Abstract
Endogenous activities of phospholipases A and C in Ureaplasma urealyticum were assayed in cellular fractions of exponential-phase cells. Enzymatic studies indicated that ATPase activity was localized in the plasma membrane fraction and NADH and NADPH dehydrogenase activities were localized in the cytosol fraction. Studies with purified ureaplasma membranes demonstrated that, of three serovars tested, endogenous phospholipase A1, A2, and C activities were localized in the plasma membrane. Very low levels of activity were observed in the cytosol fractions. Phospholipase A2 activity in the plasma membrane was 3- to 5-fold higher than the activity in the lysates and 60- to 300-fold higher than the activity of phospholipase A1. Phospholipase C was localized mainly in the plasma membrane, with 20% found in the cytosol fraction. The levels of activity were comparable among the three serovars. There was a significantly lower level of activity in cells from the stationary growth phase than in the exponential phase. Significant differences were observed in the phospholipase A activities among the U. urealyticum serovars 3, 4, and 8. Phospholipase A2 activity was twofold higher in serovar 8 membranes, and phospholipase A1 activity was twofold higher in serovar 3 membranes. These results demonstrate that endogenous activities of phospholipase A and C are localized primarily in the plasma membrane fraction of U. urealyticum. The specific activities in the membranes of the phospholipases varied among the three serovars. Phospholipase enzymes may function as virulence factors in U. urealyticum and may vary among the serovars.
Full text
PDF

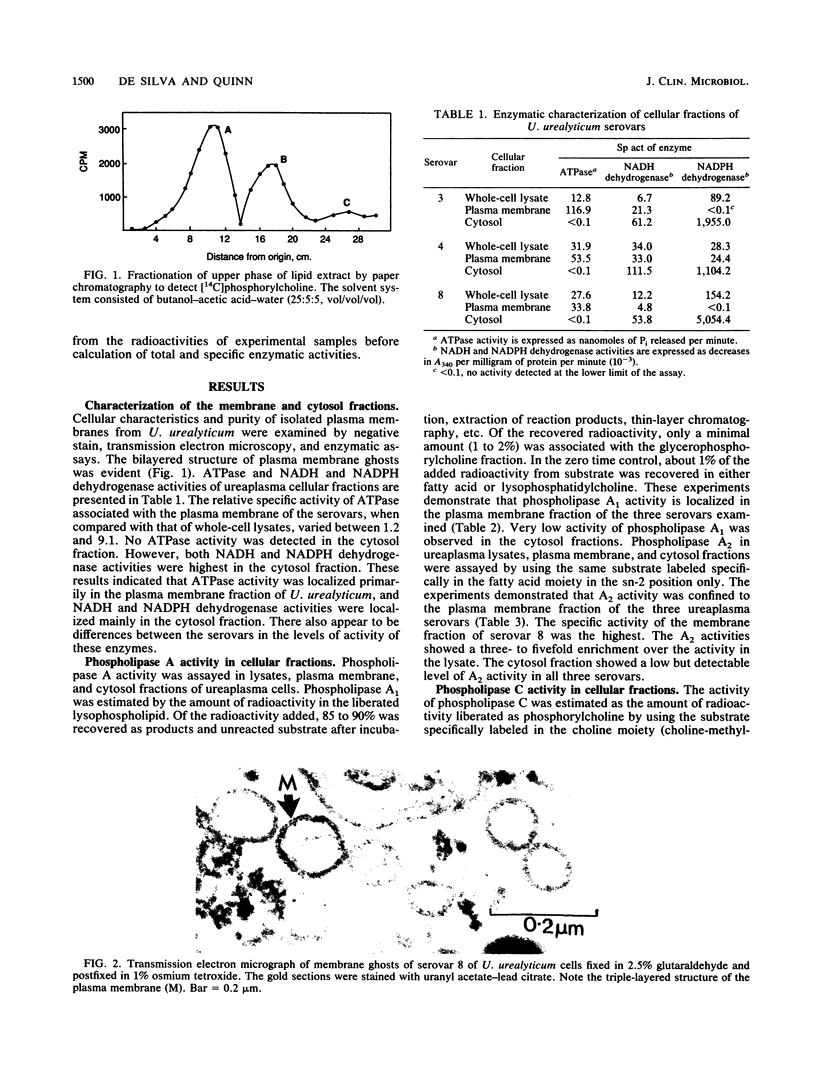
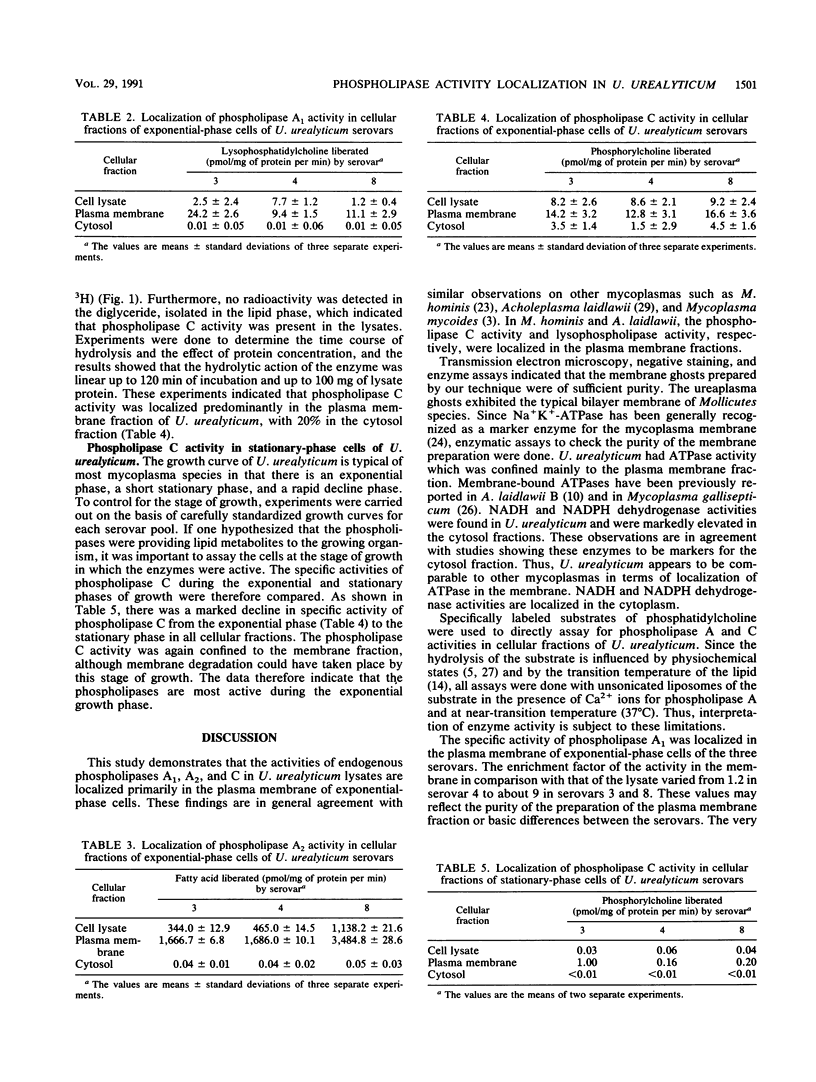


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baine W. B., Rasheed J. K., Mackel D. C., Bopp C. A., Wells J. G., Kaufmann A. F. Exotoxin activity associated with the Legionnaires disease bacterium. J Clin Microbiol. 1979 Mar;9(3):453–456. doi: 10.1128/jcm.9.3.453-456.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejar R., Curbelo V., Davis C., Gluck L. Premature labor. II. Bacterial sources of phospholipase. Obstet Gynecol. 1981 Apr;57(4):479–482. [PubMed] [Google Scholar]
- Bhandari S., Asnani P. J. Characterization of phospholipase A2 of mycoplasma species. Folia Microbiol (Praha) 1989;34(4):294–301. doi: 10.1007/BF02814471. [DOI] [PubMed] [Google Scholar]
- Busolo F., Zanchetta R. The effect of Mycoplasma hominis and Ureaplasma urealyticum on hamster egg in vitro penetration by human spermatozoa. Fertil Steril. 1985 Jan;43(1):110–114. doi: 10.1016/s0015-0282(16)48327-5. [DOI] [PubMed] [Google Scholar]
- De Silva N. S., Quinn P. A. Endogenous activity of phospholipases A and C in Ureaplasma urealyticum. J Clin Microbiol. 1986 Feb;23(2):354–359. doi: 10.1128/jcm.23.2.354-359.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva N. S., Siu C. H. Preferential incorporation of phospholipids into plasma membranes during cell aggregation of Dictyostelium discoideum. J Biol Chem. 1980 Sep 25;255(18):8489–8496. [PubMed] [Google Scholar]
- Gross Z., Rottem S. Lipid interconversions in aging Mycoplasma capricolum cultures. J Bacteriol. 1986 Sep;167(3):986–991. doi: 10.1128/jb.167.3.986-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDS W. E. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J Biol Chem. 1960 Aug;235:2233–2237. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Purification and characterization of the membrane (Na+ + Mg2+)-ATPase from Acholeplasma laidlawii B. Biochim Biophys Acta. 1983 Oct 26;735(1):113–122. doi: 10.1016/0005-2736(83)90266-3. [DOI] [PubMed] [Google Scholar]
- Lingwood C., Schramayr S., Quinn P. Male germ cell specific sulfogalactoglycerolipid is recognized and degraded by mycoplasmas associated with male infertility. J Cell Physiol. 1990 Jan;142(1):170–176. doi: 10.1002/jcp.1041420121. [DOI] [PubMed] [Google Scholar]
- Ne'eman Z., Razin S. Characterization of the mycoplasma membrane proteins. V. Release and localization of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1975 Jan 14;375(1):54–68. doi: 10.1016/0005-2736(75)90072-3. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A., de Gier J., van Deenen L. L. Hydrolysis of phosphatidylcholine liposomes by pancreatic phospholipase A2 at the transition temperature. Biochim Biophys Acta. 1974 Apr 29;345(2):253–256. doi: 10.1016/0005-2736(74)90263-6. [DOI] [PubMed] [Google Scholar]
- Pollack J. D. Metabolic distinctiveness of ureaplasmas. Pediatr Infect Dis. 1986 Nov-Dec;5(6 Suppl):S305–S307. doi: 10.1097/00006454-198611010-00023. [DOI] [PubMed] [Google Scholar]
- Quinn P. A., Gillan J. E., Markestad T., St John M. A., Daneman A., Lie K. I., Li H. C., Czegledy-Nagy E., Klein A. Intrauterine infection with Ureaplasma urealyticum as a cause of fatal neonatal pneumonia. Pediatr Infect Dis. 1985 Sep-Oct;4(5):538–543. doi: 10.1097/00006454-198509000-00020. [DOI] [PubMed] [Google Scholar]
- Quinn P. A. Mycoplasma infection of the fetus and newborn. Prog Clin Biol Res. 1988;281:107–151. [PubMed] [Google Scholar]
- Quinn P. A., Rubin S., Nocilla D. M., Read S. E., Chipman M. Serological evidence of Ureaplasma urealyticum infection in neonatal respiratory disease. Yale J Biol Med. 1983 Sep-Dec;56(5-6):565–572. [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Hasin M., Razin S. Differences in susceptibility to phospholipase C of free and membrane-bound phospholipids of Mycoplasma hominis. Biochim Biophys Acta. 1973 Nov 16;323(4):520–531. doi: 10.1016/0005-2736(73)90160-0. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Adenosine triphosphatase activity of mycoplasma membranes. J Bacteriol. 1966 Sep;92(3):714–722. doi: 10.1128/jb.92.3.714-722.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Serological typing of Ureaplasma urealyticum isolates from urethritis patients by an agar growth inhibition method. J Clin Microbiol. 1978 Nov;8(5):566–574. doi: 10.1128/jcm.8.5.566-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvan M. H., Schuldiner S., Rottem S. Control of sodium fluxes in Mycoplasma gallisepticum. Isr J Med Sci. 1987 May;23(5):384–388. [PubMed] [Google Scholar]
- van Golde L. M., McElhaney R. N., van Deenen L. L. A membrane-bound lysophospholipase from Mycoplasma laidlawii strain B. Biochim Biophys Acta. 1971 Feb 2;231(1):245–249. doi: 10.1016/0005-2760(71)90275-x. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]




