Abstract
Maurocalcine (MCa), initially identified from a tunisian scorpion venom, defines a new member of the family of cell penetrating peptides by its ability to efficiently cross the plasma membrane. The initiating mechanistic step required for the cell translocation of a cell penetrating peptide implicates its binding onto cell surface components such as membrane lipids and/or heparan sulfate proteoglycans. Here we characterized the interaction of wild-type MCa and MCa K20A, a mutant analogue with reduced cell-penetration efficiency, with heparin (HP) and heparan sulfates (HS) through surface plasma resonance. HP and HS bind both to MCa, indicating that heparan sulfate proteoglycans may represent an important entry route of the peptide. This is confirmed by the fact that (i) both compounds bind with reduced affinity to MCa K20A and (ii) the cell penetration of wild-type or mutant MCa coupled to fluorescent streptavidin is reduced by about 50% in mutant Chinese hamster ovary cell lines lacking either all glycosaminoglycans (GAGs) or just HS. Incubating MCa with soluble HS, HP, or chondroitin sulfates also inhibits the cell penetration of MCa-streptavidin complexes. Analyses of the cell distributions of MCa/streptavidin in several Chinese hamster ovary cell lines show that the distribution of the complex coincides with the endosomal marker Lyso-Tracker red and is not affected by the absence of GAGs. The distribution of MCa/streptavidin is not coincident with that of transferrin receptors nor affected by a dominant-negative dynamin 2 K44A mutant, an inhibitor of clathrin-mediated endocytosis. However, entry of the complex is greatly diminished by amiloride, indicating the importance of macropinocytosis in MCa/streptavidin entry. It is concluded that (i) interaction of MCa with GAGs quantitatively improves the cell penetration of MCa, and (ii) GAG-dependent and -independent MCa penetration rely similarly on the macropinocytosis pathway.
Maurocalcine (MCa)4 is a 33-mer peptide isolated from the venom of the scorpion Scorpio maurus palmatus. MCa is a highly basic peptide, as 12 of 33 residues are positively charged including the amino-terminal Gly residue, seven Lys residues, and four Arg residues. Because it contains only four negatively charged residues, the net global charge of the peptide is also positive. MCa possesses three disulfide bridges connected according to the pattern Cys3-Cys17, Cys10-Cys21, and Cys16-Cys32. 1H NMR analysis further indicates that MCa folds along an inhibitor cystine knot motif (1). MCa contains three β-strands running from amino acid residues 9 to 11 (strand 1), 20 to 23 (strand 2), and 30 to 33 (strand 3), respectively, with β-strands 2 and 3 forming an antiparallel β-sheet. MCa has proven to be a highly potent modulator of the skeletal muscle ryanodine receptor type 1 (RyR1), an intracellular calcium channel. The addition of MCa to the extracellular medium of cultured myotubes induces Ca2+ release from the sarcoplasmic reticulum into the cytoplasm within seconds, as shown using a calcium-imaging approach (2, 3). These observations suggested that MCa is able to cross the plasma membrane to reach its pharmacological target. This was first demonstrated when a biotinylated analogue of MCa was coupled to a fluorescent derivative of streptavidin, and the complex was shown to cross the plasma membrane (4). Cell penetration of this MCa-based complex is rapid, reaches saturation within minutes, and occurs at concentrations as low as 10 nm (5). Furthermore, an alanine scan of MCa indicates the importance of basic amino acid residues in the cell penetration mechanism. Reducing the net positive charge of the molecule appears to decrease its cell penetration efficiency. In parallel, MCa analogues exhibiting decreased penetration efficiency were also found to present reduced affinity for negatively charged lipids of the plasma membrane (6).
Over the past years several peptides have been characterized for their ability to cross the plasma membrane (7-11). Cell penetration of peptides obeys three fundamental steps; (i) binding to some components of the plasma membrane, (ii) the cell entry process per se, and (iii) the subsequent release into the cytoplasm. Obviously, none of these steps are well understood, and conflicting reports have emerged that may well arise from differences in the nature of the cell-penetrating peptide (CPP) considered, cell preparations, experimental conditions, type of linkage to cargoes, or even cargo nature. Two non-competing mechanisms have been proposed for the cell entry of CPP. One is direct translocation through the plasma membrane by the CPP-induced reorganization of the membrane after several possible structural alterations (7-11). According to some investigators, this mechanism implies a direct CPP interaction with negatively charged lipids of the plasma membrane. This mechanism of penetration would be independent of both cell metabolic energy and membrane receptor presence. For instance, it was proposed that penetratin binds to the polar heads of lipids leading to the formation of inverted micelles followed by a subsequent opening of these micelles inside the cell, and the release of the peptide into the cytoplasm (12). A second mechanism involves a form of endocytosis by which the CPP gets localized into late endosomes from where it may eventually leak out partially toward the cytoplasm. Endocytosis can be initiated by binding of CPPs to HS along with binding to negatively charged moieties on the cell surface, such as lipids (13). Lipid-raft dependent macropinocytosis has been evidenced as one endocytosis pathway for the cell entry of CPPs (14, 15). For instance, cellular uptake of a recombinant glutathione S-transferase-TAT-green fluorescent protein fusion protein depends on the presence of HS proteoglycans (HSPG) at the cell surface (16). Nevertheless, the role of GAGs in the cell penetration of CPPs remains debated. Stereochemistry, chain length, patterns of sulfation, and negative charge distribution of GAGs lead to a great variety of protein binding motifs. Furthermore, the CPP structure also appears to determine its specificity for HSPG (17).
In the present study we show that MCa interacts with GAGs such as HS and HP with apparent affinities in the micromolar range. A less penetrating analogue of MCa (MCa K20A) also shows a reduced apparent affinity for these GAGs, suggesting a direct link between GAG interaction and cell penetration. Cell penetration of MCab-streptavidin complex is strongly inhibited by an inhibitor of macropinocytosis indicating that this route of entry is responsible for MCa penetration. However, use of GAG-deficient cell lines indicates that half of the cell penetration of the complex is conserved and still relies on macropinocytosis. We conclude that GAG-dependent and -independent entries of MCa use similar pathways. Cell surface GAGs appear important to specify a higher cell penetration level, but penetration still can occur in their absence presumably because binding onto lipids can also activate macropinocytosis.
EXPERIMENTAL PROCEDURES
Equipment and Reagents—The Biacore 3000 apparatus, CM4 sensor chips, amine coupling kit, and HBS-P buffer (10 mm HEPES, 150 mm NaCl, 3 mm EDTA, 0.005% surfactant P20, pH 7.4) were from Biacore AB. Biotin-LC-hydrazide was from Pierce. Streptavidin, 6-kDa HP, and 35-45-kDa chondroitin 4 sulfate (CS-A, here abbreviated CS) were from Sigma, streptavidin-Cy5 or -Cy3 was from Amersham Biosciences, and 9-kDa HS was from Celsus. Concerning the 6-kDa HP, smaller molecular species that this material may contain were removed through a filtration column. This material was routinely used for Biacore analysis (18). This material was preferred to unfractionated heparin because it is less polydisperse. CS-A contains on average one sulfate group by disaccharide. The molecular weight of the HS used in this study was 9000 g/mol as determined by sedimentation-diffusion analysis. Its sulfur and nitrogen contents, determined by elemental analysis, were 6.96 and 2.15%, respectively.5 LysoTracker red DND-99 and Alexa Fluor® 488- or 594-conjugated transferrin were from Invitrogen. Size-defined HP-derived oligosaccharides (dp6 (hexa)-, dp12 (dodeca)-, and dp18 octadecasaccharide) were prepared from porcine mucosal HP as described (19). These HP-derived oligosaccharides were obtained by size fractionation. Because the starting material was HP and HS, these samples were relatively homogenous and highly sulfated. Strong anion exchange high performance liquid chromatography analysis of the HP-derived octasaccharide gave rise to three major peaks.
MCa, MCab, MCa K20A, and MCab K20A Peptide Syntheses—Chemical syntheses of MCa and MCa K20A or biotinylated MCab and MCab K20A were performed as previously described (2, 6). The molecular weights of the peptides are 3858.62 (MCa) and 3801.52 (MCa K20A). Their pI values are 9.46 (MCa) and 9.30 (MCa K20A), indicating that they are basic at physiological pH 7.4. Primary structures of MCa and MCa K20A are shown in Fig. 1A. The position of biotin in MCab and MCab K20A is on an extra amino-terminal lysine residue.
FIGURE 1.
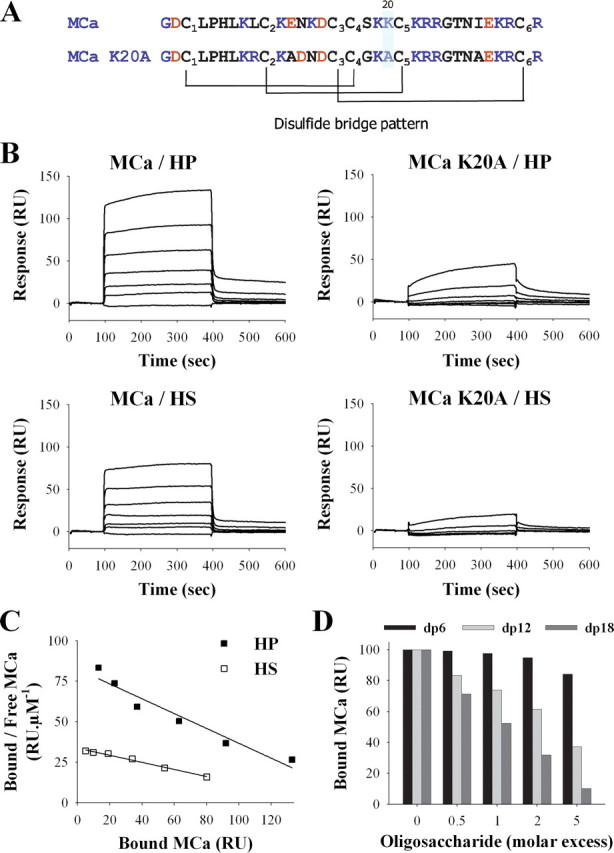
Binding of wild-type MCa and MCa K20A to HP or HS immobilized on SPR sensorchip. A, primary structure of MCa and MCa K20A. Basic and acidic residues are in blue and red, respectively. The disulfide bridge patterns of both molecules are shown. B, sensorgrams of the interactions. Various concentrations of MCa or MCa K20A were injected over a HP- or HS-activated surface at a flow rate of 20 μl/min during 5 min. After this peptide injection time, running buffer was injected to monitor the wash off reaction. All responses were recorded and subtracted from the control surface online as a function of time (in RUs). Each set of sensorgrams was obtained with MCa at (from top to bottom) 5, 2.5, 1.25, 0.62, 0.31, 0.15, and 0 μm. C, Scatchard plots of the equilibrium binding data measured on the sensorgrams corresponding to the injection of MCa over HP or HS SPR surfaces. Data were fitted with a linear equation of the type y = a × x + b, where a =-0.46 (HP) or -0.22 (HS), and b = 82.5 (HP) or 33.7 (HS). Calculated binding affinities are Kd = 2.1 μm (HP) and Kd = 4.6 μm (HS). D, inhibition of the MCa/HP binding by HP-derived oligosaccharides. MCa (1 μm) was coincubated with increasing molar excess of dp6, dp12, or dp18 for 45 min, then injected over a HP-activated sensorchip for 5 min. Responses (in RU) were recorded and plotted as the percentage of maximum responses obtained without preincubation (70-80 RU).
Formation of MCab- or MCab K20A-Strep-Cy5/3 Complexes—Soluble streptavidin-Cy5 or -Cy3 (Amersham Biosciences) was mixed with 4 mol eq of MCab or MCab K20A for 2 h at 37 °C in the dark in phosphate-buffered saline (PBS): 136 mm NaCl, 4.3 mm Na2HPO4, 1.47 mm KH2PO4, 2.6 mm KCl, 1 mm CaCl2, 0.5 mm MgCl2, pH 7.2. In some experiments, where indicated, various molar ratios of MCab and streptavidin-Cy3 were used to prepare the MCab-Strep complex, the concentration of streptavidin-Cy3 being kept constant (1 μm).
Surface Plasmon Resonance Binding Experiments—6-kDa HP and HS were biotinylated at their reducing end with biotin-LC-hydrazide. The biotinylation procedure was checked by streptavidin-peroxidase labeling after blotting of the material onto zetaprobe membrane. These molecules have been widely used to study HP or HS binding onto several other proteins, such as RANTES (regulated on activation normal T cell expressed and secreted), gp120, or CXCL12 (18, 20-22). For the purpose of immobilization of biotinylated HP and HS on a Biacore sensorchip, flow cells of a CM4 sensorchip were activated with 50 μl of 0.2 m N-ethyl-N′-(diethylaminopropyl)-carbodiimide and 0.05 m N-hydroxysuccinimide before injection of 50 μl of streptavidin (0.2 mg/ml in 10 mm acetate buffer, pH 4.2). The remaining activated groups were blocked with 50 μl of ethanolamine 1 m, pH 8.5. Typically, this procedure permitted coupling of ∼3.000-3.500 resonance units (RU) of streptavidin. Biotinylated HP (5 μg/ml) or HS (10 μg/ml) in HBS-P buffer was then injected over a one-surface flow cell to obtain an immobilization level of ∼50 RU. Flow cells were then conditioned with several injections of 2 m NaCl. One-flow cells were left untreated and served as negative control. For binding assays, different MCa concentrations in HBS-P and at 25 °C were simultaneously injected at 20 μl/min onto the control, HP, and HS surfaces for 5 min, after which the formed complexes were washed with running buffer. The sensorchip surfaces were regenerated with a 5-min pulse of 2 m NaCl in HBS-P buffer. For competition assays, MCa at 2 μm was preincubated for at least 45 min with various molar excesses of HP-derived oligosaccharides (dp6, dp12, and dp18) and then injected over the HP surface as described above.
Cell Culture and Transfection—The wild-type Chinese hamster ovary (CHO-K1) cell line and mutant CHO cell lines lacking all GAGs (pgsB-618) or HS (pgsD-677) (ATCC) were maintained at 37 °C in 5% CO2 in F-12K nutrient medium (Invitrogen) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Invitrogen) and 10,000 units/ml streptomycin and penicillin (Invitrogen). For transfection experiments with FuGENE® HD (Roche Applied Science), wild-type and mutant pgsB-618 CHO cells were transfected with a plasmid that encodes a dominant-negative form of dynamin 2 (dynamin 2 K44A) in fusion with enhanced green fluorescent protein (pEGFP-N1 vector from Clontech). 24 h after transfection, cells were incubated with 1 μm MCab-Strep-Cy3 or transferrin-conjugated to Alexa Fluor-594 (25 μg/ml).
Flow Cytometry—MCab/MCab K20A-Strep-Cy5 complexes were incubated for 2 h in phosphate-buffered saline with CHO and mutant cells to allow cell penetration. The cells were then washed twice with PBS to remove the excess extracellular complexes. Next, the cells were treated with 1 mg/ml trypsin (Invitrogen) for 10 min at 37 °C to remove remaining membrane-associated extracellular cell surface-bound complexes. After trypsin incubation, the cell suspension was centrifuged at 500 × g and suspended in PBS. For inhibition studies, MCab-Strep-Cy5 complexes were preincubated with PBS containing variable concentrations (as indicated) of CS-A, HP, or HS for 45 min, and the mixture was incubated with cells for 2 h to investigate cell penetration. Washing and trypsination steps were also applied in these conditions. For experiments concerning endocytosis inhibitors, wild-type and mutant CHO cells were initially washed with F12K and preincubated for 30 min at 37 °C with different inhibitors of endocytosis: (i) 5 mm amiloride, (ii) 5 μm cytochalasin D, (iii) 5 mm nocodazole, or (iv) 5 mm methyl-β-cyclodextrin (all from Sigma). The cells were then incubated for 2 h at 37 °C with 1 μm MCab-Strep-Cy5 or with 25 μg/ml transferrin-Alexa Fluor 488 in the presence of each drug. For all these experimental conditions, flow cytometry analyses were performed with live cells using a BD Biosciences FACSCalibur flow cytometer. Data were obtained and analyzed using CellQuest software (BD Biosciences). Live cells were gated by forward/side scattering from a total of 10,000 events.
Confocal Microscopy—For analysis of the subcellular localization of MCab-Strep-Cy5 complexes in living cells, CHO and mutant cells were incubated with the complexes for 2 h and then washed with Dulbecco's modified Eagle's medium alone. Immediately after washing, the nucleus was stained with 1 μg/ml dihydroethidium (Molecular Probes) for 20 min and then washed again with Dulbecco's modified Eagle's medium. After this step the plasma membrane was stained with 5 μg/ml fluorescein isothiocyanate-conjugated concanavalin A (Sigma) for 3 min. Cells were washed once more, but with PBS. Live cells were then immediately analyzed by confocal laser scanning microscopy using a Leica TCS-SP2 operating system. Fluorescein isothiocyanate (Ex = 488 nm), Cy5 (Ex = 642 nm), or Cy3 (Ex = 543 nm) fluorescence emission were collected in z-confocal planes of 10-15 nm. Images were merged in Adobe Photoshop 7.0. For studies on endocytosis, wild-type and mutant CHO cells were incubated with 1 μm MCab-Strep-Cy5 along with 25 μg/ml transferrin conjugated to Alexa Fluor 488 (a marker of clathrin-mediated endocytosis) for 2 h, and the distribution was analyzed through confocal microscopy. In parallel studies cells were first incubated for 2 h with 1 μm MCab-Strep-Cy5, washed with PBS, and incubated with 50 nm LysoTracker red DND-99 for 20 min at 37 °C. Cells were then washed again with PBS and visualized alive by confocal microscopy.
Effect of HP on the Interaction of MCab with Lipids—Strips of nitrocellulose membranes containing spots with different phospholipids and sphingolipids were obtained from Molecular Probes. These membranes were first blocked with TBS-T (150 mm NaCl, 10 mm Tris-HCL, pH 8.0, 0.1% (v/v) Tween 20) supplemented with 0.1% free bovine serum albumin (BSA) for about 1 h at room temperature and then incubated for 2 h at room temperature in TBS-T, 0.1% free BSA with either 100 nm MCab alone or a MCab·HP complex, resulting from a 45-min preincubation of 100 nm MCab with 10 μg/ml HP. Incubation of the membranes with 100 nm biotin alone was used as a negative control condition. The membranes were then washed a first time with TBS-T, 0.1% free bovine serum albumin using a gentle agitation for 10 min. In all conditions MCab or biotin binding onto the lipid spots was detected by a 30-min incubation with 1 μg/ml streptavidin horseradish peroxidase (Vector labs, SA-5704) followed by a second wash with TBS-T 0.1% free bovine serum albumin and an incubation with horseradish peroxidase substrate (Western Lightning, PerkinElmer Life Science) for 1 min. Lipid membranes were then exposed to a Biomax film (Kodak). The intensity of interaction with the lipids was analyzed with Image J (National Institutes of Health).
RESULTS
MCa Interacts with HS and HP—Preincubation of MCab with HP was found to partially inhibit its penetration in HEK293 cells (5). To evaluate the binding of MCa to HSPGs, HP or HS was coupled to a Biacore sensorchip, and the MCa binding was monitored by SPR (Fig. 1). Injection of a range of MCa concentrations (up to 5 μm) over HP- or HS-coupled sensorchips gave rise to increasing binding amplitudes as shown in Fig. 1B. A mutated analogue of MCa (MCa K20A) showed impaired binding activity, indicating the importance of residue Lys-20 for glycosaminoglycan recognition. This finding is in agreement with the role of HSPGs in the cell entry of CPPs and with the observation that the MCa K20A has impaired cell penetration (6). The data could not be fitted to a binding model, presumably because all binding curves had a “square” shape with sharp edges, suggesting high association and dissociation rates. We were, thus, not able to extract reliable kinetic values from curve-fitting. Equilibrium data, plotted according to the Scatchard representation, were used to determine affinity (Fig. 1C). The straight lines obtained show that MCa recognizes a single class of binding site, characterized by an affinity constant of Kd = 2.1 μm (HP) or 4.6 μm (HS). Because HP (6 kDa) and HS (9 kDa), immobilized at a level of 55 and 45 RU, respectively, both permitted a maximum binding of 155 RU of MCa (3859 Da), we calculated that each HP molecule bound an average of 4.4 MCa, and each HS molecule bound an average of 8 MCa. These two molecules contain, respectively, an average of 20 and 36 saccharidic units (using an approximate Mr of 600 for the HP-derived disaccharides and 500 for the HS-derived disaccharides); thus, it can be estimated conversely that each MCa should occupy in both cases an average of 4.5 monosaccharide units (20/4.4 or 36/8) along the GAG chain.
HP is formed by the polymerization of a various number of disaccharide units. To study the effect of the size of the polymer on its interaction with MCa, we performed competition experiments using HP-derived oligosaccharides of a defined degree of polymerization (dp) (Fig. 1D). For this purpose wild-type MCa was preincubated with different HP-derived oligosaccharides (dp6, dp12, or dp18), as mentioned under “Experimental Procedures” and then injected over the HP-conjugated sensorchip. As shown, the oligosaccharides caused a dose-dependent inhibition of the interaction of MCa with HP. dp18 was the most active oligosaccharide with an IC50 close to 1 μm. In contrast, dp6 had almost no effect at 5 μm.
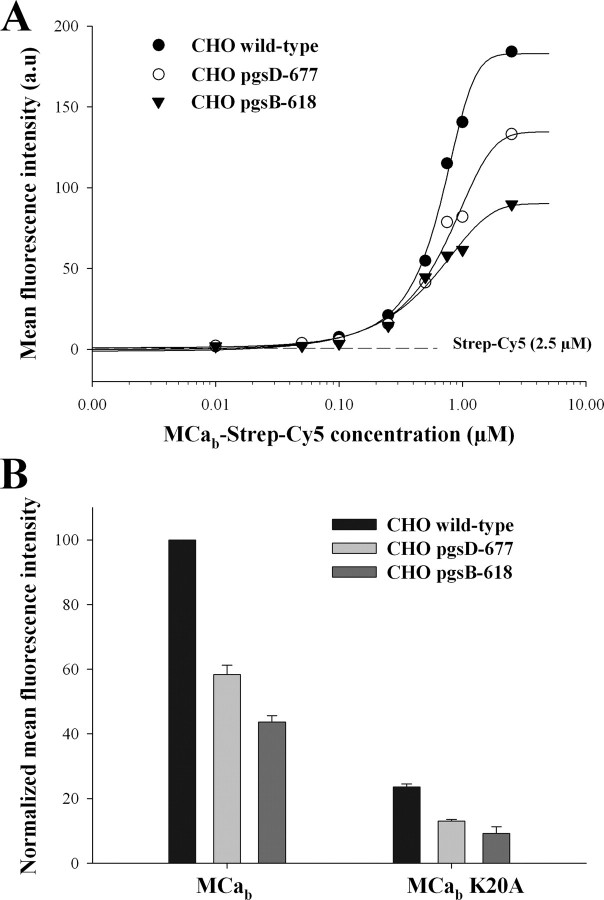
Dose-dependent Penetration of MCab-Strep-Cy5 in Wild-type and Mutant CHO Cell Lines—Results presented in Fig. 1 indicate that MCa interacts with negatively charged HP and HS. To challenge the implication of HP and HS in the cell penetration of MCa, the cell penetration efficacy of MCab-Strep-Cy5 was assessed using wild-type CHO cells (CHO wild-type) and mutant CHO cells lacking either HS (CHO pgsD-677) or all GAGs (CHO pgsB-618). Each CHO cell line was incubated for 2 h in the presence of variable concentrations of MCab-Strep-Cy5 complexes, and the amount of internalized complex was measured by FACS. Fig. 2A represents the dose-response curves for MCab-Strep penetration in the three CHO cell lines. Half saturation of MCa penetration (PC50) was only slightly modified by the absence of GAG, with PC50 values of 0.46, 0.56, and 0.71 μm in CHO wild-type, CHO pgsD-677, and CHO pgsB-618, respectively. In contrast, the maximum amount of incorporated MCab-Strep-Cy5, measured in the presence of 1 μm of complex, was strongly reduced in both the pgsD-677 and pgsB-618 CHO lines compared with the wild-type CHO, 43.0 ± 3.0% (n = 3) and 57.0 ± 2.5% (n = 3) reduction in pgsD-677 and pgsB-618 CHO, respectively (Fig. 2B). Similar experiments were done using the mutant MCab K20A that was previously shown to exhibit reduced penetration compared with the wild-type MCa (6). Results presented on Fig. 2B show that although already strongly reduced in wild-type CHO, the penetration of this mutant was further reduced in the HS- or GAGs-deficient CHO, resulting in similar reductions in cell entry; 45 ± 2% (n = 3) and 60 ± 4% (n = 3) reduction in pgsD-677 and pgsB-618 CHO, respectively. This result indicates that the mechanism of cell penetration of the K20A mutant is identical to that of wild-type MCa despite the reduction in cell entry induced by the mutation. Therefore, MCab K20A also relies on GAG-dependent and GAG-independent mechanisms for cell penetration. This observation is consistent with the fact that the K20A mutation in MCa only reduces the PC50 value (6). In addition, the significant amount of MCab-Strep-Cy5 taken up by GAG-deficient cells indicates that a significant fraction of MCab-Strep-Cy5 penetration is GAG-independent, likely relying on the contribution of plasma membrane lipids.
FIGURE 2.
Cell penetration of MCab-Strep-Cy5 and MCab K20A-Strep-Cy5 in wild-type and HSPG mutant CHO cells. A, dose-dependent cell penetration of wild-type MCab-Strep-Cy5 in wild-type and mutant CHO cells, as assessed quantitatively by FACS. Results are from a representative experiment. The indicated concentrations are for streptavidin-Cy5 (from 10 nm to 2.5 μm). Data were fitted by a sigmoid equation providing the following half effective concentrations: PC50 = 0.46 ± 0.01 μm (wild-type CHO), 0.56 ± 0.01 μm (pgsD-677), and 0.71 ± 0.01 μm (pgsB-618). a.u., absorbance units. B, comparative cell penetration of 1 μm MCab-Strep-Cy5 and MCab K20A-Strep-Cy5 in CHO and CHO mutant cell lines. Results are from a representative experiment of n = 3. Values are normalized with mean fluorescence intensity of wild-type CHO cells.
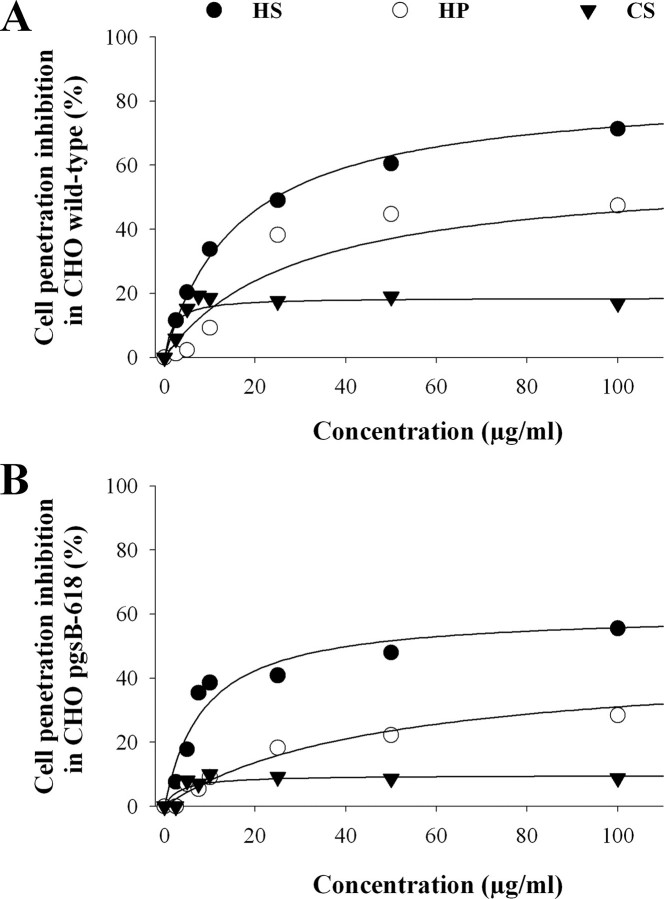
Inhibition of Cell Penetration of MCab-Strep-Cy5 by Soluble HSPGs—According to the two observations described above, (i) interaction of MCa with HSPGs and (ii) reduction in MCa cell penetration in GAG-deficient cells, one would expect that incubation of MCa with soluble GAGs also reduces the penetration of MCab-Strep-Cy5. To challenge this point, MCab-Strep-Cy5 was preincubated with various concentrations of HS, HP, or CS for 45 min before incubation with wild-type or GAG-deficient CHO cells for 2 h. The total amount of MCab-Strep-Cy5 inside the cell was then measured by flow cytometry (Fig. 3). In wild-type CHO cells, HS (250 μg/ml) produced the most potent inhibition of cell penetration (84 ± 2%, n = 3, Fig. 3A). HP and CS were less efficient than HS with a mean inhibition of 59 ± 10% (n = 3) and 19 ± 2% (n = 3) for HP and CS, respectively (at 250 μg/ml). Linking inhibition with the global negative charge of the GAG tested remains hazardous because charge relationship follows the rule HP > CS > HS, and here we observe HS > HP > CS. Interestingly, all three GAGs also reduced the cell penetration of MCab-Strep-Cy5 in GAG-deficient cells with the same rank as observed for wild-type CHO cells (Fig. 3B). Incubation of MCab-Strep-Cy5 with HS, HP, or CS induced a significant inhibition in GAG-deficient cells (61 ± 9% (n = 3), 45 ± 4% (n = 3), and 10 ± 1% (n = 3) in the presence, respectively, of 250 μg/ml HS, HP, and CS) although lower than in wild-type CHO cells. Therefore, these data indicate that binding of soluble GAGs to MCa inhibits the cell penetration of MCab-Strep-Cy5 not only by preventing its interaction with CHO cell surface GAGs but also with non-GAG cell surface components.
FIGURE 3.
Inhibition of MCab-Strep-Cy5 cell penetration by soluble GAGs in wild-type and GAG-deficient CHO cell lines. A, dose-dependent inhibition of MCab-Strep-Cy5 cell penetration by soluble GAGs in wild-type CHO cells. Results are from a representative experiment of n = 3. Data were fitted by an hyperbola equation of the type y = (a × x)/(b + x), where a = 83.9 ± 1.7% (HS), 58.7 ± 9.8% (HP), and 18.7 ± 1.7% (CS) and b = 16.6 ± 1.2 μg/ml (HS), 29.1 ± 14.5 μg/ml (HP), and 1.8 ± 1.0 μg/ml (CS). B, dose-dependent inhibition of MCab-Strep-Cy5 cell penetration by soluble GAGs in GAG-deficient CHO cells. Results are from a representative experiment of n = 3. Fitting values are a = 60.6 ± 8.6% (HS), 45.4 ± 3.5% (HP), and 9.8 ± 1.3% (CS) and b = 8.6 ± 2.0 μg/ml (HS), 46.2 ± 8.9 μg/ml (HP), and 3.3 ± 2.1 μg/ml (CS).
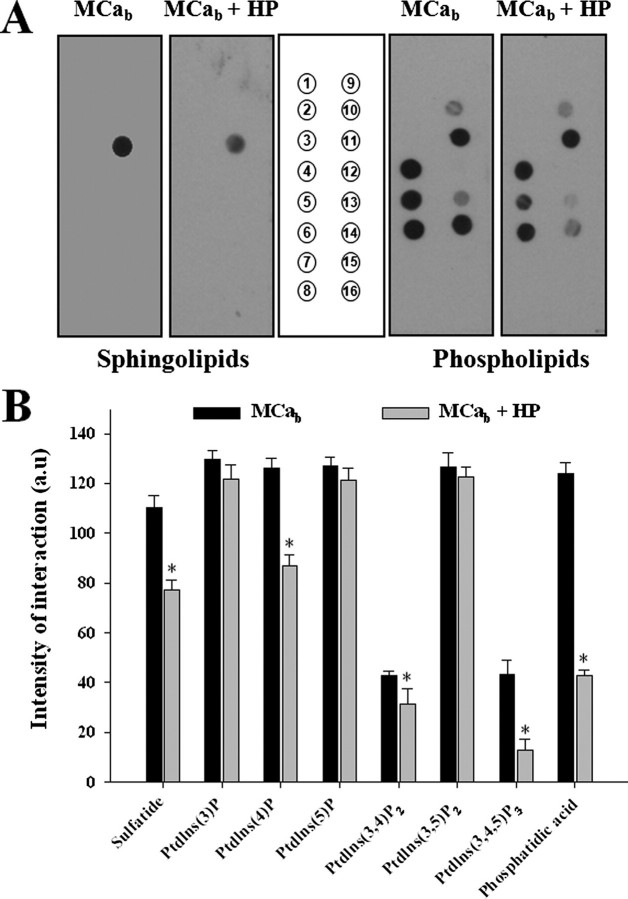
To check whether GAGs could inhibit the interaction of MCa with membrane lipids, we investigated the effect of HP on MCab interaction with lipids immobilized on strips (Fig. 4A). MCab (100 nm) was incubated for 45 min in the presence or absence of HP (10 μg/ml) before incubation with lipid strips, as described under “Experimental Procedures.” As shown in Fig. 4, HP significantly decreased the interaction of MCab with phosphatidic acid (66%), sulfatide (30%), phosphatidylinositol (Ptdlns) (4)P (31%), Ptdlns(3,4)P2 (26%), and Ptdlns(3,4,5)P3 (72%) but not with lipids such as Ptdlns(3)P, Ptdlns(5)P, or Ptdlns(3,4,5)P2. These results provide a clear explanation of the fact that the interaction of MCab with soluble GAGs may also lead to an inhibition of the GAG-independent MCab cell penetration.
FIGURE 4.
Effect of HP on the interaction of MCab with membrane lipids. A, 100 nm MCab alone or MCab preincubated for 45 min with 10 μg/ml of HP was incubated for 2 h with lipid strips. Each dot corresponds to 100 pmol of lipid immobilized on the strip. Left panel, interaction with various sphingolipids. 1, sphingosine; 2, sphingosine 1-phosphate; 3, phytosphingosine; 4, ceramide; 5, sphingomyelin; 6, sphingosylphosphophocholine; 7, lysophosphatidic acid; 8, myriocin; 9, monosialoganglioside; 10, disialoganglioside; 11, sulfatide; 12, sphingosylgalactoside; 13, cholesterol; 14, lysophosphatidyl choline; 15, phosphatidylcholine; 16, blank). Right panel, interaction with various phospholipids. The lipids are identified by their numbered positions on the strip (middle panel). 1, lysophosphatidic acid; 2, lysophosphatidylcholine; 3, Ptdlns; 4, Ptdlns(3)P; 5, Ptdlns(4)P; 6, Ptdlns(5)P; 7, phosphatidylethanolamine; 8, phosphatidylcholine; 9, sphingosine 1-phosphate; 10, Ptdlns(3,4)P2; 11, Ptdlns(3,5)P2; 12, Ptdlns(4,5)P2; 13, Ptdlns(3,4,5)P3; 14, phosphatidic acid; 15, phosphatidylserine; 16, blank. B, Intensity of the interaction of MCab or MCab + HP with various lipids as analyzed by Image J (NIH). The data are quantified in arbitrary units, and the results are presented as histograms. These experiments were repeated three times; data shown as triplicates. Significance is provided as a deviation of three times the S.D. value from 100% (denoted as asterisks). a.u., arbitrary units.
Effect of HSPGs on the Cell Distribution of MCab-Strep-Cy5—To examine the contribution of cell surface GAGs to the cell distribution of MCab-Strep-Cy5, MCab-Strep-Cy5 localization within the cell was defined using confocal microscopy and compared between wild-type and GAG-deficient CHO cell lines. For these experiments the plasma membrane, the nucleus, and MCab-Strep were labeled with concanavalin A (green), dihydroethidium (red), and Cy5 (blue), respectively. Images presented on Fig. 5 were obtained 2 h after the start of the cell incubation. Living cells were used to avoid possible cell distribution artifacts that may occur during the fixation procedure (5, 9, 23). As shown, MCab coupling to Strep-Cy5 is required for the cell penetration of Strep-Cy5 into CHO cells (Fig. 5A). The MCab-Strep-Cy5 complex is exclusively present as punctuate dots in the cytoplasm of living CHO cells. A similar cell distribution is observed in living CHO cell mutants lacking just HS (pgsD-677) or all GAGs (pgsB-618), suggesting that GAG-dependent and GAG-independent cell entries produce similar cell distributions (Fig. 5B). A Strep-Cy5 complex made with the MCab K20A analogue produces a similar subcellular distribution than MCab-Strep-Cy5 in wild-type and HS-deficient CHO cells, suggesting that the mechanism of cell penetration is not altered by point mutation of MCab (data not shown). Punctuate staining of MCab-Strep-Cy5 is indicative of a form of endosomal localization. This point was further investigated.
FIGURE 5.
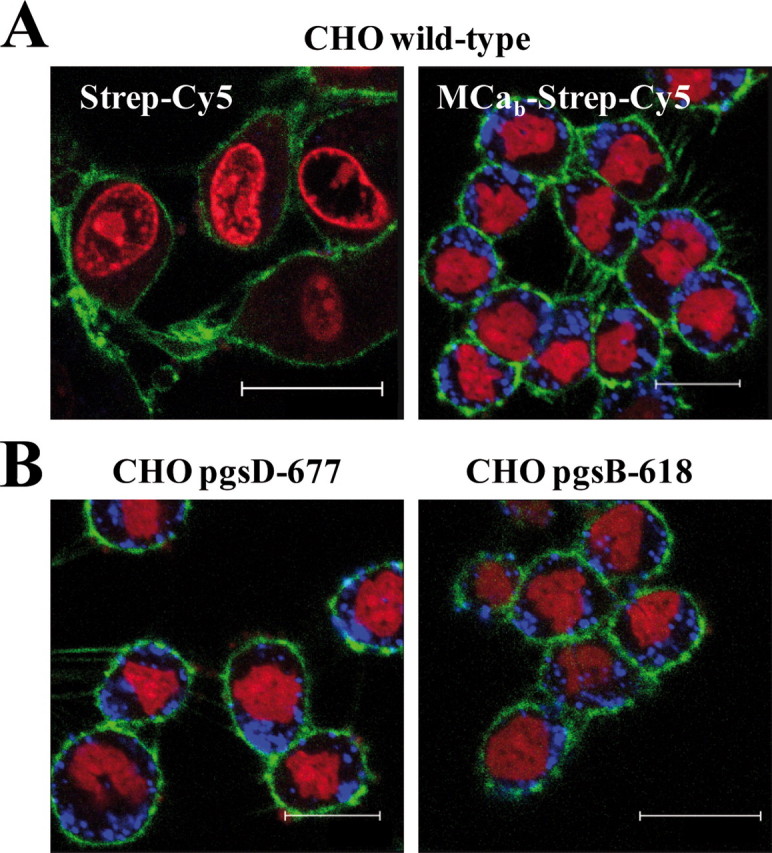
Cell distribution of MCab-Strep-Cy5 in living wild-type or mutant CHO cells. A, confocal images showing the cell penetration of Strep-Cy5 (2 h of incubation) in the absence (left panel) or presence (right panel) of MCab (4 μm) in wild-type CHO cells. Scale bars, 25 μm (left) and 15 μm (right bar). B, cell distribution of MCab-Strep-Cy5 in mutant CHO cells. Scale bars, 15 μm (left) and 20 μm (right bar). Blue, Strep-Cy5; red, dihydroethidium, nuclei; green, concanavalin A, plasma membrane.
MCab-Strep-Cy5 Localizes to Endosomal Structures That Do Not Originate from Clathrin-mediated Endocytosis—Using confocal microscopy, the cell distribution of MCab-Strep-Cy5 was compared with that of endosomal structures as revealed by LysoTracker red staining. As shown on Fig. 6A, there is a very good co-localization between MCab-Strep-Cy5 and Lyso-Tracker red fluorescence in all cell lines used (CHO wild-type, CHO pgsB-618, and CHO pgsD-677). These data clearly indicate that the lack of GAGs does not alter the subcellular localization of MCab-Strep-Cy5, suggesting that both GAG-dependent and GAG-independent cell penetration rely on endocytosis. To determine whether the type of endocytosis involved in MCab-Strep-Cy5 entry could be altered in GAG-deficient cells, we first analyzed whether it had common features with clathrin-mediated endocytosis. Transferrin is known to enter cells via transferrin receptors through clathrin-mediated endocytosis (24, 25). As shown here, there was an almost complete absence of co-localization between MCab-Strep-Cy5 and transferrin-labeled with Alexa Fluor 488 in wild-type as well as in mutant CHO cells (Fig. 6B). These data indicate that the route of entry of Strep-Cy5 when coupled to MCab is not through clathrin-mediated endocytosis. It also indicates that the absence of GAGs at the cell surface does not favor clathrindependent endocytosis for the cell entry of MCab-Strep-Cy5 over other mechanisms of endocytosis. Expression of a dominant-negative mutant of dynamin 2, dynamin 2 K44A, is known to prevent normal clathrin-mediated endocytosis (26). As shown in Fig. 7A, expression of dynamin 2 K44A prevents the entry of transferrin-Alexa Fluor-594 in both wild-type and GAG-deficient CHO cells, confirming that transferrin receptors get internalized by clathrin-mediated endocytosis. In contrast, MCab-Strep-Cy3 entry was not prevented by the expression of dynamin 2 K44A (Fig. 7B), clearly indicating that clathrin-mediated endocytosis was not required for the entry of MCab when coupled to streptavidin.
FIGURE 6.
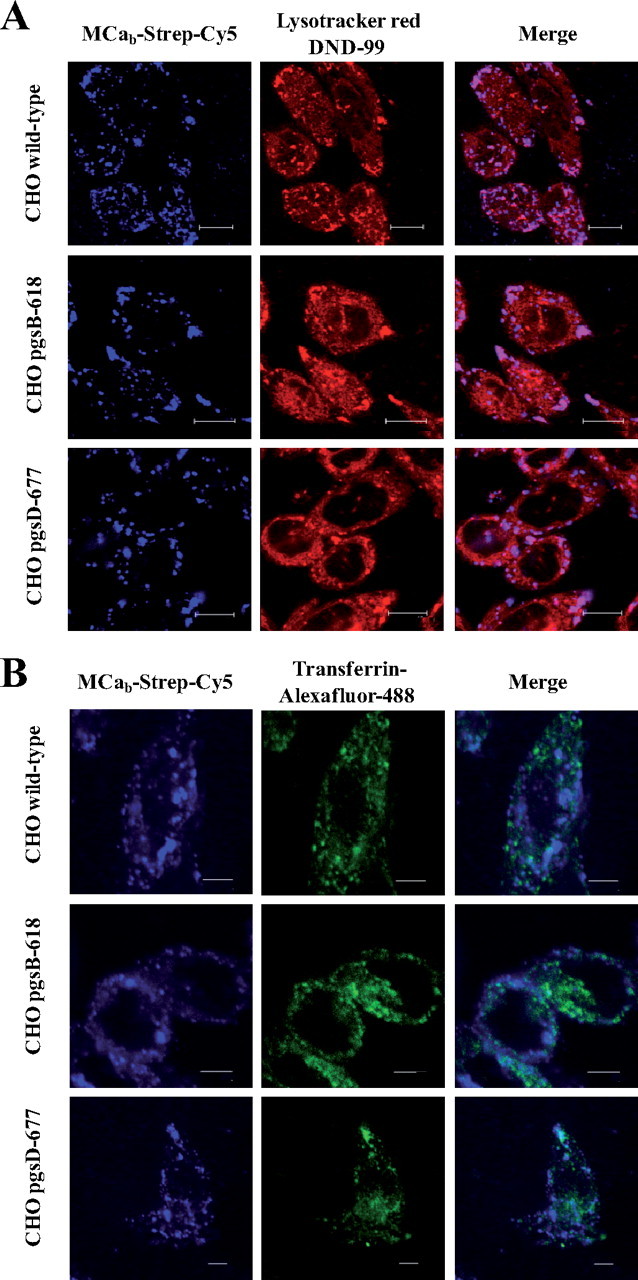
MCab-Strep-Cy5 entry and endocytosis. A, endocytic route of entry of MCab-Strep-Cy5. Various CHO cell lines (upper panels, wild type; middle panels, pgsB-618; lower panels, pgsD-677,) were incubated 2 h with 1 μm of MCab-Strep-Cy5, washed, and incubated with 50 nm LysoTracker red DND-99 for 20 min right before confocal acquisition. Scale bars, 10 μm (upper panels) and 11 μm (middle and lower panels). B, different endocytic entry pathways for transferrin-Alexa Fluor 488 and MCab-Strep-Cy5. Confocal immunofluorescence images of living wild-type or mutant CHO cells to compare the cell distribution of transferrin-Alexa Fluor 488 and MCab-Strep-Cy5. Cells were incubated 2 h with 1 μm MCab-Strep-Cy5 (blue) along with 25 μg/ml transferrin-Alexa Fluor 488 (green), washed, and immediately analyzed by confocal microscopy. Scale bars, 5 μm.
FIGURE 7.
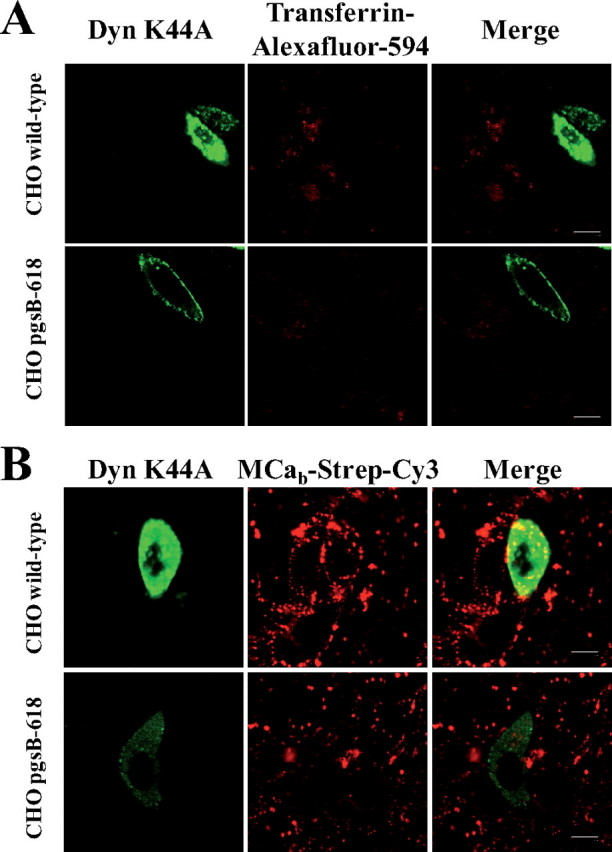
Expression of the dominant-negative dynamin 2 K44A mutant blocks clathrin-mediated endocytosis and transferring entry but spares MCab-Strep-Cy3 entry. A, transferrin entry is inhibited in dynamin 2 K44A transfected wild-type and pgsB-618 mutant CHO cells. B, MCab-Strep-Cy3 entry is preserved in dynamin 2 K44A-transfected wild-type and pgsB-618 mutant CHO cells. Scale bars, 10 μm. Note that expression of dynamin 2 K44A mutant was always less in pgsB-618 mutant CHO cells than in wild-type CHO cells, possibly due to a role of GAGs in plasmid entry.
Lack of Alteration of the Main Endocytosis Entry Pathway in GAG-depleted Cells—To further identify the route of entry of MCab-Strep-Cy5 and analyze the impact of GAG depletion on this process, several inhibitors were tested by FACS on the entry of MCab-Strep-Cy5 in wild-type (Fig. 8A) and pgsB-618 CHO cells (Fig. 8B). These inhibitors were also tested on the entry of transferrin-Alexa Fluor 488 for comparison. Amiloride was tested to block macropinocytosis, methyl-β-cyclodextrin to deplete membrane cholesterol and inhibit lipid raft-dependent pathways, nocodazole to inhibit microtubule formation, and cytochalasin D to inhibit F-actin elongation, required for macropinocytosis and clathrin-dependent endocytosis (27). Chlorpromazine, an inhibitor of clathrin-mediated endocytosis, could not be tested because it produced cell dissociation from the plastic dish surface (data not shown). In wild-type CHO cells, transferrin-Alexa Fluor 488 endocytosis was not affected by amiloride, methyl-β-cyclodextrin, or nocodazole, as expected for clathrin-mediated endocytosis. Cytochalasin D was found to produce a curious 37% increase in the cell entry of transferrin, indicating an alteration in clathrin-dependent endocytosis. In contrast, with the exception of methyl-β-cyclodextrin, all drugs tested were found to inhibit partially the entry of MCab-Strep-Cy5 in wild-type CHO cells (Fig. 8A). The lack of effect of methyl-β-cyclodextrin indicates that caveolae-mediated endocytosis is not involved in the entry of MCab-Strep-Cy5. The fact that both amiloride and cytochalasin D inhibit MCab-Strep-Cy5 cell entry by 80 and 30%, respectively, indicates a significant contribution of macropinocytosis pathway. Cytochalasin D probably acts exclusively on macropinocytosis for the cell entry of MCab-Strep-Cy5 as the involvement of the other major endocytic pathway affected by this drug, clathrin-mediated endocytosis, can be ruled out. Nocodazole, which has a wide range of effects on various endocytosis pathways, also had a great effect, inducing a 63% reduction of MCab-Strep-Cy5 cell entry. Thus, the rather segregated effects of endocytosis inhibitors on cell penetration of transferrin and MCab-Strep-Cy5 is coherent with their lack of colocalization inside cells (Fig. 6B). These data stress the importance of macropinocytosis as the main entry pathway of Strep-Cy5 when coupled to MCab. The same set of drugs was then tested for the cell entry of both transferrin and MCab-Strep-Cy5 in GAG-deficient pgsB-618 CHO cells (Fig. 8B). Interestingly, in the absence of GAGs, the effects of the endocytosis inhibitors on MCab-Strep-Cy5 entry were not altered, indicating that in the absence of cell surface GAGs, macropinocytosis is still the main route of entry of the complex. This is in perfect agreement with the data shown in Figs. 5 and 6, suggesting similar cell distribution of the complex, colocalization with LysoTracker red, and identical effects of dynamin 2 K44A expression. Surprisingly, the absence of GAGs had an impact on the effects of the drugs on transferrin entry (Fig. 8B). Although no clear explanation can be provided for this observation, it may suggest that in the absence of GAGs inhibition of alternative endocytosis pathways favors somehow clathrin-mediated endocytosis. These effects remain, however, outside the focus of this study, namely the entry pathways of MCa, but are clear indications of the potential importance of GAGs in endocytosis.
FIGURE 8.
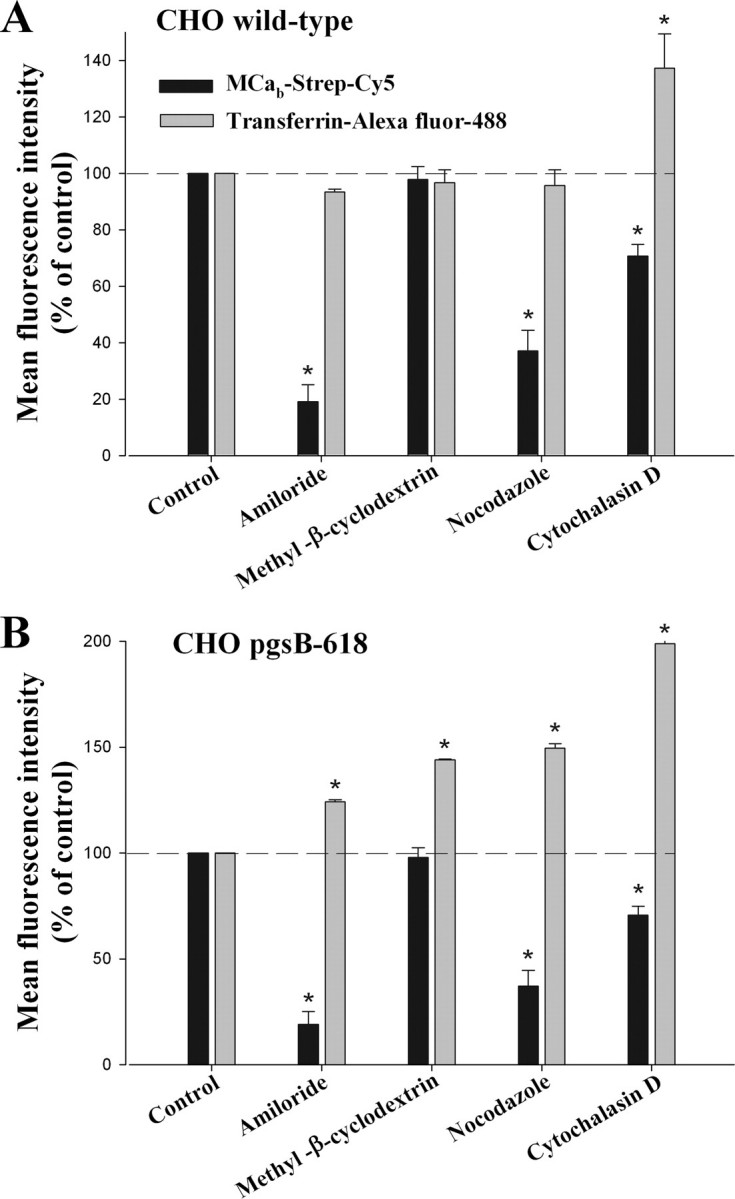
Effect of endocytic inhibitors on the entry of transferrin-Alexa Fluor 488 and MCab-Strep-Cy5. Data are expressed in percentage of mean control fluorescence as assessed by FACS. Average data are from three experiments. A, data for wild-type CHO cells. B, data for pgsB-618 CHO cells. Significance is provided as a deviation of three times the S.D. value from 100% (denoted as asterisks).
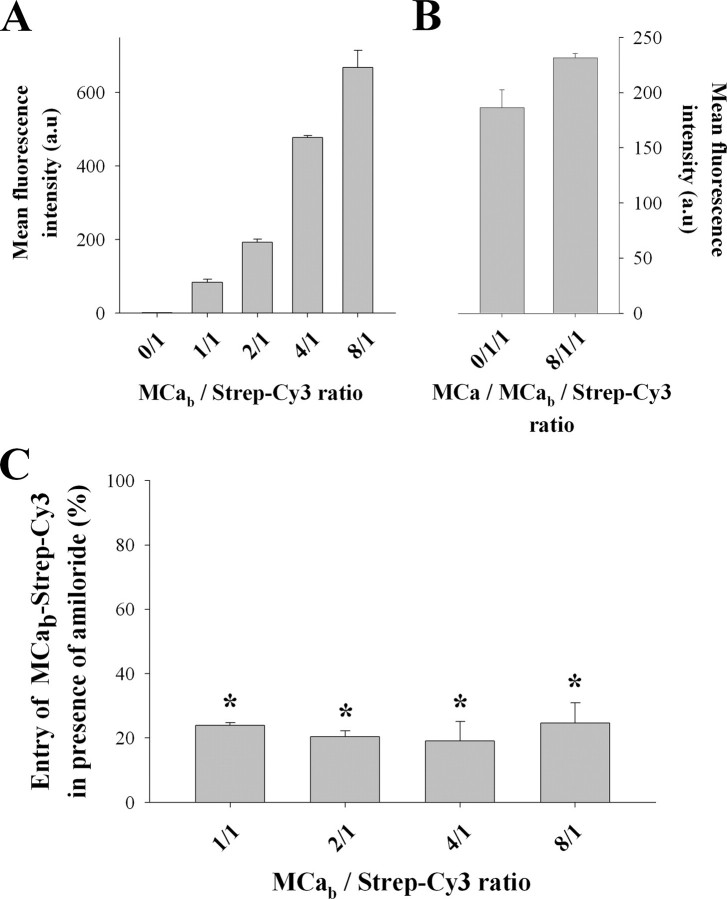
The Molar Ratio MCab/Strep-Cy3 Does Not Influence the Type of Endocytosis—Streptavidin molecules are tetramers that can bind up to four MCab peptides. It is, therefore, possible that the number of bound peptides may somehow affect the residency time of the complex at the cell surface and thereby influence the mode of cell penetration. To test this hypothesis, various molar ratios of MCab and Strep-Cy3 were mixed together to prepare complexes with increased numbers of MCab immobilized on Strep-Cy3. The exact molar ratio between MCab and Strep-Cy3 can, however, not be warranted by simply mixing various molar ratios of the two molecules. Once these complexes were prepared, their cell entry along with the effect of amiloride was quantified by FACS (Fig. 9). Increasing the molar ratio of MCab over Strep-Cy3 from 1:1 to 8:1 dramatically increased the amount of Strep-Cy3 that penetrates into wild-type CHO cells (Fig. 9A). These data indicate that immobilizing an increasing number of MCab onto streptavidin greatly favors the entry of the complex, possibly by multiplying the number of contacts with cell surface components and/or increasing the residency time at the cell surface. In contrast, using increased amounts of non-biotinylated MCa, unable to bind Strep-Cy3, in place of MCab did not produce any increase in Strep-Cy3 penetration, indicating that coupling of MCa to Strep-Cy3 was required (Fig. 9B). This result also shows that the association of MCa to cell surface components does not trigger a generalized increase in cell endocytosis that would indirectly favor the penetration of MCab-Strep-Cy3 complexes. Finally, Fig. 9C indicates that coupling several MCab peptides to streptavidin does not quantitatively alter the effect of 5 mm amiloride, indicating that macropinocytosis remains the predominant mode of entry of the complex regardless of the MCab/Strep-Cy3 molar ratio used.
FIGURE 9.
Effect of MCab/strep ratio on the cell penetration of strep. A, effect of MCab/Strep-Cy3 ratio on the total entry of Strep-Cy3 in wild-type CHO cells. B, effect of an 8-fold molar excess of non-biotinylated MCa on the penetration of MCab-Strep-Cy3 at a 1:1 molar ratio. C, effect of 5 mm amiloride on Strep-Cy3 entry into wild-type CHO cells as a function of MCab/Strep-Cy3 ratio. Significance is provided as a deviation of 3× the S.D. value from 100% (denoted as asterisks). Strep-Cy3 concentration was kept constant at 1 μm in all experiments. a.u., arbitrary units.
DISCUSSION
HSPGs Are New Cell Surface Targets of MCa That Are Involved in Cell Penetration of This Peptide—Using a Biacore system, we have demonstrated that MCa, a member of a new family of CPPs, directly interacts with HP and HS with affinities in the low micromolar range (between 2 and 5 μm). These values are more or less well correlated to the PC50 values of MCab-Strep-Cy5 in CHO cells (around 0.5 μm), suggesting a contribution of HSPGs to the cell penetration of this complex. This slight difference could be related to the fact that each streptavidin molecule has the ability to bind four MCa molecules, thereby increasing the local concentration of the CPP in the vicinity of the cell surface receptors. Indeed, we show here that increasing the molar ratio of MCab over streptavidin during complex formation produces an increase in cell penetration efficiency. Alternatively, differences may also be related to the exact nature of the cell surface HSPG involved in MCa interaction. By using HS- and GAG-deficient CHO cell lines, we conclusively demonstrate that HSPGs quantitatively contribute to more than 57% of the cell entry of Strep-Cy5 when coupled to MCa. HS represents the most important GAG since it is responsible for 75% of the GAG contribution. However, because a significant fraction of the total cell entry is conserved in GAG-deficient cells, the entry of MCab-Strep-Cy5 does not solely rely on GAGs but also on other cell surface components, with apparent affinities closely related to that of MCa for HSPGs since the PC50 values varied only mildly in GAG-deficient CHO cells. Data presented here and in previous manuscripts (5, 6) indicate that membrane lipids are also cell surface receptors for MCa. For instance, MCa was found to interact with the ganglioside GD3 with a closely related apparent affinity of 0.49 μm. Another important conclusion that can be made from these data is that the increase or the decrease of the penetration efficiency observed with specific mutants of MCa, such as MCa K20A tested herein, results from a modification of the apparent affinity of these MCa mutants for the cell surface components with which they interact. For instance, MCa K20A was found to have reduced apparent affinity for both HS and HP (present data) but also for membrane lipids (6). There is, thus, an interesting parallel to pursue on the structural determinants of CPP interaction with HSPGs and negatively charged lipids that may ultimately result in the design of better CPP analogues. This observation appears particularly pertinent since the cell entry process of MCa-Strep-Cy5 or MCa-Strep-Cy3 complex, i.e. macropinocytosis, seems independent of the type of membrane receptor involved in MCa binding (HSPGs versus lipids).
HP Inhibition of the Cell Penetration of MCa-Strep Complex Is Not Limited to the Interaction of This CPP to Cell Surface HSPGs—HP-induced inhibition of CPPs cell entry is generally interpreted as being due to an inhibition of CPP interaction with cell surface HSPGs (28). However, an alternative possibility is that, by neutralizing the basic face of MCa, the interaction of HP with MCa also inhibits the subsequent interaction of MCa with negatively charged lipids of the cells, another surface component for the route of entry of MCa. Three sets of evidence indicate that this interpretation is likely to be correct. First, both soluble HS and HP inhibit the cell entry of MCab-Strep-Cy5 to levels beyond that measured for MCab-Strep-Cy5 entry in HS- and GAG-deficient CHO cells. Second, soluble HS and HP still produce significant reductions of MCab-Strep-Cy5 entry in GAG-deficient CHO cells, clearly indicating an inhibition through an alternate mode of inhibition. Third, incubation of HS with MCab produces a reduction in the interaction of MCab with several negatively charged lipids, the most dramatic effects being observed for PtdIns(3,4,5)P3 and phosphatidic acid. These observations indicate that interpretation of the involvement of cell surface HSPGs in the penetration of CPPs based on soluble HP inhibition should be performed carefully. They also confirm the importance of the basic face of CPPs in the mechanism of cell penetration. Finally, the presence of a residual MCa-Strep complex penetration in GAG-deficient CHO cells in the presence of HS or HP might indicate that either the interaction between HSPGs is rapidly reversible or that this interaction does not fully cover the entire molecular surface of MCa required for cell penetration. Further detailed biochemical experiments will be needed to sort out the molecular determinants of MCa involved in HP or HS interaction. Such an investigation will determine to what extent the basic surface of MCa is involved in an interaction with HSPGs.
Macropinocytosis Is the Main Endocytic Pathway Used by MCa When Coupled to Streptavidin in GAG-positive and GAG-deficient CHO Cells—In previous work, we reported that MCa-Strep complex penetration in HEK293 cells was also observed in the presence of amiloride or nystatin, suggesting that a non-endocytic pathway was involved in the penetration process (4). Here, we provide a quantitative analysis of the effects of endocytosis inhibitors on the entry of MCab-Strep-Cy5 in CHO cells using a FACS method and show that endocytosis represents the major route of penetration, whereas only 20% of MCab-Strep-Cy5 penetration is still observed in the presence of endocytosis inhibitors (amiloride). Interestingly, the amount of MCab-Strep-Cy5 taken up in the presence of amiloride is close to the amount of complex taken up in GAG-deficient CHO cells in the presence of HS or HP. The apparent discrepancy between the two studies is likely due to the fact that confocal analysis used in the previous work was not quantitative enough to allow the calculation of the relative importance of each mechanism. Moreover, we cannot rule out the possibility that some endocytosis pathway, insensitive to amiloride or nystatin, may be present in the previously studied HEK293 cell line. This indicates that one needs to be cautious with regard to confocal images that are unfortunately not quantitative enough to rule out one or several cellular mechanisms for cell entry. Use of a marker of endosomes demonstrates that MCab-Strep-Cy5 is distributed within endosomes after cell entry, supporting the fact that endocytosis is a predominant route of entry of the cargo when coupled to MCab. The total lack of co-localization between transferrin-Alexa Fluor 488 and MCab-Strep-Cy5 clearly indicates that clathrin-mediated endocytosis is not at play in the entry of MCab-Strep-Cy5. This was further proven by (i) the lack of effect of expression of dynamin 2 K44A, a dominant negative construct that inhibits clathrin-mediated endocytosis but does not prevent MCab-Strep-Cy3 entry and (ii) the differential effects of various endocytosis blockers on transferrin-Alexa Fluor 488 and MCab-Strep-Cy5 cell entries. The effects of cytochalasin D and of amiloride indicate that macropinocytosis is predominantly involved in the cell entry of MCab-Strep-Cy5. This observation is consistent with many other reports that indicate a role of macropinocytosis in cell entry of other CPPs (14, 29). However, the lack of effect of methyl-β-cyclodextrin appears to indicate that endocytosis of MCab-Strep-Cy5 is not dependent on lipid rafts or at least on cholesterol availability. Because macropinocytosis appears to be responsible for the uptake of MCab-Strep-Cy5/3 in wild-type and GAG-deficient CHO cells alike, it seems that all surface components able to bind MCa, negatively charged HSPGs, and lipids are involved in macropinocytosis. Because of the nature of macropinosomes, which do not fuse with lysosomes and are leaky, it is likely that release of CPPs in the cytosol may occur very slowly. In the case of the Strep-Cy5 cargo, this leakage was, however, not observed when coupled to MCab.
Cargo Dependence of MCa Mode of Penetration and/or Release in the Cytosol?—The mechanism of cell penetration of CPPs remains highly debated. There are pro and con arguments in favor of membrane translocation, a process whereby the peptide would flip from the outer face of the plasma membrane to the inner face then be released free into the cytoplasm. Here we do not provide compelling evidence for a translocation mechanism for Strep-Cy5 entry when coupled to MCab. On the contrary, the data strongly emphasize the importance of endocytosis in the penetration of the vector/cargo complex. Nevertheless, the issue of the mode of penetration of MCa itself remains open to a large extent. First, there is compelling evidence that MCa has a near-complete pharmacological effect when applied at the extracellular face of cells. Second, the pharmacological site of MCa on the ryanodine receptor has been localized to the cytosol face of the calcium channel. Taken together, these results suggest that MCa must reach the cytosol within seconds or minutes. Two possibilities can be envisioned; (i) MCa may be released within the cytosol from leaky macropinosomes immediately after uptake, but the time scale seems inappropriate, or (ii) when “free”, i.e. not coupled to a cargo, MCa may indeed simply translocate through the membrane. This raises immediately the question of the contribution of the cargo to the mode of entry of MCa. Streptavidin is a cargo that can bind four different MCab molecules. Linking multiple vectors to a single cargo molecule could theoretically complicate the mode of entry of MCa. Intuitively, one could imagine that multiple attachment points to cell surface components might hamper the translocation of the peptide through the plasma membrane, increase the residency time at the cell surface, and thereby strongly promote macropinocytosis over direct translocation. Experimentally, this is, however, not observed. The fact that amiloride inhibits penetration of MCab-Strep-Cy5 complex, prepared with 1 MCab for 1 Strep-Cy5, as efficiently as the penetration of MCab-Strep-Cy5 complex, prepared with 8 MCab for 1 Strep-Cy5, indicates that macropinocytosis is not influenced by the presence of multiple MCab molecules. Therefore, the putative difference between free MCab and MCab-cargo complex might be due to the nature of the cargo rather than its specific properties of MCab binding. In addition, the size of the cargo may itself represent a problem for simple diffusion of the complex from “leaky” macropinosomes to the cytosol. Further studies will be required to investigate the contribution of cargo size and nature in the mode of entry and cell distribution (cytosol versus endosomes) of the vector. Nevertheless, these data are coherent with many other studies on CPPs, and macropinocytosis is likely to be the main entry route of many other cargoes that will be attached to maurocalcine. Although streptavidin is used as a reporter cargo here (fluorescence property), it is worth mentioning that its ability to bind to several different biotinylated molecules at a time should be considered as a significant advantage for the cell delivery of multiple cargoes with a single MCa vector.
Acknowledgments
We thank Rabia Sadir for the kind preparation of oligosaccharides. We also thank Dr. McNiven MA for providing the cDNA encoding dynamin 2 K44A mutant.
This work was supported by INSERM, Université Joseph Fourier, and Commissariat à l'Energie Atomique (Saclay, France). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
A fellow of 1'Agence Francaise contre les Myopathies. The abbreviations used are: MCa, maurocalcine; MCab, biotinylated MCa; CHO, Chinese hamster ovary; CPP, cell penetrating peptide; CS, chondroitin sulfate; dp, degree of polymerization; FACS, fluorescence-activated cell sorter; HEK293, human embryonic kidney 293 cells; HS, heparan sulfate; HSPG, HS proteoglycans; HP, heparin; PC50, half-maximal penetration concentration; PBS, phosphate-buffered saline; PtdIns, phosphatidylinositol; Strep-Cy5 (Cy3), streptavidin-cyanine 5 (cyanine 3); RU, response units.
G. Pavlov and C. Ebel, personal communication.
References
- 1.Mosbah, A., Kharrat, R., Fajloun, Z., Renisio, J. G., Blanc, E., Sabatier, J. M., El Ayeb, M., and Darbon, H. (2000) Proteins 40 436-442 [DOI] [PubMed] [Google Scholar]
- 2.Esteve, E., Smida-Rezgui, S., Sarkozi, S., Szegedi, C., Regaya, I., Chen, L., Altafaj, X., Rochat, H., Allen, P., Pessah, I. N., Marty, I., Sabatier, J. M., Jona, I., De Waard, M., and Ronjat, M. (2003) J. Biol. Chem. 278 37822-37831 [DOI] [PubMed] [Google Scholar]
- 3.Chen, L., Esteve, E., Sabatier, J. M., Ronjat, M., De Waard, M., Allen, P. D., and Pessah, I. N. (2003) J. Biol. Chem. 278 16095-16106 [DOI] [PubMed] [Google Scholar]
- 4.Esteve, E., Mabrouk, K., Dupuis, A., Smida-Rezgui, S., Altafaj, X., Grunwald, D., Platel, J. C., Andreotti, N., Marty, I., Sabatier, J. M., Ronjat, M., and De Waard, M. (2005) J. Biol. Chem. 280 12833-12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boisseau, S., Mabrouk, K., Ram, N., Garmy, N., Collin, V., Tadmouri, A., Mikati, M., Sabatier, J. M., Ronjat, M., Fantini, J., and De Waard, M. (2006) Biochim. Biophys. Acta 1758 308-319 [DOI] [PubMed] [Google Scholar]
- 6.Mabrouk, K., Ram, N., Boisseau, S., Strappazzon, F., Rehaim, A., Sadoul, R., Darbon, H., Ronjat, M., and De Waard, M. (2007) Biochim. Biophys. Acta 1768 2528-2540 [DOI] [PubMed] [Google Scholar]
- 7.Derossi, D., Calvet, S., Trembleau, A., Brunissen, A., Chassaing, G., and Prochiantz, A. (1996) J. Biol. Chem. 271 18188-18193 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki, T., Futaki, S., Niwa, M., Tanaka, S., Ueda, K., and Sugiura, Y. (2002) J. Biol. Chem. 277 2437-2443 [DOI] [PubMed] [Google Scholar]
- 9.Vives, E., Richard, J. P., Rispal, C., and Lebleu, B. (2003) Curr. Protein Pept. Sci. 4 125-132 [DOI] [PubMed] [Google Scholar]
- 10.Vives, E., Brodin, P., and Lebleu, B. (1997) J. Biol. Chem. 272 16010-16017 [DOI] [PubMed] [Google Scholar]
- 11.Terrone, D., Sang, S. L., Roudaia, L., and Silvius, J. R. (2003) Biochemistry 42 13787-13799 [DOI] [PubMed] [Google Scholar]
- 12.Berlose, J. P., Convert, O., Derossi, D., Brunissen, A., and Chassaing, G. (1996) Eur. J. Biochem. 242 372-386 [DOI] [PubMed] [Google Scholar]
- 13.Magzoub, M., and Graslund, A. (2004) Q. Rev. Biophys. 37 147-195 [DOI] [PubMed] [Google Scholar]
- 14.Wadia, J. S., Stan, R. V., and Dowdy, S. F. (2004) Nat. Med. 10 310-315 [DOI] [PubMed] [Google Scholar]
- 15.Foerg, C., Ziegler, U., Fernandez-Carneado, J., Giralt, E., Rennert, R., BeckSickinger, A. G., and Merkle, H. P. (2005) Biochemistry 44 72-81 [DOI] [PubMed] [Google Scholar]
- 16.Sandgren, S., Cheng, F., and Belting, M. (2002) J. Biol. Chem. 277 38877-38883 [DOI] [PubMed] [Google Scholar]
- 17.Nakase, I., Tadokoro, A., Kawabata, N., Takeuchi, T., Katoh, H., Hiramoto, K., Negishi, M., Nomizu, M., Sugiura, Y., and Futaki, S. (2007) Biochemistry 46 492-501 [DOI] [PubMed] [Google Scholar]
- 18.Laguri, C., Sadir, R., Rueda, P., Baleux, F., Gans, P., Arenzana-Seisdedos, F., and Lortat-Jacob, H. (2007) PLoS ONE 2 e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadir, R., Baleux, F., Grosdidier, A., Imberty, A., and Lortat-Jacob, H. (2001) J. Biol. Chem. 276 8288-8296 [DOI] [PubMed] [Google Scholar]
- 20.Vives, R. R., Imberty, A., Sattentau, Q. J., and Lortat-Jacob, H. (2005) J. Biol. Chem. 280 21353-21357 [DOI] [PubMed] [Google Scholar]
- 21.Vives, R. R., Sadir, R., Imberty, A., Rencurosi, A., and Lortat-Jacob, H. (2002) Biochemistry 41 14779-14789 [DOI] [PubMed] [Google Scholar]
- 22.Sarrazin, S., Bonnaffe, D., Lubineau, A., and Lortat-Jacob, H. (2005) J. Biol. Chem. 280 37558-37564 [DOI] [PubMed] [Google Scholar]
- 23.Richard, J. P., Melikov, K., Vives, E., Ramos, C., Verbeure, B., Gait, M. J., Chernomordik, L. V., and Lebleu, B. (2003) J. Biol. Chem. 278 585-590 [DOI] [PubMed] [Google Scholar]
- 24.Kurten, R. C. (2003) Adv. Drug Delivery Rev. 55 1405-1419 [DOI] [PubMed] [Google Scholar]
- 25.Conner, S. D., and Schmid, S. L. (2003) Nature 422 37-44 [DOI] [PubMed] [Google Scholar]
- 26.Sun, T. X., Van Hoek, A., Huang, Y., Bouley, R., McLaughlin, M., and Brown, D. (2002) Am. J. Physiol. Renal Physiol. 282 998-1011 [DOI] [PubMed] [Google Scholar]
- 27.Mano, M., Teodosio, C., Paiva, A., Simoes, S., and Pedroso de Lima, M. C. (2005) Biochem. J. 390 603-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nascimento, F. D., Hayashi, M. A., Kerkis, A., Oliveira, V., Oliveira, E. B., Radis-Baptista, G., Nader, H. B., Yamane, T., Tersariol, I. L., and Kerkis, I. (2007) J. Biol. Chem. 282 21349-21360 [DOI] [PubMed] [Google Scholar]
- 29.Rinne, J., Albarran, B., Jylhava, J., Ihalainen, T. O., Kankaanpaa, P., Hytonen, V. P., Stayton, P. S., Kulomaa, M. S., and Vihinen-Ranta, M. (2007) BMC Biotechnol. 7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]