Summary
Retinoic acid (RA) is thought to be a key signaling molecule involved in limb bud patterning along the proximodistal or anteroposterior axes functioning through induction of Meis2 and Shh, respectively [1]. Here, we utilize Raldh2-/- and Raldh3-/- mouse embryos lacking RA synthesis [2] to demonstrate that RA signaling is not required for limb expression of Shh and Meis2. We demonstrate that RA action is required outside the limb field in the body axis during forelimb induction, but that RA is unnecessary at later stages when hindlimb budding and patterning occurs. We provide evidence for a model of trunk mesodermal RA action in which forelimb induction requires RA repression of Fgf8 in the developing trunk similar to how RA controls somitogenesis [3, 4] and heart development [5]. We demonstrate that pectoral fin development in RA-deficient zebrafish embryos can be rescued by an FGF receptor antagonist SU5402. In addition, embryo ChIP assays demonstrate that RA receptors bind the Fgf8 promoter in vivo. Our findings suggest that RA signaling is not required for limb proximodistal or anteroposterior patterning but that RA inhibition of FGF8 signaling during the early stages of body axis extension provides an environment permissive for induction of forelimb buds.
Results and Discussion
Retinoic acid (RA) is an important cell-cell signaling molecule that directly regulates genes through a nuclear RA receptor (RAR) bound to a RA response element (RARE) [2]. RA has been proposed to control chick limb anteroposterior patterning by inducing Shh posteriorly [6, 7]. However, studies in mice carrying a RA-reporter transgene demonstrated that limb RA activity is distributed equally along the anteroposterior axis, although RA is located differentially along the proximodistal axis with highest activity proximally [8]. Genetic studies in mice have demonstrated that RA synthesis is controlled by retinaldehyde dehydrogenase-2 (Raldh2) expressed in trunk mesoderm lying proximal to the limb bud, but not in the limb bud itself [9, 10]. Further studies in chick embryos suggested that RA may control the limb proximodistal axis through a mechanism in which RA generated by Raldh2 proximally in the flank induces Meis1 and Meis2 (proximal limb markers), and that fibroblast growth factor (FGF) generated distally in the apical ectodermal ridge (AER) represses these Meis genes [11]. However, gene inactivation studies have shown that Meis genes are not essential for normal limb development, at least individually [1, 12]. Although genetic studies have demonstrated the requirement for a distal FGF signaling center during proximodistal patterning [13] and the requirement for a distal region of RA degradation controlled by Cyp26b1 to prevent RA-induced limb teratogenesis [14], there is no clear evidence that a proximal RA signaling center is required to establish the limb proximodistal axis [1]. Furthermore, Raldh2-/- mouse embryos lacking RA synthesis fail to undergo forelimb induction suggesting that RA plays a role in limb development prior to limb patterning [9, 10]; also, zebrafish raldh2 mutants lack pectoral fins [15]. Raldh2-/- embryos rescued by maternal dietary RA supplementation undergo limb induction resulting in forelimbs that are undersized but hindlimbs appear relatively normal [8, 16]; as mutants rescued with a low RA dose express Meis2 in forelimb buds despite a lack of RA-reporter activity in limb mesoderm, a role for RA induction of Meis during proximodistal patterning is questionable [16]. Although rescued Raldh2-/- embryos display normal hindlimb buds, another potential source of RA exists near the hindlimb provided by Raldh3 expressed in the mesonephros [10]. Thus, the requirement of RA signaling for limb induction or patterning remains unclear, particularly as forelimb and hindlimb buds may differ in this regard. Here, we explore the role of RA during limb development by examining mouse embryos lacking either Raldh2 or both Raldh2 and Raldh3 to eliminate all endogenous RA synthesis during induction and patterning of forelimbs and hindlimbs.
Hindlimb Budding and Patterning does not Require RA
As Raldh2-/- embryos fail to grow beyond E8.5, mutants were rescued using maternal dietary RA supplementation [4]; the low doses of dietary RA used here (0.1-0.25 mg RA per g food) have been shown to provide embryos an amount of RA in the normal physiological range [17]. In order to detect RA activity in rescued Raldh2-/- embryos, we used embryos carrying the RARE-lacZ RA-reporter transgene [18]. We found that Raldh2-/- embryos provided brief RA treatment (0.1 mg RA per g food from E6.75-E8.25), then analyzed at E10.5, display RA activity in the neural tube and in the mesonephros adjoining the proximal hindlimb bud (Figure 1A-D; n = 6/6). We examined transverse sections of E10.5 wild-type embryos and found that Raldh3 mRNA is expressed in the mesonephric duct adjacent to the hindlimb bud (Figure 1E-F). Raldh3-/- embryos have normal limb buds [19], so we tested the potential role of Raldh3 in providing RA for hindlimb development by generating Raldh2-/-;Raldh3-/- double mutants. As Raldh2-/-;Raldh3-/- embryos exhibit early lethality similar to Raldh2-/- mutants [19], we examined rescued Raldh2-/-;Raldh3-/- embryos at E10.5 following brief RA treatment from E6.75-E8.25. Hindlimb buds of a normal size were observed in rescued Raldh2-/-;Raldh3-/- embryos (n = 7/7) despite a complete absence of RA activity (monitored by RARE-lacZ expression) in the mesonephros and hindlimb mesoderm (Figure 1G; n = 3/3). Similar to Raldh2-/- embryos, rescued Raldh2-/-;Raldh3-/- embryos always exhibited forelimbs smaller than their hindlimbs (Figure 1G-H). These findings demonstrate that Raldh3 is a source of RA for the mesonephros, but that RA synthesized by RALDH3 is not required in rescued Raldh2-/- embryos for hindlimb induction or early outgrowth.
Figure 1.

RA is unnecessary for hindlimb budding and patterning. (A-B) RARE-lacZ expression in E10.5 wild-type (WT) and Raldh2-/- embryos rescued by brief RA treatment (res -/-); mutant forelimb is smaller than hindlimb which has RA activity nearby in mesonephros. (C-D), Transverse sections through the hindlimbs of the embryos shown in panels A and B. (E-F) Raldh2 and Raldh3 mRNA in transverse sections through wild-type hindlimbs demonstrates that Raldh3 co-localizes with mesonephric RA activity. (G-H) RARE-lacZ expression in a rescued Raldh2-/-;Raldh3-/- embryo compared to a rescued Raldh2-/- embryo demonstrates that the hindlimb develops without mesonephric RA. (I-T), E10.5 wild-type and rescued Raldh2-/-;Raldh3-/- (R2-/-;R3-/-) embryos hybridized with probes for Fgf8 (I-J), Shh (K-L), Tbx4 (M-N), Pitx1 (O-P); Meis2 (Q-R); and Hoxa11 (S-T); anterior to left, posterior to right. f, forelimb bud; h, hindlimb bud; lpm, lateral plate mesoderm; m, mesonephros; md, mesonephric duct; mm, mesonephric mesenchyme; n, neural tube; s, somite.
RA activity was also absent at earlier stages in the hindlimb field of rescued Raldh2-/- embryos. In an E9.5 RA-rescued Raldh2-/- embryo (25-somite stage when the hindlimb field is forming), RA activity was not observed in hindlimb mesoderm (adjacent to somites 23-25) but a small region of RARE-lacZ expression was seen in the mesonephros likely due to Raldh3 expressed in that tissue (Figure. 2D; see Figure S1 for transverse sections).
Figure 2.
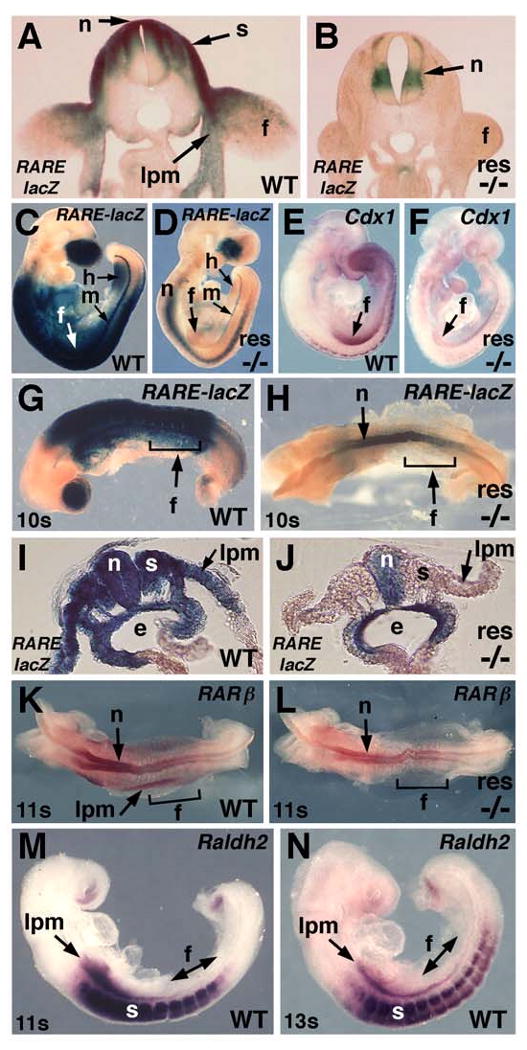
Forelimb induction requires RA signaling in the body axis but not limb mesoderm. (A-B) Transverse sections through the forelimbs of the E10.5 embryos shown in Figure 1a-b. RARE-lacZ expression (C-D) and RA-responsive Cdx1 mRNA (E-F) in E9.5 rescued Raldh2-/- embryos; wild-type and mutant embryos were stained for the same length of time here and in all other studies. (G-H) RARE-lacZ expression in E8.5 (10-somite) rescued Raldh2-/- embryo; in the mutant no RA activity is detected in somites or eye which normally express Raldh2 but no other RA-synthesizing enzyme at this stage; brackets indicate the beginning of the forelimb field lying parallel to somites 6-10. (I-J) Transverse section through forelimb field of embryos shown in panels G and H showing no RA activity in limb-field lpm of mutant. (K-L) RARβ is not expressed in lateral plate mesoderm of E8.5 (11-somite) rescued Raldh2-/- embryo. (M-N) Raldh2 expression at 11-13 somite stages. e, endoderm; f, forelimb field; lpm, lateral plate mesoderm; n, neural tube; s, somite.
Genes required for hindlimb induction and patterning were examined in Raldh2-/-;Raldh3-/- embryos including Fgf8 [13], Shh [20], Tbx4 [21], and Pitx1 [21]. Following brief RA treatment, Raldh2-/-;Raldh3-/- hindlimbs at E10.5 displayed relatively normal expression of Fgf8 in the AER needed for proximodistal patterning (Figure 1I-J; Figure S2) and normal expression of Shh in the zone of polarizing activity (ZPA) needed for anteroposterior patterning (Figure 1K-L; Figure S2). We also detected normal hindlimb expression of Tbx4 and Pitx1 which function in hindlimb induction (Figure 1M-P). We also analyzed early proximodistal patterning of the hindlimb which can be visualized with probes for expression of Meis2 (stylopod) and Hoxa11 (zeugopod) [1]. Analysis of these markers at E10.5 in RA-rescued Raldh2-/-;Raldh3-/- hindlimbs showed that these segments of the limb are present indicating that early proximodistal patterning does not depend on RA signaling (Figure 1Q-T). Moreover, although Meis2 expression is proposed to be dependent on RA from the flank [11], our data do not support this hypothesis. As rescued Raldh2-/-;Raldh3-/- hindlimb buds lack RA activity but undergo normal induction and patterning, these results suggest that RA is not required to establish limb patterning along either the anteroposterior or proximodistal axes. This conclusion is further supported by mutation of retinol dehydrogenase Rdh10 (acting upstream of Raldh2 for RA synthesis) which results in a phenotype with small forelimbs and normally-patterned hindlimbs reminiscent of rescued Raldh2 mutants [22]; the Rdh10 mutant does not require a small dose of RA to survive until hindlimb budding occurs, but nevertheless displays the same RARE-lacZ pattern as a rescued Raldh2-/-;Raldh3-/- embryo with RA activity detected in the neural tube but not the limb buds.
Forelimb Induction Requires RA Signaling in the Body Axis but not Limb Mesoderm
Although we find no requirement for RA during hindlimb budding or patterning, forelimb buds do appear to require RA for normal development. We further investigated RA signaling during budding of rescued forelimbs using RARE-lacZ and found that RA activity was not present in the small forelimb buds of E10.5 rescued Raldh2-/- embryos but RA was detected in the neural tube (Figure 2A-B; n = 6/6); RA activity was also not present in the small forelimb bud at E9.25 when it is first morphologically detectable (Figure 2C-D; Figure S3; n = 5/5). To complement this analysis of RA activity, we also examined expression of Cdx1 in rescued forelimbs. Although Cdx1 is not required for forelimb development [23], the Cdx1 promoter contains a highly sensitive RARE that functions in vivo and therefore serves as an endogenous reporter of RA activity [24]. Cdx1 was highly expressed in wild-type forelimbs, but was not expressed in small forelimb buds of rescued Raldh2-/- embryos suggesting they lack RA activity (Figure 2E-F; Figure S3; n = 3/3). At E8.5 (8-10 somites) the forelimb field has already been determined as evidenced by expression of Tbx5, the earliest known marker of the mammalian forelimb [25]. Examination of E8.5 rescued Raldh2-/- embryos revealed that RA activity was undetectable in the lateral plate mesoderm that gives rise to the forelimb field although RA was detected in neuroectoderm and endoderm (Figure 2G-J; n = 7/7). To further test whether RA signaling is absent in lateral plate mesoderm of rescued Raldh2-/- embryos, we examined expression of RARβ which possesses a potent RARE and is expressed in lateral plate mesoderm and neuroectoderm [26]; in rescued Raldh2-/- embryos, RARβ expression was detected in neuroectoderm but not lateral plate mesoderm (Figure 2K-L; n = 4/4). These findings demonstrate that our low-dose dietary method of RA administration to Raldh2-/- embryos provides less RA than Raldh2 normally generates. Even though we presume that RA is entering the embryo uniformly during rescue (by diffusion from the uterus since there is no placenta at this early stage), when RA is provided at such a low level it does not stimulate gene expression in all cells where it normally would perhaps due to tissue-specific differences in expression of RA-binding proteins or RARs [4]. The normal source of RA during forelimb induction is Raldh2 expressed in the somites and lateral plate mesoderm (Figure 2M-N). Thus, even though RA generated by Raldh2 in wild-type embryos is normally present in somites and lateral plate mesoderm fated to become limb, and can induce Cdx1 in limb mesoderm, RA is not acting there to initiate forelimb development but instead RA is functioning in a paracrine fashion elsewhere in the body axis to permit forelimb induction.
RA is Required for Induction of Forelimb Buds but not for Anteroposterior or Proximodistal Patterning
We explored whether RA acts early during forelimb induction to establish the forelimb field by examining the effect of RA rescue on expression of Tbx5 encoding a T-box transcription factor that is the earliest known marker of the mouse forelimb field [25]. Unrescued Raldh2-/- embryos failed to initiate Tbx5 expression in the lateral plate mesoderm posterior to the heart indicating a failure in forelimb induction at the 13-somite (13s) stage (Figure 3A-B; n = 2/2). Rescued Raldh2-/- embryos exhibited Tbx5 expression at 18s, although the size of the forelimb field was much smaller than normal (Figure 3C-D; n = 6/6). Double-staining for expression of Tbx5 and the somite marker Uncx4.1 [4] revealed that rescued Raldh2-/- embryos have no Tbx5 expression at 10s (Figure 3E-F; n = 4/4), but that a small domain of Tbx5 expression was observed at 13s (Figure 3G-H; n = 3/3). These findings indicate that brief RA treatment allows a domain of Tbx5 expression to arise after a delay of a few hours, potentially leading to the small forelimbs observed later. However, the effect of RA on Tbx5 must be indirect as we do not detect RA in the cells where Tbx5 is induced (Figure 2H,J,L).
Figure 3.
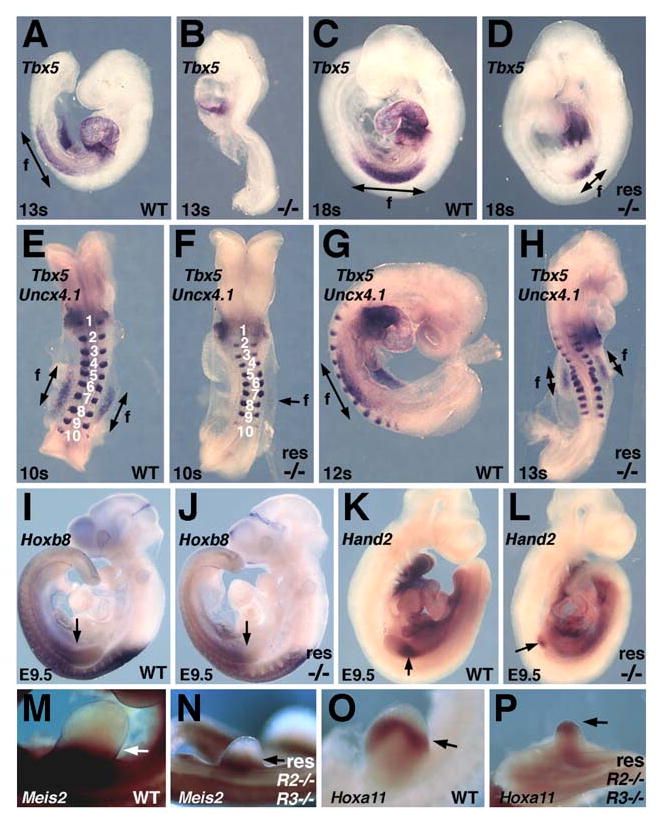
RA is required for induction but not A-P patterning of forelimb buds. Tbx5 mRNA in Raldh2-/- embryos that are unrescued (A-B) or rescued with brief RA treatment (C-D); note smaller forelimb field in rescued mutant compared to wild-type. (E-H) Tbx5 and Uncx4.1 mRNA double-staining in rescued Raldh2-/- embryos; note delay in Tbx5 expression in rescued mutant. (I-J) Hoxb8 mRNA in rescued Raldh2-/- embryo; note similar anteroposterior boundary of expression in rescued mutant and wild-type. (K-L) Hand2 mRNA in rescued Raldh2-/- embryo; note small expression domain in rescued mutant. (M-N) Meis2 mRNA and (O-P) Hoxa11 mRNA in E10.5 wild-type and rescued Raldh2-/-;Raldh3-/- embryos. f, forelimb field.
We find that forelimbs lacking RA activity still express Shh even though expression appears distally rather than posteriorly as normal (Figure 1L; Figure S2). However, exogenous RA treatment (high-dose beads) has been reported to induce expression of Shh, Hoxb8, and Hand2 leading to the conclusion that RA is needed to establish anteroposterior patterning of chick limb buds; thus, we examined rescued E9.5 Raldh2-/- embryos for expression of Hoxb8 and Hand2 which are expressed prior to Shh [20]. In E9.5 rescued Raldh2-/- embryos we found that Hoxb8 was still expressed in the posterior portion of forelimb buds (Figure 3I-J; Figure S4; n = 3/3), and that a small domain of Hand2 was still expressed in the small forelimb bud that develops even though expression is localized distally rather than posteriorly (Figure 3K-L; Figure S4; n = 2/2). We examined proximodistal markers in E10.5 rescued Raldh2-/-;Raldh3-/- embryos and found that Meis2 (previously suggested to require RA for induction) was expressed in the proximal portion of the small forelimb that develops, and Hoxa11 was expressed more distally as expected (Fig. 3M-P). Thus, taken together with our findings above demonstrating that rescued Raldh2-/- embryos lack forelimb RA activity, RA signaling is not required in the forelimb bud for induction of Hoxb8, Hand2, Shh, and Meis2. However, RA signaling is required outside the forelimb field during induction to obtain the correct size bud and the correct posterior expression domains of Hand2 and Shh; we suggest that posterior domains may not be able to form properly when forelimb growth is retarded, thus resulting in distal expression.
Previous studies using pharmacological treatment of chick embryos with combined RAR/RXR antagonists to block RA receptor activity [6] or the RA synthesis inhibitor disulfiram [7] have shown down-regulation of Shh and Hoxb8. However, these chemicals may have non-specific effects as RXRs are heterodimer partners for at least 10 nuclear receptors other than RARs, and disulfiram inhibits the enzymatic activity of many if not all 19 members of the aldehyde dehydrogenase family to which RALDH2 belongs. By removing RALDH2 genetically our findings suggest that endogenous RA action is not required to induce genes needed for limb anteroposterior or proximodistal patterning, but that RA action is required at an earlier stage when forelimb induction occurs.
RA Inhibits FGF Signaling in the Body Axis Near the Forelimb Field
Our studies suggest RA does not play an instructive role in forelimb development, but rather a permissive role through action in the body axis near the forelimb field at the 8-somite stage when forelimb induction occurs [25]. During the 1-10 somite stages, RA functions permissively during development of the body axis by repressing Fgf8 posteriorly at the neuroectoderm/epiblast junction to prevent Fgf8 expression from extending anteriorly into neuroectoderm [3, 4], and by repressing Fgf8 anteriorly in cardiac lateral plate mesoderm to prevent Fgf8 expression from extending posteriorly into trunk lateral plate mesoderm [5]. By limiting the cardiac and epiblast Fgf8 domains, we suggest that RA permits an Fgf8-free zone to develop in between needed for proper development of the trunk including the forelimb fields. This hypothesis is supported by previous studies demonstrating that expression of a constitutively active FGF receptor (Fgfr1) in zebrafish results in expansion of the heart field and loss or reduction of pectoral fins [27]. To further test this hypothesis, we examined the effect of the FGF receptor antagonist SU5402 on pectoral fin development in RA-deficient zebrafish embryos using the RA synthesis inhibitor DEAB as previously described [15]. Zebrafish embryos treated with DEAB to inhibit RA synthesis from the bud stage (∼9.5-10 hpf) to somite 12-13 (∼15 hpf) were found to always lack pectoral fins when observed at 96 hpf (n = 0/8 fin positive with 4 μM DEAB; n = 0/7 fin positive with 5 μM DEAB; n = 17/17 fin positive with 0.1% DMSO vehicle). Treatment during the same time period with 3 μM SU5402 resulted in yolk sac edema, but pectoral fins always developed (n = 14/14 fin positive). Importantly, treatment with both DEAB and SU5402 during this time period often rescued pectoral fin development (n = 6/17 fin positive with 4 μM DEAB + 3 μM SU5402; n = 3/8 fin positive with 5 μM DEAB + 3 μM SU5402). Rescued pectoral fins were smaller than those present in vehicle-treated embryos (Fig. S5). These findings suggest that loss of RA synthesis results in an increase in FGF signaling which inhibits pectoral fin development. Treatment with both inhibitors also resulted in less yolk sac edema in most embryos (including all those that were fin positive), suggesting that DEAB may be reducing the toxicity of SU5402, consistent with a loss of RA leading to increased Fgf expression as previously reported in mouse.
In order to determine if excessive Fgf8 expression observed in Raldh2-/- mouse embryos results in excessive FGF signaling, we examined expression of Sprouty2 (Spry2) which is induced by FGF signaling and functions to regulate transmission of the FGF signal [28]. Whereas wild-type embryos exhibited anterior and posterior domains of Spry2 mRNA separated by a large negative region in the trunk, Raldh2-/- embryos at 6-8 somites exhibited ectopic Spry2 mRNA encroaching into the trunk where the forelimb field normally develops (Figure 4C; n = 3/3). Thus, a loss of RA signaling leads to a large increase in trunk FGF signaling.
Figure 4.
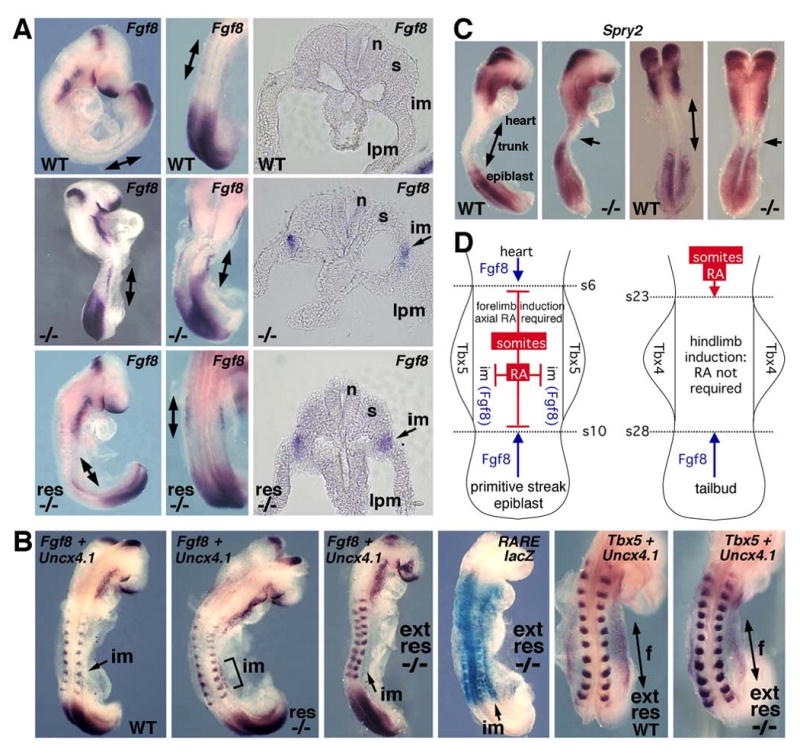
Ectopic Fgf8 expression near the forelimb field following loss of RA. (A) Fgf8 mRNA in 13-somite embryos: wild-type (top panels), unrescued Raldh2-/- (middle panels), and rescued Raldh2-/- with brief RA treatment (bottom panels); in mutants note abnormal domain of Fgf8 mRNA in the intermediate mesoderm adjacent to the forelimb field marked by double-arrow in whole-mount and arrow in transverse sections. (B) Fgf8 and Uncx4.1 (somite marker) expression in 10-somite Raldh2-/- embryos following brief RA rescue (res -/-) or extended RA rescue (ext res -/-); note loss of Fgf8 mRNA in intermediate mesoderm following extended RA rescue and higher levels of RARE-lacZ expression showing that RA activity has been stimulated in intermediate mesoderm. Extended RA treatment also results in comparable Tbx5 mRNA domains in the forelimb fields of 12-somite wild-type and Raldh2-/- embryos. (C) Expression of Spry2 (a marker of FGF signaling) in 7-somite wild-type and unrescued Raldh2-/- embryos; arrows in mutants point out expansion of FGF signaling into trunk domain where forelimbs develop. (D) Model for RA signaling based on studies presented here as well as previous findings [4, 5] suggesting that RA acts in the body axis to repress Fgf8 during the 1-10 somite stages to provide an environment permissive for forelimb induction; at the 23-28 somite stages RA signaling has retracted anteriorly and is not involved in hindlimb induction. e, endoderm; f, forelimb bud; h, hindlimb bud; im, intermediate mesoderm (mesonephros); lpm, lateral plate mesoderm; n, neural tube; s, somite.
Previous studies on chick embryos suggested that Fgf8 expressed in intermediate mesoderm might be needed for limb initiation due to the ability of FGF-beads to induce extra limbs in the interlimb flank [29]. However, studies on mouse embryos have shown that Tbx5 expression in the forelimb field precedes Fgf8 expression in the intermediate mesoderm by about 18 hours [25], and conditional mutagenesis has demonstrated that Fgf8 expression in the intermediate mesoderm is unnecessary for limb induction although it is required at later stages for kidney development [30]. Also, further studies on chick embryos demonstrated that ablation of the intermediate mesoderm [31] or neuroectoderm [32] does not affect limb initiation. Thus, we futher pursued the hypothesis that Fgf8 expression may normally inhibit rather than stimulate forelimb induction. Here, we found that wild-type mouse embryos at 10-13s have no Fgf8 expression detectable in the intermediate mesoderm (Figure 4A; n = 7/7). However, unrescued Raldh2-/- embryos at 12-13s always exhibited an abnormal domain of Fgf8 expression in the intermediate mesoderm adjacent to the region where the forelimb field has failed to develop (Figure 4A; n = 4/4). Interestingly, Raldh2-/- embryos at 11-13s rescued by brief RA treatment still exhibited ectopic Fgf8 expression in the intermediate mesoderm although this domain was now well-separated from the Fgf8 expression domain observed posteriorly at the neuroectoderm/epiblast junction which has retracted posteriorly compared to the unrescued mutant (Figure 4A; n = 3/3). We compared Fgf8 expression in Raldh2-/- embryos at 10-11s treated with either brief RA treatment (0.1 mg RA per g food from E6.75-E8.25) or extended RA treatment (similar to brief treatment except 0.25 mg RA per g of food from E7.75-E8.5), and found that extended RA treatment eliminated the ectopic domain of Fgf8 expression in the intermediate mesoderm parallel to somites 6-10 (Figure 4B; n = 4/4). Extended RA treatment resulted in RARE-lacZ expression (RA activity) not only in the neuroectoderm (as found for brief treatment) but also in the somitic, intermediate, and lateral plate mesoderm of Raldh2-/- embryos (Figure 4B). Extended RA treatment of E8.5 Raldh2-/- embryos resulted in a significant increase in the size of the forelimb field marked by Tbx5 expression (Figure 4B; n = 2/2) compared to brief RA treatment (Figure 3H). Previous RA rescue studies of Raldh2-/- embryos demonstrated that extended RA treatment increases the size of the forelimb bud at E10.5, in some cases close to normal size [8, 16]. Taken together, these findings suggest that brief RA treatment eliminates excessive FGF8 signaling emanating from the neuroectoderm/epiblast junction which may allow the forelimb field to initiate, but that the field may be delayed and undersized due to excessive FGF8 signaling emanating from the intermediate mesoderm which requires higher levels of RA to repress Fgf8.
Support for a direct role of RA in regulation of Fgf8 comes from studies suggesting that a nearby RARE represses the major isoform (Fgf8b) when RAR and RA are both present, but allows Fgf8b expression when RAR is unliganded [33]. We provide further evidence that RA regulation of Fgf8 is direct using chromatin immunoprecipitation (ChIP). We show that a conserved RARE near the Fgf8 promoter binds all three RAR isoforms (RARa, RARb, and RARg) using ChIP with RAR antibodies and chromatin from 5-somite mouse embryos (Figure S6). This finding is important since it shows that the mouse Fgf8 gene can bind RAR in vivo just prior to induction of Tbx5 in the forelimb field.
Conclusion
Previous studies of limb RA action proposed that RA acts instructively in both forelimb and hindlimb bud mesoderm to induce genes needed for limb anteroposterior and proximodistal patterning such as Shh, Hoxb8, Hand2, and Meis1/2. However, our findings indicate that RA signaling in limb mesoderm is dispensable for expression of these patterning genes in their normal locations and at normal levels, and our RA-reporter data using RARE-lacZ, Cdx1, and RARβ strongly support this critical point by demonstrating that RA activity is absent. We point out that our hypothesis is based upon the assumption that these are indeed very good markers of RA activity with well-characterized RAREs. Although previous studies indicate administration of excess RA can lead to induction of Shh, Hoxb8, Hand2, and Meis1/2, we suggest this is an abnormal response to a teratogenic dose of RA and does not reflect the normal function of RA; these genes have not been reported to possess bona fide RAREs but may contain DNA sequences loosely resembling a RARE that bind RARs weakly and function only during excess RA treatment.
Although our studies suggest that hindlimb patterning does not require RA, other studies on vitamin A deficient quails suggested that hindlimb patterning was affected [34]; we suggest that the quail findings were due to a general effect on embryonic health (maybe secondary to cardiac defects that occur in those embryos) as it is clear that a complete loss of RA in mouse Raldh2 mutants leads to severe cardiac and body axis defects that prevent embryos from developing to the stage when hindlimbs arise. Cyp26b1 expression in forelimb and hindlimb bud mesenchyme stimulates RA degradation and prevents distal limb teratogenesis which was suggested to be important for proximodistal patterning of the limb buds [14]. Our findings suggest that the function of Cyp26b1 is not to provide a gradient of RA needed for patterning, but to eliminate an RA signal unnecessary for limb patterning that is teratogenic for distal limb patterning; in the fetal testis, the function of Cyp26b1 is simply to eliminate RA so that meiosis does not occur until after birth [35].
Our findings reported here suggest a different model of limb RA action that does not involve RA induction of limb genes, but which incorporates the concept of RA antagonism of FGF signaling. In our model, RA acts outside the forelimb field in the body axis in neuroectoderm [4], cardiac mesoderm [5], and intermediate mesoderm (reported here) to repress expression of Fgf8 in order to prevent excessive secretion of FGF8 that enters the forelimb field and inhibits forelimb induction (Figure 4D). Thus, instead of RA counteracting distal FGF signaling emanating from the AER after forelimb budding has occurred, as originally proposed [11], our findings suggest that RA acts prior to forelimb induction to reduce FGF signaling in the developing trunk, thus providing an environment permissive for induction of forelimbs. This conclusion is firmly supported by our zebrafish studies demonstrating that an FGF receptor antagonist can rescue pectoral fin development in RA-deficient embryos. Further support comes from studies on the Fgf8 promoter where we have demonstrated the existence of a RARE in vivo by ChIP analysis of mouse embryo chromatin, and where others have shown with cell line assays that the Fgf8 RARE represses the major isoform (Fgf8b) when RAR and RA are both present [33]. We further suggest that limb proximodistal patterning either does not require a proximal signal or uses one distinct from RA.
Our findings also support a model in which RA signaling is required for induction of forelimbs but not hindlimbs based upon the observation that embryos lacking limb bud RA activity exhibit relatively normal hindlimb buds but small forelimb buds (Figure 4D). This temporal model is supported by previous studies demonstrating that Raldh2 expression during somite stages 1-10 is present in presomitic mesoderm located just anterior to the border of Fgf8 expression at the epiblast/neuroectoderm junction, but that Raldh2 expression later retracts anteriorly (lost in presomitic mesoderm and newly formed somites) such that by the time hindlimb induction is occurring (23-28 somite stage) RA signaling does not reach the tailbud/neuroectoderm junction and is no longer required for posterior Fgf8 regulation or somitogenesis [4]. We suggest that the role of RA in forelimb induction is part of a more fundamental event in which RA repression of Fgf8 helps establish the trunk as a distinct region during a brief period of body axis extension when the primitive streak still exists. This regulatory event does not continue when the primitive streak gives way to the tailbud and the hindlimbs arise; in the tailbud another mechanism presumably exists to limit caudal Fgf8 expression. Future studies on this unique temporal action of RA should shed more light on the signaling mechanisms underlying early organogenesis.
Experimental Procedures
Generation of Raldh Null Mutant Embryos
Raldh2-/- embryos [10] and Raldh2-/-;Raldh3-/- double homozygous embryos [19] were previously described. All mouse studies conformed to the regulatory standards adopted by the Animal Research Committee at the Burnham Institute for Medical Research.
Rescue with a Physiological Dose of RA
Rescue of embryos by maternal dietary RA supplementation was performed as previously described [4] with low RA doses demonstrated to provide embryos an amount of RA in the normal physiological range [17]. For brief treatment, the final RA concentration was 0.1 mg/g food and treatment was from E6.75-E8.25. For extended treatment, an RA concentration of 0.1 mg/g food was used from E6.75-E7.75 followed by an RA concentration of 0.25 mg/g food from E7.75-E8.5. For embryos analyzed at time points after RA treatment was ended, mice were returned to standard mouse chow until the point of analysis.
In situ Hybridization and Retinoic Acid Detection
Whole-mount in situ hybridization was performed as described previously; wild-type and mutant embryos were treated identically and stained for the same length of time [10]. The RARE-lacZ RA-reporter transgene, which places lacZ (encoding β-galactosidase) under the control of a RARE, was used to detect RA activity in embryos [18]; wild-type and mutant embryos were stained for the same length of time. Stained embryos were embedded in 3% agarose and sectioned at 30 μm with a vibratome.
Supplementary Material
Acknowledgments
We thank the following for mouse cDNAs used to prepare in situ hybridization probes: M. Capecchi (Hoxb8), P. Gruss (Meis2), D. Lohnes (Cdx1), M. Logan (Pitx1), A. Mansouri (Uncx4.1), G. Martin (Fgf8, Spry2), A. McMahon (Shh), E. Olson (Hand2), S. Potter (Hoxa11), and V. Papaioannou (Tbx4, Tbx5). We also thank J. Rossant for providing RARE-lacZ mice. This work was funded by Deutsche Forschungsgemeinschaft grant Si1381/1-1 (I.O.S.) and National Institutes of Health grant GM062848 (G.D.).
Footnotes
[Additional information is available online in the supplemental material].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tabin C, Wolpert L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes & Development. 2007;21:1433–1442. doi: 10.1101/gad.1547407. [DOI] [PubMed] [Google Scholar]
- 2.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Corral RD, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 4.Sirbu IO, Duester G. Retinoic acid signaling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol. 2006;8:271–277. doi: 10.1038/ncb1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu HC, Revelli JP, Goering L, Thaller C, Eichele G. Retinoid signaling is required for the establishment of a ZPA and for the expression of Hoxb-8, a mediator of ZPA formation. Development. 1997;124:1643–1651. doi: 10.1242/dev.124.9.1643. [DOI] [PubMed] [Google Scholar]
- 7.Stratford T, Horton C, Maden M. Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Curr Biol. 1996;6:1124–1133. doi: 10.1016/s0960-9822(02)70679-9. [DOI] [PubMed] [Google Scholar]
- 8.Mic FA, Sirbu IO, Duester G. Retinoic acid synthesis controlled by Raldh2 is required early for limb bud initiation and then later as a proximodistal signal during apical ectodermal ridge formation. J Biol Chem. 2004;279:26698–26706. doi: 10.1074/jbc.M401920200. [DOI] [PubMed] [Google Scholar]
- 9.Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nature Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 10.Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129:2271–2282. doi: 10.1242/dev.129.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercader N, Leonardo E, Piedra ME, Martínez-A C, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- 12.Azcoitia V, Araci LM, Martinez-A C, Torres M. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol. 2005;280:307–320. doi: 10.1016/j.ydbio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401–405. doi: 10.1038/nature06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing limb. Dev Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- 15.Gibert Y, Gajewski A, Meyer A, Begemann G. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development. 2006;133:2649–2659. doi: 10.1242/dev.02438. [DOI] [PubMed] [Google Scholar]
- 16.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- 17.Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci USA. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- 19.Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 21.Logan M, Tabin CJ. Role of Pitx1 upstream of Tbx4 in specification of hindlimb identity. Science. 1999;283:1736–1739. doi: 10.1126/science.283.5408.1736. [DOI] [PubMed] [Google Scholar]
- 22.Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes & Development. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian V, Meyer BI, Gruss P. Disruption of the murine homeobox gene Cdx1 affects skeletal identities by altering the mesodermal expression domains of Hox genes. Cell. 1995;83:641–653. doi: 10.1016/0092-8674(95)90104-3. [DOI] [PubMed] [Google Scholar]
- 24.Houle M, Sylvestre JR, Lohnes D. Retinoic acid regulates a subset of Cdx1 function in vivo. Development. 2003;130:6555–6567. doi: 10.1242/dev.00889. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- 26.Mendelsohn C, Ruberte E, LeMeur M, Morriss-Kay G, Chambon P. Developmental analysis of the retinoic acid-inducible RAR-β2 promoter in transgenic animals. Development. 1991;113:723–734. doi: 10.1242/dev.113.3.723. [DOI] [PubMed] [Google Scholar]
- 27.Marques SR, Lee Y, Poss KD, Yelon D. Reiterative roles for FGF signaling in the establishment of size and proportion of the zebrafish heart. Developmental Biology. 2008;321:397–406. doi: 10.1016/j.ydbio.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 29.Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 30.Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132:3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Teran M, Piedra ME, Simandl BK, Fallon JF, Ros MA. Limb initiation and development is normal in the absence of the mesonephros. Dev Biol. 1997;189:246–255. doi: 10.1006/dbio.1997.8680. [DOI] [PubMed] [Google Scholar]
- 32.Rong PM, Teillet MA, Ziller C, Le Douarin NM. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development. 1992;115:657–672. doi: 10.1242/dev.115.3.657. [DOI] [PubMed] [Google Scholar]
- 33.Brondani V, Klimkait T, Egly JM, Hamy F. Promoter of FGF8 reveals a unique regulation by unliganded RARa. J Mol Biol. 2002;319:715–728. doi: 10.1016/S0022-2836(02)00376-5. [DOI] [PubMed] [Google Scholar]
- 34.Stratford T, Logan C, Zile M, Maden M. Abnormal anteroposterior and dorsoventral patterning of the limb bud in the absence of retinoids. Mech Dev. 1999;81:115–125. doi: 10.1016/s0925-4773(98)00231-7. [DOI] [PubMed] [Google Scholar]
- 35.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


