Abstract
AIM: To evaluate long-term follow-up of minimum-sized hepatocellular carcinoma (HCC) treated with percutaneous ethanol injection (PEI).
METHODS: PEI was applied to 42 lesions in 31 patients (23 male and eight female) with HCC < 15 mm in diameter, over the past 15 years.
RESULTS: Overall survival rate was 74.1% at 3 years, 49.9% at 5 years, 27.2% at 7 years and 14.5% at 10 years. These results are superior to, or at least the same as those for hepatic resection and radiofrequency ablation. Survival was affected only by liver function, but not by sex, age, etiology of Hepatitis B virus or Hepatitis C virus, α-fetoprotein levels, arterial and portal blood flow, histological characteristics, and tumor multiplicity or size. Patients in Child-Pugh class A and B had 5-, 7- and 10-years survival rates of 76.0%, 42.2% and 15.8%, and 17.1%, 8.6% and 0%, respectively (P = 0.025).
CONCLUSION: Treatment with PEI is best indicated for patients with HCC < 15 mm in Child-Pugh class A.
Keywords: Percutaneous ethanol injection, Interventional ablation, Ultrasound, Hepatocellular carcinoma, Prognosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the major malignancies worldwide[1–4]. With recent advances in diagnostic imaging, particularly in ultrasound (US), an increasing number of small or early-stage HCCs have been detected. In patients with early-stage HCC, percutaneous ethanol injection (PEI) has been a second choice when surgical techniques have been precluded, although PEI has been used as a first-line treatment option in some centers in Italy and Japan. Over the past few years, several methods for thermal tumor destruction through localized heating or freezing, including radiofrequency ablation (RFA), laser ablation, microwave ablation, and cryoablation, have been developed and clinically tested. Among these, RFA has recently emerged as a real competitor to PEI.
At this point, there are no unequivocal data to back up percutaneous ablation as a replacement for resection as first-line treatment for patients with early-stage HCC. Therefore, whether percutaneous ablation replaces resection as first-line option for very early HCC will be resolved by launching a large international randomized controlled trial.
In this study, early-stage HCC (< 15 mm diameter) is referred to as minimum-sized HCC[5]. The present study is a long-term follow-up of minimum-sized HCC treated with PEI.
MATERIALS AND METHODS
Subjects
We analyzed the clinical features of consecutive patients treated with PEI in the 1990s at Kobe Asahi Hospital. The diagnosis of HCC was made by imaging, including US and computed topography (CT) and confirmed by tumor-targeted biopsies. Thirty-one patients with liver cirrhosis and HCC were treated with PEI as the first-line anticancer treatment. The characteristics of the patients are summarized in Table 1. The criteria for treatment with PEI were as follows: (1) Uninodular HCC ≤ 15 mm in diameter, or multinodular HCC lesions ≤ 15 mm in diameter (in one or both hepatic lobes); (2) absence of portal vein thrombosis and extra-hepatic metastases; (3) age < 75 years; (4) liver cirrhosis of Child-Pugh class A or B; and (5) prothrombin time ratio (normal/patient) > 40% and platelet count > 40 000/μL. The number of tumorous nodules and portal vein patency were established by US and CT. Maximum tumor diameter was measured by US. The absence of extrahepatic metastases was ascertained by chest X-ray, and abdominal CT and US.
Table 1.
Characteristics of 31 patients with liver cirrhosis and small HCC
| Parameter | |
| Sex (male:female) | 23:8 |
| Age (yr) (mean ± SD) | 63.8 ± 8.9 |
| Cause of cirrhosis | |
| HCV | 29 |
| Non-HCV | 2 |
| Liver dysfunction | |
| Child-Pugh A | 17 |
| Child-Pugh B | 14 |
| AFP level (μg/L) | |
| ≤ 20 | 15 |
| > 20 | 16 |
| Tumor multiplicity | |
| Uninodular | 21 |
| Multinodular | 10 |
| Tumor diameter (mm) (mean ± SD) | 11.9 ± 2.4 |
| ≤ 10 | 12 |
| 11-15 | 19 |
| Histological characteristics | |
| Well-differentiated | 18 |
| Well to moderately differentiated | 13 |
| Arterial blood flow | |
| Positive | 9 |
| Negative | 13 |
| Portal blood flow | |
| Positive | 12 |
| Negative | 9 |
Liver cirrhosis was diagnosed histologically, radiologically or clinically. Serum hepatitis B surface antigen (HBsAg) was positive in two patients, and anti-hepatitis C virus antibody (anti-HCV) was positive in 29. Arterial blood flow was confirmed by CO2 US-angiography or CT during arteriography (CTA); portal blood flow was confirmed by CT during arterial portography (CTAP) (Table 1). Surgery was contraindicated in most patients because of liver dysfunction, presence of lesions in locations that made hepatic resection inappropriate, advanced age, coexistence of another disease, or a combination of these factors. However, during the past few years, some patients, including possible candidates for surgery, were treated with PEI. Informed consent was obtained from all patients after the nature of the procedure had been fully explained.
The diameter of the tumors ranged between 8 and 15 (mean 11.9 ± 2.4) mm. The diagnosis of HCC was established by histological biopsy with the needle guided by sonography in 31 patients.
Treatment schedule and follow-up protocol
PEI[6] was administered to each patient (3-6 sessions; once or twice weekly) by one or two injections of 95% sterile ethyl alcohol (1.6-73.9 mL, mean 17.6 ± 16.7 mL) delivered to each lesion with a multiple-side-hole 21-gauge needle (Et-hanoject, TSK, Tokyo, Japan), depending on the size of the lesion and the distribution of the injected ethanol within the tumor.
One month after the end of PEI treatment, the α-fetoprotein (AFP) level was measured, CT was repeated, and multiple percutaneous biopsies of the treated lesions were carried out to evaluate treatment outcome. Lesions appearing as hypoattenuated, non-enhanced areas on CT scans were diagnosed as necrotic, whereas enhanced areas were suspected of being persistent tumors. The biopsies under US guidance were carried out by placing the needle in the enhanced areas of the tumor. Histological samples were obtained from all of the patients.
The treatment was terminated and the patients entered the follow-up protocol in the absence of residual tumors, confirmed by CT and biopsies, and of suspected persistent tumors, confirmed by AFP levels. Such patients were given additional PEIs targeted in areas where viable tumors had previously been observed, and were examined again by CT and biopsies after 1 mo. The follow-up protocol included clinical assessment, measurement of hepatic functional serum indexes and AFP levels, and US examinations conducted at 3-mo intervals. The duration of the follow-up was calculated from the beginning of PEI and lasted for 2-167 (mean ± SD, 57.5 ± 37.7) mo.
Statistical analysis
Student’s t test and χ2 test were used to identify differences in patient characteristics in the various subgroups with prognostic factors. The Kaplan-Meier method was used to analyze the factors associated with post-PEI survival of patients with HCC and distant intrahepatic recurrence of HCC, and the difference was determined by log rank test. Stepwise regression analysis was used to identify factors that affected the survival rate of post-PEI patients. P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
All patients completed the planned treatment course. No major treatment-related complication had occurred by the end of the study. Overall survival rate was 74.1% at 3 years, 49.9% at 5 years, 27.2% at 7 years and 14.5% at 10 years. The longest survival period was 13 years 11 mo, and three patients lived longer than 10 years after PEI treatment. Up to December 2003, 23 post-PEI patients died: Four from cancer (17.4%), 15 from hepatic failure (65.2%) and four from other causes (17.4%). According to the Child-Pugh classification, two (20%) died from cancer and seven (70%) from hepatic failure (class A), and two (15.3%) from cancer and eight (61.5%) from hepatic failure (class B) (Table 2).
Table 2.
Cause of death of post-PEI patients with small HCC, according to Child-Pugh classification and number of treatment sessions
|
Child-Pugh classification |
Number of treatment sessions |
||||||
| A (n = 10) | B (n = 13) | 1st | 2nd | 3rd | 4th | 5th | |
| Cancer growth | 2 | 2 | 0 | 3 | 0 | 0 | 0 |
| Hepatic failure | |||||||
| Cancer (+) | 7 | 6 | 3 | 2 | 2 | 1 | 5 |
| Cancer (–) | 0 | 2 | |||||
| Other | 1 | 3 | 3 | 0 | 0 | 0 | 0 |
Prognostic factors
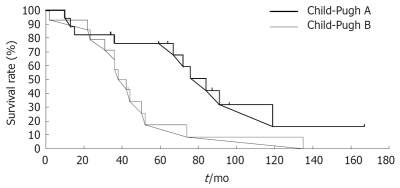
The influence of patient- and tumor-related factors on survival is shown in Table 3. Survival was affected by liver function, but not by sex, age, etiology of cirrhosis (HCV or non-HCV), AFP level, arterial blood flow, portal blood flow, histological characteristics, and tumor multiplicity or size. The final step of stepwise variable showed function was significantly associated with survival rate. Child-Pugh class A patients showed a higher survival rate than Child-Pugh class B (P = 0.011, Table 4). The 5-, 7- and 10-years survival rates of 76.0%, 42.2% and 15.8%, respectively, for patients in Child-Pugh class A were significantly higher than those for patients in Child-Pugh class B (17.1%, 8.6% and 0%, respectively) (P = 0.025, Figure 1). Patient characteristics by Child-Pugh classification showed that prognosis of class A and class B patients was affected by sex and not by any other patient- and tumor-related factors (Table 5). The two groups were similar with respect to the other patient- and tumor-related factors.
Table 3.
Analysis of the prognostic value of patient- and tumor-related factors
|
Probability of survival (%) |
||||||
| Factor | n | 3-yr | 5-yr | 7-yr | 10-yr | P value |
| Sex | 0.595 | |||||
| Male | 23 | 73.7 | 59.5 | 29.7 | 11.1 | |
| Female | 8 | 62.5 | 33.3 | 0 | 0.0 | |
| Age (yr) | 0.475 | |||||
| ≤ 65 | 18 | 77.4 | 59.5 | 29.8 | 9.9 | |
| > 65 | 13 | 61.5 | 35.9 | 23.9 | 0.0 | |
| Cause of cirrhosis | ||||||
| HCV | 29 | 72.2 | 46.2 | 23.1 | 15.4 | |
| Non-HCV | 2 | - | - | - | - | |
| Liver dysfunction | 0.025 | |||||
| Child-Pugh A | 17 | 76.0 | 76.0 | 42.2 | 15.8 | |
| Child-Pugh B | 14 | 64.3 | 17.1 | 8.6 | 0.0 | |
| AFP level (μg/L) | 0.139 | |||||
| ≤ 20 | 15 | 79.4 | 56.9 | 42.6 | 21.3 | |
| > 20 | 16 | 62.5 | 37.5 | 18.7 | 0.0 | |
| Tumor multiplicity | 0.751 | |||||
| Uninodular | 21 | 75.9 | 50.6 | 36.1 | 13.5 | |
| Multinodular | 10 | 60.0 | 37.5 | 12.5 | 12.5 | |
| Tumor diameter (mm) | 0.336 | |||||
| ≤ 10 | 12 | 74.1 | 64.8 | 32.4 | 21.6 | |
| 11-15 | 19 | 68.4 | 40.9 | 24.6 | 12.2 | |
| Histological characteristics | 0.119 | |||||
| Well-differentiated | 18 | 83.3 | 58.0 | 33.2 | 22.1 | |
| Well to moderately differentiated | 13 | 61.5 | 38.5 | 19.2 | 0.0 | |
| Arterial blood flow | 0.269 | |||||
| Positive | 9 | 64.8 | 38.9 | 38.9 | 38.9 | |
| Negative | 13 | 61.5 | 30.8 | 10.2 | 0.0 | |
| Portal blood flow | 0.458 | |||||
| Positive | 12 | 58.3 | 33.3 | 16.7 | 0.0 | |
| Negative | 9 | 64.8 | 38.9 | 38.9 | 0.0 | |
Table 4.
Factors affecting survival of patients with HCC treated by PEI, determined by stepwise regression analysis
| Factors | Hazard ratio | 95% CI | P value |
| Child-Pugh class | |||
| A | 1 | ||
| B | 5.89 | 1.50-23.15 | 0.011 |
Figure 1.
Survivial of patients with HCC treated by PEI according to Child-Pugh classification.
Table 5.
Characteristics of patients according to Child-Pugh class
| Parameter | Class A (n = 17) | Class B (n = 14) | P value |
| Sex (male:female) | 16:1 | 7:7 | 0.017 |
| Age (yr) (mean ± SD) | 61.2 ± 8.3 | 66.9 ± 8.9 | NS |
| Cause of cirrhosis | |||
| HCV | 15 | 14 | NS |
| Non- HCV | 2 | 0 | |
| AFP level | |||
| ≤ 20 μg/L | 9 | 6 | NS |
| > 20 μg/L | 8 | 8 | |
| Tumor multiplicity | |||
| Uninodular | 11 | 10 | NS |
| Multinodular | 6 | 4 | |
| Tumor diameter (mm) (mean ± SD) | |||
| ≤ 10 | 7 | 5 | NS |
| 11-15 | 10 | 9 | |
| Histological characteristics | |||
| Well-differentiated | 9 | 9 | NS |
| Well to moderately differentiated | 8 | 5 | |
| Arterial blood flow | |||
| Positive | 5 | 4 | NS |
| Negative | 5 | 8 | |
| Portal blood flow | |||
| Positive | 7 | 5 | NS |
| Negative | 4 | 5 |
NS: Not significant.
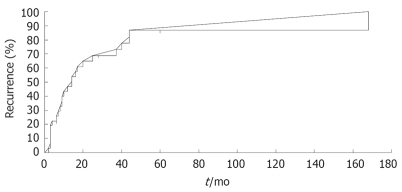
Lesions in intrahepatic areas other than the site treated by PEI were observed in 25 patients (81.8%), and cumulative recurrence rates at 1, 3, 5, 7 and 10 years after PEI were 47.0%, 73.4%, 87.7%, 87.7% and 87.7%, respectively (Figure 2). The frequency of initial recurrence according to segment was 32.0% in segments other than the initially treated segment (other segments), 32.0% in the same segment as the initially treated segments, and 28.0% in both the same and other segments. The tumor diameter of recurrent lesions was < 20 mm in 84.0% of cases, 21-30 mm in 8.0%, and no tumor was > 30 mm at detection (Table 6). The recurrent lesions that occurred in 25 post-PEI patients after initial treatment were managed as follows: PEI in 15 (60.0%); transcatheter chemoembolization (TACE) in two (8.0%); TACE + PEI in four (16.0%); transcatheter arterial infusion chemotherapy in one (4.0%); and other methods in two (8.0%). One patient (4.0%) was untreatable because of severe liver dysfunction or poor general condition. Second, third, fourth and fifth recurrent lesions were treated with PEI in eight (42.1%), five (41.6%), two (25.0%) and three (42.8%) patients, respectively (Figure 3).
Figure 2.
Recurrence of HCC treated by PEI at remote site from initial treated site.
Table 6.
Recurrence of HCC initially treated by PEI (n = 25)
| Location of recurrence | |
| Same segment | 8 (32.0%) |
| Same and other segments | 7 (28.0%) |
| Other segments | 8 (32.0%) |
| Others1 | 2 (8.0%) |
| Tumor diameter at detection (mm) | |
| ≤ 10 | 8 (32.0%) |
| 11-15 | 9 (36.0%) |
| 16-20 | 4 (16.0%) |
| 21-30 | 2 (8.0%) |
| ≥ 31 | 0 |
| Others | 2 (8.0%) |
In two cases, tumor thrombus was seen in portal vein and HCC metastasizing to distant site (sacrum and lumbar vertebra), respectively.
Figure 3.
Treatment for repeated recurrences in patients with HCC initially treated by PEI.
DISCUSSION
In a multicenter trial conducted in Italy[7], the 3-, 5- and 7-years survival rates after PEI in patients with a single HCC ≤ 30 mm in diameter have been reported as 78%, 54% and 28%, respectively; those in patients with a single HCC 30.1-50 mm as 61%, 32% and 16%, respectively; and those in patients with multiple lesions as 51%, 21% and 0%, respectively. In a series of 270 patients in Japan with fewer than three small lesions (≤ 30 mm in diameter) of HCC, overall 3- and 5-years survival rates after PEI were 81.6% and 60.3%, respectively, but the rates were higher, 87.3% and 78.3% in Child-Pugh class A patients with a solitary tumor ≤ 20 mm in diameter[8]. The overall 3-, 5-, 7- and 10-years survival rates after PEI in the present study were 74.1%, 49.9%, 27.2% and 14.5%, respectively, in our population with minimum-sized HCC. Patients of Child-Pugh class A had 5-, 7- and 10-years survival rates of 76.0%, 42.2% and 15.8%, respectively. The outcome in this study was superior, or at least equal to that reported in other studies.
The superiority or equality of our results, including those for multinodular HCC, compared with other studies can be explained by tumor size alone. Generally speaking, the therapeutic effect of PEI is largely dependent on tumor size[9]. Strictly speaking, the difference between tumors 15 and 16-20 mm in diameter is very important. Pathological events identified in 106 small resected HCCs < 20 mm in diameter have demonstrated local metastases (located ≤ 10 mm from the nodule), and microscopic portal invasion among the most frequently occurring tumors (the so-called distinctly nodular types). The frequency of portal invasion has been reported as significantly higher in HCC 16-20 mm in diameter (40%) than in HCC 11-15 mm in diameter (25%, P < 0.01)[9,10]. In the current study, survival was not influenced by sex, age, HBsAg positivity, anti-HCV positivity, AFP levels, arterial blood flow, portal blood flow, histological characteristics, tumor multiplicity, or tumor size. However, a statistically significant difference was observed in long-term survival probability attributed only to liver dysfunction.
PEI-treated patients of Child-Pugh class A had longer survival than those of class B, which was comparable with the Italian and Japanese studies[7,8,11]. Generally speaking, in treating HCC, prognosis depends not only on the grade of cancer spread (tumor stage)[12], but also on the grade of residual liver function (liver disease stage). Kudo et al[13] have proposed a prognostic staging system for HCC called the Japan Integrated Staging Score (JIS score), and have suggested that the prognosis of stageIHCC (solitary, < 20 mm in diameter, no vascular invasion) depends on liver function. Here, the long-term results of PEI were equivalent to those achieved with patients treated by hepatic resection. According to the Liver Cancer Study Group of Japan[14], the 5- and 7-years survival rates among 3674 patients with single, clinical stageIHCC lesions < 20 mm in diameter, treated by hepatic resection, reached 65.4% and 47.1%, respectively. In our study, the respective rates of 77.3% and 43.0% were obtained by restricting the final analysis to a selected group of 20 patients with single or multiple HCC nodules ≤ 15 mm in diameter and with Child-Pugh class A cirrhosis. Hence, although no prospective randomized trials comparing PEI versus surgery have been conducted, the long-term results of the two treatments seem to be quite similar.
Recently, new thermal therapeutic techniques for HCC have been developed, including RFA, laser microwaves and cryotherapy. Among these, RFA has attracted much international interest and is now widely used in clinical practice. In a comparison of PEI and RFA in 86 patients with 112 HCCs[15], a complete response was reached in 90.3% by RFA and 80% by PEI, with an average of 1.2 sessions for RFA and 4.8 sessions for PEI. However, more complications arose by RFA: one severe (hemothorax that required drainage) and four minor (intraperitoneal bleeding, hemobilia, pleural effusion and cholecytitis), compared with none by PEI.
Recently, three randomized studies which compared RFA versus PEI for first-line treatment of early-stage HCC have been published[16–18]. European groups have failed to show a statistically significant difference in overall survival between patients who received RFA and PEI[16]. On the other hand, survival advantages have been identified in studies in Japan and Taiwan[17,18]. In Japan, Shiina et al[17] have described 232 patients, 118 treated by RFA and 112 by PEI. Four-year survival rate was 74% (95% CI: 65-84) for RFA and 57% (95% CI: 45-71) for PEI. RFA had a 46% smaller risk of death [adjusted relative risk, 0.54 (95% CI: 0.33-0.89), P = 0.02], a 43% smaller risk of overall recurrence [adjusted relative risk 0.57 (95% CI: 0.41-0.80), P = 0.0009], and an 88% smaller risk of local tumor progression [relative risk, 0.12 (95% CI: 0.03-0.55), P = 0.006] than PEI. Similarly, benefits in survival were also suggested in a subgroup analysis of a trial in Taiwan[18].
Most trials comparing RFA and PEI for treatment of small HCC have yielded better survival, local efficacy, local recurrence and duration of treatment in favor of RFA; the only advantage in favor of PEI being a slightly lower rate of complications[16]. In the present study, the frequency of HCC recurrence was considered to be high, with new lesions appearing in 25 of the 31 patients. However, after PEI, almost all recurrence was caused by the emergence of new nodular lesions in hepatic segments other than at the locations of the treated tumors, and were therefore probably unrelated to the original tumor. Repeat PEI alone was feasible for recurrence in 60.0%, 42.1%, 41.6%, 25.0% and 42.8% of these cases at detection of the first to fifth recurrence, respectively. As the number of lesions also increases whenever a tumor recurs, local treatment such as PEI is of limited value, and PEI must be replaced by another form of treatment such as TACE or transcatheter infusion chemotherapy.
All the studies mentioned above confirm the high efficacy of PEI in the treatment of minimum-sized HCC. In conclusion, to achieve the best possible prognosis in its treatment, early detection of HCC < 15 mm in diameter by imaging and histological diagnoses, and early treatment by PEI are essential.
COMMENTS
Background
In treatment of hepatocellular carcinoma (HCC), only 20%-30% of patients are candidates for surgery. Thus, various non-surgical therapies, such as percutaneous ethanol injection (PEI), microwave coagulation and radiofrequency ablation (RFA) have been widely used for small HCC. Although PEI was used as first-line treatment in some Japanese and Italian centers in the 1980s and 1990s when surgical techniques were precluded, RFA has recently emerged as a real competitor to PEI. At this time, there are no unequivocal data to back up PEI as a replacement for resection as a first-line treatment for patients with early-stage HCC.
Research frontiers
PEI is a standard therapy. However, there has been a drastic shift from PEI to RFA since the introduction of the latter into clinical practice, because efficacy seems more reproducible in RFA than in PEI and microwave coagulation. Most trials comparing RFA and PEI for the treatment of small HCC have yielded not only local efficacy but also survival in favor of RFA. In addition, RFA requires shorter hospitalization than PEI, which improves quality of life. However, severe complications arise with RFA, such as hemothorax, intraperitoneal bleeding, liver abscess, liver infarction and diaphragmatic hernia, compared with none with PEI.
Innovations and breakthroughs
PEI can be safely performed. In fact, severe complications such as intraperitoneal bleeding, liver abscess, liver infarction and diaphragmatic hernia did not occur in our study. After PEI however, almost all recurrence was caused by the emergence of new nodular lesions in hepatic segments other than at the locations of the treated tumors. In those cases, PEI was used not only for the initial treatment of small HCC, but also for recurrent lesions at untreated sites after treatment. The post-PEI survival rates in our patients with Child-Pugh class A cirrhosis were at least equal to those in the post-surgery group. Tumor size (< 15 mm in diameter) and liver function (Child-Pugh class A cirrhosis) were significant survival predictors, and such patients were the best candidates for percutaneous ablation. PEI is considered not to compete with but to be complementary to RFA in the treatment of small HCC, because of its excellent safety and efficacy.
Applications
RFA is superior to PEI in the treatment of small HCC from the viewpoint of treatment response and long-term survival. PEI however, seems feasible, efficacious and is very safe. RFA is difficult with tumors located near the gall bladder, bile ducts and diaphragm. Therefore, the usefulness and importance of PEI for HCC, especially for small-sized (< 15 mm in diameter) HCC, should be emphasized. Early detection of HCC < 15 mm by imaging and histological diagnosis and early treatment by PEI are essential.
Terminology
Small HCC: < 15 mm in diameter. Local ablation therapy: non-surgical imaging-guided therapy (using US and/or CT) such as PEI, microwave coagulation and RFA. PEI: absolute ethanol is injected directly into lesions through 21-22-G needles, which are inserted under US guidance. It can destroy a considerably large volume of tissue in one session. RFA: electrodes are inserted into the tumor under imaging guidance. Radiofrequency energy is emitted from the exposed portion of the electrode, which is converted into heat and causes necrosis of the tumor. Child-Pugh class A liver cirrhosis: Cirrhosis with relatively good liver function (bilirubin < 2 mg/dL, albumin > 3.5 g/dL, and prothrombin time > 80%), without ascites and encephalopathy.
Peer review
This study reported a small cohort of 31 patients with HCC < 15 mm in diameter treated by PEI. Overall survival of the patients was equal or possibly slightly superior to that with treatment by hepatic resection or radioablation. Although a small number of patients was analyzed, it represents an interesting and potentially important clinical finding.
Peer reviewer: Reinhard Buettner, Professor, Institute of Pathology, University Hospital Bonn, Sigmund-Freud-Str. 25, D-53127 Bonn, Germany
S- Editor Liu JN L- Editor Kerr C E- Editor Ma WH
References
- 1.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Okuda K, Peters RL, Simson IW. Gross anatomic features of hepatocellular carcinoma from three disparate geographic areas. Proposal of new classification. Cancer. 1984;54:2165–2173. doi: 10.1002/1097-0142(19841115)54:10<2165::aid-cncr2820541017>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Trevisani F, Caraceni P, Bernardi M, D'Intino PE, Arienti V, Amorati P, Stefanini GF, Grazi G, Mazziotti A, Fornale L. Gross pathologic types of hepatocellular carcinoma in Italian patients. Relationship with demographic, environmental, and clinical factors. Cancer. 1993;72:1557–1563. doi: 10.1002/1097-0142(19930901)72:5<1557::aid-cncr2820720512>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K. Early recognition of hepatocellular carcinoma. Hepatology. 1986;6:729–738. doi: 10.1002/hep.1840060432. [DOI] [PubMed] [Google Scholar]
- 5.Kim SR, Kang KB, Soh CG, Kim JH, Hayashi Y, Hanioka K, Itoh H. Clinicopathological study of minimum-sized hepatocellular carcinoma: an approach to the definition of early hepatocellular carcinoma. J Gastroenterol Hepatol. 1995;10:498–508. doi: 10.1111/j.1440-1746.1995.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartolozzi C, Lencioni R. Ethanol injection for the treatment of hepatic tumours. Eur Radiol. 1996;6:682–696. doi: 10.1007/BF00187673. [DOI] [PubMed] [Google Scholar]
- 7.Lencioni R, Pinto F, Armillotta N, Bassi AM, Moretti M, Di Giulio M, Marchi S, Uliana M, Della Capanna S, Lencioni M, et al. Long-term results of percutaneous ethanol injection therapy for hepatocellular carcinoma in cirrhosis: a European experience. Eur Radiol. 1997;7:514–519. doi: 10.1007/s003300050194. [DOI] [PubMed] [Google Scholar]
- 8.Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458–464. doi: 10.1016/j.jhep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Vilana R, Bruix J, Bru C, Ayuso C, Sole M, Rodes J. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology. 1992;16:353–357. doi: 10.1002/hep.1840160212. [DOI] [PubMed] [Google Scholar]
- 10.Kojiro M. The evolution of pathologic features of hepato-cellular carcinoma. In: Tabor E, editor. Viruses and liver cancer. Amsterdam: Elsevier; 2002. pp. 113–122. [Google Scholar]
- 11.Lencioni R, Caramella D, Bartolozzi C. Hepatocellular carcinoma: use of color Doppler US to evaluate response to treatment with percutaneous ethanol injection. Radiology. 1995;194:113–118. doi: 10.1148/radiology.194.1.7997536. [DOI] [PubMed] [Google Scholar]
- 12.Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538–543. doi: 10.1002/(sici)1097-0142(20000201)88:3<538::aid-cncr7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 14.Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224–1229. doi: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- 15.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 17.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004;127:1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]