Abstract
Little is known about the specific functional contribution of the human orbitofrontal cortex with regard to memory processing, although there is strong evidence from lesion studies in monkeys that it may play an important role. The present investigation measured changes in regional cerebral blood flow with positron emission tomography in normal human subjects who were instructed to commit to memory abstract visual patterns. The results indicated that the rostral orbitofrontal region (area 11), which is primarily linked with the anterior medial temporal limbic region and lateral prefrontal cortical areas, is involved in the process of encoding of new information.
Damage to the limbic medial temporal region yields a severe amnesic syndrome (1–3). Recent work in the monkey has shown that the rhinal region, i.e., the entorhinal and perirhinal cortex of the medial temporal lobe, is critical for recognition memory (4, 5). Anatomical studies of the connections of the entorhinal cortex have demonstrated that it is most strongly linked with the orbital and ventromedial frontal cortex (6, 7). For instance, Insausti et al. (6), in their extensive study of entorhinal afferents, noted that, in the frontal lobe, the greatest number of labeled cells after injection of retrograde tracers in the entorhinal cortex were observed in the orbitofrontal region. Only a few labeled neurons were found in lateral frontal areas. More recent studies have also noted only sparse connections between the entorhinal cortex and the lateral frontal cortex (7, 8). This finding is in marked contrast to the strong connections of the mid-dorsolateral prefrontal areas with the retrosplenial cortex (7–9). Thus, the orbitofrontal region is the part of the frontal cortex that is most strongly linked with the rhinal cortical region, which recent monkey studies have shown to be critical for recognition memory (4, 5).
The aim of the present experiment was to investigate whether the orbitofrontal cortical region is a key area in the processing of new information. In addition to the above mentioned strong connections between this region and the rhinal cortex, bilateral lesions to the orbitofrontal region in nonhuman primates result in a severe recognition memory impairment (10, 11). In humans, the evidence is not clear cut, because lesions of the orbitofrontal cortex often include damage to the basal forebrain region. For instance, ruptured aneurysms of the anterior communicating artery can give rise to memory impairments, and damage to the orbitofrontal cortex can be demonstrated in these cases. However, because there is also involvement of the basal forebrain region, the memory deficits cannot be ascribed unequivocally to the orbitofrontal region (see ref. 12 for review). A recent study (13) has shown that patients with lesions of the posterior ventromedial region were impaired on an analogue of the delayed nonmatching-to-sample test that had been used with monkeys (10, 11). Furthermore, these impairments could be dissociated from the effects of more rostral lesions that seem to give rise to decision-making problems (13).
Lesions of the lateral prefrontal cortex, either in monkey (10, 11, 14) or in human subjects (12), typically leave recognition memory intact. Lesions of the dorsolateral prefrontal cortex in monkeys and humans give rise to severe problems in the monitoring and manipulation of multiple events in working memory (14–16). Lateral frontal lesions can also give rise to free recall impairments (17), and functional neuroimaging evidence has shown that free recall is associated with increased activity in the ventrolateral frontal region (18, 19).
When human subjects attempt to learn new material (verbal and nonverbal), they often use organizational strategies (e.g., classifying the material into meaningful groups and creating narratives or explicit verbal associations) to improve learning of the material. Unless these organizational strategies are the explicit aim of a study, their existence creates problems when designing functional neuroimaging studies of basic recognition memory, because one might observe activity patterns related to these organizational strategies and not necessarily the basic encoding of the material, i.e., the noticing of the novel information and the setting in motion of processing to enter this information into long-term memory. It is for this reason that, in the present study, we have opted for the use of abstract visual material that is difficult to verbalize and is therefore less likely to provoke semantic associations. Pictures of meaningful objects with the verbalization and semantic associations that they inevitably trigger, might have set in motion several cognitive processes requiring close monitoring and organization of the information and therefore activity in the lateral prefrontal cortex related to these processes. In addition, because imaging the orbitofrontal region with functional MRI is often problematic because of field inhomogeneities near sinus cavities, we have carried out this study by using positron emission tomography (PET). The present PET study was aimed at testing the hypothesis, derived from anatomy (6, 7, 20, 21) and lesion studies in monkeys (10, 11) and to a lesser extent from lesion studies in patients (12, 13), that the orbitofrontal cortex may have a major involvement in the encoding of new information.
We set up an encoding task that minimizes organizational and monitoring processes that are known to lead to an increase in activity in the lateral frontal cortex. Our hope was that, in this manner, the orbitofrontal activity (which was the focus of our study) could be demonstrated selectively. The present experiment evaluated changes in regional cerebral blood flow (CBF) with PET in normal volunteer subjects as they learned new visual information. There were two conditions in this study. In the encoding condition, the subjects viewed abstract images that they had not seen before and were required to commit these to memory. In the control condition, the subjects were simply required to view familiar abstract designs that required no learning. We used abstract visual designs (Fig. 1), because these stimuli cannot be verbalized easily and were therefore not likely to provoke semantic associations.
Figure 1.
Examples of four abstract color images that were used during the encoding test condition.
Materials and Methods
The present experiment was approved by the Ethics Committee of the Montreal Neurological Institute, and informed consent was obtained from all participants after the nature and possible risks of the study were explained. The subjects were scanned for 60 s with PET under each condition of testing. In addition to the PET scans, each subject underwent a high-resolution MRI study that was used to align data sets stereotactically for within- and between-subject averaging of the functional data obtained with PET. The subjects were scanned with a Scanditronix (Uppsala) PC-2048 system, which produces 15 image slices at an intrinsic resolution of 5.0 × 5.0 × 6.0. The distribution of CBF was measured during the 60-s scan by means of the water bolus H215O methodology (22). The CBF images were reconstructed with a 18-mm Hanning filter, normalized for differences in global CBF, coregistered with the individual MRIs (23), and transformed into the Talairach and Tournoux proportional stereotaxic space (24) by means of an automated feature-matching algorithm (25). The statistical significance of focal changes was tested by a method based on three-dimensional Gaussian random field theory (26). For an exploratory search involving all peaks within the gray matter volume of 600 ml, the threshold for reporting a peak as significant was set at t = 4.27, corresponding to a corrected probability of P < 0.05. For predicted blood flow changes within the orbitofrontal region, the threshold for significance was set at t = 3.00, corresponding to a corrected probability of P < 0.05 based on a search region of a 2-cm-diameter sphere centered over the orbitofrontal cortex (27).
PET Experiment.
Right-handed male subjects (n = 12) participated in this study (mean age, 22.6; range, 19–29 years). In the encoding condition, subjects were presented with 20 novel visual abstract images during the scan (Fig. 1). The subjects were asked to memorize the stimuli simply by looking at them and without attaching any verbal labels to them. A computer program displayed the stimuli one by one in the middle of the screen for a duration of 4 s each. The subjects were told that, after the scan, they would be tested for recognition of the presented stimuli. After the scan, the experimenter presented all of the images that the subject had previously seen along with novel images in a random order. The mean correct performance for all of the subjects was 83.3% (range, 64–100%). In the control condition, the subjects viewed three familiar visual abstract images presented in a random order. Each image was presented separately in the middle of the screen for a duration of 4 s each, similar to the encoding condition. The subjects were told that there was no memory component involved in this condition and that they should simply view each image. These two scanning conditions (active encoding and control condition) were part of an eight-scan PET session. The active encoding and control conditions were presented in a counterbalanced manner across the 12 subjects. They were always presented in either the middle or the last part of the eight-scan session. In the control condition, the three visual abstract images used were very familiar to the subjects, because these images were included in three prior PET scanning conditions and were presented again just before the control scanning condition. The six other PET conditions (not included in the present study) looked specifically at how monitoring and organizing skills, with different stimulus material, tax the lateral frontal cortex.
Results
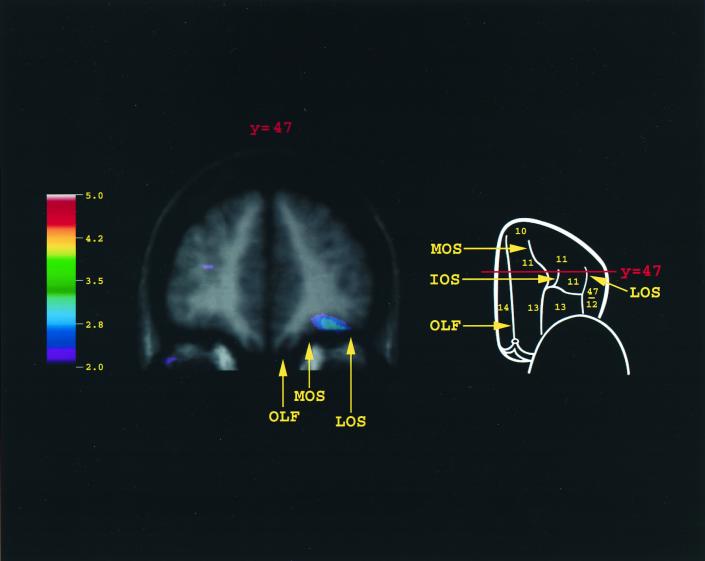
When activity in the control condition was subtracted from the encoding condition, there was a single peak of increased activity in the right orbitofrontal cortex (x = 34, y = 46, z = −17, t = 3.07). As can be seen (Figs. 2 and 3), the activity was located between the rostral parts of the medial and lateral orbital sulci, the part of the orbitofrontal cortex where area 11 is located. Outside the frontal cortex, increases in activity were observed in the left (x = −28, y = −78, z = −12, t = 6.15) and right (x = 17, y = −83, z = −14, t = 5.47) fusiform cortex and in the entorhinal/perirhinal cortex of the medial temporal lobe (x = −35, y = −13, z = −33, t = 3.59).
Figure 2.
Merged PET-MRI section illustrating the average regional CBF increase for all 12 subjects in orbitofrontal area 11. The schematic outline of the brain indicates the level of the coronal section (y = 47). Note that the activity was localized within area 11, lying between the medial and the lateral orbital sulci in the anterior part of the orbitofrontal cortex. A point must be made with regard to our definition of area 11. In the monkey, the orbital frontal cortex has been parcellated into a number of distinct architectonic areas over the years (42–44). In the human brain, however, area 11 was used loosely by Brodmann to refer to the entire orbital frontal cortex (except for the orbital extension of the ventrolateral frontal cortex). Brodmann's parcellation was adopted in the Talairach and Tournoux stereotaxic atlas (24), which is commonly used in the functional neuroimaging field. However, the human orbital frontal cortex that Brodmann referred to globally as area 11 is not cytoarchitectonically homogeneous and has recently been subdivided into different architectonic areas that correspond to areas 13, 14, and 11 of the orbital frontal cortex of the macaque monkey (45). As can be seen in the present experiment, the activation was confined within the human homologue of area 11. There was no activation within the caudal-most part of the orbitofrontal cortex (area 13) or in the gyrus rectus (area 14), which had been included previously as part of area 11 by Brodmann. IOS, intermediate orbital sulcus; LOS, lateral orbital sulcus; MOS, medial orbital sulcus; OLF, olfactory sulcus.
Figure 3.
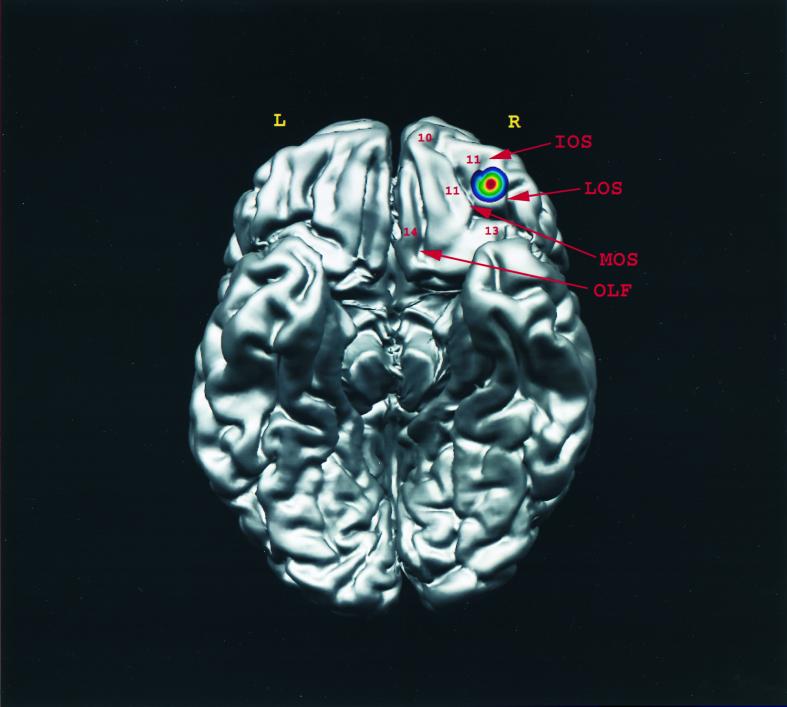
Summary diagram indicating the location of the peak in area 11 after the encoding of abstract visual information was subtracted from its control condition in the present experiment. The activation was projected on the right side of the orbital view of the brain, R and L representing the right and left hemispheres, respectively. IOS, intermediate orbital sulcus; LOS, lateral orbital sulcus; MOS, medial orbital sulcus; OLF, olfactory sulcus.
When CBF in the right orbitofrontal focus (area 11), which showed increased activity during the encoding of the new visual information, was correlated with CBF in the rest of the brain, there were positive correlations with the right caudal orbitofrontal cortex (x = 13, y = 17, z = −23, t = 4.43) and the right entorhinal/perirhinal cortex (area 28/35; x = 21, y = −28, z = −17, t = 3.76).
Discussion
The question addressed in the present study was whether there would be significant functional activation of the orbitofrontal cortex when subjects were required to encode information about novel abstract visual stimuli in the context of a task with minimal requirements for executive processing, such as monitoring and organizational strategies, known to involve lateral frontal cortex (28). When activation in the control condition, in which minimal memorization was required, was subtracted from the encoding condition, a significant increase in activity was observed in area 11 of the right orbitofrontal cortex (see Figs. 2 and 3). It is important to note that the comparison between the encoding and control tasks did not reveal differences in activity within the lateral prefrontal cortex, suggesting that the encoding and the control tasks were successfully matched in terms of the executive processes known to depend on the lateral prefrontal cortex.
The lateralization of the activity within the right orbitofrontal cortex is consistent with the general role of the right hemisphere in the processing of nonverbal information, which is also reflected as impaired processing in studies of the effects of lesions in memory-related regions (1). In functional neuroimaging studies, the frequently observed lateralization of activity related to encoding in the left hemisphere, as expressed by the hemispheric asymmetry principle (29), is most probably due to the fact that human subjects tend to use verbal strategies to organize and to learn a wide variety of stimuli, including nonverbal ones. In the present study, an effort was made to control the contribution of verbal strategies by asking subjects to encode abstract visual material that was difficult to verbalize and therefore less likely to activate areas of the left frontal lobe.
Orbitofrontal activity, along with lateral frontal activity, was observed in some earlier studies requiring the encoding of new material (30–32). In these PET studies (30–32), however, the emphasis was placed on the lateral frontal activity, and the orbitofrontal activity could not be related unequivocally to the learning of new material. The demonstration in the present study of a selective increase in activity in the orbitofrontal cortex (when activity in the lateral frontal cortex is carefully controlled) provides powerful evidence, in the normal human brain, that this region of the frontal cortex plays a key role in the processing of new information. As such, these results are consistent with work in monkeys (10, 11) that has suggested that the orbitofrontal cortex may be a critical frontal component of the limbic circuit underlying the acquisition of new information. In a recent PET study, with a separate group of normal volunteer subjects, we extended the present results by demonstrating that area 11 is also involved in the encoding of auditory information (33).
The results of the correlation analyses showed that, when the subjects were attempting to commit to memory novel abstract images, the orbitofrontal cortex (area 11) was in close functional interaction with the right entorhinal/perirhinal cortex. It is interesting that this particular portion of the medial temporal memory system was shown recently to be the most important one for visual recognition memory in the monkey (4, 5). It should be noted, however, that the direct comparison between the encoding and the control condition did not demonstrate increased medial temporal activity. Some studies failed to observe such changes (34–36), whereas others did make such observations (31, 37–39). Several explanations have been proposed to account for this inconsistent activation of medial temporal areas in encoding tasks. It has been suggested that these areas may be continually active and thus do not show differential activation in relation to the encoding of novel information. Another possible explanation is that encoding processes may involve relatively sparse changes within the medial temporal region that are difficult to detect with current neuroimaging techniques (35, 36).
The present experiment investigated changes in relation to the encoding of visual stimuli in a situation where both the experimental and the control conditions made minimal demands on various executive processes, such as monitoring of information, subserved by lateral prefrontal cortical areas (28). Under these particular conditions, area 11, which maintains connections both with the caudal orbitofrontal cortex and, importantly, with the lateral frontal areas, was the only orbitofrontal area that demonstrated increased activity during an attempt to memorize new information. Thus, the orbitofrontal cortex in association with the rhinal region of the medial temporal lobe plays a major role in the noticing of novel information and the setting in motion of processes to enter this information into long-term memory. Depending on the complexity of the materials and the need to employ more complex strategies to organize material and monitor its memorization, various lateral frontal areas will be engaged to different degrees. Consistent with this interpretation is the observation that, in other functional neuroimaging studies in which subjects were required to encode a series of words or complex visual scenes, encouraging organization of the material and thus improve learning, several foci within the lateral prefrontal cortex have shown increased activity (e.g., refs. 30, 31, 34, 40, and 41).
Acknowledgments
We thank the staff of the McConnell Brain Imaging Center for their technical assistance. This work was supported by grants from the Medical Research Council of Canada.
Abbreviations
- CBF
cerebral blood flow
- PET
positron emission tomography
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140543497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140543497
References
- 1.Milner B. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 2.Mishkin M. Philos Trans R Soc London B. 1982;298:85–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- 3.Squire L R, Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki W, Zola-Morgan S, Squire L R, Amaral D G. J Neurosci. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meunier M, Bachevalier J, Mishkin M, Murray E A. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insausti R, Amaral D G, Cowan W M. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- 7.Barbas H, Blatt G J. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- 8.Petrides M, Pandya D N. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 9.Morris R, Pandya D N, Petrides M. J Comp Neurol. 1999;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Bachevalier J, Mishkin M. Behav Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- 11.Meunier M, Bachevalier J, Mishkin M. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- 12.Petrides M. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Amsterdam: Elsevier; 2000. , in press. [Google Scholar]
- 13.Bechara A, Damasio H, Tranel D, Anderson S W. J Neurosci. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrides M. J Neurosci. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrides M, Milner B. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 16.Petrides M. Proc R Soc London Ser B. 1991;246:299–306. doi: 10.1098/rspb.1991.0158. [DOI] [PubMed] [Google Scholar]
- 17.Incisa Della Rocchetta A, Milner B. Neuropsychologia. 1993;31:503–524. doi: 10.1016/0028-3932(93)90049-6. [DOI] [PubMed] [Google Scholar]
- 18.Petrides M, Alivisatos B, Evans A C. Proc Natl Acad Sci USA. 1995;92:5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmichael S T, Price J L. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 21.Carmichael S T, Price J L. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Raichle M E, Martin W R W, Herscovitch P, Mintun M A, Markham J. J Nucl Med. 1983;24:790–798. [PubMed] [Google Scholar]
- 23.Woods R P, Mazziotta J C, Cherry S R. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Georg Thieme; 1988. [Google Scholar]
- 25.Collins D L, Neelin P, Peters T M, Evans A C. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 26.Worsley K J, Evans A C, Marrett S, Neelin P. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 27.Worsley K J, Marrett S, Neelin P, Vandal A C, Friston K J, Evans A C. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Petrides M. Philos Trans R Soc London B. 1996;351:1455–1462. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- 29.Tulving E, Kapur S, Craik F I M, Moscovitch M, Houle S. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tulving E, Markowitsch H J, Kapur S, Habib R, Houle S. NeuroReport. 1994;5:2525–2528. doi: 10.1097/00001756-199412000-00030. [DOI] [PubMed] [Google Scholar]
- 31.Haxby J V, Ungerleider L G, Horwitz B, Maisog J M, Rapoport S I, Grady C L. Proc Natl Acad Sci USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtney S M, Ungerleider L G, Keil K, Haxby J V. Cereb Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Frey S, Kostopoulos P, Petrides M. Neuroimage. 1999;9:S944. doi: 10.1016/j.neuroimage.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Kapur S, Craik F I M, Tulving E, Wilson A A, Houle S, Brown G M. Proc Natl Acad Sci USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fletcher P C, Frith C D, Grasby P M, Shallice T, Frackowiak R S J, Dolan R J. Brain. 1995;118:401–416. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- 36.Buckner R L, Koutstaal W. Proc Natl Acad Sci USA. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley W M, Miezin F M, McDermott K B, Buckner R L, Raichle M E, Cohen N J, Ollinger J M, Akbudak E, Conturo T E, et al. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 38.Grady C L, McIntosh A R, Horwitz B, Miasog J M, Ungerleider L G, Mentis M J, Pietrini P, Schapiro M B, Haxby J V. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- 39.Dolan R J, Fletcher P C. Nature (London) 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- 40.Brewer J B, Zhao Z, Desmond J E, Glover G H, Gabrieli J D E. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 41.Wagner A D, Schacter D L, Rotte M, Koutstaal W, Maril A, Dale A M, Rosen B R, Buckner R L. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 42.Walker A E. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- 43.Barbas H, Pandya D H. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 44.Carmichael S T, Price J L. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 45.Petrides M, Pandya D N. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 9. Amsterdam: Elsevier; 1994. pp. 17–58. [Google Scholar]