Abstract
S-Adenosylmethionine (SAMe) and its metabolite 5′-methylthioadenosine (MTA) inhibit mitogen-induced proliferative response in liver and colon cancer cells. SAMe and MTA are also proapoptotic in liver cancer cells by selectively inducing Bcl-xS expression. The aims of this work were to assess whether these agents are proapoptotic in colon cancer cells, and if so, to elucidate the molecular mechanisms. We found that both SAMe and MTA are proapoptotic in HT-29 and RKO cells in a dose- and time-dependent manner. Gene microarray uncovered down-regulation of cellular FLICE inhibitory protein (cFLIP). SAMe and MTA treatment led to a decrease in the mRNA and protein levels of both the long and short cFLIP isoforms. This required de novo RNA synthesis and was associated with activation of procaspase-8, Bid cleavage, and release of cytochrome c from the mitochondria. Inhibiting caspase 8 activity or overexpression of cFLIP protected against apoptosis, whereas supplementing with polyamines did not. SAMe and MTA treatment sensitized RKO cells to tumor necrosis factor α-related apoptosis-inducing ligand-induced apoptosis. Although SAMe and MTA are proapoptotic in colon cancer cells, they have no toxic effects in NCM460 cells, a normal colon epithelial cell line. In contrast to liver cancer cells, SAMe and MTA had no effect on Bcl-xS expression in colon cancer cells. In conclusion, SAMe and MTA are proapoptotic in colon cancer cells but not normal colon epithelial cells. One molecular mechanism identified is the inhibition of cFLIP expression. SAMe and MTA may be attractive agents in the chemoprevention and treatment of colon cancer.
S-adenosylmethionine (SAMe) is the principal biological methyl donor and a precursor for polyamine biosynthesis in mammalian cells (Lu and Mato, 2005). SAMe is metabolized from methionine and ATP by methionine adenosyltransferase (MAT) (Lu and Mato, 2005). In mammals, there are three MAT enzymes: MATI, MATII, and MATIII (Kotb et al., 1997). MAT1A and MAT2A are genes that encode for the catalytic subunits of MATI/MATIII and MATII, respectively (Kotb et al., 1997). In the normal liver, in which most of the SAMe is produced by MATI/MATIII, MAT1A is expressed, whereas MAT2A expression is absent (Lu and Mato, 2005). MAT2A is expressed in all other nonhepatic tissues, including colon (Horikawa and Tsukada, 1992; Kotb et al., 1997).
Aberrant MAT2A expression in the liver has been linked to increased growth and dedifferentiation (Cai et al., 1996, 1998; Huang et al., 1998, 1999). In hepatocellular carcinoma, MAT2A is transcriptionally induced (Cai et al., 1996; Lu and Mato, 2005). In addition, we recently showed that MAT2A expression is increased in colon cancer cell lines, mouse intestinal polyps, and human colon cancer tissue samples compared with normal adjacent tissue (Chen et al., 2007). Mitogens such as leptin, insulin-like growth factor-1 (IGF-1), and epidermal growth factor induced MAT2A expression in colon cancer cell lines (Chen et al., 2007). SAMe and 5′-methylthioadenosine (MTA), a byproduct of polyamine synthesis from SAMe catabolism as well as a spontaneous breakdown product of SAMe, lowered MAT2A expression in colon cancer cell lines (Chen et al., 2007). SAMe and MTA also prevented leptin, IGF-1 and epidermal growth factor from inducing MAT2A expression and exerting their mitogenic effect in colon cancer cells (Chen et al., 2007). SAMe and MTA also exerted an inhibitory effect on MAT2A expression in proximal small intestine when administered orally for 6 days (Chen et al., 2007).
In addition to growth modulatory effects, SAMe and MTA regulate hepatocyte apoptotic response (Ansorena et al., 2002; Yang et al., 2004). It is noteworthy that although both agents are antiapoptotic in normal hepatocytes, they are proapoptotic in liver cancer cells (Ansorena et al., 2002). A key molecular mechanism for their differential proapoptotic effect in liver cancer cells is the selective induction of Bcl-xS expression (Yang et al., 2004). This effect required the participation of protein phosphatase 1, which enhanced the alternative splicing of Bcl-x (Yang et al., 2004). Although we have shown that SAMe and MTA can inhibit mitogen-induced cell growth in colon cancer cells, whether they also modulate apoptosis in colon cancer cells remains unknown.
The goals of this study were 1) to examine the apoptotic potential of SAMe and MTA in colon cancer cells; 2) to identify novel gene targets that may be associated with the SAMe/MTA proapoptotic effect; and 3) to test the feasibility of SAMe and MTA as potential therapeutic agents in colon cancer treatment. Our studies revealed that indeed, SAMe and MTA are proapoptotic in colon cancer cells but not in normal colon epithelial cells. It is noteworthy that the molecular mechanism is quite different from that in liver cancer cells. Our findings also support the notion that these agents may be useful in colon cancer chemotherapy.
Materials and Methods
Materials. Cell culture media for HT-29 and RKO cells were obtained from the University of Southern California Norris Cancer Center Cell Culture Core. Fetal bovine serum (FBS) was obtained from Omega Scientific (Tarzana, CA), and M3 Base culture media were from INCELL Corporation (San Antonio, TX). SAMe in the form of disulfate p-toluene sulfonate dried powder was generously provided by Gnosis SRL (Cairate, Italy), and MTA was purchased from Sigma-Aldrich (St. Louis, MO). Caspase-8 and cFLIP antibodies were purchased from Alexis Biochemicals (San Diego, CA). cFLIP-long (cFLIPL) and cFLIP-short (cFLIPS) expression vectors were a kind gift from Dr. Jürg Tschopp (Department of Biochemistry, University of Lausanne, Lausanne, Switzerland). All other reagents were of analytical grade and were obtained from commercial sources.
Cell Culture and SAMe, MTA, Caspase-8 Inhibitor, Polyamines, 5-Fluorouracil, and Recombinant Tumor Necrosis Factor α-Related Apoptosis-Inducing Ligand Treatments. HT-29 and RKO colon cancer cells were obtained from the Cell Culture Core of the University of Southern California Research Center for Liver Diseases (Los Angeles, CA). These cell lines were grown according to the conditions specified by the American Type Culture Collection (Manassas, VA). NCM460 normal colon epithelial cells (Moyer et al., 1996) were from INCELL Corporation and were grown in M3:Base cell culture medium supplemented with 10% FBS at 37°C in a 5% CO2 humidified incubator.
Cells were plated, grown to 50 to 60% confluence on six-well plates, and changed to media containing 1% FBS (RKO and HT-29) or 0% FBS (NCM460 cells) for 24 h before treatment with either SAMe or MTA. Cells were treated with either SAMe (0.25-5 mM) or MTA (0.25-2 mM) in 0% FBS for 1 to 24 h.
To test the effect of inhibiting caspase-8 on SAMe- or MTA-mediated apoptosis, RKO cells were treated with 2 mM SAMe, 1 mM MTA, or vehicle in the presence or absence of 100 μM benzyloxycarbonyl-Ile-Glu(OMe)-Thr-Asp(OMe)-fluoromethylketone, a caspase-8 inhibitor (Calbiochem, San Diego, CA). Cells were changed to 1% FBS for 24 h and then to 0% FBS when treated with these agents.
In some experiments, RKO cells were treated with 1 mM MTA or vehicle in the presence or absence of increasing amounts of polyamines (putrescine/spermidine/spermine) in a 1:18.5:86.8 ratio for 24 h. The ratios are to recapitulate intracellular polyamine ratios in control colon cancer cells (Chen et al., 2007). Cells were changed to 1% FBS for 24 h and then to 0% FBS when treated with these agents.
In other experiments, RKO cells were treated with 2 mM SAMe, 1 mM MTA, or 5 μM 5-fluorouracil (5-FU; Sigma-Aldrich) alone or in combination with 100 ng/ml recombinant tumor necrosis factor α-related apoptosis-inducing ligand (rTRAIL) (EMD Biosciences, San Diego, CA) for 24 h. Cells were changed to 1% FBS for 24 h before treatment and then to 0% FBS when treated with these agents.
cFLIP Overexpression Studies. RKO cells (5 × 105 cells/well) were plated to 60 to 70% confluence in media containing 1% FBS. Two micrograms of cFLIPL or cFLIPS or 1 μg each of both isoforms was transfected using 3 μl of Transmax (a generous gift from Dr. Z. Z. Wang, University of Southern California) transfection reagent for a total of 48 h in 1% FBS. Twenty-four hours after transfection, the media were replaced with 0% FBS, and the cells were also treated with 2 mM SAMe or 1 mM MTA for 24 h. Cells were either harvested to check for cFLIP protein expression or used for Hoechst staining to detect the level of apoptosis.
Apoptosis Detection. Apoptosis was measured using Hoechst staining and flow cytometry using the Apo-direct Kit (BD Pharmingen, San Diego, CA), which uses the terminal deoxynucleotidyl transferase dUTP nick-end labeling assay as we described previously (Yang et al., 2008). Hoeschst staining and terminal deoxynucleotidyl transferase dUTP nick-end labeling yielded comparable results and were pooled for analysis.
The ApoAlert Cell Fractionation Kit (Clontech, Mountain View, CA) was used to isolate mitochondrial and cytosolic fractions of HT-29 cells treated with either 2 mM SAMe or 1 mM MTA for 24 h. Western blot analysis was performed to detect cytochrome c release. Antibodies to cytochrome c oxidase 4 and α tubulin (Cell Signaling Technology, Danvers, MA) were used as loading controls and to check the purity of the mitochondrial and cytosolic fractions, respectively. The assay was performed according to the manufacturer's suggested protocol.
RNA Isolation and Microarray Analysis. HT-29 cells were treated with either 2 mM SAMe or 1 mM MTA for 6 h, and total RNA was extracted using the ArrayGrade Total RNA Isolation Kit (SABiosciences, Frederick, MD). One microgram of RNA was labeled, and cRNA was synthesized using TrueLabeling-AMP 2.0 kit (SABiosciences). cRNA cleanup was done using ArrayGrade cRNA Cleanup kit (SABiosciences). Four micrograms of cRNA was used to hybridize to an Oligo GEArray Human Apoptosis Array membrane containing apoptosis-related genes (SABiosciences) at 60°C overnight. Chemiluminescent detection was done using Nonrad-GEArray Q series Detection Kit (SABiosciences) and exposed to autoradiographic film. Densitometric analysis was performed on the apoptosis Oligo GEArray using Quantity-One software (Bio-Rad, Hercules, CA).
RNA Isolation and Quantitative Gene Expression Analysis. RNA isolation, cDNA synthesis, and real-time polymerase chain reaction (PCR) analysis were done as described previously (Chen et al., 2007). Taqman probes to cFLIPL, cFLIPS, bifunctional apoptosis regulator (BFAR), tumor necrosis factor (TNF), IGF-1R, Bcl-xS, and the housekeeping genes (ubiquitin C or hypoxanthine phosphoribosyl-transferase-1) were purchased from Applied Biosystems (Foster City, CA).
Effects of Actinomycin D and Cycloheximide on cFLIP Expression. RKO cells were pretreated for 1 h with 5 μg/ml actinomycin D to inhibit de novo RNA synthesis or 10 μg/ml cycloheximide to inhibit de novo protein synthesis followed by treatment with 1 mM MTA or vehicle control for up to 4 h. RNA was extracted for the determination of cFLIP mRNA levels at different time points. Regression analysis using the best-fit line was used to calculate half-lives of the cFLIP isoforms.
Cell Viability Assay. Cell viability was assayed using the 3-(4,5-dimethylthiazolyl-2-yl)-2,5-diphenyltetrazolium bromide-based cell growth determination kit (Sigma-Aldrich). RKO or NCM460 cells were treated with 1 mM SAMe or 1 mM MTA for 24 h in serum-free media. The assay was performed according to the manufacturer's suggested protocol.
Western Blot Analysis. For cFLIP, Bid, and caspase-8 expression, RKO cells were treated with either SAMe (0.5-2 mM) or MTA (0.25-2 mM) for 6 to 24 h or were transfected with cFLIP expression vectors for 48 h. Western blot analysis was done as we described previously (Chen et al., 2004) using anti-cFLIP, Bid (Cell Signaling Technology), and caspase-8 antibodies.
Statistical Analysis. Data are given as mean ± S.E.M. Statistical analysis was performed using analysis of variance and Fisher's test. For mRNA and protein levels, ratios of genes and proteins to respective housekeeping densitometric values were compared. Significance was defined by p < 0.05.
Results
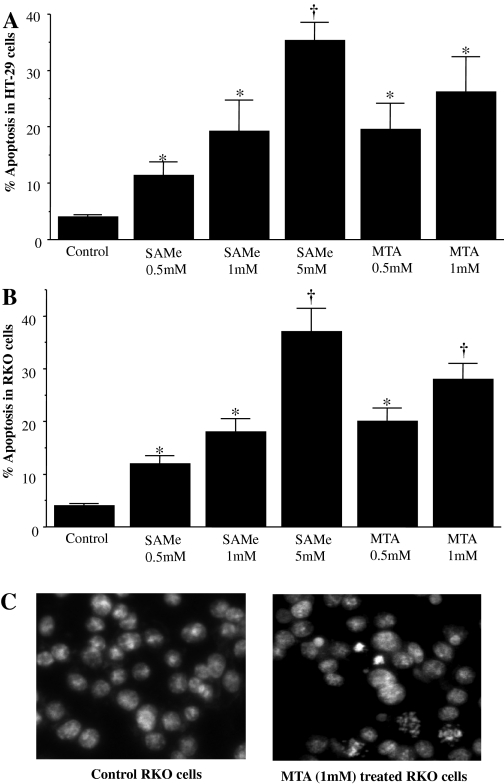
SAMe and MTA Are Proapoptotic in Colon Cancer Cells. Similar to their effects in liver cancer cells, SAMe and MTA exerted a proapoptotic effect in both HT-29 (Fig. 1A) and RKO (Fig. 1B) cells in a dose-dependent manner after 24 h of treatment. The proapoptotic effect was noted after 16 h (data not shown). Figure 1C shows the typical appearance of RKO cells treated for 24 h with 1 mM MTA, with approximately a 3-fold increase in apoptotic cells.
Fig. 1.
SAMe and MTA are proapoptotic in HT-29 and RKO cells. HT-29 and RKO cells were treated with SAMe and MTA at varying doses for 24 h, and apoptosis was measured as described under Materials and Methods. Effect in HT-29 cells (A), effect in RKO cells (B), and Hoechst staining after 24 h of MTA treatment in representative RKO cells (C). *, p < 0.05 versus control; †, p < 0.01 versus control from three to four experiments.
Molecular Targets of SAMe and MTA. Because SAMe and MTA can selectively induce Bcl-xS in liver cancer cells, we measured the mRNA levels of Bcl-xS after SAMe and MTA treatment in colon cancer cells. Unlike their effects in liver cancer cells, SAMe and MTA had no effect on the Bcl-xS mRNA level in colon cancer cells (data not shown).
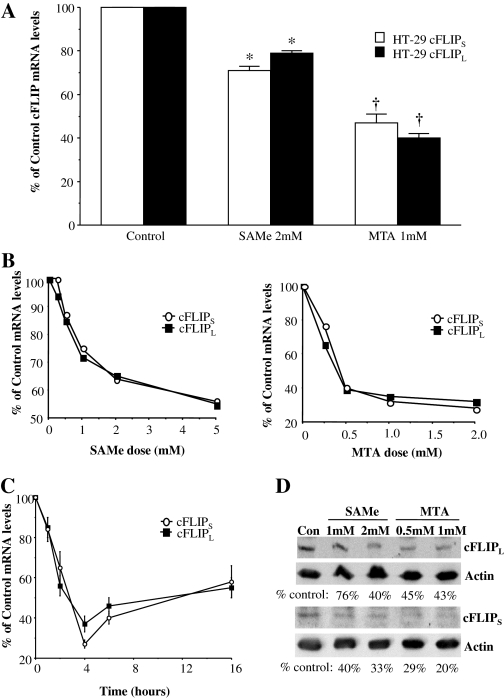
To elucidate the molecular targets of SAMe and MTA in their proapoptotic effects, we used a microarray approach to see their effects on the expression of proapoptotic or antiapoptotic genes. No significant change in the mRNA level of proapoptotic genes was detected (data not shown), but down-regulation in the expression of several antiapoptotic genes was noted (Table 1). Of the antiapoptotic genes included in the array, more than 40% reduced expression with either SAMe or MTA occurred with BFAR, baculoviral inhibitor of apoptosis repeat-containing 7 (BIRC7), CFLAR (same as cFLIP), and TNF, and 34% reduced expression was observed with IGF-1R (Table 1). Real-time PCR analysis confirmed only CFLAR expression. It is noteworthy that only cFLIPS was included on the microarray. Using real-time PCR, we confirmed that in HT-29 cells, 2 mM SAMe treatment lowered the mRNA levels of both cFLIP isoforms by 25 to 30% after 6 h. One micromolar MTA was more potent, decreasing the mRNA levels of the same genes by 50 to 60% after 6 h (Fig. 2A). SAMe and MTA also exerted a dose-dependent (Fig. 2B) and time-dependent (Fig. 2C) inhibitory effect on the mRNA levels of both cFLIP isoforms in RKO cells. Western blot analysis performed after 6 h of treatment with either SAMe or MTA showed lower protein levels of both cFLIPL and cFLIPS (Fig. 2D).
TABLE 1.
Effects of SAMe and MTA treatment on the expression of antiapoptotic genes in HT-29 cells
HT-29 cells were treated with 2 mM SAMe or 1 mM MTA for 6 h and subjected to microarray analysis as described under Materials and Methods. See list of genes in Apoptotic Array (SABiosciences, Bioscience).
| Genes | 2 mM SAMe | 1 mM MTA |
|---|---|---|
| -fold of untreated control | ||
| BAG1 | 1.05 | 0.90 |
| BAG3 | 1.214 | 1.08 |
| BAG4 | 1.03 | 1.02 |
| BCL2L2 | 0.97 | 0.91 |
| BCL2L10 | 1.38 | 0.93 |
| BFAR | 0.55 | 0.60 |
| BIRC1 | 0.97 | 0.96 |
| BIRC6 | 0.95 | 0.86 |
| BIRC7 | 0.82 | 0.56 |
| BRAF | 0.99 | 0.72 |
| CFLAR | 1.19 | 0.45 |
| IGF1R | 0.74 | 0.66 |
| MCL1 | 1.12 | 0.98 |
| TNF | 0.80 | 0.36 |
Fig. 2.
SAMe and MTA inhibit cFLIP expression in colon cancer cells. A, HT-29 cells were treated with 2 mM SAMe or 1 mM MTA, and cFLIP isoform mRNA levels were determined by real-time PCR as described under Materials and Methods. *, p < 0.01, †, p < 0.001 versus control from three to four experiments. B, RKO cells were treated with SAMe (0.25-5 mM) (left) or MTA (0.25-2 mM) (right) for 6 h, and cFLIP isoform mRNA levels were measured using real-time PCR. C, RKO cells were treated with 1 mM MTA, and cFLIP isoform mRNA levels were determined after 1 to 16 h. D, RKO cells were treated with SAMe or MTA at the given doses, and protein levels of cFLIP isoforms were determined by Western blot analysis using 80 μg of protein per lane. The numbers below each blot are densitometric values expressed as the percentage of control after normalizing to actin. Representative blots from three separate experiments are shown.
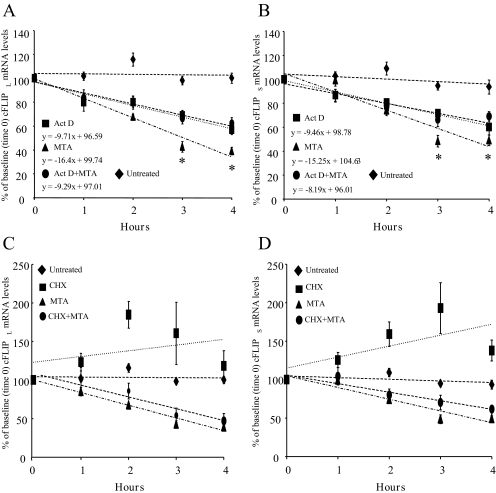
The Inhibitory Effect of MTA on cFLIP Expression in Colon Cancer Cells Requires De Novo RNA but Not Protein Synthesis. To identify the molecular mechanism of MTA's inhibitory effect on cFLIP expression, RKO cells were pretreated with either actinomycin D or cycloheximide for 1 h before 1 mM MTA treatment for 1 to 4 h. The half-lives of cFLIPL and cFLIPS mRNAs are 4.8 and 5.2 h, respectively. MTA treatment led to a more rapid decrease in the mRNA levels of both cFLIP isoforms (t½ for cFLIPL = 3.0 h and cFLIPS = 3.6 h) compared with actinomycin D alone (Fig. 3, A and B). However, in the presence of actinomycin D, MTA did not exert any additional inhibitory effect. Cycloheximide pretreatment increased the mRNA levels of both cFLIP isoforms but had no effect on the reduction of cFLIP mRNA levels in MTA-treated RKO cells (Fig. 3, C and D).
Fig. 3.
MTA's effect on cFLIP mRNA expression in colon cancer cells requires de novo RNA synthesis but not de novo protein synthesis. A and B, RKO cells were pretreated with either 5 μg/ml actinomycin D (Act D) or vehicle (EtOH) for 1 h before either 1 mM MTA treatment or vehicle control (dimethyl sulfoxide) for 1 to 4 h. RNA was extracted at each time point, and cFLIP expression was assayed by quantitative real-time PCR analysis for cFLIPL (A) and for cFLIPS (B). Results are expressed as the average ± S.E. of five independent experiments. Linear regression analysis with bestfit lines provided formulas for the calculation of t½. *, p < 0.01 versus Act D + MTA. C and D, de novo protein synthesis is not required for cFLIP mRNA down-regulation by MTA in colon cancer cells. RKO cells were pretreated with either 10 μg/ml cycloheximide (CHX) or vehicle (EtOH) for 1 h before treatment with either 1 mM MTA or vehicle control (dimethyl sulfoxide) for 1 to 4 h. RNA was extracted for each time point, and cFLIP expression levels were determined by quantitative real-time PCR for cFLIPL (C) and for cFLIPS (D). Results are expressed as the average ± S.E. of three to five experiments.
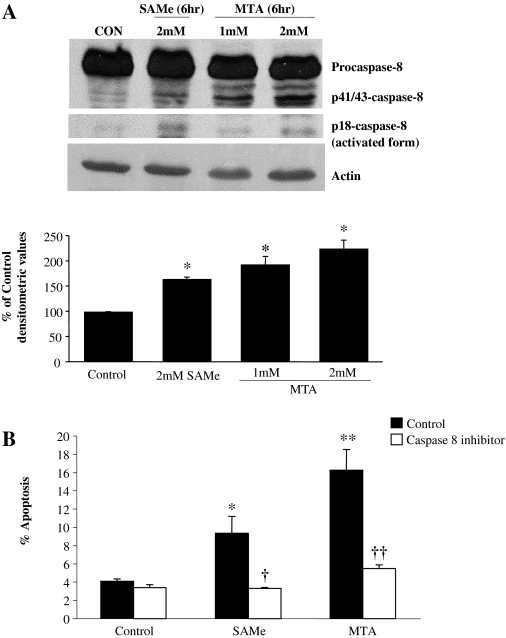
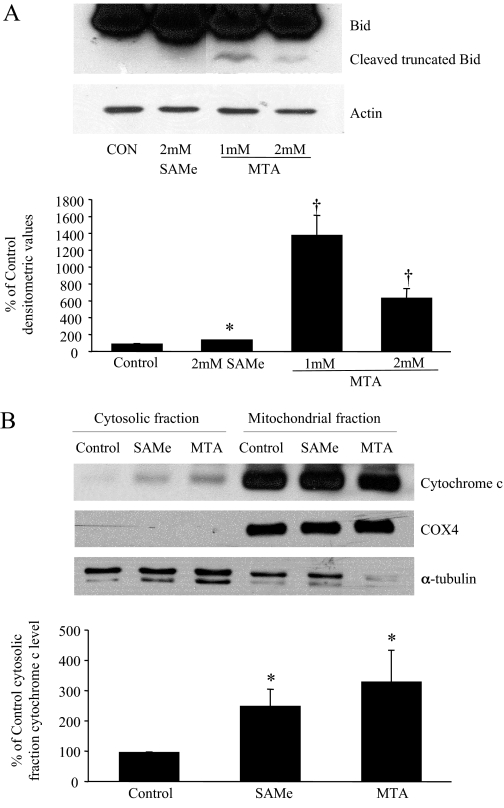
Activation of Procaspase-8 and Release of Cytochrome c. cFLIP is structurally related to procaspase-8 but lacks enzymatic activity (Sharp et al., 2005). It is antiapoptotic by preventing caspase-8 activation (Sharp et al., 2005). Consistent with this, treatment of either SAMe or MTA for 6 h led to the activation of procaspase-8 (Fig. 4A) and the release of cytochrome c from mitochondria (Fig. 5B). In addition, an inhibitor of caspase-8 was able to significantly block the SAMe- or MTA-mediated apoptosis in RKO cells (Fig. 4B). Truncated Bid, cleaved by active caspase-8, can lead to the release of cytochrome c (Yin, 2006). Figure 5A shows that MTA treatment led to a clear increase in Bid cleavage.
Fig. 4.
SAMe and MTA activates procaspase-8 in colon cancer cells and is required for SAMe or MTA-mediated apoptosis of colon cancer cells. A, RKO cells were treated with SAMe or MTA for 6 h, and Western blot analysis was performed for various forms of caspase-8 using 80 μg of protein per lane. Densitometric values (mean ± S.E.) are the percentage of control, which are normalized to actin for loading control (bar graph below the Western blot). Blot is representative of three independent experiments. *, p < 0.005 versus control. B, RKO cells were cotreated with either 2 mM SAMe or 1 mM MTA with 100 μM caspase-8 inhibitor for 24 h. Apoptosis was determined as described under Materials and Methods. Results are the mean ± S.E. from three to six experiments. *, p < 0.05 versus control; **, p < 0.001 versus control; †, p < 0.05 versus SAMe; ††, p < 0.05 versus MTA.
Fig. 5.
SAMe or MTA can affect downstream components of the apoptotic pathway. A, RKO cells were treated with either SAMe or MTA at the indicated doses for 24 h. Western blot analysis against Bid was done with 80 μg of protein per lane. The graph below represent mean ± S.E. densitometric values from three experiments. *, p < 0.05 versus control; †, p < 0.005 versus control. B, HT-29 cells were treated with 2 mM SAMe or 1 mM MTA for 24 h, and cytoplasmic and mitochondrial cytochrome c levels were measured by Western blot analysis using 10 μg of protein per lane as described under Materials and Methods. The bar graph below represents mean ± S.E. densitometric values expressed as the percentage of control cytosolic fraction cytochrome c levels from three experiments. *, p < 0.05 versus control.
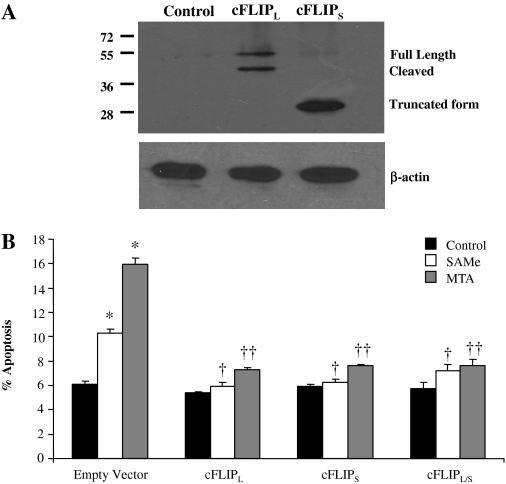
Role of cFLIP on SAMe and MTA-Induced Apoptosis. To determine whether down-regulation of cFLIP is the cause of SAMe and MTA-induced apoptosis in colon cancer cells, cells were transfected with overexpression vector for cFLIPL and/or cFLIPS for 24 h before treatment with SAMe or MTA. Figure 6 shows that overexpression of either cFLIPL or cFLIPS alone or together prevented the ability of SAMe and MTA to induce apoptosis.
Fig. 6.
cFLIP overexpression protects against SAMe- and MTA-induced apoptosis. RKO cells were transfected with empty vector (control), cFLIPL, cFLIPS, or cFLIPL/S vectors for 48 h as described under Materials and Methods. A, the effect of overexpression on cFLIP protein levels on Western blot analysis using 20 μg of protein per lane. B, treatment with 2 mM SAMe or 1 mM MTA was started 24 h after transfection for another 24 h. *, p < 0.001 versus control + empty vector; †, p < 0.001 versus SAMe + empty vector; ††, p < 0.001 versus MTA + empty vector from six to nine experiments.
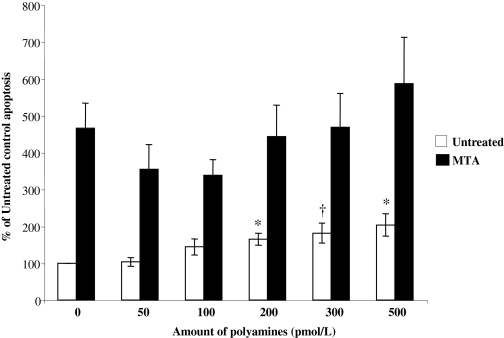
Role of Polyamines in MTA-Induced Apoptosis in Colon Cancer Cells. We have shown previously that 6-h MTA treatment of colon cancer cells reduced polyamine levels (Chen et al., 2007). To determine whether MTA's proapoptotic effect is due to a reduction of polyamine levels, we added polyamines to MTA-treated RKO cells for 24 h. There is a dose-dependent significant proapoptotic effect of the polyamines in RKO cells, but lower doses of polyamines (50 or 100 pM polyamines with respect to putrescine) had a tendency (not statistically significant) to reduce the level of apoptosis in MTA-treated RKO cells (Fig. 7).
Fig. 7.
Effects of polyamine addition on MTA's proapoptotic effect in colon cancer cells. RKO cells were treated with either 1 mM MTA or vehicle control with increasing amounts of polyamines concurrently for 24 h as described under Materials and Methods. Apoptosis levels were determined by Hoechst staining. Results represent an average ± S.E. of six independent experiments. *, p < 0.01 versus untreated control; †, p < 0.05 versus untreated control.
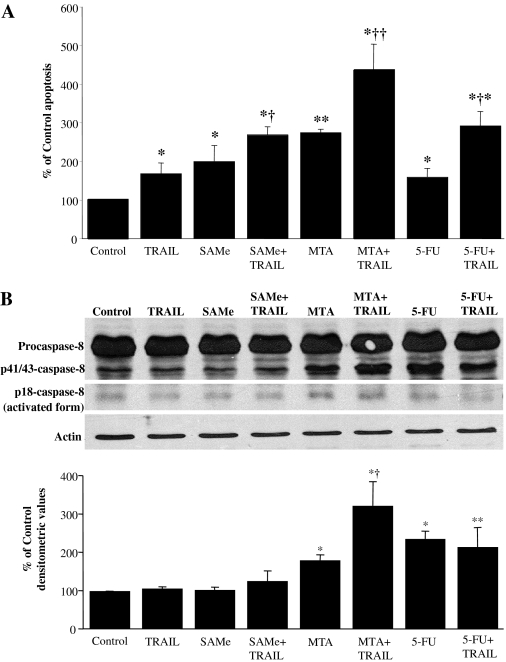
SAMe and MTA Sensitize RKO Cells to Tumor Necrosis Factor α-Related Apoptosis-Inducing Ligand-Induced Apoptosis. Colon cancer is known to have overexpression of cFLIP (Zhou et al., 2005), and cFLIPL inhibited chemotherapy-induced colorectal cancer cell death (Longley et al., 2006). To determine whether SAMe and MTA can enhance the ability of rTRAIL to induce apoptosis, we compared the effect of SAMe and MTA with that of 5-FU on rTRAIL-induced apoptosis. Figure 8A shows that although rTRAIL by itself had a small inductive effect on apoptosis, cotreatment with SAMe, MTA, or 5-FU all enhanced the proapoptotic effect. This enhanced proapoptotic effect correlates with increased activation of procaspase-8, especially with MTA (Fig. 8B). Of the three agents, MTA in particular had the most dramatic effect on promoting the apoptotic effect.
Fig. 8.
SAMe and MTA sensitize RKO cells to TRAIL-induced apoptosis. A, RKO cells were treated with 2 mM SAMe, 1 mM MTA, 100 ng/ml TRAIL, or 5 μM 5-FU alone or in combination for 24 h, and apoptosis was determined as described under Materials and Methods. Results are expressed as the mean ± S.E. from four to seven experiments. *, p < 0.05 versus control; **, p < 0.001 versus control; †, p < 0.05 versus TRAIL; ††, p < 0.05 versus MTA or TRAIL; †*, p < 0.05 versus 5-FU or TRAIL. B, Western blot analysis of caspase-8 using 80 μg of total protein of the various treatments as above. The bar graph below the blot represent densitometric results shown as mean ± S.E. from three experiments. *, p < 0.01 versus control or TRAIL; **, p < 0.05 versus control or TRAIL; †, p < 0.05 versus MTA.
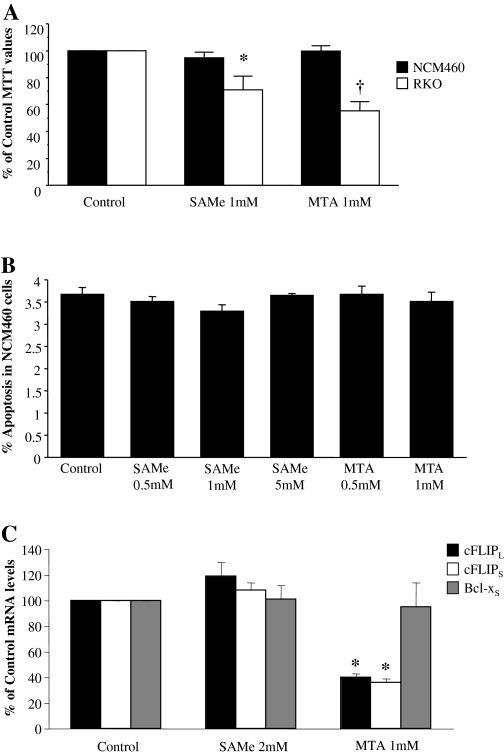
SAMe and MTA Are Not Toxic to NCM460 Cells. NCM460 cells are normal colon epithelial cells (Moyer et al., 1996). We examined the effect of SAMe and MTA at doses that are toxic to colon cancer cells in this cell line. Figures 1 and 9, A and B, show that SAMe and MTA are selectively toxic to colon cancer cells but have no harmful effect on NCM460 cells. Neither agent any no effect on Bcl-xS expression, but interestingly, MTA but not SAMe reduced cFLIP expression (Fig. 9C).
Fig. 9.
SAMe and MTA are selectively toxic to colon cancer cells. A, NCM460 and RKO cells were treated with 1 mM SAMe or MTA for 24 h, and 3-(4,5-dimethylthiazolyl-2-yl)-2,5-diphenyltetrazolium bromide assay was used to measure cell viability as described under Materials and Methods. Results are the mean ± S.E. from four experiments. *, p < 0.05 versus control; †, p < 0.01 versus control. B, NCM460 cells were treated with varying doses of SAMe or MTA for 24 h. Apoptosis levels were determined as the mean ± S.E. from three experiments as described under Materials and Methods. C, NCM460 cells were treated with either 2 mM SAMe or 1 mM MTA for 4 h, and total RNA was isolated as described under Materials and Methods. Real-time PCR analysis was done using Taqman probes to Bcl-xS and both cFLIP isoforms. Results are mean ± S.E. from three experiments. *, p < 0.001 versus control.
Discussion
SAMe is essential for life as the principal biological methyl donor and precursor for polyamines (Lu and Mato, 2005). In contrast to SAMe, MTA inhibits transmethylation and polyamine biosynthesis (Clarke, 2006). SAMe is highly unstable with significant conversion to MTA when delivered exogenously (Chen et al., 2007). Given the ready availability of SAMe as a nutritional supplement in the United States and as a therapeutic agent elsewhere, it is important to juxtapose the actions of SAMe and MTA. MTA has thus far recapitulated SAMe's effect on MAT2A and TNFα expression (Veal et al., 2004; Chen et al., 2007), growth, and apoptosis (Ansorena et al., 2002; Yang et al., 2004; Ramani et al., 2008) at doses lower than SAMe. Our speculation is that many pharmacological actions of SAMe may actually be mediated by MTA.
In this work, we have examined the actions of SAMe and MTA on apoptosis in colon cancer cell lines RKO and HT-29 compared with the normal colon epithelial cell line NCM460. This work was prompted by our findings that these agents modulate growth in liver and colon cancer cells and are selectively proapoptotic in liver cancer cells (Ansorena et al., 2002; Yang et al., 2004; Chen et al., 2007; Ramani et al., 2008). If they are also selectively proapoptotic in colon cancer cells, then they might be of great value in either chemoprevention or treatment of colon neoplasia.
Both SAMe and MTA exerted a proapoptotic effect in HT-29 and RKO cells in a dose- and time-dependent manner. HT-29 and RKO differ in p53 status: HT-29 cells express inactive p53, whereas RKO cells express wild-type p53 (American Type Culture Collection). Thus, the proapoptotic effects of SAMe and MTA are independent of p53. SAMe and MTA treatment increased procaspase-8 cleavage, and MTA in particular increased Bid cleavage. Treatment with SAMe and MTA also resulted in cytochrome c release, implicating mitochondrial involvement in the process. It is noteworthy that both agents are not toxic to NCM460 cells, which was originally established in 1996 as the first nonmalignant colon epithelial cell line derived from the transverse colon (Moyer et al., 1996). NCM460 cells are nontumorigenic, have features similar to those of normal human colonocytes in primary culture, and have served as an excellent in vitro model of normal colon epithelial cells (Moyer et al., 1996). The fact that SAMe is nontoxic to normal colon epithelial cells is consistent with its excellent safety profile and lack of significant side effects (Mato and Lu, 2005). Although MTA reduced cFLIP mRNA expression, it did not induce apoptosis. This suggests that NCM460 cells may have protective mechanism(s) to prevent the induction of apoptosis with reduced cFLIP expression.
SAMe and MTA's selective proapoptotic effects in liver cancer cells are a result of inducing Bcl-xS in cancerous but not normal hepatocytes (Yang et al., 2004). However, these agents had no effect on Bcl-xS expression in colon cancer cell lines, suggesting that the molecular mechanisms differ in liver versus colon cancer cells.
To gain insight into the mechanism of exogenous SAMe- and MTA-induced apoptosis, we used a microarray approach focusing on apoptosis-related genes. Of the several genes altered, the one that intrigued us is cFLIP, which was down-regulated particularly by MTA. Only 3 of the 11 splice variants of the cFLIP gene are translated (cFLIPL, cFLIPS, and cFLIPR) (Tschopp et al., 1998; Golks et al., 2005). cFLIPR was recently found to be expressed exclusively in the Raji B cell line (Golks et al., 2005), whereas cFLIPL and cFLIPS are widely expressed. cFLIPL is a catalytically inactive version of procaspase-8 (Budd et al., 2006). cFLIPS is a truncated form that lacks the caspase-like domain (Budd et al., 2006). cFLIP proteins are important in lymphocyte activation and development, and they interact with caspase-8 to modulate the death receptor-mediated apoptotic signal (Sharp et al., 2005; Budd et al., 2006). cFLIPS can act as a dominant-negative to compete with procaspase-8 for recruitment to the death-inducing signaling complex (DISC) (Budd et al., 2006). cFLIPL can compete with procaspase-8 for binding to the DISC, but it also forms heterodimers with procaspase-8 at the DISC (Budd et al., 2006). This interaction allows for partial cleavage of procaspase-8 but prevents its full activation, thus limiting its proapoptotic activity in the cell (Budd et al., 2006). Therefore, the balance between caspase-8 and cFLIP is crucial in determining the apoptotic potential of a cell.
Both SAMe and MTA treatment down-regulated the expression of both cFLIP isoforms at the mRNA and protein levels in a dose- and time-dependent manner, with MTA exerting a much more potent effect at comparable doses. The effect requires de novo synthesis of RNA (but not protein), because in the presence of actinomycin D, MTA no longer exhibited an inhibitory effect. Although this may signal a transcriptional mechanism, it is interesting to note that cFLIP isoform mRNA levels fell more rapidly after MTA treatment than actinomycin D alone. This suggests that MTA also accelerated the cFLIP mRNA degradation, but this requires de novo RNA synthesis. The exact molecular mechanism of this effect will require additional work to elucidate. Cycloheximide treatment increased the mRNA levels of both cFLIP isoforms. This is consistent with the ability of cycloheximide to prevent degradation of labile mRNAs (Ohh and Takei, 1995).
Consistent with cFLIP's known role in preventing procaspase-8 activation, treatment with SAMe or MTA led to procaspase-8 activation. It is noteworthy that inhibition of caspase-8 attenuated SAMe and MTA's proapoptotic effect on colon cancer cells, thus confirming the importance of caspase-8 in SAMe/MTA-mediated apoptosis in these cells. In addition, overexpression with either cFLIP isoform prevented apoptosis induced by either SAMe or MTA, supporting an important role for cFLIP down-regulation in the apoptotic event.
We previously reported that 6 h of MTA treatment in colon cancer cells depleted intracellular polyamine levels (Chen et al., 2007), which can lead to apoptosis (Seiler and Raul, 2005). We cotreated RKO cells with MTA and varying doses of polyamines to determine whether the proapoptotic effect of MTA can be blocked. Polyamines increased apoptosis in a dose-dependent manner. Although the role of polyamines in growth is well accepted, their role in apoptosis is often contradictory. Polyamine depletion has been shown to both enhance and protect against apoptosis in a cell line- and apoptotic stimulus-dependent manner (Seiler and Raul, 2005). Complicating this further, excessive polyamines can also activate apoptosis (Seiler and Raul, 2005). Taken together, although polyamine depletion may have contributed to MTA's proapoptotic effect, it is unlikely to be a dominant mechanism.
cFLIP is overexpressed in many cancers, including colon cancer (Ryu et al., 2001; Zhou et al., 2005; Longley et al., 2006). A number of studies have demonstrated that overexpression of cFLIP protects cancer cells from apoptosis induced by a wide variety of chemotherapeutic drugs (Kruyt, 2008). In one study, cFLIPL overexpression in HCT116 cells reduced the apoptotic potential of 5-FU, oxaliplatin, and irinotecan (CPT-11), whereas small-interfering RNA knockdown of cFLIP in RKO and HCT116 cells increased the potency of these same chemotherapeutic drugs (Longley et al., 2006). Many recent studies have focused on the potential use of TRAIL as a chemotherapeutic agent in treating cancer. TRAIL, a ligand for the TNF superfamily and a potent activator of the extrinsic apoptosis pathway, is currently in phase I clinical trials (Zhang and Fang, 2005; Kruyt, 2008). TRAIL is attractive as a cancer therapy drug because it causes apoptosis in cancer cells but has minimal toxicity in most normal cells (Wiley et al., 1995; Pitti et al., 1996). However, a number of cancer cell lines are chemoresistant to TRAIL, which might also translate to human tumors (Zhang and Fang, 2005; Kruyt, 2008). Increased expression of cFLIP may be a major factor in chemoresistance to TRAIL (Zhang and Fang, 2005; Kruyt, 2008). 5-FU, oxaliplatin, and CPT-11 have been shown to enhance the sensitivity and potency of TRAIL in HCT116 cells (Longley et al., 2006) by reducing the expression of both cFLIP isoforms (Galligan et al., 2005). This makes cFLIP an increasingly attractive target for improving TRAIL-mediated apoptosis in resistant cancers.
To examine whether SAMe and MTA can also sensitize TRAIL-resistant colon cancer cell lines to apoptosis, we used TRAIL-resistant RKO cells (Vasilevskaya and O'Dwyer, 2005). Simultaneous treatment of either 2 mM SAMe or 1 mM MTA with 100 ng/ml TRAIL increased cellular apoptosis by 60 and 160%, respectively, compared with TRAIL alone. MTA seems to have a greater effect on TRAIL-mediated apoptosis in RKO cells, which may be due to the greater effect MTA has on cFLIP expression and procaspase-8 activation. The magnitude of apoptosis was even higher than the combination treatment of 5-FU and TRAIL. However, 5-FU is indiscriminate in killing either cancer or normal cells, whereas SAMe and MTA, like TRAIL, only target cancer cells. In addition, SAMe and MTA are naturally occurring molecules found in every person, thus increasing the likelihood of having fewer toxic side effects.
In summary, SAMe or MTA was able to induce apoptosis in colon cancer cell lines but not in normal colon epithelial cells. This mimics the effect of SAMe and MTA on liver cancer cells but with different molecular mechanism(s). The apoptotic signal may be due to reduced cFLIP expression in the treated cells because cFLIP overexpression was able to prevent SAMe- and MTA-induced apoptosis. Finally, our results suggest that SAMe and MTA can enhance the ability of TRAIL to induce apoptosis in colon cancer cells. Overall, the results suggests that SAMe and MTA via the down-regulation of cFLIP can be potentially effective and specific chemopreventive and chemotherapeutic agents in the treatment of colonic neoplasia.
Acknowledgments
RKO and HT-29 cells were provided by the Cell Culture Core, and confocal microscope was provided by the Cell Imaging Core of the University of Southern California Research Center for Liver Diseases.
This work was supported by the National Institutes of Health National Center for Complementary and Alternative Medicine [Grant AT004896] and by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK44510, DK48522].
ABBREVIATIONS: SAMe, S-adenosylmethionine; 5-FU, 5-fluorouracil; cFLIP, cellular FLICE inhibitory protein; cFLIPL, cellular FLICE inhibitory protein-long; cFLIPS, cellular FLICE inhibitory protein-short; DISC, death-inducing signaling complex; BIRC7, baculoviral inhibitor of apoptosis repeat-containing 7; BFAR, bifunctional apoptosis regulator; FBS, fetal bovine serum; IGF, insulin-like growth factor; MAT, methionine adenosyltransferase; MTA, methylthioadenosine; PCR, polymerase chain reaction; rTRAIL, recombinant tumor necrosis factor α-related apoptosis-inducing ligand; TNFα, tumor necrosis factor-α; TRAIL, tumor necrosis factor-α-related apoptosis-inducing ligand; CFLAR, caspase-8 and Fas-associated protein with death domain-like apoptosis regulator; DMSO, CPT-11, irinotecan.
References
- Ansorena E, García-Trevijano ER, Martínez-Chantar ML, Huang ZZ, Chen L, Mato JM, Iraburu M, Lu SC, and Avila MA (2002) S-adenosylmethionine and methylthioadenosine are antiapoptotic in cultured rat hepatocytes but proapoptotic in human hepatoma cells. Hepatology 35 274-280. [DOI] [PubMed] [Google Scholar]
- Budd RC, Yeh WC, and Tschopp J (2006) cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol 6 196-204. [DOI] [PubMed] [Google Scholar]
- Cai J, Mao Z, Hwang JJ, and Lu SC (1998) Differential expression of methionine adenosyltransferase genes influences the rate of growth of human hepatocellular carcinoma cells. Cancer Res 58 1444-1450. [PubMed] [Google Scholar]
- Cai J, Sun WM, Hwang JJ, Stain SC, and Lu SC (1996) Changes in S-adenosylmethionine synthetase in human liver cancer: molecular characterization and significance. Hepatology 24 1090-1097. [DOI] [PubMed] [Google Scholar]
- Chen H, Xia M, Lin M, Yang H, Kuhlenkamp J, Li T, Sodir NM, Chen YH, Josef-Lenz H, Laird PW, Clarke S, Mato JM, and Lu SC (2007) Role of methionine adenosyl-transferase 2A and S-adenosylmethionine in mitogen-induced growth of human colon cancer cells. Gastroenterology 133 207-218. [DOI] [PubMed] [Google Scholar]
- Chen L, Zeng Y, Yang H, Lee TD, French SW, Corrales FJ, García-Trevijano ER, Avila MA, Mato JM, and Lu SC (2004) Impaired liver regeneration in mice lacking methionine adenosyltransferase 1A. FASEB J 18 914-916. [DOI] [PubMed] [Google Scholar]
- Clarke S (2006) Inhibition of mammalian protein methyltransferases by 5′-methylthioadenosine (MTA): a mechanism of action of dietary SAMe?, in The Enzymes (Tamanoi F ed) pp 467-493, Academic Press. [DOI] [PubMed]
- Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, and Johnston PG (2005) Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther 4 2026-2036. [DOI] [PubMed] [Google Scholar]
- Golks A, Brenner D, Fritsch C, Krammer PH, and Lavrik IN (2005) c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem 280 14507-14513. [DOI] [PubMed] [Google Scholar]
- Horikawa S and Tsukada K (1992) Molecular cloning and developmental expression of a human kidney S-adenosylmethionine synthetase. FEBS Lett 312 37-41. [DOI] [PubMed] [Google Scholar]
- Huang ZZ, Mao Z, Cai J, and Lu SC (1998) Changes in methionine adenosyltransferase during liver regeneration in the rat. Am J Physiol 275 G14-21. [DOI] [PubMed] [Google Scholar]
- Huang ZZ, Mato JM, Kanel G, and Lu SC (1999) Differential effect of thioacetamide on hepatic methionine adenosyltransferase expression in the rat. Hepatology 29 1471-1478. [DOI] [PubMed] [Google Scholar]
- Kotb M, Mudd SH, Mato JM, Geller AM, Kredich NM, Chou JY, and Cantoni GL (1997) Consensus nomenclature for the mammalian methionine adenosyltransferase genes and gene products. Trends Genet 13 51-52. [DOI] [PubMed] [Google Scholar]
- Kruyt FA (2008) TRAIL and cancer therapy. Cancer Lett 263 14-25. [DOI] [PubMed] [Google Scholar]
- Longley DB, Wilson TR, McEwan M, Allen WL, McDermott U, Galligan L, and Johnston PG (2006) c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene 25 838-848. [DOI] [PubMed] [Google Scholar]
- Lu SC and Mato JM (2005) Role of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancer. Alcohol 35 227-234. [DOI] [PubMed] [Google Scholar]
- Mato JM and Lu SC (2005) S-adenosylmethionine, in Encyclopedia of Dietary Supplements (Coates PM BM, Levine M, Moss J, White JD, eds) pp 1-6, Marcel Dekker, Inc., New York.
- Moyer MP, Manzano LA, Merriman RL, Stauffer JS, and Tanzer LR (1996) NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim 32 315-317. [DOI] [PubMed] [Google Scholar]
- Ohh M and Takei F (1995) Regulation of ICAM-1 mRNA stability by cycloheximide: role of serine/threonine phosphorylation and protein synthesis. J Cell Biochem 59 202-213. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, and Ashkenazi A (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 271 12687-12690. [DOI] [PubMed] [Google Scholar]
- Ramani K, Yang H, Xia M, Ara AI, Mato JM, and Lu SC (2008) Leptin's mitogenic effect in human liver cancer cells requires induction of both methionine adenosyltransferase 2A and 2beta. Hepatology 47 521-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BK, Lee MG, Chi SG, Kim YW, and Park JH (2001) Increased expression of cFLIP(L) in colonic adenocarcinoma. J Pathol 194 15-19. [DOI] [PubMed] [Google Scholar]
- Seiler N and Raul F (2005) Polyamines and apoptosis. J Cell Mol Med 9 623-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DA, Lawrence DA, and Ashkenazi A (2005) Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J Biol Chem 280 19401-19409. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Irmler M, and Thome M (1998) Inhibition of fas death signals by FLIPs. Curr Opin Immunol 10 552-558. [DOI] [PubMed] [Google Scholar]
- Vasilevskaya IA and O'Dwyer PJ (2005) 17-Allylamino-17-demethoxygeldanamycin overcomes TRAIL resistance in colon cancer cell lines. Biochem Pharmacol 70 580-589. [DOI] [PubMed] [Google Scholar]
- Veal N, Hsieh CL, Xiong S, Mato JM, Lu S, and Tsukamoto H (2004) Inhibition of lipopolysaccharide-stimulated TNF-alpha promoter activity by S-adenosylmethionine and 5′-methylthioadenosine. Am J Physiol Gastrointest Liver Physiol 287 G352-G362. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, and Smith CA (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3 673-682. [DOI] [PubMed] [Google Scholar]
- Yang H, Ara AI, Magilnick N, Xia M, Ramani K, Chen H, Lee TD, Mato JM, and Lu SC (2008) Expression pattern, regulation, and functions of methionine adenosyltransferase 2beta splicing variants in hepatoma cells. Gastroenterology 134 281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Sadda MR, Li M, Zeng Y, Chen L, Bae W, Ou X, Runnegar MT, Mato JM, and Lu SC (2004) S-adenosylmethionine and its metabolite induce apoptosis in HepG2 cells: Role of protein phosphatase 1 and Bcl-xS. Hepatology 40 221-231. [DOI] [PubMed] [Google Scholar]
- Yin XM (2006) Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene 369: 7-19. [DOI] [PubMed] [Google Scholar]
- Zhang L and Fang B (2005) Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 12 228-237. [DOI] [PubMed] [Google Scholar]
- Zhou XD, Yu JP, Chen HX, Yu HG, and Luo HS (2005) Expression of cellular FLICE-inhibitory protein and its association with p53 mutation in colon cancer. World J Gastroenterol 11 2482-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]