Abstract
Copper deficiency lowers brain copper and iron during development. The reduced iron content could be due to hypoferremia. Experiments were conducted to evaluate plasma iron and “ferroxidase” hypotheses by determining copper and iron status of Holtzman albino rats following gestational/lactational copper deficiency. Copper deficient (Cu−) dams on treatment for 5 weeks, two of gestation and three of lactation, had markedly lower copper content of milk and mammary tissue, and lower milk iron. Newborn pups from Cu− dams had lower copper and iron concentrations. Compared to Cu+ pups, Cu− pups, analyzed between postnatal age (P) 0 and P26, were smaller, anemic, had lower plasma iron, cardiac hypertrophy, and near zero ceruloplasmin activity. Liver copper in Cu+ pups increased then decreased during development and major reductions were evident in Cu− pups. Liver iron in Cu+ pups decreased with age while nursing but increased after eating solid food. Liver iron was lower in Cu− pups at P0 and P13 and normal at P20 and P26. Small intestinal copper decreased with age in Cu+ pups and was lower in Cu− pups. Intestinal iron levels in Cu- pups were higher than Cu+ pups postweaning in some experiments. Reduction in plasma iron in Cu− pups is likely due to a decreased “ferroxidase” function leading to lower placental iron transport, a lower milk iron diet, and partial block in iron uptake from intestine but is not due to failure to mobilize hepatic iron, in contrast to older rats eating diet with adequate iron.
Keywords: Copper deficiency, Rats, Plasma iron, Ceruloplasmin, Milk, Intestine
Introduction
Development of the central nervous system requires an optimal nutrient environment including several trace minerals. Trace mineral deficiencies can have long-term effects on biochemical and behavioral abnormalities (Keen et al. 2003). For example, iron status has a profound impact on neural functioning mediated via its role in myelination, energy metabolism and catecholamine homeostasis (Beard and Connor 2003). Interestingly, copper status can also impact myelination, energy metabolism and catecholamine homeostasis (O’Dell and Prohaska 1983). Recently, we showed that perinatal copper deficiency in rats was associated with iron deficiency in brain (Prohaska and Gybina 2005). The mechanism for this observation is not known but it was shown that plasma iron was lower in a single pooled sample from copper deficient rat pups, suggesting that iron availability to the brain could be rate limiting. This has not been proven.
The explanation for lower plasma iron in copper deficient pups, if it can be confirmed, has not been investigated. It is possible that gestational copper deficiency limits iron delivery to the fetus (Andersen et al. 2007). Alternatively, it is possible that milk iron concentration is impacted by dietary copper (Cohen et al. 1985). Additionally, iron transport across the absorptive enterocyte could be compromised by decreased hephaestin activity and protein levels (Reeves et al. 2005; Chen et al. 2006). Hephaestin is a copper-dependent ferroxidase essential for baso-lateral iron transport in conjunction with ferroportin (SLC40A1) (Andrews and Schmidt 2007). Finally, efflux of iron from macrophage storage sites in liver and spleen could be impaired in copper deficient animals due to reduction in ceruloplasmin (Cp) (ferroxidase) activity as proposed several decades previously (Osaki et al. 1966). Cp is thought necessary to facilitate iron efflux in concert with ferroportin in macrophages and astrocytes by analogy to hephaestin in enterocytes (De Domenico et al. 2007).
However, there are many unanswered questions that “ferroxidase” hypotheses cannot explain that suggest that the mammalian phenotype of copper deficiency is not just a lack of plasma iron. Dietary supplementation or injection with iron fails to reverse anemia of copper-deficient rats with lower plasma iron (Williams et al. 1983; Reeves and DeMars 2006). Copper deficient rats have normal whole body iron, challenging whether hephaestin is limiting in iron uptake (Failla and Seidel 1988). Copper deficient rats with low ceruloplasmin function that limits iron efflux from liver do not accumulate iron in spleen, an organ rich in macrophages (Williams et al. 1983). Ceruloplasmin null mice (Cp −/−) do not develop anemia or have impaired release of iron from liver or spleen (Cherukuri et al. 2005).
The dependency of adequate copper for proper iron biology may be different in young mammals. Iron absorption in the suckling rat is developmentally regulated (Leong et al. 2003a). Thus, dietary copper restriction during gestation and lactation may impact iron uptake across the intestine differently than a postweanling model of dietary copper limitation. Clearly, additional research is needed on the copper iron interaction.
The purpose of the current studies was to determine if lower plasma iron occurs in copper-deficient rat pups and to identify the mechanism(s) for this putative reduction by evaluation of pup and dam copper and iron status.
Materials and methods
Animal care and diets
Pregnant Holtzman rats were purchased commercially (Harland Sprague Dawley, Indianapolis, IN, USA). Rats were offered a copper-deficient diet (Teklad Laboratories, Madison, WI, USA) similar to the AIN-76A diet but modified by omitting cupric carbonate from the AIN-76A mineral mix as described previously (Kuo et al. 2006). This diet contained 0.36 mg Cu/kg and 53 mg Fe/kg by chemical analysis. Copper adequate (Cu+) rats were given deionized water with copper sulfate (20 mg Cu/l) to drink. Copper deficient (Cu−) rats drank copper-free deionized water.
A perinatal nutritional paradigm was studied. Dams were placed on the diet treatment at embryonic day 7 (E7) similar to established recent protocols (Prohaska and Gybina 2005). On postnatal day 2 (P2), litter sizes were culled to 10 pups. At P20, rat pups were weaned and placed on the same dietary treatment as their dams. Two replicate experiments were conducted. Ten dams (n = 5 of each treatment group) were sampled in Experiment 1 and eight dams (n = 4 per group) were sampled in Experiment 2. Pups, one per litter, were sampled at specific ages. All rats had free access to diet and drinking water and were maintained at 24°C with 55% relative humidity on a 12-h light cycle (0700–1900 h light). All protocols were approved by the University of Minnesota Animal Care Committee.
Male offspring from the perinatal experiments were sampled at P0, P13, P20, and P26. All rats were weighed then lightly anesthetized with diethyl ether and killed by decapitation. Upper small intestine (15 cm), livers, and blood were harvested from rat pups. Trunk blood was collected in a heparinized tube. Intestinal lumens were flushed with saline to remove contents, blotted with tissue paper and dried to constant weight prior to metal analyses.
At the time of weaning (P20), dams were weighed and anesthetized with xylazine/ketamine and injected with oxytocin for milking (Kelleher and Lonnerdal 2001). Blood was collected via cardiac puncture into a heparinized syringe. Portions of liver and mammary tissue were collected. Tissues were weighed and either processed for biochemical analysis or frozen in liquid nitrogen and stored at −75°C until used.
Biochemical analyses
Hemoglobin was determined spectrophotometrically as metcyanhemoglobin and plasma activity of the cuproprotein ceruloplasmin (EC 1.16.3.1) was measured by following oxidation of o-dianisidine at 37°C (Prohaska 1983). Tissues and diet were wet-digested with HNO3 (Trace Metal Grade, Fischer Scientific) and residue was dissolved in 0.1 mol/l HNO3. Samples were analyzed for metals by flame atomic absorption spectroscopy (AAS) (model 1100 B, Perkin-Elmer). Plasma iron was measured by flame AAS following treatment with trichloroacetic acid (TCA) (Olson and Hamlin 1969). Briefly, plasma was treated with one volume of 200 g/l TCA and heated to precipitate contaminating hemoglobin and release transferrin bound iron. More than 95% of hemoglobin bound iron is removed by this technique. Following centrifugation, the supernatant was diluted with five volumes of water and then analyzed by AAS. To confirm the validity of the TCA method, selected plasma samples were also analyzed for iron using Ferene-S, a colorimetric method (Eskelinen et al. 1983).
PCR methods
Enterocytes were isolated from the upper small intestine of four P21 male Cu− and Cu+ rats using an EDTA treatment method (Chen et al. 2003). Total RNA was isolated and purity established spectrophotometrically and by RNA gels (Prohaska and Brokate 1999). The mRNA content of enterocyte ferroportin (Fpn) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) were determined by qRT-PCR using a Roche Light Cycler™. Rat Fpn primer pairs were 5′ GGT GGT GGC AGG CTC TGT 3′ (forward) and 5′ TTT GAA CCA CCA GGG ACG TC 3′ (reverse). Gapdh primer pairs were 5′ TTC CTA CCC CCA ATG TAT CCG 3′ (forward) and 5′ ACC ACC CTG TTG CTG TAG CCA 3′ (reverse). cDNA was synthesized using Omniscript Reverse Transcripase (Qiagen) and amplified with a Roche SYBR Green I kit. The rat Fpn primers amplified a DNA product with Tm of 87.5°C.
Statistics
Dietary treatment effects were evaluated by Student’s t-test after variance equality was established by F-test, α = 0.05. Data were analyzed using Microsoft Excel™.
Results
Rat dam biochemical indices
Six characteristics were measured in rat dams in both perinatal model experiments to determine the effect of dietary treatment during late gestation and lactation (Table 1). There was no significant difference in body weight or hemoglobin level due to copper deficiency in either experiment. However, ceruloplasmin activity was reduced by 95% and over 99% in Experiments 1 and 2, respectively. Liver copper was reduced by 67 and 78% in Experiments 1 and 2, respectively, and plasma iron was reduced 64 and 50%, respectively. Liver iron concentration was approximately double in Cu− dams in Experiment 2, but not statistically higher in Experiment 1, P = 0.11. Together this data indicate that Cu− dams exhibited signs consistent with copper deficiency and that dams in Experiment 2 may have been slightly more deficient.
Table 1.
Characteristics of rat dams following lactation
| Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|
| Characteristic | Cu-adequate | Cu-deficient | Cu-adequate | Cu-deficient |
| Body weight (g) | 333 ± 14.5 | 335 ± 24.0 | 332 ± 5.7 | 337 ± 6.5 |
| Hemoglobin (g/l) | 148 ± 4.3 | 147 ± 4.2 | 188 ± 5.2 | 169 ± 13.8 |
| Ceruloplasmin (units/l) | 383 ± 36.5 | 19.6 ± 2.1* | 278 ± 26.6 | 0.03 ± 0.02* |
| Liver copper (µg/g) | 4.25 ± 0.48 | 1.40 ± 0.12* | 4.57 ± 0.51 | 1.03 ± 0.13* |
| Liver iron (µg/g) | 69.2 ± 10.2 | 116 ± 31.8 | 93.9 ± 5.88 | 184 ± 24.0* |
| Plasma iron (µg/ml) | 2.80 ± 0.34 | 1.01 ± 0.23* | 5.19 ± 0.41 | 2.58 ± 0.25* |
Values are means ± SEM (n = 5, Experiment 1; n = 4, Experiment 2). Dams were milked 19 days after parturition (P19) and killed 2 days later for Experiment 1 and milked then killed at P20 for Experiment 2. Dams were maintained on treatment since embryonic day 7
Difference from Cu-adequate, P<0.05 (Student’s t-test)
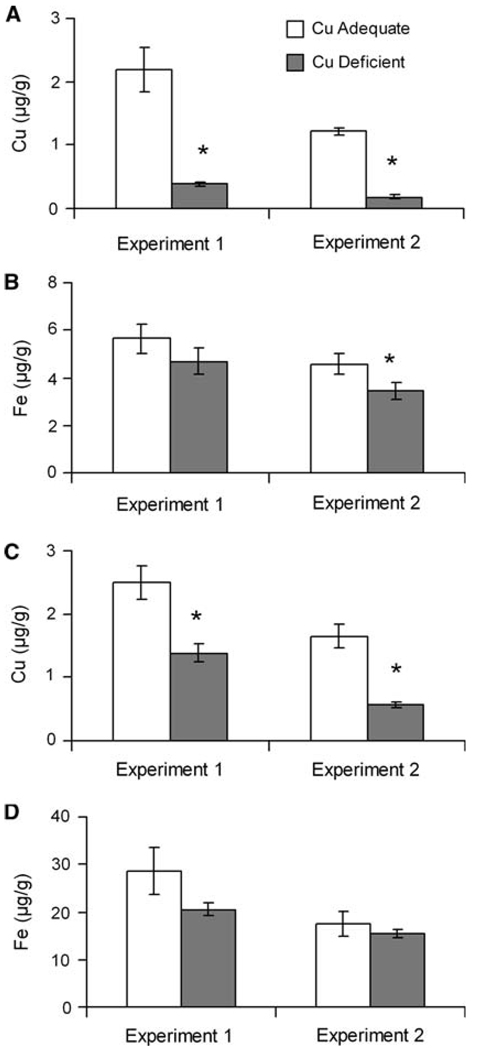
Copper and iron were measured in both milk and mammary tissue (Fig. 1). In both experiments, copper concentrations were decreased in milk and mammary tissue in the Cu− compared to Cu+ dams. Milk copper concentration was lower 82 and 85% and mammary tissue copper concentrations were lower 45 and 65%, respectively. Iron concentration in milk was unchanged by copper deficiency in Experiment 1 but was modestly lower (24%) in Experiment 2 in Cu− dams. Mammary tissue iron concentrations were not impacted by dietary copper deficiency in either experiment.
Fig. 1.
Milk copper (A) and iron (B) concentrations in Cu+ and Cu− rat dams milked at P19 (Experiment 1) and P20 (Experiment 2). Mammary tissue copper (C) and iron (D) concentrations from Cu+ and Cu− dams killed at P21 (Experiment 1) and P20 (Experiment 2). Values are means ± SEM (n = 5 in Experiment 1, n = 4 in Experiment 2). Cu− means were significantly different than Cu+, *P<0.05 (Student’s t-test)
Rat pup biochemical and anthropometric indices
Body weight, hemoglobin, ceruloplasmin, plasma iron, and heart/body weight at four ages were measured in pups in Experiment 2 to determine the impact of dietary treatment (Table 2). At P0, no difference was observed in body weight or ceruloplasmin activity, but plasma iron was markedly lower (57%) in Cu− pups compared to Cu+ pups. Hemoglobin was not measured and hearts were not removed.
Table 2.
Characteristics of male rat pups
| Age (days) | |||||
|---|---|---|---|---|---|
| Characteristic | Diet | P0 | P13 | P20 | P26 |
| Body wt (g) | Cu+ | 6.9 ± 0.17 | 35.3 ± 0.8 | 64.4 ± 3.5 | 91.1 ± 3.4 |
| Cu− | 7.4 ± 0.46 | 31.8 ± 0.8* | 53.4 ± 2.3* | 72.7 ± 6.2* | |
| Hb (g/l) | Cu+ | ND | 91.2 ± 1.3 | 81.8 ± 2.2 | 116 ± 7.2 |
| Cu− | ND | 68.6 ± 2.7* | 60.4 ± 3.0* | 67.8 ± 3.5* | |
| Cp (units/l) | Cu+ | 44.6 ± 2.5 | 51.2 ± 4.2 | 88.2 ± 5.7 | 74.2 ± 9.2 |
| Cu− | 13.8 ± 5.7* | 0.56 ± 0.20* | 0.24 ± 0.24* | 0.0 ± 0.01* | |
| P Fe (µg/ml) | Cu+ | 2.04 ± 0.23 | 0.53 ± 0.08 | 2.49 ± 0.37 | 4.34 ± 0.14 |
| Cu− | 0.87 ± 0.12* | 0.33 ± 0.05* | 0.33 ± 0.12* | 1.15 ± 0.21* | |
| Heart/BW (mg/g) | Cu+ | ND | 5.70 ± 0.13 | 5.35 ± 0.19 | 5.00 ± 0.20 |
| Cu− | ND | 6.71 ± 0.31* | 8.84 ± 0.86* | 11.3 ± 0.96* | |
Values are means ± SEM. Male pups from Experiment 2 (n = 4) were used except P0 which were pools of n = 3 or n = 4 pups per pool, gender was not identified. Body weight (BW), heart weight (H), hemoglobin (Hb), ceruloplasmin (Cp), and plasma (P) iron were determined as described in “Materials and methods”; ND = not determined
Difference from Cu-adequate (Cu+) at same age, P < 0.05 (Student’s t-test)
At P13, all measured characteristics were significantly different between groups. Cu− pups weighed less and exhibited cardiac hypertrophy compared to Cu+ rats. Cu− pups had lower hemoglobin (25%), ceruloplasmin activity (99%), and plasma iron (37%) compared to Cu+ pups.
At P20, when the pups were weaned, Cu− pups were smaller and had cardiac hypertrophy compared to Cu+ controls. Reduction in hemoglobin and ceruloplasmin activity remained at levels consistent with P13. Plasma iron concentrations remained markedly decreased in Cu− pups similar to P13 values but Cu+ P20 pups had higher iron levels compared to P13, nevertheless P20 Cu− values were 87% lower than Cu+.
At P26, when the rats had been eating solid diet for 6 days, Cu− rats remained smaller and the extent of cardiac hypertrophy increased. Reduction in hemoglobin increased to 58% compared to 25% seen at P20; ceruloplasmin activity was virtually non detectable. Plasma iron was still markedly lower (75%) in Cu− than Cu+ rats.
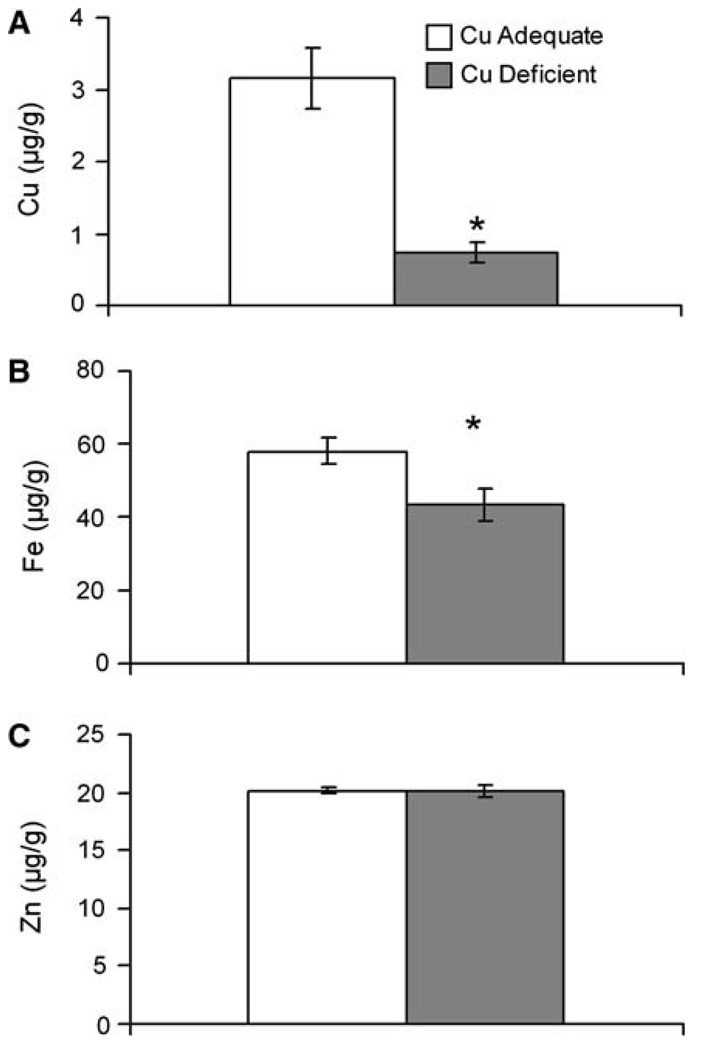
Whole body analysis of metal content was done in selected rat pups at P0 in Experiment 1 to determine if iron limitation was evident as suggested by plasma iron data at P0. Compared to newborn Cu+ pups both copper and iron concentration was significantly reduced in the Cu− pups (Fig. 2). Copper levels in Cu− pups were 77% lower and iron levels were 25% lower. Not all metals were impacted, however, as zinc concentration in the same pups was nearly identical. Similar to Experiment 2 (Table 2), dietary copper deficiency at P0 did not impact body weight of pups in Experiment 1. Average body weight of Cu+ pups was 6.3 ± 0.11 g (mean ± SEM, n = 6) compared to 6.6 ± 0.23 g for Cu− pups.
Fig. 2.
Copper (A), iron (B), and zinc (C) concentrations in Cu+ and Cu− in newborn P0 pups. Values are means ± SEM (n = 5–6, Experiment 2). Cu− means were significantly different than Cu+, *P < 0.05 (Student’s t-test)
Rat pup liver
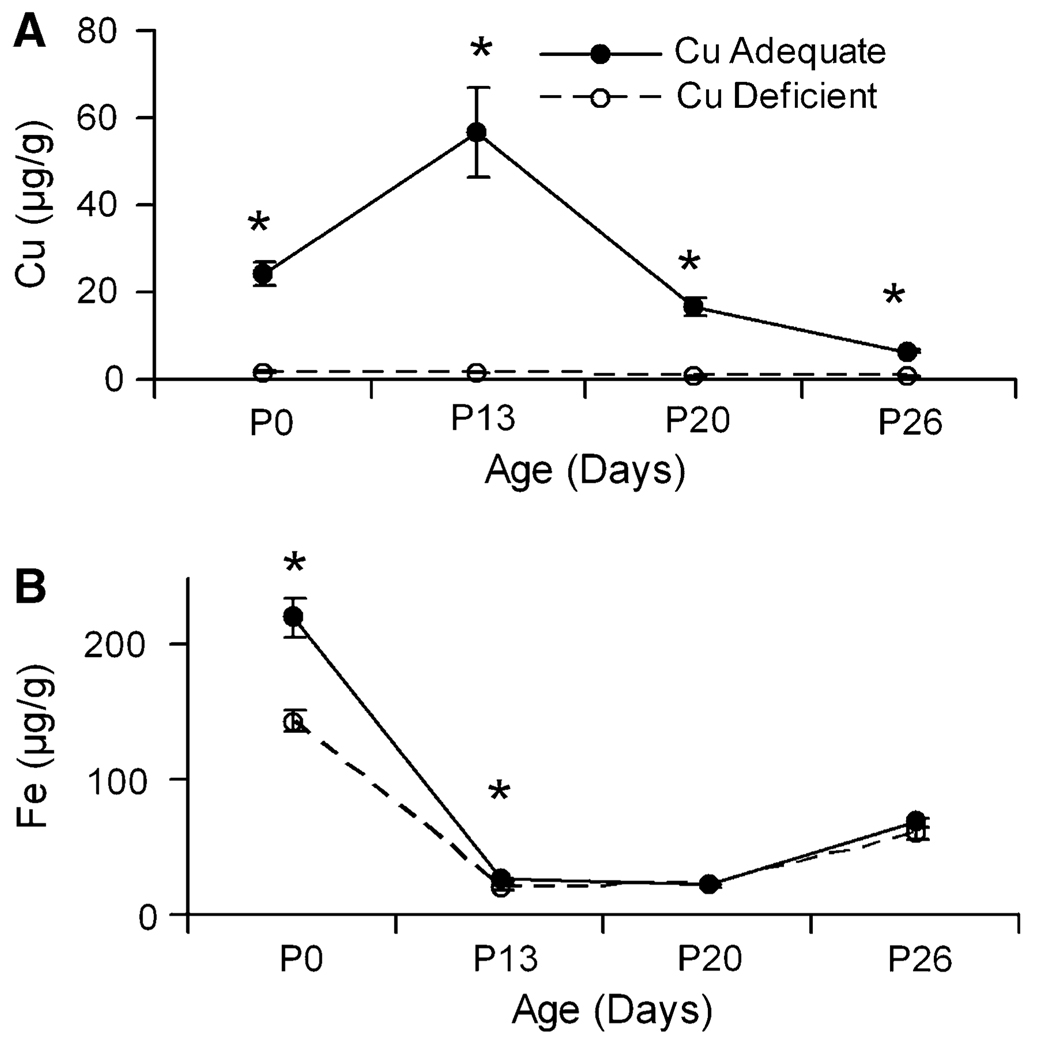
In Experiment 2, livers from pups were analyzed for total copper and iron at several ages (Fig. 3). Liver copper analyses demonstrated significant differences due to treatment at all four ages measured with Cu− pups having lower copper concentrations than Cu+ pups. Cu+ pups had mean liver copper concentrations of 24.5 µg Cu/g at P0 that peaked at 56.6 µg Cu/g at P13, and decreased to 6.5 µg Cu/g on P26. Iron concentration in both Cu+ and Cu− pups tracked similarly with the only significant difference between the Cu− and Cu+ pups detected at P0 and P13 (Fig. 3). In Cu+ rats, liver iron concentration also changed with age.
Fig. 3.
Liver copper (A) and iron (B) concentrations in Holtzman Cu+ and Cu− rat pups during development from Experiment 2. Values are means ± SEM(n = 4).Cu− means were significantly different than Cu+, *P < 0.05 (Student’s t-test)
Rat pup small intestine
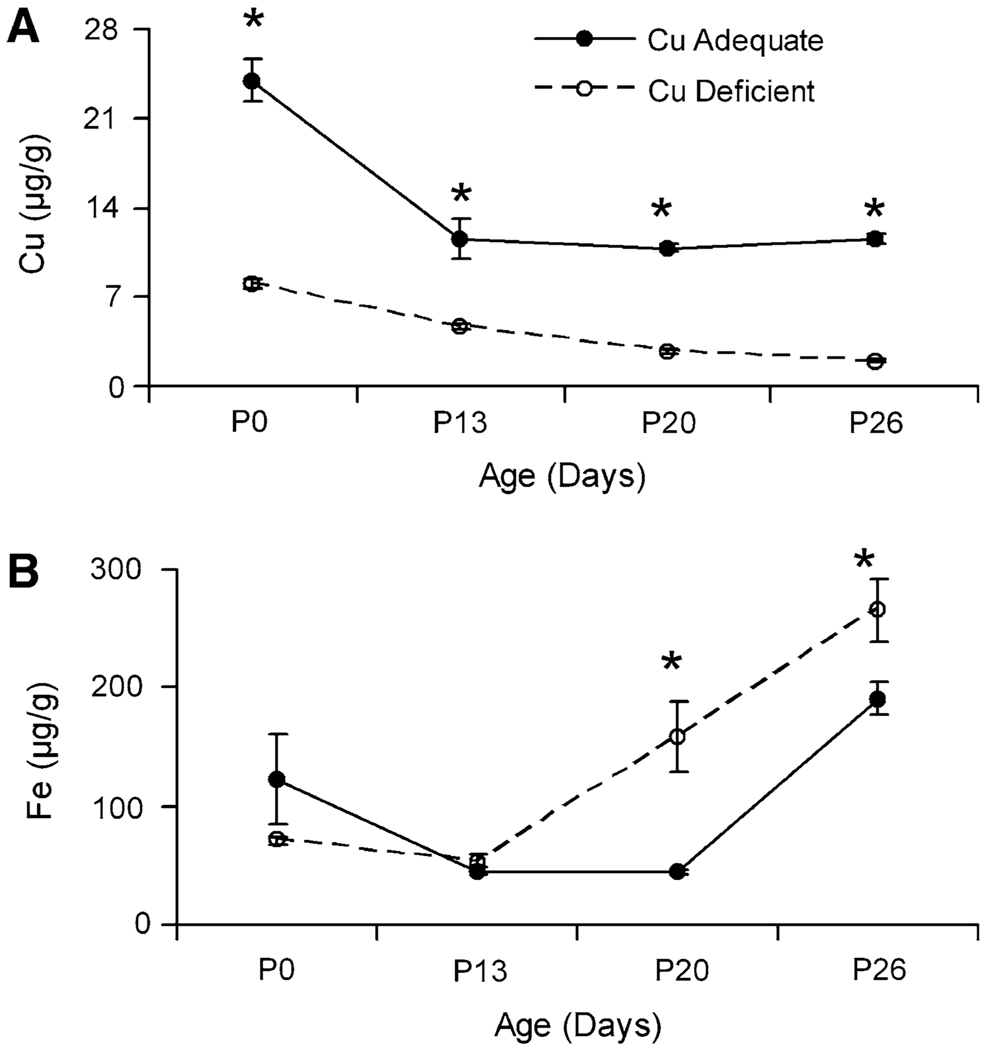
Copper and iron concentration in the upper small intestine was measured in pups from Experiment 2. Dry weights of small intestine were determined and were not altered by copper deficiency (data not shown). Copper concentration at all four ages measured was significantly lower in the Cu− rats compared to Cu+ rats (Fig. 4). Newborn Cu+ pups had a Cu concentration near 24 µg Cu/g at P0. Copper levels decreased to near 11 µg Cu/g by P13 and remained at this level at P20 and P26. In other similar experiments with Sprague Dawley rats (unpublished), we measured small intestinal copper levels during early suckling that were much higher, for example, at P12 nearly 100 µg Cu/g dry weight. Small intestinal iron concentration was significantly different at P20 and P26 (Fig. 4). At both ages, levels in Cu− rats were higher than Cu+ rats.
Fig. 4.
Small intestinal copper (A) and iron (B) concentrations in Holtzman Cu+ and Cu− rat pups during development from Experiment 2. Values are means ± SEM (n = 4), based on dry weight. Cu− means were significantly different than Cu+, *P<0.05 (Student’s t-test)
Data in our two current models yielded variable response in Cu− rats of intestinal iron levels: higher iron at later ages in the perinatal model, Experiment 2 (Fig. 4) but no elevation in P26 Cu− rats in Experiment 1. Average Cu− values for rats 193 ± 24.3 (mean ± SEM, n = 6) were not different than Cu+ values 198 ± 35.6 µg Fe/g dry weight. In the same P26 pups, however, plasma iron was lower in Cu− than Cu+ pups, 2.16 ± 0.33 µg/ml compared to 5.23 ± 0.29, P<0.01. For Cu+ P26 pups in Experiment 1 small intestinal iron values agreed well with that obtained in Experiment 2 (Fig. 4) of 191 ± 14.1.
Lack of iron elevation in small intestine, in contrast to the current results in Fig. 4, has been observed previously in male Cu− weanling rats but data were quite variable and not previously published. In six separate experiments weanling rats were always anemic but this was not always associated with iron trapping in the gut (Table 3). In duodenum statistically higher iron levels were detected in C− weanling pups (Table 3).
Table 3.
Hemoglobin and small intestinal metal levels of male weanling rat pups
| Experiment | Age (d) | n | Hemoglobin (g/l) | Copper (µg/g) | Iron (µg/g) | |||
|---|---|---|---|---|---|---|---|---|
| Cu+ | Cu− | Cu+ | Cu− | Cu+ | Cu− | |||
| A | 22 | 4 | 80.3 ± 4.3 | 62 ± 1.9* | 2.08 ± 0.16 | 0.55 ± 0.04* | 8.89 ± 0.81 | 23.4 ± 7.3 |
| B | 23 | 6 | 103 ± 3.9 | 70 ± 1.5* | 1.99 ± 0.21 | 0.53 ± 0.02* | 8.36 ± 0.76 | 8.99 ± 0.64 |
| C | 24–27 | 8 | 103 ± 3.4 | 60.3 ± 5.8* | 2.42 ± 0.07 | 0.40 ± 0.03* | 22.2 ± 1.9 | 45.4 ± 9.1* |
| D | 24 | 4 | 102 ± 3.0 | 53.9 ± 3.5* | 1.79 ± 0.11 | 0.45 ± 0.05* | 11.2 ± 1.2 | 26.1 ± 8.6 |
| E | 19 | 5 | 112 ± 5.9 | 70.9 ± 2.1* | 3.35 ± 0.35 | 1.05 ± 0.09* | 7.92 ± 0.41 | 14.8 ± 5.5 |
| F | 26 | 4 | 116 ± 7.2 | 67.8 ± 3.5* | 2.32 ± 0.10 | 0.39 ± 0.01* | 38.2 ± 2.52 | 52.0 ± 5.4 |
Values are means ± SEM. The average dietary copper content of six experiments (A–F) was 0.38 ± 0.08 (mean ± SD) mg/kg and for iron 46.7 ± 2.59 mg/kg. In all but Experiment C, approximately 15 cm of intestine was harvested starting at the pyloric sphincter. In Experiment C only the duodenum, the first 5 cm, was digested. Hemoglobin and metal levels, fresh weight, were determined as described in “Materials and methods”
Difference from Cu-adequate (Cu+) P < 0.05 (Student’s t-test)
To address a potential criticism that whole tissue was used in these experiments as opposed to measuring iron only in absorptive enterocytes, we analyzed enterocyte metal content of cells isolated from the duodenum of P25 male pups (using a very similar paradigm). Iron concentration in the Cu− samples 471 ± 126 ng Fe/mg protein was not statistically higher than values from Cu+ pups 436 ± 47.4 (P>0.05). However, copper levels were markedly lower in Cu− than Cu+ samples, 17.7 ± 7.3 ng Cu/mg protein compared to 109 ± 14.3 (P<0.01). Cu− pups were anemic with hemoglobin levels averaging 74.6 g/l compared to 116 g/l for the Cu+ pups. Thus, anemia can occur without iron entrapment in the intestine.
Enterocyte mRNA levels of Fpn were determined since this exporter, if lower in content, might also explain the higher intestinal iron levels in Cu− rat intestine. Cu− P21 anemic male rats had lower liver copper levels, µg/g mean ± SEM (n = 4), than Cu+ rats, 0.65 ± 0.04 compared to 15.6 ± 3.24, P<0.01. However, the Fpn/Gapdh content of their enterocytes was not different, Cu− 0.27 ± 0.04 versus Cu+ 0.34 ± 0.06, P>0.05.
Discussion
Dietary restriction of copper employed in these perinatal studies produced suckling rats with clear evidence of classical signs of copper deficiency including impaired growth, anemia and cardiac hypertrophy. The pups born to and nursed by Cu− dams had profound reductions in plasma iron throughout lactation and even when switched from nursing to solid food where iron concentration rose 10-fold. Plasma iron was 50% lower in Holtzman male Cu− rats compared to controls in a postnatal model with older rats using the same diet (Prohaska and Gybina 2005). Others have reported that following weaning copper deficiency in Sprague Dawley rats leads to lower plasma iron (Reeves et al. 2005). Thus, dietary copper restriction results in lower plasma iron concentration in suckling rats as well as older rats eating solid food with adequate (50 mg Fe/kg) iron.
We found high intestinal copper in younger rats and this level dropped during lactation, in agreement with prior work (Bauerly et al. 2005). Iron in the small intestine demonstrated a similar but less drastic drop. This pattern also agrees well with previous work in rat pups (Leong et al. 2003b). As pups mature and evolve from drinking milk to eating solid food their metal transport machinery changes. This may account for some of the interesting intestinal concentration patterns observed during lactation. The developmental pattern of liver copper concentration (bell-shaped) and iron concentration (U-shaped) in control rat pups agrees well with previous work (Linder and Munro 1973).
What copper-dependent mechanism is responsible for the lower plasma iron of pups? One theory suggests hephaestin is involved. In a series of elegant experiments with a postweanling model Reeves et al. demonstrated that copper deficient Sprague-Dawley rats had a reduction in iron absorption (Reeves and DeMars 2004); that hephaestin protein level was reduced and duodenal mucosa iron levels were higher (Reeves et al. 2005); and that copper repletion reversed this observation (Reeves and Demars 2005). In the current studies with younger male Holtzman pups there was evidence of limiting hephaestin as pups aged but clearly no evidence in suckling pups that also had lower plasma iron concentrations. Furthermore, even in some weaning Cu− anemic rats there was no elevation in intestinal iron concentration.
Recent studies in mice also suggested that hephaestin activity was responsible for iron deficiency (Chen et al. 2006). That study reported higher iron levels in liver and intestine of copper deficient C57BL mice. In contrast, in a similar study done earlier, also with C57BL mice, and with similar dietary copper restriction we found no evidence for iron trapping in small intestine even though Cu− mice were anemic and had liver iron overload (Prohaska and Lukasewycz 1990). Clearly, the hephaestin issue remains unresolved as the primary reason why mammals develop anemia. Iron transport from the intestine could also be limited if the efflux transporter ferroportin (Fpn) were limiting. Data from P21 male rats in these studies and our previous work with Cu− albino mice failed to detect any regulation of intestinal Fpn as the mRNA levels were similar between treatment groups (Chung et al. 2004). Another group reported higher enterocyte Fpn mRNA and protein levels in copper deficient C57BL mice (Chen et al. 2006). Thus, it appears unlikely that Fpn is limiting iron egress from the enterocyte of copper deficient rodents.
Lower plasma iron was observed in newborn Cu− rat pups prior to nursing. This implies that iron was limiting via placental transfer. Supporting data demonstrated that Cu− newborn pups had a reduction in total body iron and in a second study lower liver iron. Both these observations are consistent with recent data by others showing that gestational copper deficiency in rats limits both fetal liver copper and iron (Andersen et al. 2007). This suggests that gestational copper deficiency, even when limited to the last two-thirds of gestation, limits iron transport to pups. This defective transfer of iron is consistent with limitation in a copper-dependent placental “ferroxidase” described previously (Danzeisen et al. 2002).
Liver iron concentration was lower in P13 Cu− pups, and not elevated even at P26. Previous work from our lab with similar Cu− rat pups demonstrated lower liver iron earlier, at P3 and P12 (Prohaska and Broderius 2006). This suggests that the accumulation of liver iron associated with copper deficiency occurs only in older mammals.
Is lower plasma iron related to loss of ceruloplasmin function? The liver iron data in Cu− rat pups suggests that although ceruloplasmin activity was nearly undetectable this limitation did not result in liver iron trapping. Previously we demonstrated the Cu− rat plasma with low diamine oxidase activity also had low ferroxidase activity (Prohaska 1981). Even the tenet that Cp is necessary for iron release from liver has been challenged recently (Cherukuri et al. 2005). Cp −/− mice, with low ferroxidase activity, have liver iron overload but sometimes have normal plasma iron (Harris et al. 1999). Thus, the role of ceruloplasmin in explaining decreased plasma iron in young Cu− rat pups remains unknown.
It seems likely that a copper-dependent ferroxidase is involved in transport of iron from mammary gland to milk. Fpn is located in mammary epithelial tissue and likely required for iron transport into secretory vesicles for export to milk (Kelleher and Lonnerdal 2005). A major diversion of newly ingested copper is delivered to the mammary gland, as opposed to liver, in lactating rats (Donley et al. 2002). This may be to provide copper for milk but also to supply copper for other functions such as a putative “mammary ferroxidase”. Thus, analogous to placenta, dietary copper restriction may limit transport of iron to milk. Previous work in both copper-deficient rat and mouse dams reported modestly lower milk iron levels (Cohen et al. 1985; Prohaska 1989). In one of the current studies were we able to detect a significant reduction in milk iron concentration in Cu− dams at P20. In contrast for both experiments, milk copper was markedly reduced in Cu− compared to Cu+ dams. Importantly, others reported that iron limitation in the diet and iron supplementation to the diet had no impact on milk iron of rats sampled at the end of lactation (Kochanowski and Sherman 1983; Leong and Lonnerdal 2005). Thus, it is possible that dietary copper is as important as dietary iron in establishing milk iron content. Furthermore, the reduction in milk iron associated with copper deficiency in our studies likely contributes to the lower plasma iron in pups during lactation. However, if this was the only factor in limiting plasma iron one would have predicted at P26 (6 days after eating solid food containing 10-fold higher iron than milk) that the plasma iron deficit in Cu− rats would have rebounded. Thus, limiting iron in the diet (milk) may be only one of several factors contributing to hypoferremia.
Also somewhat surprising was no alteration in mammary tissue iron. Presumably if copper deficiency limited this “ferroxidase” lowering its ability to mobilize iron to milk, iron trapping would have been detected. Since liver iron was higher in Cu− dams, we assume that limiting ceruloplasmin may have been responsible for that observation. Importantly though, others reported that placental iron of copper-deficient dams was also not “trapped” by copper deficiency even though fetal iron suggested an impairment in iron delivery (Andersen et al. 2007).
Taken together current data suggest that the reason plasma iron is lower in Cu− pups is a combination of reduced placental iron transport creating an iron deficient pup at birth, a lower dietary intake from milk during suckling, and a modest entrapment of iron in the upper small intestine in older Cu− pups. Though consistent with a role of copper in “ferroxidases” in general, our current data in pups do not support a role for ceruloplasmin in explaining lower plasma iron.
If the Cu− rat pups are really iron deficient, then iron repletion should correct the phenotype. In P7 Cu− mice iron injection reversed anemia without altering ceruloplasmin (Prohaska 1984). However, in older Cu− mice iron injection had no effect, but copper injection did rescue anemia (Prohaska et al. 1985). Furthermore, iron repletion by diet or injection of older Cu− rats also did not reverse anemia (Williams et al. 1983; Reeves and DeMars 2006). This suggests that age of the pups is a major factor to consider in the copper-iron interaction phenomena. It also suggests that reduction in plasma iron is not the only factor limiting the anemia that develops following copper deficiency.
Future work will be directed at evaluating if iron repletion of Cu− pups can reverse plasma iron deficits and anemia as was observed for young mice. Importantly, can iron repletion restore brain iron deficits in Cu− pups? If possible, this will aid in determining if the neurological abnormalities observed in both copper and iron deficiency, such as hippocampus immaturity, are independent or related (Hunt and Idso 1995; Jorgenson et al. 2003).
Acknowledgements
We appreciate the excellent technical assistance of Margaret Broderius. Research was supported by funding from US Public Health Service NIH HD-39708. We thank Dr. Mitchell Knutson for providing the rat Fpn primer pair sequences.
References
- Andersen HS, Gambling L, Holtrop G, et al. Effect of dietary copper deficiency on iron metabolism in the pregnant rat. Br J Nutr. 2007;97:239–246. doi: 10.1017/S0007114507239960. [DOI] [PubMed] [Google Scholar]
- Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- Bauerly KA, Kelleher SL, Lonnerdal B. Effects of copper supplementation on copper absorption, tissue distribution, and copper transporter expression in an infant rat model. Am J Physiol. 2005;288:G1007–G1014. doi: 10.1152/ajpgi.00210.2004. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Chen H, Su T, Attieh ZK, et al. Systemic regulation of Hephaestin and Ireg1 revealed in studies of genetic and nutritional iron deficiency. Blood. 2003;102:1893–1899. doi: 10.1182/blood-2003-02-0347. [DOI] [PubMed] [Google Scholar]
- Chen H, Huang G, Su T, et al. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr. 2006;136:1236–1241. doi: 10.1093/jn/136.5.1236. [DOI] [PubMed] [Google Scholar]
- Cherukuri S, Potla R, Sarkar J, et al. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab. 2005;2:309–319. doi: 10.1016/j.cmet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Chung J, Prohaska JR, Wessling-Resnick M. Ferroportin-1 is not upregulated in copper-deficient mice. J Nutr. 2004;134:517–521. doi: 10.1093/jn/134.3.517. [DOI] [PubMed] [Google Scholar]
- Cohen NL, Keen CL, Hurley LS, et al. Determinants of copper-deficiency anemia in rats. J Nutr. 1985;115:710–725. doi: 10.1093/jn/115.6.710. [DOI] [PubMed] [Google Scholar]
- Danzeisen R, Fosset C, Chariana Z, et al. Placental ceruloplasmin homolog is regulated by iron and copper and is implicated in iron metabolism. Am J Physiol. 2002;282:C472–C478. doi: 10.1152/ajpcell.00019.2001. [DOI] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, di Patti MC, et al. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26:2823–2831. doi: 10.1038/sj.emboj.7601735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donley SA, Ilagan BJ, Rim H, et al. Copper transport to mammary gland and milk during lactation in rats. Am J Physiol. 2002;283:E667–E675. doi: 10.1152/ajpendo.00115.2002. [DOI] [PubMed] [Google Scholar]
- Eskelinen S, Haikonen M, Raisanen S. Ferene-S as the chromogen for serum iron determinations. Scand J Clin Lab Invest. 1983;43:453–455. [PubMed] [Google Scholar]
- Failla ML, Seidel KE. Total body content of copper and other essential metals in rats fed fructose or starch. Nutr Res. 1988;8:1379–1389. [Google Scholar]
- Harris ZL, Durley AP, Man TK, et al. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CD, Idso JP. Moderate copper deprivation during gestation and lactation affects dentate gyrus and hippocampal maturation in immature male rats. J Nutr. 1995;125:2700–2710. doi: 10.1093/jn/125.10.2700. [DOI] [PubMed] [Google Scholar]
- Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- Keen CL, Hanna LA, Lanoue L, et al. Developmental consequences of trace mineral deficiencies in rodents: acute and long-term effects. J Nutr. 2003;133:1477S–1480S. doi: 10.1093/jn/133.5.1477S. [DOI] [PubMed] [Google Scholar]
- Kelleher SL, Lonnerdal B. Long-term marginal intakes of zinc and retinol affect retinol homeostasis without compromising circulating levels during lactation in rats. J Nutr. 2001;131:3237–3242. doi: 10.1093/jn/131.12.3237. [DOI] [PubMed] [Google Scholar]
- Kelleher SL, Lonnerdal B. Molecular regulation of milk trace mineral homeostasis. Mol Aspects Med. 2005;26:328–339. doi: 10.1016/j.mam.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kochanowski BA, Sherman AR. Iron status of suckling rats as influenced by maternal diet during gestation and lactation. Br J Nutr. 1983;49:51–57. doi: 10.1079/bjn19830010. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Gybina AA, Pyatskowit JW, et al. Copper transport protein (ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr. 2006;136:21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong WI, Lonnerdal B. Iron transporters in rat mammary gland: effects of different stages of lactation and maternal iron status. Am J Clin Nutr. 2005;81:445–453. doi: 10.1093/ajcn.81.2.445. [DOI] [PubMed] [Google Scholar]
- Leong WI, Bowlus CL, Tallkvist J, et al. DMT1 and FPN1 expression during infancy: developmental regulation of iron absorption. Am J Physiol. 2003a;285:G1153–G1161. doi: 10.1152/ajpgi.00107.2003. [DOI] [PubMed] [Google Scholar]
- Leong WI, Bowlus CL, Tallkvist J, et al. Iron supplementation during infancy—effects on expression of iron transporters, iron absorption, and iron utilization in rat pups. Am J Clin Nutr. 2003b;78:1203–1211. doi: 10.1093/ajcn/78.6.1203. [DOI] [PubMed] [Google Scholar]
- Linder MC, Munro HN. Iron and copper metabolism during development. Enzyme. 1973;15:111–138. [PubMed] [Google Scholar]
- O’Dell BL, Prohaska JR. Biochemical aspects of copper deficiency in the nervous system. In: Dreosti IE, Smith RM, editors. Neurobiology of the trace elements. Clifton: Humana Press; 1983. pp. 41–81. [Google Scholar]
- Olson AD, Hamlin WB. A new method for serum iron and total iron-binding capacity by atomic absorption spectrophotometry. Clin Chem. 1969;15:438–444. [PubMed] [Google Scholar]
- Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746–2751. [PubMed] [Google Scholar]
- Prohaska JR. Comparison between dietary and genetic copper deficiency in mice: copper-dependent anemia. Nutr Res. 1981;1:159–167. [Google Scholar]
- Prohaska JR. Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J Nutr. 1983;113:2048–2058. doi: 10.1093/jn/113.10.2048. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Repletion of copper-deficient mice and brindled mice with copper or iron. J Nutr. 1984;114:422–430. doi: 10.1093/jn/114.2.422. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Effect of diet on milk copper and iron content of normal and heterozygous brindled mice. Nutr Res. 1989;9:353–356. [Google Scholar]
- Prohaska JR, Broderius M. Plasma peptidylglycine alpha-amidating monooxygenase (PAM) and ceruloplasmin are affected by age and copper-status in rats and mice. Comp Biochem Physiol B. 2006;143:360–366. doi: 10.1016/j.cbpb.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR, Brokate B. Copper deficiency alters rat dopamine beta-monooxygenase mRNA and activity. J Nutr. 1999;129:2147–2153. doi: 10.1093/jn/129.12.2147. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Gybina AA. Rat brain iron concentration is lower following perinatal copper deficiency. J Neurochem. 2005;93:698–705. doi: 10.1111/j.1471-4159.2005.03091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR, Lukasewycz OA. Effects of copper deficiency on the immune system. Adv Exp Med Biol. 1990;262:123–143. doi: 10.1007/978-1-4613-0553-8_11. [DOI] [PubMed] [Google Scholar]
- Prohaska J, Bailey W, Cox D. Failure of iron injection to reserve copper-dependent anemia in mice. In: Mills CF, Bremner I, Chesters JK, editors. Trace elements in man and animals. vol. TEMA 5. Farnham Royal: Commonwealth Agricultural Bureaux; 1985. pp. 27–32. [Google Scholar]
- Reeves PG, DeMars LC. Copper deficiency reduces iron absorption and biological half-life in male rats. J Nutr. 2004;134:1953–1957. doi: 10.1093/jn/134.8.1953. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Demars LC. Repletion of copper-deficient rats with dietary copper restores duodenal hephaestin protein and iron absorption. Exp Biol Med. 2005;230:320–325. doi: 10.1177/153537020523000505. [DOI] [PubMed] [Google Scholar]
- Reeves PG, DeMars LC. Signs of iron deficiency in copper-deficient rats are not affected by iron supplements administered by diet or by injection. J Nutr Biochem. 2006;17:635–642. doi: 10.1016/j.jnutbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Demars LC, Johnson WT, et al. Dietary copper deficiency reduces iron absorption and duodenal enterocyte hephaestin protein in male and female rats. J Nutr. 2005;135:92–98. doi: 10.1093/jn/135.1.92. [DOI] [PubMed] [Google Scholar]
- Williams DM, Kennedy FS, Green BG. Hepatic iron accumulation in copper-deficient rats. Br J Nutr. 1983;50:653–660. doi: 10.1079/bjn19830136. [DOI] [PubMed] [Google Scholar]