Abstract
Previously we showed that intracutaneous vaccination of rabbits with DNA vectors encoding ubiquitin-fused versions of the cottontail rabbit papillomavirus (CRPV) early proteins E1, E2, E6 and E7 protected against subsequent challenge with CRPV. Here we tested the immunotherapeutic activity of a vaccine composed of the four CRPV DNA vectors (designated UbE1267) in rabbits. The results show that the UbE1267 DNA vaccine, relative to empty vector DNA, virtually eliminated papilloma growth in rabbits with subclinical infection and greatly reduced papilloma volumes in rabbits bearing papillomas at the time of vaccination. These results in a physiologically relevant animal model of high-risk human papillomavirus (HPV) infection indicate that DNA vaccines targeting the early papillomavirus proteins may have a role in the treatment of HPV-associated lesions in humans.
1. Introduction
Proliferative epithelial lesions induced by human papillomaviruses (HPVs) generally resolve spontaneously due to cell-mediated immune responses. Resolution however takes several months or years to occur and, in some individuals, high-risk HPV infections persist for decades and induce lesions that would progress to carcinoma if left untreated. High-risk HPVs such as HPV16 and HPV18 initiate the development of multiple cancers in the anogenital and respiratory tracts, including virtually 100% of cervical cancers (reviewed in [1]). Since high grade HPV-associated anogenital lesions require surgical removal and surgical procedures are not universally effective [2, 3], a therapeutic HPV vaccine would be of considerable value for use in conjunction with surgery to cure lesions and prevent cancer. Therapeutic vaccines would likely provide the most benefit to patients before malignancy occurs. For such patients, the vaccine targets would logically include the E1 and E2 proteins as well as the E6 and E7 proteins, since all four are expressed in premalignant lesions. The E1 and E2 proteins are also larger than E6 or E7 and might contain larger numbers of immunogenic epitopes.
Recombinant DNA vectors are well suited for the development of therapeutic vaccines because the protein targets they encode are endogenously synthesized, processed via the MHC class I pathway, and elicit cytotoxic T cell responses which are critical for the elimination of infected and transformed cells (reviewed in [4, 5]). One way to accelerate MHC class I antigen presentation is by fusing a target gene to a ubiquitin monomer to enhance trafficking of the encoded protein through the proteasome, an organelle that generates short peptides for presentation via the MHC class I pathway (reviewed in [6]). Using the CRPV/rabbit model we previously showed that a DNA vaccine encoding a ubiquitin-fused CRPV E6 protein (UbE6) provided significant protection and a combination of DNA vaccines individually encoding UbE1, UbE2 and UbE7 proteins completely prevented papilloma formation following CRPV challenge [7].
We hypothesized that the ubiquitin-fused CRPV DNA vaccines could also induce therapeutic efficacy in rabbits with established CRPV infections. The current study was undertaken to test this hypothesis.
2. Methods
2.1 CRPV infection of rabbits
Two-kilogram female New Zealand white Pasteurella-free rabbits (Charles River, Wilmington, MA) were maintained in the animal facilities at the Yale University School of Medicine. All experiments were performed in accordance with procedures approved by the Yale Institutional Animal Care and Use Committee. Rabbits were infected with CRPV at cutaneous sites on the right flank by scarification as described previously [8]. Each rabbit was infected with 10 μl of a low, moderate or high dilution of our K216 stock of CRPV at three sites, i.e. nine sites total.
2.2 DNA vaccination
Rabbits were vaccinated once in each experiment. Prior to vaccination, rabbits were anesthetized with intramuscular Acepromazine (35 mg/kg) and fur was clipped from left flank (contralateral to the sites of CRPV infection). Rabbits were inoculated with the “UbE1267” DNA vaccine, an equimolar mixture of the four DNAs encoding ubiquitin-fused CRPV genes, or with the empty pcDNA3 vector as negative control. The DNAs were delivered with the aid of a helium-driven gene delivery device (PowderJect XR, Powderject Vaccines Inc., Madison, WI) to nine cutaneous sites on the left flank using 1μg DNA/0.5mg gold per site, as described previously [9].
2.3 Collection of clinical data
Rabbits were examined for papilloma formation 21, 28, 35, 42, 48 and 53 days after the CRPV infection in the first experiment, and 22, 28, 34, 42, 49, 55, 64, 70, 77 and 84 days after CRPV infection in the second experiment. At each examination, the number, location and dimensions (length, width, height) of each papilloma were recorded. The frequency of papilloma formation (number of positive sites/all sites), the time to detection (number of days between infection and the initial detection of a palpable papilloma). Papilloma volumes were calculated using the formula for an irregular sphere (4/3 * π * length/2 * width/2 * height/2).
2.4 Statistical analysis
The analysis of vaccine effects on papilloma volumes were based on cumulative volumes as in a previous study [10], due to the heterogeneity of the volume data. In first experiment an ANOVA model with censoring was used; censoring was used to deal with sites that did not form a papilloma. In the second experiment a similar ANOVA model with missing data was used. The missing data were mainly at the sites infected with the low doses of CRPV. In the ANOVA model, normality and independence were the assumptions for the papilloma volume distribution at each dose level and each group. The ANOVA model takes into consideration the similarity of volumes at the three sites at a given dose level within a rabbit. As in a previous study [10], log transformation was used before model fitting and SAS Version 9.1 was the software package for computing.
3. Results
3.1 Effects of UbE1267 DNA vaccination in rabbits with subclinical infection
We first tested whether a combination of four DNA vaccines individually encoding the CRPV UbE1, UbE2, UbE6 and UbE7 proteins [7] and here designated UbE1267 would reduce the severity of CRPV-induced disease when administered to rabbits during the subclinical stage of infection. Ten rabbits were each inoculated with three different dilutions of CRPV at three different cutaneous sites per dilution, i.e. a total of nine sites per rabbit, on the right flank. The CRPV dilutions were 1:50, 1:150 and 1:450, and represented high, moderate and low doses. Each infection site received 10 μl of virus (once). Five rabbits served as the vaccine cohort, and the remaining five as the control cohort. In the control rabbits, these infections produced papillomas with actual volumes at the end of the experiment of 1,219mm3 ± 254mm3, 584 ± 114mm3, and 345 ± 90mm3 at the high, moderate and low dose sites, respectively, for a total papilloma burden per rabbit at the end of the experiment of 6,446 ± 992 mm3 (mean actual volume ± standard error of the mean (SEM)).
Three days after CRPV inoculation, when the infection was well established but papillomas had not yet formed, the vaccine group was treated with an equimolar mixture of the four CRPV DNAs, and the control group with the empty pcDNA3 vector. The UbE1267 and control DNA vaccines were administered intracutaneously on the left flank, contralateral to the CRPV infection sites. No booster was given. As shown in Table 1, papillomas formed at 100% of infected sites in the control group. In contrast, three of the five UbE1267-vaccinated rabbits did not form any papilloma, one formed a single papilloma at a low dose site (it regressed within one week), and one formed papillomas at all sites, for an overall frequency of 22% (P=0.0139). Thus, UbE1267 DNA vaccination significantly suppressed papilloma outgrowth.
Table 1.
Effects of UbE1267 DNA vaccination during the CRPV incubation period
| Papilloma variable | Results obtained at sites infected with the following CRPV dilutions: | ||
|---|---|---|---|
| Group a | 1:50 | 1:150 | 1:450 |
| Frequency b | |||
| Vector DNA | 15 / 15 | 15 / 15 | 15 / 15 |
| UbE1267 DNA | 3 / 15 | 3 / 15 | 4 / 15 |
| Time to detection c | |||
| Vector DNA d | < 21.0 ± 0.0 | < 21.5 ± 0.5 | < 21.5 ± 0.5 |
| UbE1267 DNA e | 28.0 ± 4.0 | 35.0 ± 4.0 | 30.3 ± 2.3 |
Each group contained five rabbits.
Number of papilloma-forming sites/total number of infected sites. The papilloma frequency in the vaccinated rabbits is significantly lower than in the controls (P=0.0139).
Number of days from CRPV infection to the first detection of a palpable papilloma (mean ± standard error of the mean (SEM). Many papillomas in the vector control group actually formed earlier than 21 days, the first day that rabbits were examined after infection.
Data from the only vaccinee that formed papillomas (mean ± SEM).
Data from the only vaccinee that formed papillomas (mean ± SEM).
We next evaluated the effect of vaccination on the time between infection and the first detection of a palpable lesion. In the control rabbits, papillomas were first detected < 21 to 21.5 days after infection (Table 1). In the UbE1267 vaccinee with nine papillomas, detection was delayed by one to two weeks, a statistically significant difference from the controls (P=0.0139).
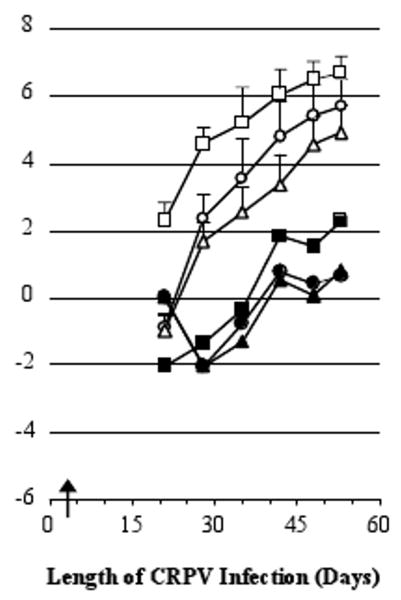
The time course of actual papilloma growth in this experiment is shown in Fig. 1. To analyze the effects of vaccination on lesion severity, cumulative papilloma volumes were used. Cumulative volume is the total papilloma burden carried by each rabbit throughout the experiment and thus is a stringent criterion for judging efficacy. Relative to the DNA vector alone, immunization with the UbE1267 DNA vaccine reduced cumulative papilloma volumes in the fifth vaccinee by ≥99.7% at the high, moderate and low dose CRPV infection sites (P < 0.0005). These results show that a single immunization with the UbE1267 DNA vaccine three days after CRPV infection inhibited all papilloma formation in three of five vaccinees, nearly inhibited all papilloma formation in a fourth vaccinee, and delayed papilloma formation and greatly reduced papilloma volumes in the fifth vaccinee.
Fig. 1.
Papilloma growth in rabbits vaccinated during the subclinical period of infection. Shown are the natural logarithms of the actual papilloma volumes in cubic millimeters (mean per site + SEM). Symbols used in this figure: (open symbols) the control group, and (closed symbols) the only vaccinated rabbit that developed more than a transient papilloma. The shapes of the symbols represent sites infected with different dilutions of CRPV virus: 1:50 (squares), 1:150 (circles) and 1:450 (triangles). The arrow marks the day of vaccination.
3.2 Effects of UbE1267 DNA vaccination in rabbits bearing papillomas
Having demonstrated strong therapeutic efficacy of the UbE1267 DNA vaccine when administered during the subclinical stage of infection, we were encouraged to test its efficacy in papilloma-bearing rabbits. Considering the severity of disease elicited in the first experiment (mean papilloma burden of >6 cm3 per rabbit) and the anticipated longer period of observation required to show efficacy in the second experiment, the doses of CRPV were lowered to 1:250, 1:1000 and 1:4000. Subsequent analysis showed no differences in papilloma outcomes at the sites infected with the two lower doses of CRPV for either group (P=0.18), suggesting that the rabbits were naturally able to control CRPV at these doses equally well. Data from these sites were therefore combined for further analysis. In the control rabbits, CRPV induced papillomas that reached volumes of 225 mm3 ± 114 mm3 and 52mm3 ± 26 mm3 at the high and lower dose sites, respectively, for a total papilloma burden per rabbit of 988mm3 ± 262mm3 at the end of the experiment (mean actual volume ± standard error).
Six rabbits were infected with CRPV and 22 days later allocated to two groups to achieve equivalent papilloma frequencies and volumes in each group, as previously described [11]. At that time, there were no significant differences between the groups with respect to either papilloma frequency or papilloma volume, as shown in Table 2. The experimental group was then treated on day 22 with theUbE1267 DNA vaccine, and the control group with the DNA vector. As in the first experiment, the vaccine was delivered to the contralateral flank relative to CRPV. No booster was given. Following vaccination, 10 additional papillomas formed in each group. Thus vaccination did not affect papilloma frequency.
Table 2.
Clinical status of rabbits prior to vaccination three weeks after CRPV infection
| Papilloma variable | Status at sites infected with the following CRPV dilutions: | ||
|---|---|---|---|
| Group a | 1:50 | 1:1000 or 1:4000 | |
| Frequency b | |||
| 1 | 9 / 9 | 16 / 18 | |
| 2 | 9 / 9 | 14 / 18 | |
| Volume c | |||
| 1 | 0.28 ± 0.17 | 0.04 ± 0.01 | |
| 2 | 0.19 ± 0.07 | 0.09 ± 0.03 | |
The rabbits were subsequently treated as controls (group 1) or vaccinees (group 2). Each group contained three rabbits.
Number of papilloma-forming sites/total number of infected sites.
Actual volume in cubic millimeters (mean ± SEM)
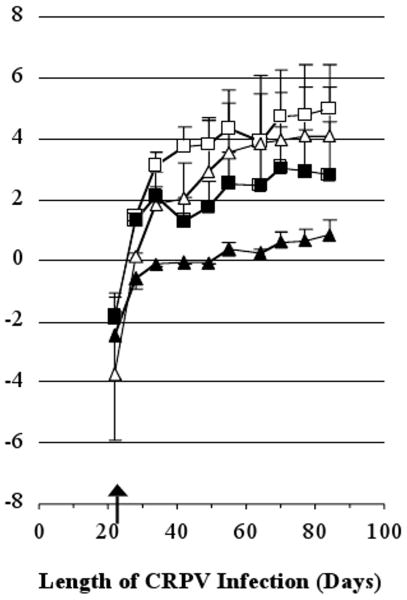
Finally, we analyzed the effect of UbE1267 DNA vaccination on papilloma volumes. Interestingly, vaccination conferred the best control over papilloma growth (greatest differences between the vaccinated and control groups) three to four weeks after immunization (Fig. 2), a reasonable time frame for the induction of immunity. Thereafter papilloma volumes remained smaller than in the vaccinees than the controls, but the relative difference did not increase. Analysis of cumulative papilloma volumes showed that the UbE1267 DNA vaccine reduced volumes by 63.0% at the high dose sites and 94.5% at the lower dose sites relative to the vector-treated controls. Statistical analysis of the differences in cumulative papilloma volumes confirmed the superiority of the UbE1267 DNA vaccine (p = 0.027). Thus, the results show that one immunization of papilloma-bearing rabbits with the UbE1267 DNA vaccine strongly suppressed papilloma growth, especially at sites infected with the lower doses of virus.
Fig. 2.
Papilloma growth in rabbits vaccinated after papilloma formation. Shown are the natural logarithms of the actual papilloma volumes in cubic millimeters (mean per site + SEM). Symbols used in this figure: (open symbols) the control group and (closed symbols) the vaccinated group. The shapes of the symbols represent sites infected with different dilutions of CRPV virus: 1:250 (squares) and either 1:1000 or 1:4000 (triangles). The arrow marks the day of vaccination.
4. Discussion
A number of therapeutic vaccine candidates targeting the HPV E6 or E7 antigens have been developed in mouse tumor transplantation models. However, when brought to clinical trials, such vaccines have provided little if any clinical benefit to human patients [16-23]. These findings indicate the need for more faithful preclinical models of high risk HPV-associated disease. The CRPV/rabbit model is a most relevant preclinical model because CRPV, like high-risk HPVs, induces viral papillomas that persist for long periods of time before progressing to squamous cell carcinoma. Previously we used this model to show that DNA vaccines encoding ubiquitin-fused versions of the CRPV early proteins E1, E2, E6 and E7 could protect rabbits against subsequent CRPV challenge [7]. The objective of the current study was to determine whether a combination of the four ubiquitin-fused CRPV genes could induce therapeutic immunity in rabbits. Despite the genetic heterogeneity among outbred animals such as rabbits and the small group sizes in the experiments, the results show that a single vaccination with a relatively small amount of UbE1267 DNA (9μg) virtually suppressed all papilloma growth in four of five immunized rabbits versus none of the controls when administered three days after CRPV infection under conditions that induced a final papilloma burden per control rabbit of >6 cm3.
It also reduced papilloma volumes in the fifth vaccinee by >99% relative to the controls. Since CRPV infection is well established within three days, the therapeutic effects were undoubtedly mediated via cytotoxic T cell (CTL) responses, although their exact nature awaits the development of in vitro CTL assays for rabbit cells.
The high level of therapeutic efficacy induced by UbE1267 DNA vaccination during the subclinical period of viral infection suggested that it might also be immunotherapeutic in rabbits with more advanced infections. To evaluate this possibility, rabbits were allowed to form papillomas prior to vaccination. They were also infected with lower CRPV doses than in the first experiment. Nevertheless, the controls still developed a mean papilloma burden of nearly 1 cm3 per rabbit. Under these conditions, UbE1267 DNA vaccination reduced papilloma volumes by 63% at the high dose sites and 94% at the lower dose sites. Clearly, the UbE1267 DNA vaccine induced therapeutic immunity. The vaccine's efficacy against lesions of longer duration or more severe pathology can be assessed in future studies.
The therapeutic responses to UbE1267 vaccination were achieved without any deliberate boosting of the immune response. However, boosting probably occurred as a natural consequence of vaccine-induced cytotoxicity, via the release of previously inaccessible viral (and cellular) antigens. We speculate that cross-presentation of such antigens [12, 13] stimulated the development of new T cell clones while simultaneously expanding existing T cell populations. One can imagine multiple rounds of “natural boosting” occurring in this fashion, each potentially enhancing the immunotherapeutic effects of the primary vaccination. Since immune responses are systemic, the extent of immune stimulation provided by natural boosting in any rabbit likely depended on the sum total amount of antigen released from its papillomas. The nature of that response, as well as its ultimate therapeutic efficacy, likely also depended on the level and particular combination of proinflammatory, immunosuppressive and tolerigenic regulatory signals in the local environment of individual papillomas. Our results are consistent with those of Han et al who treated rabbits with four DNA vaccines encoding the wild-type CRPV E1, E2, E6 and E7 proteins, also using a gene delivery device [14]. Differences between their study and ours include the time of therapeutic intervention (four months after CRPV infection vs. three days or three weeks), the nature of the vaccine genes (wild-type vs. ubiquitin-fused), their inoculation (to separate sites vs. as a mixture), the dose of DNA (80ug vs. 9ug per immunization) and the number of immunizations (six in their study vs. one in ours). Despite these differences, both studies found that DNA vaccination against the CRPV E1, E2, E6 and E7 proteins significantly reduced papilloma volumes but did not induce papilloma regression. Their study additionally showed that DNA vaccination inhibited the subsequent development of carcinomas. While our rabbits were not held long enough to develop carcinomas, they probably would have been at least partially protected because smaller lesions progress less rapidly and less frequently to carcinoma than larger lesions ([15] and unpublished data).
Therapeutic vaccination could be an ideal compliment to surgical ablation/excision of HPV-associated premalignant lesions because such lesions recur in a significant percent of treated patients [3]. The ability to induce therapeutic immunity with a single inoculation of the UbE1267 DNA vaccine provides opportunities to increase its efficacy by deliberate boosting, either with the same vaccine as done by Han et al [14] or with heterologous vectors encoding the same vaccine targets. DNA vectors can be particularly useful priming agents for other vaccines based on viral vectors [24]. Future studies will assess the ability of CRPV early gene DNA vaccines to augment therapeutic immune responses induced by recombinant adenoviruses [25] and vesicular stomatitis viruses (VSVs) [10, 11] encoding the same protein(s).
Acknowledgments
This work was supported by grants from the American Cancer Society (#TURPG MBC-100103) and the National Cancer Institute (RO1-98355).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. Journal of the National Cancer Institute. 2000;92(9):690–8. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 2.van Hamont D, van Ham MAPC, Struik-van der Zanden PHTH, Keijser KGG, Bulten J, Melchers WJG, et al. Long-term follow-up after large-loop excision of the transformation zone: evaluation of 22 years treatment of high-grade cervical intraepithelial neoplasia. International Journal of Gynecological Cancer. 2006;16(2):615–9. doi: 10.1111/j.1525-1438.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell MF, Tortolero-Luna G, Cook E, Whittaker L, Rhodes-Morris H, Silva E. A randomized clinical trial of cryotherapy, laser vaporization, and loop electrosurgical excision for treatment of squamous intraepithelial lesions of the cervix. Obstetrics & Gynecology. 1998;92(5):737–44. [PubMed] [Google Scholar]
- 4.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annual Review of Immunology. 1997;15:617–48. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll DM, Beckerleg AM. Exposing the immunology of naked DNA vaccines. Immunity. 1995;3(2):165–9. doi: 10.1016/1074-7613(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez F, Whitton JL. Enhancing DNA immunization. Virology. 2000;268(2):233–8. doi: 10.1006/viro.2000.0209. [DOI] [PubMed] [Google Scholar]
- 7.Leachman SA, Shylankevich M, Slade MD, Levine D, Sundaram RK, Xiao W, et al. Ubiquitin-fused and/or multiple early genes from cottontail rabbit papillomavirus as DNA vaccines. Journal of Virology. 2002;76(15):7616–24. doi: 10.1128/JVI.76.15.7616-7624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandsma JL. The cottontail rabbit papillomavirus model of high-risk HPV-induced disease. Methods in Molecular Medicine. 2005;119:217–35. doi: 10.1385/1-59259-982-6:217. [DOI] [PubMed] [Google Scholar]
- 9.Sundaram P, Tigelaar RE, Xiao W, Brandsma JL. Intracutaneous vaccination of rabbits with the E6 gene of cottontail rabbit papillomavirus provides partial protection against virus challenge. Vaccine. 1998;16(6):613–23. doi: 10.1016/s0264-410x(97)84510-0. [DOI] [PubMed] [Google Scholar]
- 10.Brandsma JL, Shlyankevich M, Su Y, Roberts A, Rose JK, Zelterman D, et al. Vesicular stomatitis virus (VSV)-based therapeutic vaccination targeted to the E1, E2, E6 and E7 proteins of cottontail rabbit papillomavirus (CRPV) Journal of Virology. 2007;81(11):5749–58. doi: 10.1128/JVI.02835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandsma JL, Shlyankevich M, Buonocore L, Roberts A, Becker SM, Rose JK. Therapeutic efficacy of vesicular stomatitis virus-based E6 vaccination in rabbits. Vaccine. 2007;25(4):751–62. doi: 10.1016/j.vaccine.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nature Reviews Immunology. 2001;1(2):126–34. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 13.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunological Reviews. 2005;207:166–83. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 14.Han R, Peng X, Reed CA, Cladel NM, Budgeon LR, Pickel MD, et al. Gene gun-mediated intracutaneous vaccination with papillomavirus E7 gene delays cancer development of papillomavirus-induced skin papillomas on rabbits. Cancer Detection & Prevention. 2002;26(6):458–67. doi: 10.1016/s0361-090x(02)00125-3. [DOI] [PubMed] [Google Scholar]
- 15.Rous P, K JG, B JW. Observations of the relation fo the virus causing rabbit papillomas to the cancers deriving therefrom. J Exp Med. 1936;64:385–400. doi: 10.1084/jem.64.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Hernandez E, Gonzalez-Sanchez JL, Andrade-Manzano A, Contreras ML, Padilla S, Guzman CC, et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Therapy. 2006;13(6):592–7. doi: 10.1038/sj.cgt.7700937. [DOI] [PubMed] [Google Scholar]
- 17.Palefsky JM, Berry JM, Jay N, Krogstad M, Da Costa M, Darragh TM, et al. A trial of SGN-00101 (HspE7) to treat high-grade anal intraepithelial neoplasia in HIV-positive individuals. AIDS. 2006;20(8):1151–5. doi: 10.1097/01.aids.0000226955.02719.26. [DOI] [PubMed] [Google Scholar]
- 18.Frazer IH, Quinn M, Nicklin JL, Tan J, Perrin LC, Ng P, et al. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine. 2004;23(2):172–81. doi: 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Davidson EJ, Faulkner RL, Sehr P, Pawlita M, Smyth LJC, Burt DJ, et al. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7) Vaccine. 2004;22(2122):2722–9. doi: 10.1016/j.vaccine.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 20.Hallez S, Simon P, Maudoux F, Doyen J, Noel JC, Beliard A, et al. Phase I/II trial of immunogenicity of a human papillomavirus (HPV) type 16 E7 protein-based vaccine in women with oncogenic HPV-positive cervical intraepithelial neoplasia. Cancer Immunology, Immunotherapy. 2004;53(7):642–50. doi: 10.1007/s00262-004-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldwin PJ, van der Burg SH, Boswell CM, Offringa R, Hickling JK, Dobson J, et al. Vaccinia-expressed human papillomavirus 16 and 18 E6 and E7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clinical Cancer Research. 2003;9(14):5205–13. [PubMed] [Google Scholar]
- 22.Muderspach L, Wilczynski S, Roman L, Bade L, Felix J, Small LA, et al. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clinical Cancer Research. 2000;6(9):3406–16. [PubMed] [Google Scholar]
- 23.Steller MA, Gurski KJ, Murakami M, Daniel RW, Shah KV, Celis E, et al. Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clinical Cancer Research. 1998;4(9):2103–9. [PubMed] [Google Scholar]
- 24.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends in Immunology. 2004;25(2):98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Brandsma JL, Shlyankevich M, Zhang L, Slade MD, Goodwin EC, Peh W, et al. Vaccination of rabbits with an adenovirus vector expressing the papillomavirus E2 protein leads to clearance of papillomas and infection. Journal of Virology. 2004;78(1):116–23. doi: 10.1128/JVI.78.1.116-123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]