Abstract
Increased longevity and population aging will increase the number of men with late onset hypogonadism. It is a common condition, but often underdiagnosed and undertreated. The indication of testosterone-replacement therapy (TRT) treatment requires the presence of low testosterone level, and symptoms and signs of hypogonadism. Although controversy remains regarding indications for testosterone supplementation in aging men due to lack of large-scale, long-term studies assessing the benefits and risks of testosterone-replacement therapy in men, reports indicate that TRT may produce a wide range of benefits for men with hypogonadism that include improvement in libido and sexual function, bone density, muscle mass, body composition, mood, erythropoiesis, cognition, quality of life and cardiovascular disease. Perhaps the most controversial area is the issue of risk, especially possible stimulation of prostate cancer by testosterone, even though no evidence to support this risk exists. Other possible risks include worsening symptoms of benign prostatic hypertrophy, liver toxicity, hyperviscosity, erythrocytosis, worsening untreated sleep apnea or severe heart failure. Despite this controversy, testosterone supplementation in the United States has increased substantially over the past several years. The physician should discuss with the patient the potential benefits and risks of TRT. The purpose of this review is to discuss what is known and not known regarding the benefits and risks of TRT.
Keywords: hypogonadism, testosterone replacement therapy, erectile dysfunction, osteoporosis, cardiovascular disease
Introduction
Hypogonadism is a clinical condition in which low levels of serum testosterone are found in association with specific signs and symptoms. When hypogonadism occurs in an older man, the condition is often called andropause or androgen deficiency of the aging male or late onset hypogonadism (LOH).1 The most easily recognized clinical signs of relative androgen deficiency in older men are a decrease in muscle mass and strength, a decrease in bone mass and osteoporosis, and an increase in central body fat. However, symptoms such as a decrease in libido and sexual desire, forgetfulness, loss of memory, anemia, difficulty in concentration, insomnia, and a decreased sense of well-being are more difficult to measure and differentiate from hormone-independent aging. This condition may result in significant detriment to quality of life and adversely affect the function of multiple organ systems.1–3 A health factor-independent, age-related longitudinal decrease in serum testosterone levels has been reported.4 This LOH is important since it features many potentially serious consequences that can be readily avoided or treated, and the affected sector of the population is currently expanding in number. Prospective population-based studies reported in the past decade indicate that low testosterone levels are associated with an increase in the risk for developing type 2 diabetes mellitus and metabolic syndrome and possibly a reduction in survival. Results were similar for bioavailable testosterone.5–7 In men, endogenous testosterone concentrations are inversely related to mortality due to cardiovascular disease and all causes. Low testosterone may be a predictive marker for those at high risk of cardiovascular disease.8 Also, low testosterone levels were associated with increased mortality in male veterans9 but this association could not be confirmed in the Massachusetts Male Aging Study10 or the New Mexico Aging Study.11
As the clinical symptoms of hormone deficiency in older males may be nonspecific, and since a substantial number of relatively asymptomatic elderly men have testosterone levels outside the normal range for young adults, investigators have suggested that testosterone replacement therapy is only warranted in the presence of both clinical symptoms suggestive of hormone deficiency and decreased hormone levels.12 Restoring serum testosterone levels to the normal range using testosterone replacement therapy results in clinical benefits in some of these areas. Successful management of testosterone replacement therapy requires appropriate evaluation and an understanding of the benefits and risks of treatment.
Aging and hypogonadism
Due to the baby boom that occurred after World War II, the percentage of population in the older age group in developed countries is increasing. Testosterone deficiency is a common disorder in middle-aged and older men but it is underdiagnosed and often untreated. Clinicians tend to overlook it, and the complaints of androgen-deficient men are merely considered part of aging. Hypogonadism affects an estimated 2 million to 4 million men in the United States. Many patients can derive significant benefits from treatment. Testosterone supplementation in the United States has increased substantially over the past several years.13 However, it has been estimated that only 5% of affected men currently receive treatment.
The decline of serum testosterone levels appears to be a gradual, age-related process resulting in an approximate 1% annual decline after age 30. In cross-sectional and longitudinal studies of men aged 30 or 40 years and above, total, bioavailable and free testosterone concentrations fall with increasing age with a steeper decline in bioavailable and free compared with total testosterone concentrations.4,14,15 In older men above the age of 65 or 70 years, the changes in total testosterone are overshadowed by a more significant decline in free testosterone levels.16,17 This is a consequence of the age-associated increase of the levels of sex hormone binding globulin (SHBG) demonstrated by cross-sectional studies, and has now been confirmed by longitudinal studies.18,19 Although the fall is gradual, by the eighth decade, according to the Baltimore Longitudinal Study, 30% of men had total testosterone values in the hypogonadal range, and 50% had low free testosterone values. The rate of age-related decline in serum testosterone levels varies in different individuals and is affected by chronic disease and medications.20 There is evidence that many of these men are not symptomatic.21
Multiple mechanisms are likely to influence the decline in testosterone levels in aging men.22 Lower testosterone levels may result from reduced testicular responses to gonadotrophin stimuli with aging, coupled with incomplete hypothalamo–pituitary compensation for the fall in total and free testosterone levels.23,24 Whether the age-dependent decline in androgen levels leads to health problems in older men is being debated vigorously.21,25
Diagnosis
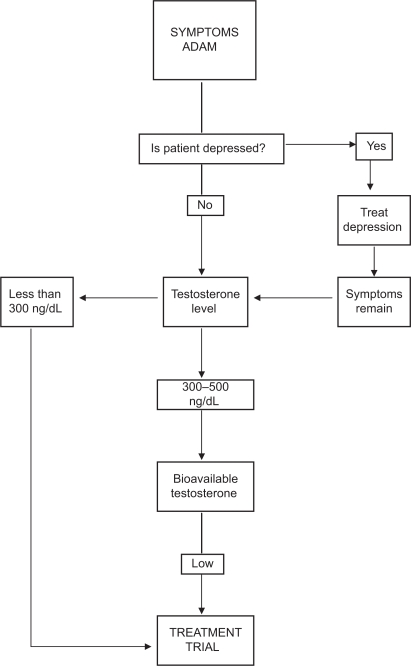
At present, the diagnosis of hypogonadism requires the presence of symptoms and signs suggestive of testosterone deficiency.1,26 The symptom most associated with hypogonadism is low libido.27–29 Other manifestations of hypogonadism include erectile dysfunction, decreased muscle mass and strength, increased body fat, decreased bone mineral density and osteoporosis, mild anemia, breast discomfort and gynecomastia, hot flushes, sleep disturbance, body hair and skin alterations, decreased vitality, and decreased intellectual capacity (poor concentration, depression, fatigue).30 The problem is many of the symptoms of late life hypogonadism are similar in other conditions31,32 or are physiologically associated with the aging process.33 One or more of these symptoms must be corroborated with a low serum testosterone level.21,25,34 Depression, hypothyroidism and chronic alcoholism should be excluded, as should the use of medications such as corticosteroids, cimetidine, spironolactone, digoxin, opioid analgesics, antidepressants and antifungal drugs. Of course, diagnosis of LOH should never be undertaken during an acute illness, which is likely to result in temporarily low testosterone levels (Figure 1).
Figure 1.
Approach to the diagnosis and treatment of late onset hypogonadism (ADAM = St. Louis University Androgen Deficiency in Aging Males Questionnaire).
The problem with symptoms associated with low serum testosterone is that they may be found in other conditions, which become increasingly common as men age. While the questionnaires, the Androgen Deficiency in Aging Male (ADAM)35,36 and the Aging Male Symptoms Scale (AMS)37,38 (Table 1), may be sensitive markers of the low testosterone state (97% and 83%, respectively), they are not tightly correlated with low testosterone (specificity 30% and 39%), particularly in the borderline low serum testosterone range. Therefore, questionnaires are not recommended for screening of androgen deficiency in men receiving health care for unrelated reasons.26 Moreover, healthy ambulatory elderly males over 70, assessed by the AMS, had a high perception of sexual symptoms with mild psychological and mild to moderate somatovegetative symptoms.39 Note also that there is marked inter-individual variation of the testosterone level at which symptoms occur.28,34,40 A low libido by itself is insufficient to allow the diagnosis of hypogonadism.41
Table 1.
Androgen Deficiency In Ageing Male (ADAM) questionnaire
|
A positive ADAM questionnaire was defined as “yes” for question 1 and 7, and 2–4 for all other items.
Laboratory diagnosis
A thorough physical and biochemical work-up is necessary. Transient decreases of serum testosterone levels such as those due to acute illnesses should be excluded by careful clinical evaluations and repeated hormone measurement. Hypogonadism (primary or secondary) can occur at all ages, including in elderly men. Risk factors for hypogonadism in older men may include chronic illnesses (including diabetes mellitus, chronic obstructive lung disease, and inflammatory arthritic, renal, and HIV-related diseases), obesity, metabolic syndrome, and hemachromatosis.26 Such chronic diseases should be investigated and treated.42 Primary hypogonadism is characterized by raised levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in response to diminished testosterone (and estradiol and inhibin B) feedback. Secondary hypogonadism is characterized by low levels of testosterone associated with low or normal levels of FSH and/or LH.43 However, in older men, the figure is less clear-cut, testosterone deficiency being both primary and secondary.
Serum testosterone has a diurnal variation and levels peak between 08.00 and 10.00 h, a serum sample should be obtained between 07.00 and 11.00 h.44 The most widely accepted parameters to establish the presence of hypogonadism is the measurement of serum total testosterone. Total testosterone levels above 500 ng/dL do not require substitution; patients with serum total testosterone levels below 300 ng/dL will usually benefit from testosterone treatment. If the serum total testosterone level is between 230 and 350 ng/dL in males under 50 years or between 300 and 500 ng/dL in older males, repeating the measurement of total testosterone with sex hormone binding globulin (SHBG) to calculate free testosterone or measuring free testosterone by equilibrium dialysis or bioavailable testosterone is recommended.45 Note that SHBG is elevated in elderly patients and effected by many diseases.30 Measurements of serum LH will assist in differentiating between primary and secondary hypogonadism and serum prolactin is indicated when the serum testosterone is lower than 150 ng/dl46–49 or when secondary hypogonadism is suspected.50,51 Measurement of free or bioavailable testosterone (BT, free plus albumin bound) should be considered when the serum total testosterone concentration is not diagnostic of hypogonadism, particularly in obese men. Bioavailable testosterone appears to correlate better with potential hypogonadal symptoms than does total testosterone.52 A free testosterone level below 65 pg/mL can provide supportive evidence for testosterone treatment.53,54 The gold standard for bioavailable testosterone measurement is by sulphate precipitation and equilibrium dialysis for free testosterone. However, both techniques are not usually available in local laboratories so that calculated values seem preferable. Salivary testosterone, a proxy for unbound testosterone, has also been shown to be a reliable substitute for free testosterone measurements.55–57 Diagnosis of late life hypogonadism requires both symptoms and low testosterone (Figure 1).
Treatment and delivery systems
Testosterone replacement therapy aims at restoring hormone levels in the normal range of young adults and should, in theory, approximate the natural, endogenous production of the hormone produces and maintains physiologic serum concentrations of the hormone and its active metabolites without significant side effects or safety concerns and, more importantly, alleviates the symptoms suggestive of the hormone deficiency. However, the ultimate goals are to maintain or regain the highest quality of life, to reduce disability, to compress major illnesses into a narrow age range, and to add life to years.
Several different types of testosterone replacement exist including tablets, injections, transdermal systems, oral, pellets, and buccal preparations of testosterone. Selective androgen receptor modulators (SARMs) are under development but not yet clinically available.
The selection of the preparation should be a joint decision of an informed patient and physician.58 Short-acting preparations may be preferred over long-acting depot preparations in the initial treatment of patients with LOH.59 It has been recommended that the optimal serum testosterone level for efficacy and safety should be in the mid range to lower young-adult-male serum testosterone levels as the therapeutic goal.60 However, older males need higher levels to obtain a therapeutic benefit.
Oral agents
The oral form of 17-alpha-alkylated androgen should not be used because of their potential liver toxicity including the development of benign and malignant neoplasm61 in addition deleterious effects on levels of both LDL cholesterol (LDL-C) and HDL cholesterol (HDL-C).62 Testosterone undecanoate, which is absorbed predominantly through the lymphatic system, is widely used outside the United States and has the best safety data. However, it can rarely raise testosterone levels above the mid-range.
Intramuscular injection
Until recently, testosterone cypionate and enanthate were frequently used by intramuscular injection of short-acting testosterone esters that usually produces supraphysiological peaks and hypogonadal troughs in testosterone levels. These fluctuations in testosterone levels may yield variations in libido, sexual function, energy, and mood.63 A “roller coaster” effect can also occur, characterized by alternating periods of symptomatic benefit and a return to base-line symptoms, corresponding to the fluctuations in serum testosterone levels.64,65 However, in our experience many males eventually prefer this form of therapy to the others. Compared with conventional testosterone enanthate or cypionate treatment requiring injection intervals of 2 to 3 weeks, and resulting in supraphysiological serum testosterone levels, injections of testosterone undecanoate (TU) at intervals of up to 3 months offer an excellent alternative for substitution therapy of male hypogonadism.66 The long duration of action creates a problem if there are complications of testosterone therapy.
Transdermal systems
Currently, transdermal testosterone is available in either a scrotal or a nonscrotal skin patch and more recently as a gel preparation, allowing a single application of this formulation to provide continuous transdermal delivery of testosterone for 24 hours, producing circulating testosterone levels that approximate the normal levels (eg, 300 to 1000 ng/dL) seen in healthy men. Daily application is required for each of these. They are designed to deliver 5 to 10 mg of testosterone per day. Scrotal patches produce high levels of circulating dihydrotestosterone (DHT) due to the high 5-alpha-reductase enzyme activity of scrotal skin. The advantages include ease of use and maintenance of relatively uniform serum testosterone levels over time,67 in addition to their efficacy in providing adequate testosterone replacement therapy.68 A reservoir-type transdermal delivery system of testosterone (TS) was developed using an ethanol/water (70:30) cosolvent system as the vehicle. This device is available in Europe as a body patch without reservoir and applied every 2 days.69
Skin irritation may be associated with the use of transdermal systems especially with testosterone patches but is uncommon with gel preparations.70 At present the two testosterone gel preparations (Androgel® and Testim®) are the most commonly used in the United States.
Sublingual and buccal
Cyclodextrin-complexed testosterone sublingual formulation is absorbed rapidly into circulation, where testosterone is released from the cyclodextrin shell.71 This formulation has been suggested to have a good therapeutic potential, after adjustment of its kinetics, to produce physiologic levels of testosterone.
A novel sustained-release mucoadhesive buccal testosterone tablet (Striant®; Columbia Laboratories, Inc.), delivering 30 mg, applied to the upper gum region above the lateral incisors twice daily, in the morning and evening, approximately 12 hours apart. This formulation has been shown to restore serum testosterone concentrations to the physiological range within 4 hours of application, with steady-state concentrations achieved within 24 hours of twice-daily dosing72 and achieves testosterone levels within the normal range.73 Studies indicate that Striant® is an effective, well-tolerated, convenient and discreet treatment for male hypogonadism.74 However, it has had minimal clinical uptake, due to the difficulty of maintaining the buccal treatment in the mouth.
Subdermal implants
Subdermal testosterone implants still offer the longest duration of action with prolonged zero-order, steady-state delivery characteristics lasting 4 to 7 months.75,76 The standard dosage is four 200 mg pellets (800 mg) implanted subdermally at intervals of 5 to 7 months.77 Yet the in vivo testosterone release rate of these testosterone pellets and its determinants have not been studied systematically. However the risk of infection at the implant site and extrusion of the pellets which occurs in 5% to 10% of cases even with the most experienced limit their use.
Benefits of testosterone replacement therapy
Restoring testosterone levels to within the normal range by using testosterone replacement therapy can improve many of the effects of hypogonadism. Most importantly, these include beneficial effects on mood, energy levels and patients’ sense of well-being, sexual function, lean body mass and muscle strength, erythropoiesis and bone mineral density (BMD), cognition and some benefits on cardiovascular risk factors. These are summarized in Table 2. Testosterone is well known to help in libido, bone density, muscle mass, body composition, mood, erythropoiesis, and cognition. All these benefits made testosterone replacement therapy in the United States increase substantially over the past several years, with an increase of more than 500% in prescription sales of testosterone products since 1993.
Table 2.
Potential benefits of testosterone replacement therapy
| Improve sexual desire and function |
| Increase bone mineral density |
| Improve mood, energy and quality of life |
| Change body composition and improve muscle mass and strength |
| Improve cognitive function |
Improved sexual desire, function and performance
The prevalence of erectile dysfunction increases markedly with age.78,79 Serum-free testosterone was significantly correlated with erectile and orgasmic function domains of the International Index of Erectile Function (IIEF) questionnaire. Men with greater sexual activity had higher bio-T levels than men with a lower frequency and androgen deficiency may contribute to the age-related decline in male sexuality; correspondingly low levels of bio T were associated with low sexual activity.80,81 Compared with younger men, elderly men require higher levels of circulating testosterone for libido and erectile function.82,83 However, erectile dysfunction and/or diminished libido with or without a testosterone deficiency, might be related to other comorbidities or medications.84
Men with erectile dysfunction (ED) and/or diminished libido and documented testosterone deficiency are candidates for testosterone therapy. Adequate testosterone treatment can restore venous leakage in the corpus cavernosum which is a frequent etiological factor in ED in elderly men.85 Overviews of randomized controlled clinical trials indicate some benefit of testosterone therapy on sexual health-related outcomes; however further investigation in this area is warranted particularly in older men who are not clearly hypogonadal.86,87 Long-term follow-up of testosterone replacement in hypogonadal males and a control group indicates that self-assessment of libido was significantly higher in the testosterone-treated group.88 Testosterone replacement has also been shown to enhance libido and the frequency of sexual acts and sleep-related erections.89,90 Wang et al67 also reported improvement of sexual function; however, their data suggest that there is a threshold level of T above which there is no further enhancement of response. On the other hand, Two placebo-controlled trials reported on the effect of testosterone on overall sexual satisfaction, yielding imprecise results.91,92 Transdermal testosterone replacement therapy, in particular, has been linked to positive effects on fatigue, mood, and sexual function, as well as significant increases in sexual activity.93 1% hydroalcoholic testosterone gel is an effective and safe treatment option in subjects with ED.94
In the presence of a clinical picture of testosterone deficiency and borderline serum testosterone levels, a short therapeutic trial may be tried.95 An inadequate response to testosterone treatment requires reassessment of the causes of the erectile dysfunction.45 There is evidence that the combined use of testosterone and phosphodiesterase type 5 inhibitors in hypogonadal or borderline eugonadal men have a synergetic effect.96–101 The combination treatment should be considered in hypogonadal patients with erectile dysfunction (ED) failing to respond to either treatment alone. Testosterone produces this effect by enhancing the production of nitric oxide synthase. It is unclear whether PDE5-I, testosterone, or the combination of the two should be started first in men with hypogonadism and ED.45 All men with ED should be screened for hypogonadism before treating for erectile dysfunction.102
In addition to improvement in sexual function, testosterone therapy may also improve lower urinary tract symptomatology (LUTS)/bladder functions by increasing bladder capacity and compliance and decreasing detrusor pressure at maximal flow in men with SLOH.103 However, the role of testosterone supplementation in men with erectile dysfunction who are not androgen deficient or in the low to normal range needs further investigation to determine whether testosterone therapy will improve erectile function in older men and to weigh the risk–benefit ratio for testosterone therapy in this setting.86
Bone mineral density
Chronically ill men may have both apparent androgen deficiency and low bone mass.104 Osteopenia, osteoporosis, and fracture prevalence rates are greater in hypogonadal younger and older men.105 The prevalence of osteoporosis in testosterone deficient males is double that of those with normal testosterone level.106,107 Testosterone plays a major role in BMD.108 Bone density in hypogonadal men of all ages increases under testosterone substitution provided the dose is high enough;109–111 although normal adult bone mass is not reached.112 Testosterone produces this effect by increasing osteoblastic activity and through aromatization to estrogen reducing osteoclastic activity. The role of the partial androgen deficiency in aging males in bone fracture rate remains to be established.113 Patients with prostate cancer treated with androgen deprivation therapy have an increased risk of osteoporotic fracture.114 The long-term benefit of testosterone requires further investigation. However, the correlation with bioestradiol, the levels of which decline in elderly males, was even stronger, suggesting that part of the androgen effects on bone are at least partially indirect, mediated via their aromatization.115,116 An increase in osteocalcin levels, an index of osteoblast activity was observed,90 and a decrease of hydroxyproline excretion, an index of bone resorption also was noted.117
Assessment of bone density at 2-year intervals is advisable in hypogonadal men, and serum testosterone measurements should be obtained in all men with osteopenia.118,119 Trials of the effects of testosterone replacement therapy on bone mineral density yielded mixed results.120 Increases in spinal bone density have been realized in hypogonadal men,121 with most treated men maintaining bone density above the fracture threshold.122 An improvement in both trabecular and cortical bone mineral density of the spine was seen, independent of age and type of hypogonadism; in addition, a significant increase in paraspinal muscle area has been observed, emphasizing the clinical benefit of adequate replacement therapy for the physical fitness of hypogonadal men.123 The pooled results of a meta-analysis suggest a beneficial effect on lumbar spine bone density and equivocal findings on femoral neck BMD. Trials of intra muscular testosterone reported significantly larger effects on lumbar bone density than trials of transdermal testosterone, particularly among patients receiving chronic glucocorticoids.124 Also in eugonadal men with osteoporosis, Testosterone esters (250 mg/2 weeks) increased BMD;125 again, the effects in elderly men are less convincing, though the 3 year study of Amory et al126 showed a significant increase in hip BMD. None of studies have been large enough to show a fracture risk reduction with testosterone replacement therapy.
Improved body composition and muscle mass and strength
The aging process is accompanied by significant changes in body composition characterized by decreased fat free mass and increased and redistributed fat mass. These changes can impose functional limitations and increase morbidity.127,128 Maximal muscle strength correlates with muscle mass independently of age.129 In men, declining testosterone levels that occur with aging can be a contributing factor to these changes by direct effect on muscle cells by testosterone or by stimulating IGF-1 expression directly and indirectly leading to increased muscle protein synthesis and growth.130 Epidemiologic studies have demonstrated a correlation between bioavailable testosterone concentrations and fat-free mass;131 however the correlation with grip strength is not clear.131,132
Testosterone replacement may be effective in reversing age-dependent body composition changes and associated morbidity.133 Testosterone administration improves body composition decrease of fat mass, increase of lean body mass.134–136 In most of these studies, the body weight change did not differ significantly. After androgen supplementation to elderly men, generally at a biweekly dose of 200 mg T enanthate, there is a relatively modest increase in muscle mass (±2 kg)117 and/or arm circumference and grip strength, whereas fat mass was decreased modestly.137–139
Testosterone therapy was associated with a greater improvement in grip strength than placebo.135,139,140 Changes in lower-extremity muscle strength and measures of physical function were reported in only a few studies and were inconsistent. Recent cross-sectional studies showed that in aging men there are also positive correlations between testosterone and muscle strength parameters of upper and lower extremities, as measured by leg extensor strength and isometric hand grip strength.141 Moreover, testosterone was positively associated with functional parameters, including the doors test as well as “get up and go” test, and 5-chair sit/stand test.142 On the other hand, some reported an increase in lean body mass (LBM) but no change in physical function,92 or an increase in strength of knee extension or flexion.
Although there is a potential role of testosterone in the management of frailty, we do not know whether testosterone replacement improves physical function and other health-related outcomes, or reduces the risk of disability, falls, or fractures in older men with low testosterone levels in addition to the long-term risks and benefits of testosterone supplementation in older men.143
Mood and energy and quality of life
Men older than 50 years with low free testosterone levels had poorer quality of life. Hypogonadal men commonly complain of loss of libido, dysphoria, fatigue, and irritability.67,70,144,145 These symptoms overlap with signs and symptoms of major depression. There is significant inverse correlation between bioavailable testosterone and a depression score in elderly men, independent of age and weight but not with total testosterone levels.146 There was a reduced libido and reduced feelings of well being and minimal effect on mood in patients with induced testosterone deficiency; the depressive symptoms during the hypogonadal state were reversed by testosterone replacement.147 One study showed that the relationship between testosterone level and depression was nonlinear and may be idiosyncratic.148 The risk of depression was increased for hypogonadal and hypergonadal men, but this effect was only detectable in underweight and obese men, not in ‘normal’ weight men in whom a linear decrease in the risk of depression with increasing testosterone level was noted.149
Testosterone replacement therapy has variable effects on mood, energy and sense of well being. The results of placebo-controlled randomized trials on testosterone’s effect on quality of life and depressive mood were inconsistent across trials and imprecise.150–152 Testosterone administered to nondepressed eugonadal men at physiological doses, did not result in significant effects on mood.153,154 Administration of supraphysiological doses of testosterone to eugonadal men has been associated with mania in a small proportion of men.155,156
In hypogonadal men, testosterone replacement was associated with improved mood and well-being, and reduced fatigue and irritability.157–159 Randomized controlled trials of testosterone therapy in men without or with underlying chronic illness using a variety of testosterone formulations report equivocal improvements in quality of life measures, including general well-being and fatigue.41,86,160 Testosterone replacement of hypogonadal men with MDD might be an effective antidepressant161,162 or augmentation to partially effective antidepressant,163 Testosterone gel had significantly greater improvement as augmentation therapy in depressive symptoms than subjects receiving placebo in hypogonadal men with selective serotonin reuptake inhibitor (SSRI) partial response.164 In one study, the improvements in mood persisted.67 These significant correlations with T levels were only observed when T levels were below the normal range, which suggests that once a minimally adequate T/dihydrotestosterone (DHT) level was achieved, further increase did not further contribute to improvement of mood.67,157 However, the studies reported tend to be of limited size and duration, with a lack of large-scale trials with extended long-term follow-up. In HIV-infected men, testosterone therapy was well tolerated and effective in ameliorating symptoms of hypogonadism. For patients with major depression and/or dysthymia, improvement was equal to that achieved with standard antidepressants with significant improvement in depression inventory score. This effect may be a direct effect of testosterone or related to positive effects of testosterone on weight and/or other anthropometric indices. Additional studies are needed to assess the effects of testosterone on clinical depression indices in human immunodeficiency virus-infected patients.165,166
On the other hand, several placebo-controlled testosterone replacement studies did not show a testosterone-placebo difference distinguishable with respect to mood.150,164,167,168 No relationship between testosterone level and depressive symptoms was found in the Massachusetts Male Aging Study (MMAS).14 This discrepancy in the results of the effects of testosterone replacement therapy on mood may be explained by the genetic polymorphism in the androgen receptor which defines a vulnerable group in whom depression is expressed when testosterone levels fall below a particular threshold.169,170
Finally, the available controlled studies using exogenous testosterone for depression in elderly men are limited, and testosterone treatment must be considered experimental. The best candidates for treatment may be hypogonadal men who are currently taking an existing antidepressant with inadequate response.171–173 Additional studies have been recommended incorporating vitality, well-being and/or quality of life as end points.
Cognitive function
Subclinical androgen deficiency was hypothesized to enhance the expression of AD related peptides in vivo.174 Age-related decreases in bioavailable T predicted age-related decline in visual and verbal memory.175 Men with a higher ratio of total testosterone to SHBG predict a reduced incidence of Alzheimer’s disease176 and patients with Alzheimer’s disease had a lower ratio of total testosterone to SHBG compared with age-matched controls.177 In the Baltimore Longitudinal Study of Aging (BLSA), a prospective longitudinal study176 risk for AD was reduced by 26% for each 10 unit (nmoL/nmoL) increase in free T at 2, 5 and 10 years prior to AD diagnosis. Altered T levels in AD may precede rather than follow diagnosis.
There is good evidence for a strong correlation between T levels and cognitive performance such as spatial abilities or mathematical reasoning.178 Higher bioavailable and free testosterone concentrations have each been associated with better performance in specific aspects of memory and cognitive function, with optimal processing capacity found in men ranging from 35 to 90 years of age even after adjustment for potential confounders including age, educational attainment and cardiovascular morbidity;179–181 whereas total testosterone was not.182 However, contradictory findings have also been reported, two cross-sectional studies did not show a relationship between total or free testosterone and measures of working memory, speed/attention or spatial relations in men aged from 48 to 80 years.184 In another cross-sectional analyses of similarly aged men, no association was found between lower free testosterone levels and higher performance on spatial visualization tasks, and between higher free and total testosterone levels and poorer verbal memory and executive performance; however there is correlation with faster processing speed.184,185 A possible source of conflicting results in these studies may stem from interactions between testosterone levels and other risk factors for cognitive impairment such as apolipoprotein E 4 genotype186 and systemic illness which cause low testosterone.24,177,187
In men undergoing hormonal therapy for prostate cancer, suppression of endogenous testosterone synthesis and blockade of the androgen receptor resulted in a beneficial effect on verbal memory but an adverse effect on spatial ability188 and visuomotor slowing and slowed reaction times in several attentional domains;189 plasma amyloid levels increased as T levels decreased.190 Discontinuation of treatment resulted in improved memory but not visuospatial abilities.190 One of the possible protective mechanisms of action of T would be through its conversion into estradiol (E2), the most potent estrogen. Both serum E2 and T levels were lower in men with AD compared to age-matched controls.191,192 E2 could exert protective effects on the brain structures in aging patients.193
Trials of testosterone therapy in men to evaluate its effects on measures of cognitive function and memory to date were all relatively small and of a relatively short duration and have shown mixed results.177,194 Small-scale T intervention trials in elderly men suggest that some cognitive deficits may be reversed, at least in part, by short term T supplementation.178Androgen supplementation in elderly hypogonadal men improves spatial cognition117,195 and verbal fluency196,197 and in elderly men without dementia, it may reduce working memory errors.198 In addition, transdermal testosterone or DHT treatment in men aged 34 to 70 years improved verbal memory and spatial memory respectively,199 and intramuscular testosterone improved verbal and spatial memory and constructional abilities in non-hypogonadal men with mild cognitive impairment and Alzheimer’s disease.200 In one study of healthy men aged 50 to 90 years, intramuscular testosterone alone or in combination with anastrozole improved spatial memory, whereas verbal memory only improved in testosterone-treated men in the absence of anastrozole, raising the possibility that part of the effect of exogenous testosterone is mediated by its aromatization to estradiol.200
However, in men with Alzheimer’s disease testosterone treatment appeared to improve quality of life without impacting on measures of cognition.201 In a randomized, placebo-controlled crossover trial intramuscular testosterone therapy resulted in decreased verbal memory.202 In other placebo-controlled randomized trials, one of which studied patients with Alzheimer’s dementia and low testosterone levels,203 reported imprecise effects on several dimensions of cognition, none of which was significant after pooling.203,204
Therefore, although the evidence from observational studies is not uniform, lower free testosterone appears to be associated with poorer outcomes on measures of cognitive function, particularly in older men and testosterone therapy in hypogonadal men may have some benefit for cognitive performance.
Improving metabolic syndrome and diabetes type 2, cardiovascular disease
Many of the components of the metabolic syndrome (obesity, hypertension, dyslipidemia impaired glucose regulation, and insulin resistance) are also present in hypogonadal men. Lower testosterone levels are associated with surrogate markers for cardiovascular disease, including less favorable carotid intima medial thickness205–207 ankle/brachial index as a measure of peripheral arterial disease208 and calcific aortic atheroma.209 Endogenous testosterone concentrations are inversely related to mortality due to cardiovascular disease and all causes. Low testosterone may be a predictive marker for those at high risk of cardiovascular disease.8 Thus, lower testosterone levels, erectile dysfunction and conditions associated with higher cardiovascular risk appear to be interrelated.210,211 Obesity induces a decrease of T levels via a decrease in SHBG levels, and morbid obesity also induces a decrease of FT.212 There is evidence to suggest that an inverse relationship exists between serum testosterone levels and the degree of obesity in men.213,214 There is a close relationship between obesity and low serum testosterone levels in healthy men.215 Twenty percent to 64% of obese men have a low serum total or free testosterone level.216 Visceral obesity is more strongly inversely related to total and free testosterone levels than other forms of obesity.217,218 The Relationship between reduced testosterone and obesity in men can be explained by the hypogonadal-cytokine-obesity cycle219,220 and it exhibits antiatherogenic effects at the tissue level, whether mediated by classical or nonclassical pathways.221,222
Initially cross-sectional studies but later also longitudinal studies were able to confirm that low testosterone levels and sex hormone-binding globulin (SHBG) were predictive of the metabolic syndrome, not only in obese men but also in men with a body mass index (BMI) <25 kg/m 2.223,224 The metabolic syndrome and type 2 diabetes mellitus are associated with low plasma testosterone34,225,226 and insulin sensitivity.34,227,228 There is positive correlation between serum testosterone levels and insulin sensitivity in men across the full spectrum of glucose tolerance229 and an improvement of insulin sensitivity was noted after replacement.230 Although Abate et al 231 found no association between low plasma levels of bioavailable testosterone and insulin resistance in eugonadal men; a recent larger study (NHANES [National Health and Nutrition Examination Survey]) showed that low free and bioavailable testosterone levels, even in the normal range, are associated with diabetes, independent of adiposity.216 Serum testosterone should be measured in men with type 2 diabetes mellitus with symptoms suggestive of testosterone deficiency. The effects of testosterone administration on glycemic control of men with diabetes mellitus are much less certain.232–234 By increasing lean body mass and reducing fat mass, testosterone therapy modulates insulin resistance and risk of metabolic syndrome.232
Studies on androgen replacement in elderly men on LDL-C and HDL-C are controversial.235,236 The correlation between testosterone levels and HDL-C and insulin sensitivity is only observed within the physiologic male concentration range of testosterone.237 A physiologic dose of androgens did not cause significant change in lipids.238 A recent randomized controlled trial showed decrease in high-density lipoprotein cholesterol.239 A 24-week, multi-center, randomized, parallel-group study by Dobs et al240 of transdermal and intramuscular administration of androgens in 58 men did not detect any significant change in HDL levels or in the ratio of total cholesterol to HDL in either group, apart from the mode of therapy. In addition, testosterone replacement decreases lipoprotein (a).19 The mechanism of the fall in lipids might be related to the decrease in the visceral abdominal fat mass241 under the influence of androgens, which inhibit lipoprotein lipase activity and increase lipolysis242,243 with improvement of insulin sensitivity and mobilization of triglycerides from abdominal fat tissue.244 Supraphysiological T levels induce an increase in LDL-C and a decrease of HDL-C245–247 and may increase the risk of cardiovascular disease.
Low androgen levels increase the risk of cardiovascular disease in men.236 Data are lacking as to whether higher testosterone levels predict reduced incidence of combined nonfatal and fatal major cardiovascular events.101 The inverse correlation between T levels and the severity of coronary artery disease248 may be related to the fact that low androgen levels are accompanied by an accumulation of abdominal visceral fat,249–251 which is known to be associated with increased cardiovascular risk factors252 impaired glucose tolerance, and non-insulin-dependent diabetes mellitus (syndrome X).253
No consistent relationship between the levels of free or total testosterone and coronary atherosclerosis in men undergoing coronary angiography has been observed.151,254 Testosterone-replacement therapy does not increase the incidence of cardiovascular disease or events such as myocardial infarction, stroke, or angina.88 A meta-analysis255 in 2007 concluded that the current available evidence shows no association between testosterone replacement therapy and cardiac events. Transdermal testosterone replacement therapy was found to be beneficial for men with chronic stable angina as they had greater angina-free exercise tolerance than placebo-treated controls.151
However, trials of testosterone therapy generally have not been designed or adequately powered to detect effects on clinically significant cardiovascular events.86,256 The outcome of most studies in men report either a favorable or neutral effect of normal T levels on cardiovascular disease in men. The administration of T in physiological concentration increases coronary blood flow in patients with coronary heart disease.257 Beneficial effects on endothelial function258 and myocardial ischemia have also been demonstrated,248,259,260 but not with cardiovascular mortality.257,258 In addition, testosterone has a negative correlation with fibrinogen, plasminogen activator inhibitor-1261 and a direct effect on several vasoactive factors such as endothelin,262 prostacyclin, thromboxane A2 and platelet aggregation,263 blood coagulation, and fibrinolysis, respectively.248,261
Thus, although lower testosterone levels are associated with higher cardiovascular risk and to an extent with mortality in aging men, randomized controlled clinical trials of adequate size and duration are needed to determine whether testosterone therapy will reduce morbidity and mortality from cardiovascular disease in hypogonadal or eugonadal men.
Improving anemia
Endogenous androgens are known to stimulate erythropoiesis; increase reticulocyte count, blood hemoglobin levels and bone marrow erythropoietic activity in mammals, whereas castration has opposite effects. Testosterone deficiency results in a 10% to 20% decrease in the blood hemoglobin concentration, which can result in anemia.264,265 Young hypogonadal men usually have fewer red blood cells and lower hemoglobin levels than age-matched controls, whilst healthy older men also may have lower hemoglobin than normal young men. The main androgen involvement in the mechanism of normal hematopoiesis is thought to involve direct stimulation of renal production of erythropoietin by testosterone. Moreover, the latter may also act directly on erythropoietic stem cells.265
Risks of testosterone replacement therapy
The risks of testosterone replacement therapy depend upon age, life circumstances, and other medical conditions.26 There is a risk for prostate cancer and worsening symptoms of benign prostatic hypertrophy, liver toxicity and tumor, worsening symptoms of sleep apnea and congestive heart failure, gynecomastia, infertility and skin diseases. Testosterone replacement therapy is not appropriate for men who are interested in fathering a child because exogenous testosterone will suppress the HPT axis. The risks of testosterone replacement therapy are summarized in Table 3.
Table 3.
Potential risks for testosterone replacement therapy
| Stimulate growth of prostate cancer and breast cancer |
| Worsen symptoms of benign prostatic hypertrophy |
| Cause liver toxicity and liver tumor |
| Cause gynecomastia |
| Cause erythrocytosis |
| Cause testicular atrophy and infertility |
| Cause skin diseases |
| Cause or exacerbate sleep apnea |
The prostate and testosterone replacement therapy
In aging men with late-onset hypogonadism, TRT may normalize serum androgen levels but appears to have little effect on prostate tissue androgen levels and cellular functions266 and causes no significant adverse affects on the prostate.267 At the present time, there is no conclusive evidence that testosterone therapy increases the risk of prostate cancer or benign prostatic hyperplasia (BPH).45,268,269
Benign prostatic hyperplasia
It is well known that the presence of androgen is required for the development of benign prostatic hyperplasia. Testosterone supplements increase prostate volume with, eventually mild increase in prostate specific antigen (PSA) levels in old men.90,117,270 Although a meta-analysis58 showed that the total number of prostate events combined was significantly greater in testosterone-treated men than in placebo-treated men, the majority of events are due to prostate biopsy; and the marked reduction in serum testosterone caused by chemical or surgical castration causes reduced prostate volume. At the same time, many studies271–273 have failed to show significant exacerbation of voiding symptoms attributable to benign prostatic hyperplasia during testosterone supplementation, and complications such as urinary retention have not occurred at higher rates than in controls receiving placebo nor has there been any difference in the urine flow rates, postvoiding residual urine volumes, and prostate voiding symptoms with patients receiving treatment in these studies. The poor correlation between prostate volume and urinary symptoms explain this illogicality.
Finally, although there are no compelling data to suggest that testosterone treatment exacerbates lower urinary tract symptoms (LUTS) or promotes acute urinary retention, severe LUTS, due to BPH, represent a relative contraindication which is no longer applicable after successful treatment of lower urinary tract obstruction.45
Prostate cancer
Prostate cancer is well known to be, in the majority of cases, an androgen sensitive disease, and prostate cancer has been treated in patterns designed to lower testosterone levels.270 Androgen replacement therapy is an absolute contraindication. There is also no good evidence that testosterone treatment will convert subclinical prostate cancer to clinically detectable prostate cancer,45 although some case reports reported this correlation.274,275 In a retrospective analysis of hypogonadal men who underwent prostate biopsy prior to testosterone replacement therapy, no increase in the risk of prostate cancer in men was revealed compared to those without prostatic intraepithelial neoplasia at baseline.276
The prevalence of prostate cancer in many studies receiving testosterone replacement therapy was similar to that in the general population.67,277 So far, there is no compelling evidence that testosterone has a causative role in prostate cancer.271,272,278,279 In fact, it should be recognized that prostate cancer becomes more prevalent exactly at the time of a man’s life when testosterone levels decline. At a median of 19 months of TRT hypogonadal patients with a history of prostate cancer had no PSA recurrence and had statistically significant improvements in total testosterone and hypogonadal symptoms. In highly select patients after radical retropubic prostatectomy TRT can be administered carefully and with benefit to hypogonadal patients with prostate cancer.280 Hormonal replacement in patients that underwent castration seems to be feasible in improving intense symptoms associated with androgen deprivation. After 18 months, no evidence of recurrence was noted. It is an experimental alternative for highly symptomatic patients, but the short follow-up and the small number of patients cannot allow for definitive conclusions and should be studied further.281 A recent prospective study282 did not show definitive data to confirm the relationship between serum sex hormones and prostate cancer. There is, however, unequivocal evidence that testosterone can stimulate growth and aggravate symptoms in men with locally advanced and metastatic prostate cancer.283,284 Men successfully treated for prostate cancer and diagnosed with hypogonadism are candidates for testosterone replacement after a prudent interval if there is no clinical or laboratory evidence of residual cancer.285,286 In addition, no effect was found of testosterone replacement therapy on PSA levels287–289 and the change in PSA were not influenced by the mode of testosterone replacement therapy, patient age, or baseline levels of PSA or testosterone.290
The online prostate cancer risk calculator is one of the most commonly used tools to assist the clinician in assessing the risk of prostate cancer.291–293 All men who present for TRT should undergo prostate biopsy if they have abnormal PSA level or abnormal result on digital rectal examination with low threshold to do or repeat prostate biopsy if the PSA level or digital rectal exam changes. Prostatic biopsy or referral to a urologist is recommended if PSA rises above 4.0 ng/mL or if it increases either by more than 1.5 ng/mL/year or by more than 0.75 ng/mL/year over 2 years,294 or if PSA rises by more than 1.0 ng/mL in the first 6 months of treatment or by more than 0.4 ng/mL/year thereafter.295
In summary, there is no convincing evidence that the normalization of testosterone serum levels in men with low levels is deleterious. TRT can be cautiously considered in selected hypogonadal men treated with curative intent for prostate and without evidence of active disease.296
Liver problems
Benign and malignant hepatic tumors, intrahepatic cholestasis, hepatotoxicity, and liver failure have been reported with testosterone replacement therapy.297,298 These unfavorable hepatic effects do not appear to be associated with transdermal or intramuscular injections. For this reason the oral forms of testosterone, with the exception of testosterone undecanoate, are discouraged. Other liver abnormalities associated with TRT include Peliosis hepatis, hepatocellular adenoma, and carcinoma.299
The risk of polycythemia
There is a correlation between high testosterone levels and high hemoglobin, most likely because testosterone stimulates erythropoiesis. Erythrocytosis can develop during testosterone treatment, especially in older men treated by injectable testosterone preparations.45 Hemoglobin at puberty increases 15% to 20% in boys, men have higher levels of hemoglobin than women, and hypogonadism causes a decline in hemoglobin levels that can be restored with testosterone replacement therapy.139,300 But the elevation in hemoglobin above certain levels may have bad outcomes, particularly in elderly, because the increase in blood viscosity could exacerbate vascular disease in the coronary, cerebrovascular, or peripheral vascular circulation, especially in people with other diseases that cause secondary polycythemia, ie, chronic obstructive pulmonary disease.301–303 Injection is associated with higher potentials for erythrocytosis than topical preparations.240 Snyder et al noticed that erythrocytosis occurred in 5.5% of scrotal transdermal users, and the majority of changes took place over the first three months of treatment.92 It has been demonstrated that testosterone dosage correlates with the incidence of erythrocytosis,67 The frequency of polycythemia (hematocrit over 51%) is related most of the time to supra-physiological levels.240 The resolution of erythrocytosis (hematocrit >52%), untreated obstructive sleep apnea, or untreated severe congestive heart failure, is required before starting on testosterone treatment.19,58,304
Periodic hematological assessment is indicated (ie, before treatment, then at 3 to 4 months and at 12 mo in the first year of treatment and annually thereafter). While it is not yet clear what critical threshold is a desirable, dose adjustment and/or periodic phlebotomy may be necessary to keep hematocrit below 52% to 55%.45,58,305
Other effects of testosterone replacement therapy
Psychotic symptoms, excessive libido and aggression, in addition to physical and psychological dependence and withdrawal syndromes have been rarely described with testosterone treatment,306,307 though the validity of testosterone or the cause is uncertain. Kenny et al204 did not find that significant changes in behavior, function, depression, or cognitive performance occurred following 12 weeks of testosterone replacement in men with low testosterone levels and early-to-moderate cognitive impairment.
Gynecomastia is a benign complication of testosterone treatment. It is related to aromatization of testosterone into estradiol in peripheral fat and muscle tissue. Even the ratio of estradiol to testosterone usually remains normal. It occurs especially with testosterone enanthate or cypionate. Dose adjustment may be necessary.
Diminished testicular size and compromised fertility during testosterone replacement therapy occur because of the down-regulation of gonadotropins.308 The administration of exogenous testosterone as a means of male contraception is under study.309 In these men, azoospermia usually results within approximately 10 weeks of beginning therapy. Rebound of the sperm count to baseline levels occurs within six to 18 months of cessation, and subsequent fertility has been demonstrated.310 Supraphysiologic doses of androgens may cause decreased testicular size, and azoospermia.311
Testosterone-replacement therapy has been associated with exacerbation of sleep apnea.312,313 The effect of testosterone is not on the dimensions of the upper airway, but it most likely contributes to sleep disorder breathing by central mechanisms.314 The development of signs and symptoms of obstructive sleep apnea during testosterone therapy warrants a formal sleep study and treatment with continuous positive airway pressure (CPAP) if necessary. If the patient is unresponsive or cannot tolerate CPAP, the testosterone must be reduced or discontinued.
Transdermal testosterone-replacement therapy is associated with a variety of skin reactions, mainly erythema or pruritus, which are more common with patches than with gel preparations.67 Intramuscular injections of testosterone can cause local pain, soreness, bruising, erythema, swelling, nodules, or furuncles.315 Supraphysiologic doses of androgens may cause acne.311
Testosterone is anabolic, and it will cause some nitrogen, sodium and water retention. Edema may be worsened in patients with preexisting cardiac, renal, or hepatic disease. Hypertension has rarely been reported.316
Hyperandrogenism was reported in a female whose partner was using testosterone gel.317
Monitoring patients on testosterone replacement
Laboratory parameters that should be monitored before and during treatment include PSA, hemoglobin, hematocrit, lipid profiles, and liver function tests. Patients should also be monitored for signs of edema, gynecomastia, sleep apnea, lower urinary tract symptoms, and low BMD. There are clinical practice guidelines from the Endocrine Society for monitoring patients receiving TRT.45 Testosterone level, digital rectal exam, PSA, hematocrit, BMD, lipids, and liver function tests should be checked at baseline then evaluate the patient 3 and 6 months after treatment starts and then annually to assess whether symptoms have responded to treatment and whether the patient is suffering from any adverse effects.
Testosterone levels should be monitored 3 months after initiation of testosterone therapy. A mid-morning total serum testosterone level should be obtained. A target range of 400 to 500 ng/dL (14.0 to 17.5 nmol/L) for older men is suggested. However, if there is no symptomatic response, higher levels may be necessary. For injectable testosterone, the serum level can be measured between injections. For men treated with a transdermal testosterone patch, the serum level should be measured 3 to 12 hours after patch application. In patients receiving buccal testosterone tablets, the serum level should be measured immediately before application of a fresh system. Patients on testosterone gel may have levels checked anytime after at least 1 week of therapy.45 In all cases, bioavailable testosterone levels should also be monitored as testosterone therapy lowers SHBG.
The hematocrit should be checked at baseline, at 3 and 6 months, and then annually. If hematocrit is more than 54%, stop therapy until hematocrit decreases to a safe level; evaluate the patient for hypoxia and sleep apnea; reinitiate therapy with a reduced dose. BMD of lumbar spine and/or femoral neck should be measured at baseline every 1 to 2 years of testosterone therapy in hypogonadal men with osteoporosis or low trauma fracture. A digital rectal examination and PSA level should be obtained before initiating treatment, at 3 months, and then in accordance with guidelines for prostate cancer screening, depending on the age and race of the patient. A urologic consultation is mandatory if any of the following is present (Table 4).
Table 4.
When a urological consultation is needed
|
Abbreviation: PSA, prostate specific antigen.
The use of testosterone preparations should be discussed with patients and then closely monitored for efficacy and toxicities.3,318–320 Failure to benefit from clinical manifestations should result in discontinuation of treatment after 3 months for libido and sexual function, muscle function, and improved body fat; and a longer interval for bone mineral density. Further investigation for other causes of symptoms is then mandatory.45
Contraindications to testosterone replacement therapy
Before starting patients on TRT, health care providers must rule out contraindications to treatment (Table 5). The presence of a clinical prostatic carcinoma is an absolute contraindication for HRT and should be carefully excluded by PSA, rectal examination and, eventually, biopsy before starting any therapy.45 There is also no clear recommendation for men successfully treated for prostate cancer who would be potential candidates for testosterone substitution after a ‘prudent’ interval if there is no clinical or laboratory evidence of residual cancer.45
Table 5.
Contraindications to testosterone replacement therapy
| Very high risk of serious adverse outcomes |
| Prostatic carcinoma |
| Breast cancer |
| Prostate nodules or indurations |
| Unexplained prostate-specific antigen (PSA) elevation |
| Erythrocytosis (hematocrit > 50%) |
| Severe lower urinary tract symptoms with benign prostatic hyperplasia with an International Prostate Symptom Score (IPSS) >19 |
| Unstable congestive heart failure (class III or IV) |
| Severe untreated sleep apnea |
The presence of breast cancer is also a contraindication for TRT as well as a prolactinoma, as their growth may be stimulated by HRT.26 Very high risk of serious adverse outcomes, undiagnosed prostate nodules or indurations, unexplained PSA elevation, erythrocytosis (hematocrit > 50%), severe lower urinary tract symptoms with benign prostatic hyperplasia with an International Prostate Symptom Score (IPSS) >19, and unstable congestive heart failure (class III or IV), untreated obstructive sleep apnea are considered as moderate- to high-risk factors for potential adverse outcomes.26,45,319
Conclusion
Male hypogonadism and its treatment is a rapidly evolving area. While male hypogonadism has previously been underdiagnosed, the apparently increasing incidence and expanding range of treatment options may facilitate greater awareness of the condition. The symptoms in the elderly have a complex origin. It may be reasonably assumed that the age-associated decrease in T levels is in part responsible for the symptoms of aging. The benefits and risks of testosterone therapy must be clearly discussed with the patient and assessment of prostate and other risk factors considered before commencing testosterone treatment. There is some benefit from testosterone replacement therapy on muscle mass and strength, fat mass, BMD, sexual function, mood and general sense of well-being. Therefore, it seems logical to consider that in elderly men with subnormal T levels and clinical symptoms suggestive of androgen deficiency, hormone replacement therapy in combination with physical activity (resistance training) and adequate nutrition will result in an optimal increase in muscle strength, BMD, and general sense of well-being. However, data on clinical effects of androgen substitution, such as cardiovascular morbidity and mortality, falls and bone fracture rates are not yet available. Response to testosterone treatment should be assessed. If there is no improvement of symptoms and signs, treatment should be discontinued and the patient investigated for other possible causes of the clinical presentations. The major contraindication for androgen supplementation is the presence of a prostatic carcinoma.
Future of TRT
Hypogonadism may present to a range of specialties. Previously, late-onset hypogonadism has not been well understood. Studies conducted to date have been too small to address potential long-term adverse effects, and there are risks in extrapolating benefit from epidemiological studies. Many questions in the treatment of hypogonadism remain unanswered, and there is a need for large clinical trials; or at least a meta-analysis of the extensive short-term data combined with analysis of long-term clinical experience which many physicians working in the field now have; to assess the long-term benefits and risks of TRT in older men with late-onset hypogonadism.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Nieschlag E, Swerdloff R, Behre HM, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2005;8:56–58. doi: 10.1080/13685530500130969. [DOI] [PubMed] [Google Scholar]
- 2.Morales A, Schulman CC, Tostain J, Wu FCW. Testosterone deficiency syndrome (TDS) needs to be named appropriately – the importance of accurate terminology. Eur Urol. 2006;50:407–409. doi: 10.1016/j.eururo.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350(5):482–492. doi: 10.1056/NEJMra022251. [DOI] [PubMed] [Google Scholar]
- 4.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 5.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 6.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 8.Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study Circulation 2007. 4116232694–2701. [DOI] [PubMed] [Google Scholar]
- 9.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 10.Araujo A, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, McKinlay JB. Sex steroids and all-cause mortality and cause-specific mortality in men. Arch Intern Med. 2007;167:1252–1260. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 11.Lindeman RD, Yau CL, Baumgartner RN, Morley JE, Garry PJ. New Mexico Aging Process Study. Longitudinal study of fasting serum glucose concentrations in healthy elderly. The New Mexico Aging Process Study. J Nutr Health Aging. 2003;7(3):172–177. [PubMed] [Google Scholar]
- 12.Vermeulen A. Androgen replacement therapy in the aging male – a critical evaluation. J Clin Endocrinol Metab. 2001;86:2380–390. doi: 10.1210/jcem.86.6.7630. [DOI] [PubMed] [Google Scholar]
- 13.Bhasin S, Buckwalter JG. Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl. 2001;22:718–731. [PubMed] [Google Scholar]
- 14.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 15.Liu PY, Beilin J, Meier C, Nguyen TV, Center JR, Leedman PJ, et al. Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab. 2007;92:3599–3603. doi: 10.1210/jc.2007-0862. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll E, Lambert LC, Marshall LM, et al. Testosterone and estradiol in older men. J Clin Endocrinol Metab. 2006;91:1336–1344. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- 17.Yeap BB, Almeida OP, Hyde Z, et al. In men older than 70 years, total testosterone remains stable while free testosterone declines with age. The Health in Men Study. Eur J Endocrinol. 2007;156:585–594. doi: 10.1530/EJE-06-0714. [DOI] [PubMed] [Google Scholar]
- 18.Krithivas K, Yurgalevitch SM, Mohr BA, et al. Evidence that the CAG repeat in the androgen receptor is associated with age related decline in serum androgens levels in men. J Endocrinol. 1999;162:137–142. doi: 10.1677/joe.0.1620137. [DOI] [PubMed] [Google Scholar]
- 19.Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle age men: a 13 year follow-up of former Multiple Risk Factors Intervention Trial participants. Am J Epidemiol. 1997;46:609–617. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]
- 20.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 21.Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 22.Wu FCW, Tajar A, Pye SR, et al. Hypothalamic–pituitary–testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: The European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 23.Veldhuis JD. Aging and hormones of the hypothalamo–pituitary axis: gonadotropic axis in men and somatotropic axes in men and women. Ageing Res Rev. 2008;7:189–208. doi: 10.1016/j.arr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 25.Morales A, Spevack M, Emerson L, et al. Adding to the controversy: pitfalls in the diagnosis of testosterone deficiency syndromes with questionnaires and biochemistry. Aging Male. 2007;10:57–65. doi: 10.1080/13685530701342686. [DOI] [PubMed] [Google Scholar]
- 26.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 27.Schiavi RC, Schreiner-Engel P, White D, Mandeli J. The relationship between pituitary-gonadal function and sexual behavior in healthy aging men. Psychosom Med. 1991;53:363–374. doi: 10.1097/00006842-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Travison TG, Morley JE, Araujo AB, O’Donnell AB, McKinlay JB. The relationship between libido and testosterone levels in aging men. J Clin Endocrinol Metab. 2006;91:2509–2513. doi: 10.1210/jc.2005-2508. [DOI] [PubMed] [Google Scholar]
- 29.Morley JE, Kim MJ, Haren MT, Kevorkian R, Banks WA. Frailty and the aging male. Aging Male. 2005;8:135–140. doi: 10.1080/13685530500277232. [DOI] [PubMed] [Google Scholar]
- 30.Baum N, Candace A, Crespi CA. Testosterone replacement in elderly men. Testosterone replacement in elderly men. Geriatrics. 2007;62:15–18. [PubMed] [Google Scholar]
- 31.Shores MM, Moceri VM, Sloan KL, Matsumoto AM, Kivlahan DR. Low testosterone levels predict incident depressive illness in older men: effects of age and medical morbidity. J Clin Pyschiatry. 2005;66:7–14. doi: 10.4088/jcp.v66n0102. [DOI] [PubMed] [Google Scholar]
- 32.Lunenfeld B, Saad F, Hoesl CE. ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late-onset hypogonadism in males: scientific background and rationale. Aging Male. 2005;8:59–74. doi: 10.1080/13685530500163416. [DOI] [PubMed] [Google Scholar]
- 33.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 34.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–4343. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 35.Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239–1242. doi: 10.1053/meta.2000.8625. [DOI] [PubMed] [Google Scholar]
- 36.Morley JE, Perry HM, III, Kevorkian RT, Patrick P. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas. 2006;53:424–429. doi: 10.1016/j.maturitas.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Heinemann LA, Saad F, Heinemann K, Thai DM. Can results of the Aging Males’ Symptoms (AMS) scale predict those of screening scales for androgen deficiency. Aging Male. 2004;7:211–218. doi: 10.1080/13685530400004223. [DOI] [PubMed] [Google Scholar]
- 38.Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol. 2004;46:80–87. doi: 10.1016/j.eururo.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 39.T’Sjoen G, Goemaere S, De Meyere M, Kaufman JM. Perception of males’ aging symptoms, health and well-being in elderly community-dwelling men is not related to circulating androgen levels. Psychoneuroendocrinology. 2004;29:201–214. doi: 10.1016/s0306-4530(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 40.Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab. 2004;89:3813–3817. doi: 10.1210/jc.2004-0143. [DOI] [PubMed] [Google Scholar]
- 41.Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol. 2005;63:601–602. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- 42.Morley JE, Melmed S. Gonadal dysfunction in systematic disorders. Metabolism. 1979;28:1051–1073. doi: 10.1016/0026-0495(79)90010-6. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto AM. Andropause: Clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol Med Sci. 2002;57:76–99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- 44.Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf) 2003;58:710–717. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159:507–514. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Citron JT, Ettinger B, Rubinoff H, et al. Prevalence of hypothalamic-pituitary imaging abnormalities in impotent men with secondary hypogonadism. J Urol. 1996;155:529–533. [PubMed] [Google Scholar]
- 47.Bunch TJ, Abraham D, Wang S, Meikle AW. Pituitary radiographic abnormalities and clinical correlates of hypogonadism in elderly males presenting with erectile dysfunction. Aging Male. 2002;5:38–46. [PubMed] [Google Scholar]
- 48.Rhoden EL, Estrada C, Levine L, Morgentaler A. The value of pituitary magnetic resonance imaging in men with hypogonadism. J Urol. 2003;170:795–798. doi: 10.1097/01.ju.0000082960.84075.54. [DOI] [PubMed] [Google Scholar]
- 49.Buvat J, Lemaire A. Endocrine screening in 1, 022 men with erectile dysfunction clinical significance and cost effective strategy. J Urol. 1997;158:1764–1767. doi: 10.1016/s0022-5347(01)64123-5. [DOI] [PubMed] [Google Scholar]
- 50.Araujo AB, O’Donnell A, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89:5920–5926. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 51.Vermeulen A. Hormonal cut-offs of partial androgen deficiency: a survey of androgen assays. J Endocrinol Invest. 2005;28:28–31. [PubMed] [Google Scholar]
- 52.van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- 53.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–13. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 54.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Plymate S, Nieschlag E, Paulsen CA. Salivary testosterone in men: further evidence of a direct correlation with free serum testosterone. J Clin Endocrinol Metab. 1981;53:1021–1024. doi: 10.1210/jcem-53-5-1021. [DOI] [PubMed] [Google Scholar]
- 56.Morley JE, Perry HM, III, Patrick P, Dollbaum CM, Kells JM. Validation of salivary testosterone as a screening test for male hypogonadism. Aging Male. 2006;9:165–169. doi: 10.1080/13685530600907993. [DOI] [PubMed] [Google Scholar]
- 57.Gonacharov N, Katsya G, Dobracheva A, Nizhnik A, Kolesnikova G, Herbst V, Westermann J. Diagnostic significance of free salivary testosterone measurement using a direct luminescence immunoassay in healthy men and in patients with disorders of androgenic status. Aging Male. 2006;9:111–122. doi: 10.1080/13685530600713060. [DOI] [PubMed] [Google Scholar]
- 58.Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 59.Parsons JK, Carter HB, Platz EA, Wright EJ, Landis P, Metter EJ. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005;14:2257–2260. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 60.Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92:3844–3853. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- 61.Velazquez I, Alter BP. Androgens and liver tumors: Fanconi’s anemia and non-Fanconi’s conditions. Am J Hematol. 2004;77(3):257–267. doi: 10.1002/ajh.20183. [DOI] [PubMed] [Google Scholar]
- 62.Imamoto T, Suzuki H, Fukasawa S, et al. Pretreatment serum testosterone level as a predictive factor of pathological stage in localized prostate cancer patients treated with radical prostatectomy. EurUrol. 2005;47:308–312. doi: 10.1016/j.eururo.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 63.McClure RD, Oses R, Ernest ML. Hypogonadal impotence treated by transdermal testosterone. Urology. 1991;37(3):224. doi: 10.1016/0090-4295(91)80289-j. [DOI] [PubMed] [Google Scholar]
- 64.Bhasin S, Bremner WJ. Emerging issues in androgen replacement therapy. J Clin Endocrinol Metab. 1997;82:3–8. doi: 10.1210/jcem.82.1.3640. [DOI] [PubMed] [Google Scholar]
- 65.Comhaire FH. Andropause: hormone replacement therapy in the aging male. EurUrol. 2000;38:655–662. doi: 10.1159/000020358. [DOI] [PubMed] [Google Scholar]
- 66.Von Eckardstein, Nieschlag E. Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: a phase II study. J Androl. 2002;23(3):419–425. [PubMed] [Google Scholar]
- 67.Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2839–2853. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- 68.Yu Z, Gupta SK, Hwang SS, et al. Testosterone pharmacokinetics after application of an investigational transdermal system in hypogonadal men. J Clin Pharmacol. 1997;37:1139–1145. doi: 10.1002/j.1552-4604.1997.tb04298.x. [DOI] [PubMed] [Google Scholar]
- 69.Kim MK, Zhao H, Lee CH, Kim DD. Formulation of a reservoir-type testosterone transdermal delivery system. Int J Pharm. 2001;219(1–2):51–59. doi: 10.1016/s0378-5173(01)00631-7. [DOI] [PubMed] [Google Scholar]
- 70.McNicholas TA, Dean JD, Mulder H, Carnegie C, Jones NA. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU Int. 2003;91:69–74. doi: 10.1046/j.1464-410x.2003.04016.x. [DOI] [PubMed] [Google Scholar]
- 71.Salehian B, Wang C, Alexander G, et al. Pharmacokinetics, bioefficacy, and safety of sublingual testosterone cyclodextrin in hypogonadal men: comparison to testosterone enanthate – a clinical research center study. J Clin Endocrinol Metab. 1995;80:3567–3575. doi: 10.1210/jcem.80.12.8530600. [DOI] [PubMed] [Google Scholar]
- 72.Korbonits M, Slawik M, Cullen D, Ross RJ. A comparison of a novel testosterone bioadhesive buccal system, striant, with a testosterone adhesive patch in hypogonadal males. J Clin Endocrinol Metab. 2004;89:2039–2043. doi: 10.1210/jc.2003-030319. [DOI] [PubMed] [Google Scholar]
- 73.Ross CN, French JA, Patera KJ. Intensity of aggressive interactions modulates testosterone in male marmosets. Physiol Behav. 2004;83(3):437–445. doi: 10.1016/j.physbeh.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 74.Wang C, Swerdloff R, Kipnes M, Matsumoto AM. New testosterone buccal system (Striant) delivers physiological testosterone levels: pharmacokinetics study in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3821–3829. doi: 10.1210/jc.2003-031866. [DOI] [PubMed] [Google Scholar]
- 75.Jockenhovel F, Vogel E, Kreutzer M, Reinhardt W, Lederbogen S, Reinwein D. Pharmacokinetics and pharmacodynamics of subcutaneous testosterone implants in hypogonadal men. Clin Endocrinol. 1996;45:61–71. doi: 10.1111/j.1365-2265.1996.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 76.Kelleher S, Conway AJ, Handelsman DJ. Influence of implantation site and track geometry on the extrusion rate and pharmacology of testosterone implants. Clin Endocrinol. 2001;55:531–536. doi: 10.1046/j.1365-2265.2001.01357.x. [DOI] [PubMed] [Google Scholar]
- 77.Handelsman DJ. Clinical pharmacology of testosterone pellet implants. In: Nieschlag E, Behre HM, editors. Testosterone action deficiency substitution. 2nd ed. Berlin: Springer-Verlag; 1998. pp. 349–364. [Google Scholar]
- 78.Lyngdorf P, Hemmingsen L. Epidemiology of erectile dysfunction and its risk factors: a practice-based study in Denmark. Int J Impot Res. 2004;16:105–111. doi: 10.1038/sj.ijir.3901184. [DOI] [PubMed] [Google Scholar]
- 79.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–157. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 80.Schiavi RC, Schreiner-Engel P, White D, Mandeli J. Pituitary-gonadal function during sleep in men with hypoactive sexual desire and in normal controls. Psychosomat Med. 1988;50:304–318. doi: 10.1097/00006842-198805000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Nilsson P, Moller L, Solkad K. Adverse effects of psychosocial stress on gonadal function and insulin levels in middle aged males. J Intern Med. 1995;237:479–486. doi: 10.1111/j.1365-2796.1995.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 82.Schiavi RC, Rehman J. Sexuality and aging. Urol Clin North Am. 1995;22:711–726. [PubMed] [Google Scholar]
- 83.Gray PB, Singh AB, Woodhouse LJ, et al. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J Clin Endocrinol Metab. 2005;90:3838–3846. doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- 84.Morales A, Buvat J, Gooren LJ, et al. Endocrine aspects of sexual dysfunction in men. J Sex Med. 2004;1:69–81. doi: 10.1111/j.1743-6109.2004.10111.x. [DOI] [PubMed] [Google Scholar]
- 85.Yassin AA, Saad F. Dramatic improvement of penile venous leakage upon testosterone administration. A case report and review of literature. Andrologia. 2006;38:34–37. doi: 10.1111/j.1439-0272.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 86.Liverman CT, Blazer DG, editors. Testosterone and aging: clinical research directions. Washington, DC: Institute of Medicine. The National Academies Press; 2004. [PubMed] [Google Scholar]
- 87.Krause W, Mueller U, Mazur A. Testosterone supplementation in the aging male: which questions have been answered? Aging Male. 2005;8:31–38. doi: 10.1080/13685530500048872. [DOI] [PubMed] [Google Scholar]
- 88.Hajjar RR, Kaiser FE, Morley JE. Outcomes of long-term testosterone replacement in older hypogonadal males: a retrospective analysis. J Clin Endocrinol Metab. 1997;82:3793. doi: 10.1210/jcem.82.11.4387. [DOI] [PubMed] [Google Scholar]
- 89.Shabsigh R. The effects of testosterone on the cavernous tissue and erectile function. World J Urol. 1997;15:21–26. doi: 10.1007/BF01275152. [DOI] [PubMed] [Google Scholar]
- 90.Morley JE, Perry HM, Kaiser FE, et al. Effect of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. 1993;41:149–152. doi: 10.1111/j.1532-5415.1993.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 91.Cavallini G, Caracciolo S, Vitali G, Modenini F, Biagiotti G. Carnitine versus androgen administration in the treatment of sexual dysfunction, depressed mood, and fatigue associated with male aging. Urology. 2004;63:641–646. doi: 10.1016/j.urology.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 92.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 93.Meikle AW, Arver S, Dobs AS, Sanders SW, Mazer NA. Androderm: A permeation-enhanced, non-scrotal testosterone transdermal system for the treatment of male hypogonadism. In: Bhasin, editor. Pharmacology, biology, and clinical applications of androgens. New York, NY: Wiley Liss, Inc; 1996. pp. 449–457. [Google Scholar]
- 94.Corona G, Petrone L, Fisher AD, Mansani R, Bandini E. Six-month administration of 1% testosterone gel is able to restore erectile function in hypogonadal patients with erectile dysfunction. Arch Ital Urol Androl. 2008;80(3):103–108. [PubMed] [Google Scholar]
- 95.Black AM, Day AG, Morales A. The reliability of clinical and biochemical assessment in symptomatic late-onset hypogonadism: Can a case be made for a 3-month therapeutic trial? BJU Int. 2004;94:1066–1070. doi: 10.1111/j.1464-410X.2004.05105.x. [DOI] [PubMed] [Google Scholar]
- 96.Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol. 2004;172:658–663. doi: 10.1097/01.ju.0000132389.97804.d7. [DOI] [PubMed] [Google Scholar]
- 97.Greenstein A, Mabjeesh NJ, Sofer M, Kaver I, Matzkin H, Chen J. Does sildenafil combined with testosterone gel improve erectile dysfunction in hypogonadal men in whom testosterone supplement therapy alone failed? J Urol. 2005;173:530–532. doi: 10.1097/01.ju.0000149870.36577.05. [DOI] [PubMed] [Google Scholar]
- 98.Behre HM. Testosterone and erection. In: Nieschlag E, Behre HM, editors. Testosterone: action, deficiency, substitution. Cambridge: Cambridge University Press; 2004. pp. 333–346. [Google Scholar]
- 99.Kalintchenko SY, Kozlov GI, Gontcharov NP, et al. Oral testosterone undecanoate reverses erectile dysfunction associated with diabetes mellitus in patients failing on sildenafil citrate therapy alone. Aging Male. 2003;6:94–99. [PubMed] [Google Scholar]
- 100.Greco EA, Spera G, Aversa A. Combining testosterone and PDE5 inhibitors in erectile dysfunction: basic rationale and clinical evidences. Eur Urol. 2006;50:940–947. doi: 10.1016/j.eururo.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 101.Aversa A, Isidori AM, Greco EA, Giannetta E, Gianfrilli D, Spera E, et al. Hormonal supplementation and erectile dysfunction. Eur Urol. 2004;45:535–538. doi: 10.1016/j.eururo.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Miner M, Canty DJ, Shabsigh R. Testosterone replacement therapy. Postgrad Med. 2008;120(3):130–153. doi: 10.3810/pgm.2008.09.1914. [DOI] [PubMed] [Google Scholar]
- 103.Karazindiyanoğlu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male. 2008;11(3):146–149. doi: 10.1080/13685530802290438. [DOI] [PubMed] [Google Scholar]
- 104.Fairfield WP, Finkelstein JS, Klibanski A, Grinspoon SK. Osteopenia in eugonadal men with acquired immune deficiency syndrome wasting syndrome. J Clin Endocrinol Metab. 2001;86:2020–2026. doi: 10.1210/jcem.86.5.7515. [DOI] [PubMed] [Google Scholar]
- 105.Meier C, Nguyen TV, Handelsman DJ, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008;168:47–54. doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 106.Kenny AM, Prestwood KM, Marcello KM, Raisz LG. Determinants of bone density in healthy older men with low testosterone levels. J Gerontol A Biol Sci Med Sci. 2000;55:M492–M497. doi: 10.1093/gerona/55.9.m492. [DOI] [PubMed] [Google Scholar]
- 107.Fink HA, Ewing SK, Ensrud KE, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91(10):3908–39015. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 108.Compston JE. Sex steroids and bone. Physiol Rev. 2001;81(1):419–447. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- 109.Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 110.Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res. 2008;20(4):378–387. doi: 10.1038/ijir.2008.19. [DOI] [PubMed] [Google Scholar]
- 111.Behre HH, Kliesch S, Leifke E, Link TM, Nieschlag E. Long term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2386–2390. doi: 10.1210/jcem.82.8.4163. [DOI] [PubMed] [Google Scholar]
- 112.Saggese G, Bertelloni S, Baroncelli GI. Sex steroids and acquisition of bone mass. Horm Res. 1997;48:65–72. doi: 10.1159/000191331. [DOI] [PubMed] [Google Scholar]
- 113.Center JR, Nguyen TV, Sambrook PN, Eisman JA. Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. J Clin Endocrinol Metab. 1999;84:3626–3635. doi: 10.1210/jcem.84.10.6051. [DOI] [PubMed] [Google Scholar]
- 114.Holzbeierlein JM, Castle E, Thrasher JB. Complications of androgen deprivation therapy: prevention and treatment. Oncology (Williston Park) 2004;18(3):303–309. [PubMed] [Google Scholar]
- 115.Van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85(9):3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- 116.Michael H, Härkönen PL, Väänänen HK, Hentunen TA. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res. 2005;20(12):2224–2232. doi: 10.1359/JBMR.050803. [DOI] [PubMed] [Google Scholar]
- 117.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75:1092–1098. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 118.Freitas SS, Barrett-Connor E, Ensrud KE, et al. Rate and circumstances of clinical vertebral fractures in older men. Osteoporos Int. 2008;19:615–623. doi: 10.1007/s00198-007-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schousboe JT, Taylor BC, Fink HA, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA. 2007;298:629–637. doi: 10.1001/jama.298.6.629. [DOI] [PubMed] [Google Scholar]
- 120.Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:266–272. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- 121.De Rosa M, Paesano L, Nuzzo V, et al. Bone mineral density and bone markers in hypogonadotropic and hypergonadotropic hypogonadal men after prolonged testosterone treatment. J Endocrinol Invest. 2001;24(4):246–252. doi: 10.1007/BF03343854. [DOI] [PubMed] [Google Scholar]
- 122.Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2386. doi: 10.1210/jcem.82.8.4163. [DOI] [PubMed] [Google Scholar]
- 123.Leifke E, Korner HC, Link TM, Behre HM, Peters PE, Nieschlag E. Effects of testosterone replacement on cortical and trabecular bone mineral density, vertebral body area and paraspinal muscle area in hypogonadal men. Eur J Endocrinol. 1991;38:51–58. doi: 10.1530/eje.0.1380051. [DOI] [PubMed] [Google Scholar]
- 124.Michal J, Tracz, Sideras K, Bolon ERA. Clinical review: testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 91(6):2011–2016. doi: 10.1210/jc.2006-0036. [DOI] [PubMed] [Google Scholar]
- 125.Anderson FH, Francis RM, Faulkner K. Androgen supplementation in eugonadal men with osteoporosis. Effects of 6 months of treatment on bone mineral density and cardiovascular risk factors. Bone. 1996;18:171–178. doi: 10.1016/8756-3282(95)00441-6. [DOI] [PubMed] [Google Scholar]
- 126.Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrniol Metab. 2004;89:503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 127.Bhasin S. regulation of body composition by androgens. J Endocrinol Invest. 2003;26(9):814–822. doi: 10.1007/BF03345230. [DOI] [PubMed] [Google Scholar]
- 128.Frontera WR, Hughes VV, Fiatarone MA, Fielding RA. Aging and skeletal muscle: a 12 year longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 129.Reed RL, Pearlmutter L, Jochum K, Meredith KE, Mooradian AD. The relationship between muscle mass and muscle strength in the elderly. J Am Geriatr Soc. 1991;39:555–591. doi: 10.1111/j.1532-5415.1991.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 130.Mauras N, Hayes V, Welch S, et al. Testosterone deficiency in young men; marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;38(6):1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 131.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 132.Verhaar HJJ, Samson MM, Aleman A, de Vries WR, de Vreede PL, Koppeschaar HPF. The relationship between indices of muscle function and circulating anabolic hormones in healthy. Aging Male. 2000;3:75–80. [Google Scholar]
- 133.Mudali S, Dobs AS. Effects of testosterone on body composition of the aging male. Mech Ageing Dev. 2004;125(4):297–304. doi: 10.1016/j.mad.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 134.Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003;88:358–362. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- 135.Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 136.Harman SM, Blackman MR. The effects of growth hormone and sex steroid on lean body mass, fat mass, muscle strength, cardiovascular endurance and adverse events in healthy elderly women and men. Horm Res. 2003;60:121–124. doi: 10.1159/000071236. [DOI] [PubMed] [Google Scholar]
- 137.Tenover JS. Androgen administration in aging men. Endocrinol Metab Clin. 1994;23:877–889. [PubMed] [Google Scholar]
- 138.Urban RJ, Bodenburg C, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 139.Sih R, Morley JE, Kaiser FE, Perry HM, III, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12 months randomized controlled study. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 140.Morley JE, Perry HM, 3rd, Kaiser FE, et al. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. 1993;41:149–152. doi: 10.1111/j.1532-5415.1993.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 141.Perry HM, Miller DK, Patrick P, et al. Testosterone and leptin in older African-American men: relationship to age, strength, function, and season. Metabolism. 2000;49:1085–1091. doi: 10.1053/meta.2000.7710. [DOI] [PubMed] [Google Scholar]
- 142.Breuer B, Trungold S, Martucci C, et al. Relationship of sex hormone levels to dependence of daily living in the frail elderly. Maturitas. 2001;39:147–159. doi: 10.1016/s0378-5122(01)00208-0. [DOI] [PubMed] [Google Scholar]
- 143.Bhasin S. Testosterone supplementation for aging-associated sarcopenia. J Gerontol A Biol Sci Med Sci. 2003;58(11):1002–1008. doi: 10.1093/gerona/58.11.m1002. [DOI] [PubMed] [Google Scholar]
- 144.Steidle C, Schwartz S, Jacoby K, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 145.Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- 146.Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 147.Schmidt PJ, Berlin KL, Danaceau MA, et al. The effects of pharmacologically induced hypogonadism on mood in healthy men. Arch Gen Psychiatry. 2004;61:997–1004. doi: 10.1001/archpsyc.61.10.997. [DOI] [PubMed] [Google Scholar]
- 148.Booth A, Johnson DR, Granger DA. Testosterone and men’s depression: the role of social behavior. J Health Soc Behav. 1999;40:130–140. [PubMed] [Google Scholar]
- 149.Kratzik CW, Schatzl G, Lackner JE, et al. Mood changes, body mass index and bioavailable testosterone in healthy men: results of the Androx Vienna Municipality Study. BJU Int. 2007;100:614–618. doi: 10.1111/j.1464-410X.2007.07010.x. [DOI] [PubMed] [Google Scholar]
- 150.Seidman SN, Spatz E, Rizzo C, Roose SP. Testosterone replacement therapy for hypogonadal men with major depressive disorder: a randomized, placebo-controlled clinical trial. J Clin Psychiatry. 2001;62(6):406–412. doi: 10.4088/jcp.v62n0602. [DOI] [PubMed] [Google Scholar]
- 151.English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 152.Reddy P, White CM, Dunn AB, Moyna NM, Thompson PD. The effect of testosterone on health-related quality of life in elderly males: a pilot study. J Clin Pharm Ther. 2000;25:421–426. doi: 10.1046/j.1365-2710.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- 153.Tricker R, Casaburi R, Storer TW, et al. The effects of supraphysiological doses of testosterone on angry behavior in healthy eugonadal men: a clinical research center study. J Clin Endocrinol Metab. 1996;81:3754–3758. doi: 10.1210/jcem.81.10.8855834. [DOI] [PubMed] [Google Scholar]
- 154.Haren MT, Wittert GA, Chapman IM, et al. Effect of oral testosterone undecanoate on visuospatial cognition, mood and quality of life in elderly men with low–normal gonadal status. Maturitas. 2005;50:124–133. doi: 10.1016/j.maturitas.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 155.Pope HGJ, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry. 2000;57:133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- 156.Su TP, Pagliaro M, Schmidt PJ, et al. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269:2760–2764. [PubMed] [Google Scholar]
- 157.Wang CW, Alexander G, Berman N, et al. Testosterone replacement therapy improves mood in hypogonadal menóa clinical research center study. J Clin Endocrinol Metab. 1996;81:3578. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- 158.Lunenfeld B, Nieschlag E. Testosterone therapy in the aging male. Aging Male. 2007;10:139–153. doi: 10.1080/13685530701485998. [DOI] [PubMed] [Google Scholar]
- 159.Morley JE. Testosterone replacement in older men and women. J Gend Specif Med. 2001;4:49–53. [PubMed] [Google Scholar]
- 160.Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–115. doi: 10.1034/j.1601-5215.2002.51018.x. [DOI] [PubMed] [Google Scholar]
- 161.Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatr Res. 1999;33b:379–387. doi: 10.1016/s0022-3956(99)00017-5. [DOI] [PubMed] [Google Scholar]
- 162.Heuser I, Hartmann A, Oertel H. Androgen replacement in a 48, XXYY-male patient. Arch Gen Psychiatry. 1999;56:194–195. doi: 10.1001/archpsyc.56.2.194. [DOI] [PubMed] [Google Scholar]
- 163.Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord. 1998;48(2–3):157–161. doi: 10.1016/s0165-0327(97)00168-7. [DOI] [PubMed] [Google Scholar]
- 164.Orengo CA, Fullerton L, Kunik ME. Safety and efficacy of testosterone gel 1% augmentation in depressed men with partial response to antidepressant therapy. J Geriatr Psychiatry Neurol. 2005;18:20–24. doi: 10.1177/0891988704271767. [DOI] [PubMed] [Google Scholar]
- 165.Rabkin JG, Wagner GJ, Rabkin R. Testosterone therapy for HIV+ men with and without clinical hypogonadism. J Clin Psychopharmacol. 1999;19:19–27. doi: 10.1097/00004714-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 166.Grinspoon S, Corcoran C, Stanley T, et al. Effects of hypogonadism and testosterone administration on depression indices in HIV-infected men. J Clin Endocrinol Metab. 2000;85:60–65. doi: 10.1210/jcem.85.1.6224. [DOI] [PubMed] [Google Scholar]
- 167.Seidman SN, Miyazaki M, Roose SP. Intramuscular testosterone supplementation to selective serotonin reuptake inhibitor in treatment-resistant depressed men: randomized placebo-controlled clinical trial. J Clin Psychopharmacol. 2005;25:584–588. doi: 10.1097/01.jcp.0000185424.23515.e5. [DOI] [PubMed] [Google Scholar]
- 168.Perry PJ, Yates WR, Williams RD, et al. Testosterone therapy in late-life major depression in males. J Clin Psychiatry. 2002;63:1096–1101. doi: 10.4088/jcp.v63n1202. [DOI] [PubMed] [Google Scholar]
- 169.Seidman SN, Araujo AB, Roose SP, McKinlay JB. Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol Psychiatry. 2001;50:371–376. doi: 10.1016/s0006-3223(01)01148-9. [DOI] [PubMed] [Google Scholar]
- 170.Harkonen K, Huhtaniemi I, Makinen J, et al. The polymorphic androgen receptor gene CAG repeat, pituitary-testicular function and andropausal symptoms in ageing men. Int J Androl. 2003;26:187–194. doi: 10.1046/j.1365-2605.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 171.Amiaz RA, Seidman SN, Stuart NB. Testosterone and depression in men. Curr Opin Endocrinol Diabetes Obes. 2008;15(3):278–283. doi: 10.1097/MED.0b013e3282fc27eb. [DOI] [PubMed] [Google Scholar]
- 172.Shamlian NT, Cole MG. Androgen treatment of depressive symptoms in older men: a systematic review of feasibility and effectiveness. Can J Psychiatry. 2006;51:295–299. doi: 10.1177/070674370605100505. [DOI] [PubMed] [Google Scholar]
- 173.Kanayama G, Amiaz R, Seidman SN, Pope HGJ. Testosterone supplementation for depressed men: current research and suggested treatment guidelines. Exp Clin Psychopharmacol. 2007;15:529–538. doi: 10.1037/1064-1297.15.6.529. [DOI] [PubMed] [Google Scholar]
- 174.Gillett MJ, Martins RN, Clarnette RM, Chubb SA, Bruce DG, Yeap BB. Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis. 2003;5:267–269. doi: 10.3233/jad-2003-5401. [DOI] [PubMed] [Google Scholar]
- 175.Morley JE, Kaiser F, Raum WJ, et al. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci U S A. 1997;94:7537–7542. doi: 10.1073/pnas.94.14.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- 177.Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 178.McKeever WF, Deyo A. Testosterone, dihydrotestosterone and spatial task performance of males. Bull Psychonomic Soc. 1990;28:305–308. [Google Scholar]
- 179.Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- 180.Yaffe K, Lui L-Y, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- 181.Thilers PP, MacDonald SWS, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35–90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 182.Yeap BB, Almeida OP, Hyde Z, Chubb SAP, Hankey GJ, Jamrozik K, et al. Higher serum free testosterone is associated with better cognitive function in older men, whilst total testosterone is not. The Health In Men Study. Clin Endocrinol. 2008;68:404–412. doi: 10.1111/j.1365-2265.2007.03055.x. [DOI] [PubMed] [Google Scholar]
- 183.Fonda SJ, Bertrand R, O’Donnell A, Longcope C, McKinlay JB. Age, hormones, and cognitive functioning among middle aged and elderly men: cross-sectional evidence from the Massachusetts Male Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:385–390. doi: 10.1093/gerona/60.3.385. [DOI] [PubMed] [Google Scholar]
- 184.Yonker JE, Eriksson E, Nilsson L-G, Herlitz A. Negative association of testosterone on spatial visualisation in 35 to 80 year old men. Cortex. 2006;42:376–386. doi: 10.1016/s0010-9452(08)70364-2. [DOI] [PubMed] [Google Scholar]
- 185.Martin DM, Wittert G, Burns NR, Haren MT, Sugarman R. Testosterone and cognitive function in ageing men: data from the Florey Adelaide Male Ageing Study (FAMAS) Maturitas. 2007;57:182–194. doi: 10.1016/j.maturitas.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 186.Burkhardt MS, Foster JK, Clarnette RM, Chubb SAP, Bruce DG, Drummond PD, et al. Interaction between testosterone and apolipoprotein E e4 status on cognition in healthy older men. J Clin Endocrinol Metab. 2006;91:1168–1172. doi: 10.1210/jc.2005-1072. [DOI] [PubMed] [Google Scholar]
- 187.Chu LW, Tam S, Lee PW, et al. Bioavailable testosterone is associated with a reduced risk of amnestic mild cognitive impairment in older men. Clin Endocrinol (Oxf) 2008;68(4):589–598. doi: 10.1111/j.1365-2265.2007.03094.x. [DOI] [PubMed] [Google Scholar]
- 188.Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J Urol. 2003;170:1808–1811. doi: 10.1097/01.ju.0000091640.59812.83. [DOI] [PubMed] [Google Scholar]
- 189.Salminen EK, Portin RI, Koskinen A, Helenius H, Nurmi M. Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clin Cancer Res. 2004;10:7575–7582. doi: 10.1158/1078-0432.CCR-04-0750. [DOI] [PubMed] [Google Scholar]
- 190.Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29(8):1071–1081. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 191.Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer’s disease. Neuro Endocrinol Lett. 2001;22(3):163–168. [PubMed] [Google Scholar]
- 192.Bowen RL, Isley JP, Atkinson RL. An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol. 2000;12(4):351–354. doi: 10.1046/j.1365-2826.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- 193.Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74(5):637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- 194.Beauchet O. Testosterone and cognitive function: current clinical evidence of a relationship. Eur J Endocrinol. 2006;155:773–781. doi: 10.1530/eje.1.02306. [DOI] [PubMed] [Google Scholar]
- 195.Orwoll ES, Oviatt SK, Biddle J, et al. Transdermal testosterone supplementation in normal older men. Proc 74th Meeting of The Endocrine Soc; San Antonio, TX. 1992. p. 319. [Google Scholar]
- 196.Alexander GM, Swerdloff RS, Wang C, et al. Androgen-behavior correlations in hypogonadal men and eugonadal men. Horm Behav. 1998;33:85–94. doi: 10.1006/hbeh.1998.1439. [DOI] [PubMed] [Google Scholar]
- 197.Janowski SC, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 198.Janowsky JS, Chavez BJ, Orwoll E. Sex steroids modify working memory. Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- 199.Cherrier MM, Craft S, Matsumoto AH. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. J Androl. 2003;24:568–576. doi: 10.1002/j.1939-4640.2003.tb02708.x. [DOI] [PubMed] [Google Scholar]
- 200.Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 201.Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, et al. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch Neurol. 2006;63:177–185. doi: 10.1001/archneur.63.2.nct50002. [DOI] [PubMed] [Google Scholar]
- 202.Maki PM, Ernst M, London ED, Mordecai KL, Perschler P, Durso SC, et al. Intramuscular testosterone treatment in elderly men: evidence of memory decline and altered brain function. J Clin Endocrinol Metab. 2007;92:4107–4114. doi: 10.1210/jc.2006-1805. [DOI] [PubMed] [Google Scholar]
- 203.Tan RS, Culberson JW. An integrative review on current evidence of testosterone replacement therapyfor the andropause. Maturitas. 2003;45:15–27. doi: 10.1016/s0378-5122(03)00083-5. [DOI] [PubMed] [Google Scholar]
- 204.Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J Gerontol A Biol Sci Med Sci. 2004;59:75–78. doi: 10.1093/gerona/59.1.m75. [DOI] [PubMed] [Google Scholar]
- 205.van den Beld AW, Bots ML, Janssen JA, Pols HA, Lamberts SW, Grobbee DE. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol. 2003;157:25–31. doi: 10.1093/aje/kwf160. [DOI] [PubMed] [Google Scholar]
- 206.Makinen J, Jarvisalo MJ, Pollanen P, Perheentupa A, Irjala K, Koskenvuo M, et al. Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol. 2005;45:1603–1608. doi: 10.1016/j.jacc.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 207.Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, vander Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109:2074–2079. doi: 10.1161/01.CIR.0000125854.51637.06. [DOI] [PubMed] [Google Scholar]
- 208.Tivesten A, Mellstrom D, Jutberger H, Fagerberg B, Lernfelt B, Orwoll E, et al. Low serum testosterone and high serum estradiol associate with lower extremity peripheral arterial disease in elderly men. The MrOS Study in Sweden. J Am Coll Cardiol. 2007;50:1070–1076. doi: 10.1016/j.jacc.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 209.Hak AE, Witteman JCM, de Jong FH, Geerlings MI, Hofman A, Pols HAP. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–3639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 210.Kapoor D, Clarke S, Channer KS, Jones TH. Erectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetes. Int J Androl. 2007;30:500–507. doi: 10.1111/j.1365-2605.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 211.Ma RC-W, So W-Y, Yang X, Yu LW-L, Kong AP-S, Ko GT-C, et al. Erectile dysfunction predicts coronary heart disease in type 2 diabetes. J Am Coll Cardiol. 2008;51:2045–2050. doi: 10.1016/j.jacc.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 212.Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indices on the sex hormone binding globulin and androgens in aging and obese men. J Clin Endocrinol Metab. 1996;81:1821–1827. doi: 10.1210/jcem.81.5.8626841. [DOI] [PubMed] [Google Scholar]
- 213.Zumoff B, Strain GW, Miller LK, et al. Plasma free and non sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab. 1990;71:929–931. doi: 10.1210/jcem-71-4-929. [DOI] [PubMed] [Google Scholar]
- 214.Pasquali R, Casimirri F, Cantobelli S, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991;40:101–104. doi: 10.1016/0026-0495(91)90199-7. [DOI] [PubMed] [Google Scholar]
- 215.Kalyani RR, Dobs AS. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes. 2007;14:226–234. doi: 10.1097/MED.0b013e32814db856. [DOI] [PubMed] [Google Scholar]
- 216.Selvin E, Feinleib M, Zhang L, et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 217.Haffner SM, Valdez RA, Stern MP, et al. Obesity, body fat distribution and sex hormones in men. Int J Obes. 1993;17:634–639. [PubMed] [Google Scholar]
- 218.Phillips GB. Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism. 2003;52:784–790. doi: 10.1016/s0026-0495(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 219.Cohen PG. The hypogonadal-obesity cycle. Med Hypotheses. 1999;52:49–51. doi: 10.1054/mehy.1997.0624. [DOI] [PubMed] [Google Scholar]
- 220.Jones TH. Testosterone associations with erectile dysfunction, diabetes and the metabolic syndrome. Eur Urol (Suppl) 2007;6:847–857. [Google Scholar]
- 221.Er F, Michels G, Gassanov N, Rivero F, Hoppe UC. Testosterone induces cytoprotection by activating ATPsensitive Kþ channels in the cardiac mitochondrial inner membrane. Circulation. 2004;110:3100–3107. doi: 10.1161/01.CIR.0000146900.84943.E0. [DOI] [PubMed] [Google Scholar]
- 222.Nettleship JE, Jones TH, Channer KS, Jones RD. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse. Circulation. 2007;116:2427–2434. doi: 10.1161/CIRCULATIONAHA.107.708768. [DOI] [PubMed] [Google Scholar]
- 223.Kalme T, Seppala M, Qiao Q, et al. Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J Clin Endocrinol Metab. 2005;90:1550–1556. doi: 10.1210/jc.2004-0762. [DOI] [PubMed] [Google Scholar]
- 224.Kupelian V, Page ST, Araujo AB, et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–850. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 225.Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 2006;65:125–131. doi: 10.1111/j.1365-2265.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- 226.Rodriguez A, Muller DC, Metter EJ, et al. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92:3568–3572. doi: 10.1210/jc.2006-2764. [DOI] [PubMed] [Google Scholar]
- 227.Simon D, Preziosi P, Barrett-Connor E, et al. Interrelation between plasma testosterone and plasma insulin in healthy adult men: the Telecom Study. Diabetologia. 1992;35:173–177. doi: 10.1007/BF00402551. [DOI] [PubMed] [Google Scholar]
- 228.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men: a prospective population-based study. Circulation. 1988;78:539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 229.Pitteloud N, Mootha VK, Dwyer AA, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636–1642. doi: 10.2337/diacare.28.7.1636. [DOI] [PubMed] [Google Scholar]
- 230.Münzer T, Harman SM, Christmas C, et al. Effects of administration of testosterone and/or GH in healthy aged men [abstract]. Aging Male; Presented at 2nd World Congress on the Aging Male; Geneva, Switzeland. 2000; 2000. p. 3. [Google Scholar]
- 231.Abate N, Haffner SM, Garg A, et al. Sex steroid hormones, upper body obesity and insulin resistance. J Clin Endocrinol Metab. 2002;87:4522–4527. doi: 10.1210/jc.2002-020567. [DOI] [PubMed] [Google Scholar]
- 232.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 233.Corrales JJ, Burgo RM, Garca-Berrocal B, et al. Partial androgen deficiency in aging type 2 diabetic men and its relationship to glycemic control. Metabolism. 2004;53:666–672. doi: 10.1016/j.metabol.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 234.Basu R, Dalla MC, Campioni M, et al. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care. 2007;30:1972–1978. doi: 10.2337/dc07-0359. [DOI] [PubMed] [Google Scholar]
- 235.Tenover JL. Effects of androgen supplementation in aging males. In: Oddens B, Vermeulen A, editors. Androgens and the aging male. New York, London: Parthenon Publishing Group; 1996. pp. 191–204. [Google Scholar]
- 236.Haffner JM. Androgens in relation to cardiovascular disease and insulin resistance in aging men. In: Oddens B, Vermeulen A, editors. Androgens and the aging male. New York, London: Parthenon Publishing Group; 1996. pp. 65–84. [Google Scholar]
- 237.Moorjani S, Dupont A, Labrie F, et al. Increase in plasma high density lipoprotein concentration following complete androgen blockade in men with prostatic carcinoma. Metabolism. 1987;36:244–250. doi: 10.1016/0026-0495(87)90183-1. [DOI] [PubMed] [Google Scholar]
- 238.Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med. 2001;111:261–269. doi: 10.1016/s0002-9343(01)00833-6. [DOI] [PubMed] [Google Scholar]
- 239.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(6):634. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 240.Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84:3469–3478. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 241.Marin P, Holmäng S, Gustafson C, et al. Androgen treatment of abdominally obese men. Obesity Res. 1993;1:245–248. doi: 10.1002/j.1550-8528.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 242.Marin P, Lonn B, Andersson B, Oden B, Olbe L, Bengtsson BA. Assimilation of triglycerides in subcutaneous and intra-abdominal adipose tissues in vivo in men. J Clin Endocrinol Metab. 1996;81:1018–1022. doi: 10.1210/jcem.81.3.8772568. [DOI] [PubMed] [Google Scholar]
- 243.Xu X, De Pergola G, Bjorntorp P. Testosterone increases lipolysis and the number ß-adrenoreceptors in male rat adipocytes. Endocrinology. 1991;128:379–381. doi: 10.1210/endo-128-1-379. [DOI] [PubMed] [Google Scholar]
- 244.Marin P, Oden B, Björntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab. 1995;80:239–243. doi: 10.1210/jcem.80.1.7829619. [DOI] [PubMed] [Google Scholar]
- 245.Zmuda JM, Fahrenbach MC, Younkin BT, et al. The effect of testosterone aromatization on high-density lipoprotein cholesterol level and postheparin lipolytic activity. Metabolism. 1993;42:446–450. doi: 10.1016/0026-0495(93)90101-s. [DOI] [PubMed] [Google Scholar]
- 246.Jacobs DR, Jr, Mebane IL, Bangdiwala SI, Criqui MH, Tyroler HA. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow-up study of the Lipid Research Clinics Prevalence Study. Am J Epidemiol. 1990;131:32–47. doi: 10.1093/oxfordjournals.aje.a115483. [DOI] [PubMed] [Google Scholar]
- 247.Singh AB, Hsia S, Alaupovic P, et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87:136–143. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 248.Phillips G, Pinkernell BH, Jing TY. The association between hypotestosteronemia and coronary heart disease in men. Arterioscler Thromb. 1994;14:701–706. doi: 10.1161/01.atv.14.5.701. [DOI] [PubMed] [Google Scholar]
- 249.Vermeulen A, Goemaere S, Kaufman JM. Sex hormones, body composition and aging. Aging Male. 1999;2:8–15. [PubMed] [Google Scholar]
- 250.Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 251.Tchernof A, Labrie F, Belanger A, et al. Relationships between endogenous sex steroid hormones, sex hormone binding globulin and lipoprotein levels in men: contribution of visceral obesity, insulin levels and other metabolic variables. Atherosclerosis. 1997;133:235–244. doi: 10.1016/s0021-9150(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 252.Kannell WB, Cupples LA, Ramaswami R, Stokes J, Jr, Kreger BE, Higgings M. Regional obesity and the risk of coronary disease: The Framingham Study. J Clin Epidemiol. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 253.Björntorp P. Visceral obesity: a civilisation syndrome. Obes Res. 1993;1:206–222. doi: 10.1002/j.1550-8528.1993.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 254.Kabakci G, Yildirir A, Can I, Unsal I, Erbas B. Relationship between endogenous sex hormone levels, lipoproteins and coronary atherosclerosis in men undergoing coronary angiography. Cardiology. 1999;92:221–225. doi: 10.1159/000006977. [DOI] [PubMed] [Google Scholar]
- 255.Haddad RM, Kennedy CC, Caples SM. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):11–13. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 256.Allan CA, McLachlan RI. Age-related changes in testosterone and the role of replacement therapy in older men. Clin Endocrinol. 2004;60:653–670. doi: 10.1111/j.1365-2265.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- 257.Haffner JE, Moss SE, Klein BEK, Klein R. Sex hormones and DHEASO4 in relation to ischemic heart disease in diabetic subjects. The WESDR Study. Diabetes Care. 1996;19:1045–1050. doi: 10.2337/diacare.19.10.1045. [DOI] [PubMed] [Google Scholar]
- 258.Barrett-Connor E, Khaw KS. Endogenous sex hormone levels and cardiovascular disease in men: a prospective population based study. Circulation. 1988;788:539–543. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 259.Cauley JA, Gutai JP, Kuller LH, Dai WS. Usefulness of sex steroid hormone levels in predicting coronary artery disease in men. Am J Cardiol. 1987;60:771–777. doi: 10.1016/0002-9149(87)91021-6. [DOI] [PubMed] [Google Scholar]
- 260.Goldberg RB, Rabin AN, Alexander AN, Doelle GC, Getz GS. Suppression of plasma testosterone leads to an increase in serum total and high density lipoprotein cholesterol and Apo A and B. J Clin Endocrinol Metab. 1985;60:203–207. doi: 10.1210/jcem-60-1-203. [DOI] [PubMed] [Google Scholar]
- 261.Caron P, Bennet A, Camare L, Louvet JP, Boneu S, Sie P. Plasminogen activator inhibitor in plasma is related to testosterone in man. Metabolism. 1989;38:1010–1013. doi: 10.1016/0026-0495(89)90014-0. [DOI] [PubMed] [Google Scholar]
- 262.Polderman KH, Stehouwer CDA, van de Kamp GJ, Dekker GH, Verheugt FWA, Gooren LJG. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118:429–431. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 263.Ajayi AA. Testosterone increases platelet thromboxane A2 receptor density. Circulation. 1995;91:2740–2747. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 264.Spivak JL. The blood in systemic disorders. Lancet. 2000;355:1707–1712. doi: 10.1016/S0140-6736(00)02249-2. [DOI] [PubMed] [Google Scholar]
- 265.Zitzmann M, Nieschlag E. Androgens and erythropoiesis. In: Nieschlag E, Behre HM, editors. Testosterone: action, deficiency, substitution. Cambridge: Cambridge University Press; 2004. pp. 283–296. [Google Scholar]
- 266.Marks LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2369–2371. doi: 10.1001/jama.296.19.2351. [DOI] [PubMed] [Google Scholar]
- 267.Holyoak JD, Crawford ED, Meacham RB. testosterone and the testosterone: implications for the treatment of hypogonadal men. Curr Urol Rep. 2008;9:500–505. doi: 10.1007/s11934-008-0085-1. [DOI] [PubMed] [Google Scholar]
- 268.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–83. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Carpenter WR, Robinson WR, Godley PA. Getting over testosterone: postulating a fresh start for etiologic studies of prostate cancer. J Natl Cancer Inst. 2008;100:158–159. doi: 10.1093/jnci/djm329. [DOI] [PubMed] [Google Scholar]
- 270.Holmäng S, Marin P, Lindstedt G, Hedelin H. Effect of long-term oral testosterone-undecanoate treatment on prostatic volume and serum prostate specific antigen in eugonadal middle-aged men. Prostate. 1993;23:99–106. doi: 10.1002/pros.2990230203. [DOI] [PubMed] [Google Scholar]
- 271.Krieg M, Nass R, Tunn S. Effect of aging on endogenous level of 5a-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab. 1993;77:375–381. doi: 10.1210/jcem.77.2.7688377. [DOI] [PubMed] [Google Scholar]
- 272.Slater S, Oliver RTD. Testosterone: its role in development of prostate cancer and potential risk from use as hormone replacement therapy. Drugs Aging. 2000;17:431–439. doi: 10.2165/00002512-200017060-00001. [DOI] [PubMed] [Google Scholar]
- 273.Pechersky AV, Mazurov VI, Semiglazov VF, Karpischenko AI, Mikhailichenko VV, Udintsev AV. Androgen administration in middle-aged and ageing men: effects of oral testosterone undecanoate on dihydrotestosterone, oestradiol and prostate volume. Int J Androl. 2002;25:119–125. doi: 10.1046/j.1365-2605.2002.00335.x. [DOI] [PubMed] [Google Scholar]
- 274.Curran MJ, Bihrle W., III Dramatic rise in prostate-specific antigen after androgen replacement in a hypogonadal man with occult adenocarcinoma of the prostate. Urology. 1999;53:423–424. doi: 10.1016/s0090-4295(98)00348-3. [DOI] [PubMed] [Google Scholar]
- 275.Loughlin KR, Richie JP. Prostate cancer after exogenous testosterone treatment for impotence. J Urol. 1997;157:1845. [PubMed] [Google Scholar]
- 276.Rhoden EL, Morgentaler A. Testosterone replacement therapy in hypogonadal men at high risk for prostate cancer: results of 1 year of treatment in men with prostatic intraepithelial neoplasia. J Urol. 2003;170(6 pt 1):2348–2351. doi: 10.1097/01.ju.0000091104.71869.8e. [DOI] [PubMed] [Google Scholar]
- 277.Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 278.Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of serum androgen levels in men with and without prostate cancer. Prostate. 1995;27:25–31. doi: 10.1002/pros.2990270106. [DOI] [PubMed] [Google Scholar]
- 279.Heikkila R, Aho K, Heliovaara M, et al. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: a longitudinal study. Cancer. 1999;86:312–315. [PubMed] [Google Scholar]
- 280.Agarwal PK, Oefelein MG. Testosterone replacement therapy after primary treatment for prostate cancer. J Urol. 2005;173:533–536. doi: 10.1097/01.ju.0000143942.55896.64. [DOI] [PubMed] [Google Scholar]
- 281.Ferreira U, Leitao VA, Denardi F, Matheus WE, Stopiglia RM, Netto NR., Jr Intermittent androgen replacement for intense hypogonadism symptoms in castrated patients. Prostate Cancer Prostatic Dis. 2006;9:39–41. doi: 10.1038/sj.pcan.4500833. [DOI] [PubMed] [Google Scholar]
- 282.Weiss JM, Huang WY, Rinaldi S. Endogenous sex hormones and the risk of prostate cancer: a prospective study. Int J Cancer. 2008;122(10):2345–2350. doi: 10.1002/ijc.23326. [DOI] [PubMed] [Google Scholar]
- 283.Fowler JE, Jr, Whitmore WF., Jr Considerations for the use of testosterone with systemic chemotherapy in prostatic cancer. Cancer. 1982;49:1373–1377. doi: 10.1002/1097-0142(19820401)49:7<1373::aid-cncr2820490712>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 284.McConnell JD. Prostatic growth: new insights into hormonal regulation. Br J Urol. 1995;76(Suppl 1):5–10. [PubMed] [Google Scholar]
- 285.Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer. 2007;109:536–541. doi: 10.1002/cncr.22438. [DOI] [PubMed] [Google Scholar]
- 286.Khera M, Lipshultz LI. The role of testosterone replacement therapy following radical prostatectomy. Urol Clin North Am. 2007;34:549–553. doi: 10.1016/j.ucl.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 287.Swerdloff RS, Wang C. Three-year follow-up of androgen treatment in hypogonadal men: preliminary report with testosterone gel. Aging Male. 2003;6:207–211. [PubMed] [Google Scholar]
- 288.El-Sakka AI, Hassoba HM, Elbakry AM, Hassan HA. Prostatic specific antigen in patients with hypogonadism: effect of testosterone replacement. J Sex Med. 2005;2:235–240. doi: 10.1111/j.1743-6109.2005.20233.x. [DOI] [PubMed] [Google Scholar]
- 289.Rhoden EL, Morgentaler A. Influence of demographic factors and biochemical characteristics on the prostate-specific antigen (PSA) response to testosterone replacement therapy. Int J Impot Res. 2006;18:201–205. doi: 10.1038/sj.ijir.3901394. [DOI] [PubMed] [Google Scholar]
- 290.Kuhnert B, Byrne M, Simoni M, et al. Testosterone substitution with a new transdermal, hydroalcoholic gel applied to scrotal or non-scrotal skin: a multicentre trial. Eur J Endocrinol. 2005;153:317–326. doi: 10.1530/eje.1.01964. [DOI] [PubMed] [Google Scholar]
- 291.Parekh DJ, Ankerst DP, Higgins BA, et al. External validation of the Prostate Cancer Prevention Trial risk calculator in a screened population. Urology. 2006;68:1152–1155. doi: 10.1016/j.urology.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 292.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 293.Thompson IM, Carroll PR, Carducci MA. Recommendations for defining and treating high risk localized prostate cancer. J Urol. 2006;176:S6–S10. doi: 10.1016/j.juro.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 294.Summary from the second annual andropause consensus meeting; Chevy Chase, Md: Endocrine Society; 2001. [Google Scholar]
- 295.Bhasin S, Singh AB, Mac RP, Carter B, Lee MI, Cunningham GR. Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl. 2003;24:299–311. doi: 10.1002/j.1939-4640.2003.tb02676.x. [DOI] [PubMed] [Google Scholar]
- 296.Rhoden EL, Averbeck MA, Teloken PE. Androgen replacement in men undergoing treatment for prostate cancer. J Sex Med. 2008;5(9):2202–2208. doi: 10.1111/j.1743-6109.2008.00925.x. [DOI] [PubMed] [Google Scholar]
- 297.Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet. 1977;2:262–263. [PubMed] [Google Scholar]
- 298.Gurakar A, Caraceni P, Fagiuoli S, Van Thiel DH. Androgenic/anabolic steroid-induced intrahepatic cholestasis: a review with four additional case reports. J Okla State Med Assoc. 1994;87:399–404. [PubMed] [Google Scholar]
- 299.Soe KL, Soe M, Gluud C. Liver pathology associated with the use of anabolic-androgenic steroids. Liver. 1992;12:73–79. doi: 10.1111/j.1600-0676.1992.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 300.Basaria S, Dobs AS. Risks versus benefits of testosterone therapy in elderly men. Drugs Aging. 1999;15:131–142. doi: 10.2165/00002512-199915020-00006. [DOI] [PubMed] [Google Scholar]
- 301.The Endocrine Society Clinical bulletins in andropause: benefits and risks of treating hypogonadism in the aging male. Endocr Rep. 2002;2:1–6. [Google Scholar]
- 302.Kim YC. Testosterone supplementation in the aging male. Int J Impot Res. 1999;11:343–352. doi: 10.1038/sj.ijir.3900446. [DOI] [PubMed] [Google Scholar]
- 303.Viallard JF, Marit G, Mercie P, Leng B, Reiffers J, Pellegrin JL. Polycythaemia as a complication of transdermal testosterone therapy. Br J Haematol. 2000;110:237–238. doi: 10.1046/j.1365-2141.2000.02072-3.x. [DOI] [PubMed] [Google Scholar]
- 304.Drinka PJ, Jochen AL, Cuisinier M, Bloom R, Rudman I, Rudman D. Polycythemia as a complication of testosterone replacement therapy in nursing home men with low testosterone levels. J Am Geriatr Soc. 1995;43:899–901. doi: 10.1111/j.1532-5415.1995.tb05534.x. [DOI] [PubMed] [Google Scholar]
- 305.Nieschlag E. Testosterone treatment comes of age: new options for hypogonadal men. Clin Endocrinol (Oxf) 2006;65:275–281. doi: 10.1111/j.1365-2265.2006.02618.x. [DOI] [PubMed] [Google Scholar]
- 306.Uzych L. Anabolic-androgenic steroids and psychiatric-related effects: a review. Can J Psychiatry. 1992;37:23–28. doi: 10.1177/070674379203700106. [DOI] [PubMed] [Google Scholar]
- 307.Bahrke MS, Yesalis CE, III, Wright JE. Psychological and behavioural effects of endogenous testosterone levels and anabolic-androgenic steroids among males: a review. Sports Med. 1990;10:303–337. doi: 10.2165/00007256-199010050-00003. [DOI] [PubMed] [Google Scholar]
- 308.Bagatell CJ, Bremner WJ. Androgens in men – uses and abuses. N Engl J Med. 1996;334:707–714. doi: 10.1056/NEJM199603143341107. [DOI] [PubMed] [Google Scholar]
- 309.World Health Organization Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet. 1990;336:955. [PubMed] [Google Scholar]
- 310.World Health Organization Contraceptive efficacy of testosterone-induced azoospermia and oligozoopermia in normal men. Fertil Steril. 1996;65:821. [PubMed] [Google Scholar]
- 311.Wilson JD. Androgen abuse by athletes. Endocr Rev. 1988;9:181–199. doi: 10.1210/edrv-9-2-181. [DOI] [PubMed] [Google Scholar]
- 312.Luboshitzky R, Aviv A, Hefetz A, Herer P, Shen-Orr Z, Lavie L, Lavie P. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab. 2002;87:3394–3398. doi: 10.1210/jcem.87.7.8663. [DOI] [PubMed] [Google Scholar]
- 313.Schneider BK, Pickett CK, Zwillich CW, et al. Influence of testosterone on breathing during sleep. J Appl Physiol. 1986;61:618–623. doi: 10.1152/jappl.1986.61.2.618. [DOI] [PubMed] [Google Scholar]
- 314.Matsumoto AM, Sandblom RE, Schoene RB, et al. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnea, respiratory drives, and sleep. Clin Endocrinol (Oxf) 1985;22:713–721. doi: 10.1111/j.1365-2265.1985.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 315.von Eckardstein S, Nieschlag E. Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: a phase II study. J Androl. 2002;23:419–425. [PubMed] [Google Scholar]
- 316.Tangredi JF, Buxton LL. Hypertension as a complication of topical testosterone therapy. Ann Pharmacother. 2001;35:1205–1207. doi: 10.1345/aph.1A020. [DOI] [PubMed] [Google Scholar]
- 317.de Ronde W. Hyperandrogenism after transfer of topical testosterone gel: case report and review of published and unpublished studies. Hum Reprod. 2009;24(2):425–428. doi: 10.1093/humrep/den372. [DOI] [PubMed] [Google Scholar]
- 318.Cunningham GR. Testosterone replacement therapy for late-onset hypogonadism. Nat Clin Pract Urol. 2006;3(5):260–267. doi: 10.1038/ncpuro0479. [DOI] [PubMed] [Google Scholar]
- 319.Cunningham GR. Male hypogonadism: management strategies. News and views on male testosterone deficiency. A Supplement to Renal &Urology News. 2006;1(3) [Google Scholar]
- 320.Kalyani RR, Gavini S, Dobs AS. Male hypogonadism in systemic disease. Endocrinol Metab Clin North Am. 2007;36(6):333–348. doi: 10.1016/j.ecl.2007.03.014. [DOI] [PubMed] [Google Scholar]