Abstract
The nicotinic acetylcholine receptors (nAChRs) are targets for human and veterinary medicines as well as insecticides. Subtype-selectivity among the diverse nAChR family members is important for medicines targeting particular disorders, and pest-insect selectivity is essential for the development of safer, environmentally acceptable insecticides. Neonicotinoid insecticides selectively targeting insect nAChRs have important applications in crop protection and animal health. Members of this class exhibit strikingly diverse actions on their nAChR targets. Here we review the chemistry and diverse actions of neonicotinoids on insect and mammalian nAChRs. Electrophysiological studies on native nAChRs and on wild-type and mutagenized recombinant nAChRs have shown that basic residues particular to loop D of insect nAChRs are likely to interact electrostatically with the nitro group of neonicotinoids. In 2008, the crystal structures were published showing neonicotinoids docking into the acetylcholine binding site of molluscan acetylcholine binding proteins with homology to the ligand binding domain (LBD) of nAChRs. The crystal structures showed that 1) glutamine in loop D, corresponding to the basic residues of insect nAChRs, hydrogen bonds with the NO2 group of imidacloprid and 2) neonicotinoid-unique stacking and CH-π bonds at the LBD. A neonicotinoid-resistant strain obtained by laboratory-screening has been found to result from target site mutations, and possible reasons for this are also suggested by the crystal structures. The prospects of designing neonicotinoids that are safe not only for mammals but also for beneficial insects such as honey bees (Apis mellifera) are discussed in terms of interactions with non-α nAChR subunits.
Sustainable agriculture aims to supply sufficient food for the world population while minimizing environmental impact. Neonicotinoids, targeting insect nicotinic acetylcholine receptors (insect nAChRs), have veterinary and crop protection applications, with their fast actions providing economic benefits. However, their target-selectivity is important to ensure safety and to limit adverse effects on beneficial insects such as honeybees.
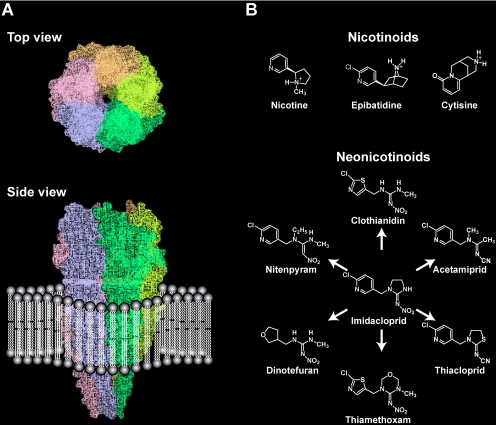
The nAChRs (Fig. 1A) are pentameric membrane proteins that rapidly transduce the actions of the chemical neurotransmitter acetylcholine (ACh) to membrane depolarization at synapses. Nicotine (Fig. 1B), a major alkaloid of the tobacco plant Nicotiana tabacum, is a nonhydrolyzable agonist of nAChRs and remains much longer at the synapses than ACh, which is hydrolyzed by acetylcholine esterase, inducing complex modifications to neural signaling. Human drugs targeting nAChRs are clinically important because they may offer therapeutic approaches for nicotine addiction, Alzheimer's disease and schizophrenia as well as treatment for some neuropathies resulting from mutations in nAChRs (Arneric et al., 2007; Dani and Bertrand, 2007; Levin and Rezvani, 2007; Changeux and Taly, 2008). Effective control of insect pests and helminth parasites has been achieved by targeting invertebrate nAChRs (Matsuda et al., 2001, 2005; Tomizawa and Casida, 2003, 2005; Brown et al., 2006b). This road from nicotine to neonicotinoids was long and tortuous. In general, compounds require appropriate lipophilicity to show high insecticidal actions because they can only access nAChRs after traversing waxy cuticle membranes and cells enveloping the nervous system. However, nicotine is protonated at neutral or lower pH, yielding a water-soluble ammonium that lowers its insect toxicity, although this ammonium form is recognized by nAChRs. In addition, the low field stability and adverse mammalian toxicity mean that nicotine is of historical interest only in pest control. The development of insecticides acting on insect nAChRs has posed a challenge. Indeed, until recently, the only successes were cartap, bensultap, and thiocyclam based on a marine worm (Lumbriconereis heteropoda) toxin nereistoxin. Cartap was shown to undergo hydrolytic activation to nereistoxin (Lee et al., 2004), which exerts toxicity by blocking nAChRs (Eldefrawi et al., 1980; Sattelle et al., 1985; Raymond Delpech et al., 2003; Lee et al., 2004). Although these nereistoxin derivatives are used for crop protection, their current market share is much smaller than that of organophosphates and pyrethroids.
Fig. 1.
The nAChR (A) and ligands (nicotinoids and neonicotinoids) (B). The model of the well characterized muscle nAChR was generated based on a PDB file of 2BG9 (Unwin, 2005) using Sybyl (version 7.1; Tripos Associates, Inc., St. Louis, MO).
The major commercial insecticides targeting nAChRs were not derived from natural products but rather from the discovery of synthetic nitromethylene heterocyles (Soloway et al., 1979; Kagabu, 1997). Although the leading compounds were not in the first wave, introducing the 6-chloro-3-pyridylmethyl and nitroimine moieties led to the development of the first new-type of nicotinic insecticide imidacloprid (Moriya et al., 1992; Kagabu, 1997). In parallel with synthesis, mode-of-action studies have been conducted to show that both early chemotypes, the nitromethylene heterocycles (Schroeder and Flattum, 1984; Sattelle et al., 1989; Leech et al., 1991) and imidacloprid (Bai et al., 1991) act on insect nAChRs. Imidacloprid rapidly expanded its share of the market, and several analogs followed. Because the chloronicotinyl (6-chloro-3-pyridylmethyl) moiety is seen in the first generation of imidacloprid analogs, they were once called chloronicotinyl insecticides. However, neither this moiety nor the imidazolidine ring features in the second generation of neonicotinoids (Fig. 1B). The generic name “neonicotinoids” has been adopted now for all members of this class to show that they are new in terms of their mode of action and their structural features that are clearly different from those of nicotine and nicotine-related compounds, “nicotinoids.”
Neonicotinoids show selective actions on insect nAChRs (Matsuda et al., 2001, 2005; Tomizawa and Casida, 2003, 2005; Thany et al., 2007). Electrophysiology, computational chemistry, and site-directed mutagenesis, in conjunction with homology modeling of the nAChR ligand binding domain (nAChR LBD)-imidacloprid complexes, have been used to elucidate the nature and the diversity of their actions. To understand the structural factors involved in the selectivity and diversity of neonicotinoid actions, we crystallized the molluscan Lymnaea stagnalis (Ls) acetylcholine binding protein (AChBP) in complex with neonicotinoids (imidacloprid and clothianidin) (Ihara et al., 2008). At about the same time, the crystal structures of the Aplysia californica (Ac)-AChBP in complex with imidacloprid and thiacloprid were elucidated (Talley et al., 2008). From these crystal structures, a common concept for the nAChR LBD-neonicotinoid interactions can be derived, which clearly differs from the binding modes of nicotinoids. Despite these achievements, two major problems may threaten the future of neonicotinoids: 1) the development of resistance in pests, and 2) the adverse effects on beneficial insect species. Structural insights relevant to these two problems are also discussed here.
Neonicotinoids and Nicotinic Ligands Defined by Computational Chemistry
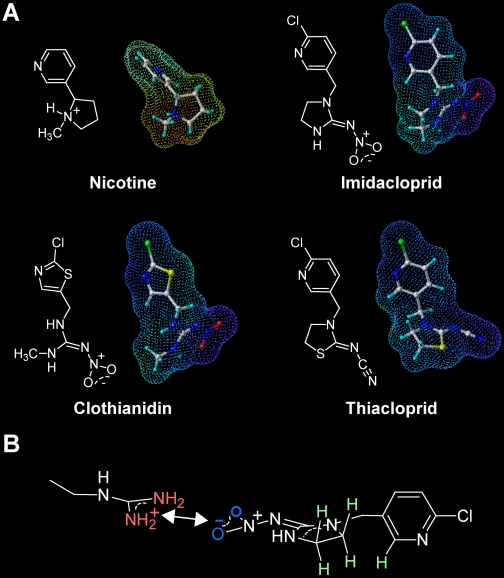
Nicotine possesses two nitrogens, one in the pyridine ring and another in the pyrrolidine ring. The basicity of the pyridine nitrogen is low because its lone-pair electrons participate in the aromatic system, whereas the pyrrolidine nitrogen can accept a proton to become a positively charged ammonium, mimicking the quaternary ammonium of ACh (Fig. 2).
Fig. 2.
Electrostatic potentials (EPs) surrounding nicotinoids and neonicotinoids (A), and interactions of imidacloprid with the arginine residue in insect nAChRs predicted by computational chemistry (B). In A, the EPs were calculated by the MNDO semiempirical molecular orbital method using the Sybyl (version 7.1; Tripos Associates, Inc.). Red dots indicate positive EPs, whereas blue dots indicate negative EPs. In B, the NO2 oxygens are highlighted in blue, whereas the protonated guanidine moiety of the arginine is highlighted in red. The hydrogens on the CH2-CH2 moiety in the imidazolidine ring and on the C2 carbon in the pyridine ring are colored light green because they are more electron-deficient than those on usual alkyl carbons. A part of these hydrogens form CH-π hydrogen bonds with the tryptophan ring in loop B in the crystal structures of acetylcholine binding proteins (see Fig. 4 for details).
Unlike nicotine, neonicotinoids (Fig. 1) are largely devoid of protonation. In the case of imidacloprid, two nitrogens in its imidazolidine ring are conjugated through a C=N bond with the electron-withdrawing nitro (NO2) group. Such a push-pull conjugation results in a coplanarity of the imidazolidine ring with the nitroimino (C=N-NO2) group (Kagabu, 1997). Positive electrostatic potentials surround the ammonium form of pyrrolidine in nicotine and similar properties hold for other nicotinic ligands such as epibatidine (Fig. 1). In contrast, such strongly positive regions are not seen in neonicotinoids. Instead, the NO2 oxygens and the CN nitrogen are negatively charged in neonicotinoids. In addition to their electrostatic nature, both groups can hydrogen-bond with local hydrogen donors.
The changes in atomic charge of the imidazolidine ring before and after the NO2 group-ammonium complex formation have been calculated (Matsuda et al., 2001). When complexed with ammonium, the imidazolidine ring, notably hydrogens of the CH2-CH2 moiety, become more electron-deficient, resulting in enhanced positive charges. Another calculation has shown that the NO2 group forms a stronger complex with a methylammonium than with phenol and methanol (Ihara et al., 2003). The following predictions for imidacloprid can be made from these calculations: 1) basic or hydrogen bondable residues, which are selectively present in the ACh binding region of insect nAChRs, contact the NO2 group of imidacloprid to strengthen the nAChR-neonicotinoid binding; 2) the complex formation also strengthens the electron-deficient nature of hydrogens in the imidazolidine CH2-CH2 as well as the π-electron nature of the lone pairs on the imidazolidine nitrogens; 3) these electron-deficient hydrogens are predicted to interact with electron-rich amino acid residues. This three-step binding consisting of 1) first contact, 2) changing electrostatic properties, and 3) attracting electron-rich residue is believed to be a kind of induced fit. In the case of ACh (Zhong et al., 1998) and nicotine (Cashin et al., 2005), cation-π interactions of the ammonium nitrogens with the aromatic ring of the tryptophan residue in loop B determine the binding affinity. Thus, it was predicted that the imidazolidine and related moieties may contact by cation-π electrostatic interactions the tryptophan residue in loop B (Tomizawa et al., 2003; Ihara et al., 2007). Another model was proposed based on ab initio molecular orbital calculations showing that the imidazolidine ring is likely to interact with the tryptophan by a π-π stacking (Wang et al., 2007). Conclusions from this in silico-based approach have been harder to reconcile with the subsequent crystal structures.
Differential Binding of Nicotine and Neonicotinoids
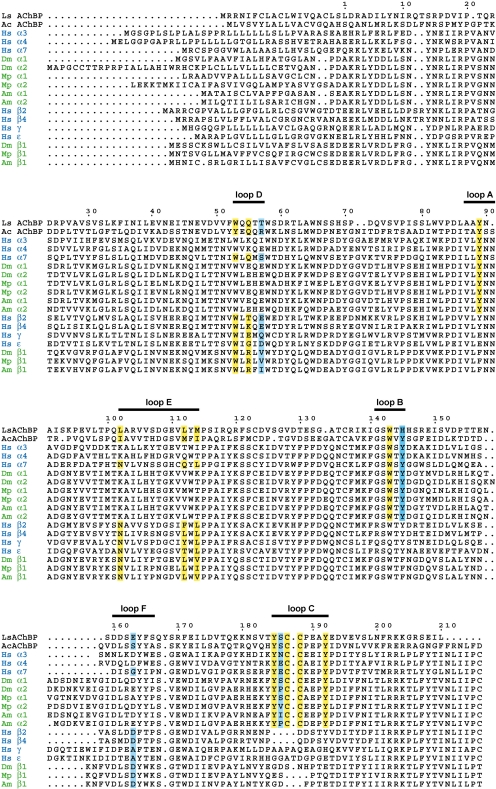
The nAChRs possess a long extracellular N-terminal LBD and four transmembrane (TM) regions with the C terminus also located extracellularly. Two classes of subunit are present among nAChRs (α and non-α), the α subunits, possessing a pair of adjacent cysteines in loop C of the ACh binding site (Karlin, 2002). The integral, cation-selective ion channel opens transiently upon binding of ACh. In the case of heteromeric nAChRs, ACh binds at the interface of the N-terminal regions of α and non-α subunits. However, in the case of either homomers (α7, α8, and α9) (Couturier et al., 1990; Elgoyhen et al., 1994; Gerzanich et al., 1994) or hetero-α-dimers such as α9/α10 (Elgoyhen et al., 2001) and Caenorhabditis elegans DEG-3/DES-2 (Treinin et al., 1998), the ACh binding site is formed at the interface of two adjacent α subunits. The α and non-α subunits, respectively, donate loops A to C and loops D to F to generate the ACh binding site (Corringer et al., 2000; Karlin, 2002). Site-directed mutagenesis and photoaffinity-labeling of amino acids that contact directly with agonists and antagonists have been deployed extensively in the case of vertebrate α7 nAChRs (Corringer et al., 2000). A general principle derived from these “wet” experiments requires confirmation by crystallization of nAChRs, but it has not yet been achieved, although exciting progress has been made in crystallizing bacterial ligand-gated ion channels (Bocquet et al., 2009; Hilf and Dutzler, 2008a,b). However, water-soluble AChBPs from molluscs Ls, Ac, and Bulinus truncatus have added considerably to our understanding of nAChR-ligand interactions. The AChBPs are homologous to the N-terminal ligand binding domain of α7 and also form a pentamer. Unlike nAChRs, the AChBPs lack the TM regions and are thus water-soluble. They act as an ACh-sink at molluscan synapses (Smit et al., 2001). The first AChBP crystal structure showed that the six binding site loops (A-F) (Fig. 3) are all located at subunit interfaces (Brejc et al., 2001) and AChBPs proved to be profitable surrogates of nAChRs with respect to exploring ligand interactions (Celie et al., 2004, 2005; Bourne et al., 2005, 2006; Hansen et al., 2005; Hansen and Taylor, 2007).
Fig. 3.
Multiple sequence alignments of AChBPs with nicotinic acetylcholine receptor α and non-α subunits. Amino acids that have been shown to interact directly with nicotine and neonicotinoids are highlighted with a yellow background, whereas those indirectly determining neonicotinoid sensitivity are shown with a light blue background (The X residue in the YXCC motif is tentatively highlighted with blue). The six loops comprising the ligand binding domain are shown above the sequences. Ls, Lymnaea stagnalis; Ac, Aplysia californica; Hs, Homo sapiens; Dm, Drosophila melanogaster; Mp, Myzus persicae; Am, Apis mellifera.
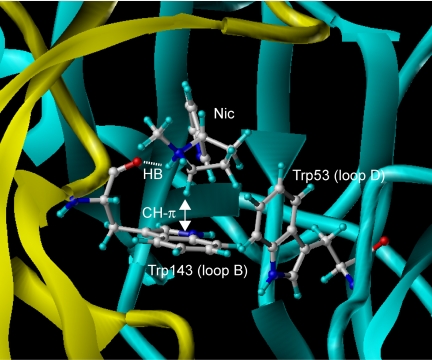
In the crystal structure of Ls-AChBP with nicotine bound (Celie et al., 2004), the proton on the pyrrolidine nitrogen of nicotine forms a hydrogen bond with the backbone C=Oof Trp143 (loop B), whereas N-CH3 in the pyrrolidine ring points to the center of the tryptophan ring, forming a CH-π hydrogen bond (Fig. 4). The CH-π hydrogen bond involves not only the London's dispersion force but also electrostatic interaction (Nishio, 2005). This interaction resembles a conventional hydrogen bond and therefore should not be referred to simply as a hydrophobic contact. The proton of N-CH3 also makes a CH-π interaction with Tyr192 (loop A; not shown in Fig. 4 to facilitate the view of nicotine-tryptophan interactions). Trp53 in loop D is located close to nicotine but only contributes to building a hydrophobic wall. In addition to these interactions, the cationic center of epibatidine, namely the protonated nitrogen, undergoes a cation-π interaction (Cashin et al., 2005), and the OH of Tyr93 (loop A) and the backbone C=O of Trp147 (loop B) form hydrogen bonds with the hydrogens on the bridge head nitrogen (Hansen et al., 2005). The pyridine nitrogen of nicotine and epibatidine forms a water bridge with the backbone C=O of two amino acids in loop E (Leu102 in both Ls- and Ac-AChBPs; Met114 in Ls-AChBP and Ile118 in Ac-AChBP).
Fig. 4.
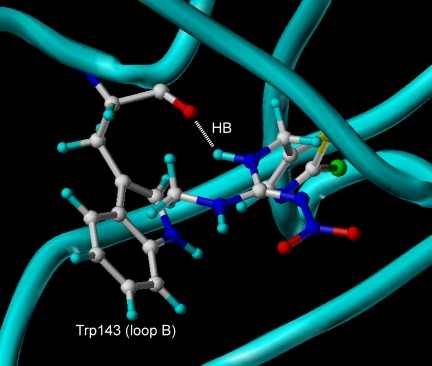
Amino acids playing a critical role in the interactions with nicotine in the crystal structure of Ls acetylcholine binding protein (PDB, 1UV6). The picture was generated using Sybyl (version 7.1; Tripos Associates, Inc.). Note that nicotine is captured by a hydrogen bond between NH and the backbone of Trp143 as well as a CH-π interaction between N+CH2-H and the tryptophan ring. The backbone of the principal side donating loops A to C is colored yellow, whereas that of the complementary side donating loops D to F is colored cyan. Nic, nicotine; HB, hydrogen bond.
The crystal structures of Ls-AChBP (Fig. 5A) (Ihara et al., 2008) and Ac-AChBP (Fig. 5B) (Talley et al., 2008) in complex with imidacloprid were elucidated almost at the same time. The five binding pockets are fully occupied with imidacloprid in Ls-AChBP, whereas in the crystal structure of Ac-AChBP, four of five sites are occupied, with the remaining site being complexed with an isopropyl alcohol molecule. Furthermore, one binding pocket of Ac-AChBP was bound by both imidacloprid and isopropyl alcohol. Although neonicotinoids show higher binding affinity for Ac-AChBP versus Ls-AChBP (Tomizawa et al., 2008), the binding modes of imidacloprid in these two crystals are quite similar. The pyridine ring forms a water bridge (Ihara et al., 2008; Talley et al., 2008) with the backbones of two amino acids in loop E similar to the binding seen for both nicotine (Celie et al., 2004) and epibatidine (Hansen et al., 2005). This result is in accord with photoaffinity-labeling results obtained using azidopyridine analogs (Tomizawa et al., 2007). However, because the pyridine ring recognition pattern is conserved in nicotinoids and neonicotinoids, this cannot explain the selectivity of neonicotinoids.
Fig. 5.
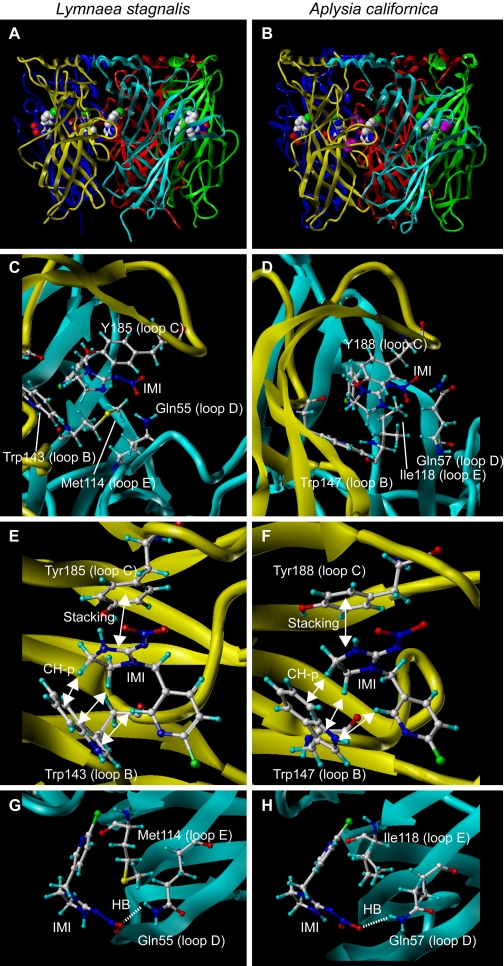
Amino acids interacting with a neonicotinoid imidacloprid in Ls- and Ac-AChBPs. A and B, side views of crystal structures of Ls- (A) and Ac- (B) AChBPs. All were prepared using Sybyl (version 7.1; Tripos Associates, Inc.). In A and B, imidacloprid and isopropyl alcohol (colored magenta) are generated in spheres to highlight. In C and D, amino acids interacting with imidacloprid in Ls- and Ac-AChBPs are shown, respectively. Irrespective of the mollusc species, a common mechanism is involved in the neonicotinoid recognition by the ligand binding domain of AChBPs. The main chain donating loops A to C is colored yellow, whereas the main chain giving loops D to F is shown cyan. In E (Ls-AChBP) and F (Ac-AChBP), amino acids from loops B and C are shown, whereas in G and H, those from loops D and E are shown in orientations facilitating view of interactions. Carbon, hydrogen, nitrogen, oxygen, and chlorine atoms are colored white, light blue, blue, red, and blue-green, respectively. IMI, imidacloprid; HB, hydrogen bond.
Interactions particular to imidacloprid are observed for the 2-nitroimino-imidazolidine moiety (Fig. 5, C and D). This group stacks with Tyr185 and Tyr188 in loop C of Ls- and Ac-AChBPs, respectively, whereas two protons in the CH2-CH2 moiety of the imidazolidine ring and a proton on the C2 of the pyridine ring of imidacloprid form CH-π hydrogen bonds with the tryptophan ring in loop B (Fig. 5, C-F). Because the tyrosine residue corresponding to Tyr185 of Ls-AChBP and Tyr188 of Ac-AChBP is conserved throughout vertebrate and invertebrate nAChRs (Fig. 3), its presence in itself is not the cause for selectivity. The nitro group of imidacloprid forms a hydrogen bond with a glutamine residue (Gln55 and Gln57 in loop D of Ls- and Ac-AChBPs, respectively) (Fig. 5, C, D, G, and H), and the corresponding residues of insect nAChRs are basic (Fig. 3). Thus, they are able to tether the nitro group of neonicotinoids by an electrostatic force. Furthermore, if the distance between the NO2 group and the basic residues is short, hydrogen bonds will add to the interaction. Therefore, the loop D basic residue (glutamine in AChBPs) plays a role in capturing neonicotinoids to strengthen the stacking and CH-π hydrogen bonds. Consistent with this, mutations of the corresponding loop D residues to basic residues were found to dramatically enhance the neonicotinoid sensitivity of the chicken α7 (Shimomura et al., 2002) and α4β2 nAChRs (Shimomura et al., 2006; Toshima et al., 2009). The selectivity-determining role of this residue can also explain, at least in part, why α7 having a glutamine (Gln89) residue in loop D is more neonicotinoid-sensitive than α4β2 (Ihara et al., 2003). In this context, AChBPs, from L. stagnalis or A. californica, resemble insect nAChRs because they possess this important residue. At first sight, the finding that human β4 has a lysine, at this otherwise highly conserved residue, is surprising, yet interestingly, β4-containing nAChRs are also less sensitive to imidacloprid than insect nAChRs (Lansdell and Millar, 2000). This too can be resolved by consideration of electrostatic interference, in this case involving a glutamate residue corresponding to Thr57 of Ls-AChBP, which is located very close to the basic residue (Ihara et al., 2008).
In the crystal structure of the Ls-AChBP-clothianidin complex, the NO2-Gln55 distance was outside the hydrogen bondable range. However, in the Q55R mutant, the basic residue contacts electrostatically with NO2 in Ls-AChBP (M. Ihara and K. Matsuda, unpublished data). For thiacloprid (Fig. 1), its thiazolidine ring stacks with Tyr188 in loop C, the CN group pointing to Ser189 in Ac-AChBP (Talley et al., 2008). Although this seems to indicate that loop D is not essential for selectivity, in its Q57R mutant, the CN group may point to the introduced arginine residue. In addition, appropriate care is required in the interpretation of the crystal data because isopropyl alcohol used for crystallization binds in the vicinity of thiacloprid (see the PDB file 3C84). Thus, for Ac-AChBP, isopropyl alcohol-free crystals with all five LBDs filled with neonicotinoids are desirable for detailed comparison and homology modeling.
Loop D alone is not the only determinant of selective neonicotinoid actions. Using the fruit fly Drosophila melanogaster α2(Dα2)/chicken β2 hybrid nAChR (Bertrand et al., 1994) and the chicken α4β2 nAChR, mutations of the X residue in the α-defining YXCC motif in loop C were found to strongly influence neonicotinoid sensitivity of the nAChRs (Shimomura et al., 2004). The D. melanogaster Dα2 subunit has a proline at this position, whereas in vertebrate α4 subunits this is a glutamate (see Fig. 3). The E219P mutation enhanced the response amplitude of the chicken α4β2 nAChR to imidacloprid, whereas a reverse mutation P242E markedly reduced the affinity and the efficacy of the Dα2β2 hybrid nAChR. The crystallographic data offer, at least in part, an explanation of these findings. In both Ls- and Ac-AChBPs, the corresponding residue is a serine (Ser186 in Ls-AChBP and Ser189 in Ac-AChBP). Ser186 in loop C contacts with Glu163 in loop F in Ls-AChBP (Fig. 3), whereas Ser189 forms a hydrogen bond with the NO2 of imidacloprid in Ac-AChBP. It is conceivable from the crystal structure of the Ls-AChBP-imidacloprid complex that vertebrate α2 and α4 subunits with a glutamate residue in this motif (YECC) (Fig. 3) will lead to an electrostatic repulsion when in contact with acidic residues in loop F, corresponding to Glu163 of Ls-AChBP. As a consequence of loop C-F repulsion, an intersubunit bridge is broken, resulting in a reduced affinity or efficacy of neonicotinoids. Supporting this hypothesis, neither Glu219 in loop C nor Thr77 in loop D contacts with the NO2 of imidacloprid in the homology model of the wild-type α4β2 LBD with imidacloprid bound (Toshima et al., 2009). An alternative explanation based on the crystal structure of Ac-AChBP-imidacloprid complex is that the acidic residue in loop C may directly repel the NO2 or CN groups of neonicotinoids, lowering affinity. We have found that the addition of serine to the YXCC motif of the chicken α4β2 nAChR scarcely influences the response to imidacloprid, and that, when combined together with the mutations in loop D, the X residue mutations to insect nAChR-type amino acids result in enhanced efficacy but not affinity of imidacloprid (Toshima et al., 2009). Thus, it is apparent that the YXCC motif affects the neonicotinoid sensitivity of nAChRs, yet a serine residue in this motif alone is not sufficient for the selective neonicotinoid actions on insect nAChRs, whether or not it contacts with the NO2 or the CN group of neonicotinoids.
Structural Factors and the Diverse Actions of Neonicotinoids
Voltage-clamp electrophysiology has shown that neonicotinoids act as partial, full and, in particular cases, super agonists on nAChRs. Imidacloprid is a partial agonist of native nAChRs expressed by insect neurons (Nagata et al., 1996, 1998; Déglise et al., 2002; Brown et al., 2006a) as well as the recombinant Dα2β2 hybrid nAChRs expressed in Xenopus laevis oocytes (Matsuda et al., 1998; Ihara et al., 2003). Opening of the imidazolidine ring leads to an enhanced efficacy (Ihara et al., 2003, 2004; Tan et al., 2007). For example, dinotefuran (Kagabu et al., 2002) and nitenpyram (Ihara et al., 2003) (Fig. 1) are full or nearly full agonists of the Dα2β2 hybrid nAChR. On the other hand, clothianidin and its analog both show higher agonist efficacy than ACh on the Dα2β2 hybrid nAChR (Ihara et al., 2004) and native D. melanogaster nAChRs (Brown et al., 2006a). Patch-clamp electrophysiology has been used to demonstrate that the clothianidin analog opened the native nAChRs at the highest conductance state more frequently than ACh, offering a possible explanation for its super agonist action. The crystal structure of Ls-AChBP in complex with clothianidin shows that the NH of the guanidine moiety of clothianidin forms a hydrogen bond with the backbone C=O of Trp143 in loop B (Fig. 6), which is not seen in the AChBP-imidacloprid complex (Ihara et al., 2008). It has been demonstrated that the agonist binding to LBD is likely to induce a global twist of nAChRs to gate the ion channels (Miyazawa et al., 2003; Taly et al., 2005; Unwin, 2005; Cheng et al., 2006). In this event, the agonist-binding-induced inward motion of loop C is transmitted to the cysteine loop through a structural rearrangement of loops D and A, resulting finally in the interaction of the cysteine loop and β2-β3 linker with the TM2-TM3 linker for the channel opening. For neonicotinoids interacting not only with loops B and C but also with loop D, this structural rearrangement is likely to cause its release from the binding site. The NH backbone hydrogen bond particular to clothianidin may help capture the ligand even after this structural rearrangement, thereby leading to enhanced channel opening.
Fig. 6.
The ligand binding domain of Ls acetylcholine binding protein in complex with clothianidin. The figure was generated using Sybyl (version 7.1; Tripos Associates, Inc.). Carbon, hydrogen, nitrogen, oxygen, and chlorine and sulfur atoms of clothianidin are colored white, light blue, blue, red, blue-green, and yellow, respectively. Note that the NH of the guanidine moiety of clothianidin forms a hydrogen bond (HB) with the backbone C=O of Trp143 in loop B. Such a clothianidin-unique hydrogen bond may be involved in the super agonist actions of clothianidin and its analog on native D. melanogaster nAChRs (Brown et al., 2006a) as well as recombinant D. melanogaster Dα2/chicken β2 hybrid nAChRs expressed in X. laevis oocytes (Ihara et al., 2004).
Met114 (Fig. 5G) in loop E of Ls-AChBP and corresponding Ile118 in Ac-AChBP (Fig. 5H) are located in the vicinity of the nitroimine moiety of imidacloprid. Because these amino acids are predicted to play a role in the agonist recognition, the effects of Leu118 mutations on the responses to ACh and imidacloprid were investigated. The L118E mutation almost completely blocked the response to imidacloprid, leaving the response to ACh, whereas the reverse was the case for L118K and L118R mutations (Amiri et al., 2008), suggesting a contribution to efficacy. Some insect nAChR α subunits possess a basic residue at this position. The possibility that such residues are also involved in the selective actions of neonicotinoids cannot be excluded because some α nAChRs are functional when they serve as partners for another α subunit (e.g., α10, which partners α9). The special case of loop E in α/α heteromers remains to be clarified.
Target-Based Neonicotinoid Resistance: A Structural Interpretation
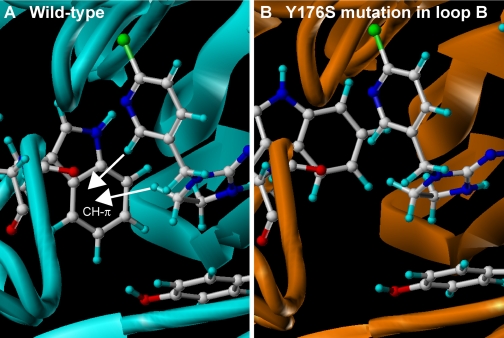
Two issues may limit the long-term utility of neonicotinoids: 1) resistance in pest species; and 2) adverse effects on beneficial insect species. Neonicotinoid resistance has been well described in rice plant hoppers (Matsumura et al., 2008; Wang et al., 2008), peach potato aphids (Foster et al., 2008), and whiteflies (Nauen et al., 2008). Neonicotinoid resistance is often the result of enhanced metabolism (Karunker et al., 2008; Nauen et al., 2008), but there are examples of reduced sensitivity to neonicotinoids at the target site. In the case of eastern U.S. field populations of the Colorado potato beetle Leptinotarsa decemlineata, imidacloprid sensitivity of the central nervous system in terms of excitation blocking action was found to be significantly reduced in resistant insects (Tan et al., 2008). Equally interesting is the study on the laboratory-selected neonicotinoid-resistant brown planthopper Nilaparvata lugens. Binding assays using [3H]imidacloprid show a reduced binding affinity for membrane preparations from the resistant population. A comparison of α and non-α subunit genes from susceptible and resistant population has shown that one point mutation, Y151S in loop B, can account for the reduced imidacloprid sensitivity (Liu et al., 2005). To understand the mechanism and to examine whether this kind of mutation lowers the neonicotinoid sensitivity in other insect species, wild-type and mutant α2β1 nAChRs from peach potato aphid Myzus persicae (Mpα2β1 nAChR) have been modeled using the crystal structures of Ls-AChBP in complex with imidacloprid (Fig. 7). This subunit combination was adopted for the following reasons: 1) both α2 and β1 subunits are important subunits and have a similar distribution in the D. melanogaster central nervous system (Jonas et al., 1994); 2) the D. melanogaster Dα2 and Dβ1 subunits were copurified with the Dα1 and Dα3 subunits from the fly heads by α-bungarotoxin-affinity column (Chamaon et al., 2002); 3) the Mpα2/rat β2 hybrid nAChR is much more sensitive to imidacloprid than the Mpα1/rat β2 hybrid nAChR (Huang et al., 1999), and the Mpα2 subunit coassembles with the Mpβ1 subunit in the D. melanogaster S2 cell (Huang et al., 2000); and 4) the Mpα2 subunit has a valine at position X in the YXCC motif (Fig. 3), which obviates the need to consider possible hydrogen bonding with NO2. In the wild-type Mpα2β1 nAChR, the imidazolidine ring stacks with loop C tyrosine, whereas two protons in the CH2-CH2 bridge make CH-π contacts with loop B tryptophan as seen in the AChBPs (Fig. 7A). The M. persicae tyrosine residue (Tyr176) corresponding to Tyr151 of the N. lugens α subunit is tightly packed in a hydrophobic groove (data not shown), thereby indirectly fixing the tryptophan residue in loop B. The tyrosine-to-serine mutation resulted in a reduced residue size, making the tryptophan residue wobble. As a consequence, the tryptophan residue has a reduced probability of proximity to the imidazolidine ring, thereby reducing the CH-π contacts with the imidazolidine ring and resulting in reduced neonicotinoid sensitivity. Among the commercial neonicotinoids, dinotefuran was found to act on the Y151S mutant of N. lugens nAChR as effectively as on the wild type (Liu et al., 2006). It will be of interest to examine in the future whether dinotefuran can compensate for the movement of the tryptophan residue by particular contacts with the mutant nAChR. This could lead to a strategy for rational design of novel neonicotinoids effective on target-based resistant pests.
Fig. 7.
LBD homology models of wild-type α2β1 nicotinic acetylcholine receptor from the peach potato aphid M. persicae (A) and Y176S mutant (B). Models were constructed according to Toshima et al. (2009). Modeling of the N-terminal region of M. persicae α2β1 and its Y176S mutant nAChRs was carried out using the molecular modeling software package Sybyl (version 7.3; Tripos Associates, Inc.) and the homology modeling software PDFAMS Ligands & Complex (version 2.1; In-Silico Sciences, Inc., Tokyo, Japan) in the ligand and complex mode. Both α2 and β1 subunits were aligned with the Ls-AChBP bound by imidacloprid (PDB code 2ZJU). In the second step, the three-dimensional structures of the wild-type and the Y176S mutant LBD-imidacloprid complexes were generated based on the sequence alignment and the coordinates of the AChBP and imidacloprid using the simulated annealing method (Kirkpatrick et al., 1983). The coordinates of imidacloprid were fixed during the simulated annealing. The receptor model constructed in this way was energy-minimized for 5000 iterations of conjugated gradients using the force field and partial charges of the molecular mechanics MMFF94 (Halgren 1999a,b) using Sybyl. Residues within a 10-Å radius of the centrally located imidacloprid, as well as imidacloprid itself, were treated as flexible entities except the C=N-NO2 moiety, which was fixed during energy minimization. In addition, residues within 10 to 15 Å radius of the ligand were considered rigid entities to speed up the computation. Other residues were ignored in energy minimization. Carbon, hydrogen, nitrogen, oxygen, and chlorine atoms of amino acids and imidacloprid are colored white, light blue, blue, red, and blue-green, respectively. Note that the CH-π hydrogen bonds with the tryptophan residue in loop B are reduced by this mutation, consistent with enhanced imidacloprid-resistance in pests.
Prospects for Design of Species-Specific Insecticides
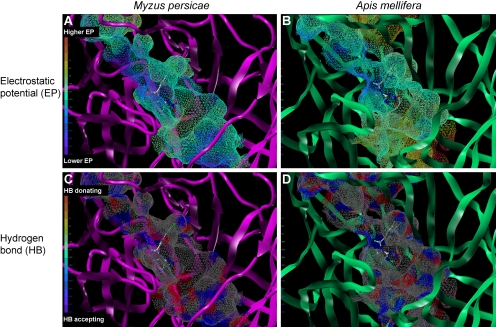
Another issue confronting neonicotinoids is the adverse effects on honeybees (Guez et al., 2001, 2003; Decourtye et al., 2003, 2004; Faucon et al., 2005; Yang et al., 2008), although they are safe to mammals. To explore a solution to this issue, we have modeled in complex with imidacloprid, cotton peach aphid (M. persicae), and honeybee (A. mellifera) α2β1 nAChRs (Fig. 8). The stacking and CH-π interactions (Fig. 5) are conserved irrespective of insect species, yet marked differences between the binding sites of the two insect species are seen at a hidden groove extending from the NO2 binding site, formed mainly by loops D and F, with particular reference to electrostatic (Fig. 8, A and B) and hydrogen-bond accepting/donating features (Fig. 8, C and D). First, the groove in the aphid nAChR is broader than that of the bee receptor. Second, higher electrostatic regions distribute more broadly in the bee receptor (Fig. 8A) than in the aphid receptor (Fig. 8B). Finally, the aphid nAChR groove (Fig. 8C) contains more hydrogen bond-forming hooks than the bee nAChR groove (Fig. 8D). These predictions suggest a concept insecticide generation, in which designing a molecular fragment for optimal fit to the groove is the first step. Then, linking this fragment with a traditional neonicotinoid framework using a functional group that is isosteric to the nitro group may yield new insect control chemicals highly selective for pest species nAChRs. Alternatively, “Crick Chemistry” (Kolb et al., 2001) may be applied to link the two fragments. By this mean, one fragment with an alkynyl end is reacted with another fragment containing an azide group on the pest nAChRs in aqueous solution at ambient temperature. Such pest target-selective neonicotinoids could help resolve a major issue in crop protection.
Fig. 8.
Electrostatic (A and B) and hydrogen bonding (C and D) fields extended from the ligand binding site of the peach potato aphid M. persicae (A and C) and the honeybee A. mellifera (B and D). Models were constructed according to Toshima et al. (2009). In A and B, regions with high (positive) and low (negative) electrostatic potentials are colored red and blue, respectively. In C and D, the hydrogen-bond-accepting atoms such as nitrogens and oxygens are colored blue, whereas the hydrogen atoms attached to these hetero-atoms are colored red. In all, carbon, hydrogen, nitrogen, oxygen, and chlorine atoms of imidacloprid are colored white, light blue, blue, red, and blue-green, respectively. The grooves extending from the NO2 binding site in the pest and beneficial species nAChRs differ in terms of the size, electrostatic, and hydrogen bonding properties, which may lead to a generation of pest target-selective insecticides.
Concluding Remarks
We have discussed the structural basis of how nAChRs recognize nicotinoids and neonicotinoids. Several hooks play important roles in either capturing or repelling small but important features of these ligands. So far, the importance of non-α subunits in the interactions of nicotinic ligands with heteromeric nAChRs seems to have been underestimated. The interactions with loops A to C are common for nicotinic and neonicotinic ligands, and selectivity is often donated by interactions with loops D to F. For nicotinoids or neonicotinoids, the hidden grooves and hydrogen bonding options offer a treasure trove of possibilities for generating novel ligands selective to nAChR subtypes. For rapid progress, the crystallization of an entire nAChR molecule is needed. Now that bacterial pentameric ligand-gated ion channels have been crystallized (Bocquet et al., 2009; Hilf and Dutzler, 2008a,b), a new era with rational design of a key component is an exciting and perhaps a not-too-distant prospect.
This work was supported by the “Academic Frontier” Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology [Grant 04F011]; a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science [Grant 21380039]; the Integrated Research Project for Plant, Insect and Animal using Genome Technology from the Ministry of Agriculture, Forestry and Fisheries of Japan [Grant 1302]; and by The Medical Research Council of the United Kingdom.
ABBREVIATIONS: nAChR, nicotinic acetylcholine receptor; Ac, Aplysia californica; ACh, acetylcholine; AChBP, acetylcholine binding protein; LBD, ligand binding domain; Ls, Lymnaea stagnalis; TM, transmembrane region; EP, electrostatic potential; PDB, Protein Data Bank.
References
- Amiri S, Shimomura M, Vijayan R, Nishiwaki H, Akamatsu M, Matsuda K, Jones AK, Sansom MS, Biggin PC, and Sattelle DB (2008) A role for Leu118 of loop E in agonist binding to the α7 nicotinic acetylcholine receptor. Mol Pharmacol 73 1659-1667. [DOI] [PubMed] [Google Scholar]
- Arneric SP, Holladay M, and Williams M (2007) Neuronal nicotinic receptors: a perspective on two decades of drug discovery research. Biochem Pharmacol 74 1092-1101. [DOI] [PubMed] [Google Scholar]
- Bai D, Lummis S, Leicht W, Breer H, and Sattelle D (1991) Actions of imidacloprid and a related nitromethylene on cholinergic receptors of an identified insect motor neurone. Pestic Sci 33 197-204. [Google Scholar]
- Bertrand D, Ballivet M, Gomez M, Bertrand S, Phannavong B, and Gundelfinger ED (1994) Physiological properties of neuronal nicotinic receptors reconstituted from the vertebrate β2 subunit and Drosophila a subunits. Eur J Neurosci 6 869-875. [DOI] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, and Corringer PJ (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457 111-114. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Hansen SB, Sulzenbacher G, Talley TT, Huxford T, Taylor P, and Marchot P (2006) Structural comparison of three crystalline complexes of a peptidic toxin with a synaptic acetylcholine recognition protein. J Mol Neurosci 30 103-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y, Talley TT, Hansen SB, Taylor P, and Marchot P (2005) Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake alphaneurotoxins and nicotinic receptors. EMBO J 24 1512-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, and Sixma TK (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411 269-276. [DOI] [PubMed] [Google Scholar]
- Brown LA, Ihara M, Buckingham SD, Matsuda K, and Sattelle DB (2006a) Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. J Neurochem 99 608-615. [DOI] [PubMed] [Google Scholar]
- Brown LA, Jones AK, Buckingham SD, Mee CJ, and Sattelle DB (2006b) Contributions from Caenorhabditis elegans functional genetics to antiparasitic drug target identification and validation: nicotinic acetylcholine receptors, a case study. Int J Parasitol 36 617-624. [DOI] [PubMed] [Google Scholar]
- Cashin AL, Petersson EJ, Lester HA, and Dougherty DA (2005) Using physical chemistry to differentiate nicotinic from cholinergic agonists at the nicotinic acetylcholine receptor. J Am Chem Soc 127 350-356. [DOI] [PubMed] [Google Scholar]
- Celie PH, Kasheverov IE, Mordvintsev DY, Hogg RC, van Nierop P, van Elk R, van Rossum-Fikkert SE, Zhmak MN, Bertrand D, Tsetlin V, Sixma TK, and Smit AB (2005) Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an α-conotoxin PnIA variant. Nat Struct Mol Biol 12 582-588. [DOI] [PubMed] [Google Scholar]
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, and Sixma TK (2004) Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41 907-914. [DOI] [PubMed] [Google Scholar]
- Chamaon K, Smalla KH, Thomas U, and Gundelfinger ED (2002) Nicotinic acetylcholine receptors of Drosophila: three subunits encoded by genomically linked genes can co-assemble into the same receptor complex. J Neurochem 80 149-157. [DOI] [PubMed] [Google Scholar]
- Changeux JP and Taly A (2008) Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med 14 93-102. [DOI] [PubMed] [Google Scholar]
- Cheng X, Lu B, Grant B, Law RJ, and McCammon JA (2006) Channel opening motion of α7 nicotinic acetylcholine receptor as suggested by normal mode analysis. J Mol Biol 355 310-324. [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Le Novère N, and Changeux JP (2000) Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol 40 431-458. [DOI] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, and Ballivet M (1990) A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 5 847-856. [DOI] [PubMed] [Google Scholar]
- Dani JA and Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47 699-729. [DOI] [PubMed] [Google Scholar]
- Decourtye A, Devillers J, Cluzeau S, Charreton M, and Pham-Delègue MH (2004) Effects of imidacloprid and deltamethrin on associative learning in honeybees under semifield and laboratory conditions. Ecotoxicol Environ Saf 57 410-419. [DOI] [PubMed] [Google Scholar]
- Decourtye A, Lacassie E, and Pham-Delègue MH (2003) Learning performances of honeybees (Apis mellifera L) are differentially affected by imidacloprid according to the season. Pest Manag Sci 59 269-278. [DOI] [PubMed] [Google Scholar]
- Déglise P, Grünewald B, and Gauthier M (2002) The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci Lett 321 13-16. [DOI] [PubMed] [Google Scholar]
- Eldefrawi AT, Bakry NM, Eldefrawi ME, Tsai MC, and Albuquerque EX (1980) Nereistoxin interaction with the acetylcholine receptor-ionic channel complex. Mol Pharmacol 17 172-179. [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, and Heinemann S (1994) α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79 705-715. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, and Boulter J (2001) α10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A 98 3501-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucon JP, Aurières C, Drajnudel P, Mathieu L, Ribière M, Martel AC, Zeggane S, Chauzat MP, and Aubert MF (2005) Experimental study on the toxicity of imidacloprid given in syrup to honey bee (Apis mellifera) colonies. Pest Manag Sci 61 111-125. [DOI] [PubMed] [Google Scholar]
- Foster SP, Cox D, Oliphant L, Mitchinson S, and Denholm I (2008) Correlated responses to neonicotinoid insecticides in clones of the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae). Pest Manag Sci 64 1111-1114. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Anand R, and Lindstrom J (1994) Homomers of α8 and α7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties. Mol Pharmacol 45 212-220. [PubMed] [Google Scholar]
- Guez D, Belzunces LP, and Maleszka R (2003) Effects of imidacloprid metabolites on habituation in honeybees suggest the existence of two subtypes of nicotinic receptors differentially expressed during adult development. Pharmacol Biochem Behav 75 217-222. [DOI] [PubMed] [Google Scholar]
- Guez D, Suchail S, Gauthier M, Maleszka R, and Belzunces LP (2001) Contrasting effects of imidacloprid on habituation in 7- and 8-day-old honeybees (Apis mellifera). Neurobiol Learn Mem 76 183-191. [DOI] [PubMed] [Google Scholar]
- Halgren TA (1999a) MMFF VI. MMFF94s option for energy minimization studies. J Comput Chem 20 720-729. [DOI] [PubMed] [Google Scholar]
- Halgren TA (1999b) MMFF VII. Characterization of MMFF94, MMFF94s, and other widely available force fields for conformational energies and for intermolecular-interaction energies and geometries. J Comput Chem 20 730-748. [DOI] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, and Bourne Y (2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J 24 3635-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SB and Taylor P (2007) Galanthamine and non-competitive inhibitor binding to ACh-binding protein: evidence for a binding site on non-α-subunit interfaces of heteromeric neuronal nicotinic receptors. J Mol Biol 369 895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf RJ and Dutzler R (2008a) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457 115-118. [DOI] [PubMed] [Google Scholar]
- Hilf RJ and Dutzler R (2008b) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452 375-379. [DOI] [PubMed] [Google Scholar]
- Huang Y, Williamson MS, Devonshire AL, Windass JD, Lansdell SJ, and Millar NS (1999) Molecular characterization and imidacloprid selectivity of nicotinic acetylcholine receptor subunits from the peach-potato aphid Myzus persicae. J Neurochem 73 380-389. [DOI] [PubMed] [Google Scholar]
- Huang Y, Williamson MS, Devonshire AL, Windass JD, Lansdell SJ, and Millar NS (2000) Cloning, heterologous expression and co-assembly of Mpβ1, a nicotinic acetylcholine receptor subunit from the aphid Myzus persicae. Neurosci Lett 284: 116-120. [DOI] [PubMed] [Google Scholar]
- Ihara M, Matsuda K, Otake M, Kuwamura M, Shimomura M, Komai K, Akamatsu M, Raymond V, and Sattelle DB (2003) Diverse actions of neonicotinoids on chicken α7, α4β2 and Drosophila-chicken SADβ2 and ALSβ2 hybrid nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Neuropharmacology 45 133-144. [DOI] [PubMed] [Google Scholar]
- Ihara M, Matsuda K, Shimomura M, Sattelle DB, and Komai K (2004) Super agonist actions of clothianidin and related compounds on the SADβ2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Biosci Biotechnol Biochem 68 761-763. [DOI] [PubMed] [Google Scholar]
- Ihara M, Okajima T, Yamashita A, Oda T, Hirata K, Nishiwaki H, Morimoto T, Akamatsu M, Ashikawa Y, Kuroda S, Mega R, Kuramitsu S, Sattelle DB, and Matsuda K (2008) Crystal structures of Lymnaea stagnalis AChBP in complex with neonicotinoid insecticides imidacloprid and clothianidin. Invert Neurosci 8 71-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Shimomura M, Ishida C, Nishiwaki H, Akamatsu M, Sattelle DB, and Matsuda K (2007) A hypothesis to account for the selective and diverse actions of neonicotinoid insecticides at their molecular targets, nicotinic acetylcholine receptors: catch and release in hydrogen bond networks. Invert Neurosci 7 47-51. [DOI] [PubMed] [Google Scholar]
- Jonas PE, Phannavong B, Schuster R, Schröder C, and Gundelfinger ED (1994) Expression of the ligand-binding nicotinic acetylcholine receptor subunit Dα2 in the Drosophila central nervous system. J Neurobiol 25 1494-1508. [DOI] [PubMed] [Google Scholar]
- Kagabu S (1997) Chloronicotinyl insecticides—discovery, application and future perspective. Rev Toxicol 1 75-129. [Google Scholar]
- Kagabu S, Matsuda K, and Komai K (2002) Preparation of dinotefuran related compounds and agonistic actions on SADβ2 hybrid nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. J Pestic Sci 27 375-377. [Google Scholar]
- Karlin A (2002) Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci 3 102-114. [DOI] [PubMed] [Google Scholar]
- Karunker I, Benting J, Lueke B, Ponge T, Nauen R, Roditakis E, Vontas J, Gorman K, Denholm I, and Morin S (2008) Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem Mol Biol 38 634-644. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick S, Gelatt CD Jr, and Vecchi MP (1983) Optimization by simulated annealing. Science 220 671-680. [DOI] [PubMed] [Google Scholar]
- Kolb HC, Finn MG, and Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl 40 2004-2021. [DOI] [PubMed] [Google Scholar]
- Lansdell SJ and Millar NS (2000) The influence of nicotinic receptor subunit composition upon agonist, α-bungarotoxin and insecticide (imidacloprid) binding affinity. Neuropharmacology 39 671-679. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Caboni P, Tomizawa M, and Casida JE (2004) Cartap hydrolysis relative to its action at the insect nicotinic channel. J Agric Food Chem 52 95-98. [DOI] [PubMed] [Google Scholar]
- Leech CA, Jewess P, Marshall J, and Sattelle DB (1991) Nitromethylene actions on in situ and expressed insect nicotinic acetylcholine receptors. FEBS Lett 290 90-94. [DOI] [PubMed] [Google Scholar]
- Levin ED and Rezvani AH (2007) Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol 74 1182-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Williamson MS, Lansdell SJ, Denholm I, Han Z, and Millar NS (2005) A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc Natl Acad Sci U S A 102 8420-8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Williamson MS, Lansdell SJ, Han Z, Denholm I, and Millar NS (2006) A nicotinic acetylcholine receptor mutation (Y151S) causes reduced agonist potency to a range of neonicotinoid insecticides. J Neurochem 99 1273-1281. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Buckingham SD, Freeman JC, Squire MD, Baylis HA, and Sattelle DB (1998) Effects of the α subunit on imidacloprid sensitivity of recombinant nicotinic acetylcholine receptors. Br J Pharmacol 123 518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, and Sattelle DB (2001) Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22 573-580. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Shimomura M, Ihara M, Akamatsu M, and Sattelle DB (2005) Neonicotinoids show selective and diverse actions on their nicotinic receptor targets: electrophysiology, molecular biology, and receptor modeling studies. Biosci Biotechnol Biochem 69 1442-1452. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Takeuchi H, Satoh M, Sanada-Morimura S, Otuka A, Watanabe T, and Van Thanh D (2008) Species-specific insecticide resistance to imidacloprid and fipronil in the rice planthoppers Nilaparvata lugens and Sogatella furcifera in East and South-east Asia. Pest Manag Sci 64 1115-1121. [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, and Unwin N (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423 949-955. [DOI] [PubMed] [Google Scholar]
- Moriya K, Shibuya K, Hattori Y, Tsuboi S, Shiokawa K, and Kagabu S (1992) 1-(6-Chloronicotinyl)-2-nitroimino-imidazolidines and related compounds as potential new insecticides. Biosci Biotechnol Biochem 56 364-365. [Google Scholar]
- Nagata K, Aistrup GL, Song JH, and Narahashi T (1996) Subconductance-state currents generated by imidacloprid at the nicotinic acetylcholine receptor in PC 12 cells. Neuroreport 7 1025-1028. [DOI] [PubMed] [Google Scholar]
- Nagata K, Song JH, Shono T, and Narahashi T (1998) Modulation of the neuronal nicotinic acetylcholine receptor-channel by the nitromethylene heterocycle imidacloprid. J Pharmacol Exp Ther 285 731-738. [PubMed] [Google Scholar]
- Nauen R, Bielza P, Denholm I, and Gorman K (2008) Age-specific expression of resistance to a neonicotinoid insecticide in the whitefly Bemisia tabaci. Pest Manag Sci 64 1106-1110. [DOI] [PubMed] [Google Scholar]
- Nishio M (2005) CH/π hydrogen bonds in organic reactions. Tetrahedron 61 6923-6950. [Google Scholar]
- Raymond Delpech V, Ihara M, Coddou C, Matsuda K, and Sattelle DB (2003) Action of nereistoxin on recombinant neuronal nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Invert Neurosci 5 29-35. [DOI] [PubMed] [Google Scholar]
- Sattelle DB, Buckingham SD, Wafford KA, Sherby SM, Bakry NM, Eldefrawi AT, Eldefrawi ME, and May TE (1989) Actions of the insecticide 2(nitromethylene)tetrahydro-1,3-thiazine on insect and vertebrate nicotinic acetylcholine receptors. Proc R Soc Lond B Biol Sci 237 501-514. [DOI] [PubMed] [Google Scholar]
- Sattelle DB, Harrow ID, David JA, Pelhate M, Callect JJ, Gepner JI, and Hall LM (1985) Nereistoxin: action on a CNS acetylcholine receptor/ion channel in the cockroach Periplaneta americana. J Exp Biol 118 37-52. [Google Scholar]
- Schroeder M and Flattum R (1984) The mode of action and neurotoxic properties of the nitromethylene heterocycle insecticides. Pestic Biochem Physiol 22 148-160. [Google Scholar]
- Shimomura M, Okuda H, Matsuda K, Komai K, Akamatsu M, and Sattelle DB (2002) Effects of mutations of a glutamine residue in loop D of the α7 nicotinic acetylcholine receptor on agonist profiles for neonicotinoid insecticides and related ligands. Br J Pharmacol 137 162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura M, Yokota M, Ihara M, Akamatsu M, Sattelle DB, and Matsuda K (2006) Role in the selectivity of neonicotinoids of insect-specific basic residues in loop D of the nicotinic acetylcholine receptor agonist binding site. Mol Pharmacol 70 1255-1263. [DOI] [PubMed] [Google Scholar]
- Shimomura M, Yokota M, Matsuda K, Sattelle DB, and Komai K (2004) Roles of loop C and the loop B-C interval of the nicotinic receptor α subunit in its selective interactions with imidacloprid in insects. Neurosci Lett 363 195-198. [DOI] [PubMed] [Google Scholar]
- Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, Lodder H, van der Schors RC, van Elk R, Sorgedrager B, Brejc K, Sixma TK, and Geraerts WP (2001) A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature 411 261-268. [DOI] [PubMed] [Google Scholar]
- Soloway SB, Henry AC, Kollmeyer WD, Padgett WM, Powell JE, Roman SA, Tieman CH, Corey RA and Horne CA (1979) Nitromethylene insecticides, in Advances in Pesticide Science Part 2 (Geissbühler H, Brooks GT and Kearney PC eds) pp 206-217, Pergamon, Oxford.
- Taly A, Delarue M, Grutter T, Nilges M, Le Novère N, Corringer PJ, and Changeux JP (2005) Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys J 88 3954-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley TT, Harel M, Hibbs RE, Radic Z, Tomizawa M, Casida JE, and Taylor P (2008) Atomic interactions of neonicotinoid agonists with AChBP: molecular recognition of the distinctive electronegative pharmacophore. Proc Natl Acad Sci U S A 105 7606-7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Galligan JJ, and Hollingworth RM (2007) Agonist actions of neonicotinoids on nicotinic acetylcholine receptors expressed by cockroach neurons. Neurotoxicology 28 829-842. [DOI] [PubMed] [Google Scholar]
- Tan J, Salgado VL, and Hollingworth RM (2008) Neural actions of imidacloprid and their involvement in resistance in the Colorado potato beetle, Leptinotarsa decemlineata (Say). Pest Manag Sci 64 37-47. [DOI] [PubMed] [Google Scholar]
- Thany SH, Lenaers G, Raymond-Delpech V, Sattelle DB, and Lapied B (2007) Exploring the pharmacological properties of insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 28 14-22. [DOI] [PubMed] [Google Scholar]
- Tomizawa M and Casida JE (2003) Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol 48 339-364. [DOI] [PubMed] [Google Scholar]
- Tomizawa M and Casida JE (2005) Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 45 247-268. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Maltby D, Talley TT, Durkin KA, Medzihradszky KF, Burlingame AL, Taylor P, and Casida JE (2008) Atypical nicotinic agonist bound conformations conferring subtype selectivity. Proc Natl Acad Sci U S A 105 1728-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa M, Talley TT, Maltby D, Durkin KA, Medzihradszky KF, Burlingame AL, Taylor P, and Casida JE (2007) Mapping the elusive neonicotinoid binding site. Proc Natl Acad Sci U S A 104 9075-9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa M, Zhang N, Durkin KA, Olmstead MM, and Casida JE (2003) The neonicotinoid electronegative pharmacophore plays the crucial role in the high affinity and selectivity for the Drosophila nicotinic receptor: an anomaly for the nicotinoid cation-π interaction model. Biochemistry 42 7819-7827. [DOI] [PubMed] [Google Scholar]
- Toshima K, Kanaoka S, Yamada A, Tarumoto K, Akamatsu M, Sattelle DB, and Matsuda K (2009) Combined roles of loops C and D in the interactions of a neonicotinoid insecticide imidacloprid with the α4β2 nicotinic acetylcholine receptor. Neuropharmacology 56 264-272. [DOI] [PubMed] [Google Scholar]
- Treinin M, Gillo B, Liebman L, and Chalfie M (1998) Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc Natl Acad Sci U S A 95 15492-15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol 346 967-989. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen J, Zhu YC, Ma C, Huang Y, and Shen J (2008) Susceptibility to neonicotinoids and risk of resistance development in the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Pest Manag Sci 64 1278-1284. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng J, Qian X, and Li Z (2007) Actions between neonicotinoids and key residues of insect nAChR based on an ab initio quantum chemistry study: hydrogen bonding and cooperative π-π interaction. Bioorg Med Chem 15 2624-2630. [DOI] [PubMed] [Google Scholar]
- Yang EC, Chuang YC, Chen YL, and Chang LH (2008) Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J Econ Entomol 101 1743-1748. [DOI] [PubMed] [Google Scholar]
- Zhong W, Gallivan JP, Zhang Y, Li L, Lester HA, and Dougherty DA (1998) From ab initio quantum mechanics to molecular neurobiology: a cation-π binding site in the nicotinic receptor. Proc Natl Acad Sci U S A 95 12088-12093. [DOI] [PMC free article] [PubMed] [Google Scholar]