Abstract
AIM: To determine the effect of Prepacol®, a combination of sodium phosphate and bisacodyl, on transit and quality of capsule endoscopy (CE).
METHODS: Fivety two consecutive patients were included in this prospective study. CE was performed following a 12 h fasting period. Twenty six patients were randomized for additional preparation with Prepacol®. The quality of CE was assessed separately for the proximal and the distal small bowel by 3 experienced endoscopists on the basis of a graduation which was initially developed with 20 previous CE.
RESULTS: Preparation with Prepacol® accelerated small bowel transit time (262 ± 55 min vs 287 ± 97 min), but had no effect on the quality of CE. Visibility was significantly reduced in the distal compared to the proximal small bowel.
CONCLUSION: The significantly reduced visibility of CE in the distal small bowel allocates the need for a good preparation. Since Prepacol® has no beneficial effect on CE the modality of preparation and the ideal time of application remains unclear. Further standardized examinations are necessary to identify sufficient preparation procedures and to determine the impact of the volume of the preparation solution.
Keywords: Small bowel, Capsule endoscopy, Preparation, Laxative, Visibility, Transit time
INTRODUCTION
Capsule endoscopy (CE) is a well accepted tool for evaluation of small bowel pathologies[1–4]. However, it has some limitations due to restricted recording time and reduced visibility by air and residual material especially in the distal small bowel. Therefore, prokinetic drugs, laxatives and defoaming agents have been tested to improve the quality of the examination.
Prokinetic drugs were used to avoid incomplete small bowel examinations due to long gastric retention and slow bowel transit of the capsule. It was shown that domperidone shortened gastric emptying time of the capsule[5]. The results on metoclopramide were inconsistent: Keuchel et al[5] found no effect, whereas Selby[6] demonstrated an increased gastric emptying time. Erythromycin accelerated gastric emptying[7,8], however, this treatment had no effect on the visibility in one study[7] and led even to an impaired visibility in another study[8].
In order to clean the small bowel from residual material different laxatives were tested. Sodium phosphate improved the view in some studies[9–11]. Preparation with polyethylenglycol produced controversial results: In two studies the visibility was improved[12,13], whereas in others it was unchanged[14–16].
Preparation with simethicone, a defoaming agent, resulted in fewer air bubbles and better visibility in one study[17].
However, since the data is scanty and partially inconsistent, to date no standardized protocol has been recommended for bowel preparation for CE.
Prepacol® (Guerbet GmbH, Sulzbach, Germany) is a combination of a saline (sodium phosphate) and a stimulant laxative (bisacodyl). It consists of 30 mL of a sodium phosphate solution (containing 6.9 sodiummonohydrogenphosphatedodecahydrate and 16.4 mg sodiumdihydrogenphosphatedihydrate) and 4 tablets (5 mg bisacodyl each). Prepacol® is available in several European countries and mainly applied for preparation before gastrointestinal operations, radiological and endoscopic bowel examinations.
The sodium phosphate solution is poorly absorbable. Water absorption from the gut is therefore impeded by the osmotic gradient. Besides its effects on colonic motor and secretory function, bisacodyl changes the net absorption of sodium and water in the small bowel into a net secretion[18] and accelerates small intestinal transit[18,19]. It was shown that bisacodyl elicits propulsive contractions of the terminal ileum[20,21]. The combination of the osmotic purgative effect of sodium phosphate with the secretory and prokinetic effect of bisacodyl makes Prepacol® at least theoretically an ideal candidate for small bowel preparation for CE. However, its effect on the quality and gastrointestinal transit of CE has not been studied yet.
MATERIALS AND METHODS
Fifty two consecutive patients receiving capsule endoscopy were included. The patients were prospectively randomized into two groups. Group A fasted at least 8 h before the examination; group B received additionally Prepacol®. A written informed consent was obtained from all patients. The research protocol was approved by the ethics committee of the University of Heidelberg. All patients fasted from 7 p.m. the day before CE, patients in group B received additionally at 7 p.m. 30 mL of the sodium phosphate-solution diluted with 70 mL of tap water. Subsequently, they drank 250 mL of water. At 10 p.m. the patients received 4 tablets Prepacol® (20 mg Bisacodyl totally) again with 250 mL of water. All patients were allowed to drink water until 2 h before the examination. The capsule was swallowed at 10 a.m. with 250 mL of plain water.
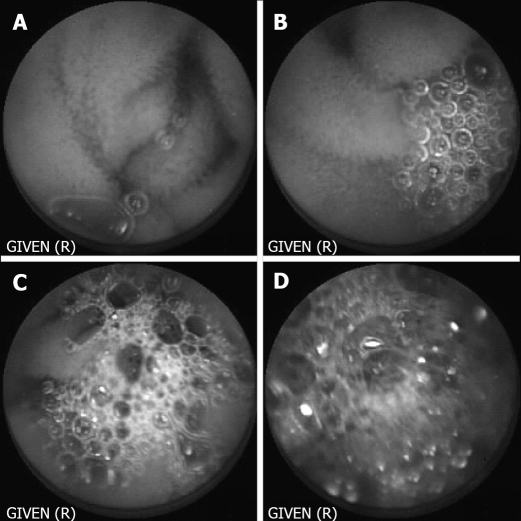
Capsule endoscopy films were evaluated by three independent, endoscopically experienced investigators who were blinded concerning the kind of preparation. In a run-in-phase the three investigators corporately generated the appraisal factors and their graduation on the basis of 20 retrospective CE examinations. The following parameters were assessed: total quality of the film, visibility of small bowel mucosa, velocity of the capsule and occurrence of foam, air and residual food. Every parameter was graduated from 1 to 4, accordingly, excellent, good, limited and poor quality. Graduation for occurrence of foam is shown in Figure 1. To evaluate the effect of the preparation with Prepacol® two one-hour-lasting periods were evaluated. The first period started one hour after the capsule left the stomach, the second period ended when the capsule reached the ileocecal valve. Investigators examined the films at a rate of 20 pictures per second.
Figure 1.
Graduation of visibility concerning occurrence of foam. A: Excellent; B: Good; C: Limited; D: Poor visibility.
Quantitative data are expressed as mean ± SEM and were analyzed by student’s t-test for significant differences. Categorical data were evaluated by Chi2 and Fisher exact test. P < 0.05 was chosen as the level of statistical significance.
RESULTS
Both groups were not different concerning age, weight, length and gender of the patients. Obscure gastrointestinal (GI) bleeding was the main indication for CE in both groups (Table 1). Other indications were suspicion for or follow-up in IBD, celiac disease, small bowel polyps or malignancy and no difference was observed between the groups (Table 1).
Table 1.
Patient’s data and indications for CE: Group A (fasting) and group B (additionally Prepacol®)
| Group A | Group B | P value | |
| Gender (w/m) | 13/13 | 10/16 | NS |
| Age (yr) | 54 ± 17 | 56 ± 20 | NS |
| Weight (kg) | 71 ± 15 | 79 ± 17 | NS |
| Length (cm) | 170 ± 10 | 171 ± 8 | NS |
| Indication | |||
| GI-bleeding | 17 (65%) | 19 (73%) | NS |
| Inflammatory bowel disease | 4 (15%) | 3 (12%) | NS |
| Miscellaneous | 5 (19%) | 4 (15%) | NS |
Data are mean ± SEM; NS: Not significant.
There were no differences in gastric emptying time of the capsule between the two groups (Table 2). Small bowel transit time was slightly but significantly shorter in the Prepacol®-group (262 ± 55 min vs 287 ± 97 min, P = 0.05) (Table 2). Recording time was not different between both groups.
Table 2.
GI transit times: Group A (fasting) and group B (additionally Prepacol®)
| Group A | Group B | P value | |
| Gastric retention (min) | 38 ± 23 | 44 ± 47 | NS |
| Small bowel transit (min) | 287 ± 97 | 262 ± 55 | 0.05 |
| Total recording time (min) | 441 ± 36 | 424 ± 49 | NS |
Data are mean ± SEM; NS: Not significant.
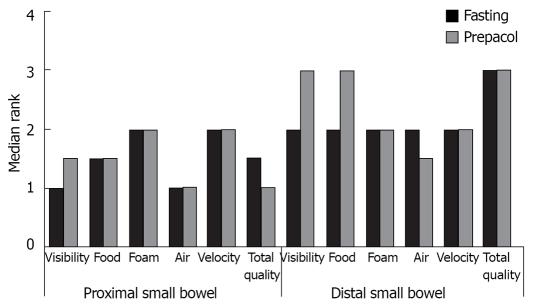
Figure 2 demonstrates median assessment of investigators concerning visibility of small bowel mucosa, occurrence of foam, air and residual food, as well as velocity of the capsule and total quality of the film separately for the proximal and distal small bowel. Compared to exclusive fasting additional preparation with Prepacol® did not improve any parameter.
Figure 2.
Quality of CE as assessed by three independent investigators, separately shown for proximal and distal small bowel, data are median of 52 patients, n = 26 for each group.
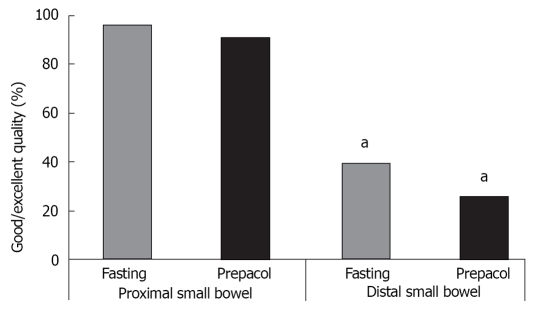
The quality was significantly more frequently judged as excellent or good in the proximal compared to the distal small bowel (Figure 3).
Figure 3.
Percentage of good or excellent quality as assessed by three independent investigators, separately shown for proximal and distal small bowel, data are median of 52 patients, n = 26 for each group, aP < 0.05 vs proximal small bowel.
Concordance in the assessment between each of the investigators was good (82%, 78% and 87%, respectively).
DISCUSSION
An adequately cleaned bowel is an important precondition for any gastrointestinal endoscopic procedure. Turbid fluid due to intestinal secretion and food residues in the small bowel may limit visibility and therewith the information obtained by capsule endoscopy. Although in some studies preparation with prokinetic agents or laxatives improved quality of CE[5–16], no standard procedure has been reached since the data is scanty and partially inconsistent. The selection of an appropriate preparation is further aggravated by the fact that the evaluation of the quality of capsule endoscopy is subjective. Therefore we chose three independent investigators to estimate the influence of Prepacol® on the quality of CE.
In the present study preparation with Prepacol® had no advantages concerning visibility of the mucosa, occurrence of foam, frequency and extent of air filled segments, food residues, velocity of the capsule and total quality as compared to exclusive overnight fasting.
Quality was significantly inferior in distal small bowel segments compared to proximal segments. This demonstrates that sufficient preparation would be of great help to obtain best possible conditions throughout the whole small bowel.
Prepacol® is not effective as a preparation for capsule endoscopy. This may be due to the pharmacological effect, the dosage and the time of application in relation to CE. Bisacodyl is mainly activated by bacterial metabolism in the colon. Although both, sodium phosphate and bisacodyl increase luminal fluid in the small bowel, their main effect is documented in colon preparation[22,23]. The dose of the sodium phosphates could be too low. Niv and colleagues could show that 90 mL of sodium phosphate in combination with 2 liters of water improved CE quality[9,10]. Those studies showing a positive effect of PEG on CE visibility also used volumes of at least 2 liters[12,15]. Therefore, not only the pharmacological effect may be responsible, but also the volume itself. Patients in our study were allowed to drink until 2 h before the examination, but no minimum volume was recommended. The volume was not documented, it was therefore not possible to examine if the individual amount of fluid intake had any effect on CE quality.
Double balloon enteroscopy (DBE) is another recent tool for examination of the small bowel[24–26]. Requirement for more manpower and a slightly increased complication rate are some disadvantages of DBE compared to CE[27,28]. However, DBE has a working channel which enables the examiner for example to take biopsies or intervene in bleedings[29]. In contrast to CE no other preparation than fasting is essential when DBE is performed orally, since the small bowel content can be cleaned during the examination by means of the suction channel[27,28].
CE is an expensive and also time consuming examination. An effective preparation is essential to minimize false results at the best possible rate. Therefore, additional studies are necessary to identify sufficient preparation procedures and to determine the impact of the volume of the preparation solution. This has to be performed in consideration of the patient’s compliance, which might be reduced by the taste of the preparation solution[30].
COMMENTS
Background
Capsule endoscopy (CE), a well accepted tool for evaluation of small bowel pathologies, has some limitations due to reduced visibility by air and residual material especially in the distal small bowel.
Research frontiers
Several forms of preparation (e.g. prokinetic drugs, laxatives and defoaming agents) have been tried to improve the quality of the examination. However, since the data is scanty and partially inconsistent, to date no standardized protocol has been recommended for bowel preparation for CE.
Innovations and breakthroughs
The effect of Prepacol® (Guerbet GmbH, Sulzbach, Germany), a combination of a saline (sodium phosphate) and a stimulant laxative (bisacodyl), on the quality and transit time of CE was tested. It has been shown that preparation with Prepacol® accelerated small bowel transit time, but had no effect on the quality of CE.
Applications
Since Prepacol® has no beneficial effect on CE the modality of preparation and the ideal time of application remains unclear.
Peer review
Interesting effort to do proper research on SB-preparation.
Peer reviewer: Chris JJ Mulder, Professor, Department of Gastroenterology, VU University Medical Center, PO Box 7057, Amsterdam1007 MB, The Netherlands
S- Editor Li DL L- Editor Alpini GD E- Editor Yin DH
References
- 1.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 2.Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999–1005. doi: 10.1053/gast.2002.35988. [DOI] [PubMed] [Google Scholar]
- 3.Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34:685–689. doi: 10.1055/s-2002-33446. [DOI] [PubMed] [Google Scholar]
- 4.Fireman Z, Mahajna E, Broide E, Shapiro M, Fich L, Sternberg A, Kopelman Y, Scapa E. Diagnosing small bowel Crohn's disease with wireless capsule endoscopy. Gut. 2003;52:390–392. doi: 10.1136/gut.52.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keuchel M, Vorderholzer W, Schenk G, Csomos G, Hagenmuller F, Lochs H. Domperidone shortens gastric transit time of video capsule endoscope. Endoscopy. 2003;35 (Suppl II):A185. [Google Scholar]
- 6.Selby W. Complete small-bowel transit in patients undergoing capsule endoscopy: determining factors and improvement with metoclopramide. Gastrointest Endosc. 2005;61:80–85. doi: 10.1016/s0016-5107(04)02462-9. [DOI] [PubMed] [Google Scholar]
- 7.Leung WK, Chan FK, Fung SS, Wong MY, Sung JJ. Effect of oral erythromycin on gastric and small bowel transit time of capsule endoscopy. World J Gastroenterol. 2005;11:4865–4868. doi: 10.3748/wjg.v11.i31.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fireman Z, Paz D, Kopelman Y. Capsule endoscopy: improving transit time and image view. World J Gastroenterol. 2005;11:5863–5866. doi: 10.3748/wjg.v11.i37.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niv Y, Niv G, Wiser K, Demarco DC. Capsule endoscopy - comparison of two strategies of bowel preparation. Aliment Pharmacol Ther. 2005;22:957–962. doi: 10.1111/j.1365-2036.2005.02647.x. [DOI] [PubMed] [Google Scholar]
- 10.Niv Y, Niv G. Capsule endoscopy: role of bowel preparation in successful visualization. Scand J Gastroenterol. 2004;39:1005–1009. doi: 10.1080/00365520410003209. [DOI] [PubMed] [Google Scholar]
- 11.Lapalus M, Saurin J, Mion F, Ponchon T. Prospective randomized single-blind trial on oral sodium phosphate efficacy for small intestine preparation before capsule endoscopy. Endoscopy. 2003;35 (Suppl II):A183. [Google Scholar]
- 12.Viazis N, Sgouros S, Papaxoinis K, Vlachogiannakos J, Bergele C, Sklavos P, Panani A, Avgerinos A. Bowel preparation increases the diagnostic yield of capsule endoscopy: a prospective, randomized, controlled study. Gastrointest Endosc. 2004;60:534–538. doi: 10.1016/s0016-5107(04)01879-6. [DOI] [PubMed] [Google Scholar]
- 13.Fireman Z, Kopelman Y, Fish L, Sternberg A, Scapa E, Mahaina E. Effect of oral purgatives on gastric and small bowel transit time in capsule endoscopy. Isr Med Assoc J. 2004;6:521–523. [PubMed] [Google Scholar]
- 14.Ben-Soussan E, Savoye G, Antonietti M, Ramirez S, Ducrotte P, Lerebours E. Is a 2-liter PEG preparation useful before capsule endoscopy? J Clin Gastroenterol. 2005;39:381–384. doi: 10.1097/01.mcg.0000159271.43233.45. [DOI] [PubMed] [Google Scholar]
- 15.Dai N, Gubler C, Hengstler P, Meyenberger C, Bauerfeind P. Improved capsule endoscopy after bowel preparation. Gastrointest Endosc. 2005;61:28–31. doi: 10.1016/s0016-5107(04)02444-7. [DOI] [PubMed] [Google Scholar]
- 16.Lee HS, Um SH, Lee SW, Choi JH. Kim CD, Ryu HS, Hyun JH, Uhm CS. Comparison of two bowel prep-aration for capsule endoscopy: NPO only versus PEG. Endoscopy. 2003;35 (Suppl II):A117. [Google Scholar]
- 17.Albert J, Gobel CM, Lesske J, Lotterer E, Nietsch H, Fleig WE. Simethicone for small bowel preparation for capsule endoscopy: a systematic, single-blinded, controlled study. Gastrointest Endosc. 2004;59:487–491. doi: 10.1016/s0016-5107(04)00003-3. [DOI] [PubMed] [Google Scholar]
- 18.Ewe K. Effect of bisacodyl on intestinal electrolyte and water net transport and transit. Perfusion studies in men. Digestion. 1987;37:247–253. doi: 10.1159/000199508. [DOI] [PubMed] [Google Scholar]
- 19.Ewe K, Ueberschaer B, Press AG, Kurreck C, Klump M. Effect of lactose, lactulose and bisacodyl on gastrointestinal transit studied by metal detector. Aliment Pharmacol Ther. 1995;9:69–73. doi: 10.1111/j.1365-2036.1995.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 20.Saunders DR, Sillery J, Rachmilewitz D, Rubin CE, Tytgat GN. Effect of bisacodyl on the structure and function of rodent and human intestine. Gastroenterology. 1977;72:849–856. [PubMed] [Google Scholar]
- 21.Pescatori M. Myoelectric and motor activity of the terminal ileum after pelvic pouch for ulcerative colitis. Dis Colon Rectum. 1985;28:246–253. doi: 10.1007/BF02554045. [DOI] [PubMed] [Google Scholar]
- 22.Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum. 1994;37:229–233; discussion 233-234. doi: 10.1007/BF02048160. [DOI] [PubMed] [Google Scholar]
- 23.Ker TS. Comparison of reduced volume versus four-liter electrolyte lavage solutions for colon cleansing. Am Surg. 2006;72:909–911. [PubMed] [Google Scholar]
- 24.May A, Nachbar L, Wardak A, Yamamoto H, Ell C. Double-balloon enteroscopy: preliminary experience in patients with obscure gastrointestinal bleeding or chronic abdominal pain. Endoscopy. 2003;35:985–991. doi: 10.1055/s-2003-44582. [DOI] [PubMed] [Google Scholar]
- 25.May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62–70. doi: 10.1016/s0016-5107(05)01586-5. [DOI] [PubMed] [Google Scholar]
- 26.Ell C, May A, Nachbar L, Cellier C, Landi B, di Caro S, Gasbarrini A. Push-and-pull enteroscopy in the small bowel using the double-balloon technique: results of a prospective European multicenter study. Endoscopy. 2005;37:613–616. doi: 10.1055/s-2005-870126. [DOI] [PubMed] [Google Scholar]
- 27.Fujimori S, Seo T, Gudis K, Tanaka S, Mitsui K, Kobayashi T, Ehara A, Yonezawa M, Tatsuguchi A, Sakamoto C. Diagnosis and treatment of obscure gastrointestinal bleeding using combined capsule endoscopy and double balloon endoscopy: 1-year follow-up study. Endoscopy. 2007;39:1053–1058. doi: 10.1055/s-2007-967014. [DOI] [PubMed] [Google Scholar]
- 28.Kaffes AJ, Siah C, Koo JH. Clinical outcomes after double-balloon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. 2007;66:304–309. doi: 10.1016/j.gie.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 29.May A, Nachbar L, Pohl J, Ell C. Endoscopic interventions in the small bowel using double balloon enteroscopy: feasibility and limitations. Am J Gastroenterol. 2007;102:527–535. doi: 10.1111/j.1572-0241.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 30.Szojda MM, Mulder CJ, Felt-Bersma RJ. Differences in taste between two polyethylene glycol preparations. J Gastrointestin Liver Dis. 2007;16:379–381. [PubMed] [Google Scholar]