Abstract
Recombinant μ and δ opioid receptors expressed in cell lines can form heterodimers with distinctive properties and trafficking. However, a role for opioid receptor heterodimerization in neurons has yet to be identified. The inhibitory coupling of opioid receptors to voltage-dependent Ca2+ channels (VDCCs) is a relatively inefficient process and therefore provides a sensitive assay of altered opioid receptor function and expression. We examined μ-receptor coupling to VDCCs in dorsal root ganglion neurons of δ(+/+), δ(+/-), and δ(-/-) mice. Neurons deficient in δ receptors exhibited reduced inhibition of VDCCs by morphine and [d-Ala2,Phe4,Gly5-ol]-enkephalin (DAMGO). An absence of δ receptors caused reduced efficacy of DAMGO without affecting potency. An absence of δ receptors reduced neither the density of VDCCs nor their inhibition by either the GABAB receptor agonist baclofen or intracellular guanosine 5′-O-(3-thio)triphosphate. Flow cytometry revealed a reduction in μ-receptor surface expression in δ(-/-) neurons without altered DAMGO-induced internalization. There was no change in μ-receptor mRNA levels. d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2-sensitive μ-receptor-coupling efficacy was fully restored to δ(+/+) levels in δ(-/-) neurons by expression of recombinant δ receptors. However, the dimerization-deficient δ-15 construct expressed in δ(-/-) neurons failed to fully restore the inhibitory coupling of μ receptors compared with that seen in δ(+/+) neurons, suggesting that, although not essential for μ-receptor function, μ-δ receptor dimerization contributes to full μ-agonist efficacy. Because DAMGO exhibited a similar potency in δ(+/+) and δ(-/-) neurons and caused similar levels of internalization, the role for heterodimerization is probably at the level of receptor biosynthesis.
Opioid receptors are the focus of intensive investigation because of their participation in nociception, mood, analgesia, and the rewarding effects of opioids and other abused substances. Opioid (μ, δ, and κ) receptors use common signaling mechanisms and have overlapping distributions in the central and peripheral nervous systems (Williams et al., 2001; Chen et al., 2008; Christie, 2008). However, despite these similarities, activation of different opioid receptor subtypes has distinctive behavioral consequences (Rees, 1992; Coop and Rice, 2000; Gavériaux-Ruff and Kieffer, 2002).
The generation of μ-receptor knockout [μ(-/-)] mice led to the demonstration of the paramount importance of μ receptors in the analgesic and rewarding actions of morphine (Matthes et al., 1996). Systemic morphine administration had no discernible antinociceptive or rewarding effects in μ(-/-) mice. Furthermore, μ(-/-) mice lack the analgesic response to δ receptor agonists seen in wild-type animals, suggesting that much of this activity is mediated through partial activation of μ receptors (Scherrer et al., 2004). In contrast to μ(-/-) mice, δ receptor [δ(-/-)] and κ receptor [κ(-/-)] knockout mice exhibit intact morphine analgesia (Simonin et al., 1998; Scherrer et al., 2004). There is, however, evidence that δ receptors modulate μ receptor-mediated analgesia. Analgesia by intrathecal morphine is potentiated by a δ receptor antagonist (Gomes et al., 2004). Furthermore, δ(-/-) mice exhibit a reduced propensity for the development of morphine tolerance (Zhu et al., 1999). These findings suggest that there is an interaction between μ and δ receptors in vivo that could contribute to the analgesic actions of μ agonists.
When expressed in cell lines, recombinant μ receptors can interact with either δ or κ receptors producing novel pharmacology and, in some cases, altered signaling mechanisms (Jordan and Devi, 1999; George et al., 2000; Charles et al., 2003). Furthermore, immunoaffinity purification and Western analysis demonstrate that opioid receptors can combine to form heteromeric receptor complexes. Heterodimerization is a requirement for efficient trafficking of some G-protein-coupled receptors from the endoplasmic reticulum to the cell membrane (Gurevich and Gurevich, 2008). The same may also be true for the opioid receptors. However, despite ample evidence for the formation of opioid receptor heterodimers in recombinant studies, there is a dearth of evidence of a requirement for heterodimer formation for the normal function of μ or δ receptor activity in neurons.
Opioid receptors couple to adenylyl cyclase, inwardly rectifying K+ channels, and high-threshold voltage-dependent Ca2+ channels (VDCCs) (Williams et al., 2001). Inhibitory coupling of opioid receptors to VDCCs is an inefficient process compared with coupling of opioid receptors to adenylyl cyclase (Prather et al., 2000). Therefore VDCC activity provides a sensitive assay for studying coupling efficiency (Walwyn et al., 2005, 2007).
In this study, we examined the effect of a deficit of δ receptors on μ-receptor coupling to VDCCs in primary afferent dorsal root ganglion (DRG) neurons. Both μ receptor surface expression and the efficacy of μ-receptor coupling to VDCCs were attenuated in δ(-/-) DRG neurons and restored by recombinant δ receptor expression. Thus, δ receptors are required for normal functional expression of μ receptors in DRG neurons. We explored whether this interaction between μ and δ receptors required the formation of heterodimers using a dimerization-deficient truncated δ construct (δ-15) that lacks 15 C-terminal amino acids (Cvejic and Devi, 1997; Fan et al., 2005). Expression of either the full-length δ or δ-15 in δ(-/-) neurons caused the appearance of similarly robust DPDPE evoked inhibitions of VDCCs. However, the δ-15 construct only partially restored coupling of μ receptors to VDCCs compared with that seen in δ(+/+) neurons suggesting that the formation of μ-δ heterodimers is required for full functional expression of μ receptors in DRG neurons.
Materials and Methods
Dorsal Root Ganglion Neuron Cultures. Dorsal root ganglia were harvested from early postnatal mice (p0-p2), which contained both [δ(+/+)], one [δ(+/-)], or neither [δ(-/-)] of the δ-opioid receptor alleles in the C57BL/6 background (Filliol et al., 2000). This line has been fully backcrossed to the C57BL/6 background, and the pups used were littermates or within two generations of δ(+/-) mating. The DRGs were enzymatically and physically dissociated, and neurons were plated as described previously (Walwyn et al., 2007) for electrophysiology and flow cytometry experiments. Cells were cultured in Neurobasal A, B27 (2%), GlutaMAX (0.5 mM), and antibiotic-antimycotic (12 U/ml penicillin, 12 U/ml streptomycin, and 30 ng/ml Fungizone) media (Invitrogen, Carlsbad, CA) containing nerve growth factor (10 μg/ml; Roche, Indianapolis, IN) and kept for 2 to 3 days in vitro at 37°C and 5% CO2.
Fluorescent μ and δ Viral Constructs and Lentiviral Packaging. Polymerase chain reaction was used to introduce an EcoRI site at the stop codon of the mouse δ receptor cDNA and to generate the δ-cerulean fluorescent protein (CFP) C-terminal fusion protein. The linker nucleotide sequence was ggtcagaattccggcggcagagccaccgcccgg. The construct was sequenced subsequent to amplification and subcloning. A similar approach was used to generate a truncated δ-15 construct that lacks amino acids 357 to 372. The C terminus of the truncation construct was fused to CFP using the sequence cagaattccgacacgctcacgcgtggtggc. The constructs were tested in HEK 293 cells to confirm correct color and fusion protein status before making the lentivirus vectors. DNA constructs encoding the fusion proteins were then inserted into lentiviral expression vectors behind the phosphoglycerokinase promoter, and their orientation and expression were confirmed by transfection in mammalian cell lines. These expression constructs were cotransfected (Fugene 6; Roche) with viral packaging and envelope constructs kindly supplied by Dr. D. Trono (University of Geneva, Geneva, Switzerland) in HEK293FT cells (Invitrogen). The supernatant was harvested after 48 h, concentrated through Amicon 100-kDa molecular mass filters (Millipore, Billerica, MA), and pelleted by ultracentrifugation. The viral pellet was resuspended overnight, frozen in 10-μl aliquots at -70°C, and titered using the p24 enzyme-linked immunosorbent assay. Titers were 1.5 × 105 ng of p24/ml for δ-CFP and 1.6 × 105 ng of p24/ml for δ-15-CFP. DRGs were transduced with each virus applying 50 ng of p24 per plate to 1 × 105 cells 2 h after plating and were recorded from after 3 days in vitro. This protocol resulted in a neuronal transduction efficiency of 90 to 100%.
Electrophysiology. VDCC activity was recorded from cultured DRG neurons using the whole-cell patch-clamp technique (Axopatch 200A amplifier; Molecular Devices, Sunnyvale, CA). The external solution contained 130 mM tetraethylammonium chloride, 10 mM CaCl2, 5 mM HEPES, 25 mM d-glucose, and 0.25 mM tetrodotoxin, pH 7.2. Recording electrodes contained 105 mM CsCl, 40 mM HEPES, 5 mM d-glucose, 2.5 mM MgCl2, 10 mM EGTA, 2 mM Mg2+-ATP, and 0.5 mM Na+-GTP, pH 7.2. No compensation was made for the cancellation of liquid junction potential. Voltage steps from -80 to 10 mV for 100 ms at 20-s intervals were used to activate Ca2+ currents. Currents were low-pass-filtered at 2 kHz and digitized (Digidata; Molecular Devices) at 10 kHz for storage on the hard drive of a Pentium personal computer. Leak currents were nulled using the P/4 subtraction method. Neurons were rapidly and continuously superfused with external solution as described previously (Walwyn et al., 2007). Opioid agonists, opioid antagonists, ω-conotoxin GVIA, and baclofen (all from Sigma, St. Louis, MO) were diluted into external solution on the day of the experiment and applied through the perfusion system. Experiments were performed at room temperature (22-24°C). Mean current amplitudes were measured (pCLAMP 9.0; Molecular Devices) between 5 and 10 ms after initiating the depolarizing step. Recordings that exhibited marked rundown were discarded. Stable recordings were fitted by a linear function to compare, by extrapolation, control current amplitude to the current amplitude recorded in the presence of agonists and/or antagonists.
Statistical Analysis. Data are expressed as mean ± S.E.M. Statistical comparisons of multiple values were made using analysis of variance with the post hoc Scheffe test. When only two data sets were compared, the Student's t test was used. Values were considered statistically different when p < 0.05.
Flow Cytometry. After 2 days, cultured δ(+/+), δ(+/-), and δ(-/-) DRG neurons were harvested in ice-cold PBS/EDTA and spun at 300g for 5 min at 4°C. Cells were washed in ice-cold PBS containing 2% fetal bovine serum and 0.1% sodium azide and incubated with an anti-μ-receptor antibody raised against the third extracellular loop for 60 min at 4°C (1:100 dilution in PBS containing 2% fetal bovine serum and 0.1% sodium azide; MBL International, Woburn, MA). Antibodies to this region of the μ receptor do not label μ(-/-) DRG neurons (Guarna et al., 2003). Thereafter, the cells were washed and incubated in the secondary antibody [allophycocyanin (APC)-conjugated rabbit IgG, 1:100; BD Biosciences, San Jose, CA] for 60 min at room temperature. After a final wash, 5000 neurons per sample were acquired on a FACScalibur flow cytometer (BD Immunocytochemistry Systems, Mountain View, CA) and analyzed using FCS Express version 3.0 (DeNovo Software, Los Angeles, CA).
Analysis. The neuronal population in each sample for each experiment was defined by size (forward scatter, FSC-H) and granularity (side scatter, SSC-H) as described previously (Walwyn et al., 2004). The APC fluorescence of this population was measured in channel FL4-H. Nonspecific fluorescence was subtracted, and the mean fluorescence intensity (FI) was subsequently obtained for each sample. Such mean FI values were normalized to the FI of the untreated δ(+/+) sample for each experiment. Experiments were repeated five times, and the data were analyzed using the Student's t test. Values were considered statistically different when p < 0.05. Data are expressed as mean ± S.E.M.
Quantitative Polymerase Chain Reaction. Cultured DRG neurons from δ(-/-) and δ(+/+) p0-2 pups were harvested in PBS/EDTA, spun (300g) for 5 min at 4°C, and lysed. mRNA was isolated (RNAqueous; Ambion, Austin, TX) and reverse-transcribed (Superscript III; Invitrogen). Sequence-specific primer/probe sets (Invitrogen) for the mouse μ receptor (NM_011013) and the control gene, synaptophysin (NM_009305) (Chen et al., 2001), were used to determine the relative transcript level, quantified by the cycle number or count threshold (CT) at which the gene-specific fluorescence reached mid-linear levels, using the Taqman 7700 (Applied Biosystems, Union City, CA). The data are presented as the mean ± S.E.M. of the ΔCT between the target and control genes for three separate experiments.
Results
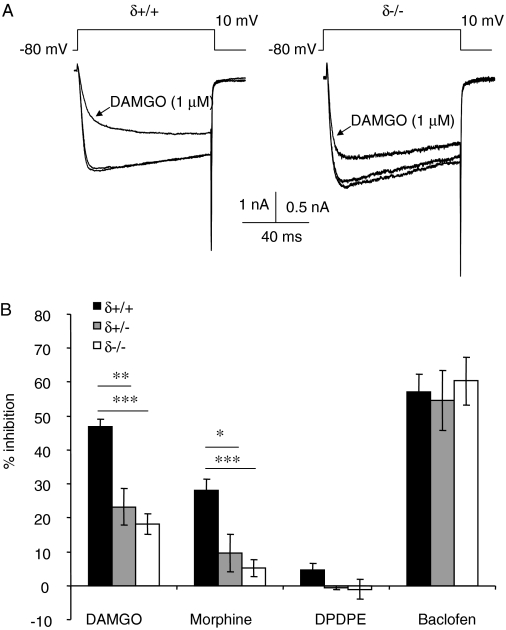
The Efficacy of μ-Receptor Coupling to VDCCs Is Reduced in δ-Receptor-Deficient DRG Neurons. Consistent with our previous report (Walwyn et al., 2005), the selective δ receptor agonist DPDPE (1 μM) caused a negligible inhibition of Ca2+ currents evoked by depolarizing δ(+/+) DRG neurons from -80 to 0 mV. There was a complete absence of inhibition in δ(+/-) and δ(-/-) neurons (Fig. 1B). An absence of μ receptors from DRG neurons of μ(-/-) mice leads to an up-regulation of surface δ receptors and the appearance of more robust δ-receptor inhibitory coupling to VDCCs (Walwyn et al., 2005). On the basis of this finding, we anticipated that a lack of δ receptors in DRG neurons from δ(-/-) mice would lead to an increase in μ-receptor coupling to VDCCs. However, contrary to our expectations, a lack of δ receptors attenuated μ-receptor inhibitory coupling to VDCCs in δ(-/-) DRG neurons (Fig. 1). Consistent with our previous recordings from wild-type DRG neurons, DAMGO (1 μM) inhibited Ca2+ current amplitude in all δ(+/+) neurons tested, with an average inhibition of 47 ± 2% (range, 36-58%, n = 11). The coapplication with the δ receptor agonists DPDPE (1 μM) or deltorphin II (1 μM) did not significantly affect the amplitude of Ca2+ current inhibition by DAMGO (1 μM) in δ(+/+) neurons (Supplementary Fig. 1). However, DAMGO (1 μM) caused a significantly smaller (p < 0.0001) inhibition (19 ± 4%) of Ca2+ currents recorded from δ(-/-) compared with δ(+/+) (Fig. 1B). Of the 23 δ(-/-) neurons tested, 4 lacked a significant inhibitory response to DAMGO, and 5 exhibited responses within the range of those recorded from δ(+/+) neurons; the inhibitory responses to DAMGO in the remainder was 4 to 28% (mean = 15 ± 2). Coupling of μ receptors to VDCCs was also attenuated in neurons from δ(+/-) heterozygous mice (p < 0.005). DAMGO caused a 23 ± 6% inhibition of Ca2+ currents recorded from δ(+/-) DRG neurons (Fig. 1B). Likewise, morphine applied to either δ(+/-) or δ(-/-) neurons caused a smaller inhibition (10 ± 5 and 5.7 ± 2.6%, respectively) than when applied to δ(+/+) neurons (28 ± 3%). The reduction in the inhibition of VDCCs in δ-receptor-deficient DRG neurons was selective to μ-receptor activation. The GABAB receptor agonist baclofen (50 μM) caused a similar inhibition of Ca2+ current amplitudes in δ(+/+), δ(+/-), and δ(-/-) DRG neurons (Fig. 1B).
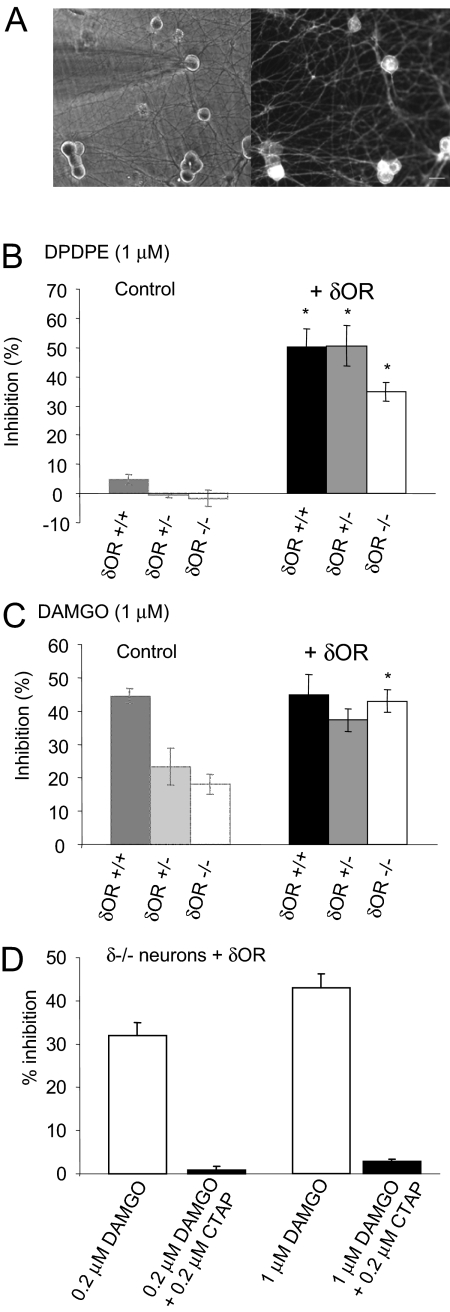
Fig. 1.
A deficiency of δ receptors causes a reduction in μ-receptor inhibitory coupling to VDCCs. A, exemplar superimposed Ca2+ currents evoked by depolarizing δ(+/+) and δ(-/-) DRG neurons from -80 to 0 mV, recorded in the absence and presence of DAMGO (1 μM). B, bar graph indicating the mean Ca2+ current inhibition by μ, δ, and GABAB receptor-selective agonists, DAMGO (1 μM), morphine (1 μM), DPDPE (1 μM), and baclofen (50 μM). Data points are averages of between 5 and 22 recordings. Vertical lines represent ± S.E.M. ANOVA with a post hoc Scheffe test was used to determine the statistical significance, across genotypes, of differences in the mean inhibitions: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
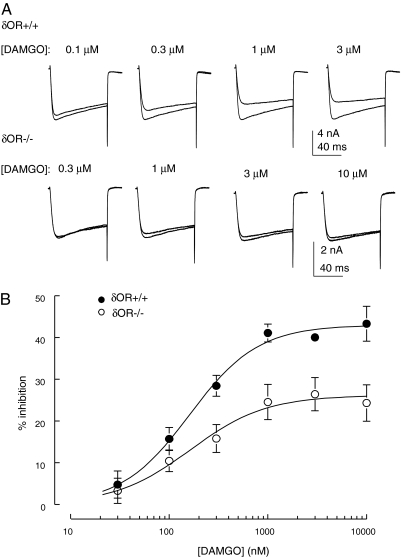
We investigated the concentration-response relationship for the inhibition of Ca2+ currents by DAMGO in δ(+/+) and δ(-/-) neurons to determine whether the reduction in coupling in the latter occurred through altered potency and/or efficacy of DAMGO (Fig. 2). The IC50 values for DAMGO as an inhibitor of VDCC activity in δ(+/+) and δ(-/-) neurons were 161 ± 20 and 163 ± 36 nM, respectively (Fig. 2B). Thus, a lack of δ receptors has no significant effect on potency but instead leads to a reduction in the efficacy of DAMGO as an inhibitor of VDCCs in DRG neurons.
Fig. 2.
A lack of δ receptors causes a reduction in the efficacy of DAMGO without affecting potency. A, exemplar superimposed Ca2+ currents evoked by depolarizing δ(+/+) and δ(-/-) DRG neurons from -80 to 0 mV, recorded in the absence and presence of DAMGO at the concentrations indicated. Each series of traces was recorded from the same DRG neuron. B, graph of DAMGO concentration versus Ca2+ current inhibition. Data points (averages of between 4 and 10 observations) were fitted with a logistic function yielding IC50 values for DAMGO of 161 ± 20 and 163 ± 36 nM in δ(+/+) and δ(-/-) neurons, respectively (Hill slopes were 1.3 ± 0.2 and 1.1 ± 0.3, respectively). Vertical lines represent ± S.E.M.
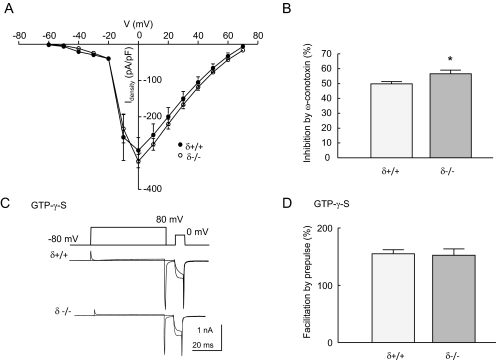
There Is No Deficit in VDCC Function or Their Inhibition by G Protein Activation in δ(-/-) Neurons. There is evidence for coordinated trafficking of GPCRs with G proteins and their effectors (Dong et al., 2007). Therefore, it is possible that reduced μ-receptor coupling in δ(-/-) neurons could be caused by a deficit at the level of the requisite G proteins or VDCCs. We examined the profile of VDCC activation at voltages from -60 to 70 mV in δ(+/+) and δ(-/-) DRG neurons (Fig. 3A). The threshold for current activation in neurons of both genotypes was ∼-40 mV, and current amplitudes peaked at 0 mV. There was no significant difference in current densities at any voltage tested. N-type VDCCs are the prevalent form of G protein-sensitive VDCC in DRG neurons (Rusin and Moises, 1995). There was a small increase in the level of VDCC inhibition by the N-type channel antagonist ω-conotoxin GVIA (100 nM) in δ(-/-) compared with δ(+/+) neurons (Fig. 3B). These data demonstrate that reduced inhibitory coupling of μ receptors in the absence of δ receptors is not caused by a deficit in the expression of VDCCs.
Fig. 3.
A lack of δ receptors causes neither a deficit in VDCC function nor inhibition by G proteins. A, the relationship between voltage and Ca2+ current density is similar in δ(+/+) and δ(-/-) DRG neurons. Cells were depolarized from -80 mV to between -60 and 70 mV (10-mV increments). Data points are average current densities (Idensity) determined for δ(+/+) (n = 5) and δ(-/-) (n = 7) neurons by normalizing peak current amplitudes to cell capacitance (pA/pF). B, the bar graph illustrates the inhibition by ω-conotoxin (100 nM) of Ca2+ currents evoked in δ(+/+) (n = 10), and δ(-/-) (n = 12) DRG neurons by depolarizing from -80 to 0 mV. There was a significant increase in the amplitude of inhibition by ω-conotoxin in δ(-/-) compared with δ(+/+) neurons (*, p < 0.05, Student's t test). C, superimposed Ca2+ currents evoked by depolarizing δ(+/+) and δ(-/-) neurons from -80 to 0 mV either with or without a prepulse to 80 mV. Currents were recorded with GTPγS (500 μM) in the recording electrode to enhance voltage-dependent Gβγ subunit-mediated VDCC inhibition. D, the graph displays the average ratios of Ca2+ current amplitudes recorded from δ(+/+) (n = 8) and δ(-/-) (n = 5) neurons in the absence and presence of depolarizing prepulses using the protocol illustrated in C. The ratio is provided as the percentage of facilitation by the prepulse and represents the level of voltage-dependent block in the presence of intracellular GTPγS. In all graphs, vertical lines represent ± S.E.M.
The nonhydrolyzable analog of GTP, GTPγS, increases the activity of G proteins by binding irreversibly to the α subunit and preventing its reassociation with and inactivation of the βγ subunits. GTPγS (500 μM) was included in the recording electrode, and the level of inhibition of VDCCs was established using an 80-mV depolarizing prepulse to reverse inhibition by activated βγ subunits. The level of GTPγS-mediated inhibition can be quantified by measuring the level of facilitation caused by the depolarizing prepulse. There was no significant difference between the amplitude of prepulse-evoked facilitation recorded from δ(+/+) and δ(-/-) neurons (Fig. 3D). These data suggest that there is no deficit in the inhibitory coupling of G proteins to VDCCs in the absence of δ receptors.
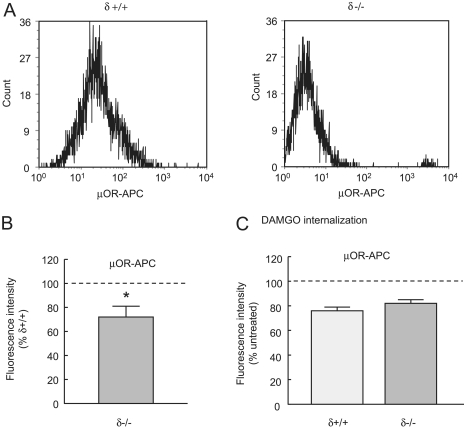
A Lack of δ Receptors Causes Reduced Cell Surface μ-Receptor Expression. The simplest explanation for the loss of μ receptor coupling to VDCCs in δ(-/-) neurons is a decrease in μ-receptor levels on the cell membrane. Indeed, an autoradiographic study of δ(-/-) mouse brains demonstrated a reduction in DAMGO binding consistent with a loss of cell surface μ receptors (Goody et al., 2002). We used flow cytometry to quantify the level of cell surface μ receptors in δ(+/+) and δ(-/-) neurons (Walwyn et al., 2007), an example of which is shown in Fig. 4A. Surface μ-receptor labeling of δ(-/-) neurons was reduced to 72 ± 11% of that in δ(+/+) neurons (p < 0.05, Fig. 4B), which probably accounts for the decreased inhibition by μ-receptor agonists of VDCCs in δ(-/-) neurons (Fig. 1B). Despite this difference in cell surface expression, there was no effect of genotype on μ-receptor internalization measured after 20 min of exposure of δ(+/+) and δ(-/-) DRG neurons to DAMGO (1 μM), all genotypes showing ∼20% internalization at this time point (Fig. 4C).
Fig. 4.
Cell surface μ-receptor levels are decreased in δ(-/-) neurons. A, exemplar histograms showing the distribution and numbers of δ(+/+) (left) and δ(-/-) cells (right) labeled with an antibody against the third extracellular loop of the μ receptor. The secondary antibody was conjugated to APC (see Materials and Methods). The population of cells is a subset of the total population, identified as neurons by their size and granularity. B, the graph illustrates the relative cell surface levels of the μ receptors in δ(-/-) compared with δ(+/+) DRG neurons (n = 6). There was significantly less surface labeling with the μ receptor antibody in δ(-/-) neurons, *, p < 0.05 determined by the Student's t test. C, the graph illustrates the loss of cell surface receptors caused by exposure to DAMGO (1 μM for 10 min). Average data (n = 3) are expressed as a percentage of μ-receptor labeling without exposure to DAMGO. There were similar levels of μ-receptor internalization in δ(+/+) and δ(-/-) neurons. In B and C, vertical lines represent ± S.E.M.
We used quantitative real-time polymerase chain reaction to compare μ-receptor transcript levels in δ(+/+) and δ(-/-) DRG neurons. There was no difference in the ΔCT values for μ-receptor mRNA, when normalized to that of synaptophysin, in δ(+/+) (9.6 ± 0.7) and δ(-/-) (10.5 ± 0.5) DRG neuronal cultures (Supplementary Fig. 2). Thus, the absence of δ receptors reduces cell surface μ-receptor surface expression without affecting the level of μ-receptor mRNA.
Restoration of Full μ-Receptor Coupling to VDCCs by Recombinant δ Receptor Expression in δ(-/-) Neurons. Because δ(+/-) and δ(-/-) neurons exhibit reduced μ-receptor coupling to VDCCs (Fig. 1B), we attempted to recover full coupling by restoring δ-receptor expression. We overexpressed δ receptors in δ(-/-), δ(+/-), and δ(+/+) DRG neurons using a lentiviral vector encoding a CFP-tagged δ-receptor construct and recorded from fluorescent neurons (Fig. 5A). Expression of recombinant δ receptors in DRG neurons produced robust inhibition of VDCC activity by DPDPE (Fig. 5B). DPDPE (1 μM) inhibited Ca2+ currents recorded from δ(+/+) and δ(+/-) neurons expressing recombinant δ receptors by 50 ± 6 and 51 ± 7%, respectively. The inhibition of Ca2+ currents by DPDPE in δ(-/-) neurons expressing recombinant δ receptors was somewhat smaller (41 ± 3%).
Fig. 5.
Expression of recombinant δ receptors in δ-receptor-deficient neurons restores full μ-receptor coupling. A, Left, electrode, viewed using transmitted light, attached to a cultured DRG neuron. Right, the same neurons viewed using fluorescence microscopy. Labeled neurons contain recombinant δ receptor fused to CFP. B, the bar graph depicts the mean Ca2+ current inhibitions evoked by DPDPE (1 μM) in δ(+/+), δ(+/-), and δ(-/-) DRG neurons expressing recombinant δ receptor (+δOR). Bars on the left provide control data (in the absence of δOR) for comparison (from Fig. 1B). Expression of recombinant δ receptors in neurons of all three genotypes caused a significant increase in the inhibition of VDCCs by DPDPE, *, p < 0.001, Student's t test. C, the bar graph illustrates the mean Ca2+ current inhibitions evoked by DAMGO (1 μM) in neurons expressing recombinant δ receptors. Bars on the left provide the relevant control data for comparison (from Fig. 1B). Expression of recombinant δ receptors in δ(-/-) neurons caused a significant increase in the inhibition of VDCCs by DAMGO, *, p < 0.001, Student's t test. D, the bar graph shows the average inhibition of Ca2+ currents by DAMGO applied to neurons in the presence or absence of the μ-receptor-selective antagonist CTAP. The inhibition of Ca2+ currents by DAMGO, recorded from δ(-/-) neurons expressing recombinant δ receptors, was attenuated by coapplication with the CTAP. In all graphs, the vertical lines represent ± S.E.M.
The appearance of δ-receptor coupling upon the introduction of recombinant δ receptors was associated with a restoration of full μ-receptor coupling to VDCCs in fluorescently labeled δ(-/-) and δ(+/-) neurons (Fig. 5C). DAMGO (1 μM) inhibited Ca2+ currents recorded from δ(+/+), δ(+/-), and δ(-/-) neurons expressing recombinant δ receptors by 45 ± 6, 37 ± 4, and 44 ± 3%, respectively. These were similar to the DAMGO-evoked inhibition of VDCCs recorded from control δ(+/+) neurons (47 ± 2%, Fig. 5C).
DAMGO is a selective μ-receptor agonist; however, it is possible that DAMGO (1 μM) could inhibit VDCC activity by binding to recombinant δ receptors overexpressed in DRG neurons. Therefore, we tested the sensitivity to the selective μ-receptor antagonist CTAP of the inhibition of Ca2+ currents by DAMGO. CTAP (200 nM) almost abolished DAMGO-mediated inhibitions (200 nM, 0.9 ± 0.8%; and 1 μM, 2.9 ± 0.5% of control) of VDCC activity in DRG neurons overexpressing recombinant δ receptors (Fig. 5D). Therefore, DAMGO inhibits Ca2+ currents through μ-receptor activation even in neurons overexpressing δ receptors.
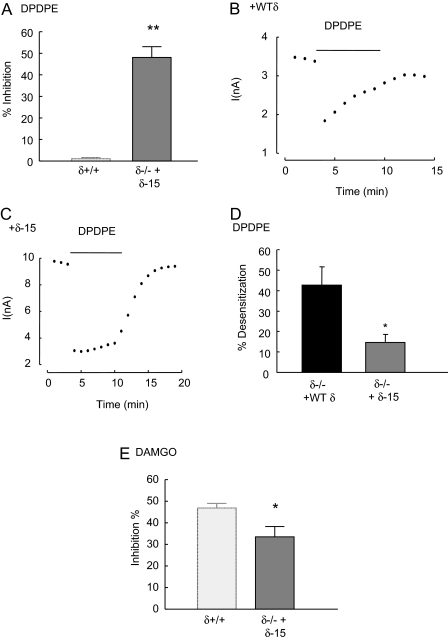
A Dimerization-Defective δ Receptor Construct Does Not Fully Restore μ-Receptor Function to δ(-/-) Neurons. Recombinant expression studies demonstrate that μ and δ receptors can combine to form heterodimers (Jordan and Devi, 1999; George et al., 2000). Thus it is possible that the reduced inhibitory coupling of μ receptors to VDCCs in δ(-/-) neurons reflects an absence of μ/δ heterodimers. Fifteen amino acids at the C terminus of the δ receptor (Val357 to Ala372) are necessary for efficient heterodimerization with the μ receptor (Cvejic and Devi, 1997; Fan et al., 2005). Therefore, we tested the ability of the truncated δ-15 receptor construct (that lacked 15 C-terminal amino acids) to restore μ-receptor coupling to VDCCs in δ(-/-) neurons.
First, we established that the δ-15 construct was functional when introduced, by lentiviral vector, into δ(-/-) neurons. DPDPE (1 μM) inhibited Ca2+ currents recorded from δ(-/-) neurons expressing δ-15 receptors (Fig. 6A). In addition to their role in dimerization, C-terminal amino acids of the δ receptor also participate in agonist-induced phosphorylation and internalization, mechanisms associated with desensitization (Trapaidze et al., 1996; Guo et al., 2000). The inhibition of VDCCs by DPDPE, recorded from δ(-/-) neurons expressing wild-type δ receptors, exhibited rapid desensitization (Fig. 6B). DPDPE (1 μM) caused less desensitization of inhibitory coupling to VDCCs when applied to δ(-/-) neurons expressing the δ-15 construct compared with neurons expressing wild-type δ receptors (Fig. 6C). After 120 s of DPDPE (10 μM) exposure, the inhibition of VDCC activity decreased by 43 ± 9 and 15 ± 4% in δ(-/-) neurons expressing recombinant wild-type δ receptors and δ-15 receptors, respectively (Fig. 6D).
Fig. 6.
Expression of a heterodimerization-defective δ receptor construct in δ(-/-) neurons does not fully restore μ-receptor inhibitory coupling. A, the bar graph illustrates the mean inhibition by DPDPE (1 μM) of Ca2+ currents recorded from δ(-/-) DRG neurons expressing the dimerization-deficient δ-15 construct that lacks the last 15 amino acids of the δ receptor. The left bar represents the mean inhibition of Ca2+ currents when DPDPE was applied to δ(+/+) neurons (data from Fig. 1B) and is included here for comparison. Like recombinant full-length δ receptors (Fig. 5B), δ-15 receptors mediate robust inhibitory coupling to VDCCs. The dot plots illustrate the time course of the inhibition of Ca2+ currents by DPDPE (1 μM) in δ(-/-) neurons expressing the full-length recombinant δ receptor (+WTδ; B) and the truncated δ-15 construct (+δ-15; C). Note that the full-length δ receptor undergoes desensitization leading to a reduction in the inhibitory effect of DPDPE with time. D, the bar graph depicts the mean percentage of desensitization during 5-min exposure to DPDPE of δ(-/-) neurons expressing the full-length δ receptor or the δ-15 construct. The loss of DPDPE-evoked inhibition after 5 min of exposure was expressed as a percentage of the peak inhibition in each cell. E, the bar graph illustrates the mean inhibition evoked by DAMGO (1 μM) in δ(-/-) neurons expressing the δ-15 receptor. The bar on the left provides for comparison the mean inhibition by DAMGO of Ca2+ currents recorded from δ(+/+) neurons (data from Fig. 1B). The DAMGO-evoked inhibition in cell expressing the δ-15 is less than that of δ(+/+) neurons (p < 0.05) but greater than observed in δ(-/-) (p < 0.05) neurons. In all graphs, the vertical lines represent ± S.E.M. Significance was determined using the Student's t test: **, p < 0.01; *, p < 0.05.
Having established that the δ-15 construct functionally couples to VDCCs when expressed in δ(-/-) neurons, we examined whether it restores full inhibitory coupling of μ receptors compared with the full-length δ receptor. The mean inhibition evoked by DAMGO in δ(-/-) neurons expressing the δ-15 receptor was only 34 ± 5%, significantly less (p < 0.05) than that seen in δ(+/+) neurons (Fig. 6E). Thus, the δ-15 construct mediated a robust inhibition of VDCCs by DPDPE (Fig. 6A) but failed to fully restore μ-receptor inhibitory coupling in δ(-/-) neurons to levels seen in δ(+/+) neurons. This contrasts with the full-length δ receptor, which fully restored μ-receptor coupling to VDCCs to levels seen in recordings from δ(+/+) neurons (Fig. 5C).
Interestingly, the presence of the δ-15 construct in δ(-/-) neurons did not affect the level of desensitization evoked by DAMGO (10 μM). The inhibitory coupling of μ receptors to VDCCs decreased by 10 ± 4% (n = 6) after exposure of δ(-/-) neurons expressing the δ-15 construct. Likewise, the response to DAMGO (10 μM) desensitized by 11 ± 3% (n = 4) in δ(-/-) neurons expressing recombinant wild-type δ receptors (Supplementary Fig. 3).
Discussion
Consistent with our previous report, DPDPE had little effect on VDCCs in wild-type DRG neurons (Walwyn et al., 2005). Not surprisingly, there was also a lack of inhibition by DPDPE in δ(-/-) and δ(+/-) DRG neurons. Overexpression of δ receptors caused robust VDCC inhibition by DPDPE application to δ(-/-), δ(+/-), and δ(+/+) neurons. This is consistent with the idea that under basal conditions, there are insufficient δ receptors for either functional coupling to VDCCs (Walwyn et al., 2005) or robust analgesia in response to δ agonists (Zhang et al., 2006).
More surprising was our observation that, compared with δ(+/+) neurons, there was diminished inhibition of VDCCs when DAMGO or morphine was applied to δ(+/-) and δ(-/-) neurons. A lack of δ receptors reduced DAMGO efficacy without causing a deficit in VDCC function or inhibition by either the GABAB agonist baclofen or intracellular GTPγS. Reduced DAMGO efficacy can be explained by a reduction in the surface expression of μ receptors in δ(-/-) neurons. These findings are consistent with a previous study in which reduced μ-receptor binding was observed in the brains of δ(-/-) mice using autoradiography (Goody et al., 2002).
The deficit in inhibitory coupling of μ receptors to VDCCs in δ(+/-) neurons implies that a 50% reduction in δ receptors has an impact on μ-receptor functional expression. Opioid receptor coupling to VDCCs is inefficient, and relatively small changes in the number of available receptors can cause large changes in coupling efficacy (Prather et al., 2000; Walwyn et al., 2005). Indeed, heterozygous μ(+/-) neurons exhibit a complete lack of detectable μ-receptor coupling to VDCCs.
Full μ-receptor coupling to VDCCs was restored to wild-type levels in δ(-/-) and δ(+/-) neurons expressing recombinant δ receptors. Therefore, δ receptors are necessary for full functional expression of μ receptors in some DRG neurons. Because the absence of δ receptors caused no change in μ-receptor transcript levels, the deficit seems to be caused by reduced μ-receptor trafficking to and/or increased removal from the DRG surface membrane. Because μ and δ receptors can form heterodimers when expressed in cell lines (Cvejic and Devi, 1997; Gomes et al., 2000; Fan et al., 2005), it is plausible that μ-δ complexes are required for maximal μ-receptor surface expression.
Expression of a dimerization-deficient δ-15 construct with μ receptors diminishes heterodimerization in cell lines (Cvejic and Devi, 1997; Fan et al., 2005). Expression of the δ-15 construct in δ(-/-) neurons caused the appearance of a robust DPDPE-evoked inhibition of VDCCs similar to that seen upon expression of full-length δ receptors. This suggests that truncation does not deter biosynthesis or coupling to VDCCs. The final 15 amino acids in the δ receptor have been implicated in agonist-induced desensitization (Trapaidze et al., 1996; Guo et al., 2000), and, consistent with this, δ-15 receptors desensitized more slowly in δ(-/-) neurons than did full-length δ receptors. Although there was a much higher level of δ receptor function in δ(-/-) neurons expressing δ-15 compared with wild-type neurons, the construct only partially restored μ-receptor function. These data are consistent with the hypothesis that μ-δ dimerization is required for full μ-receptor functional expression. We hypothesize that the failure of δ-15 to support full DAMGO-mediated inhibition is caused by incomplete restoration of surface μ-receptor levels. To directly test this hypothesis, in future studies it will be necessary to use techniques with a higher resolution than flow cytometry to discern small differences in surface μ-receptor expression between DRG neurons expressing wild-type and mutant δ constructs.
Heterodimers formed between μ and δ receptors in cell lines have distinctive properties. They are activated by μ- and δ-receptor agonists. However, compared with μ-receptors expressed alone, DAMGO binds to μ-δ dimers with reduced affinity and has a lower potency as an inhibitor of adenylyl cyclase activity (Cvejic and Devi, 1997; George et al., 2000; Fan et al., 2005). Furthermore, when μ-δ dimers are present, coapplication with deltorphin II increases the potency and efficacy of DAMGO (Gomes et al., 2000). Although the absence of δ receptors reduced the efficacy of DAMGO in δ(-/-) neurons, there was no change in DAMGO's potency relative to δ(+/+) neurons. In addition, coapplication of deltorphin II did not affect the efficacy of DAMGO in δ(+/+) neurons. These data suggest that μ-δ dimers do not couple to VDCCs in δ(+/+) DRG neurons, and therefore, a requirement for δ receptors for full DAMGO efficacy can not be explained by heterodimerization at the level of the surface membrane. Furthermore, despite a striking difference in desensitization of δ-15 and full-length δ receptors by DPDPE, DAMGO-evoked desensitization was similar in δ(-/-) neurons expressing either construct. These data suggest that, even when overexpressed, the δ receptor does not directly contribute to the inhibition of VDCCs by DAMGO.
GPCRs do not necessarily exist in the same state of oligomerization from biosynthesis to degradation. Indeed, they may exist as monomers, homodimers, or heterodimers in the Golgi, cell surface membrane, and/or during internalization (Gurevich and Gurevich, 2008). Dimerization may occur after receptor activation in the surface membrane, and this process may influence surface expression in neurons that express both μ and δ receptors. Indeed, interactions of recombinant opioid receptors with the trafficking protein β-arrestin2 are enhanced by μ-δ dimerization (Rozenfeld and Devi, 2007). However, we found that DAMGO evoked a similar stimulation of μ-receptor internalization in δ(-/-) and δ(+/+) neurons, suggesting that an absence of μ-δ dimerization has little effect on receptor removal from the DRG membrane initiated by the agonist.
Heterodimerization is a prerequisite for export of GABAB receptors and other GPCRs from the endoplasmic reticulum into the Golgi and out to the surface membrane (Margeta-Mitrovic et al., 2000; Terrillon et al., 2003; Hague et al., 2004). Because a lack of δ receptors in δ(-/-) DRG neurons affects neither the potency of DAMGO nor DAMGO-induced μ-receptor internalization, we hypothesize that μ-δ dimerization increases the efficiency of μ receptor biosynthesis.
Studies in cell lines suggest that increased levels of recombinant δ receptor expression lead to higher intracellular retention of μ-δ heterodimers, reducing μ-receptor cell surface expression (Law et al., 2005; Décaillot et al., 2008). However, coexpression of μ and δ receptors with the Golgi chaperone protein RTP4 in HEK cells prevents intracellular retention of μ-δ heterodimers by reducing ubiquitination. RTP4 and/or other chaperone proteins may participate in the heterodimer-specific component of μ-receptor biosynthesis and transport to the DRG surface membrane.
Immunohistochemical analysis of acutely dissociated adult rat neurons demonstrates heterogeneous expression of μ, δ, and κ receptors in different DRG subtypes (Rau et al., 2005). Nine subtypes were classified electrophysiologically, eight exhibited μ-receptor immunoreactivity, and six of these also bound antibodies to both the δ and κ receptors, although labeling of some subtypes was variable. Therefore, although many DRG neurons express both μ and δ receptors, some do not, suggesting that a role for heterodimers in μ-receptor biosynthesis is not universal. Our cultured neonatal mouse DRG neurons all responded to μ-receptor agonists, and it is known that neonatal DRG neurons have more homogeneous expression of opioid receptors than do adult neurons (Beland and Fitzgerald, 2001). However, even in neonatal neurons, it is clear that heterodimerization is not essential for μ-receptor biosynthesis because an absence of δ receptors caused less than a 30% reduction in μ-receptor surface expression in δ(-/-) compared with δ(+/+) neurons. This relatively modest reduction in μ-receptors represents an average effect across all DRG subtypes. Electrophysiological recordings demonstrate that, although an absence of δ receptors causes either a complete loss of or a dramatic reduction in μ-receptor-mediated inhibition, some δ(-/-) neurons exhibit inhibitions of Ca2+ currents by DAMGO that lie within the range observed in δ(+/+) neurons, these neurons may represent subtypes that in wild-type animals lack δ receptors and have alternative mechanisms for efficient μ-receptor biosynthesis, perhaps involving other assembly partners.
In addition to affecting the surface expression of μ receptors and thereby influencing coupling to VDCCs, δ receptors modulate morphine analgesia and tolerance. Stimulation of δ-receptor cell surface expression in DRG neurons is associated with a reduction in morphine tolerance (Guan et al., 2005). It is noteworthy that the human δ receptor F27C polymorphism, which reduces receptor maturation efficiency and cell surface expression, is associated with risk of substance dependence and acute thermal pain sensitivity (Kim et al., 2004; Zhang et al., 2008; Leskelä et al., 2009). Additional studies are required to determine whether this polymorphism disrupts μ-δ dimerization and hinders μ receptor biosynthesis.
Because a deficit of δ receptors reduces the efficacy of μ-receptor agonists in DRG neurons, pharmacological enhancement of δ receptor surface expression may provide a method for increasing the efficacy of morphine analgesia and combating tolerance. Membrane-permeable ligands that decrease δ-receptor ubiquitination act as pharmacological chaperones increasing cell surface expression of both δ and μ receptors associated with μ-δ dimers (Petäjä-Repo et al., 2002; Décaillot et al., 2008). It remains to be seen whether the approach will prove beneficial for enhancing μ-receptor-mediated analgesia.
Supplementary Material
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA05010, DA00484].
ABBREVIATIONS: VDCC, voltage-dependent Ca2+ channel; DAMGO, [d-Ala2,Phe4,Gly5-ol]-enkephalin; DPDPE, [d-Pen2,Pen5]-enkephalin; CTAP, d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2; GTPγS, guanosine 5′-O-(3-thio)triphosphate; DRG, dorsal root ganglion; CFP, δ-cerulean fluorescent protein; PBS, phosphate-buffered saline; APC, allophycocyanin; FI, fluorescence intensity; CT, count threshold; HEK, human embryonic kidney.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
References
- Beland B and Fitzgerald M (2001) Mu- and delta-opioid receptors are down-regulated in the largest diameter primary sensory neurons during postnatal development in rats. Pain 90 143-150. [DOI] [PubMed] [Google Scholar]
- Charles AC, Mostovskaya N, Asas K, Evans CJ, Dankovich ML, and Hales TG (2003) Co-expression of δ opioid receptors with μ receptors in GH3 cells changes the functional response to μ agonists from inhibitory to excitatory. Mol Pharmacol 63 89-95. [DOI] [PubMed] [Google Scholar]
- Chen J, Sochivko D, Beck H, Marechal D, Wiestler OD, and Becker AJ (2001) Activity-induced expression of common reference genes in individual CNS neurons. Lab Invest 81 913-916. [DOI] [PubMed] [Google Scholar]
- Chen YL, Law PY, and Loh HH (2008) The other side of the opioid story: modulation of cell growth and survival signaling. Curr Med Chem 15 772-778. [DOI] [PubMed] [Google Scholar]
- Christie MJ (2008) Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 154 384-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coop A and Rice KC (2000) Role of δ-opioid receptors in biological processes. Drug News Perspect 13 481-487. [PubMed] [Google Scholar]
- Cvejic S and Devi LA (1997) Dimerization of the δ opioid receptor: implication for a role in receptor internalization. J Biol Chem 272 26959-26964. [DOI] [PubMed] [Google Scholar]
- Décaillot FM, Rozenfeld R, Gupta A, and Devi LA (2008) Cell surface targeting of μ-δ opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A 105 16045-16050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Filipeanu CM, Duvernay MT, and Wu G (2007) Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta 1768 853-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Varghese G, Nguyen T, Tse R, O'Dowd BF, and George SR (2005) A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of μ and δ opioid receptor hetero-oligomers. J Biol Chem 280 38478-38488. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, et al. (2000) Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25 195-200. [DOI] [PubMed] [Google Scholar]
- Gavériaux-Ruff C and Kieffer BL (2002) Opioid receptor genes inactivated in mice: the highlights. Neuropeptides 36 62-71. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, and O'Dowd BF (2000) Oligomerization of μ- and δ-opioid receptors. Generation of novel functional properties. J Biol Chem 275 26128-26135. [DOI] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, and Devi LA (2004) A role for heterodimerization of μ and δ opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A 101 5135-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, and Devi LA (2000) Heterodimerization of μ and δ opioid receptors: a role in opiate synergy. J Neurosci 20 RC110(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody RJ, Oakley SM, Filliol D, Kieffer BL, and Kitchen I (2002) Quantitative autoradiographic mapping of opioid receptors in the brain of δ-opioid receptor gene knockout mice. Brain Res 945 9-19. [DOI] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, et al. (2005) Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell 122 619-631. [DOI] [PubMed] [Google Scholar]
- Guarna M, Bartolini A, Ghelardini C, Galeotti N, Bracci L, Stefano GB, and Bianchi E (2003) Anti-μ opioid antiserum against the third external loop of the cloned μ-opioid receptor acts as a μ receptor neutral antagonist. Brain Res Mol Brain Res 119 100-110. [DOI] [PubMed] [Google Scholar]
- Guo J, Wu Y, Zhang W, Zhao J, Devi LA, Pei G, and Ma L (2000) Identification of G protein-coupled receptor kinase 2 phosphorylation sites responsible for agonist-stimulated δ-opioid receptor phosphorylation. Mol Pharmacol 58 1050-1056. [DOI] [PubMed] [Google Scholar]
- Gurevich VV and Gurevich EV (2008) GPCR monomers and oligomers: it takes all kinds. Trends Neurosci 31 74-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague C, Uberti MA, Chen Z, Hall RA, and Minneman KP (2004) Cell surface expression of α1D-adrenergic receptors is controlled by heterodimerization with α1B-adrenergic receptors. J Biol Chem 279 15541-15549. [DOI] [PubMed] [Google Scholar]
- Jordan BA and Devi LA (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399 697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, and Dionne RA (2004) Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 109 488-496. [DOI] [PubMed] [Google Scholar]
- Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, and Loh HH (2005) Heterodimerization of μ- and δ-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J Biol Chem 280 11152-11164. [DOI] [PubMed] [Google Scholar]
- Leskelä TT, Markkanen PM, Alahuhta IA, Tuusa JT, and Petäjä-Repo UE (2009) Phe27Cys polymorphism alters the maturation and subcellular localization of the human δ opioid receptor. Traffic 10 116-129. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, and Jan LY (2000) A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron 27 97-106. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, et al. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ opioid-receptor gene. Nature 383 819-823. [DOI] [PubMed] [Google Scholar]
- Petäjä-Repo UE, Hogue M, Bhalla S, Laperrière A, Morello JP, and Bouvier M (2002) Ligands act as pharmacological chaperones and increase the efficiency of δ opioid receptor maturation. EMBO J 21 1628-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather PL, Song L, Piros ET, Law PY, and Hales TG (2000) δ Opioid receptors are more efficiently coupled to adenylyl cyclase than to L-type Ca2+ channels in transfected rat pituitary cells. J Pharmacol Exp Ther 295 552-562. [PubMed] [Google Scholar]
- Rau KK, Caudle RM, Cooper BY, and Johnson RD (2005) Diverse immunocytochemical expression of opioid receptors in electrophysiologically defined cells of rat dorsal root ganglia. J Chem Neuroanat 29 255-264. [DOI] [PubMed] [Google Scholar]
- Rees DC (1992) Chemical structures and biological activities of nonpeptide selective κ opioid ligands. Prog Med Chem 29 109-139. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R and Devi LA (2007) Receptor heterodimerization leads to a switch in signaling: β-arrestin2-mediated ERK activation by μ-δ opioid receptor heterodimers. FASEB J 21 2455-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusin KI and Moises HC (1995) μ-Opioid receptor activation reduces multiple components of high-threshold calcium current in rat sensory neurons. J Neurosci 15 4315-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, and Kieffer BL (2004) The δ agonists DPDPE and deltorphin II recruit predominantly μ receptors to produce thermal analgesia: a parallel study of μ, δ and combinatorial opioid receptor knockout mice. Eur J Neurosci 19 2239-2248. [DOI] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, and Kieffer BL (1998) Disruption of the κ-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective κ-agonist U-50,488H and attenuates morphine withdrawal. EMBO J 17 886-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Durroux T, Mouillac B, Breit A, Ayoub MA, Taulan M, Jockers R, Barberis C, and Bouvier M (2003) Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol Endocrinol 17 677-691. [DOI] [PubMed] [Google Scholar]
- Trapaidze N, Keith DE, Cvejic S, Evans CJ, and Devi LA (1996) Sequestration of the δ opioid receptor. J Biol Chem 271 29279-29285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, and Hales TG (2007) β-Arrestin2 and c-Src regulate the constitutive activity and recycling of μ opioid receptors in dorsal root ganglion neurons. J Neurosci 27 5092-5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, Maidment NT, Sanders M, Evans CJ, Kieffer BL, and Hales TG (2005) Induction of δ opioid receptor function by up-regulation of membrane receptors in mouse primary afferent neurons. Mol Pharmacol 68 1688-1698. [DOI] [PubMed] [Google Scholar]
- Walwyn WM, Keith DE Jr, Wei W, Tan AM, Xie CW, Evans CJ, Kieffer BL, and Maidment NT (2004) Functional coupling, desensitization and internalization of virally expressed μ opioid receptors in cultured dorsal root ganglion neurons from μ opioid receptor knockout mice. Neuroscience 123 111-121. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, and Manzoni O (2001) Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81 299-343. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kranzler HR, Yang BZ, Luo X, and Gelernter J (2008) The OPRD1 and OPRK1 loci in alcohol or drug dependence: OPRD1 variation modulates substance dependence risk. Mol Psychiatry 13 531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, and Guan JS (2006) Role of delivery and trafficking of δ-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci 27 324-329. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, and Pintar JE (1999) Retention of supraspinal δ-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron 24 243-252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.