Abstract
Two new, rapid, precise, accurate and specific chromatographic methods were described for the simultaneous determination of olmesartan medoxomil and hydrochlorothiazide in combined tablet dosage forms. The first method was based on reversed phase liquid chromatography using an Eurosphere 100 RP C18 column (250 × 4.6 mm ID, 5 μm). The mobile phase was methanol–0.05% o-phosphoric acid (60:40 v/v) at a flow rate of 1.0 mL min−1. Commercially available tablets and laboratory mixtures containing both drugs were assayed and detected using a UV detector at 270 nm. The second method involved silica gel 60 F254 high performance thin layer chromatography and densitometric detection at 254 nm using acetonitrile–ethyl acetate–glacial acid (7:3:0.4 v/v/v) as the mobile phase. Calibration curves ranged between 200–600 and 125–375 ng spot−1 for olmesartan and hydrochlorothiazide, respectively.
Keywords: Column liquid chromatography, High performance thin layer chromatography, Hydrochlorothiazide, Olmesartan medoxomil
Introduction
Olmesartan medoxomil (OLM) is a prodrug and hydrolyzed to olmesartan during absorption from the gastrointestinal tract [1–4]. OML is a selective AT1 subtype angiotensin II receptor antagonist. OLM is described chemically as the (5-methyl-2-oxo-1,3-dioxol-4-yl) methyl ester of 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl}-1H-imidazole-5-carboxylic acid [5, 6]. Hydrochlorothiazide (HCT), 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulphonamide 1,1-dioxide, one of the oldest and widely used thiazide diuretics [7] (Fig. 1).
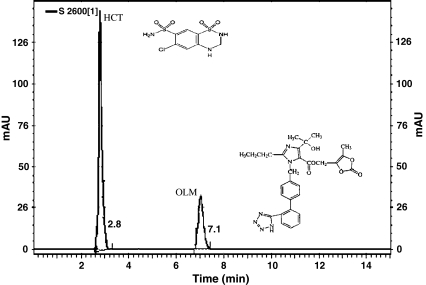
Fig. 1.
Representative HPLC chromatogram and structures of olmesartan medoxomil OLM (20 μg mL−1) and hydrochlorothiazide HCT (12.5 μg mL−1)
OLM has not yet been officially described in any pharmacopoeia. A literature survey revealed that several analytical methods were reported for the determination of OML in biological fluids including liquid chromatography tandem mass spectrometry (LC–MS–MS), capillary electrophoresis (CE), LC and high performance thin layer chromatography (HPTLC) [8–13]. OLM determination has been reported for single preparations or in combination with other antihypertensive drugs [14, 15]. The USP describes an RP-HPLC method for the determination of HCT in tablets. Several analytical methods have been reported for the determination of HCT in pharmaceutical formulations including polarography, LC, HPTLC, and spectrofluorometry [16–26]. A literature survey revealed that analytical methods have not been reported for the determination of OML and HCT in a combined tablet formulation. The present study was therefore aimed to provide such an economically viable RP-LC and HPTLC method.
Experimental
Apparatus
The HPLC system consisted of a LC Knauer pump smartline 1000 and a Knauer manual injector using a 20 μL fixed loop. A UV/VIS detector was set at 270.0 nm and peak areas were integrated automatically using Chromgate software. Samples were applied as 8 mm bands by means of a Camag Linomat V automatic sample applicator (Muttenz Switzerland) equipped with a 100 μL syringe. The distance between the bands was 11.4 mm. Silica gel 60 F254 HPTLC plates (20 × 10 cm, aluminum) were from Merck (Darmstadt, Germany). Densitometric scanning was performed at 254 nm with a Camag TLC scanner 3 equipped with Camag Wincats software 1.4.2 using the deuterium light source and slit dimensions of 6.00 mm × 0.45 mm.
Chemicals
Olmesartan medoxomil (OML) and hydrochlorothiazide (HCT) were kindly supplied by Ajanta Pharmaceutical Pvt. (Paithan) and Glen Mark Pharma (Nashik). All chemicals were of HPLC grade, methanol and acetonitrile (S d fine-chem, Mumbai, India), water and ethyl acetate (Merck, Mumbai, India), o-phosphoric acid (Qualigens, Mumbai, India). Commercially available tablets (Olmesar-H of Macleod, Gujarat, India), containing 20 mg OML and 12.5 mg HCT per tablet, 12.5 mg tablet−1) were used for analysis.
Stock Solutions of Standard OLM and HCT for RP-LC and HPTLC
OLM and HCT stock solutions (1.0 mg mL−1) were prepared in methanol. The standard working concentrations of mixed OLM (20 μg mL−1) and HCT (12.5 μg mL−1) were prepared in the mobile phase using methanol–0.05% o-phosphoric acid (60:40 v/v). This solution was subjected to LC analysis. A 10 mL standard working solution containing 200 ng OML and 125 ng HCT was spotted on the TLC plate.
Reversed Phase High Performance Liquid Chromatography
Chromatographic Conditions
Solutions and mobile phases were freshly prepared prior to use. The mobile phase consisted of methanol–0.05% o-phosphoric acid (60:40 v/v). The analytical column was an Eurosphere 100 C18 column (250 × 4.6 mm, Knauer, Berlin, Germany). UV detection was carried out at 270 nm. Analyses were performed under isocratic conditions at a flow-rate of 1.0 mL min−1. All solvents were filtered through 0.45 μm membrane filters and degassed in an ultrasonic bath.
Calibration
For calibration purposes, a range of 4–24 μg mL−1 OLM and 2.5–15 μg mL−1 HCT solutions were prepared and 20 μL injections were carried out in triplicate. OLM and HCT sample concentrations were calculated based on calibration curves. System suitability tests were also carried out.
Analysis of Tablet Formulations
Ten tablets were weighed accurately and powdered. Powder equivalent to 100 mg OLM and 62.5 mg HCT was weighed and transferred to a 100 mL volumetric flask. It was dissolved in 50 mL methanol by shaking the flask for 15 min and filled to volume with methanol, followed by filtration through a 0.45 μm membrane filter. A final concentration of 20 μg mL−1 of OLM and 12.5 μg mL−1 of HCT were prepared and concentrations of the OLM and HCT were calculated from the calibration graph.
Recovery Study
The accuracy of the proposed method was evaluated by the addition of a standard drug solution to a pre-analysed tablet sample solution at three different concentration levels at 80, 100 and 120% of linearity for both drugs.
High Performance Thin Layer Chromatography
Chromatographic Conditions
Chromatography was performed on 20 cm × 10 cm aluminum HPTLC plates coated with 0.2 mm layers of silica gel 60 F254 (Merck). Before use plates were washed with methanol and dried in an oven at 120 °C for 20 min. Ascending development of the plate with a migration distance of 70 mm was performed at 23 ± 2 °C using acetonitrile–ethyl acetate–glacial acid (7:3:0.4, v/v/v) as the mobile phase and a Camag twin-trough chamber previously saturated with mobile phase for 20 min. The average development time was 20 min.
Calibration
Mixed working standard solutions equivalent to 20, 30, 40, 50, 60 μL were separately spotted on the TLC plate in order to obtain final OML concentrations at 200, 300, 400, 500, 600 ng spot−1 and HCT at 125, 187.5, 250, 312.5, 375 ng spot−1, respectively. The plates were developed in a 20 × 10 cm twin through chamber using 20 mL freshly prepared mobile phase.
Analysis of Tablet Formulation
Ten tablets were weighed, triturated and the average tablet weight was calculated. A 1.0 mg mL−1 solution was prepared in methanol and filtered through Whatman filter paper no. 41. Five mL tablet stock solution were diluted to 50 mL with methanol which was used as the working standard solution. This solution was spotted (5 μL) on the HPTLC plate to give a concentration of 500 ng spot−1 of OLM equivalent to 312.5 ng spot−1 of HCT. Both concentrations were calculated from the calibration graph.
Recovery Study
The accuracy of the proposed method was evaluated by the addition of a standard drug solution to a pre-analysed tablet sample solution at three different concentration levels at 80, 100 and 120% of linearity for both drugs.
Results and Discussion
Reversed Phase High Performance Liquid Chromatography
A satisfactory separation was obtained when using methanol–0.05% o-phosphoric acid (60:40 v/v) under isocratic conditions and a flow rate of 1.0 mL min−1. Peaks were well defined, resolved and almost free from tailing. Retention times for OLM and HCT were observed at 2.8 min and 7.1 min, respectively (Fig. 1) and the optimum wavelength was determined to be 270.0 nm.
System suitability tests were also carried out to verify reproducibility and results are summarised in Table 1. For quantitative applications linear calibration graphs were obtained with correlation coefficients of 0.9998 and 0.9999 for OLM and HCT, respectively. Limits of detection (LOD) were 0.44 μg mL−1 for OML and 0.21 μg mL−1 for HCT limits of quantitation (LOQ) 1.32 μg mL−1 for OML and 0.63 μg mL−1 for HCT, which showed good precision for the proposed RP-LC method [26]. The proposed method was used for the determination of both drugs in tablets and results are shown in Table 2 indicating satisfactory recoveries and high precision.
Table 1.
Calibration graphs and system suitability (n = 5) of olmesartan medoxomil and hydrochlorothiazide by RP-LC and HPTLC
| Parameter | RP-LC | HPTLC | ||
|---|---|---|---|---|
| OLM | HCT | OLM | HCT | |
| Linearity range | 4–24 μg mL−1 | 2.5–15 μg mL−1 | 200–600 ng spot−1 | 125–375 ng spot−1 |
| Regression equation | ||||
| Slope | 24,055 | 104,515 | 8.79 | 9.18 |
| Intercept | 688.3 | −1,915.7 | 184.12 | 441.13 |
| Coefficient of correlation | 0.9998 | 0.9999 | 0.9992 | 0.994 |
| Limit of detection (LOD) | 0.44 μg mL−1 | 0.21 μg mL−1 | 20.20 ng spot−1 | 18.65 ng spot−1 |
| Limit of quantitation (LOQ) | 1.32 μg mL−1 | 0.63 μg mL−1 | 61.07 ng spot−1 | 57.24 ng spot−1 |
| System suitability | ||||
| Asymmetry | 1.75 | 1.22 | – | – |
| No. of theoretical plates | 2,313 | 4,976 | – | – |
| Capacity factor | 12.8 | 34.33 | – | – |
Table 2.
Results of analysis of commercially available tablets containing olmesartan medoxomil and hydrochlorothiazide by RP-LC and HPTLC
| Method | RP-LC | HPTLC | ||
|---|---|---|---|---|
| OLM | HCT | OLM | HCT | |
| Labeled claim mg tablet−1 | 20 | 12.5 | 20 | 12.5 |
| % mean (n = 4) | 100.24 | 100.1 | 100.09 | 99.77 |
| Standard deviation | 0.2926 | 0.3354 | 1.3123 | 1.6030 |
| SE | 0.1462 | 0.1677 | 0.6562 | 0.8015 |
| RSD (%) | 0.2919 | 0.3351 | 1.3112 | 1.6067 |
SE standard error
High Performance Thin Layer Chromatography
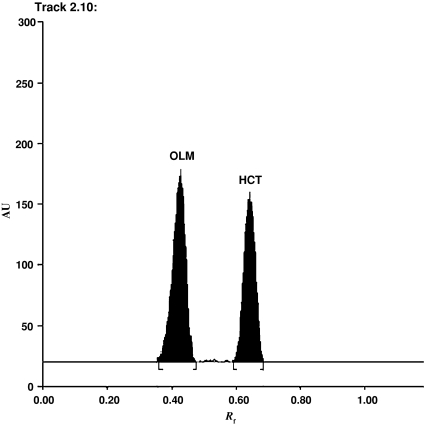
A number of experimental parameters, such as mobile phase composition, scan modes and detection wavelengths, were optimized during method development in order to provide accurate, precise and reproducible results for the simultaneous determination of OML and HCT. Maximum separation (OML Rf 0.44, HCT Rf 0.64) and minimum tailing were obtained when using a mobile phase composition of acetonitrile–ethyl acetate–glacial acid (7:3:0.5 v/v/v), respectively (Fig. 2).
Fig. 2.
Densitogram of olmesartan medoxomil (200 ng spot−1, Rf 0.44) and hydrochlorothiazide (125 ng spot−1, Rf 0.64)
Table 1 shows that correlation coefficients were 0.999 for OML and 0.994 for HCT. The LOD values were 20 ng spot−1 for OML and 19 ng spot−1 for HCT while LOQ values were 61 and 57 ng spot−1 for OML and HCT, respectively. The proposed method was used for the determination of both drugs in tablets and results are also shown in Table 2. Good recoveries and standard deviations were observed.
Conclusion
The selected methods were found to be sensitive, reproducible and accurate for the analysis of olmesartan medoxomil and hydrochlorothiazide in tablets. In general, both methods were found to be suitable for routine quality control analysis of the drugs in a combined tablet dosage form.
Acknowledgments
The authors express their gratitude to Ajanta Pharmaceutical Pvt. Ltd. (Paithan) and Glen mark Pharma Ltd. (Nashik) for providing gift samples of pure olmesartan medoxomil and hydrochlorothiazide, respectively. Thanks are also extended to Management and Principal, MGV’s Pharmacy College for providing necessary facilities.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Koike H, Konse T, Sada T, Ikeda T, Hyogo S, Hinman D, Saito H, Yanagisawa H (2003) Annu Rep Snakyo Res Lab 55:1–91
- 2.Mire DE, Silfani TN, Pugsley MK (2005) J Cardiovasc Pharmacol 46:585–593. doi:10.1097/01.fjc.0000180902.78230.fd [DOI] [PubMed]
- 3.Kobayashi N, Fujimori I, Watanabe M, Ikeda T (2000) Anal Biochem 287:272–278. doi:10.1006/abio.2000.4840 [DOI] [PubMed]
- 4.Yanagisawa H, Amemiya Y, Kanazaki T, Shimoji Y, Fujimoto K, Kitahara Y, Sada T, Mizuno M, Ikeda M, Miyamoto S, Furukawa Y, Koike H (1996) J Med Chem 39:323–338. doi:10.1021/jm950450f [DOI] [PubMed]
- 5.Unger T, McInnes GT, Neutel JM, Bohm M (2004) Drugs 64:2731–2739. doi:10.2165/00003495-200464240-00002 [DOI] [PubMed]
- 6.Reynolds JGF (ed) (1989) Martindale: the extra pharmacopoeia, 29th edn. The Pharmaceutical Press, London, pp 977–978
- 7.Dongyang L, Pei H, Nobuko M, Xiaoming L, Li L, Ji J (2007) J Chromatogr B 856:190–197. doi:10.1016/j.jchromb.2007.05.049 [DOI]
- 8.Vaidya VV, Roy MN, Yetal SM, Joshi SS, Parekh SA (2008) Chromatographia 67:147–150. doi:10.1365/s10337-007-0453-x [DOI]
- 9.Mustafa C, Sacide A (2007) Chromatographia 66:929–933. doi:10.1365/s10337-007-0424-2 [DOI]
- 10.Sagirli O, Onal A, Toker SE, Ensoy DS (2007) Chromatographia 66:213–218. doi:10.1365/s10337-007-0304-9 [DOI]
- 11.Tomonori M, Hidetoshi K, Naoto F, Michinobu O, Takao K, Fumiyo K (2008) J Pharm Biomed Anal 47:553–559. doi:10.1016/j.jpba.2008.02.021 [DOI] [PubMed]
- 12.Shah NJ, Suhagia BN, Shah RR, Patel NM (2007) Ind J Pharm Sci 69:834–836. doi:10.4103/0250-474X.39447 [DOI]
- 13.British Pharmacopoeia (2001) Her Majesty Stationary Office, London, pp 2144 (II)
- 14.The United States Pharmacopoeia, NF (2003) pp 911 (24)
- 15.Farthing D, Fakhry I, Ripley E, Sica D (1998) J Pharm Biomed Anal 17:1455–1459. doi:10.1016/S0731-7085(98)00021-1 [DOI] [PubMed]
- 16.Martin ME, Hernandez OM, Jimenez AI, Arias JJ, Jimenez F (1999) Anal Chim Acta 381:247–256. doi:10.1016/S0003-2670(98)00732-6 [DOI]
- 17.Erk N, Onur F (1996) Anal Lett 29:1963–1974
- 18.Martin E, Hernandez O, Jimenez F, Arias JJ (1995) Anal Lett 28:1449–1464
- 19.Erk N (1999) J Pharm Anal 20:155–167. doi:10.1016/S0731-7085(99)00009-6 [DOI]
- 20.Panderi IE (1999) J Pharm Biomed Anal 21:257–265. doi:10.1016/S0731-7085(99)00134-X [DOI] [PubMed]
- 21.Erk N, Onur F (1997) Analysis 25:161–167
- 22.Ouyang J, Baeyens WRG, Delanghe J, Van Der Weken G, De Keukeleire D (1999) Anal Chim Acta 386:257–264. doi:10.1016/S0003-2670(99)00020-3 [DOI]
- 23.Shetkar PB, Shinde VM (1997) Anal Lett 30:1143–1152
- 24.Erk N (2001) J Pharm Biomed Anal 24:603–611. doi:10.1016/S0731-7085(00)00434-9 [DOI] [PubMed]
- 25.Gindy AE, Ashour A, Fattah LA, Shabana MM (2001) J Pharm Biomed Anal 25:171–179. doi:10.1016/S0731-7085(00)00480-5 [DOI] [PubMed]
- 26.ICH Guideline for Industry (1996) Q2 R1:9