Abstract
NF-κB signaling has been implicated in neurodegenerative disease, epilepsy, and neuronal plasticity. However, the cellular and molecular activity of NF-κB signaling within the nervous system remains to be clearly defined. Here we show that the NF-κB and IκB homologues Dorsal and Cactus surround postsynaptic glutamate receptor (GluR) clusters at the Drosophila NMJ. We then show that mutations in dorsal, cactus and IRAK/pelle kinase specifically impair GluR levels, assayed immunohistochemically and electrophysiologically, without affecting NMJ growth, the size of the postsynaptic density, or homeostatic plasticity. Additional genetic experiments support the conclusion that cactus functions in concert with, rather than in opposition to, dorsal and pelle in this process. Finally, we provide evidence that Dorsal and Cactus act post-transcriptionally, outside the nucleus, to control GluR density. Based upon our data we speculate that Dorsal, Cactus and Pelle could function together, locally at postsynaptic density, to specify GluR levels.
INTRODUCTION
NF-κB is the founding member of a highly conserved family of Rel-homology domain containing transcription factors that have been ascribed diverse functions ranging from immunity and host defense to apoptosis, embryonic patterning and neural plasticity (Ghosh et al., 1998; Hacker and Karin, 2006; Meffert and Baltimore, 2005). The NF-κB transcription factor is embedded within a signaling cascade that conveys receptor signaling at the cell surface to NF-κB-dependent gene regulation in the cell nucleus. At the heart of the NF-κB signaling system is a core group of proteins that control NF-κB activity including the IκB/Cactus family of inhibitory molecules and the IRAK/Pelle protein kinase. The basic organization of NF-κB signaling is conserved from fly to human (Ghosh et al., 1998). Signaling is induced by ligand binding to cell surface receptors such as the Toll-like receptors involved in recognition of microbes, antigen receptors (B-cell and T-cell receptors) and cytokine receptors such as the TNF-α super family receptors (Baker and Reddy, 1998). In general, receptor activation initiates receptor-associated intracellular signaling, including activation of IRAK/Pelle kinase, followed by phosphorylation and subsequent degradation of IκB/Cactus (Haker and Karin, 2006). In the absence of signaling, IκB/Cactus binds and sequesters cytoplasmic NF-κB. In response to signaling activation, the degradation of IκB/Cactus releases NF-κB/Dorsal into the cytoplasm allowing it to translocate into the cell nucleus where it functions as a transcriptional regulator capable of increasing or decreasing target gene transcription.
Although NF-κB has been studied intensively in the context of immunity, inflammation and cancer, far less is understood about the function of NF-κB in the nervous system. NF-κB is highly expressed in both the vertebrate and invertebrate central and peripheral nervous systems. The function of NF-κB in the vertebrate central nervous system can be divided into two categories. In the first category, NF-κB is thought to regulate processes related to disease and injury. For example, NF-κB has been implicated in the cellular response to brain injury, seizure and neurodegenerative diseases such as Alzheimer’s and Parkinson’s (Mattson and Camandola, 2001; Mattson et al., 2000a). Additional related functions could include the regulation of cellular anti-oxidation and neuronal apoptosis (Mattson and Camandola, 2001). Second, NF-κB has been suggested to function during neural development and synaptic plasticity (Meffert and Baltimore, 2005; Meffert et al., 2003; Schmidt-Ullrich et al., 1996). For example, NF-κB knock-out mice have behavioral learning deficits (Meffert et al., 2003) and NF-κB has been suggested to play a role in the mechanisms of long-term synaptic plasticity (Albensi and Mattson, 2000; Mattson and Camandola, 2001; O’Mahony et al., 2006). NF-κB is also highly expressed at the vertebrate and invertebrate neuromuscular junctions (Baghdiguian et al., 1999; Cantera et al., 1999a). At the vertebrate NMJ, activation of NF-κB has been implicated in mechanisms of muscle wasting associated with disease (dystrophies and cachexia) and denervation (Cai et al., 2004; Guttridge et al., 2000). In these studies, enhanced NF-κB signaling has been shown to be deleterious. However, in both the central and peripheral nervous systems, the function of endogenous NF-κB is not well understood.
To date, studies of NF-κB signaling in the nervous system have highlighted a transcriptional function for NF-κB signaling that is consistent with the known activity of this signaling pathway in other systems. Core components of the NF-κB signaling system are present both pre- and postsynaptically and it is hypothesized that NF-κB/Dorsal can translocate from the synapse to the neuronal nucleus to control gene expression (Albensi and Mattson, 2000; Furukawa and Mattson, 1998; Meffert and Baltimore, 2005; Meffert et al., 2003). It has been speculated that NF-κB could also function locally at the synapse (Meffert and Baltimore, 2005). However, despite being an attractive hypothesis, direct experimental evidence in favor of a local synaptic function for NF-κB has yet to be defined.
We have performed a genetic analysis of NF-κB signaling at the Drosophila NMJ and provide evidence that NF-κB/Dorsal, IκB/Cactus and IRAK/Pelle are necessary to control glutamate receptor density at the postsynaptic muscle membrane. Remarkably, we failed to find evidence in support of a nuclear function of NF-κB/Dorsal. Rather, our data suggest that NF-κB/Dorsal, IκB/Cactus and IRAK/Pelle function locally, at the postsynaptic NMJ to specify GluR density.
RESULTS
Dorsal and Cactus surround postsynaptic GluR clusters
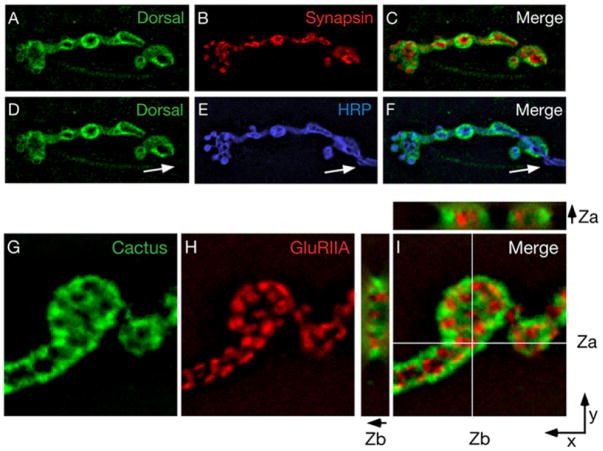
It was previously shown that NF-κB/Dorsal and IκB/Cactus are present postsynaptically at the Drosophila NMJ (Cantera et al., 1999a). We have confirmed and extended this finding. First, we demonstrate the specificity of the Cactus and Dorsal antibodies at the NMJ (Gillespie and Wasserman, 1994; Reach et al., 1996) (Supplemental Figure 1). Next, we show that Dorsal immunoreactivity surrounds presynaptic markers that delineate the neuronal membrane (anti-HRP) and define the presynaptic vesicle pool (anti-synapsin) (Figure 1A–F). In addition, Dorsal staining is absent from presynaptic axons just prior to entry into the muscle field, indicating that Dorsal protein is absent from the presynaptic motor axons (Figure 1D–F arrow). A similar analysis demonstrates that Cactus, like Dorsal, is localized postsynaptically and is absent from presynaptic axons prior to muscle innervation (Supplemental Figure 1 arrow, and data not shown). Interestingly, 3D confocal imaging of synaptic boutons at the third instar NMJ demonstrates that Cactus immunoreactivity surrounds postsynaptic GluR clusters within the muscle (Figure 1G–I). Dorsal immunoreactivity shows a similar distribution and both Cactus and Dorsal co-localize with an independent postsynaptic marker Discs-Large (Dlg) (data not shown). Based on these data, and data from previously published reports (Cantera et al., 1999a), we conclude that Dorsal and Cactus are concentrated within the same postsynaptic domain surrounding the GluR clusters. This is in contrast to the general cytoplasmic localization of these proteins in other cell types. In addition, consistent with another report (Cantera et al., 1999b), we find no evidence for expression of either Dif or Relish, two additional Rel homology domain transcription factors, at the Drosophila NMJ (data not shown).
Figure 1. Dorsal and Cactus surround postsynaptic GluR clusters at the NMJ.
A–F) The NMJ is triple-labeled with anti-Dorsal (A, D), anti-Synapsin (B), and anti-HRP (E). Dorsal protein is absent from the axon prior to the NMJ (arrow in D–F). G–I) High magnification of a synaptic bouton co-labeled with anti-Cactus (G) and anti-GluRIIA (H). A three-dimensional reconstruction shows that Cactus surrounds GluRIIA. The large panel in (I) is a single optical section of the synapse. The smaller panels on top (Za) and on left (Zb) show the Z dimension reflected along the thin vertical and horizontal lines.
Dorsal controls GluR abundance at the NMJ
To study the function of Dorsal at the NMJ we have taken advantage of a series of mutations that have been previously shown to disrupt the dorsal gene during embryonic patterning and innate immunity (Table 1). Importantly, homozygous dorsal mutants derived from heterozygous females have sufficient quantities of maternally supplied Dorsal protein to allow for normal embryonic development. This allows us to analyze the function of dorsal mutations during larval development.
Table 1.
Molecular characterization of dorsal, pelle, and cactus alleles
| Allele | Description | Molecular Lesion | References |
|---|---|---|---|
| Dorsal | |||
| dl H | Protein null | X-ray rearrangement | Roth et al., 1989 |
| dl 1 | Protein null | unsequenced EMS allele | Roth et al., 1989 |
| dl PZ | Unstable protein | R310H, altered PKA consensus site | Isoda et al., 1992 |
| dl 2 | Point mutation | G68Q, altered Rel Homology Domain | Isoda et al., 1992 |
| dl U5 | Truncated protein | Q488Stop, in Transactivation Domain | Isoda et al., 1992 |
| Pelle | |||
| Df(3R)D605 | Protein null | Large deficiency | Hecht and Anderson, 1993 |
| pll 25 | Protein null | Q93Stop in Death Domain | Towb et al., 2001 |
| pll RM8 | Protein null | Q422Stop in Kinase Domain | Towb et al., 2001 |
| pll 078 | Kinase dead | G225E in Kinase Domain | Towb et al., 2001 |
| Cactus | |||
| cactD13 | Protein null | V188Stop | Bergmann et al., 1996 |
| cact255 | Protein null | P-element in first intron | Bergmann et al., 1996, Nicolas et al., 1998 |
| cactRN | Point mutation | A196Y, near Signal-Dependent Domain | Schupbach et al., 1991, this study |
| cactHE | Point mutation | C315Y, altered Ankyrin Repeat | Schupbach et al., 1989, this study |
We first demonstrate that Dorsal is not required for synaptic growth or synapse morphology. All dorsal mutations analyzed in this study, including null mutant combinations, survive as healthy, normally sized third instar animals. We quantified bouton numbers at the NMJ of muscles 6 and 7 as done previously (Eaton and Davis, 2005). Bouton numbers are normal in a dorsal trans-heterozygous zygotic null mutant combination (dorsal1/dorsalH) and in a second trans-heterozygous mutant combination (dorsal2/doralH) compared to wild type controls (wild-type = 100±7.8, n=19; dl1/dlH = 109 ± 6.3, n=28; dl2/dlH = 98 ± 6.7, n=17; data represent % wild-type bouton number on muscles 6/7).
In the vertebrate nervous system, NF-κB signaling has been implicated in forms of neuronal plasticity that are typically associated with changes in the abundance of postsynaptic GluRs (Furukawa and Mattson, 1998; Meffert and Baltimore, 2005; Meffert et al., 2003; O’Mahony et al., 2006). Therefore, we assayed GluR abundance at the NMJ of dorsal mutant animals. To do so, we quantified the fluorescence intensity of GluRs at the NMJ using previously published methodology (Albin and Davis, 2004). GluRIIA levels are significantly decreased in dorsal null mutant animals (dl1/dlH) compared to wild type and heterozygous controls (Figure 2). A similar decrease in GluRIIA levels is also observed in a second strong loss-of-function dorsal mutant, dlPZ/dlPZ (Figure 2; Table 1; Isoda et al., 1992; Norris and Manley, 1992). Next we expressed UAS-dorsal-RNAi specifically in muscle. Dorsal protein levels, assayed by synaptic immunostaining, were decreased to 38±3.4% (n=8) of control levels demonstrating a significant protein knockdown in muscle. In these animals we find a statistically significant decrease in GluRIIA levels (66±4.8% of wild type GluRIIA levels; n=8, p<0.001 compared to control). These data support the conclusion that dosal is required in muscle for normal GluRIIA abundance. Finally, over-expression of dorsal in muscle did not change GluRIIA levels compared to wild type indicating that increased Dorsal protein levels are not sufficient to enhance GluRIIA abundance beyond that observed in wild type (Figure 2D). In all experiments, the level of a control antigen, anti-HRP, did not consistently differ between genotypes (Figure 2E). Additionally, we find a small, but statistically significant, change in Dlg levels (wild-type = 100+/−0.9, n=19; dl1/dlH = 93+/− 0.9, n=18, p<0.05, data represent % wild-type synaptic anti-DLG fluorescence). The change in synaptic Dlg is far less than that observed in other mutations that control postsynaptic morphology suggesting that Dorsal is primarily required for normal GluR abundance (Albin and Davis, 2004).
Figure 2. Decreased GluRIIA abundance in dorsal mutants.
A–C) Pseudocolor images of GluRIIA staining in wild-type, dl2/dl2, and dl1/dlH (bar at left indicates color scale). D) Quantification of GluRIIA staining at the NMJ shows that GluRIIA is reduced in all dorsal alleles. Values represent % of average wt synaptic anti-GluRIIA fluorescence levels. Values for each genotype are as follows: wt 102 ± 2.0 n=170; dl2/dl2 41 ± 2.8 n=22; dl2/dlH 56 ± 2.4 n=22; dlPZ/dlPZ 76 ± 6.2 n=10; dl1/dlH 77 ± 3.4 n=40; dl2/+ 70 ± 6.7 n=10; dlPZ/+ 93 ± 4.2 n=18; dlH/+ 91 ± 6.2 n=12; dlUY2278/+; MHC-Gal4/+ 103 ± 3.1 n=20. E) Synaptic HRP levels are not decreased in the same dorsal mutant synapses compared to wild type, except in dl2/dl2. F) Synaptic GluRIIC fluorescence level in dorsal alleles is significantly reduced (wt = 100 ± 1.8 n=44, dlPZ/dlPZ = 86 ± 5.5 n=16, dl1/dlH = 86 ± 3.5 n=21. Data represent % of average wt synaptic GluRIIC fluorescence levels). In all figures data are presented as the mean value (± standard error of the mean). Significance is shown according to **p<0.01.
Next we took advantage of existing mutations to examine the domain requirements of Dorsal/NF-κB for the regulation of GluR abundance. All NF-κB family members contain a highly conserved Rel Homology Domain (RHD). The RHD of Dorsal mediates signal-dependent phosphorylation, homodimerization, DNA binding, nuclear localization as well as interaction with other NF-κB signaling proteins such as Cactus and Pelle (Ghosh et al., 1998; Govind, 1999; Isoda et al., 1992). The dorsal2 allele (dl2) harbors a point mutation that specifically disrupts the RHD (Figure 3E, Table 1). Mutant Dorsal protein in the dl2 background remains concentrated at the NMJ (Figure 3F). Quantification of GluRIIA fluorescent intensity demonstrates that GluRIIA levels are strongly reduced in the dl2 mutant (Figure 2B, D; p<0.001 compared to wt). Surprisingly, we also observe a significant decrease in GluRIIA levels in the dl2/+ heterozygous animal (Figure 2D). Since the heterozygous null mutations show normal GluRIIA levels (dlH/+; Figure 2D), our data demonstrate that the dl2 mutation acts in a dominant-negative manner to impair GluR abundance. This is consistent with Dorsal functioning as part of a protein complex that controls GluR levels.
Figure 3. Disruption of the Dorsal transactivation domain does not impair GluRIIA abundance at the NMJ.
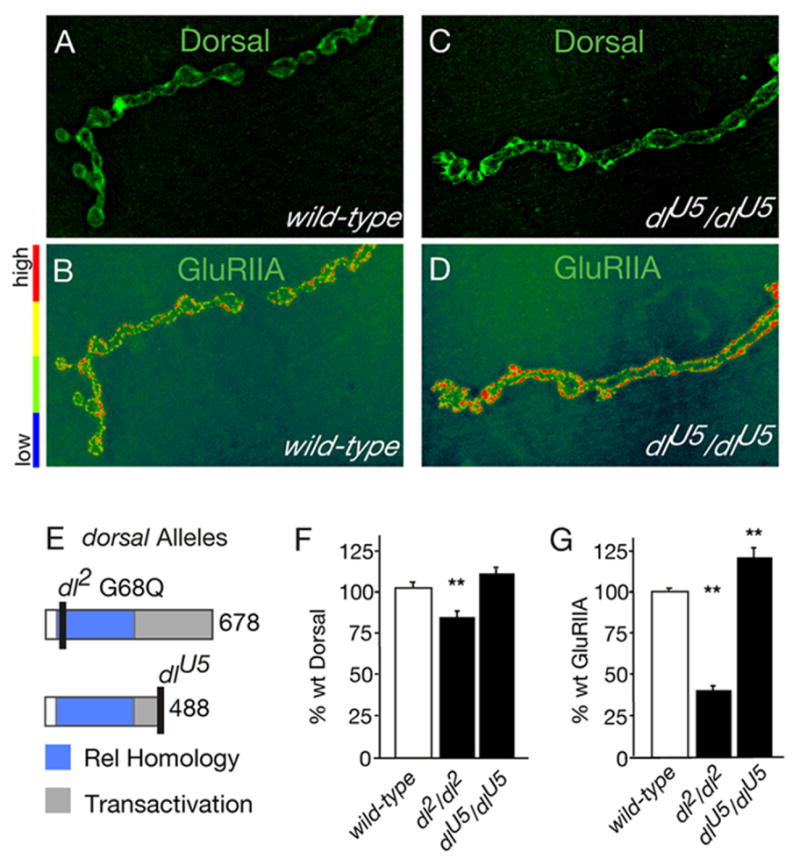
A–D) NMJ co-stained for Dorsal and GluRIIA are shown for wild-type (A, B) and dlU5/dlU5 mutants (C, D). E) Schematic of the Dorsal protein showing that dl2 is a point mutation in the Rel Homology Domain (RHD), and dlU5 is a truncation of the Transactivation Domain. F) Both dl2 and dlU5 leave Dorsal protein at the synapse (wt = 100 ± 2.5 n=51; dl2/dl2 = 82 ± 2.7 n=17; dlU5/dlU5 = 110 ± 3.5 n=15; values show % wt synaptic Dorsal fluorescence level, **p<0.01). G) Disruption of the RHD causes a decrease in synaptic GluRIIA levels (wt = 102 ± 2.0 n=170, dl2/dl2 41 ± 2.8 n=22). Disruption of the transactivation domain results in a significant increase in GluRIIA (dlU5/dlU5 120 ± 5.5 n=17 % wt synaptic GluRIIA fluorescence level, ** p<0.01).
Dorsal also contains a transactivation domain (Figure 3E) that mediates interactions between Dorsal and nuclear co-factors that are required for Dorsal-dependent transcriptional regulation (Flores-Saaib et al., 2001; Isoda et al., 1992). The dlU5 mutation truncates the transactivation domain and disrupts Dorsal-dependent transcriptional regulation in the embryo (Isoda et al., 1992). First, we demonstrate that mutant protein remains at the NMJ in dlU5 animals (Figure 3C, F). However, unlike dl2 mutants, GluRIIA levels are not decreased in dlU5 mutants (indeed there is a statistically significant increase in abundance compared to wild type, p<0.05; Figure 3A–D, G). These data are surprising since both the dl2 and dlU5 mutations cause mutant phenotypes in embryonic patterning consistent with disrupted Dorsal-mediated transcription (Isoda et al., 1992). At a minimum, our data demonstrate that mutation of protein domains necessary for Dorsal-dependent transcription in the embryo do not always correlate with decreased GluR abundance. However, we cannot rule out the possibility that there are other mechanisms of Dorsal-mediated transactivation in the context of the larval NMJ.
Mutations in dorsal Alter Synaptic Function
To determine whether the observed changes in receptor abundance correlate with altered synaptic efficacy, we performed an electrophysiological analysis of synaptic function in dorsal mutants. We find a significant reduction in the average amplitude of spontaneous miniature excitatory postsynaptic amplitudes (mEPSP) in the dorsal null mutation (dl1/dl1) compared to wild type (Figure 4A, B, G). Importantly, there was no change in the average resting membrane potential or the average muscle input resistance (Figure 4C, D). Similar observations were made for a separate allele (dlPZ/dlPZ; data not shown). Thus, the observed decrease in GluR abundance assayed by fluorescence microscopy is correlated with decreased GluR sensitivity assayed electrophysiologically.
Figure 4. Decreased mEPSP amplitude correlates with decreased GluR abundance.
A) There is a significant decrease in the average mEPSP amplitude in the dl1 mutant compared to wild type (p<0.01). There is a significant decrease in mEPSP amplitude in the dl1, GluRIIA double mutation compared to GluRIIA mutant alone (p<0.01). B) Cumulative frequency distributions show that the entire mEPSP distribution is shifted toward smaller values in mutant backgrounds that include the dl1 mutation. C) There is no difference in average resting membrane potential across genotypes. D) There is no difference in muscle input resistance comparing wild-type with dl1 or when comparing the dl1, GluRIIA double mutation to GluRIIA alone. E) There is no difference in EPSP amplitude comparing wild-type with dl1 or when comparing the dl1, GluRIIA double mutation to GluRIIA alone. F) Quantal content is increased in dl1 compared to wild type and in dl1, GluRIIA double mutation compared to GluRIIA alone. G) Representative traces showing mEPSP events in each indicated genotype.
At least five different subunits can contribute to the composition of multimeric GluR complexes at the Drosophila NMJ (Featherstone et al., 2005; Marrus et al., 2004; Petersen et al., 1997; Qin et al., 2005). To investigate whether Dorsal specifically controls the levels of the GluRIIA subunit, we examined GluRIIA-dorsal double mutant animals. We find that that mEPSP amplitudes in the GluRIIA-dorsal double mutant animals are decreased beyond that observed in the GluRIIA mutant alone (Figure 4A, B, G). This observation suggests that Dorsal may influence the abundance of GluR subunits in addition to the GluRIIA subunit. Consistent with this possibility, we find a statistically significant decrease in the levels of the GluRIIC subunit in the dorsal mutant background (Figure 2F), though there is not a statistically significant change in GluRIIB levels (dl1/dlH 98 ± 8.8 %, n=4; dl1, GluRIIAsp16/dl1, GluRIIAsp16 102±4.0, n=28 % wt GluRIIB levels). Finally, EPSP amplitudes are normal in both dorsal mutants and GluRIIA-dorsal double mutants indicating that the homeostatic, retrograde modulation of presynaptic release (Davis, 2006) is not perturbed in dorsal mutations. In conclusion, our genetic analysis demonstrates that Dorsal is required to establish or maintain normal GluR abundance at the Drosophila NMJ.
Pelle is Necessary for Normal GluR Abundance and Synaptic Function
In canonical NF-κB signaling, Pelle kinase (IRAK homologue) acts upstream of Dorsal to convey signaling from activated receptors to the Cactus/Dorsal complex (Edwards et al., 1997; Hecht and Anderson, 1993). Therefore, mutations in pelle are predicted to phenocopy the effects of the dorsal mutants on GluR abundance. We used several pelle mutant alleles to examine Pelle function at the NMJ including null mutations (pll25 and pllRM8 and Df(3R)D605) and a mutation that specifically impairs the kinase domain (pll078) (Table 1). The mutant combinations that we examine here survive as normally-sized, third instar larvae with normal bouton numbers (wild-type = 100±7.8 and pll078/pll078 = 100 ± 8.6, n=19; data represent % wild type bouton numbers).
In pelle mutant animals we find a significant decrease in GluRIIA fluorescence intensity demonstrating that Pelle is necessary for normal GluRIIA abundance (Figure 5A–C). There is no change in a control antigen (anti-HRP; Figure 5D) and no change in the levels of postsynaptic Dlg (wild-type = 100+/−0.9, n=19; pll078/pll078 = 94 +/− 6.0, n=24; data represent % wild-type synaptic anti-DLG fluorescence). Importantly, the kinase dead mutation (pll078/pll078) causes a decrease in GluRIIA levels that is as severe as observed in the null mutant conditions (pllRM8/pllRM8 or pll25/Df(3R)D605). Thus, Pelle kinase activity is required for the regulation of GluR abundance. We also observe a significant reduction in GluRIIA levels in the heterozygous null (pllRM8/+) and heterozygous kinase dead (pll078/+) animals (Figure 5C). It has been previously shown that the kinase activity of Pelle is sensitive to Pelle protein levels (Shen and Manley, 1998; Shen and Manley, 2002). Thus, our data also suggest that GluRIIA abundance is sensitive to the levels of postsynaptic Pelle kinase activity at the NMJ. We also demonstrate that muscle specific expression of a UAS-GFP-Pelle transgene restores GluRIIA fluorescence intensity to wild type levels in a pelle mutant supporting the conclusion that Pelle functions postsynaptically to specify normal GluR abundance (Figure 5C). In this experiment, overexpressed Pelle-GFP is found throughout the muscle, surrounding muscle nuclei and at the postsynaptic membranes where Dorsal and Cactus reside (data not shown). Finally, to further support a postsynaptic function for Pelle we expressed a UAS-pelle-RNAi transgene specifically in muscle (24B-GAL4), and find that GluRIIA levels are decreased to 59±6.1% of wild type levels (n=8, p<0.001). Together, our data indicate that Pelle kinase activity is required postsynaptically to control GluRIIA levels.
Figure 5. Pelle kinase regulates GluRIIA abundance at the NMJ.
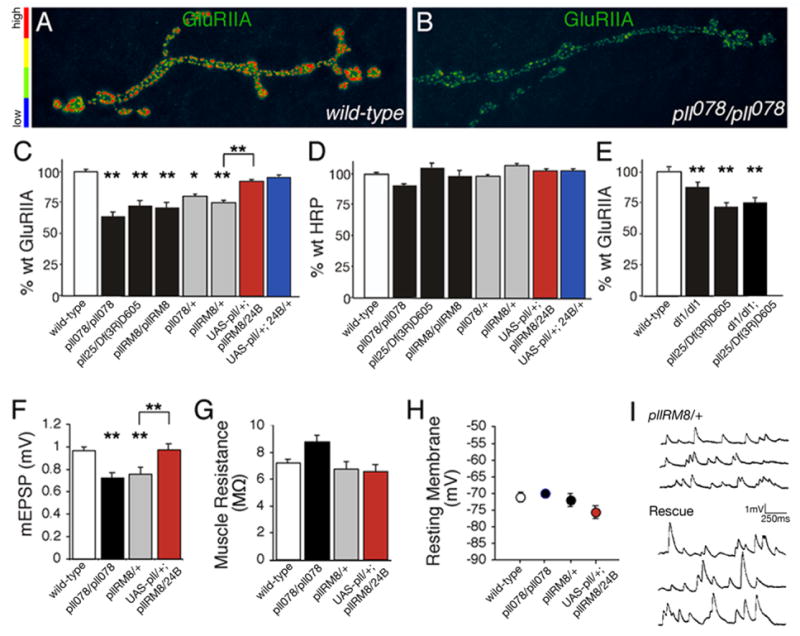
A, B) Pseudocolored images reveal that GluRIIA levels are reduced in pelle mutant synapses compared to wild type. C) Synaptic GluRIIA fluorescence intensity is reduced in all pelle alleles examined. This reduction can be rescued by muscle-specific expression of a UAS-GFP-pelle transgene (wt = 100 ± 2.1 n=104; pll078/pll078 = 56 ± 3.4 n=24; pllRM8/pllRM8 = 68 ± 4.0 n=24; pll25/Df(3R)D605 = 72 ± 2.6 n=45; pll078/+ = 80 ± 4.4 n=38; pllRM8/+ = 68 ± 2.0 n=24; UAS-Pelle/+; pllRM8/24B-Gal4 = 94 ± 1.6 n=22; UAS-Pelle/+; 24B-Gal4/+ = 96 ± 1.4 n=21). Values represent % wt synaptic GluRIIA fluorescence level. D) HRP fluorescence intensity is unaltered at the same pelle synapses. E) pll and dl null mutations are not additive (wt = 100 ± 2.7 n=42, dl1/dl1 = 83 ± 2.9 n=38, pll25/Df(3R)D605 = 72 ± 2.6 n=45, dl1/dl1; pll25/Df(3R)D605 = 75 ± 3.5 n=20). F) A significant decrease in average mEPSP amplitude in pll078/pll078 and pllRM8/+ correlates with decreased GluRIIA in these mutant backgrounds. Decreased mEPSP amplitudes are restored to wild type levels by muscle-specific expression of UAS-pelle-GFP (red). G) The decrease in mEPSP amplitude is not correlated with a decrease in muscle input resistance. H) There is no difference in resting membrane potential of the muscle across genotypes. I) Representative recordings show the rescue of mEPSP amplitudes comparing a pelle mutant (top) to the pelle mutant expressing UAS-pelle-GFP postsynaptically (bottom, “rescue”). Significance is shown according to * p<0.05; **p<0.01.
We next performed an electrophysiological analysis of synaptic function in the pelle mutant background. As in the dorsal mutants we find a significant decrease in mEPSP amplitude in pelle mutants that correlates with the observed decrease in GluRIIA levels (Figure 5F–I). Again, there is no significant change in the average muscle input resistance or resting membrane potential that could account for this change (Figure 5G, H). In addition, we demonstrate that the muscle specific expression of UAS-pelle restores mEPSP amplitude to wild type levels (Figure 5F–I). Thus, pelle mutations are similar to the dorsal mutation with respect to GluRIIA levels and synaptic electrophysiology.
We next pursued genetic epistasis experiments to examine the genetic relationship of dorsal and pelle at the NMJ. First, to formally test whether Pelle and Dorsal function in the same postsynaptic signaling cascade at the NMJ, we examined GluRIIA levels in a pelle; dorsal double null mutant animal (dl1/dl1; pll25/Df(3R)D605). In these animals the decrease in GluRIIA levels is no greater than that observed in the pelle or dorsal mutants alone (Figure 5E). These genetic data indicate that pelle and dorsal function together in the same postsynaptic signaling pathway to control GluR abundance at the NMJ. Second, to define the order of these two genes in this genetic pathway we overexpressed UAS-GFP-pelle in the dorsal null mutant background (dorsal1/dorsalH; UAS-GFP-Pelle/24B-GAL4). GluRIIA levels remain significantly decreased, in this experiment, compared to wild type controls (GluRIIA levels are 69±2.9% of wild type, p<0.01; n=8). These data are consistent with pelle functioning upstream of dorsal in the control of GluRIIA levels. In addition these data raise the possibility that the function of Pelle at the NMJ is similar to the function of Pelle kinase during embryonic patterning where it triggers the dissociation of the Cactus-Dorsal complex. If Pelle functions in a similar manner at the NMJ, we expected to see increased Dorsal and Cactus protein levels pelle mutant animals. However, in pelle mutant animals we find that Cactus is not different from wild type and Dorsal levels are slightly, but statistically significantly, reduced compared to wild type (p<0.01; Supplemental Figure 2). Thus, although Pelle is constitutively required for normal GluRIIA levels, Pelle is not constitutively acting to regulate the abundance of Dorsal and Cactus at the NMJ.
Finally, as done for our analysis of the dorsal mutation, we have tested whether disruption of pelle kinase affects the levels of other GluR subunits at the NMJ. In the pll078/pll078 animals we find that GluRIIC levels are not statistically different from controls (GluRIIC = 108±10% compared to wild type control; p>0.1, n=11) and that GluRIIB levels are slightly, but statistically significantly, increased compared to controls (GluRIIB = 119±7.5% compared to wild type control; p<0.05, n=22). It has previously been shown that mutations in the essential GluR subunits (GluRIIC, D and E) eliminate the presence of both GluRIIA and GluRIIB at the NMJ (Featherstone et al., 2005, Marrus et al., 2005, Qin et al., 2005). If the primary effect of dorsal or pelle were a change in the levels of one of the essential GluR subunits, we might expect to see parallel changes in GluRIIA and B. This is not what we observe, suggesting a more complex regulation of subunit composition by NF-κB signaling at the NMJ.
Mutations in cactus Impair GluR Abundance
In canonical NF-κB signaling IκB/Cactus binds to and sequesters NF-κB/Dorsal protein in the cytoplasm (Edwards et al., 1997; Isoda and Nusslein-Volhard, 1994). As such, IκB/Cactus acts to inhibit NF-κB/Dorsal-dependent transcription until IκB/Cactus is degraded in a signal-dependent manner (Belvin et al., 1995; Bergmann et al., 1996; Reach et al., 1996). Consistent with Cactus acting to inhibit Dorsal in Drosophila, mutations in cactus and dorsal result in opposing phenotypes during both embryonic patterning and innate immunity (Govind, 1999). Therefore, we predicted that mutations in dorsal and cactus would also result in opposing GluR phenotypes. Instead, we find that cactus mutations phenocopy the dorsal and pelle mutations.
We analyzed severe loss of function mutations in cactus including cactusE10RN and cactusA2 and observe decreased Cactus protein and decreased GluRIIA levels at the NMJ (data now shown). However, analysis of these mutations is complicated because these mutants are mid-larval lethal and have melanotic tumors associated with an essential function of Cactus in the larval hematopoietic cell lineage (Govind, 1996; Qiu et al., 1998). To circumvent mid-larval lethality, we analyzed two independent, temperature sensitive cactus alleles (cactusRN and cactusHE, Table 1; Roth et al., 1991). Both alleles cause defects in embryonic patterning similar to, but less severe than, cactus null mutations indicating that cactusRN and cactusHE are loss-of-function mutations (Schupbach et al., 1989; Schupbach et al., 1991). When we raise cactusRN and cactusHE mutants at a non-permissive temperature (29°C), these animals survive as healthy, normally sized third instar animals. This allows us to assay the function of Cactus at the mature, third instar NMJ.
We defined the molecular nature of the cactusRN and cactusHE mutations by sequence analysis and immunostaining (Table 1). CactusRN harbors an A196G mutation near the signal dependent domain, a region important for signaling-dependent degradation of Cactus protein (Bergmann et al., 1996; Govind, 1999; Reach et al., 1996). CactusHE harbors a C315Y mutation within the third ankyrin repeat of Cactus. Ankyrin repeats in Cactus mediate protein-protein interactions including interaction between Cactus and Dorsal (Govind, 1999). Both cactusRN and cactusHE have a slight, though statistically significant, decrease in levels of Cactus protein at the synapse (cactusRN/cactusRN has 93±1.1% normal Cactus protein; cactusHE/cactusHE has 88±3.7% normal Cactus protein, p<0.01; n=8) indicating that these mutations do not strongly disrupt the synaptic localization of Cactus protein.
We tested whether cactus mutations affect synaptic growth and morphology. We quantified bouton numbers in cactusRN/cactusRN and cactusHE/cactusHE animals and compared these data to control animals raised under identical conditions. We find bouton numbers in both cactus alleles are not significantly different from wild type (wild-type = 100 +/− 7.8, n=19; cactRN/cactRN = 98+/− 10.2, n=9; cactHE/cactHE = 83+/− 8.8, n=14; data represent % wild-type bouton number at m6/7). In addition, using anti-HRP to visualize synaptic boutons, we find that synapse morphology is qualitatively normal in these cactus mutant backgrounds (data not shown).
We analyzed GluR levels in homozygous cactusRN and cactusHE mutant animals as well as trans-heterozygous allelic combinations in which these alleles were placed in trans to the cactD13 null allele. We find that GluRIIA levels are significantly decreased in all four cactus mutant combinations compared to wild type (Figure 6A–C). The levels of a control antigen are unchanged (anti-HRP; Figure 6D), and there is no change in postsynaptic Dlg (wild-type = 100+/−0.9, n=19; cactRN/cactD13 = 98 +/−0.98, n=23; cactHE/cactD13 = 99 +/− 0.97, n=24; data represent % wild-type Dlg levels). Interestingly, the cactRN mutation acts as a dominant mutation for decreased GluRIIA levels (compare wild type, cactRN/+ and cactRN/cactD13, Figure 6C). Finally, GluRIIB and GluRIIC staining are also slightly, but significantly (p<0.05) reduced in these cactus mutant backgrounds indicating that multiple GluR subunits are affected (values for % wild type GluRIIC levels are cactRN/cactD13 = 85+/−1.9, n=31; cactHE/cactD13 = 89+/−1.6, n=32; values for % wild type GluRIIB are cactRN/cactRN = 87+/−5.5, n=22 p<0.05; cactHE/cactHE = 94+/−6.5, n=16).
Figure 6. GluRIIA abundance is decreased in cactus mutants.
A–B) The intensity of anti-GluRIIA fluorescence is reduced in cactus mutant synapses. C) Quantification of the decrease in GluRIIA levels observed in cactus mutants (wt = 101 ± 1.9 n= 186; cactRN/cactRN = 74 ± 6.7 n=18; cactRN/cactD13 = 59 ± 2.3 n=16; cactHE/cactHE = 60 ± 6.1 n=16; cactHE/cactD13 = 55 ± 4.8 n=17; cactRN/+ = 63 ± 3.3 n=22; cactHE/+ = 86 ± 4.9 n=22; cactD13/+ = 94 ± 7.0 n=17). D) Synaptic HRP levels are not reduced in cactus alleles. E) Average mEPSP amplitude is decreased in cactRN compared to wild type. F) EPSP amplitudes are not different comparing cactRN and wild type. G) Muscle input resistance is not different comparing cactRN and wild type. H) Resting muscle membrane potential is not different comparing cactRN and wild type. Significance is shown according to *p<0.05; **p<0.01.
To test whether dorsal and cactus function in the same genetic pathway to control GluRIIA levels we examined a double homozygous mutant combination of dorsal and cactus. We find that there is a statistically significant decrease in GluRIIA (GluRIIA is 72±2.3% of wild type; n=19, p<0.01) in this double homozygous mutant combination that is no more severe than either mutation alone. These data confirm that cactus and dorsal function in the same genetic pathway to control GluRIIA levels. To explore the possibility that cactus and dorsal might act together to control GluR levels (rather than Cactus acting to oppose Dorsal signaling) we examined GluRIIA levels in a trans-heterozygous dorsal/+ and cactus/+ null mutant combination. Heterozygous null alleles of either cactus or dorsal alone have normal GluRIIA levels (Figures 2D, 6C). However, in the trans-heterozygous animals we find a significant decrease in GluRIIA levels (cactD13/dlH = 74 +/− 6.0, n=10; data represent % wild-type synaptic GluRIIA levels, p<0.01). These genetic data are consistent with dorsal and cactus functioning together in the same genetic pathway to control GluR levels.
An electrophysiological analysis confirms that the observed decrease in GluRIIA immunostaining correlates with a decreased mEPSP amplitude in cactusRN (Figure 6E). Since there is no change in the muscle input resistance or resting membrane potential we conclude that the change in mEPSP amplitude is likely caused by the observed decrease in GluR abundance at the NMJ (Figure 6G, H). Finally, we assayed whether cactus mutations affect evoked neurotransmission. We find that EPSP amplitudes are unchanged comparing cactusRN to wild type (p>0.3; n=15, Figure 6F) demonstrating that a homeostatic increase in presynaptic release compensates for the decreased mEPSP amplitude in the cactusRN background. Thus, neither Cactus nor Dorsal appear to be involved in the mechanisms of synaptic homeostasis at the NMJ.
Mutations in cactus alter GluR levels not postsynaptic density (PSD) size
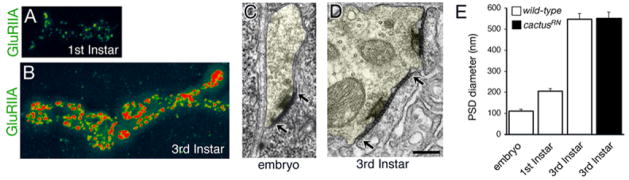
During larval development GluR abundance increases as well as the size of the presynaptic active zone and associated postsynaptic density (PSD). For example, GluRIIA antibody staining intensity at the postsynaptic membrane increases by ~350% when we compare the first instar NMJ to the third instar NMJ (Figure 7A–B). These data are consistent with the insertion of new GluRIIA receptors into pre-existing GluR clusters during synapse maturation (Rasse et al., 2005). Second, we quantified the diameter of the PSD using serial section EM at three stages of NMJ development including nascent embryonic NMJs (18 hours after egg laying), newly formed first instar NMJs (~30 hours after egg laying) and third instar NMJs (~ 4 days after egg laying). PSD size increases significantly during the first ~12 hours of synapse development and increases dramatically by the third instar (Figure 7C–E). Thus, it appears that developmental mechanisms are in place to control both GluR abundance and PSD size during this period of rapid synaptic growth.
Figure 7. Cactus mutations impair GluRIIA abundance without affecting active zone size.
A, B) NMJ from a first instar animal (A) and third instar animal (B) are shown stained for GluRIIA and imaged under identical conditions. There is a large increase in GluRIIA staining intensity per GluR cluster. C, D) Representative electron micrographs of a stage 17 embryonic synaptic bouton (C; arrows delineate an active zone and associated PSD) and a third instar synaptic bouton (arrows delineate an active zone and associated PSD). The area of the presynaptic terminal is shaded pale yellow to discriminate presynaptic terminal from postsynaptic muscle membrane. Scale is 200 nm. E) Quantification of PSD diameter demonstrates a significant increase in PSD size during development in wild type. There is no difference in PSD diameter comparing wild type and cactus third instar NMJ.
We next addressed whether postsynaptic Cactus controls PSD size with secondary effects on GluR abundance, or whether Cactus specifically controls GluR density within otherwise normally sized PSD. To do so, we compared PSD diameters measured in wild type and cactusRN mutants. Despite a ~50% decrease in GluR abundance, there is no change in PSD size in the cactusRN mutant (Figure 7E). We also analyzed GluR cluster size at the light level and find no significant difference comparing cactusRN or pelle078 to wild type (data not shown). Finally, we find no change in the extent of the SSR in the cactus mutant background at the ultrastructural level (wt SSR cross-sectional thickness: 429 nm +/− 40.3 n=16, cactRN SSR: 525 nm +/− 75.0, n=12). Since GluR abundance decreases without a change in PSD size, our data demonstrate that cactus controls GluR density within an otherwise normally sized PSD.
Dorsal functions in the cytoplasm to control GluR levels
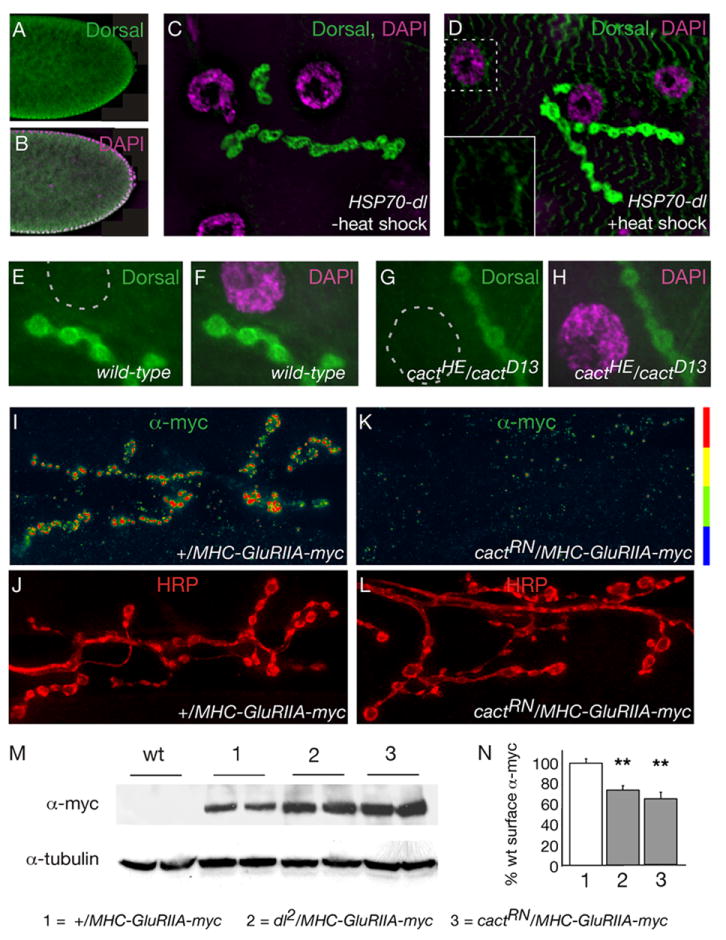
We find that mutations that render Dorsal transcriptionally incompetent in the embryo do not always correlate with decreased GluR levels (Figure 3). Furthermore, we do not observe Dorsal protein in the muscle nucleus of wild type larvae (Figure 8C–F). Therefore, we set out to examine in greater detail whether or not Dorsal functions in the muscle nucleus to control GluR density. First, we tested whether nuclear translocation of Dorsal occurs in temperature sensitive cactus mutations that cause decreased synaptic GluR levels. We stained for Dorsal protein in the cactus mutants and assayed for the loss of synaptic Dorsal and the accumulation of Dorsal in muscle nuclei. We find that synaptic Dorsal protein levels are unaltered in the cactusRN and cactusHE mutants (wild-type = 100 +/− 4.6, n=19; cactRN/+ = 91 +/− 6.5, n=20; cactRN/cactRN = 99 +/− 3.6, n=14; cactHE/cactHE = 101 +/− 4.4, n=15; data represent % wild-type Dorsal fluorescence intensity). Indeed, we find no evidence of Dorsal protein in the muscle nuclei, either in wild type or in any of the cactus mutants that we tested (Figure 8E–H). These data are consistent with prior studies using other cactus alleles (Beramendi et al., 2005). However, since Dorsal may be transiently present in the nucleus, we inhibited nuclear export of Dorsal with leptomycin B and assayed for accumulation of nuclear Dorsal protein under conditions where leptomycin B has been shown to trap other nuclear transcription factors (Ashmore et al., 2003). In this experiment we assayed both wild type and cactus mutant animals under normal conditions and following high-potassium stimulation (60mM KCl, 2mM Ca++ in HL3 saline) of the NMJ. Again, we found no evidence for nuclear Dorsal protein (Supplemental Figure 3, and data not shown).
Figure 8. Dorsal and Cactus control GluRIIA levels post-transcriptionally.
A, B) The Dorsal antibody is able to recognize nuclear Dorsal staining during embryogenesis. Stage 4 embryos, co-labeled with anti-Dorsal (A) and DAPI (B). Dorsal enters nuclei on the ventral side (bottom) of the embryo; nuclear Dorsal appears white because of co-localization with purple DAPI. C, D) Overexpression of Dorsal is not sufficient for Dorsal nuclear entry at the NMJ. Animals harboring an heat-shock-inducible dorsal transgene (HSP70-dl) were raised at room temperature, (-heat shock, C) or treated with multiple heat shocks (+heat shock, D). Synapses are co-labeled with anti-Dorsal (green) and DAPI (purple). Inset in (D) highlights anti-Dorsal accumulating around, rather than within muscle nuclei. E–H) Dorsal does not enter the nucleus in wild-type (E, F) or cactus mutants (G, H). The nucleus is indicated by a dashed line (E, G) or highlighted by purple DAPI stain (F, H). I–L) NMJ were co-labeled with anti-myc (I, K) and the synaptic membrane marker, anti-HRP (J, L) in wild type and cactus mutant animals. A myc-tagged GluRIIA transgene, driven under the control of the MHC promoter, localizes to the NMJ in wild type (I). Less myc-tagged GluRIIA reaches the synaptic surface in cactRN/+ animals (K). M) Western analysis shows that myc-tagged GluRIIA expression in dorsal and cactus is not reduced in comparison to wild type. N) There is a significant reduction in the amount of anti-myc staining in the cactRN/+ and dorsal2/+ mutations (cactRN/+ = 64 ± 6.7%, n=14; dorsal2/+ = 73 ± 4.4%, n=34) in comparison to wt (wild type = 100 ± 4.4%, n=29; ** p<0.01).
Next, since it is formally possible that small amounts of Dorsal protein in the nucleus escape our detection, we tested whether over-expression of Dorsal could generate increased Dorsal in muscle nuclei. We were able to generate a significant increase in synaptic and non-synaptic Dorsal protein, but still did not see any Dorsal immunoreactivity within the muscle nuclei (Figure 8C, D). Finally, it remains possible that we are unable to detect small but transcriptionally relevant changes in nuclear Dorsal protein. Therefore, we used a series of Dorsal transcriptional reporters (Table 2) to monitor Dorsal mediated transcription in the muscle nuclei. Because tissue specific promoter activity is well established we chose a series of reporters that respond to Dorsal in a variety of different contexts (i.e. patterning and immunity), and included a reporter that is purely synthetic, which is composed of four dorsal binding sites upstream of the core HSP70 promoter. In addition we analyzed a cactus enhancer trap since Cactus is a transcriptional target of Dorsal is every tissue examined thus far (Nicolas et al., 1998, Table 2). Since Dorsal can both activate and repress transcription, we assayed these reporters in a wild-type background, a dorsal null mutant background (dl1/dlH) and in animals over-expressing Dorsal using a heat shock activated dorsal transgene (HSP70-dl). We did not find any evidence of Dorsal activity in muscle nuclei using four separate Dorsal transcriptional reporters, despite seeing robust reporter activity in other control tissues where we observe the expected changes in reporter activity (Table 2). It should be noted that LacZ is easily detected and frequently used as reporter in Drosophila muscle (Roy and VigayRaghavan, 1997). Taken together, these data suggest that Dorsal functions in the cytosol to regulate GluR levels.
Table 2.
Dorsal-dependent transcription not detected in muscle
| Reporter name, reporter description | Genotype | Activity in muscle | Activity in other larval tissues |
|---|---|---|---|
| -920twi/lacZ, 0.9kb of twist promoter driving lacZ1 | Dl over-expression wild-type dl1/dlH | none none none |
trachea trachea trachea |
| DD1, promoters of Dpt driving lacZ and Drs driving GFP2 | Dl over-expression wild-type | none none |
trachea trachea |
| D4/hsp70, 4 Dorsal binding sites + TATA box driving lacZ3 | Dl over-expression wild-type dl1/dlH | none none none |
hemocytes hemocytes hemocytes |
| cactus255, cactus enhancer trap driving lacZ4 | cactus255/cactD13 cactus255/+ | none none |
none none |
Pan, et al., Genes and Development 1991
Manfruelli, et al., EMBO 1999
Pan, et al., EMBO 1992
Post-transcriptional regulation of GluRIIA abundance by Dorsal and Cactus
The next question that we addressed was how NF-κB signaling influences the density of postsynaptic GluRs. We first performed real-time PCR to assay whether there is a decrease in GluRIIA transcript levels in dorsal and cactus compared to wild type. Although there was a trend toward reduced levels of GluRIIA transcript in dorsal and cactus mutants compared to wild-type, these differences are not statistically significant (wild-type = 1.0 +/− 0.2; dl2/dl2 = 0.8 +/− 0.2; cactHE/cactHE = 0.7+/− 0.2; GluRIIAsp16/GluRIIAsp16 = 0.0+/− 0.02). However, it remains possible that even a small change in GluR transcription could result in a significant change in GluR levels over a period of several days. Therefore, we asked whether expression of a GluRIIA cDNA, driven from a heterologous, muscle-specific promoter, could restore normal GluR levels to the cactus or dorsal mutant NMJ. In this experiment, we expressed a myc tagged GluRIIA transgene using the myosin heavy chain promoter (MHC:GluRIIA-myc) in wild-type as well as both dorsal and cactus mutations. It is important to note that MHC:GluRIIA-myc is able to rescue the GluRIIA null mutation (Petersen et al., 1997). We assayed levels of myc tagged receptor that reached the muscle surface by staining with an anti-myc antibody in un-permeablized tissue. We find that significantly less GluRIIA-myc reaches the synaptic surface in dorsal and cactus mutants compared to wild type controls (Figure 8I–M). A decrease in total GluR-myc protein levels cannot account for the decrease in surface GluRIIA-myc staining as shown by Western (Figure 8M). This experiment was then repeated with similar results using animals harboring two copies of the GluRIIA-myc transgene in a homozygous cactusRN mutant (data not shown). These data provide further evidence that GluR levels are not controlled by Dorsal-dependent GluRIIA transcription. In combination with the observations that Dorsal protein is not detectable in the muscle nuclei (Figure 8), that a Dorsal transactivation mutation does not affect GluRIIA regulation (Figure 3) and that Dorsal reporters fail to show nuclear activity in muscle (Table 2), our data suggest that Dorsal does not function in the muscle nuclei to control GluR abundance.
Discussion
NF-κB signaling has been implicated in the mechanisms of neural plasticity, learning, epilepsy, neurodegeneration and the adaptive response to neuronal injury (Mattson and Camandola, 2001; Mattson et al., 2000b; Meffert and Baltimore, 2005). The data presented here advance our understanding of neuronal NF-κB signaling in two ways. First, we present multiple lines of evidence that NF-κB/Dorsal signaling is required for the control of GluR density at the NMJ. These data provide a synaptic function for NF-κB signaling that may be directly relevant to the diverse activities ascribed to NF-κB in the nervous system. Second, we provide molecular and genetic evidence that Dorsal, Cactus and Pelle may function post-transcriptionally, at the postsynaptic side of the NMJ, to specify GluR density during postembryonic development.
GluR density is specified by synaptic NF-κB
Several independent lines of experimentation suggest that Cactus, Dorsal and Pelle function together at the PSD to specify GluR density. We provide evidence that Cactus and Dorsal localize to a similar postsynaptic domain. In addition, over-expression of a GFP-tagged Pelle protein that is sufficient to rescue a pelle mutation, can traffic to the PSD where Cactus and Dorsal reside. Next we present genetic evidence that cactus, dorsal and pelle function together, in the same genetic pathway to control GluR density. It is particularly surprising that mutations in cactus behave similarly to dorsal and pelle. In other systems (embryonic patterning and immunity), Cactus inhibits Dorsal-mediated transcription by binding and sequestering cytoplasmic Dorsal protein. As a result, in these other systems, cactus mutations cause phenotypes that are opposite to those observed in dorsal mutations. Here we have used the same cactus and dorsal mutations that previously have been observed to generate the predicted opposing phenotypes during embryonic patterning (Govind, 1999), and yet we observe that cactus phenocopies the dorsal mutations. In addition, genetic epistasis experiments indicate that these genes function together to facilitate GluR density. Thus, at the NMJ, Cactus functions in concert with, rather than in opposition to, Dorsal.
One explanation for this observation could be that Dorsal does not function as a nuclear transcription factor during the control of GluR levels. In support of this idea we demonstrate that: 1) Dorsal protein is not detected in the nucleus, 2) reporters of Dorsal-dependent transcription fail to show activity in muscle nuclei, and 3) mutation of the Dorsal transactivation domain, dlU5 does not impair GluR abundance even though this same mutation has been shown to impair transcription-dependent patterning during embryogenesis. An alternative explanation for the observation that dorsal and cactus have similar phenotypes at the NMJ could be that Cactus and Dorsal act synergistically to control the transcription of GluRs at the NMJ. Indeed, there is evidence in other systems that I-κB can shuttle with NF-κB to the nucleus (Ghosh and Karin, 2002). A previous study shows Cactus accumulation in Drosophila larval muscle nuclei in a dorsal mutant background (Cantera et al., 1999a). However, we have been unable to repeat this result despite having examined Cactus localization in five allelic combinations of dorsal. Importantly, we are confident of the specificity of the anti-Cactus antibody that we use because staining is eliminated in the cactus null mutant (Supplemental Figure 1; also see Supplemental Text for a further comparison with previously published data on cactus mutant phenotypes at the Drosophila NMJ). Furthermore, the data from vertebrate systems suggests that I-κB should shuttle into the nucleus with NF-κB, not in its absence (Ghosh and Karin, 2002). Thus, we favor a model in which Dorsal and Cactus function together at the postsynaptic membrane to facilitate GluR abundance during development.
Post-transcriptional control of GluR density by NF-κB
If our model is correct, then we predict that NF-κB does not control GluR density through transcriptional regulation. This prediction is supported by two experimental observations. First, GluR transcript levels (assessed by QT PCR) are not statistically different from wild type in dorsal and cactus mutations that cause a ~50% decrease in GluR abundance. Second, we demonstrate that over-expression of a myc-tagged GluRIIA cDNA using a heterologous, muscle-specific promoter is not able to restore synaptic GluRIIA levels in either the cactus or dorsal mutant backgrounds. These data are consistent with Dorsal and Cactus acting post-transcriptionally to control GluR density at the NMJ. There are two general mechanisms by which GluR levels could be controlled post-transcriptionally: 1) altered receptor delivery to the NMJ or 2) altered receptor internalization/degradation. If receptor internalization/degradation were enhanced in the cactus, dorsal or pelle mutant backgrounds, one might expect GluRIIA-myc overexpression to overcome this change and restore normal receptor levels. In addition, we might see less myc-tagged protein produced in mutant in comparison to wild type. This is not what we observed. Therefore, we favor the hypothesis that Cactus, Dorsal and Pelle function together to promote the delivery of glutamate receptors to the NMJ during development.
The possibility that Cactus, Dorsal and Pelle act post-transcriptionally to control GluR density raises many questions. For example, do Dorsal and Cactus exist as a protein complex at the PSD? If so, is this complex regulated and how might such a complex influence GluR density? Since pelle kinase dead mutants impair GluR density, it is possible that Dorsal and Cactus recruit Pelle to the PSD. If so, what are the targets of Pelle Kinase that are relevant to establishing or maintaining the proper density of glutamate receptors at the PSD? Finally, the demonstration that cytoplasmic NF-κB/Dorsal can influence GluR density does not rule out the possibility that NF-κB/Dorsal may also translocate to the muscle nucleus at the Drosophila NMJ under certain stimulus conditions. Indeed, in both the vertebrate central and peripheral nervous systems NF-κB is found within neuronal and muscle nuclei, and nuclear translocation can be stimulated by neuronal activity, glutamate, injury and disease (Mattson and Commandola, 2001). For nuclear entry of Dorsal, two events must occur: 1) Cactus must be degraded and 2) Dorsal must be phosphorylated (Govind, 1999; Nicolas et al., 1998). It remains possible that one or both of these criteria are not met during the normal development of the Drosophila NMJ, but could be met under as yet to be identified stimulus conditions. The possibility that NF-κB acts both locally at the synapse and globally via the nucleus is not unique to this signaling pathway. A similar organization has been documented for wingless/wnt signaling where non-canonical cytoplasmic signaling can impact cytoskeletal organization while canonical signaling involves the nuclear translocation of downstream beta-catenin and TCF-dependent gene transcription (Moon et al., 2002).
Intercellular signaling, NF-κB activation and the control of GluR density
It remains unknown how NF-κB signaling is activated at the Drosophila NMJ. In Drosophila embryonic patterning and innate immunity, NF-κB signaling is initiated through activation of Toll or Toll-like receptors. There are nine Toll and Toll-like receptors encoded in the Drosophila genome. However, none of these receptors appear to be present in Drosophila larval muscle. The Toll receptor is expressed in a subset of embryonic muscle fibers (Halfon et al., 1995; Halfon and Keshishian, 1998), but is absent from larval muscle (Nose et al., 1992). None of the Toll-like receptors are expressed in Drosophila embryonic muscle (Kambris et al., 2002) and none appear to be expressed in larval muscle (data not shown). An alternative possibility is that TNF-α receptors activate NF-κB in Drosophila muscle as has been observed in vertebrate skeletal muscle (Ladner et al., 2003). Indeed, a TNF-α receptor homolog has been identified, and it is expressed in Drosophila skeletal muscle (Kauppila et al., 2003). The possibility that TNF-α signaling is mediated via NF-κB is intriguing given the recent demonstration that TNF-α regulates GluR abundance in the vertebrate central nervous system (Stellwagen and Malenka, 2006). In both cultured neurons and hippocampal slices glial-derived TNF-α signaling is required for the increase in postsynaptic AMPA receptor abundance observed following chronic activity blockade (Stellwagen and Malenka, 2006). Thus, our data in combination with work in the vertebrate CNS raise the possibility that a conserved TNFα/NF-κB signaling system controls GluR abundance at both neuromuscular and central synapses during development and in response to chronic activity blockade.
MATERIALS AND METHODS
Genetics
Drosophila mutant stocks were obtained from the following sources: Df(3R)D605, dl1 and dlUY2278 from the Bloomington Stock Center (Bloomington, IN); pllRM8, pll078, dlH, dl2, dlPZ, dlU5, cactD13, cact255, cactHE, and the double mutant dl1, cactPD from the Max-Planck-Institut (Tuebingen, Germany); pll25 from Steve Wasserman (University of California, San Diego); HSP70-dl and cactRN from Ruth Steward (Rutgers University); D4 and -920twi/lacZ from Albert Courey (University of California, Los Angeles); DD1 from Marika Olcot (Oregon State University); dlgM52 from Vivian Budnik (University of Massachusetts, Medical School). Animals for experiments involving dl2, dlPZ, cactRN and cactHE were incubated at 29°C, as were the wild-type controls. For the double mutant analysis of cactus and dorsal the double mutant dl1, cactusPD allelic combination was used after verification that in this background only 11±.13% of wild type Cactus protein remains (n=10). UAS-dorsal-RNAi animals were provided by Barry Dickson (Research Institute of Molecular Pathology, Vienna Austria). To achieve significant knock down of Dorsal protein larvae (UAS-dorsal-RNAi/dlH; 24B-Gal4/24B-Gal4) were raised at 30°C, and control genotypes were raised identically in paralle. The UAS-pelle-RNAi was provided by NIG stock center, and experiments involving this line were preformed with animals reared at 30°C.
Larval over-expression of dorsal was achieved by using a heat shock inducible dorsal transgene (HSP70-dl); vials were immersed in a 37°C water bath for one hour, three times on each day of larval development. Muscle specific over-expression was achieved by crossing dlUY2278, a UAS-containing P-element (Nicolai et al., 2003), to muscle specific Gal4 drivers. UAS-GFP-Pelle was constructed by inserting the coding sequence of pelle into the GW (N-terminal EGFP tag) Gateway vector using TOPO TA Cloning Kit for entry into Gateway Technology (Invitrogen, Carlsbad, CA).
Immunohistochemistry
Unless otherwise noted wandering third-instar larva were dissected and stained according to previously published methods (Albin and Davis, 2004). The following antibodies were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Department of Biological Sciences, Iowa City): anti-myc (9E 10) (Evan et al., 1985), anti-GluRIIA (8B4D2; 1:10)(Schuster CM, 1991), and mAb-Dlg (1:1000) (Corey Goodman, University of California, Berkeley). We obtained mouse anti-Synapsin (1:10) from Erich Buchner (University of Würzburg), rabbit anti-GluRIIC (1:2500) from Aaron DiAntonio (Washington University School of Medicine, St. Louis MO), rabbit anti-Cactus (1:1000) and rabbit anti-Dorsal (1:500) from Steve Wasserman (University of California, San Diego). Secondary antibodies (FITC labeled, TRITC labeled, Cy5 labeled) anti-mouse, and anti-rabbit as well as FITC-, TRITC- and Cy5-conjugated anti-HRP antibodies were provided by Jackson Immunoresearch Laboratories and used at 1:200. For LacZ staining, larvae were fixed in 4% paraformaldehyde (Sigma, St. Louis, MO) for seven minutes. 12.5μl of 8% X-gal and 0.5 ml staining solution (Drosophila Protocols, William Sullivan, Editor) was incubated at 65°C and then added to larvae in a 1.5 mL microfuge tube, and incubated at 37°C until staining was complete. Animals were washed in PBT and cleared in glycerol for further analysis and imaging. For Leptomycin B treatment semi-intact preparations were incubated for 6 h at 30°C in Schneider’s Drosophila Medium with or without 40 ng/ml Leptomycin B (Sigma). Animals were then processed for staining as described above.
Imaging
Unless otherwise noted, all images were acquired using a Zeiss 2000M inverted microscope, 100× 1.4nA lens and CoolSnap HQ cooled CCD camera. Image capture and analysis were performed using Intelligent Imaging Innovations (3I) software. For comparisons of fluorescent intensities across genotypes, samples from different genotypes were dissected and fixed identically, processed in the same vial and imaged at identical exposures and light intensities. For quantification of GluR fluorescence intensity, the synaptic region of interest was defined by first imaging co-stained anti-HRP. GluR fluorescence intensity was quantified by measuring the average GluR intensity across this region of interest in a 2D confocal projection image as done previously (Albin and Davis, 2004).
Western Blotting
Wandering third instar larvae were ground in Laemmli Sample Buffer (Bio-Rad) and then boiled for 5 minutes. Two larvae per lane were loaded onto pre-cast 4–15% SDS-PAGE gels (Amersham). Western blotting was preformed using anti-myc (1:10) and anti-Tubulin (1:10,000), followed by ECL detection (Amersham).
Electrophysiology
Recordings were preformed as described in (Albin and Davis, 2004) using 0.5mM calcium HL3 saline. Measurements of maximal EPSP and input resistance were done by hand using the cursor option in Clampfit (Molecular Devices). Measurements of spontaneous miniature release events were semiautomated using MiniAnalysis software (Synaptosoft, Decatur, GA). For each recording, 100–300 mEPSP events were averaged to determine the average mEPSP amplitude.
Real-time reverse transcription-PCR
Real-time reverse transcription-PCR assays were performed using an iCycler (Bio-Rad) with SYBR Green fluorescence. Real-time PCR amplification was performed after an initial denaturation of 8 min at 95°C, followed by 50 cycles of 20 sec denaturation at 95°, 30 sec annealing at 60°C, and 30 sec extension at 72°C. Fluorescent detection was performed at the annealing stage as previously described (Albin and Davis, 2004).
Electron microscopy
Larvae were prepared for electron microscopy and analysis was performed as previously described (Pielage et al., 2005).
Supplementary Material
Acknowledgments
We would like to thank Hillel Adesnik for important experimental observations at an early stage of this project. We also thank C. Andrew Frank and Dion Dickman for critical comments on this manuscript. This work was supported by an HHMI predoctoral fellowship to ESH and NIH grant number NS39313 to GWD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin SD, Davis GW. Coordinating structural and functional synapse development: postsynaptic p21-activated kinase independently specifies glutamate receptor abundance and postsynaptic morphology. J Neurosci. 2004;24:6871–6879. doi: 10.1523/JNEUROSCI.1538-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore LJ, Sathyanarayanan S, Silvestre DW, Emerson MM, Schotland P, Sehgal A. Novel insights into the regulation of the timeless protein. J Neurosci. 2003 Aug 27;23(21):7810–9. doi: 10.1523/JNEUROSCI.23-21-07810.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdiguian S, Martin M, Richard I, Pons F, Astier C, Bourg N, Hay RT, Chemaly R, Halaby G, Loiselet J, et al. Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkappaB alpha/NF-kappaB pathway in limb-girdle muscular dystrophy type 2A. Nat Med. 1999;5:503–511. doi: 10.1038/8385. [DOI] [PubMed] [Google Scholar]
- Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17:3261–3270. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]
- Belvin MP, Jin Y, Anderson KV. Cactus protein degradation mediates Drosophila dorsal-ventral signaling. Genes Dev. 1995;9:783–793. doi: 10.1101/gad.9.7.783. [DOI] [PubMed] [Google Scholar]
- Beramendi A, Peron S, Megighian A, Reggiani C, Cantera R. The inhibitor kappaB-ortholog Cactus is necessary for normal neuromuscular function in Drosophila melanogaster. Neuroscience. 2005;134(2):397–406. doi: 10.1016/j.neuroscience.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Bergmann A, Stein D, Geisler R, Hagenmaier S, Schmid B, Fernandez N, Schnell B, Nusslein-Volhard C. A gradient of cytoplasmic Cactus degradation establishes the nuclear localization gradient of the dorsal morphogen in Drosophila. Mech Dev. 1996;60:109–123. doi: 10.1016/s0925-4773(96)00607-7. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Cantera R, Kozlova T, Barillas-Mury C, Kafatos FC. Muscle Structure and Innveravtion Are Affected by Loss of Dorsal in the Fruit Fly, Drosophila melanogaster. Molecular and Cellular Neuroscience. 1999a;13:131–141. doi: 10.1006/mcne.1999.0739. [DOI] [PubMed] [Google Scholar]
- Cantera R, Roos E, Engstrom Y. DIF and Cactus are colocalized in the larval nervous system of Drosophila melanogaster. J Neurobiol. 1999b;38:16–26. [PubMed] [Google Scholar]
- Davis GW. Homeostatic Control of Neural Activity: From Phenomenology to Molecular Design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Eaton BA, Davis GW. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron. 2005;47:695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Edwards DN, Towb P, Wasserman SA. An activity-dependent network of interactions links the Rel protein Dorsal with its cytoplasmic regulators. Development. 1997;124:3855–3864. doi: 10.1242/dev.124.19.3855. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone DE, Rushton E, Rohrhough J, Liebl F, Karr J, Sheng Q, Rodesch C, Broadie K. An Essential Drosophila Glutamate Receptor Subunit that Functions in Both Central Neuropil and Neuromusclar Junction. J Neurosci. 2005;25:399–3208. doi: 10.1523/JNEUROSCI.4201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Saaib RD, Jia S, Courey AJ. Activation and repression by the C-terminal domain of Dorsal. Development. 2001;128:1869–1879. doi: 10.1242/dev.128.10.1869. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem. 1998;70:1876–1886. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002 Apr;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. Review. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Gillespie SK, Wasserman SA. Dorsal, a Drosophila Rel-like protein, is phosphorylated upon activation of the transmembrane protein Toll. Mol Cell Biol. 1994;14:3559–3568. doi: 10.1128/mcb.14.6.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind S. Rel signalling pathway and the melanotic tumour phenotype of Drosophila. Biochem Soc Trans. 1996;24:39–44. doi: 10.1042/bst0240039. [DOI] [PubMed] [Google Scholar]
- Govind S. Control of development and immunity by rel transcription factors in Drosophila. Oncogene. 1999;18:6875–6887. doi: 10.1038/sj.onc.1203223. [DOI] [PubMed] [Google Scholar]
- Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Halfon MS, Hashimoto C, Keshishian H. The Drosophila toll gene functions zygotically and is necessary for proper motoneuron and muscle development. Dev Biol. 1995;169:151–167. doi: 10.1006/dbio.1995.1134. [DOI] [PubMed] [Google Scholar]
- Halfon MS, Keshishian H. The Toll pathway is required in the epidermis for muscle development in the Drosophila embryo. Dev Biol. 1998;199:164–174. doi: 10.1006/dbio.1998.8915. [DOI] [PubMed] [Google Scholar]
- Hecht PM, Anderson KV. Genetic characterization of tube and pelle, genes required for signaling between Toll and dorsal in the specification of the dorsal-ventral pattern of the Drosophila embryo. Genetics. 1993;135:405–417. doi: 10.1093/genetics/135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda K, Nusslein-Volhard C. Disulfide cross-linking in crude embryonic lysates reveals three complexes of the Drosophila morphogen dorsal and its inhibitor cactus. Proc Natl Acad Sci U S A. 1994;91:5350–5354. doi: 10.1073/pnas.91.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda K, Roth S, Nusslein-Volhard C. The functional domains of the Drosophila morphogen dorsal: evidence from the analysis of mutants. Genes Dev. 1992;6:619–630. doi: 10.1101/gad.6.4.619. [DOI] [PubMed] [Google Scholar]
- Kambris Z, Hoffmann JA, Imler JL, Capovilla M. Tissue and stage-specific expression of the Tolls in Drosophila embryos. Gene Expr Patterns. 2002;2:311–317. doi: 10.1016/s1567-133x(02)00020-0. [DOI] [PubMed] [Google Scholar]
- Kauppila S, Maaty WS, Chen P, Tomar RS, Eby MT, Chapo J, Chew S, Rathore N, Zachariah S, Sinha SK, et al. Eiger and its receptor, Wengen, comprise a TNF-like system in Drosophila. Oncogene. 2003;22:4860–4867. doi: 10.1038/sj.onc.1206715. [DOI] [PubMed] [Google Scholar]
- Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278:2294–2303. doi: 10.1074/jbc.M207129200. [DOI] [PubMed] [Google Scholar]
- Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential Localization of Glutamate Receptor Subunits at the Drosophila Neuromuscular Junction. The Journal of Neuroscience. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Culmsee C, Yu Z, Camandola S. Roles of nuclear factor kappaB in neuronal survival and plasticity. J Neurochem. 2000a;74:443–456. doi: 10.1046/j.1471-4159.2000.740443.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000b;301:173–187. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Baltimore D. Physiological functions for brain NF-kappaB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–6. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Nicolai M, Lasbleiz C, Dura JM. Gain-of-function screen identifies a role of the Src64 oncogene in Drosophila mushroom body development. J Neurobiol. 2003;57:291–302. doi: 10.1002/neu.10277. [DOI] [PubMed] [Google Scholar]
- Nicolas E, Reichhart JM, Hoffmann JA, Lemaitre B. In vivo regulation of the IkappaB homologue cactus during the immune response of Drosophila. J Biol Chem. 1998;273:10463–9. doi: 10.1074/jbc.273.17.10463. [DOI] [PubMed] [Google Scholar]
- Norris JL, Manley JL. Selective nuclear transport of the Drosophila morphogen dorsal can be established by a signaling pathway involving the transmembrane protein Toll and protein kinase A. Genes Dev. 1992;6:1654–1667. doi: 10.1101/gad.6.9.1654. [DOI] [PubMed] [Google Scholar]
- Nose A, Van Vactor D, Auld V, Goodman CS. Development of neuromuscular specificity in Drosophila. Cold Spring Harb Symp Quant Biol. 1992;57:441–449. doi: 10.1101/sqb.1992.057.01.049. [DOI] [PubMed] [Google Scholar]
- O’Mahony A, Raber J, Montano M, Foehr E, Han V, Lu SM, Kwon H, LeFevour A, Chakraborty-Sett S, Greene WC. NF-kappaB/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity. Mol Cell Biol. 2006;26:7283–7298. doi: 10.1128/MCB.00510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006;175:491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Schwarz T, Kittel RJ, Schmid A, Rasse TM, Kappei D, Ponimaskin E, Heckmann M, Sigrist SJ. Four Different Subunits Are Essential for Expressing the Synaptic Glutamate Receptor at Neuromusclar Junctions of Drosophila. J Neurosci. 2005;25:3209–3218. doi: 10.1523/JNEUROSCI.4194-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125:1909–1920. doi: 10.1242/dev.125.10.1909. [DOI] [PubMed] [Google Scholar]
- Rasse TM, Fouquet W, Schmid A, Kittel RJ, Mertel S, Sigrist CB, Schmidt M, Guzman A, Merino C, Qin G, et al. Glutamate receptor dynamics organizing synapse formation in vivo. Nat Neurosci. 2005;8:898–905. doi: 10.1038/nn1484. [DOI] [PubMed] [Google Scholar]
- Reach M, Galindo RL, Towb P, Allen JL, Karin M, Wasserman SA. A Gradient of Cactus Protein Degradation Establishes Dorsoventral Polarity in the Drosophila Embryo. Developmental Biology. 1996;180:353–364. doi: 10.1006/dbio.1996.0308. [DOI] [PubMed] [Google Scholar]
- Roth S, Hiromi Y, Godt D, Nusslein-Volhard C. cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Development. 1991;112:371–388. doi: 10.1242/dev.112.2.371. [DOI] [PubMed] [Google Scholar]
- Roth S, Stein D, Nusslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989;59:1189–202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- Roy S, VijayRaghavan K. Homeotic genes and the regulation of myoblast migration, fusion, and fibre-specific gene expression during adult myogenesis in Drosophila. Development. 1997 Sep;124(17):3333–41. doi: 10.1242/dev.124.17.3333. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Memet S, Lilienbaum A, Feuillard J, Raphael M, Israel A. NF-kappaB activity in transgenic mice: developmental regulation and tissue specificity. Development. 1996;122:2117–2128. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- Schupbach T, Wieschaus E. Female Sterile mutations on the second chromosome of Drosophila Melanogaster I. Maternal effect mutations. Genetics. 1989;121:101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupbach T, Wieschaus EE. Female Sterile Mutations on the Second Chromosome of Drosophila Melanogaster. II. Mutations Blocking Ogenesis or Altering Egg Morphology. Genetics. 1991;129:1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CM, Ultsch A, Schloss P, Cox JA, Schmitt B, Betz H. Molecular cloning of an invertebrate glutamate receptor subunit expressed in Drosophila muscle. Science. 1991;254:112–114. doi: 10.1126/science.1681587. [DOI] [PubMed] [Google Scholar]
- Shen B, Manley JL. Phosphorylation modulates direct interactions between the Toll receptor, Pelle kinase and Tube. Development. 1998;125:4719–4728. doi: 10.1242/dev.125.23.4719. [DOI] [PubMed] [Google Scholar]
- Shen B, Manley JL. Pelle kinase is activated by autophosphorylation during Toll signaling in Drosophila. Development. 2002;129:1925–1933. doi: 10.1242/dev.129.8.1925. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Towb P, Bergmann A, Wasserman SA. The protein kinase Pelle mediates feedback regulation in the Drosophila Toll signaling pathway. Development. 2001;128:4729–36. doi: 10.1242/dev.128.23.4729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.