Summary
Various lines of evidence reveal bilateral activation of somatosensory areas after unilateral stimulation [1-6] assumed to be mediated by cross-hemispheric connections [7-11]. Despite evidence of bilateral activity in response to unilateral stimulation, neurologically intact humans do not experience bilateral percepts when stimulated on one side of the body. This may be due to active suppression of ipsilateral neural activity [12, 13] by inhibitory mechanisms whose functioning is poorly understood. We describe an individual with left fronto-parietal damage who experiences bilateral sensations in response to unilateral tactile stimulation—a rarely reported condition known as synchiria (previously described in visual [14], auditory [15] and somatosensory modalities [16-19]). Presumably the phantom sensations result from normal bilateral cross-hemispheric activation, combined with a failure of inhibitory mechanisms to prevent bilateral perceptual experiences. The disruption of these mechanisms provides a valuable opportunity to examine their internal functioning. We find that the synchiria rate is affected by hand position relative to multiple reference frames. Specifically, synchiria decreases as the hands move from right (contralesional) to left (ipsilesional) space in trunk- and head-centered reference frames and disappears when the hands are crossed. These findings provide, for the first time, evidence that the mechanisms that inhibit bilateral percepts operate in multiple reference frames [20-27].
Results and Discussion
Case Report
DLE was a 71-year old left-handed male engineer who suffered a left middle cerebral artery infarct three years before this investigation. The CVA resulted in written and spoken language production deficits and right hemiparesis affecting both upper and lower limbs. MRI revealed damage to the entire inferior frontal gyrus, insula and much of the precentral gyrus, sparing the superior frontal gyrus, a significant portion of the middle frontal gyrus and the medial surface of the frontal lobe. In the parietal lobe there was severe damage to the post-central gyrus, the supramarginal gyrus and the anterior portion of the angular gyrus, sparing the superior region of the post-central gyrus, the medial surface of the parietal lobe, the superior parietal lobule and the posterior angular gyrus. The occipital lobe was spared and damage to the temporal lobe was restricted to the posterior/superior portions of the superior temporal gyrus. There was left thalamic damage, principally affecting the lateral, anterior portion (see Figure 1 in Supplemental Data for MRI images).
Figure 1. Results from unilateral-left and bilateral stimulation trials in Experiment 1.
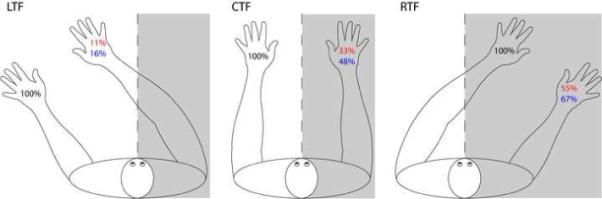
Percent correct responses for the left hand on unilateral-left trials (black), percent synchiric responses on the right hand on unilateral-left trials (red) and percent “correct” responses (including right hand stimulation) on bilateral stimulation trials (blue). Grey shading = contralesional space relative to the trunk midline. CTF (center trunk field condition) = left hand positioned to the left of the trunk midline; the right hand positioned at the symmetrical location on the right of the trunk midline, the middle finger of each hand positioned approximately 20 cm from the trunk midline. LTF (left trunk field condition) = both hands positioned approximately 30 cm left of their position in CTF condition, such that both hands were to the left of the trunk midline. RTF (right trunk field condition) = hands positioned approximately 30 cm to the right of their position in CTF condition, such that both hands were to the right of the trunk midline. The head was always aligned with the trunk midline. Stimulation: 14 blocks in LTF and RTF, 10 blocks in CTF.
Basic Evaluation: Mislocalization, synchiria and extinction
In two evaluation tasks, stimulation was always a light touch with a flat, rubber cylinder (5 mm diameter). In Task 1, stimulation was presented to the dorsal surface of the distal segment of the middle finger; in Task 2, designed to gather more detailed localization information, stimulation was presented to one of 22 locations covering the dorsal hand surface (see Supplemental Data). Trials were unilateral (left or right hand stimulation only) or bilateral; Task 1 additionally included “no stimulation” trials. Stimuli were presented to DLE (eyes closed) with hands flat on the table, on either side of his body midline and he reported stimulation location (eyes open). In Task 1, he indicated if stimulation had occurred on the right, left, both hands, or neither; in Task 2, he indicated the specific location of stimulation (see Supplemental Data for additional methodological details).
Three significant abnormalities were identified: (1) On unilateral right hand trials, while DLE was highly accurate in detecting the presence of right hand stimulation (98%, 131/134), he was highly inaccurate in his perception of its location producing a mean displacement of localization judgments of 34.8 mm along the y-axis (running distal-proximal through the stimulation point) and 29.7 mm along the x-axis (perpendicular to the y-axis through the stimulation point). In fact, virtually all right hand stimuli were perceived as originating on the 3rd and 4th fingers (see Figure 2c in the Supplemental Data). This contrasted with relatively accurate localization of left hand (ipsilesional) stimulation (mean displacement of 9.8 mm along the y-axis and 1.5 mm along the x-axis). (2) On unilateral left hand trials, DLE always reported stimulation to the left hand (134/134) but he also reported synchiric, phantom sensations on the right hand for a total of 50% synchiric trials (67/134). Synchiria was also reported subsequent to unilateral left-side stimulation of other skin surfaces: (forearm, 77%; biceps, 66%; chest under breastbone, 50%; cheek, 69%; thigh, 76%; ankle, 60%). No synchiria was reported in visual or auditory modalities. (3) On bilateral stimulation trials, although left hand stimulation was reported with 100% accuracy (178/178), right hand stimulation was not reported on 28% of trials (49/178). The failure to perceive the contralesional stimulus under bilateral stimulation conditions is referred to as “extinction”. It is worth noting that extinction rates are likely to have been underestimated because at least some apparently “correct” responses on bilateral trials may have been based on “illusory” right hand sensations. Experiments 1−3 will support and expand upon this interpretation.
Figure 2. Results from unilateral-left and bilateral stimulation trials in Experiment 2.
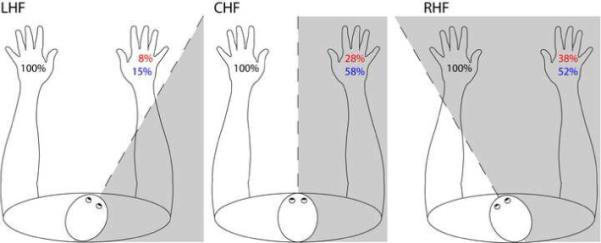
Percent correct responses for the left hand on unilateral-left trials (black), percent synchiric responses on the right hand on unilateral-left trials (red) and percent “correct” responses (including right-hand responses) on bilateral stimulation trials (blue). Grey shading = contralesional space relative to the head midline. DLE's hands and trunk remained in the same position with trunk facing forward and hands approximately 20 cm to the right or left of the trunk midline. LHF (left head field condition) = head turned approximately 30 degrees to the right of the trunk midline such that both hands were on the left side of the head midline. RHF (right head field condition) = head turned approximately 30 degrees to the left of the trunk midline such that both hands were positioned to the right of the head midline. Order of testing alternated between LHF and RHF, both within and across sessions. Stimulation: 4 blocks in the CHF, and 6 blocks each in LHF and RHF.
The mislocalization and extinction of stimulation to the right hand are consistent with damage to basic mechanisms of somatosensory representation and/or attention. The synchiric phenomena suggest disruption to inhibitory mechanisms concerned with at least some aspects of inter-hemispheric activation. We examined the frame/s of reference within which these inhibitory mechanisms operate by evaluating if the rate of synchiric experiences was modulated by the position of the hands relative to the trunk midline (Experiment 1), head midline (Experiment 2) and the position of the right hand relative to the left hand (Experiment 3). Stimulation and testing conditions were as described above for Task 1, where stimulation was limited to the dorsal segment of the middle finger.
Experiment 1: Trunk-midline reference frame
Stimuli were presented with hands in three positions relative to the trunk midline (see Figure 1). For unilateral-right and no-stimulation trials, results are combined over all three trunk field conditions as there were no significant differences between them, yielding the following accuracy levels: unilateral-right: 89%, 338/380; no-stimulation trials: 92%, 199/216. In contrast, on unilateral-left stimulation trials, synchiric errors decreased as DLE's hands moved from right to left with respect to the trunk midline (see Figure 1). Specifically, DLE experienced synchiric phantoms on 55% (77/140) of trials when his hands were positioned to the right of the trunk midline (RTF) and on only 11% (15/140) of trials when his hands were positioned to the left of the trunk midline (LTF) (LTF v center trunk field (CTF), χ2 = 18.1, p < .001; LTF v RTF, χ2 = 62.2, p < .001; CTF v RTF, χ2 = 11.4, p < .001).
With regard to bilateral stimulation trials, if, as suggested above, left hand stimulation often produces a synchiric rather than veridical percept on the right side then, as conditions favoring the generation of synchiric percepts change, accuracy on bilateral stimulation trials should also change. Specifically, as synchiria decreases, apparent accuracy on bilateral stimulation trials should also decrease. This prediction was confirmed (Figure 1) as DLE's apparent accuracy on bilateral trials with hands in the left trunk field (where synchiria was at its lowest rate) was only 16% (22/140) compared to 67% (94/140) with hands in the right trunk field (where synchiria was at its highest rate) (LTF v CTF, χ2 = 29.4 p < .001; LTF v RTF, χ2 = 75.6, p < .001; CTF v RTF, χ2 = 8.85, p = .003). This provides clear confirmation of the hypothesis that right-sided percepts on bilateral stimulation trials were often synchiric phantoms.
The coupling of head and trunk midlines in this experiment makes it impossible to determine whether the relevant reference frame was based on the trunk or head midline, or both. Additional data from Experiment 2 were used to resolve this question.
Experiment 2: Head midline frame of reference
Stimuli were presented with hands in three positions relative to the head midline (see Figure 2). Again, for unilateral-right and no-stimulation trials, results were combined over all three trunk field conditions as there were no significant differences between them, yielding the following accuracy levels: unilateral-right: 96% (153/160); no-stimulation trials: 95% (91/96). On unilateral-left trials, the rate of synchiric errors was determined by the position of the hands relative to the head midline. Figure 2 indicates that DLE reported fewer synchiric percepts on trials in which his hands were to the left of his head midline (LHF, 8%, 5/60) compared to trials on which hands straddled the head midline (CHF, 28% (11/40), χ2 = 6.56, p = .01) or when they were positioned in right head field (RHF, 38% (23/60), χ2 = 15.1, p < .001). These results provide clear evidence of the involvement of a head-based reference frame.
If we consider data from both Experiments 1 and 2 we can uncouple head and trunk midlines to determine if, in addition to a head-based frame of reference, there was also involvement of a trunk-based reference frame. To do so, we compared accuracy between RHF trials in Experiment 2 (with hands in center trunk-space and right head-space) and the RTF trials from Experiment 1 (with hands in right trunk-space and right head-space) (see Figures 1 and 2). In these two conditions, hand position relative to head is constant (hands to the right of head) while hand position relative to the trunk midline changes (hands at the center or to the right of the trunk midline). If position of the hands relative to the trunk midline is relevant, there should be significantly more synchiric errors in the right trunk-field condition compared to the right head-field condition. Consistent with this prediction, DLE made significantly more synchiric errors in the right trunk field (55%) versus right head field (38%) condition (RTF v RHF, χ2 = 4.67, p < .031). The combined results of Experiments 1 and 2, therefore, reveal the influence of both head- and trunk-centered reference frames on synchiric perception.
On bilateral trials, we observed the same pattern as was reported in Experiment 1 (Figure 2) with lower “accuracy” under conditions of low synchiria, such that accuracy was lower when hands were placed to the left relative to the head midline (15% accuracy, 9/60) than when they were straddling the head midline (58% accuracy , 23/40) or placed to the right of the head midline (52% accuracy, 31/60) (LHF v CHF, χ2 = 19.9, p < .001 and LHF v RHF, χ2 = 18.2, p < .001). Furthermore, with regard to the question of the role of both head and trunk-based reference frames, we found that on bilateral trials DLE was significantly more accurate under RHF vs. RTF conditions when hands were always to the right of the head midline but varied with respect to trunk midline (RTF v RHF, χ2 = 4.29, p < .038).
Experiment 3: Limb-relative frame of reference
Previous work has reported that tactile extinction rates may be modulated by crossing of the hands [22, 23, 28] - a manipulation that always positions the right hand to the left of the left hand in a limb-relative reference frame. Given that results from Experiments 1 and 2 indicate the sensitivity of DLE's synchiric percepts to right-left hand positioning, we examined the possible modulation of the synchiric effect by relative hand position by using the same trunk-field conditions as in Experiment 1, except that DLE's hands were crossed in all conditions. Furthermore, to determine if hand crossing per se affected synchiric perception or if effects were restricted to the crossed limbs, we included blocks of hand-crossed trials in which the upper (uncrossed) arms were stimulated.
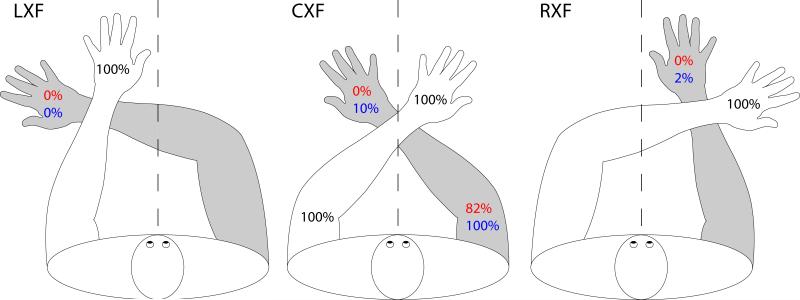
As indicated in Figure 3, when DLE's arms were crossed and stimulation was delivered to the finger, DLE demonstrated no synchiria, being 100% (140/140) accurate on every unilateral-left trial, regardless of the position of the crossed hands relative to the trunk midline. With regard to the upper-arm stimulation, DLE reported synchiric phantoms on his right (contralesional) upper arm on 83% (33/40) of trials on which the left upper arm was stimulated (unilateral-left hand versus unilateral-left forearm stimulation trials in the CXF: χ2 = 56.2, p < .001).
Figure 3. Results from unilateral-left and bilateral stimulation trials in Experiment 3.
Percent correct responses for the left hand on unilateral-left trials (black), percent synchiric responses on the right hand on unilateral-left trials (red) and percent “correct” responses (including right hand stimulation) on bilateral stimulation trials (blue). Grey shading = the contralesional arm. Hands were crossed approximately 8 cm above the wrist joint and placed on the table, with trunk and head aligned with one another in the forward-facing position. CXF (crossed center body field condition) = crossed hands straddled the body midline. LXF (crossed left) and RXF (crossed right) body field conditions = both hands were crossed and the midpoint between hands was 30 cm to the left or right of the body midline. Stimulation: 5 blocks of trials each in LXF and RXF, and 8 blocks in CXF. In CXF: 4 blocks with stimulation to the dorsal surface of the distal segment of the middle finger/s and 4 blocks with stimulation to the dorsal surface of the uncrossed upper forearms.
Given the dramatic reduction of synchiric percepts with the hand crossing manipulation, and the evidence from the previous two experiments that accuracy on bilateral trials is based, at least in part, on illusory synchiric percepts, we would predict very poor accuracy on bilateral crossed-hands stimulation trials. As indicated in Figure 3, DLE was only 4% (5/140) accurate on bilateral stimulation trials, with all but one of the responses consisting of extinction of the stimulus presented to the right hand.
Discussion
The primary and novel empirical finding of this research is that the degree of right hand synchiria and extinction are modulated by the position of the right hand as defined by multiple reference frames. In addition, we have reported disruption of localization, but not awareness of, unilateral right hand stimulation. To provide an account for these findings, we propose a schematic functional architecture of somatosensory processing and posit specific disruptions (Figure 4). All of the assumptions we make in this proposal have independent motivation, discussed earlier in this paper, here we bring them together in a way that allows us to provide an account of our findings. We acknowledge that given the still relatively scarce findings in this area, other architectures are likely to also be consistent with the current evidence. Finally, note that we refer primarily to functional processing mechanisms, making only general reference to their neural substrates, as the lesion evidence does not allow fine-grained conclusions in this regard.
Figure 4. Shift in hemispheric control of inhibition of bilateral percepts under conditions of left-hand stimulation.
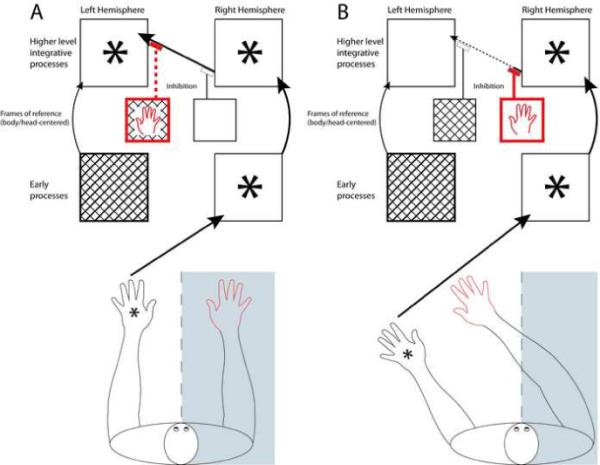
A schematic of somatosensory processes under conditions of left-hand stimulation when the right hand is in contralesional (4a) compared to ipsilesional (4b) space relative to the trunk midline. Note that we include only the mechanisms and connectivity required to highlight the shift in hemispheric control that takes place under these circumstances. In each hemisphere, the lower large box represents early cortical somatosensory processes while the upper large box represents higher level integrative processes involved in body image and tactile awareness. The small boxes represent left and right hemisphere mechanisms involved in frame of reference representation and processing; these contribute to the inhibition of bilateral percepts from cross-hemispheric transmission of somatosensory information (as indicated by flat- topped arrows). Note that for clarity, only one reference frame mechanism is shown (trunk- or head-centered), although we propose that at least three separate mechanisms (trunk-centered, head-centered, and limb-relative) operate in a similar manner. Cross-hatched boxes and dashed lines indicate damaged mechanisms; we assume that DLE has damage to early left hemisphere somatosensory processes and inhibitory processes, but that at least some higher level processes are spared. Asterisk represents a stimulus. Thick red arrows and outlining identify the critical aspects of processing related to the unstimulated right hand; thick black arrows and outlining are used for critical aspects of processing related to the stimulated left hand.
In Figure 4a, DLE's unstimulated right hand is in right space relative to trunk and head midlines. This information is represented in the contralateral (and damaged) left hemisphere. Under those conditions, the inhibitory mechanisms responsible for inhibiting the bilateral percept are likely to fail and a synchiric phantom sensation on the right hand will be experienced. In Figure 4b, the right hand is in left space relative to head and body midlines. This information is represented in the contralateral (and intact) right hemisphere, resulting in the successful inhibition of the spurious bilateral percept.
The finding that DLE can accurately detect, but not localize, stimuli presented to his contralesional (right) hand is explained by assuming that detection and localization of right hand stimulation rely on damaged left hemisphere somatosensory substrates in SI and SII (indicated in Figure 4 as “early processes”). The remaining, limited substrates may be sufficient to support detection accuracy (of above threshold stimuli), but insufficient for precise localization, producing gross distortions in the perception of stimulus location, as was proposed by Rapp et al. [29]. Furthermore, it is plausible that this damage also makes DLE susceptible to extinction of right hand percepts under conditions of bilateral stimulation.
Given previous evidence indicating bilateral activity in response to unilateral stimulation at a number of levels of the somatosensory system (including but not limited to superior and posterior regions of parietal cortex [5, 6, 30], depicted in Figure 4 as “higher level integrative processes”), we assume that the synchiric percepts experienced by DLE result, at least in part, from a failure to appropriately inhibit this bilateral activity. On the basis of our findings, we propose that these inhibitory mechanisms operate in a number of reference frames (trunk-centered, head-centered, and limb-relative). Furthermore, we propose that hemispheric control of inhibition is driven by the location of the hands as defined by these frames of reference, such that the hemisphere contralateral to the relative location of the unstimulated hand drives the inhibitory processes. As a result, when the right hand is in on the right side (as defined by a given reference frame), it relies on the contralateral left hemisphere inhibitory processes to suppress spurious percepts. In DLE's case, under these conditions, the damaged left hemisphere cannot carry out its inhibitory functions and synchiric, right-hand phantoms are experienced (see Figure 4a). In contrast, when the right hand is in left space, intact right-hemisphere inhibitory processes are utilized and are able to more successfully suppress the synchiric percepts (see Figure 4b). When the hands are crossed, the right hand is always on the left side in limb-relative space and, therefore, the intact right-hemisphere inhibitory mechanisms are engaged; this results in virtually no synchiric experiences and reveals the full extent of the extinction of right-hand percepts under conditions of bilateral stimulation. We make no specific claims regarding inputs to these inhibitory mechanisms, other than they are multiple and represent limb location in multiple frames of reference [25].
It is generally assumed that higher levels of somatosensory processing and representation produce a unified body image percept that allows us to move through space and interact with the world. Such a representation is developed on the basis of rich and complex computations that constantly integrate and update information from a variety of intra- and interhemispheric sources regarding the organism's somatosensory haptic, proprioceptive, and other sensory experiences. The results of this investigation contribute to our understanding of this complex process by providing evidence for existing claims that unilateral stimulation results in bilateral activation and that cross-hemispheric inhibitory mechanisms may be involved in preventing bilateral percepts. Furthermore, this research provides novel evidence that the hemispheric control of these inhibitory processes is determined by the position of a limb in one or more frames of reference that define tactile locations relative to head and trunk midlines as well as the position of the hands relative to one another.
Supplementary Material
Acknowledgements
The research reported was supported by NIDCD: DC006740 to BR. We would like to thank Michael McCloskey for his helpful feedback on theory and method, Erin Zaroukian for her technical assistance, and DLE and his wife for their invaluable time and dedication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hansson T, Brismar T. Tactile stimulation of the hand causes bilateral cortical activation: a functional magnetic resonance study in humans. Neuroscience Letters. 1999;271:29–32. doi: 10.1016/s0304-3940(99)00508-x. [DOI] [PubMed] [Google Scholar]
- 2.Korvenoja A, Huttunen J, Salli E, Pohjonen H, Martinkauppi S, Palva LM, Lauronen L, Virtanen J, Ilmoniemi RJ, Aronen HJ. Activation of multiple cortical areas in response to somatosensory stimulation: Combined magnetoencephalographic and functional magnetic resonance imaging. Human Brain Mapping. 1999;8:13–27. doi: 10.1002/(SICI)1097-0193(1999)8:1<13::AID-HBM2>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noachtar S, Luders HO, Dinner DS, Klem G. Ipsilateral median somatosensory evoked potentials recorded from human somatosensory cortex. Electroencephalography and Clinical Neurophysiology. 1997;104:189–198. doi: 10.1016/s0168-5597(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 4.Maldijan JA, Gottschalk A, Patel RS, Pincus D, Detre JA, Alsop DC. Mapping of secondary somatosensory cortex activation induced by vibrational stimulation: an fMRI study. Brain Research. 1999;824:291–295. doi: 10.1016/s0006-8993(99)01126-9. [DOI] [PubMed] [Google Scholar]
- 5.Ruben J, Schwiemann J, Deuchert M, Meyer R, Krause T, Curio G, Villringer K, Kurth R, Villringer A. Somatotopic organization of human secondary somatosensory cortex. Cerebral Cortex. 2001;11:463–473. doi: 10.1093/cercor/11.5.463. [DOI] [PubMed] [Google Scholar]
- 6.Polonara G, Fabri M, Manzoni T, Salvolini U. Localization of the first and second somatosensory areas in the human cerebral cortex with functional MR imaging. American Journal Of Neuroradiology. 1999;20:199–205. [PMC free article] [PubMed] [Google Scholar]
- 7.Fabri M, Polonara G, Del Pesce M, Quattrini A, Salvolini U, Manzoni T. Posterior Corpus Callosum and Interhemispheric Transfer of Somatosensory Information: An fMRI and Neuropsychological Study of a Partially Callosotomized Patient. Journal of Cognitive Neuroscience. 2001;13:1071–1079. doi: 10.1162/089892901753294365. [DOI] [PubMed] [Google Scholar]
- 8.Stancak A, Hoechstetter K, Tintera J, Vrana J, Rachmanova R, Kralik J, Scherg M. Source activity in the human secondary somatosensory cortex depends on the size of corpus callosum. Brain Research. 2002;936:47–57. doi: 10.1016/s0006-8993(02)02502-7. [DOI] [PubMed] [Google Scholar]
- 9.Iwamura Y, Iriki A, Tanaka M. Bilateral hand representation in the postcentral somatosensory cortex. Nature. 1994;369:554–556. doi: 10.1038/369554a0. [DOI] [PubMed] [Google Scholar]
- 10.Fabri M, Polonara G, Quattrini A, Salvolini U, Del Pesce M, Manzoni T. Role of the corpus callosum in the somatosensory activation of the ipsilateral cerebral cortex: an fMRI study of callosotomized patients. European Journal of Neuroscience. 1999;11:3983–3994. doi: 10.1046/j.1460-9568.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- 11.Krubitzer L, Clarey JC, Tweedale R, Calford MB. Interhemispheric Connections of Somatosensory Cortex in the Flying Fox. The Journal of Comparative Neurology. 1998;402:538–559. [PubMed] [Google Scholar]
- 12.Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. Journal Of Neuroscience. 2006;26:5819–5824. doi: 10.1523/JNEUROSCI.5536-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipton ML, Fu K-MG, Branch CA, Schroeder CE. Ipsilateral hand input to area 3b revealed by converging hemodynamic and electrophysiological analyses in macaque monkeys. The Journal of Neuroscience. 2006;26:180–185. doi: 10.1523/JNEUROSCI.1073-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halligan PW, Marshall JC, Wade DT. Left on the right - Allochiria in a case of left visuospatial neglect. Journal of Neurology Neurosurgery and Psychiatry. 1992;55:717–719. doi: 10.1136/jnnp.55.8.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond SP, Bender MB. On auditory extinction and alloacusis. The Journal of Comparative Neurology. 1965;90:154–157. [PubMed] [Google Scholar]
- 16.Janet P. Nevroses et idees fixed. Vol. 1. Felix Alcan; Paris: 1898. [Google Scholar]
- 17.Drinkwater H. Obligatory bi-manual synergia with allocheiria in a boy otherwise normal. Proceedings of the XVIIth International Congress of Medicine. 1913;11:117–124. [Google Scholar]
- 18.Kramer F. Bulbarapoplexie (Verschluss der Arteria cerebelli posterior inferior) mit Alloasthesie. Zeitschrift Fur Die Gesamte Neurologie und Psychiatre (Referate) 1917;14:58–60. [Google Scholar]
- 19.Sathian K. Intermanual referral of sensation to anesthetic hands. Neurology. 2000;54:1866–1868. doi: 10.1212/wnl.54.9.1866. [DOI] [PubMed] [Google Scholar]
- 20.Moscovitch M, Behrmann M. Coding of Spatial Information in the Somatosensory System - Evidence from Patients with Neglect Following Parietal Lobe Damage. Journal of Cognitive Neuroscience. 1994;6:151–155. doi: 10.1162/jocn.1994.6.2.151. [DOI] [PubMed] [Google Scholar]
- 21.Tinazzi M, Ferrari G, Zampini M, Aglioti SM. Neuropsychological evidence that somatic stimuli are spatially coded according to multiple frames of reference in a stroke patient with tactile extinction. Neuroscience Letters. 2000;287:133–136. doi: 10.1016/s0304-3940(00)01157-5. [DOI] [PubMed] [Google Scholar]
- 22.Smania N, Aglioti S. Sensory and Spatial Components of Somatesthetic Deficits Following Right Brain-Damage. Neurology. 1995;45:1725–1730. doi: 10.1212/wnl.45.9.1725. [DOI] [PubMed] [Google Scholar]
- 23.Aglioti S, Smania N, Peru A. Frames of reference for mapping tactile stimuli in brain-damaged patients. Journal of Cognitive Neuroscience. 1999;11:67–79. doi: 10.1162/089892999563256. [DOI] [PubMed] [Google Scholar]
- 24.Berti A, Oxbury S, Oxbury J, Affanni P, Umilta C, Orlandi L. Somatosensory extinction for meaningful objects in a patient with right hemispheric stroke. Neuropsychologia. 1999;37:333–343. doi: 10.1016/s0028-3932(98)00077-3. [DOI] [PubMed] [Google Scholar]
- 25.Lacquianiti F, Guigon E, Bianchi L, Ferraina S, Caminiti R. Representing spatial information for limb movement: Role of area 5 in the monkey. Cerebral Cortex. 1995;5:391–409. doi: 10.1093/cercor/5.5.391. [DOI] [PubMed] [Google Scholar]
- 26.Prather SC, Sathian K. Mental rotation of tactile stimuli. Cognitive Brain Research. 2002;14:91–98. doi: 10.1016/s0926-6410(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 27.Buneo CA, Andersen RA. The posterior parietal cortex: Sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Bartolomeo P, Perri R, Gainotti G. The influence of limb crossing on left tactile extinction. Journal of Neurology Neurosurgery and Psychiatry. 2004;75:49–55. [PMC free article] [PubMed] [Google Scholar]
- 29.Rapp B, Hendel SK, Medina J. Remodeling of somatosensory hand representations following cerebral lesions in humans. Neuroreport. 2002;13:207–211. doi: 10.1097/00001756-200202110-00007. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi M, Takeda K, Kaminaga T, Shimizu T, Iwata M. Neural consequences of somatosensory extinction - An fMRI study. Journal Of Neurology. 2005;252:1353–1358. doi: 10.1007/s00415-005-0865-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.