Abstract
Intercalation of drugs in the platelet membrane affects phospholipid-requiring enzymatic processes according to the drugs’ intercalation capability. We investigated effects of Promethazine, Citalopram, Ziprasidone, Risperidone, and Diazepam on phospholipase A2 (PLA2) and polyphosphoinositide (PPI) metabolism in thrombin-stimulated human platelets. We also examined effects of the drugs on monolayers of glycerophospholipids using the Langmuir technique. Diazepam did not influence PLA2 activity, had no effects on PPI cycle, and caused no change in mean molecular area of phospholipid monolayers. The remaining psychotropic drugs affected these parameters in different ways and levels of potency suggesting that they act by being intercalated between the molecules of adjacent membrane phospholipids, thus causing changes in substrate availability for phospholipid-hydrolyzing enzymes (PLA2 and Phospholipase C). We show that several psychotropic drugs can also have other cellular effects than receptor antagonism. These effects may be implicated in the psychotropic effects of the drugs and/or their side effects.
Keywords: Human platelets, PLA2, MLC, PPI cycle, Langmuir technique, Psychotrophic drugs, Diazepam, Intercalation, Substrate availability
Introduction
The treatment of psychiatric diseases presents a great challenge today, where the heaviest burden lies upon the psychiatrist who has to choose the proper medication and dosage for treatment of the different disorders, which have a very broad spectrum. In order to find the optimal drug with a very selective action on neurons, and at the same time having minimal side effects on other systems in the human body, it is essential to understand the pharmacology and pharmacokinetics of individual drugs. To study these two vital parameters, we have to gain knowledge of their action at the cellular level. They initially bind to certain receptors directly or by modification of receptor-mediated action through intercalation into the lipid bilayer of neurons or possibly a combination of both. Human platelets have long been thought to lack D2 receptors [57], which are the primary targets for antipsychotic drugs; however, very recently, [60] reported that human platelets contain D2-like receptors that mediate the potentiation of ADP-induced aggregation. Human platelets have also other receptor types like H1 [24], which is the target for Promethazine. They have also alpha-2 and serotonin receptors, which are targets for Ziprasidone [52] and other atypical antipsychotic drugs [62].
Human platelets may serve as a supplementary diagnostic probe to provide information on psychiatric diseases like schizophrenia [15, 18, 77, 80]. In this study, we have used human platelets to study the effects of five drugs on biological phenomena: PLA2 metabolism, PPI cycle in thrombin-stimulated human platelets. The drugs we have chosen in this work are applied clinically to treat the most common ailments which result in morbidity in psychiatric health care, i.e., the triad of anxiety (Diazepam, Promethazine), depression (Citalopram), and psychosis (Ziprasidone, Risperidone).
Benzodiazepine receptors (BDRs) are classified into central (two globular subunits of 200 and 400 kDa) [64] and peripheral (two subunits of 18 and 30 kDa) classes [74]. Human platelets possess peripheral BDRs [47, 48]. Peripheral BDRs that are localized in the mitochondrial outer membrane regulate steroid biosynthesis [41, 42] and mediate inhibition of mitochondrial respiratory control [27, 51].
A brief introduction to the drugs (for their chemical structures see Scheme 1) used in the study is given below:
Scheme 1.
Chemical structures of the drugs used in this work
Diazepam (DZP) is a benzodiazepine preparation, has a very broad scale of clinical application, varying from treatment of anxiety, insomnia, epilepsy, catatonic schizophrenia [32], drug-induced tardive dyskinesia (e.g., first-generation neuroleptics) [38] to its use in a rare disability as a palliative measure in Stiff Person syndrome [28]. It should not be recommended for long-term therapy because of the development of pharmacological dependency.
Citalopram is an antidepressant, which belongs to a group of selective serotonin re-uptake inhibitors (SSRI). Its main clinical use is in the treatment of depression, but it also has other applications such as treatment of painful diabetic neuropathy [20, 65], premature ejaculation [4], and poststroke pathological crying [2]. Escitalopram, which is a steric isomer of citalopram, having the same chemical structure, exhibits antiplatelet activity [3]. Citalopram is a calmodulin antagonist [34].
Promethazine is a phenothiazine derivative of the first-generation H1 receptor antagonists, used mainly as a hypnotic, as an anti-emetic, in the treatment of hyperemesis gravidarum [63], and as a preoperative medicine to counteract post-narcotic nausea [75], an antipruritic, and in a wide range of allergic situations (like allergic rhinitis, hay fever). It has an inhibitory effect on platelet aggregation induced by collagen, thrombin, or arachidonic acid (AA) [37]. Similar to other phenothiazines, promethazine antagonizes calmodulin activities [45].
Risperidone is an atypical antipsychotic for treatment of schizophrenia. It contains the functional group of benzisoxazole and piperidine as part of its molecular structure.
It is a strong D2 receptor blocker [31], it also acts as a 5HT2A antagonist [79]. It reduces platelet aggregation by different biological mechanisms [5, 13, 15].
Ziprasidone is an atypical antipsychotic, used mainly to treat schizophrenia [68]; it has also received approval for treatment of acute manic depressive psychosis [22, 78] and has a high affinity for dopamine, serotonin, and α-adrenergic receptors and a medium affinity for histamine receptors [52]. Antagonism at both histaminic and α-adrenergic receptors explains some of its side effects such as sedation and orthostasis [55].
In the present work, we have investigated the effects of these drugs on PPI metabolism and PLA2 activity in thrombin-stimulated human platelets (intact biological system in vitro). We have also employed the Langmuir monolayer technique to examine the surface pressure/mean molecular area (mma) isotherms of acidic and neutral glycerophospholipids as in our two previous studies [53, 54]. We have previously demonstrated widely varying effects of other psychotropic drugs on PPI metabolism [53] and PLA2 activity and MLC phosphorylation [54] in such stimulated platelets. Since these drugs greatly increased the mean molecular area of phosphatidylserine monolayers [53, 54], we suggested that these effects could also be a result of intercalation of these drugs in the inner leaflet of the plasma membrane occurring in a receptor-unrelated fashion. The aim of this work was to establish whether or not psychotropic drugs exert their actions in similar ways at the cellular level.
Materials and methods
Chemicals
Dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylserine (DPPS) were from Avanti Polar Lipids (Alabaster, AL, USA); 20 µl (1 mg/ml) of these lipids were used to make lipid monolayers in air/liquid interface by applying the Langmuir apparatus.
Promethazine (PRZ) was obtained from Rhone-Poulec Pharma Norden (Denmark). Diazepam (DZP) was from Sigma (St. Louis, MO, USA). Citalopram (CTM) was from Lundbeck (Denmark). Ziprasidone (ZIP) was from Pfizer. Risperidone (RSP) was from (Janssen-Cilag).
Promethazine and CTM were dissolved in 0.15 M NaCl, the stock solutions were stored in the dark at −20°C, and dilutions were made with 0.15 M NaCl just before use. Diazepam, RSP, and ZIP were dissolved in DMSO to prepare the stock solutions and stored in the dark at –20°C. Dilutions were made with 0.15 M NaCl just prior to use.
[32P] Pi as (H332PO4), specific activity as (P) 285.6 Ci/mg was from Perkin-Elmer Life and Analytical Science (Boston, MA, USA). Thrombin (bovine) was from Parke-Davis (Scarborough, ON, Canada) and stored in small portions of 100 U/ml 0.15 M NaCl at –20°C; appropriate dilutions in 0.15 M NaCl were made immediately before each experiment, and the remaining stock portion was discarded.
[5, 6, 8, 9, 11, 12, 14, 15-3H]Arachidonic acid (code TRK508, 211 Ci/mmol) in toluene was from Amersham-Pharmacia Biotech (Buckinghamshire, England).
Langmuir apparatus
The instrument used is based on a trough where the area of monolayer at the air/water interface can be modulated. A KSV Minitrough (Helsinki, Finland) of dimensions 75 (width) × 364 (length) × 5 (height) mm which is coated with (PTFE) Poly Tetra Fluoro Ethylene, a highly hydrophobic and inert material. In control experiments, the trough was filled with Milli-Q water, containing appropriate amounts of NaCl or DMSO as drug solvents, according to solubility of the drugs used .The experiments were performed with either 1 or 10 µM of drug solution. After the liquid/air interface has been cleaned, the film-forming lipid molecules could be spread on the surface. A 20-µl (1 mg/ml) volume of a lipid (either DPPC or DPPS) was carefully spread on the surface with a Hamilton syringe, and the chloroform was allowed to evaporate before starting the measurements. The film area is compressed with two barriers, one on each side of the trough. The barriers are coated with the polyacetal hydrophobic material “Delran” to prevent the monolayer from sliding under the barriers and can be driven symmetrically along the long side of the trough by a motor that is controlled by a computer. The barrier speed during compression was monitored at 5 mm/min and with the subphase thermostated at 37 °C in the dark. For more details, see [9].
Blood
Blood was collected at the blood bank, Haukeland University Hospital, Bergen, anticoagulated with acid citrate dextrose (“ACD”, 85 mM Na3citrate, 71 mM citric acid, 111 mM dextrose; 1 volume + 4 volumes of blood) from regular blood donors and hemochromatosis patients who claimed not to have taken any medications 10 days prior to blood donation. We used blood from this patient group as this blood is abundantly available to us after therapeutic phlebotomy. Platelets from these two groups of donors are the same with respect to all thrombin-induced shape change, aggregation, secretion of ATP + ADP, PPI metabolism, MLC phosphorylation, and PLA2 metabolism in resting and thrombin-stimulated cells (unpublished results). We have not found any literature reports which are to the contrary. In the experiments reported here, both categories of platelets were used interchangeably.
AA-labeled, gel-filtered platelets
Concentrated platelet-rich plasma (PRP) was prepared from the blood as described elsewhere [53, 70] and incubated with 2.5 µCi [3H]AA/ml PRP at 37 °C for 2 h and then transferred by gel-filtration into a calcium-free Tyrode’s solution, containing 0.2% bovine albumin and 5 mM glucose. The process involves passing the PRP through a column of Sepharose 2B with Tyrode solution at room temperature; the platelets are excluded from the gel particles and elute free of plasma immediately after the void volume. The platelet count in radioactive GFP was adjusted to 3.5 × 108 cells/ml with the Tyrode solution, and the cells were used immediately in the experiments.
Preparation of [32P] Pi-labelled, GFP for study of MLC phosphorylation
Concentrated PRP was prepared from the blood as described elsewhere [71] and incubated with 0.2 mCi [32P]Pi/ml at 37 °C for 1 h and then transferred by gel-filtration into calcium and phosphate-free Tyrode’s solution but containing 0.1% bovine albumin and 5 mM glucose as described in detail elsewhere [44]. The platelet count in the radioactive GFP was adjusted to 3.5 × 108 cells/ml, and the GFP was immediately used in the experiments.
Preparation of [32P] orthophosphate-labeled, gel-filtered platelets (GFP) for study of the PPI cycle
This procedure was the same for the preparation of gel-filtered platelets (GFP) for MLC phosphorylation, except that the amount of radioactive orthophosphate was 0.1 mCi/ml, and the serum bovine albumin concentration was 0.2%.
It should be noted that the Tyrode solutions used for preparation of GFP for the experiments with MLC phosphorylation and PPI cycle are different. For MLC phosphorylation, we used less albumin (0.1%) than for the PPI experiments (0.2 %) in order to avoid disturbance of protein electrophoresis by the high albumin content. Both solutions were free of [31P] phosphate in order to prevent dilution of the incorporated [32P] by [31P].
Incubation with drugs/controls and thrombin
Aliquots (in duplicate) of 500 µl of the [32P]Pi-labelled GFP were pre-equilibrated at 37 °C for 1 min, then 50 µl of drug solution (30 µM final concentration) or 0.15 M NaCl (control) was added, followed 90 s later by 50 µl of thrombin at final concentrations of 0.0, 0.03, 0.05, 0.08, 0.1, and 0.3 U/ml. These mixtures were incubated at 37 °C for another 90 s, and 500 µl aliquots of these mixtures were transferred separately to extraction tubes containing 2 ml of ice-cold chloroform/methanol/HCl (ratio 40:20:1), followed by phase separation. The chloroform phases were evaporated as described elsewhere [29]. The dried lipid extract was dissolved in 40 µl chloroform, applied onto a silica thin-layer plate (Silicagel 60, Aluminum, Merck, Darmstadt, Germany) and chromatographed for 90 min with chloroform/methanol/20% methylamine in water (ratio 60:36:10) at room temperature [71] .The radioactive spots were detected and quantified with a Packard Instant Imager (Meriden, CT, USA).
PPI cycle, a short summary
This cycle is one of the important metabolic pathways in mammalian cells including those of the brain and human platelets. The cycle starts with the phosphorylation of the 4-hydroxy group of phosphatidylinositol (PI) by ATP catalyzed by PI 4-kinase and forming phosphatidyl-4-phosphate (PIP). This phosphate, however, is split off by a phosphohydrolase. PIP is in turn phosphorylated in the 5-position by ATP, thus forming phosphatidylinositol-4,5-bisphosphate (PIP2), and this 5-phosphate is also split off by a phosphohydrolase. Thus, both PIP and PIP2 exist in the cell as typical steady-state levels and their monoester phosphate groups have high turnover. This cycle turns over slowly in non-stimulated platelets. However, it increases tremendously when the platelets are activated with an agonist-like thrombin. This cycle is explained in more detail elsewhere [53].
Expression of data
The Instant Imager expresses the relative radioactivity as pixels. The pixel values for PIP2, PIP, and PA in the control sample, that is, GFP incubated without addition of thrombin or drug (NaCl-control), were set to 100, and the pixel values in the same metabolites in the GFP samples incubated with thrombin with or without drugs were expressed relative to this value.
Thrombin-induced activation of PLA2
Portions of 500 µl of [3H] AA-labelled GFP were pre-incubated at 37 °C for 60 s. Then, 50 µl of solvents or different drug solutions were added to the separate portions, and incubation continued for a further 120 s, and after addition of 50 µl of thrombin (0.27 U/ml final concentration), incubation proceeded for a further 6 min. Aliquots (500 µl) of the incubation mixtures were added to 2 ml ice-cold chloroform/methanol (ratio 2:1). Ice-cold chloroform and water (500 µl of each) was then added, the mixture vortexed vigorously, and the samples were centrifuged at 50 rcf (5 min), so that phase separation was properly achieved. The chloroform phases (containing extracted lipids) were pipetted into separate Kimble tubes and evaporated to dryness under a N2 jet as described elsewhere [29]. The dried lipids were dissolved in 40 µl chloroform, applied to silica thin-layer plates (Silicagel 60, Aluminum, Merck, Darmstadt, Germany) and chromatographed for 90 min at room temperature with the upper phase of ethyl acetate/iso-octane/glacial acetic acid/ddH2O (ratio 90:50:20:100). In this chromatography system, the phospholipids remain at the application point, while free AA had an Rf of 0.73. Small amounts of prostaglandins and thromboxanes amounting to less than 0.6% of the total [3H] AA incorporated in the platelet phospholipids, were produced, but these were ignored. The radioactive spots were detected and quantified with a Raytest Radio-TLC Analyzer (RITA-90, Raytek Scientific, Sheffield, England).
Thrombin-induced phosphorylation of 20-kDa myosin light chain (MLC)
Portions of 500 µl [32P]Pi-labeled GFP were pre-incubated at 37 °C with 50 µl of the drugs (30 µM, final concentration) or their solvents (50 µl) for 90 s 50 µl of thrombin was added (0.45 U/ml, final concentration) and the incubation continued for another 6 min. To stop the reaction, 150 µl of the incubation mixture was mixed with 75 µl 4× Laemmli denaturing buffer [43]. The mixture was vortexed, sonicated for 2 min, centrifuged at 15.300 rcf (3 min) and the supernatants heated for 5 min at 95°C. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 12% separating gel; 4% stacking gel) at 68 V, 500 mA (250 W) for 18 h at room temperature, and the radioactive bands were visualized and quantified using a Phosphoimager (Biorad).
Langmuir monolayer experiments:
The experiments were carried out with a KSV Minitrough (Helsinki, Finland) with MilliQ water as the subphase and the calculation of per cent change in mean molecular area at surface pressures (Π) of 10, 20, and 30 mN/m (normal surface pressure at the cellular plasma membrane is between 20–30 mN/m) for the drug-containing sample relative to that of the corresponding area in the control without drug, at the same surface pressures, was performed as described elsewhere [9].
Statistics
PLA2 and arachidonate liberation A mean value per donor and drug was obtained by averaging the duplicates from three experiments. Then, a two-tailed t test comparing the means was conducted by comparing each of the values for DZP, RSP, and ZIP to the control with DMSO. The same approach was applied to PRZ and CTM by comparing them to the control with NaCl. The control values were set to 100%, and the samples using drugs were normalized to percent changes in comparison to the controls. The p values (<0.05) obtained thus indicate if there was a statistically significant change upon administration of the drug, compared to the control.
Myosin light chain The respective control value [myosin light chain (MLC) radioactivity in platelets not treated with thrombin] for each donor was subtracted from the obtained values (with thrombin) and the results obtained with DZP, RSP, and ZIP were compared to the DMSO control by a two-tailed t test comparing the means. The same approach was applied to PRZ and CTM, and these were compared to the NaCl control using the same two-tailed t test. The obtained p values (<0.05) indicate whether there was a significant difference between the drug-treated samples and the respective controls. Each drug was tested with platelets from three different donors. Again, the mean control values were set to 100%, and the mean values for samples with drugs were normalized to percent changes in comparison to the controls.
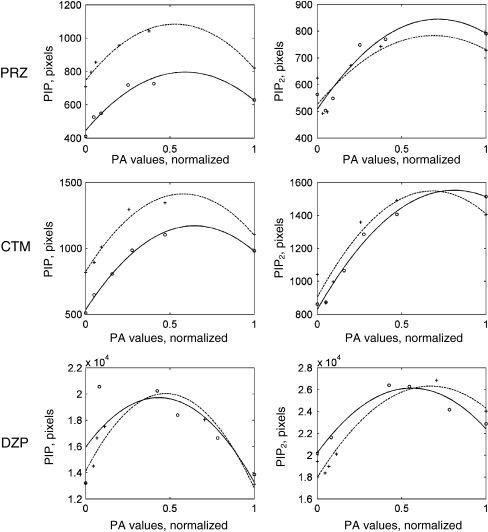
PPI cycle Descriptive statistics could not be used to calculate p values because the data points on the drug curve did not lie vertically over those of the control curve; therefore, regression analysis was applied using polynomials of second degree because we assumed that the relationships between PA and PIP or PIP2 were expected to be curved as we showed in detail in a previous paper. This procedure allowed more logical treatment of curved data because if the data were really linear, the second-degree polynomial would be reduced to a linear polynomial. For each drug, one pair of regression curves was constructed (one for control and the other drug-treated sample) as described in Fig. 5. For details, see also Fig. 5 in [53].Based on this analysis, a ranking order of means was made to arrange the drugs according to their influence on PIP and PIP2, and this is shown in rows 3 and 4 of Table 1, respectively, where the highest-ranking number is a drug with the highest impact on the tight control of PPI metabolites.
Fig. 5.
Effects of PRZ, CTP, DZP, ZIP, and RSP (30 µM) on the tight coupling of PPI cycle metabolites in platelets .The data analyzed by regression. The data shown here are from experiments with platelets from one donor, but there was a different donor for each drug. Each experiment was repeated twice for each drug with platelets from two different donors. These gave an identical pattern of results. The data were analyzed by regression using polynomials of second degree as explained in the “Statistics” section and shown in Table 1. The controls (without drugs) are shown as circles connected with solid lines, the samples with drugs are depicted as crosses connected with broken lines
Table 1.
Relative differences and ranking order of the drugs’ interference with the tight coupling of PPI metabolites
| Description | PRZ | CTM | DZP | ZIP | RSP | |
|---|---|---|---|---|---|---|
| 1. | Mean relative differences, mean (DPIP) | 2.487 | 1.843 | −0.022 | 0.071 | −0.067 |
| 2. | Mean relative differences, mean (DPIP2) | −0.341 | −0.091 | −0.074 | −0.617 | −0.707 |
| 3. | Ranking order for PIP | 5 | 4 | 1 | 3 | 2 |
| 4. | Ranking order for PIP2 | 3 | 2 | 1 | 4 | 5 |
| 5. | p Values t test, H0: mean (DPIP) = 0 | 0.000 | 0.000 | 0.638 | 0.728 | 0.85 |
| 6. | p Values t test, H0: mean (DPIP2) = 0 | 0.0392 | 0.6980 | 0.4457 | 0.0020 | 0.0010 |
| 7. | Mean ranking order | 4 | 3 | 1 | 3.5 | 3.5 |
The experiments exemplified with platelets from one donor, but different donor for each drug in Fig. 5. The experiments were repeated with platelets from two more different donors for each drug. The data from altogether three donors for each drug were analyzed by regression using polynomials of second degree, as explained in the “Materials and methods” section
Results
Langmuir isotherms
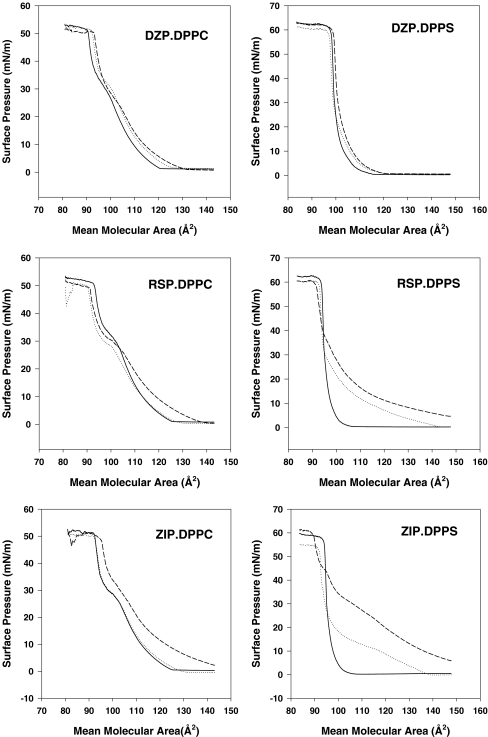
Surface pressure versus mean molecular area (mma) curves for DPPC and DPPS monolayers on distilled water at 37 °C with or without PRZ, CTM, RSP, ZIP, or DZP in the subphase are shown in Fig. 1. Without any drugs, no changes in surface pressure (Π) of the DPPC monolayer (Fig. 1, left panels) took place as the mma decreased from 145 to 105 Å2 showing that DPPC was in the gaseous phase; a further decrease in mma, produced a sharp increase in Π to about 30 mN/m at about 60 Å2 at which point the curve showed a small, but distinct, break point, and a further decrease in mma produced an even sharper increase in Π until the “collapse point” at 50 mN/m and 40 mma, after which Π remained constant by further compression of DPPC (One believes that the monolayer collapses by folding over itself). During the sharp increase in Π between 105 mma (so-called “lift-off” point) and collapse point, the glycerophospholipid is thought to go through the liquid to solid state. For pure DPPS (Fig. 1, right panels), the Π/mma isotherm resembled that of DPPC, except that lift-off took place at about 50 Å2, and the isotherm had no breakpoint in the liquid-to-solid phases.
Fig. 1.
Effects of PRZ, CTP, DZP, ZIP, and RSP/mean molecular area relations of DPPC and DPPS monolayers. DPPC and DPPS monolayers on pure MilliQ water (straight line), 1 µM (dotted line) or10 µM (dashed line) of the drugs (in MilliQ water) were compressed in the Langmuir apparatus at 37 °C and the surface pressure measured with a Wilhelmy plate as explained in [1, 9]. Three experiments were performed for each drug, and these gave identical results. The curves shown in this figure are representative for each group
PRZ, CTM, RSP, and ZIP but not DZP, drastically altered the DPPS isotherm (Fig. 1, right panels), but had little effect on the DPPC isotherm (Fig. 1, left panels). It is seen that in DPPS monolayers, PRZ, CTM, RSP, and ZIP at 1 µM concentration increased the mma at all Π levels and also lowered the surface pressure at the collapse point. We tested also the effects of 10 µM PRZ, CTM, RSP, ZIP, and DZP on DPPC and DPPS monolayers under exactly the same conditions as used in Fig. 1 and obtained very similar results, i.e., hardly any effects on DPPC isotherms, apart from PRZ which showed somewhat more effect than all the four remaining drugs, and large increases in the mma at all Π for DPPS except for DZP, which showed no increase in mma at any Π. The increases (in percent) caused by 10 µM of the drugs in mma at 10, 20, and 30 mN/m relative to the corresponding Π in the control isotherms are presented in Table 2. This table shows clearly that all five drugs have little effect on the DPPC isotherm, while they markedly increased the mma at 10, 20, and 30 mN/m of the PPS isotherm, except DZP, which showed no increase.
Table 2.
Per cent change in mean molecular area (mma) relative to control (pure MilliQ water) at 10, 20, and 30 mN/m caused by 10 µM PRZ, CTM, DZP, ZIP, RSP dissolved in MilliQ water
| PC | PS | |||
|---|---|---|---|---|
| Drug | 20 mN/m | 10 mN/m | 20 mN/m | 30 mN/m |
| PRZ | 16.66 | 154.6 | 129.41 | 95.23 |
| CTM | 5.29 | 185.41 | 140.47 | 41.66 |
| DZP | 3.88 | 2.88 | 1.49 | 1.49 |
| ZIP | 4.70 | 39.84 | 24.67 | 10.67 |
| RSP | 4.76 | 28.06 | 10.10 | 3.15 |
The experiments were performed as described in the legend to Fig. 1, and the percent change in mma at surface pressures of 10, 20, and 30 mN/m was calculated. Appropriate amounts of DMSO were included in controls for DZP, ZIP, and RSP.
Effects of psychotropic drugs on the liberation of [3H] AA from thrombin-stimulated human platelets
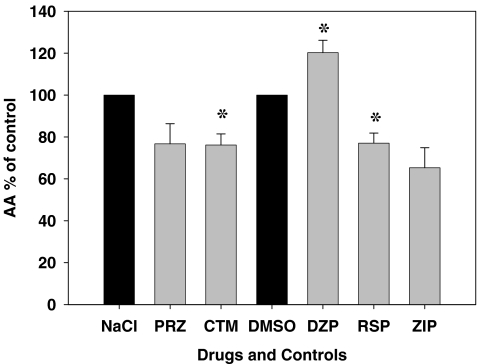
The comparison of the effects of all drugs on platelet PLA2 was measured by liberation of [3H] AA after incubation for 6 min with thrombin (0.25 U/ml final concentration) using platelets from three donors for each of all five drugs (30 µM). The results are shown in Fig. 2. It should be pointed out that since some of the drugs were only soluble in DMSO, and the others in 0.15 M NaCl, two controls with appropriate amounts of DMSO and 0.15 M NaCl, respectively, were included. Black bars in Fig. 2 represent these controls, and it can be seen that DMSO did not interfere with thrombin-induced liberation of [3H] AA. Fig. 2 clearly shows that the thrombin-induced platelet PLA2 activation was affected by these drugs. Except DZP, all other drugs decreased PLA2 activation by 24–35% (relative to the solvent controls); the decrease was significant for CTM and RSP. The effect for DZP was the opposite in that it actually increased PLA2 activity significantly (relative to DMSO solvent control). The ranking order for decreased PLA2 activation caused by these drugs was as follows:
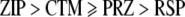 |
Fig. 2.
Comparison of the effects of all five drugs investigated on thrombin- induced [3H] arachidonate liberation in platelets. Gel-filtered, [3H] AA-labeled platelets were incubated with 0.25 U/ml of thrombin at 37°C in the absence (black bars) or presence of drugs (gray bars). After 90 s, the samples were extracted and processed as described in the “Materials and methods” section. The free [3H] AA produced is presented as percentage of a control containing no thrombin or drugs (set to 100%). The experiment was repeated three times, each time with platelets from a different donor (giving a sum of three experiments). The error bars show SEM, and the asterisks denote p < 0.05 compared to controls (i.e., without drugs)
Effects of the drugs on thrombin-induced phosphorylation of the 20 kDa myosin light chain (MLC)
Because many phenothiazines are calmodulin inhibitors [21], and the drug PRZ is also structurally a phenothiazine, to find out whether PRZ and the other remaining four psychotropic drugs also have such a property, we decided to investigate the effects of these drugs on MLC phosphorylation which is a calmodulin-dependent process in thrombin-activated human platelets [12, 25].
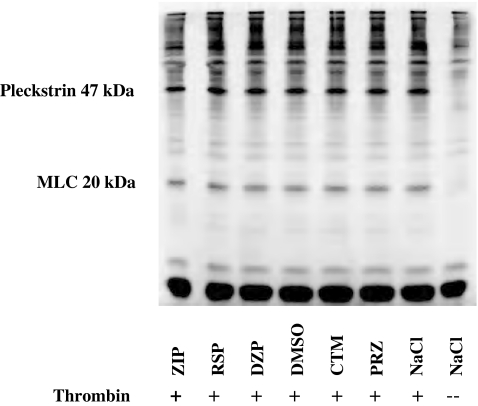
[32P] Pi-labeled GFP were incubated with or without 0.45 U/ml of thrombin in the presence of the drugs (30 μM) or their solvents for 6 min and the proteins separated by SDS-PAGE. Fig. 3 shows an autoradiogram of a representative gel where it is evident that the 20 kDa band is not labeled in the control platelets but became labeled on incubation with thrombin without addition of the drugs. The degree of labeling, however, was weaker in the presence of ZIP and RSP, and almost not affected by PRZ or DZP, while it increased in the presence of CTM. The quantification of the labeling data is discussed below. Fig. 3 also shows that a band around 47 kDa that was not labeled in control platelets became heavily labeled when the cells were incubated with thrombin. This band represents pleckstrin, a substrate for protein kinase C that is activated by thrombin [12, 17]. Ziprasidone and RSP also reduced the thrombin-induced labeling of Pleckstrin (Fig. 4).
Fig. 3.
SDS-PAGE of [32P] Pi-labeled, gel-filtered platelets treated with thrombin in the absence or presence of drugs. Aliquots (500 µl) of [32P]Pi-labeled, gel-filtered platelets were incubated with 50 µl of the drugs (30 µM, final concentration) or their solvents for 90 s at 37 °C, then 50 µl thrombin (0.45 U/ml, final concentration) or NaCl (0.15 M) were added to the samples, and incubation continued for another 6 min, then 150 µl of incubation mixture was immediately mixed with 75 µl 4× Laemli buffer as described in the “Materials and methods” section (thrombin-induced phosphorylation of 20-KDa MLC)
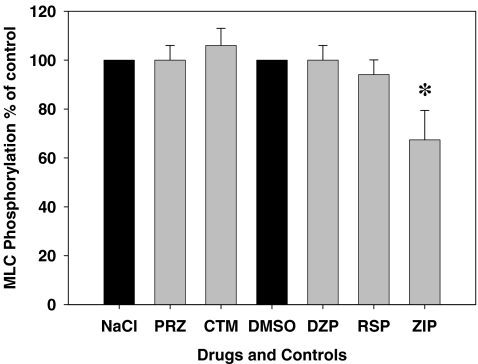
Fig. 4.
Effects of all five drugs on thrombin-induced myosin light chain phosphorylation. Myosin light chain in unstimulated samples (controls) is set to 100%, and the other values were normalized to percent of the controls. These data were obtained from the sum of three different experiments each with platelets from a different donor. See legend to Fig. 3 for experimental details. The error bars (in gray) show SEM values in samples with drugs, and the asterisk denotes p < 0.05 compared to the myosin light chain phosphorylation in the controls (i.e., black bars are without drugs)
The quantification of the level of 20 kDa MLC phosphorylation from the type of experiment shown in Fig. 3 is presented in Fig. 4. Evidently, CTM, PRZ, and DZP stimulated or had no effect on MLC phosphorylation, while ZIP and RSP reduced MLC phosphorylation by 6–33%. The reduction with ZIP was significant.
Effects of psychotropic drugs on the tight control of polyphosphoinositide metabolites in thrombin-stimulated human platelets
The rate of PA formation in the PPI cycle in thrombin-stimulated platelets was proportional to the thrombin concentration up to the dose causing maximal stimulation (0.3 U/ml). We have previously shown that changes in PIP and PIP2 were proportional to the produced PA when incubating the platelets with incremental increase in the thrombin concentration [53]. In the present work, we confirmed this observation. Since phenothiazines (neuroleptics) disturbed this tight coupling under similar conditions [53, 72], we investigated these five carefully chosen psychotropic drugs from three major different groups to find out whether they disturb the tight metabolite coupling using identical approaches as previously described [53] with platelets from three different donors for each drug. The drugs gave different patterns of change.
Regression analysis
The results of regression analysis with polynomials of second degree of the raw data obtained from the experiments with all five drugs are shown in Fig. 5. Promethazine, CTM, and RSP show obvious increases in pixel values for PIP, while DZP and RSP showed decreases when compared to the control (without drugs; see row 1 in Table 1). Concerning the changes in PIP2, all five drugs without exception showed decreases in pixel values when they were compared to the parallel controls (without drugs), see row 2 in Table 1. The ranking order for PIP using the five drugs (row 3, Table 1) was as follows;
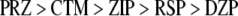 |
On the other hand, the order for PIP2 (as seen in row 4, Table 1) is;
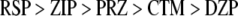 |
The mean differences between the ranking orders for PIP and PIP2 (see rows 3 and 4, Table 1) using the five drugs are shown in row 7, Table 1 and are as follows;
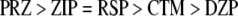 |
Discussion
In the present work, we have investigated how a sedative DZP, an antihistaminic/hypnotic PRZ, two antipsychotics (ZIP, RSP), and one antidepressive drug (CTM) interact with phospholipid monolayers and affect glycerophospholipid-hydrolyzing enzymes in human platelets, i.e., PLA2 and control of the PPI cycle metabolites. Diazepam is a highly lipophilic drug, while the remaining four are amphiphilic ones (Scheme 1), and each of them, except DZP, increased the mean molecular area of the acidic phospholipid DPPS, while they had little effect on the monolayers of the neutral phospholipid DPPC (Fig. 1, Table 2).
The greatest area-increasing effect on the DPPS monolayers was with PRZ and CTM, both of which are cationic amphiphiles (Scheme 1), and they have the same effect on DPPS monolayers as the phenothiazine drugs chlorpromazine (CPZ) [1] and trifluoperazine (TFP) [9, 67]. Solid-state 13C- and 31P-NMR studies of the interaction of CPZ and porcine brain phosphatidyl serine (PBPS) bilayers have clearly shown that the phenothiazine moiety is intercalated between the acyl chains of PBPS, while the cationic tail group is anchored electrostatically by the negatively charged phosphate and carboxyl groups [73]. Promethazine is also a phenothiazine derivative (Scheme 1) and is, like CTM, most likely intercalated in the DPPS monolayer in the same way as CPZ.
Unlike PRZ and CTM, the antipsychotics ZIP and RSP gave only a modest area-increasing effect on the DPPS monolayers, which may be explained by the virtual absence of ionizable groups in these drugs (Scheme 1). Diazepam has no ionizable group that may explain why it had no area-increasing effect on the DPPS monolayers. According to this discussion, we suggest the following “intercalation ranking order” with decreasing intercalation ability for the five drugs studied:
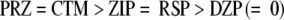 |
The main hypothesis that we have attempted to test experimentally in this work, is if intercalation of a drug into the platelet membrane occurs, then it should affect phospholipid-requiring enzymatic processes. One such process is the activation by thrombin of PLA2 measured as liberation of free [3H] AA from platelets prelabeled with [3H] AA. As shown in Fig. 2, the non-intercalating DZP increased the production of [3H] AA significantly, while the intercalating drugs PRZ, CTM, ZIP, and RSP all inhibited this process by 20–30%. There was, however, no correlation between the degrees of inhibition of PLA2 (Fig. 2) and the percent increase in DPPS molecular area (Table 2). We have previously demonstrated that 11 other psychotropic drugs also inhibited thrombin-induced PLA2 in human platelets to highly varying degrees [54]. Neither did this correlate with their area-increasing effects on DPPS monolayers [54]. One reason for the lack of correlation could be that our monolayer experiments were done with ion-free Milli-Q water of about pH 6, while the experiments with intact platelets were done in Tyrode’s solution containing 140 mM Na+ ions. This cation (and many others) counteracts the monolayer area-increasing effect of CPZ [1] and could well counteract the area-increasing effects of PRZ, ZIP, RSP, and CTM that are cations at the pH used. Nevertheless, the main purpose of our studies with monolayers was to disclose any interactions between the drugs and the phospholipids, and we therefore, on purpose, selected conditions (i.e., MilliQ water in subphase) that most sensitively would reveal such interactions.
Many phenothiazines are calmodulin antagonists [59], and this property could be involved in the inhibition of platelet PLA2 by the phenothiazine PRZ and possibly by the other drugs studied here. In order to distinguish whether or not calmodulin antagonism was involved, we have measured the effect of the drugs on myosin light chain phosphorylation, which, in human platelets, is dependent on calmodulin [25] and activated by thrombin [12] in parallel with PLA2 [30]. As shown in Fig. 4, only ZIP caused significant inhibition of myosin light chain phosphorylation, suggesting that calmodulin could be involved, whereas the inhibition caused by PRZ, CTM, and RSP was clearly calmodulin-independent.
In intact platelets, the drugs are exposed to membrane bilayers where the acyl groups in one layer face acyls from the opposite layer. In the monolayer experiments we have performed, however, the acyl groups are facing air. Thus, in bilayers the acyl groups of one layer may influence the property of the phospholipids in the other layer. One example for such a difference is that optimal intercalation of CPZ in liposomes, which are bilayers, was optimal when the mol% ratio of PC to PS was 2 [69], while intercalation of TFP was directly proportional to the PS concentration in monolayers of PS and PC [9]. Thus, the lack of correlation between the inhibition of PLA2 by PRZ, CTM, RSP, and ZIP, and the intercalation in DPPS monolayers in the present work may be due to the bilayer/monolayer difference.
The PPI cycle perturbance by psychotropic drugs has been thoroughly discussed in a previous paper [53] where we also showed that when the cycle is stimulated by thrombin, there is a tight control of the levels of PA, PIP, and PIP2; this tight coupling was markedly changed by six antipsychotic drugs. Our group has found that the phenothiazine antipsychotic CPZ also interferes with this control [72]. It was the observation of this phenomenon which actually led us to further investigate whether or not other antipsychotics (typical and atypical) could have this property as well. We found that this property is a common feature of all the antipsychotics we investigated, yet it was different from one drug to another (concerning both the pattern and intensity) [53]. In the present work, we have investigated the effects of five different psychotropic drugs (from three different groups) on the PPI cycle. Again, we confirmed that these drugs, apart from DZP, interfere with inositol phospholipid metabolism (see Fig. 5).
Promethazine gave a pattern similar to the other phenothiazines, in that it increased PIP and decreased PIP2 levels. The decrease in PIP2 level was not pronounced as we encountered with the other three phenothiazines, i.e., CPZ, TFP, and PCP [53, 72].
The difference with this phenothiazine is that it is not used as an antipsychotic drug; as a matter of fact, it is used clinically as an antihistaminic in order to treat allergy/pruritis and induce hypnosis [11, 61], so it would be expected to have a different interference potency on PPI metabolism than antipsychotic–phenothiazines. The decrease in PLA2 activity caused by PRZ was not significant compared to other phenothiazines [54]. It is also important to emphasize that PRZ (in contradiction to other phenothiazines) did not cause any decrease in MLC phosphorylation, that is to say, it did not antagonize calmodulin [54]. Citalopram, the SSRI antidepressant, disturbed the tight coupling of PPI cycle metabolites, as it increased the levels of PIP by approximately 60% over control levels, but it increased the PIP2 levels negligibly. It also decreased AA liberation (via inhibiting PLA2) significantly, similar to other antidepressants [54]. It caused a 6% increase in MLC phosphorylation in contrast to the tricyclic antidepressants which decreased MLC phosphorylation [54] more potently.
The two atypical antipsychotic drugs, RSP and ZIP, showed similar patterns concerning disturbance of PPI metabolism, in that both decreased PIP and PIP2 levels but in different potencies. This finding is quite different from the effects of other drugs investigated by our group up to now. In our previous study [53], all antipsychotic drugs tested caused an increase in PIP levels. This might give us important information about the different modes of action of these relatively new preparations. These two drugs are atypical antipsychotics; they have lower therapeutic doses than phenothiazine-derived antipsychotics; for example, the daily therapeutic dose for RSP is 2–16 mg, which is much less than the daily dose of CPZ which is 100–1,500 mg [7]. Risperidone and ZIP, similar to other antipsychotic preparations, decreased (PLA2 dependent) AA liberation; ZIP was more potent than RSP, but the decrease caused by RSP was statistically significant. Both of these two drugs decreased MLC phosphorylation, ZIP caused a more potent and significant decrease.
In the present study, DZP was different from all other drugs we have investigated up to now. It showed negligible, almost undetectable changes, in the levels of PIP and PIP2 in comparison to control values. Table 1 shows that the ranking order for changes in PIP as 1 and for PIP2 again as 1, which makes the mean ranking order for interference (change) as 1 in comparison to the effects of the other four drugs on their corresponding control samples. Diazepam did not cause any increase in mma neither with DPPC nor with DPPS, meaning that the drug has no ability to bind phospholipid monolayers. It did not decrease AA liberation like the other drugs, but actually resulted in an increase of almost 20% compared to control values (normalized to 100%).
In the literature, there are indications that DZP antagonizes calmodulin [6, 40] in mammalian cells other than human platelets. But in our experiments, which have been repeated nine times from platelets obtained from nine different donors, we did not see any decrease in MLC phosphorylation in comparison to the control sample. This finding also proves that the drug is not intercalating between the membrane phospholipids in contrast to TFP, for example [29, 30]. We can therefore explain why DZP does not interfere negatively with these three biological phenomena (PPI cycle, PLA2, and MLC phosphorylation) in human platelets. Intercalation into membrane phospholipids disturbs the substrate availability of membrane-associated phospholipases like PLC and PLA2 [8]. It is thus evident that DZP must induce its pharmacological actions through other mechanisms in human platelets (as in this work), as well as other types of mammalian cells including neurons. It is well known, and thoroughly documented, that DZP exerts its effects through binding to BDRs [14, 23, 47, 48, 66], so the drug binds to the peripheral BDRs, which are scattered in platelet membrane phospholipids, and in mitochondrial membrane phospholipids where they regulate steroid metabolism [23, 66]. Furthermore, they mediate inhibition of mitochondrial respiratory control so that they decrease metabolic rate [27, 51] and may contribute to the action of the central BDR (in neurons) to induce hypnosis and/or terminate an epileptic fit (when administered intravenously) with the anticonvulsant drug DZP [58]. Diazepam is a very lipophilic unionizable drug [16, 50] [see also Scheme 1], and this property enables the molecule to permeate readily through membrane phospholipids as this process is thermodynamically favorable and not energy demanding. Therefore, it crosses the blood–brain barrier easily similar to other addictive unionizable agents like heroin and cocaine [19]. Diazepam and other benzodiazepines are addictive drugs when they are used continuously for more than 14 days [49]. Taking into account available data in the literature and the results of the present work, we can understand why DZP exerts its effects via a receptor-dependent mechanism in contrast to the other four amphiphilic psychotropic drugs (PRZ, CTM, ZIP, and RSP). These four drugs intercalate into the membrane phospholipid bilayer in such a way that they change the alignment of distinct receptors, thus modifying their availability for agonists. In so doing, they alter the substrate availability for the enzymes catalyzing many metabolic processes [8].
When reviewing the results obtained concerning the stimulatory action of DZP on cPLA2, we can predict that this drug may deteriorate the clinical picture of psychosis and depression, besides, within conventional therapeutic doses, DZP as a sole preparation is not a first line agent in drug treatment of neither psychosis [46] nor depression. In the work described here and that reported by [54], we have shown that this drug opposes the action of all antipsychotic and antidepressant drugs tested because all these drugs (antipsychotics and antidepressives) decrease the rate of membrane phospholipid turnover by inhibiting the catalytic action of cPLA2. We have to admit, however, that up to today, there exists no clinical proof to back our claim. In contrast, benzodiazapines are still in use especially in combination with antipsychotic drugs [10, 56], and their use is still highly recommended, especially in catatonic schizophrenia, where agitation, restlessness, and stupor are cardinal symptoms besides psychosis [26, 32]. Diazepam should not be recommended for long-term applications, taking into consideration the hazards of abstinence and addiction which are actually disadvantages for DZP and all benzodiazepines. In other words, one can switch to other hypnotic/sedative drugs as an alternative, like Zopiclone which is more beneficial than DZP to induce hypnosis [35, 36] and to treat negative symptoms of chronic schizophrenic patients [39]. There is evidence indicating that Zopiclone is less risky than benzodiazepine preparations with respect to the induction of addiction [33, 76].
Since human platelets possess D2-like receptors [60], it is possible that the action of psychotropic drugs on the PPI cycle and PLA2 are mediated through antagonizing these receptors. However, due to our results from the monolayer experiments, these drugs except DZP may also induce their effects at the cellular level by modifying different receptors through intercalation in the inner leaflet of acidic membrane phospholipids. The drugs have been incubated with the platelets at 30 µM, a concentration that is considerably higher than in plasma during therapy. However, during therapy, multiple doses of drugs are administered therapeutically, and the drugs need to be available for days or even weeks in order for them to be able to induce their therapeutic pharmacological action. This is because most drugs have to accumulate to certain levels before a response is exerted. The drugs used in the present work are either amphiphilic or lipophilic; they accumulate in cellular membranes. Therefore, we believe that use of 30 µM drug for 90 s may indeed be comparable to the in vivo situation
In conclusion, the work presented here gives us valuable new information with regard to the possibility of designing new and more effective drugs than those presently available for treatment of psychiatric morbidities, a current major medical problem in western society. The aim is to develop new drugs that have no undesirable side effects and do not cause problems of addiction.
Acknowledgments
The authors wish to thank the technical staff: Sissel Rognved, Ingrid Strand, and Randi Svebak for excellent assistance. We would also like to thank Signe Hannesdal at the blood bank, Haukeland University Hospital for her cooperation in collecting blood.
Statements of conflicts of interest None.
Abbreviations
- AA
Arachidonic acid
- (BDR)s
(Benzodiazepine receptor)s
- CTM
Citalopram
- CPZ
Chlorpromazine
- DMSO
Dimethylsulfoxide
- DPPC
1,2-Dipalmitoyl phosphatidylcholine
- DPPS
1,2-Dipalmitoyl phosphatidylserine
- DZP
Diazepam
- GABAA
Gamma amino butyric acid
- GFP
Gel-filtered platelets
- PRZ
Promethazine
- mma
Mean molecular area (in Å2)
- MLC
Myosin light chain
- PC
Phosphatidylcholine
- PA
Phosphatidic acid
- PI
Phosphatidylinositol
- PIP
Phosphatidyl-4-phosphate
- PIP2
Phosphatidylinositol-4,5-bisphosphate
- PS
Phosphatidylserine
- PPI
Polyphosphoinositide
- PLA2
Phospholipase A2
- PLC
Phospholipase C
- PBPS
Porcine brain phosphatidyl serine
- PCP
Prochlorperazine
- Π
Surface pressure (in mN/m)
- RSP
Risperidone
- SSRI
Selective serotonin re-uptake inhibitor
- TFP
Trifluoperazine
- ZIP
Ziprasidone
References
- 1.Agasosler AV, Tungodden LM, Cejka D, Bakstad E, Sydnes LK, Holmsen H (2001) Chlorpromazine-induced increase in dipalmitoylphosphatidylserine surface area in monolayers at room temperature. Biochem Pharmacol 61:817–825 [DOI] [PubMed]
- 2.Andersen G, Vestergaard K, Riis JO (1993) Citalopram for post-stroke pathological crying. Lancet 342:837–839 [DOI] [PubMed]
- 3.Atar D, Malinin A, Takserman A, Pokov A, van Zyl L, Tanguay JF, Lesperance F, Serebruany V (2006) Escitalopram, but not its major metabolites, exhibits antiplatelet activity in humans. J Clin Psychopharmacol 26:172–177 [DOI] [PubMed]
- 4.Atmaca M, Kuloglu M, Tezcan E, Semercioz A (2002) The efficacy of citalopram in the treatment of premature ejaculation: a placebo-controlled study. Int J Impot Res 14:502–505 [DOI] [PubMed]
- 5.Axelsson S, Hagg S, Eriksson AC, Lindahl TL, Whiss PA (2007) In vitro effects of antipsychotics on human platelet adhesion and aggregation and plasma coagulation. Clin Exp Pharmacol Physiol 34:775–780 [DOI] [PubMed]
- 6.Babcock-Atkinson E, Norenberg LO, Norenberg MD, Neary JT (1989) Diazepam inhibits calcium, calmodulin-dependent protein kinase in primary astrocyte cultures. Brain Res 484:399–403 [DOI] [PubMed]
- 7.Boon, Colledge, Walker, Hunter (2006) Davidson’s Principles and Practice of Medicine, Chapter 10. 20th Edition edn. Churchill Livingstone: Edinburgh, London, New York, Oxford, Philadelphia, St Louis, Sydney and Toronto
- 8.Brindley DN (1984) Intracellular translocation of phosphatidate phosphohydrolase and its possible role in the control of glycerolipid synthesis. Prog Lipid Res 23:115–133 [DOI] [PubMed]
- 9.Broniec A, Gjerde AU, Olmheim AB, Holmsen H (2007) Trifluoperazine causes a disturbance in glycerophospholipid monolayers containing phosphatidylserine (PS): effects of pH, acyl unsaturation, and proportion of PS. Langmuir 23:694–699 [DOI] [PubMed]
- 10.Carpenter WT Jr, Buchanan RW, Kirkpatrick B, Breier AF (1999) Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry 156:299–303 [DOI] [PubMed]
- 11.Charlesworth EN, Massey WA, Kagey-Sobotka A, Norman PS, Lichtenstein LM (1992) Effect of H1 receptor blockade on the early and late response to cutaneous allergen challenge. J Pharmacol Exp Ther 262:964–970 [PubMed]
- 12.Daniel JL, Molish IR, Holmsen H (1981) Myosin phosphorylation in intact platelets. J Biol Chem 256:7510–7514 [PubMed]
- 13.De Clerck F, Somers Y, Mannaert E, Greenspan A, Eerdekens M (2004) In vitro effects of risperidone and 9-hydroxy-risperidone on human platelet function, plasma coagulation, and fibrinolysis. Clin Ther 26:1261–1273 [DOI] [PubMed]
- 14.Diaz-Veliz G, Butron S, Benavides MS, Dussaubat N, Mora S (2000) Gender, estrous cycle, ovariectomy, and ovarian hormones influence the effects of diazepam on avoidance conditioning in rats. Pharmacol Biochem Behav 66:887–892 [DOI] [PubMed]
- 15.Dietrich-Muszalska A, Olas B (2007) The changes of aggregability of blood platelets in schizophrenia. World J Biol Psychiatry: 1–6 [DOI] [PubMed]
- 16.Friedman H, Ochs HR, Greenblatt DJ, Shader RI (1985) Tissue distribution of diazepam and its metabolite desmethyldiazepam: a human autopsy study. J Clin Pharmacol 25:613–615 [DOI] [PubMed]
- 17.Fukami MH, Holmsen H (1995) Diacylglycerol elevations in control platelets are unaccompanied by pleckstrin phosphorylation. Implications for the role of diacylglycerol in platelet activation. Eur J Biochem 228:579–586 [DOI] [PubMed]
- 18.Gattaz WF, Schmitt A, Maras A (1995) Increased platelet phospholipase A2 activity in schizophrenia. Schizophr Res 16:1–6 [DOI] [PubMed]
- 19.Gessner P (1992) Substance Abuse Treatment. In: Smith C, Reynard A (eds) Textbook of pharmacology. 1992 edn. W.B. Saunders Company: Buffalo, New York pp 1132–1165
- 20.Giannopoulos S, Kosmidou M, Sarmas I, Markoula S, Pelidou SH, Lagos G, Kyritsis AP (2007) Patient compliance with SSRIs and gabapentin in painful diabetic neuropathy. Clin J Pain 23:267–269 [DOI] [PubMed]
- 21.Grabski R, Dewit J, De Braekeleer J, Malicka-Blaskiewicz M, De Baetselier P, Verschueren H (2001) Inhibition of T-cell invasion across cultured fibroblast monolayers by phenothiazine-related calmodulin inhibitors: impairment of lymphocyte motility by trifluoperazine and chlorpromazine, and alteration of the monolayer by pimozide. Biochem Pharmacol 61:1313–1317 [DOI] [PubMed]
- 22.Greenberg WM, Citrome L (2007) Ziprasidone for schizophrenia and bipolar disorder: a review of the clinical trials. CNS drug rev 13:137–177 [DOI] [PMC free article] [PubMed]
- 23.Greenblatt DJ, Shader RI, Abernethy DR (1983) Drug therapy. Current status of benzodiazepines. N Engl J Med 309:410–416 [DOI] [PubMed]
- 24.Hallberg T, Dohlsten M, Baldetorp B (1984) Demonstration of histamine receptors on human platelets by flow cytometry. Scand J Haematol 32:113–118 [DOI] [PubMed]
- 25.Hathaway DR, Adelstein RS (1979) Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci USA 76:1653–1657 [DOI] [PMC free article] [PubMed]
- 26.Hatta K, Miyakawa K, Ota T, Usui C, Nakamura H, Arai H (2007) Maximal response to electroconvulsive therapy for the treatment of catatonic symptoms. J Ect 23:233–235 [DOI] [PubMed]
- 27.Hirsch JD, Beyer CF, Malkowitz L, Beer B, Blume AJ (1989) Mitochondrial benzodiazepine receptors mediate inhibition of mitochondrial respiratory control. Mol Pharmacol 35:157–163 [PubMed]
- 28.Holmoy T, Horn MA, Vandvik B (2007) A stiff-legged man with a bizarre gait. Tidsskr Nor Laegeforen 127:1529–1530 [PubMed]
- 29.Holmsen H, Dangelmaier CA, Holmsen HK (1981) Thrombin-induced platelet responses differ in requirement for receptor occupancy. Evidence for tight coupling of occupancy and compartmentalized phosphatidic acid formation. J Biol Chem 256:9393–9396 [PubMed]
- 30.Holmsen H, Daniel JL, Dangelmaier CA, Molish I, Rigmaiden M, Smith JB (1984) Differential effects of trifluoperazine on arachidonate liberation, secretion and myosin phosphorylation in intact platelets. Thromb Res 36:419–428 [DOI] [PubMed]
- 31.Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Hoschl C (2006) Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS drugs 20:389–409 [DOI] [PubMed]
- 32.Huang TL (2005) Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci 59:52–55 [DOI] [PubMed]
- 33.Jaffe JH, Bloor R, Crome I, Carr M, Alam F, Simmons A, Meyer RE (2004) A postmarketing study of relative abuse liability of hypnotic sedative drugs. Addiction 99:165–173 [DOI] [PubMed]
- 34.Kagaya A, Kugaya A, Hayashi T, Okamoto Y, Takebayashi M, Uchitomi Y, Yamawaki S (1996) Effect of citalopram on the desensitization of serotonin-2A receptor-mediated calcium mobilization in rat glioma cells. Prog Neuro-Psychopharmacology Biol Psychiatry 20:157–166 [DOI] [PubMed]
- 35.Kajimura N, Kato M, Okuma T, Onuma T (1994) Effects of zopiclone on sleep and symptoms in schizophrenia: comparison with benzodiazepine hypnotics. Prog Neuropsychopharmacol Biol Psychiatry 18:477–490 [DOI] [PubMed]
- 36.Kajimura N, Kato M, Okuma T, Sekimoto M, Watanabe T, Takahashi K (1995) A quantitative sleep-EEG study on the effects of benzodiazepine and zopiclone in schizophrenic patients. Schizophr Res 15:303–312 [DOI] [PubMed]
- 37.Kanaho Y, Kometani M, Sato T, Fujii T (1983) Mechanism of inhibitory effect of some amphiphilic drugs on platelet aggregation induced by collagen, thrombin or arachidonic acid. Thromb Res 31:817–831 [DOI] [PubMed]
- 38.Kasantikul D, Kanchanatawan B (2007) Antipsychotic-induced tardive movement disorders: a series of twelve cases. J Med Assoc Thai 90:188–194 [PubMed]
- 39.Kato M, Kajimura N, Okuma T, Sekimoto M, Watanabe T, Yamadera H, Takahashi K (1999) Association between delta waves during sleep and negative symptoms in schizophrenia. Pharmaco-EEG studies by using structurally different hypnotics. Neuropsychobiology 39:165–172 [DOI] [PubMed]
- 40.Kochan LD, Churn SB, Omojokun O, Rice A, DeLorenzo RJ (2000) Status epilepticus results in an N-methyl-d-aspartate receptor-dependent inhibition of Ca2+/calmodulin-dependent kinase II activity in the rat. Neuroscience 95:735–743 [DOI] [PubMed]
- 41.Krueger KE, Papadopoulos V (1992) Mitochondrial benzodiazepine receptors and the regulation of steroid biosynthesis. Annu Rev Pharmacol Toxicol 32:211–237 [DOI] [PubMed]
- 42.Krueger KE, Papadopoulos V (1990) Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem 265:15015–15022 [PubMed]
- 43.Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed]
- 44.Lages B, Scrutton MC, Holmsen H (1975) Studies on gel-filtered human platelets: isolation and characterization in a medium containing no added Ca2+, Mg2+, or K+. J Lab Clin Med 85:811–825 [PubMed]
- 45.Lian JP, Crossley L, Zhan Q, Huang R, Coffer P, Toker A, Robinson D, Badwey JA (2001) Antagonists of calcium fluxes and calmodulin block activation of the p21-activated protein kinases in neutrophils. J Immunol 166:2643–2650 (Baltimore Md.) [DOI] [PubMed]
- 46.Lingjaerde O (1991) Benzodiazepines in the treatment of schizophrenia: an updated survey. Acta Psychiatr Scand 84:453–459 [DOI] [PubMed]
- 47.Marazziti D, Pancioli-Guadagnucci ML, Rotondo A, Giannaccini G, Martini C, Lucacchini A, Cassano GB (1994) Age-related changes in peripheral benzodiazepine receptors of human platelets. J Psychiatry Neurosci 19:136–139 [PMC free article] [PubMed]
- 48.Marazziti D, Rotondo A, Martini C, Giannaccini G, Lucacchini A, Pancioli-Guadagnucci ML, Diamond BI, Borison R, Cassano GB (1994) Changes in peripheral benzodiazepine receptors in patients with panic disorder and obsessive–compulsive disorder. Neuropsychobiology 29:8–11 [DOI] [PubMed]
- 49.Meier PJ, Ziegler WH, Neftel K (1988) Benzodiazepine—practice and problems of its use. Schweiz Med Wochenschr 118:381–392 [PubMed]
- 50.Minder S, Daniel WA, Clausen J, Bickel MH (1994) Adipose tissue storage of drugs as a function of binding competition. In vitro studies with distribution dialysis. J Pharm Pharmacol 46:313–315 [DOI] [PubMed]
- 51.Moreno-Sanchez R, Hogue BA, Bravo C, Newman AH, Basile AS, Chiang PK (1991) Inhibition of substrate oxidation in mitochondria by the peripheral-type benzodiazepine receptor ligand AHN 086. Biochem Pharmacol 41:1479–1484 [DOI] [PubMed]
- 52.Nasrallah HA (2008) Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 13:27–35 [DOI] [PubMed]
- 53.Oruch R, Hodneland E, Pryme IF, Holmsen H (2008) Psychotropic drugs interfere with the tight coupling of polyphosphoinositide cycle metabolites in human platelets: a result of receptor-independent drug intercalation in the plasma membrane? Biochim Biophys Acta 1778:2165–2176 [DOI] [PubMed]
- 54.Oruch R, Pryme IF, Holmsen H (2008) Effects of psychotropic drugs on the thrombin-induced liberation of arachidonate in human platelets. Saudi Med J 29:1397–1407 [PubMed]
- 55.Pacher P, Kecskemeti V (2004) Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm des 10:2463–2475 [DOI] [PMC free article] [PubMed]
- 56.Pilowsky LS, Ring H, Shine PJ, Battersby M, Lader M (1992) Rapid tranquillisation. A survey of emergency prescribing in a general psychiatric hospital. Br J Psychiatry 160:831–835 [DOI] [PubMed]
- 57.Ricci A, Bronzetti E, Mannino F, Mignini F, Morosetti C, Tayebati SK, Amenta F (2001) Dopamine receptors in human platelets. Naunyn Schmiedebergs Arch Pharmacol 363:376–382 [DOI] [PubMed]
- 58.Riss J, Cloyd J, Gates J, Collins S (2008) Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 118(2):69–86 [DOI] [PubMed]
- 59.Roufogalis BD, Minocherhomjee AM, Al-Jobore A (1983) Pharmacological antagonism of calmodulin. Can J Biochem Cell Biology 61:927–933 [DOI] [PubMed]
- 60.Schedel A, Schloss P, Kluter H, Bugert P (2008) The dopamine agonism on ADP-stimulated platelets is mediated through D2-like but not D1-like dopamine receptors. Naunyn Schmiedebergs Arch Pharmacol 378:431–439 [DOI] [PubMed]
- 61.Sen A, Akin A, Craft KJ, Canfield DV, Chaturvedi AK (2007) First-generation H1 antihistamines found in pilot fatalities of civil aviation accidents, 1990–2005. Aviat Space Environ Med 78:514–522 [PubMed]
- 62.Shayegan DK, Stahl SM (2004) Atypical antipsychotics: matching receptor profile to individual patient’s clinical profile. CNS Spectr 9:6–14 [DOI] [PubMed]
- 63.Sheehan P (2007) Hyperemesis gravidarum—assessment and management. Aust Fam Physician 36:698–701 [PubMed]
- 64.Sherman-Gold R, Dudai Y (1983) Heterogeneity in the physicochemical properties of deoxycholate-solubilized benzodiazepine receptors from calf cerebral cortex. Neurochem Res 8:853–864 [DOI] [PubMed]
- 65.Sindrup SH, Bjerre U, Dejgaard A, Brosen K, Aaes-Jorgensen T, Gram LF (1992) The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther 52:547–552 [DOI] [PubMed]
- 66.Skolnick P, Paul SM (1982) Benzodiazepine receptors in the central nervous system. Int Rev Neurobiol 23:103–140 [DOI] [PubMed]
- 67.Steinkopf S, Schelderup AK, Gjerde HL, Pfeiffer J, Thoresen S, Gjerde AU, Holmsen H (2008) The psychotropic drug olanzapine (Zyprexa) increases the area of acid glycerophospholipid monolayers. Biophys Chem 134:39–46 [DOI] [PubMed]
- 68.Stimmel GL, Gutierrez MA, Lee V (2002) Ziprasidone: an atypical antipsychotic drug for the treatment of schizophrenia. Clin Ther 24:21–37 [DOI] [PubMed]
- 69.Stuhne-Sekalec L, Chudzik J, Stanacev NZ (1987) Effect of chlorpromazine on the synthesis, hydrolysis, and transfer of microsomal cytidine liponucleotides and mitochondrial polyglycerophosphatides. Can J Physiol Pharmacol 65:377–384 [DOI] [PubMed]
- 70.Tharmapathy P, Fukami MH, Holmsen H (2000) The stimulatory effects of cationic amphiphilic drugs on human platelets treated with thrombin. Biochem Pharmacol 60:1267–1277 [DOI] [PubMed]
- 71.Tysnes OB, Aarbakke GM, Verhoeven AJ, Holmsen H (1985) Thin-layer chromatography of polyphosphoinositides from platelet extracts: interference by an unknown phospholipid. Thromb Res 40:329–338 [DOI] [PubMed]
- 72.Tysnes OB, Steen VM, Frolich KW, Holmsen H (1990) Evidence that chlorpromazine and prostaglandin E1 but not neomycin interfere with the inositol phospholipid metabolism in intact human platelets. FEBS Lett 264:33–36 [DOI] [PubMed]
- 73.Underhaug Gjerde A, Holmsen H, Nerdal W (2004) Chlorpromazine interaction with phosphatidylserines: a (13) C and (31) P solid-state NMR study. Biochim Biophys Acta 1682:28–37 [DOI] [PubMed]
- 74.Verma A, Snyder SH (1989) Peripheral type benzodiazepine receptors. Annu Rev Pharmacol Toxicol 29:307–322 [DOI] [PubMed]
- 75.Villars PS, Veazie MQ, Berger JS, Vu QM, Campbell-McAdory AA, Frenzel JC, Kee SS (2008) Adaptation of the OODA Loop to reduce postoperative nausea and vomiting in a high-risk outpatient oncology population. J Perianesth Nurs 23:78–86 [DOI] [PubMed]
- 76.Wagner J, Wagner ML (2000) Non-benzodiazepines for the treatment of insomnia. Sleep Med Rev 4:551–581 [DOI] [PubMed]
- 77.Walsh MT, Ryan M, Hillmann A, Condren R, Kenny D, Dinan T, Thakore JH (2002) Elevated expression of integrin alpha(IIb) beta(IIIa) in drug-naive, first-episode schizophrenic patients. Biol Psychiatry 52:874–879 [DOI] [PubMed]
- 78.Warrington L, Lombardo I, Loebel A, Ice K (2007) Ziprasidone for the treatment of acute manic or mixed episodes associated with bipolar disorder. CNS drugs 21:835–849 [DOI] [PubMed]
- 79.Weiner I, Schiller D, Gaisler-Salomon I (2003) Disruption and potentiation of latent inhibition by risperidone: the latent inhibition model of atypical antipsychotic action. Neuropsychopharmacology 28:499–509 [DOI] [PubMed]
- 80.Yao JK, van Kammen DP, Gurklis J, Peters JL (1994) Platelet aggregation and dense granule secretion in schizophrenia. Psychiatry Res 54:13–24 [DOI] [PubMed]