution. Membranes were blocked in 20 mM TBS-Tween (1%) containing 1.5% nonfat dry milk and incubated with primary antibody overnight (Caveolin-1 and Caveolin-3, Abcam – Cambridge, MA; GAPDH, Imgenex – San Diego, CA; p-Caveolin-1 Y14, Chemicon – Temecula, CA) at 4°C. Bound primary antibodies were visualized using secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology – Santa Cruz, CA) and enhanced chemiluminescence reagent (GE Healthcare/Amersham – Piscataway, NJ). All displayed bands migrated at the appropriate size, as determined by comparison with molecular weight standards (Santa Cruz Biotechnology – Santa Cruz, CA).
Electron microscopy
Control CM or CM treated with MβCD or colchicine for 1 hr were immediately fixed for 2 hr at room temperature in a solution that contained 2.5% glutaraldehyde in 0.1 M cacodylate buffer. Cardiac tissue from caveolin-3 knockout mice was excised and perfused with the same fixing solution, then postfixed in 1% OsO4 in 0.1 M cacodylate buffer (1 hr), and embedded as monolayers in LX-112 (Ladd Research –Williston, VT), as described previously [22]. Sections were stained in uranyl acetate and lead citrate and visualized with an electron microscope (JEOL 1200 EX-II or Philips CM-10). Images were taken at 8,900× and 11,500×.
Sucrose density membrane fractionation
CM were fractionated to isolate caveolae-rich domains using a detergent-free method [23]. Cells from a 10-cm2 plate were washed twice in ice-cold PBS, scraped into 3 ml of 150 mM Na2CO3 with 1 mM EDTA (pH 11.0), homogenized with a tissue grinder with three 10-sec bursts, and then sonicated with three cycles of 20-sec bursts interspersed with 1 min of incubation on ice. Whole cell lysates were equilibrated using GAPDH. 1 ml of homogenate was mixed with 1 ml of 80% sucrose in MES-buffered saline (MBS: 25 mM MES, 150 mM NaCl, 2 mM EDTA-MBS, pH 6.5) to form 40% sucrose and loaded at the bottom of an ultracentrifuge tube. A discontinuous sucrose gradient was generated by layering 6 ml of 35% sucrose prepared in MBS and then 4 ml of 5% sucrose in MBS. The gradient was centrifuged at 175,000 g using a SW41Ti rotor (Beckman Instruments – Fullerton, CA) for 3 hr at 4°C. Samples were removed in 1 ml aliquots to yield 12 fractions, which were analyzed for protein content. We defined fractions 4–6 as buoyant membrane fractions (BF) enriched in caveolae and proteins associated with caveolae. Fractions 9–12 were defined as non-buoyant fractions.
Simulated ischemia/reperfusion (SI/R) in isolated CM
CM were plated on laminin-coated 12-well plates, allowed to incubate for 24 hr, and then subjected to various experimental conditions at 37°C. Simulated ischemia was induced by replacing the air content with a 95% N2 and 5% CO2 gas mixture at 2 L/min in a metabolic chamber (Columbus Instruments – Columbus, OH) and by replacing the media with a thin film of glucose-free media (glucose-free DMEM, Invitrogen – Carlsbad, CA) covering the cells for 60 min. This was then followed by 60 min of “reperfusion” by replacing the media with normal maintenance media and by incubating the cells with 21% O2 and 5% CO2. CM were exposed to isoflurane for 30-min prior to SI/R. Isoflurane concentrations were verified continuously by sampling exhaust gas with a Datex Capnomac (SOMA Technology Inc. – Cheshire, CT). Concentrations of isoflurane (0.7%, 1.4%, and 2.8% vol/vol in air) were chosen based on the minimum alveolar concentrations (MAC) in rodents (where 1.4% vol/vol is equivalent to 1 MAC [24]). We have previously confirmed that 1.4% vol/vol isoflurane produces 0.165 ± 0.003 mM isoflurane in media in our metabolic chamber [15]. Cell death was quantified by counting trypan blue-stained cells with results expressed as a percentage of total cells counted (Figure 1). To determine the impact of intact caveolae on cardiac protection, we used MβCD and colchicine. CM were incubated under maintenance media (control conditions) or in the presence of MβCD (1 mM) or colchicine (30μM) for 1 hr before SI/R or isoflurane + SI/R.
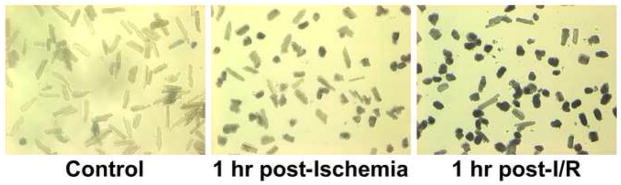
Figure 1. Simulated ischemia/reperfusion (I/R) model.
Adult cardiac myocytes were rod-shaped and viable with oxygen exposure alone averaging 3.5% cell death. Cell death increased with ischemia and reperfusion averaging 46% and 87% cell death, respectively.
In vivo ischemia/reperfusion injury
8–10 wk old caveolin-3 knockout mice (caveolin-3−/−) [13, 25] or age-matched C57BL/6 mice (controls) were studied using an in vivo ischemia/reperfusion protocol [26]. Mice were anesthetized with sodium pentobarbital (80 mg/kg IP). A 20G catheter was then inserted into the tracheae, and the mice were mechanically ventilated using a pressure-controlled ventilator (TOPO Ventilator – Kent Scientific Co., Torrington, CT, peak inspiratory pressure: 15 cmH2O, respiratory rate: 100 breaths/min, inspired O2: 100%). A thoracotomy was performed to expose the heart. Core temperature was maintained at 36°C with a heating pad and ECG leads were placed to record heart rate. After thoracotomy, baseline was established and mice were randomly assigned to experimental protocols. Mice received 1.4% isoflurane vol/vol in O2, which is equivalent to 1 MAC [27] for 30 min followed by a 15 min washout. Ischemia was produced by occluding the left coronary artery with a 7–0 silk suture on a tapered BV-1 needle (Ethicon, Inc. – Somerville, NJ). A small piece of polyethylene tubing was used to secure the silk ligature without damaging the artery. After 30 min of occlusion, the ligature was released and the heart was reperfused for 2 hr. Reperfusion was confirmed by observing return of blood flow in the epicardial coronary arteries and via electrocardiography. The area at risk (AAR) was determined by staining with 1% Evans blue (1.0 ml, Sigma – St. Louis, MO). The heart was immediately excised and placed into 1% agarose and allowed to harden. Once hardened, the heart was cut into 1 mm slices (McILwain tissue chopper; Brinkmann Instruments, Inc. – Westbury, NY). Each slice of left ventricle (LV) was counterstained with 3.0 ml of 1% 2,3,5-triphenyltetrazolium chloride (Sigma – St. Louis, MO) for 5 min at 37°C. After overnight storage in 10% formaldehyde, slices were weighed and visualized under a microscope (Leica Microsystems Inc. – Bannockburn, IL) equipped with a charge-coupled device camera (Cool SNAP-Pro, Media Cybernetics, Inc. – Silver Spring, MD). The images were analyzed (Image-Pro Plus Version 4.5, Media Cybernetics, Inc. – Silver Spring, MD) and infarct size was determined by planimetry. The AAR was expressed as a percentage of the LV (AAR/LV). Infarct size (IS) was expressed as a percentage of the AAR (IS/AAR) [28].
Cardiac troponin levels, a second measure of cardiac injury, were determined from a subset of animals using a mouse cardiac Tn-I ELISA kit (Life Diagnostics – West Chester, PA). Serum was prepared from each test group and stored at −80°C. ELISA was run per the manufacturer’s recommended protocol and absorbance was read at 405 nm using an Infinite M500 plate reader (Tecan – San Jose, CA). An additional group of caveolin-3−/− mice were used for electron microscopy and immunoblot analysis. Following anesthesia with sodium pentobarbital (80 mg/kg IP), hearts were excised and a piece of the LV apex was removed for protein analysis. The remaining LV was used for electron microscopy.
Statistical analysis
Statistical analyses were performed by one-way ANOVA followed by the Bonferroni post hoc test. All data are expressed as mean ± SEM. Statistical significance was defined as P < 0.05.
Results
Isoflurane induces cardiac protection in adult CM
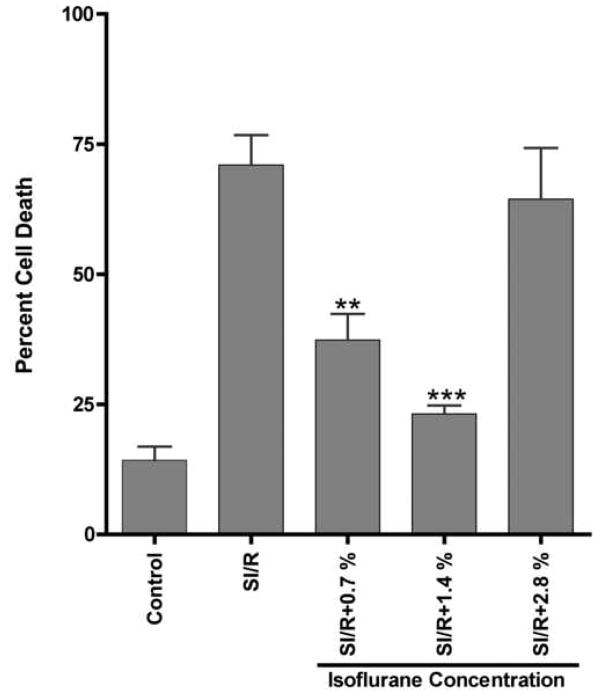
Adult CM were exposed to various concentrations of isoflurane and then to simulated ischemia/reperfusion (SI/R) (Figure 2). Exposure to 0.7% and 1.4% isoflurane before SI/R decreased cell death when compared to SI/R alone (37.4 ± 5.0%, and 23.2 ± 1.6% vs. 71.0 ± 5.8% cell death, respectively). However, at higher concentrations of isoflurane (2.8%), cardiac protection was not observed (64.4 ± 9.8% cell death).
Figure 2. Effect of isoflurane on simulated ischemia/reperfusion (SI/R) of adult cardiac myocytes (CM).
CM were plated and treated with various concentrations of isoflurane prior to exposure to SI/R. Cell death was determined by trypan blue staining. CM under control conditions had minimal cell death. Optimal protection was observed at 1.4% isoflurane. No protection was observed at 2.8%. n = 6 for all groups. ** P < 0.01 compared to SI/R, *** P < 0.001 compared to SI/R.
MβCD and colchicine abolish caveolae formation and alter caveolin expression
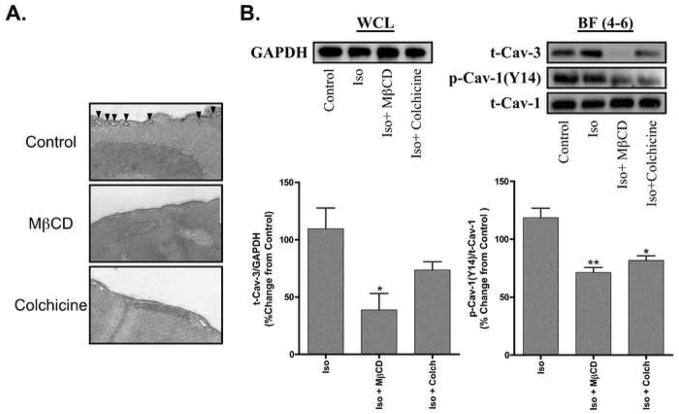
Treatment of CM with MβCD or colchicine reduced the expression of caveolae (Figure 3A). The amount of phosphorylated caveolin-1 was significantly reduced in buoyant caveolar fractions (BF, Fractions 4–6) following sucrose density fractionation of MβCD- or colchicine-treated cells (Figure 3B). Expression of caveolin-3 in BF was significantly reduced in MβCD-treated cells and to a lesser extent in colchicine-treated cells after incubation with isoflurane (Figure 3B). Expression of caveolin-3 in non-buoyant fractions (Fractions 9–12) was not significantly altered among the experimental groups (data not shown).
Figure 3. Impact of MβCD and colchicine on expression by cardiac myocytes (CM) of caveolae, caveolin-3 and phosphocaveolin-1 and on response to isoflurane.
A. Electron micrographs of CM reveal caveolae at the surface of the plasma membrane in cells under control conditions (black arrows). MβCD- and colchicine-treated myocytes have no visible caveolae n = 2 per group (magnification = 11,500×). B. Whole cell lysates (WCL) were normalized via expression of GAPDH prior to sucrose density fractionation. Sucrose gradient fractions of 1.4% isoflurane (Iso) exposed CM revealed decreased expression of pCaveolin-1 (p-Cav-1 Y14) and Caveolin-3 (t-Cav-3) in the buoyant fraction (BF = fractions 4–6) of MβCD- and colchicine (Colch)-treated cells. Densitometry revealed a significant decrease in p-Cav-1 Y14 in both MβCD- and colchicine-treated groups, whereas t-Cav-3 expression was only significantly decreased in the MβCD group. n = 3 per group. * P < 0.05 compared to Iso, ** P < 0.01 compared to Iso.
MβCD and colchicine abolish isoflurane-induced cardiac protection
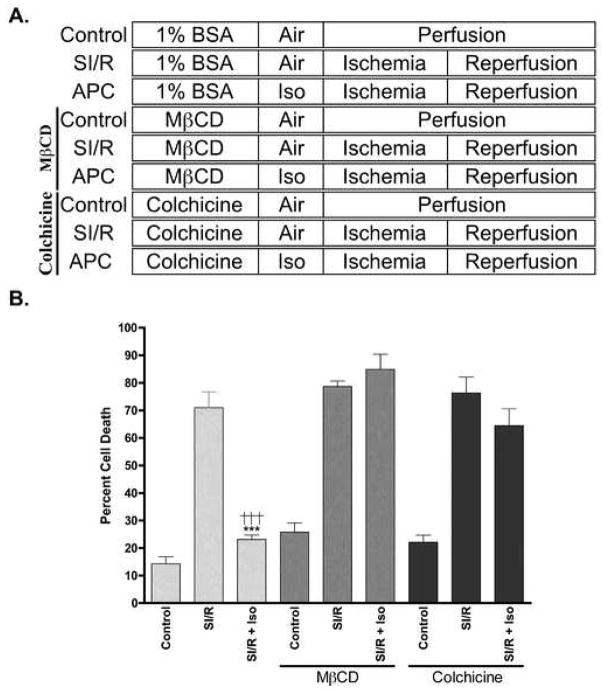
CM were incubated with 1% BSA + 0.1% penicillin/streptomycin (Control) or in control media along with MβCD (1 mM), or colchicine (30 μM) and then exposed to 1.4% isoflurane (Figure 4A). The protective effect of isoflurane was abolished in CM incubated with MβCD or colchicine (84.9 ± 5.5% and 64.5 ± 6.1% cell death, respectively). We observed no significant increase in basal cell death with the various treatments (Figure 4B).
Figure 4. Cardiac protection by isoflurane in the presence and absence of caveolae disruption.
A. Cardiac myocytes were exposed to control conditions, simulated ischemia/reperfusion (SI/R), or SI/R + isoflurane (Iso) in the presence or absence of MβCD and colchicine. MβCD- and colchicine (Colch)-treated groups were incubated with MβCD (1 mM) or Colch (30 μM) in maintenance media (1% BSA) for 1 hr, the latter of which was used for control cells. Cells were then incubated with air or 1.4% isoflurane for 30 min. SI/R was then produced by aerating a metabolic chamber with 95% N2 + 5% CO2 and changing to glucose-free media, which results in oxygen and glucose deprivation (OGD). This incubation was then followed by “reperfusion” with placement of cells in normal maintenance media at 21% O2 and 5% CO2 for 1 hr. All experiments were performed at 37°C. B. Isoflurane-induced cardiac protection was diminished in the presence of MβCD or Colch. n = 6 in all groups. *** P < 0.001 compared to SI/R + Iso + MβCD, †††P < 0.001 compared to SI/R + Iso + Colchicine.
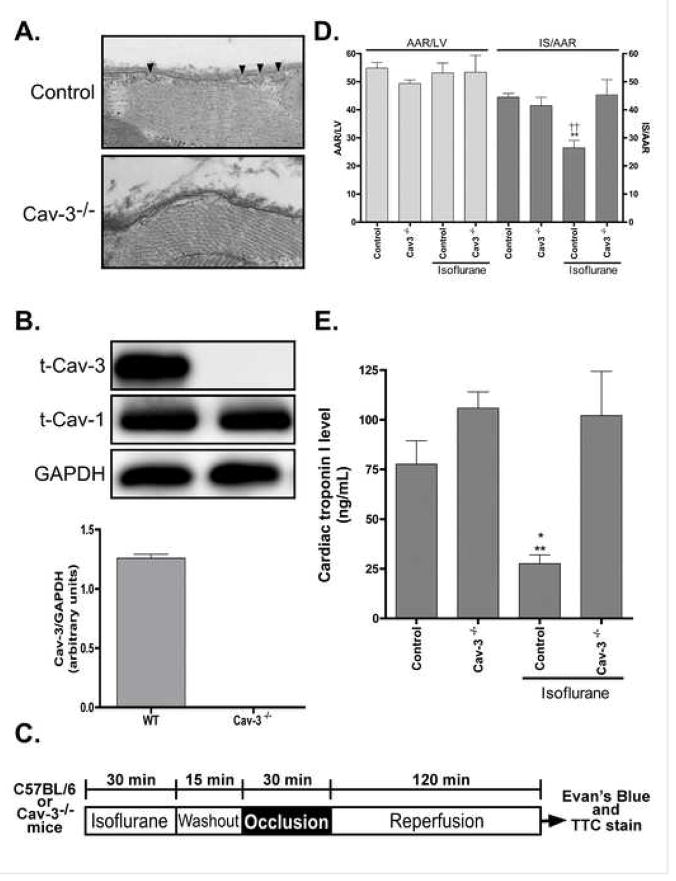
Caveolin-3 is required for isoflurane-induced cardiac protection
Electron micrographs of caveolin-3−/− mouse hearts revealed the absence of caveolae in cardiac myocyte sarcolemmal membranes (Figure 5A). The absence of caveolin-3 protein in the hearts of caveolin-3−/− mice was verified by Western immunoblot analysis; these mice had normal levels of caveolin-1 (Figure 5B). To assess the role of caveolin-3 in the protection from ischemia/reperfusion injury, we treated C57BL/6 mice or caveolin-3−/− mice with 1.4% isoflurane for 30 min, followed by 15 min washout and then exposed the mice to ischemia/reperfusion (Figure 5C). The ability of isoflurane to protect from ischemia/reperfusion injury was abolished in caveolin-3−/− mice compared to control animals [45.3 ± 5.4% and 26.5 ± 2.6% infarct size/area at risk (AAR)] even though there was a similar AAR in both groups of animals (Figure 5D; 53.2 ± 3.5% vs. 53.4 ± 6.1% AAR). Cardiac troponin I levels were significantly attenuated by isoflurane treatment in wild-type mice compared to control mice subjected to ischemia/reperfusion (27.7 ± 4.4 and 77.7 ± 11.8 ng/mL); however, isoflurane failed to reduce cardiac troponin I levels in caveolin-3−/− mice, a level similar to control caveolin-3−/− mice was observed (102.1 ± 22.3 and 105.9 ± 8.2 ng/mL).
Figure 5. Absence of isoflurane-induced cardiac protection in caveolin-3 knockout mice.
A Electron micrographs of cardiac tissue from caveolin-3 knockout (Cav-3−/−) mice reveal an absence of caveolae in the sarcolemmal membrane (8,900×). B. Western blot analysis confirmed the absence of caveolin-3 protein in the hearts of the Cav-3−/−mice but with similar levels of caveolin-1 (Cav-1), n = 6 in all groups. C. In vivo anesthetic (isoflurane)-induced cardiac protection protocol. D. Isoflurane-induced cardiac protection was abolished in Cav-3−/− mice, as shown by no significant decrease in infarct size (IS)/area at risk (AAR) when compared to control Cav-3−/−; however, a significant decrease in IS was noted between Control + Isoflurane vs. Control and Cav-3−/− + Isoflurane, n = 6 in all groups. ** P < 0.01 compared to Control. ††P < 0.01 compared to Cav-3−/− + Isoflurane. E. Following ischemia/reperfusion injury cardiac troponin I levels in Cav-3−/− mice were elevated when compared to control. In addition, no significant decrease in troponin I levels were observed in Cav-3−/− mice following isoflurane-induced cardiac protection, whereas, a significant decrease was noted in control mice that received isoflurane. Troponin I was also decreased in Control + Isoflurane vs. Cav-3−/− + Isoflurane, n = 4–6 for all groups. * P < 0.05 compared to Control, ** P < 0.01 compared to Cav-3−/− + Isoflurane.
Discussion
The current data show that the presence of caveolae and the expression of caveolin-3 in CM are essential for isoflurane-induced cardiac protection from ischemia/reperfusion (I/R) injury. Treatment with MβCD and colchicine, agents that decrease the number of caveolae and the amount of phosphorylated caveolin-1, produced an attenuation of isoflurane-induced protection in CM exposed to SI/R. Consistent with these findings, caveolin-3 knockout mice devoid of CM caveolae lack isoflurane-induced cardiac protection from I/R injury.
Volatile anesthetics are short chain halogenated alkanes and ethers that interact with cell membrane lipids and are thought to interact with membrane-bound proteins to produce cellular effects [29]. Volatile anesthetics produce cardiac protection in a number of species, including man [5]. It is not clear whether volatile anesthetics act via specific receptors or via “nonspecific” membrane effects to alter effector molecules that mediate cardiac protection. Isoflurane administration can activate opioid and adenosine receptors and blockade of these G-protein-coupled receptors can abolish cardiac protection produced by isoflurane [30]. Volatile anesthetics affect several signaling pathways implicated in preconditioning, including Src tyrosine kinase (Src), the phosphatidylinositol-3-kinase(PI3K)/protein kinase B/glycogen synthase kinase 3 beta pathway [31], protein kinase C (PKC) [32, 33], and mitogen-activated protein kinases (MAPK), including extracellular signal-regulated kinase 1 and 2 (ERK1/2) [34] and p38 MAPK [35]. In addition, volatile anesthetics modulate ATP-sensitive potassium channel activity [32, 33], the generation of reactive oxygen species, and mitochondrial permeability transition pore opening [36]. Of note, many of those signaling molecules and effector systems can either interact directly with the scaffolding domain of caveolin or are known to localize to caveolae [16, 17, 37].
Caveolae and caveolins organize signaling molecules and facilitate rapid, precise, and coordinated signal transduction [38, 39]. Furthermore, caveolae sequester many signaling proteins important in cardiac protection including the proteins activated by isoflurane. Caveolins, the structural components of caveolae, can function as chaperones and provide direct temporal and spatial regulation with numerous cardio-protective signaling molecules via their scaffolding domain including endothelial nitric oxide synthase (eNOS), Src, PKC, G-protein α, PI3K, and ERK1/2 [16, 38, 40]. Interestingly, caveolins can inhibit the activity of some of these signaling proteins such as eNOS and ERK1/2 [41–43]. However, at the same time caveolins can promote signaling via enhanced receptor-effector coupling or enhanced receptor affinity when caveolins are up regulated or overexpressed [44, 45]. This has led to the concept of a “caveolar paradox” in which caveolins may produce direct allosteric inhibition of molecules such as eNOS under basal conditions but facilitate increased signaling upon agonist stimulation through compartmentation [44, 46]. We have recently shown that isoflurane increases the recruitment and phosphorylation of Src kinase and caveolin-1 into myocardial caveolar fractions. We have also shown that this phosphorylation of Src and caveolin-1 are required for isoflurane-induced cardiac protection [15]; isoflurane increased the phosphorylation of caveolin-1 in a Src-dependent manner and caveolin-1 knockout mice were not able to be protected from myocardial ischemia/reperfusion injury by isoflurane. Importantly, myocytes isolated from these animals have normal caveolae (unpublished observations). The current results confirm and extend these findings by showing that two different pharmacological approaches that disrupt caveolae and reduce caveolin-1 phosphorylation also attenuate isoflurane-induced protection of CM. In addition, the current data involving the use of caveolin-3−/− mice define a requirement for caveolin-3 in isoflurane-induced cardiac protection.
Caveolin-3, the predominant isoform in CM, mediates interactions with cytoskeletal elements (including α-tubulin and filamin [14]) and is responsible for caveolae formation in these cells. A role for caveolin-3 in cardiac protection has not been investigated. Previous studies have shown that caveolin-3 overexpression prevents cardiac hypertrophy in isolated neonatal myocytes and may be beneficial in preventing pathological cardiac remodeling via the inhibition of Erk signaling [47]. Caveolin-3 co-localizes with opioid receptors, which can contribute to cardiac protection from ischemia [19]. The current data indicate that a decreased number of myocardial caveolae are found in caveolin-3−/− mice even though caveolin-1 expression is normal and that such mice lose the ability to undergo isoflurane-induced cardiac protection from ischemia/reperfusion injury. Collectively, these and previous data implicate a role for both caveolin-1 and -3 and the presence of caveolae in cardiac protection from ischemia/reperfusion injury.
Our results showing the absence of cardiac protection at higher concentrations of isoflurane (2.8%) are in contrast to past studies in a dog model in vivo [48] and in an adult rat CM in vitro [49] in which a concentration-dependent increase in cardiac protection was observed with isoflurane. However, Kehl et al [48], who examined the cardiac protective effects of isoflurane in an in vivo dog model, only used a maximum concentration of isoflurane of 1.6%. Although Zaugg, et al [49], showed increased cardiac protection at 2.8% isoflurane compared to lower concentrations, those studies utilized different methods of isoflurane delivery (bubbling) and simulated ischemia (mineral oil layering), and did not investigate simulated reperfusion injury, which is believed to be a major contributor to cell death following myocardial ischemia [50].
A limitation of our study is the exclusive use of isoflurane as a cardiac protective agent. Unlike isoflurane, sevoflurane is unable to increase phosphorylation of caveolin-1 in rat lung endothelial cells [51] which supports the possibility that there may be different effects of volatile anesthetics on post-translational modifications of caveolin in the myocardium. Further investigations utilizing other volatile agents such as sevoflurane or desflurane are necessary to clearly determine the role of caveolae and caveolin-3 in a generalized mechanism of action of volatile anesthetics in cardiac protection.
Based on the current results, we conclude that caveolae and caveolin-3 are essential for the protection of the heart from ischemia/reperfusion injury and in particular, for isoflurane-induced cardiac protection. The results thus suggest that treatments designed to enhance expression of caveolins and caveolae in CM have the potential to prevent ischemic damage in the heart and perhaps other tissues.
Acknowledgments
The studies conducted were supported by: American Heart Association Predoctoral Fellowship 06150217Y(YTH), American Heart Association Scientist Development Grant 060039N (HHP), American Heart Association Scientist Beginning Grant-in-Aid 0765076Y (YMT), Merit Award from the Department of Veterans Affairs (DMR), and NIHHL081400 (DMR), and HL66941 (DMR, PAI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Schultz JE, Hsu AK, Gross GJ. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ Res. 1996;78(6):1100–4. doi: 10.1161/01.res.78.6.1100. [DOI] [PubMed] [Google Scholar]
- 3.Cason BA, Gamperl AK, Slocum RE, Hickey RF. Anesthetic-induced preconditioning: previous administration of isoflurane decreases myocardial infarct size in rabbits. Anesthesiology. 1997;87(5):1182–90. doi: 10.1097/00000542-199711000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87(2):361–70. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Obal D, Preckel B, Scharbatke H, Mullenheim J, Hoterkes F, Thamer V, et al. One MAC of sevoflurane provides protection against reperfusion injury in the rat heart in vivo. Br J Anaesth. 2001;87(6):905–11. doi: 10.1093/bja/87.6.905. [DOI] [PubMed] [Google Scholar]
- 6.Preckel B, Thamer V, Schlack W. Beneficial effects of sevoflurane and desflurane against myocardial reperfusion injury after cardioplegic arrest. Can J Anaesth. 1999;46(11):1076–81. doi: 10.1007/BF03013206. [DOI] [PubMed] [Google Scholar]
- 7.Palade G. Fine structure of blood capillaries. J Appl Phsiol. 1953;24:1424–36. [Google Scholar]
- 8.Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999;11(4):424–31. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- 9.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36(8):584–95. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 10.Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994;4(7):231–5. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273(10):5419–22. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 12.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271(16):9690–7. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 13.Hagiwara Y, Nishina Y, Yorifuji H, Kikuchi T. Immunolocalization of caveolin-1 and caveolin-3 in monkey skeletal, cardiac and uterine smooth muscles. Cell Struct Funct. 2002;27(5):375–82. doi: 10.1247/csf.27.375. [DOI] [PubMed] [Google Scholar]
- 14.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, et al. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem. 2006;281(36):26391–9. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 15.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. Faseb J. 2007;21(7):1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 16.Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett. 2004;9(2):195–220. [PubMed] [Google Scholar]
- 17.Siracusano L, Girasole V, Alvaro S, Chiavarino ND. Myocardial preconditioning and cardioprotection by volatile anaesthetics. J Cardiovasc Med (Hagerstown) 2006;7(2):86–95. doi: 10.2459/01.JCM.0000199792.32479.ce. [DOI] [PubMed] [Google Scholar]
- 18.Furuchi T, Anderson RG. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK) J Biol Chem. 1998;273(33):21099–104. doi: 10.1074/jbc.273.33.21099. [DOI] [PubMed] [Google Scholar]
- 19.Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, et al. Protection of adult rat cardiac myocytes from ischemic cell death: role of caveolar microdomains and delta-opioid receptors. Am J Physiol Heart Circ Physiol. 2006;291(1):H344–50. doi: 10.1152/ajpheart.01100.2005. [DOI] [PubMed] [Google Scholar]
- 20.Goldman RD. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971;51(3):752–62. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismaeil MS, Tkachenko I, Hickey RF, Cason BA. Colchicine inhibits isoflurane-induced preconditioning. Anesthesiology. 1999;91(6):1816–22. doi: 10.1097/00000542-199912000-00036. [DOI] [PubMed] [Google Scholar]
- 22.De Vries L, Elenko E, McCaffery JM, Fischer T, Hubler L, McQuistan T, et al. RGS-GAIP, a GTPase-activating protein for Galphai heterotrimeric G proteins, is located on clathrin-coated vesicles. Mol Biol Cell. 1998;9(5):1123–34. doi: 10.1091/mbc.9.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995;92(22):10104–8. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White PF, Johnston RR, Eger EI., 2nd Determination of anesthetic requirement in rats. Anesthesiology. 1974;40(1):52–7. doi: 10.1097/00000542-197401000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Oshikawa J, Otsu K, Toya Y, Tsunematsu T, Hankins R, Kawabe J, et al. Insulin resistance in skeletal muscles of caveolin-3-null mice. Proc Natl Acad Sci U S A. 2004;101(34):12670–5. doi: 10.1073/pnas.0402053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsutsumi YM, Patel HH, Huang D, Roth DM. Role of 12-lipoxygenase in volatile anesthetic-induced delayed preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291(2):H979–83. doi: 10.1152/ajpheart.00266.2006. [DOI] [PubMed] [Google Scholar]
- 27.Deady JE, Koblin DD, Eger EI, 2nd, Heavner JE, D’Aoust B. Anesthetic potencies and the unitary theory of narcosis. Anesth Analg. 1981;60(6):380–4. [PubMed] [Google Scholar]
- 28.Tsutsumi YM, Patel HH, Lai NC, Takahashi T, Head BP, Roth DM. Isoflurane produces sustained cardiac protection after ischemia-reperfusion injury in mice. Anesthesiology. 2006;104(3):495–502. doi: 10.1097/00000542-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Keil RL, Wolfe D, Reiner T, Peterson CJ, Riley JL. Molecular genetic analysis of volatile-anesthetic action. Mol Cell Biol. 1996;16(7):3446–53. doi: 10.1128/mcb.16.7.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toller WG, Kersten JR, Gross ER, Pagel PS, Warltier DC. Isoflurane preconditions myocardium against infarction via activation of inhibitory guanine nucleotide binding proteins. Anesthesiology. 2000;92(5):1400–7. doi: 10.1097/00000542-200005000-00031. [DOI] [PubMed] [Google Scholar]
- 31.Feng J, Fischer G, Lucchinetti E, Zhu M, Bestmann L, Jegger D, et al. Infarct-remodeled myocardium is receptive to protection by isoflurane postconditioning: role of protein kinase B/Akt signaling. Anesthesiology. 2006;104(5):1004–14. doi: 10.1097/00000542-200605000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Marinovic J, Bosnjak ZJ, Stadnicka A. Preconditioning by isoflurane induces lasting sensitization of the cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel by a protein kinase C-delta-mediated mechanism. Anesthesiology. 2005;103(3):540–7. doi: 10.1097/00000542-200509000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig LM, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Protein kinase C translocation and Src protein tyrosine kinase activation mediate isoflurane-induced preconditioning in vivo: potential downstream targets of mitochondrial adenosine triphosphate-sensitive potassium channels and reactive oxygen species. Anesthesiology. 2004;100(3):532–9. doi: 10.1097/00000542-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Toma O, Weber NC, Wolter JI, Obal D, Preckel B, Schlack W. Desflurane preconditioning induces time-dependent activation of protein kinase C epsilon and extracellular signal-regulated kinase 1 and 2 in the rat heart in vivo. Anesthesiology. 2004;101(6):1372–80. doi: 10.1097/00000542-200412000-00018. [DOI] [PubMed] [Google Scholar]
- 35.da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Differential activation of mitogen-activated protein kinases in ischemic and anesthetic preconditioning. Anesthesiology. 2004;100(1):59–69. doi: 10.1097/00000542-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Krolikowski JG, Bienengraeber M, Weihrauch D, Warltier DC, Kersten JR, Pagel PS. Inhibition of mitochondrial permeability transition enhances isoflurane-induced cardioprotection during early reperfusion: the role of mitochondrial KATP channels. Anesth Analg. 2005;101(6):1590–6. doi: 10.1213/01.ANE.0000181288.13549.28. [DOI] [PubMed] [Google Scholar]
- 37.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70(2):240–53. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275(5 Pt 1):L843–51. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 39.Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143(2):235–45. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5(3):214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, et al. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428(3):205–11. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 42.Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes. Implications for the autonomic regulation of heart rate. J Biol Chem. 1998;273(46):30249–54. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- 43.Kamoun WS, Karaa A, Kresge N, Merkel SM, Korneszczuk K, Clemens MG. LPS inhibits endothelin-1-induced endothelial NOS activation in hepatic sinusoidal cells through a negative feedback involving caveolin-1. Hepatology. 2006;43(1):182–90. doi: 10.1002/hep.20940. [DOI] [PubMed] [Google Scholar]
- 44.Feron O, Balligand JL. Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc Res. 2006;69(4):788–97. doi: 10.1016/j.cardiores.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Raikar LS, Vallejo J, Lloyd PG, Hardin CD. Overexpression of caveolin-1 results in increased plasma membrane targeting of glycolytic enzymes: the structural basis for a membrane associated metabolic compartment. J Cell Biochem. 2006;98(4):861–71. doi: 10.1002/jcb.20732. [DOI] [PubMed] [Google Scholar]
- 46.Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circ Res. 2001;88(2):129–31. doi: 10.1161/01.res.88.2.129. [DOI] [PubMed] [Google Scholar]
- 47.Koga A, Oka N, Kikuchi T, Miyazaki H, Kato S, Imaizumi T. Adenovirus-mediated overexpression of caveolin-3 inhibits rat cardiomyocyte hypertrophy. Hypertension. 2003;42(2):213–9. doi: 10.1161/01.HYP.0000082926.08268.5D. [DOI] [PubMed] [Google Scholar]
- 48.Kehl F, Krolikowski JG, Mraovic B, Pagel PS, Warltier DC, Kersten JR. Is isoflurane-induced preconditioning dose related? Anesthesiology. 2002;96(3):675–80. doi: 10.1097/00000542-200203000-00025. [DOI] [PubMed] [Google Scholar]
- 49.Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Schaub MC. Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial K(ATP) channels via multiple signaling pathways. Anesthesiology. 2002;97(1):4–14. doi: 10.1097/00000542-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Gross GJ, Auchampach JA. Reperfusion injury: does it exist? J Mol Cell Cardiol. 2007;42(1):12–8. doi: 10.1016/j.yjmcc.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu G, Schwartz DE, Shajahan AN, Visintine DJ, Salem MR, Crystal GJ, et al. Isoflurane, but not sevoflurane, increases transendothelial albumin permeability in the isolated rat lung: role for enhanced phosphorylation of caveolin-1. Anesthesiology. 2006;104(4):777–85. doi: 10.1097/00000542-200604000-00023. [DOI] [PubMed] [Google Scholar]