Summary
In most eukaryotes, replication origins fire asynchronously throughout S-phase according to a precise timing programme. When replication fork progression is inhibited, an intra-S phase checkpoint is activated which blocks further origin firing and stabilises existing replication forks to prevent them undergoing irreversible collapse. We show that chromatin incubated in Xenopus egg extracts displays a replication timing programme, where firing of new replication origins during S phase depends on the continued activity of S phase-inducing cyclin-dependent kinases. We also show that low concentrations of the DNA polymerase inhibitor aphidicolin, which only slightly slow replication fork progression, strongly suppress further initiation events. This intra-S phase checkpoint can be overcome by caffeine, an inhibitor of the ATM/ATR checkpoint kinases, or by neutralizing antibodies to ATR. However, depletion or inhibition of Chk1 did not abolish the checkpoint. We could detect no significant effect on fork stability when this intra-S phase checkpoint was inhibited. Interestingly, although caffeine could prevent the checkpoint from being activated, it could not rescue replication if added after the timing programme would normally have been executed. This suggests that special mechanisms may be necessary for reversing the effects of the intra-S phase checkpoint once it has acted on particular origins.
Keywords: checkpoint, Xenopus, ATR, replication timing
Introduction
During S-phase of the cell cycle, replication forks are initiated from many replication origins that are activated in a precise temporal order (Diffley, 1998; Gilbert, 2002). In higher eukaryotes, clusters of adjacent origins typically fire near-synchronously, but with different clusters of origins firing at different times throughout S phase (Hand, 1978). In sperm chromatin replicating in cell-free extracts of Xenopus eggs, origins within these clusters are roughly spaced at 5-15 kb intervals, the spacing of origins being independent of the DNA sequence (Blow et al., 2001; Blow, 2001; Hyrien et al., 2003). It is unclear how the time of activation of any individual origin is determined.
At the end of mitosis, each origin becomes licensed for subsequent replication by the sequential loading of the Origin Recognition Complex (ORC), Cdc6, Cdt1 and multiple copies of the Mcm2-7 complex, thus forming a pre-replicative complex (pre-RC) (Blow and Hodgson, 2002; Nishitani and Lygerou, 2002). During S phase, the initiation of replication forks at licensed origins is dependent on at least two different protein kinases: S-phase inducing cyclin-dependent kinases (S-CDK) and the Cdc7-Dbf4 kinase. These kinases promote the loading of the Cdc45 protein onto origins, creating a “pre-initiation complex” (Mimura and Takisawa, 1998; Zou and Stillman, 1998; Jares and Blow, 2000).
In the yeast Saccharomyces cerevisiae, Cdc7 activity is required throughout S-phase to promote initiation at origins firing at different times (Bousset and Diffley, 1998; Donaldson et al., 1998a). CDKs have also been shown to play a role in the replication timing programme in S. cerevisiae (Donaldson et al., 1998b). CDKs are likely to be required throughout S phase to promote initiation (like Cdc7), because the CDK-dependent loading of Cdc45 onto late-firing origins only occurs late in S phase (Aparicio et al., 1999).
Genome stability is maintained by checkpoints which prevent cell cycle progression if DNA replication is blocked or if DNA is damaged (Hartwell and Weinert, 1989; Elledge, 1996). Checkpoint pathways include damage sensors, signal transducers, and effectors (Hartwell and Weinert, 1989; Latif et al., 2001; Hutchins and Clarke, 2004). At the top of the signal transduction pathway are the ATM and ATR kinases, members of the phosphoinositide 3-kinase-like kinase (PIKK) family. While ATM seems to be mainly activated by DNA double-strand breaks (Khanna and Jackson, 2001; Valerie and Povirk, 2003), ATR can be activated by a variety of DNA damaging agents or by inhibition of replication forks (Cliby et al., 1998; Guo et al., 2000; Liu et al., 2000; Wright et al., 1998). Two further protein kinases, Chk1 and Chk2 are phosphorylated by and act downstream of ATM and ATR kinases.
In S. cerevisiae, checkpoint activation has been shown to have two distinct effects on DNA replication: firstly, late-firing origins are prevented from initiating replication (Santocanale and Diffley, 1998; Shirahige et al., 1998). Secondly, pre-existing replication forks are stabilised by the checkpoint to prevent them from undergoing irreversible collapse (Lopes et al., 2001; Tercero and Diffley, 2001). Similar responses appear to operate in mammalian cells: if DNA synthesis is inhibited, a caffeine-sensitive intra-S phase checkpoint stabilizes components of existing forks and prevents initiation at late-firing origins (Dimitrova and Gilbert, 2000).
In this report we show that the replication timing programme in Xenopus requires the ongoing activity of S-CDKs and is responsive to checkpoint inhibition. Inhibition of DNA synthesis by aphidicolin leads to activation of checkpoint pathways that prevents further origin firing. However, we see no evidence for this checkpoint pathway affecting fork stability. We also show that the ATR kinase plays a major role in preventing late origin firing in this system.
Materials and Methods
Preparation and use of extracts
Xenopus egg extracts were prepared as described (Chong et al., 1997) and were supplemented with 100 μg/ml cycloheximide, 25 mM phosphocreatine and 15 μg/ml creatine phosphokinase before use. Metaphase-arrested extracts were released into interphase with 0.3 mM CaCl2. DNA synthesis was assessed by measuring incorporation of [ 32P]-dATP into acid insoluble material, assuming an endogenous dATP pool of 50 μM (Blow and Laskey, 1986; Chong et al., 1997). Final DNA concentrations in the assays were kept at 10-15 ng DNA/μl extract. All incubations were performed at 23°C.
32P]-dATP into acid insoluble material, assuming an endogenous dATP pool of 50 μM (Blow and Laskey, 1986; Chong et al., 1997). Final DNA concentrations in the assays were kept at 10-15 ng DNA/μl extract. All incubations were performed at 23°C.
Antibodies and reagents
Roscovitine, wortmannin and debromohymenialdisine (DBH) were dissolved in DMSO. Roscovitine was used at 0.5 mM, wortmannin at 400-800 nM and DBH at 2-100 μM; in all cases, final DMSO concentration was <1%. Caffeine dissolved in H2O was used at 5 mM. Neutralizing X-ATR antibody was a gift of Dr K. Cimprich and was used as described (Costanzo et al., 2003). Antibodies for immunoblotting were as follows: anti-Cdc45 antibody was a gift from Dr. H. Takisawa (Mimura and Takisawa, 1998); anti-Rad17 antibody was a gift from Dr H. Lindsay (Costanzo et al., 2003); anti-PCNA (PC10) was from Sigma; anti-human- phospho-Chk1Ser345 was from Cell Signaling Technology; anti-human-Chk1 (sc-7898) was from Santa Cruz.
The bovine His6-ΔN-Cyclin A cDNA plasmid was a gift from Dr J. Endicott (Brown et al., 1995). Cyclin A was expressed in BL21DE3 induced with 1 mM IPTG for 4 hr at 37°C. Bacteria were lysed using Bugbuster (Merck). Cyclin A was solubilised in 8 M urea and purified on Ni-NTA beads (Qiagen) following the manufacturer’s instructions. Urea was removed by dialysis against THED buffer (Hepes-KOH 20 mM, Triton 0.03%, ethyleneglycol 20%, KCl 150 mM, pH8).
Immunodepletion
Chk1 was immunodepleted from egg extracts using protein A Dynabeads (Dynal Biotechnology) according to the manufacturer’s instructions. Briefly, Dynabeads were washed with 100 mM HEPES pH 8 and saturated with anti-Chk1 antibody or non-immune rabbit IgG (control depletion). After removing excess antibody, two rounds of depletion with 50% (v/v) beads were performed at 4°C for 45 minutes each. Dynabeads were recovered and the depleted extracts were stored in liquid N2 for further use.
Chromatin and nuclear templates
Demembranated Xenopus sperm nuclei were prepared as described (Chong et al., 1997). For chromatin analysis, DNA was incubated in extract at 20 ng DNA/μl; extract was then diluted 20-fold in Nuclear Isolation Buffer (NIB: 50 mM KCl, 50 mM Hepes-KOH at pH 7.6, 5 mM MgCl2, 2mM DTT, 0.5 mM spermidine-3HCl, 0.15 mM spermine-4HCl, 1 μg/ml each aprotinin, leupeptin and pepstatin) plus 0.1% Triton X-100, and then underlayered with the same buffer containing 15% (w/v) sucrose. Chromatin was pelleted at 4,200 x g in a swing-out rotor for 5 minutes at 4°C. The supernatant was removed, and the sucrose interface washed 3x with 150 μl NIB plus 0.1% Triton X-100. The overlying cushion was removed and the chromatin re-centrifuged at 10,000 x g in a fixed-angle rotor. The chromatin pellet was resuspended in SDS sample buffer. For nuclear re-isolation assays, nuclear isolation was performed as above except that Triton X-100 was omitted and only the first centrifugation was performed, after which the chromatin was resuspended to ~100 ng DNA/μl and kept on ice for further use.
For isolation of intact nuclei (Kumagai et al., 1998), extract containing nuclei was resuspended 10-fold in buffer containing 40% (w/v) sucrose, 50 mM Hepes-KOH pH 7.5, 100 mM KCl and 2.5 mM MgCl2 and pelleted at 5,000 x g in swing-out rotor for 5 minutes at 4° C. The pellet was resuspended in 1 ml of the same buffer and re-centrifuged. The supernatant was removed and nuclear samples were resuspended in SDS sample buffer.
Biotin dUTP labelling was performed essentially as described (Blow and Watson, 1987). Biotin-11-dUTP (Roche) was added to extract at 20 μM. At the required time, the extract was diluted 10-fold with Buffer A (60 mM KCl, 15 mM Tris HCl pH 7.4, 15 mM NaCl, 2 mM DTT, 0.5 mM spermidine, 0.15 mM spermine, 1 μg/ml aprotinin, leupeptin and pepstatin) plus 10% sucrose, underlayered with 200 μl Buffer A plus 30% sucrose and spun at 5,000xg in a swing-out rotor. Most of the supernatant was removed and the nuclei gently resuspended. Nuclei were supplemented with 0.5 μl Texas Red Streptavidin (Pharmacia) and 2 μg/ml Hoechst 33258, incubated at room temperature for 10 minutes and visualized by fluorescence microscopy.
Alkaline Agarose Gels
For alkaline agarose gel analysis, sperm nuclei were incubated at 10 ng/μl in extract. Reactions were stopped with Stop N (20 mM Tris-HCl pH 8, 200 mM NaCl, 5 mM EDTA, 0.5% SDS) containing 2 μg/ml RNAse. DNA was extracted with phenol:chloroform:isoamyl alcohol (25:24:1) using Phase Lock Gel™ tubes (Eppendorf), ethanol-precipitated and resuspended in alkaline loading buffer (25 mM NaOH, 3 mM EDTA, 1.25% Ficoll, 0.0125% bromocresol green). Agarose gels were poured in 50 mM NaCl, 1 mM EDTA and then equilibrated in 50 mM NaOH, 1 mM EDTA for 3 hr. Gels were run at 2 V/cm for 14-16 h at 4°C, then fixed in 7% TCA (w/v), 1.4% (w/v) sodium pyrophosphate for 20 min. Gels were dried between sheets of 3MM paper (Whatman) and exposed to X-ray film. As DNA size standards, 25-50 ng of λ-HindIII markers (New England Biolabs) end-labelled with [α-32P]dATP, were run alongside.
Results
The Xenopus replication timing programme is driven by S-CDKs
When demembranated sperm nuclei are added to interphase Xenopus egg extract, the sperm chromatin decondenses and is assembled into interphase nuclei. S phase begins shortly after nuclear assembly is complete and continues for 20-30 minutes (Blow, 2001). Figure 1A shows a typical reaction where replication starts around 30 minutes and is completed by 60-70 min. Replication origins can fire at different times during S phase in this system (Herrick et al., 2000; Lucas et al., 2000; Blow et al., 2001). To examine the kinetics of this replication timing programme, we pulse-labelled nascent DNA with [α-32P]dATP at different times; nascent DNA was separated on alkaline agarose gels and autoradiographed (Fig 1B). The first short [α-32P]dATP-labelled strands became visible around 35 minutes and increased in size consistent with the reported fork rate of 10 nt/sec (Mahbubani et al., 1992). Small nascent DNA fragments were seen for 20-30 minutes from the start of S phase, consistent with new replication forks initiating throughout most of S phase.
Figure 1.
Replication kinetics in Xenopus egg extract.
(A) Sperm nuclei were incubated at 12 ng DNA/μl in interphase Xenopus egg extract supplemented with [α-32P]dATP. At the indicated times, total DNA synthesis was measured. (B) Sperm nuclei were incubated in extract at 12 ng DNA/μl. At the indicated times, samples were pulse labelled with [α-32P]dATP for 2 min. DNA was separated on an alkaline agarose gel and autoradiographed. The migration of end-labelled λ-Hind III DNA is also shown.
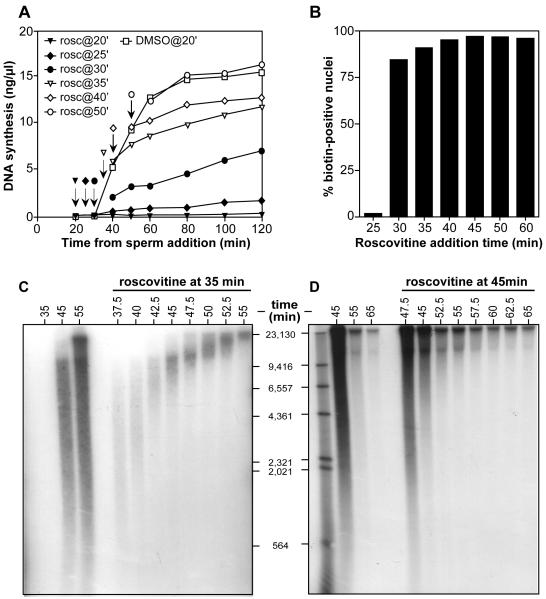
S phase-inducing CDKs (S-CDKs) such as Cdk2-cyclin E and Cdk2-cyclin A induce the initiation of DNA replication, but are not required for the elongation of forks once they have initiated (Strausfeld et al., 1994; Blow, 2001). We next investigated whether S-CDK activity was required only at the beginning of S-phase or whether it was required throughout S phase as new replication origins fired. The CDK inhibitor roscovitine (Meijer et al., 1997) was added to egg extract at different times throughout S phase and subsequent DNA synthesis measured (Fig 2A). When roscovitine was added before the start of S-phase (20-25 min; filled triangles and diamonds), there was virtually no DNA synthesis. However, addition of roscovitine during early- to mid-S phase (30-40 min; filled circles, open triangles and diamonds) allowed limited DNA synthesis to occur. Addition of roscovitine in late S phase (50 min; open circles) had no effect on subsequent DNA synthesis, all replication forks presumably having been initiated by this time.
Figure 2.
Inhibition of CDK activity prevents later origins from firing.
(A) Sperm nuclei were incubated at 15 ng DNA/μl in egg extract supplemented with [α-32P]dATP. Aliquots were supplemented with 0.5 mM roscovitine at the following times: filled triangles, 20 min; filled diamonds, 25 min; filled circles 30 min; open triangles, 35 min; open diamonds, 40 min; open circles, 50 min. Samples with no added roscovitine are shown by open squares. At the indicated times, samples were assayed for total DNA synthesis. (B) Sperm nuclei were incubated at 15 ng DNA/μl in egg extract supplemented with biotin-dUTP. 0.5 mM roscovitine was added at the indicated times. At 120 min, nuclei were isolated and stained with Texas Red streptavidin to reveal nuclei which had undergone DNA replication. The percentage of biotin-positive nuclei for each point is shown. (C, D) Sperm nuclei were incubated at 15 ng DNA/μl in egg extract. At 35 minutes (C) or 45 minutes (D), aliquots were supplemented with 0.5mM roscovitine. Samples were pulse-labelled with [α-32P]dATP for 2 minutes at the indicated times. DNA was separated on an alkaline agarose gel and autoradiographed. The migration of end-labelled λ-HindIII DNA is also shown.
In the Xenopus system, individual nuclei can assemble and initiate replication asynchronously (Blow and Watson, 1987). In order to rule out the possibility that the asynchrony in initiation was simply due to asynchrony in the times that different nuclei started to replicate, we repeated the roscovitine addition experiment using biotin-dUTP instead of [α-32P]dATP; nuclei were then isolated, the biotinylated DNA stained with fluorescent streptavidin, and the percentage of nuclei that had started to replicate was determined by fluorescence microscopy. Fig 2B shows that >80% of nuclei started replicating 25-30 minutes from the start of the incubation. This means that the asynchrony in initiation shown in Figs 1B and 2A largely resulted from asynchrony between different replication origins within individual nuclei, thus representing a replication timing programme as is seen in other eukaryotes. The partial inhibition of replication seen when roscovitine was added during early S phase (Fig 2A) suggests that CDK activity is required throughout S phase to support continuing initiation events.
In order to confirm this, roscovitine was added to extracts at early or mid S-phase (35 or 45 min) and nascent DNA was pulse-labelled with [α-32P]dATP at different times and separated on alkaline gels (Fig 2C, D). After addition of roscovitine, the smallest labelled DNA molecules moved up the gel at ~10 nt/sec (the normal fork rate; Mahbubani et al., 1992). This suggests that roscovitine inhibits new initiation events within ~2.5 minutes of being added to extract. Therefore S-CDK activity is required throughout S phase to drive continuing initiation, but is not required for elongation of replication forks (Strausfeld et al., 1994).
Late origin firing is inhibited in response to replication fork inhibition
In response to replication fork arrest in S. cerevisiae and mammals, ATM/ATR-dependent checkpoint pathways inhibit initiation from late-firing replication origins (Santocanale and Diffley, 1998; Shirahige et al., 1998; Weinert et al., 1994). We next investigated whether replication fork inhibition invokes a similar intra-S phase checkpoint in Xenopus. Figs 3A and B show the effect on DNA synthesis of supplementing Xenopus extracts with different concentrations of aphidicolin, a competitive inhibitor of replicative DNA polymerases. Replication was significantly inhibited by concentrations of aphidicolin as low as 5 μM, and at 40 μM inhibition was almost complete. Nascent strands synthesised in the presence of aphidicolin were analysed on alkaline gels (Fig 3C). Despite the significant inhibition of DNA synthesis seen with 5 μM aphidicolin, nascent strands were of high molecular weight, well above the average replicon size of ~10kb (Blow et al, 2001). This suggests that the primary cause for the inhibition of DNA synthesis at 5 μM aphidicolin is a reduction in the number of active replication forks. Only at higher aphidicolin concentrations was there a clear reduction in nascent strand length. This is consistent with aphidicolin activating an intra-S phase checkpoint that blocks initiation.
Figure 3.
Aphidicolin induces a caffeine-sensitive replication checkpoint.
(A-D) Sperm nuclei were incubated at 15 ng DNA/μl in interphase egg extract supplemented with [α-32P]dATP plus various concentrations of aphidicolin plus or minus 5 mM caffeine. (A) Total DNA synthesis was measured at 120 min. (B) DNA synthesis was measured between 15 and 120 minutes as indicated. (C, D) At 120 min, DNA was separated on an alkaline agarose gel and autoradiographed. The migration of end-labelled λ-HindIII DNA is also shown. (E, F) Sperm nuclei were incubated at 15 ng DNA/μl for 35 minutes in interphase egg extract supplemented with 7.5μM aphidicolin. The reaction was split in two, and supplemented with 0.5 mM roscovitine minus (E) or plus (F) 5 mM caffeine. At 5 minute intervals, aliquots were pulse-labelled for 2 minutes with [α-32P]dATP, then DNA was separated on an alkaline agarose gel and autoradiographed. The migration of end-labelled λ-HindIII DNA is also shown.
To test this idea, we supplemented extracts with caffeine, an inhibitor of the ATM and ATR kinases (Blasina et al., 1999; Sarkaria et al., 1999). In the absence of aphidicolin, caffeine did not significantly change the overall S phase kinetics (Fig 3B and data not shown). However, caffeine almost completely reversed the inhibition of replication observed with 5 μM aphidicolin (Fig 3A, B and D). Only at 15 μM and above, where aphidicolin had a marked effect on nascent strand length, was caffeine unable to prevent a marked reduction in DNA synthesis. The caffeine-dependent accumulation of short nascent strands in the presence of high aphidicolin concentrations has been previously reported in Xenopus (Yanow et al., 2003).
The length of nascent strands seen in extracts plus and minus caffeine was similar, though at moderate aphidicolin concentrations (10-15 μM), caffeine caused an increase in the proportion of strands >9 kb in length. This increase in strand size could be due to increased nascent strand fusion, or alternatively could be due to an increased fork rate. We therefore measured the effect of caffeine on the fork rate in the presence of aphidicolin. Sperm nuclei were incubated in extract containing 7.5 μM aphidicolin for 35 minutes (the start of S phase), and then aliquots were supplemented with 0.5 mM roscovitine (to block further initiation) plus or minus 5 mM caffeine. At various times afterwards, aliquots were pulse-labelled with [α-32P]dATP, and the nascent strands separated on alkaline agarose gels. Fig 3E shows that in the presence of 7.5 μM aphidicolin and no caffeine, nascent strands elongated to a size of ~8 kb after 90 minutes (an average fork rate of ~2.4 nt/sec). In the presence of caffeine (Fig 3F), forks elongated at a similar rate, reaching ~9kb after 90 minutes (an average fork rate of ~2.7 nt/sec). Although caffeine may therefore have a slight effect on the fork rate in the presence of aphidicolin, its major effect is to increase the number of active forks, which in turn will lead to increased strand fusion at later times. These results suggest that the inhibition of DNA synthesis by aphidicolin comprises two distinct components: a checkpoint-dependent inhibition of initiation that predominates at low aphidicolin concentrations, and direct inhibition of fork elongation that becomes significant at higher aphidicolin concentrations.
Aphidicolin reduces the number of active forks in a checkpoint-dependent manner
We next devised a method for measuring the number of active replication forks. Sperm nuclei were incubated in extract with increasing concentrations of aphidicolin; at the beginning of S-phase (35 min) the nuclei were transferred to fresh extract containing 0.5 mM roscovitine and [α-32P]dATP but lacking aphidicolin. The roscovitine present in the second extract inhibits further initiation so that the subsequent replication is dependent only on active replication forks present in the nuclei at the time of transfer. This technique showed that 7.5 μM or 15 μM aphidicolin caused a progressive decrease in the number of active replication forks (Fig 4A; a similar tendency was observed with 40 μM and 120 μM - data not shown), suggesting that aphidicolin reduced initiation in a concentration-dependent manner. Addition of caffeine to the first extract did not significantly change the number of active forks in the absence of aphidicolin. However, in the presence of caffeine, addition of aphidicolin no longer reduced the number of active forks, but instead led to an increase (Fig 4B). This result is consistent with aphidicolin inducing an intra-S phase checkpoint that inhibits further origin firing. The increase in the number of active forks seen with a combination of caffeine and aphidicolin may be a consequence of the synchronization of replication forks that this combination of inhibitors is likely to induce (when forks are synchronized near the origin, more replication can occur in the second extract). It has also been shown in yeast that inhibition of checkpoint kinases permits the activation of late-firing and dormant replication origins (Santocanale and Diffley, 1998; Shirahige et al., 1998), and this may also occur in Xenopus.
Figure 4.
The intra-S phase checkpoint reduces the number of active replication forks.
(A, B) Sperm nuclei were incubated at 10 ng DNA/μl in interphase extract with or without 7.5 μM or 15 μM aphidicolin, minus (A) or plus (B) 5 mM caffeine. At early S-phase (35min), nuclei were isolated and transferred to fresh extract supplemented with [α-32P]dATP and 0.5 mM roscovitine. At the indicated times after transfer (1-30min), total DNA synthesis was measured. (C, D) Sperm nuclei were incubated in interphase extract at 10 ng DNA/μl supplemented with 40 μM aphidicolin minus (C) or plus (D) caffeine. Sperm chromatin was incubated for 35, 45 or 60 min, and was then isolated and transferred to fresh extract supplemented with [α-32P]dATP and 0.5 mM roscovitine. Total DNA synthesis was measured at different times after transfer.
We next investigated whether the timing programme still functions in the presence of aphidicolin. Sperm nuclei were incubated for 35, 45 or 60 minutes in extract supplemented with 40 μM aphidicolin; nuclei were then isolated and transferred to fresh extract containing roscovitine and [α-32P]dATP. Since 40 μM aphidicolin is sufficient to inhibit most fork elongation under these conditions (Fig 3C and D), any forks initiated will accumulate close to the origins. The results showed a small but significant accumulation of active forks between 35 and 60 minutes in aphidicolin (Fig 4C), suggesting that the checkpoint slows (but does not abolish) the firing of new replication origins. When this experiment was repeated with both aphidicolin and caffeine in the first extract, a greater increase in the number of active forks was seen between 35 and 60 minutes (Fig 4D). This suggests that an approximately normal initiation timing programme occurs in the presence of aphidicolin when the checkpoint is abolished by caffeine.
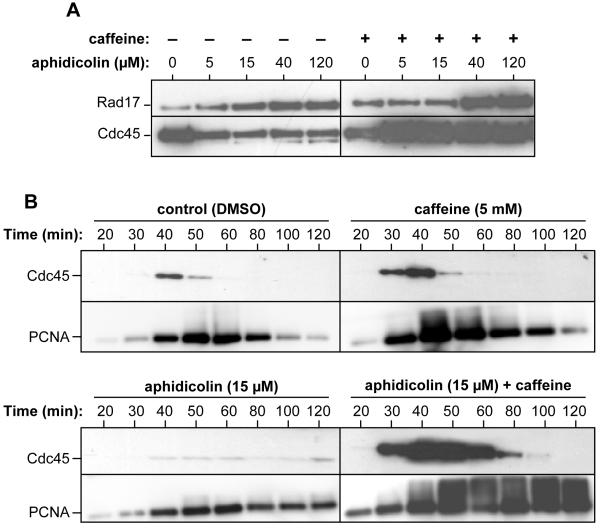
To gain direct evidence for the changes in the number of active forks, we examined proteins assembled onto chromatin in the presence of aphidicolin. Sperm nuclei were incubated in extracts containing increasing concentrations of aphidicolin, plus or minus caffeine, and at 50 minutes (mid S-phase) chromatin was isolated and immunoblotted for Rad17 (a component of the 9-1-1 or RSR damage recognition clamp loader which co-localises with DNA damage foci; Ellison and Stillman, 2003; Post et al., 2003; Stokes and Michael, 2003) and the pre-initiation/replication fork protein Cdc45. Fig 5A shows that the amount of Rad17 on chromatin increased with increasing concentrations of aphidicolin, consistent with increasing activation of the checkpoint pathway. As expected, Rad17 recruitment was not inhibited by caffeine. In contrast to Rad17, the amount of Cdc45 loaded onto chromatin was significantly reduced in the presence of 5 μM aphidicolin. This is consistent with our observations that 5 μM aphidicolin can almost fully activate the intra-S phase checkpoint (Fig 3). In the presence of both aphidicolin and caffeine, there was a dramatic increase in the amount of Cdc45 loaded onto chromatin, consistent with a previous report (Yanow et al., 2003). This increase may represent Cdc45 loading onto excess Mcm2-7 present at all origins (Mahbubani et al., 1997).
Figure 5.
The intra-S phase checkpoint reduces Cdc45 and PCNA on chromatin.
(A) Sperm nuclei were incubated at 10 ng DNA/μl in interphase extract supplemented with various concentrations of aphidicolin minus (left panel) or plus 5 mM caffeine (right panel). Chromatin was isolated and immunoblotted for XCdc45 and Rad17. (B) Sperm nuclei were incubated at 10 ng DNA/μl in interphase extract supplemented with different combinations of 15 μM aphidicolin and/or 5 mM caffeine. At the indicated times, chromatin was isolated and immunoblotted for XCdc45 and PCNA.
Fig 5B shows time-courses of Cdc45 and PCNA loading onto chromatin in the presence or absence of 15 μM aphidicolin and caffeine. PCNA is a processivity factor for DNA polymerase δ present at replication forks. Aphidicolin induced a dramatic reduction in Cdc45 loading and a less dramatic reduction in PCNA loading. The relatively high levels of PCNA seen in the presence of aphidicolin may reflect the fact that PCNA is involved in DNA repair as well as DNA replication. The reduced levels of Cdc45 and PCNA seen in the presence of aphidicolin remained approximately constant even at later times, suggesting a lack of fork termination. However, in the presence of 15 μM aphidicolin and caffeine, the higher levels of Cdc45 loaded onto chromatin declined at later times; this suggests that, consistent with the high levels of DNA synthesis observed (Fig 3), fork termination can occur under these conditions. In contrast, PCNA levels remained surprisingly high on the chromatin in the presence of aphidicolin (Fig 5B). This may reflect ongoing DNA repair that is required after DNA synthesis has occurred in the presence of aphidicolin. Some of the chromatin-bound PCNA in the presence of aphidicolin and caffeine showed a retarded electrophoretic mobility, consistent with the ubiquitination of PCNA that occurs during post-replicative repair (Hoege et al., 2002).
Neither aphidicolin nor caffeine destabilise active replication forks
Our results have so far shown that aphidicolin induces a caffeine-sensitive reduction in the number of active replication forks in Xenopus egg extracts, and are consistent with an intra-S-phase checkpoint that inhibits initiation. However, an alternative explanation might be that aphidicolin reduces the number of active forks by inactivating and disassembling them. In order to distinguish between these possibilities, sperm nuclei were incubated in extract for 40 minutes (mid S phase) to allow some origins to fire, and then 40 μM aphidicolin was added (high enough to inhibit significant fork progression) and roscovitine (to prevent further initiation); at various times afterwards, nuclei were isolated and the number of active replication forks assessed by analysing the rate of replication in fresh extracts supplemented with [α-32P]dATP and roscovitine (see scheme in Fig 6A). Figure 6B shows that the replication forks remained fairly stable in the presence of aphidicolin, with more than >75% activity remaining after a 90 minute incubation. Results consistent with this were also obtained when fork elongation in the presence of aphidicolin was analysed on alkaline agarose gels (data not shown). These results suggest that fork instability is not a major factor in our experiments and cannot explain the decreased number of active replication forks that are seen in the presence of aphidicolin.
Figure 6.
Fork stability is not significantly affected by the intra-S phase checkpoint.
(A, B, C) Sperm nuclei were incubated at 10 ng DNA/μl in Xenopus extract; after 35 minutes (early S-phase) extract was supplemented with 40 μM aphidicolin and 0.5 mM roscovitine minus (B) or plus (C) 5 mM caffeine. 0, 10, 25 or 55 minutes after this, nuclei were isolated and transferred to fresh extract supplemented with 0.5 mM roscovitine and [α-32P]dATP. Total DNA synthesis was measured at different times after transfer. A schematic outline of the experiment is shown in (A). (D) Sperm nuclei were incubated at 10 ng DNA/μl in extract. At 35 min, the extract was supplemented with 7.5 μM aphidicolin and 5 mM caffeine, minus or plus 0.5 mM roscovitine. At the indicated times, samples were pulse-labelled with [α-32P]dATP for 2 min, the DNA was isolated and analysed by agarose electrophoresis and autoradiography. The migration of end-labelled λ-HindIII DNA is also shown.
Work in yeast and mammalian cells has indicated that in addition to inhibiting further initiation, checkpoint pathways play an important role in stabilizing replication forks under conditions where replication is blocked (Dimitrova and Gilbert, 2000; Tercero and Diffley, 2001; Lopes et al., 2001; Tercero et al., 2003). S. cerevisiae cells lacking MEC1 (an ATR homologue) or RAD53 (a Chk2 homologue) are unable to recover from a replication block as replication forks collapse irreversibly (Tercero and Diffley, 2001; Lopes et al., 2001). We therefore investigated whether we could see a similar checkpoint-dependent stabilisation of replication forks in Xenopus. The experiments shown in Figs 6A and B were repeated, but this time the first extract contained caffeine in addition to aphidicolin. Figure 6C shows that following transfer to fresh extract supplemented with [α-32P]dATP and roscovitine, nuclei previously arrested for up to 45 minutes with aphidicolin and caffeine showed no significant loss of functional replication forks.
Since this experiment involved inhibiting new initiation events with the CDK inhibitor roscovitine, this formally left open the possibility that CDK activity is required to destabilise the arrested forks when the replication checkpoint is inhibited. In order to address this, we incubated sperm nuclei in untreated egg extract, and then just at the start of S phase (35 min) added 7.5 μM aphidicolin and caffeine, plus or minus roscovitine. At different times thereafter, nascent DNA was pulse-labelled with [α-32P]dATP and the nascent DNA analysed on an alkaline agarose gel (Fig 6D). In the absence of roscovitine, the nascent strands elongated at a reduced rate of ~3 nt sec (compared with 10 nt/sec in the absence of aphidicolin). This rate was maintained over the full 15 minute time-course, consistent with the replication forks remaining active over this time. In addition, new nascent strands were initiated over this time, since the checkpoint had been blocked by caffeine. When roscovitine was added to the extract at the same time as aphidicolin, the existing forks elongated at a similar rate and extent to that seen in the absence of caffeine, suggesting that CDK activity had no major effect on fork stability. However, roscovitine clearly prevented new origins from firing as evidenced by the lack of short nascent strands at later times (Fig 6D, right). Taken together, these results suggest that when inhibited by aphidicolin, replication forks remain fairly stable in Xenopus extracts irrespective of the activity of the ATM/ATR or S-CDK kinases.
The replication checkpoint involves ATR and S-CDK kinases
We have shown that the intra-S-phase checkpoint in Xenopus blocks the loading of Cdc45 onto chromatin (Fig 5B). Since S-CDK activity is required for Cdc45 recruitment to chromatin (Mimura and Takisawa, 1998; Zou and Stillman, 1998; Jares and Blow, 2000) and since we have shown that the replication timing programme in Xenopus is driven by S-CDKs (Fig 2), we investigated the potential role of S-CDK inhibition in the checkpoint response. We first checked whether caffeine-induced recovery from the checkpoint required CDK activity. Sperm nuclei were added to Xenopus egg extracts supplemented with 15 μM aphidicolin and [α-32P]dATP; then at various times, the extract was supplemented with caffeine (to block the checkpoint) plus or minus roscovitine, and the total amount of DNA synthesised at 2 hr was measured (Fig 7A). As expected, when caffeine was added to the extract prior to the onset of S phase, replication was efficiently rescued. Efficient rescue also occurred when caffeine was added up to 40 minutes (mid S phase), but rescue rapidly tailed off, so that when caffeine was added at 50 min, there was virtually no rescue of replication. The timescale of the decline in caffeine’s ability to rescue the aphidicolin-dependent block of replication is similar to that of the replication timing programme itself. This is consistent with caffeine being able to prevent activation of the checkpoint that blocks initiation, but being unable to reverse this block once it has been implemented. When both caffeine and roscovitine were added together there was virtually no rescue of replication for any time (Fig 7A). This suggests that the checkpoint inhibits replication at a stage prior to S-CDK activity, consistent with the observed inhibition of Cdc45 loading.
Figure 7.
The ability of caffeine to rescue replication time decreases with time.
(A) Sperm nuclei were incubated at 15 ng DNA/μl in extract supplemented with [α-32P]dATP and 15 μM aphidicolin. At the indicated times, 5 mM caffeine plus or minus 0.5 mM roscovitine were added to the extract and the samples incubated for a further 120 min, when total DNA synthesis was measured. As control, aphidicolin was substituted with DMSO. (B) Sperm nuclei were incubated at 15 ng DNA/μl in extract supplemented with [α-32P]dATP, 10 μM aphidicolin, plus or minus 5 mM caffeine and increasing concentrations of ΔNcyclin A. At 120 minutes total DNA synthesis was measured.
We next investigated whether we could overcome the intra-S phase checkpoint by increasing S-CDK activity. When cyclin A is added to Xenopus egg extract, it activates the large pool of free Cdk1 and provides S-CDK activity, though higher cyclin A additions induce nuclear envelope breakdown and the onset of mitosis (Strausfeld et al., 1996). We therefore performed a replication assay in the presence of 10 μM aphidicolin, with or without caffeine, and increasing concentrations of recombinant cyclin A (Fig. 7B). In the absence of caffeine, only a slight rescue of replication was detected with increasing concentrations of cyclin A (Fig 7B, diagonal shading). At the highest cyclin A concentration (1000 nM), nuclear envelope breakdown occurred, showing that functional CDK activity was generated in the extract. In the presence of both aphidicolin and caffeine, cyclin A showed a dose-dependent stimulation of DNA synthesis (Fig 7B, horizontal shading). These results suggest that although S-CDK activity is required to exit from the checkpoint response, inhibition of S-CDK activity is unlikely to be the sole cause for the checkpoint-induced inhibition of initiation.
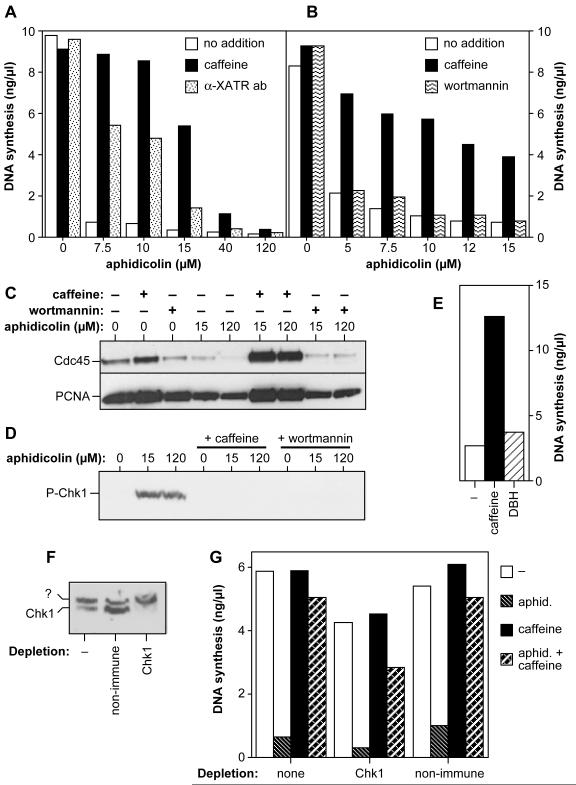
We next investigated which kinase pathways are involved in the intra-S phase checkpoint. We performed replication assays in the presence of aphidicolin and either caffeine, wortmannin or a neutralising antibody for Xenopus ATR (Costanzo et al., 2003). The ATR antibody rescued DNA replication inhibition to levels >60% of those seen with caffeine (Fig 8A), suggesting that ATR plays a major role in the intra-S checkpoint in Xenopus. In contrast, wortmannin was not able to rescue the inhibition of replication induced by even low concentrations of aphidicolin (Fig 8B) and failed to rescue the aphidicolin-induced inhibition of Cdc45 loading onto chromatin (Fig 8C). Since wortmannin inhibits ATM and DNA-PK more strongly than ATR (Sarkaria et al., 1998), the inability of wortmannin to rescue aphidicolin-dependent block of replication suggests that ATM does not play an essential role in the intra-S phase checkpoint. A combination of the anti-ATR antibody and wortmannin did not enhance the rescue above that seen with the anti-ATR antibody alone (data not shown).
Figure 8.
ATR is necessary for the aphidicolin-induced intra-S-phase checkpoint.
(A, B) Sperm nuclei were incubated at 15 ng DNA/μl in extract supplemented with [α-32P]dATP, and various concentrations of aphidicolin, plus or minus 5 mM caffeine or (A) an antibody neutralising the ATR kinase (α-XATR), or (B) 800 nM wortmannin. At 120 minutes total DNA synthesis was measured. As control, aphidicolin was substituted with DMSO. (C, D) Sperm nuclei were incubated at 15 ng DNA/μl in extract supplemented with 0, 15 or 120 μM aphidicolin, plus or minus 800nM wortmannin or 5 mM caffeine. (C) At 50 minutes (mid-S-phase) chromatin was isolated and immunoblotted for Cdc45 and PCNA. (D) At 60 min, intact nuclei were isolated and immunoblotted for phospho-Ser344-Chk1. (E) Sperm nuclei were incubated at 15 ng DNA/μl in extract supplemented with 12 μM aphidicolin plus or minus 5 mM caffeine or 5 μM DBH. At 120 minutes total DNA synthesis was measured. (F, G) Extract was immunodepleted with anti-Chk1 antibodies. (F) Immunoblotting of whole nuclei assembled in either untreated extract, mock depleted or Chk1-depleted extracted showed the removal of Chk1; ‘?’ marks an unknown cross-reacting protein. (G) Sperm nuclei were incubated at 15 ng DNA/μl in Chk1-depleted extract, non-immune-depleted extract or untreated extract, all supplemented with [α-32P]dATP and combinations of 10 μM aphidicolin and/or 5mM caffeine. At 120 minutes total DNA synthesis was measured.
The ATM/ATR kinases mediate many of their checkpoint functions by activating the downstream kinases Chk1 and Chk2. ATM/ATR activate Xenopus Chk1 by phosphorylating it on Ser344 (equivalent to Ser345 in human Chk1) (Guo et al., 2000; Liu et al., 2000; Capasso et al., 2002). We used a phospho-specific antibody capable of recognising phospho-Ser344 on Xenopus Chk1 to monitor its activation. Concentrations of aphidicolin as low as 5 μM were enough to cause phosphorylation of Chk1 on Ser344 in egg extract (Fig 8D and data not shown). Aphidicolin-induced phosphorylation of endogenous Chk1 was prevented by either caffeine or wortmannin. Since wortmannin blocks the phosphorylation of Chk1 but does not functionally inhibit the checkpoint, this suggests that Chk1 is not an essential component of the checkpoint response. Debromohymenialdisine (DBH), a relatively weak inhibitor of both Chk1 and Chk2 kinases (IC50 = 3 μM) (Curman et al., 2001), was unable to significantly restore DNA synthesis to extracts treated with 12 μM aphidicolin (Fig 8E). Finally, we immunodepleted extracts of Chk1, and examined the sensitivity of the depleted extract to aphidicolin (Figs 8F and G). DNA replication in Chk1-depeted extracts was still sensitive to 10 μM aphidicolin, and could be rescued by the addition of caffeine. Taken together, these results suggest that the intra-S checkpoint is largely mediated by ATR, not ATM, and that although there may be a minor involvement of Chk2 and/or Chk1, they are not major mediators of the response.
Discussion
Xenopus egg extracts represent a versatile system to analyse cell cycle control of DNA replication (Blow, 2001). In this report, we have taken advantage of this system to investigate the regulation of the replication timing programme. Our data show that aphidicolin induces an intra-S phase checkpoint that results in a block to late origin firing but does not significantly affect fork stability. We provide evidence that the ATR kinase is the most likely mediator of this checkpoint.
The replication timing programme in the Xenopus early embryo
In Xenopus, replication origins are spaced roughly 5-15 kb apart and are organized into small clusters (typically containing 2-10 origins) that fire almost synchronously (Blow et al., 2001). However, different clusters are activated at different times in S phase. Consistent with this, we show here that short nascent DNA strands appear throughout most of S phase. Using roscovitine, a specific CDK inhibitor, we have found that when CDK activity is inhibited during S-phase, subsets of origins fail to initiate and DNA replication cannot be completed. Moreover, we show that new initiation events are blocked within minutes of the addition of roscovitine, suggesting that the CDK execution point is very close to the time at which origins are activated. In contrast, Xenopus Cdc7 acts on replication origins prior to the S-CDKs and can act on a significant subset of replication origins prior to the onset of S phase (Jares and Blow, 2000; Walter, 2000). Taken together, these results indicate that S-CDKs play a key role in driving the replication timing programme in Xenopus. Moreover, the observation that addition of caffeine to the extract does not significantly change S phase kinetics suggests that in the absence of replication inhibitors, the normal replication programme does not require the activity of checkpoint kinases.
Aphidicolin induces a caffeine-sensitive checkpoint that prevents Cdc45 recruitment and inhibits origin firing
We show that low concentrations of aphidicolin (5-15 μM), which only slightly lower the rate of fork progression, dramatically inhibit DNA replication. This occurs as a consequence of a reduction in the total number of active forks and a reduction in the quantity of fork-associated proteins present on the DNA. All these effects can be reversed by caffeine, and appear to be due to an ATR-dependent intra-S phase checkpoint pathway. The checkpoint we describe here is clearly distinct from a previous effect noted in Xenopus, where very high concentrations of aphidicolin (~300 μM) blocked the firing of replication origins in a caffeine-insensitive manner (Marheineke and Hyrien, 2001).
In S. cerevisiae, Rad53 (a Chk2 homologue) is required to properly maintain stable replication forks, as rad53 mutants are unable to recover from a replication block (Lopes et al., 2001). Moreover, replication forks in checkpoint mutants collapse irreversibly in the presence of DNA damage (Tercero and Diffley, 2001). However, we find no evidence for significant destabilisation of forks in the Xenopus system. We have investigated fork stability by analysing nascent strands on alkaline gels, by using a nuclear re-isolation assay and by immunoblotting chromatin for replication fork proteins. In none of these experiments did we see evidence for significant destabilisation of forks, whether or not the checkpoint response had been inhibited with caffeine. It is possible, however, that replication forks transiently stall and then resume, leaving little permanent evidence of the event. We therefore conclude that the major function of the intra-S phase checkpoint in Xenopus egg extracts is to inhibit the initiation of further replication forks. It should be noted, however, that due to the limitations of working with cell-free extracts, we have only been able to investigate fork stability over a couple of hours, which is a shorter period than has been investigated for living yeast cells. It therefore remains possible that over longer time periods checkpoint kinases may play a role in stabilising forks in Xenopus.
The DNA replication intra-S checkpoint involves ATR not ATM
The intra-S phase checkpoint described in this report is sensitive to caffeine but not wortmannin. Both caffeine and wortmannin are inhibitors of the PI-3 kinase family; however wortmannin has a greater specificity for ATM than for ATR (Sarkaria et al., 1998). Even though wortmannin efficiently blocked Chk1 activation by aphidicolin, it neither significantly rescued the inhibition of DNA replication nor allowed Cdc45 to be recruited onto chromatin. In contrast, a neutralising antibody against Xenopus ATR kinase (Lupardus et al., 2002; Costanzo et al., 2003) was also able to revert the aphidicolin-induced block to DNA replication, suggesting that the intra-S phase checkpoint depends on ATR activity. This is consistent with results from other organisms suggesting that ATR mediates the checkpoint response to the inhibition of DNA replication (Zhou and Elledge, 2000; Cortez et al., 2001; Osborn et al., 2002).
Downstream from the ATR kinase in the checkpoint pathway are the checkpoint kinases Chk1 and Chk2. In Xenopus, aphidicolin-induced inhibition of S-phase leads to activation of ATR and Chk1, and inhibits entry into mitosis (Michael et al., 2000). In mammalian cells, an aphidicolin-induced checkpoint that blocks late origin firing (Dimitrova and Gilbert, 2000) could be overcome with UCN-01, an inhibitor of Chk1 (Feijoo et al., 2001). This suggests that in mammalian cells, the intra-S phase checkpoint that blocks late origin firing is mediated by Chk1. However, other related kinases inhibited by UCN-01 (e.g. Hutchins et al., 2003; Komander et al., 2003) could also be involved.
Although we detected Chk1 phosphorylation in response to low levels of aphidicolin, three lines of evidence suggest that Chk1 does not play an essential role in the intra-S phase checkpoint pathway we describe here. Firstly, Chk1 depletion showed no sign of abolishing the checkpoint. Secondly, even though wortmannin efficiently blocked Chk1 activation in response to aphidicolin, Cdc45 still failed to be recruited onto chromatin and there was little rescue of replication. Thirdly, we observed no significant rescue of the aphidicolin-induced replication block in the presence of DBH, an inhibitor of the Chk1 and Chk2 kinases (Curman et al., 2001). We therefore believe that Chk1 is not essential for the intra-S phase checkpoint in Xenopus. The lack of rescue seen with DBH, and the fact that Chk2 is typically activated by ATM not ATR, also raises doubts about whether Chk2 plays an essential role in the checkpoint we describe here. Instead, the Xenopus intra-S phase checkpoint may be mediated directly by ATR.
The targets of the intra-S phase checkpoint are currently unclear. Chk1/Chk2-dependent checkpoints inhibit the dual-specificity phosphatase Cdc25, thereby maintaining the inhibitory phosphorylation of the CDK subunit at Thr14/Tyr15 and blocking CDK activation (Costanzo et al., 2000; Falck et al., 2001). However, we failed to rescue the checkpoint by addition of high levels of cyclin A, suggesting that the checkpoint does not depend solely on inhibition of S-CDKs. CDK activity was required to exit from the checkpoint-induced arrest, suggesting that initiation had been blocked at a point upstream of CDK function. These results would be consistent with the intra-S phase checkpoint inhibiting the Cdc7-Dbf4 kinase, since in Xenopus, Cdc7 acts upstream of S-CDKs (Jares and Blow, 2000; Walter, 2000). In S. pombe, Hsk1/Cdc7 phosphorylation in response to replication inhibition by hydroxyurea is dependent on the Chk2 homologue, Cds1 (Brown and Kelly, 1999; Snaith et al., 2000). Similarly, S. cerevisiae and S. pombe Rad53/Cds1 phosphorylates Dbf4/Dfp1 and causes dissociation of the complex from the chromatin (Weinreich and Stillman, 1999; Snaith et al., 2000; Pasero et al., 1999). In Xenopus, the Topoisomerase II inhibitor etoposide induces an ATR-dependent checkpoint that inactivates the Cdc7-Dbf4 complex (Costanzo et al., 2003). Treatment of egg extracts with aphidicolin also results in accumulation of an alternative Cdc7 regulatory subunit, Drf1, onto chromatin; this accumulation is blocked by addition of caffeine and by immunodepletion of either ATR or claspin (Yanow et al., 2003). Investigation of the role of these proteins in the Xenopus intra-S phase checkpoint would therefore be of interest.
The ability of caffeine to rescue the checkpoint declines in parallel with the timing programme
We have shown that when added at the start of a reaction, caffeine could efficiently prevent the inhibition of initiation induced by low concentrations of aphidicolin. However, we also found that the ability of caffeine to rescue DNA replication declined with time. When caffeine was added in late S phase, it was almost completely unable to restore synthesis to reactions where replication had been blocked with low concentrations of aphidicolin. There was a close parallel between the period over which the ability of caffeine to rescue replication declined and the time over which the replication timing programme is executed. One possible explanation for this behaviour is that the intra-S phase checkpoint inhibits origins as they are about to be brought into play by the replication timing programme, and that although caffeine can block the pathway that inhibits initiation, caffeine cannot reverse this inhibition once it has occurred. This would be consistent with a model where the inhibition of initiation proteins by ATR is either irreversible or its reversal requires a special mechanism in addition to simply turning off the kinase activity.
Acknowledgments
We thank Alistair Thomson for providing recombinant cyclin A, James Hutchins and Paul Clarke for discussion and reagents, Matthew Michael, Howard Lindsay and Karlene Cimprich for antibodies and John Rouse for comments on the manuscript. This work was support by Cancer Research UK grants SP2385/0101, C303/A3135 and STU063/001.
References
- Aparicio OM, Stout AM, Bell SP. Differential assembly of Cdc45p and DNA polymerases at early and late origins of DNA replication. Proc. Natl. Acad. Sci. U S A. 1999;96:9130–9135. doi: 10.1073/pnas.96.16.9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasina A, Price BD, Turenne GA, McGowan CH. Caffeine inhibits the checkpoint kinase ATM. Curr. Biol. 1999;9:1135–1138. doi: 10.1016/s0960-9822(99)80486-2. [DOI] [PubMed] [Google Scholar]
- Blow JJ. Control of chromosomal DNA replication in the early Xenopus embryo. EMBO J. 2001;20:3293–3297. doi: 10.1093/emboj/20.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Gillespie PJ, Francis D, Jackson DA. Replication origins in Xenopus egg extract Are 5-15 kilobases apart and are activated in clusters that fire at different times. J. Cell Biol. 2001;152:15–25. doi: 10.1083/jcb.152.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Hodgson B. Replication licensing--defining the proliferative state? Trends Cell Biol. 2002;12:72–78. doi: 10.1016/s0962-8924(01)02203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Laskey RA. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986;47:577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Blow JJ, Watson JV. Nuclei act as independent and integrated units of replication in a Xenopus cell-free DNA replication system. EMBO J. 1987;6:1997–2002. doi: 10.1002/j.1460-2075.1987.tb02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousset K, Diffley JF. The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev. 1998;12:480–490. doi: 10.1101/gad.12.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Kelly TJ. Cell cycle regulation of Dfp1, an activator of the Hsk1 protein kinase. Proc. Natl. Acad. Sci. U S A. 1999;96:8443–8448. doi: 10.1073/pnas.96.15.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NR, Noble ME, Endicott JA, Garman EF, Wakatsuki S, Mitchell E, Rasmussen B, Hunt T, Johnson LN. The crystal structure of cyclin A. Structure. 1995;3:1235–1247. doi: 10.1016/s0969-2126(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Capasso H, Palermo C, Wan S, Rao H, John UP, O’Connell MJ, Walworth NC. Phosphorylation activates Chk1 and is required for checkpoint-mediated cell cycle arrest. J. Cell Sci. 2002;115:4555–4564. doi: 10.1242/jcs.00133. [DOI] [PubMed] [Google Scholar]
- Chong JP, Thommes P, Rowles A, Mahbubani HM, Blow JJ. Characterization of the Xenopus replication licensing system. Methods Enzymol. 1997;283:549–564. doi: 10.1016/s0076-6879(97)83043-1. [DOI] [PubMed] [Google Scholar]
- Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294:1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- Costanzo V, Robertson K, Ying CY, Kim E, Avvedimento E, Gottesman M, Grieco D, Gautier J. Reconstitution of an ATM-dependent checkpoint that inhibits chromosomal DNA replication following DNA damage. Mol. Cell. 2000;6:649–659. doi: 10.1016/s1097-2765(00)00063-0. [DOI] [PubMed] [Google Scholar]
- Costanzo V, Shechter D, Lupardus PJ, Cimprich KA, Gottesman M, Gautier J. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol. Cell. 2003;11:203–213. doi: 10.1016/s1097-2765(02)00799-2. [DOI] [PubMed] [Google Scholar]
- Curman D, Cinel B, Williams DE, Rundle N, Block WD, Goodarzi AA, Hutchins JR, Clarke PR, Zhou BB, Lees-Miller SP, et al. Inhibition of the G2 DNA damage checkpoint and of protein kinases Chk1 and Chk2 by the marine sponge alkaloid debromohymenialdisine. J. Biol. Chem. 2001;276:17914–17919. doi: 10.1074/jbc.M100728200. [DOI] [PubMed] [Google Scholar]
- Diffley JF. Replication control: choreographing replication origins. Curr. Biol. 1998;8:R771–773. doi: 10.1016/s0960-9822(07)00483-6. [DOI] [PubMed] [Google Scholar]
- Dimitrova DS, Gilbert DM. Temporally coordinated assembly and disassembly of replication factories in the absence of DNA synthesis. Nat. Cell Biol. 2000;2:686–694. doi: 10.1038/35036309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson AD, Fangman WL, Brewer BJ. Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev. 1998a;12:491–501. doi: 10.1101/gad.12.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson AD, Raghuraman MK, Friedman KL, Cross FR, Brewer BJ, Fangman WL. CLB5-dependent activation of late replication origins in S. cerevisiae. Mol. Cell. 1998b;2:173–182. doi: 10.1016/s1097-2765(00)80127-6. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Ellison V, Stillman B. Biochemical Characterization of DNA Damage Checkpoint Complexes: Clamp Loader and Clamp Complexes with Specificity for 5′ Recessed DNA. PLoS Biol. 2003;1:E33. doi: 10.1371/journal.pbio.0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001;410:842–847. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- Feijoo C, Hall-Jackson C, Wu R, Jenkins D, Leitch J, Gilbert DM, Smythe C. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 2001;154:913–923. doi: 10.1083/jcb.200104099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DM. Replication timing and transcriptional control: beyond cause and effect. Curr. Opin. Cell Biol. 2002;14:377–383. doi: 10.1016/s0955-0674(02)00326-5. [DOI] [PubMed] [Google Scholar]
- Guo Z, Kumagai A, Wang SX, Dunphy WG. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 2000;14:2745–2756. doi: 10.1101/gad.842500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978;15:317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Herrick J, Stanislawski P, Hyrien O, Bensimon A. Replication fork density increases during DNA synthesis in X. laevis egg extracts. J. Mol. Biol. 2000;300:1133–1142. doi: 10.1006/jmbi.2000.3930. [DOI] [PubMed] [Google Scholar]
- Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- Hutchins JRA, Clarke PR. Many Fingers on the Mitotic Trigger: Post-Translational Regulation of the Cdc25C phosphatase. Cell Cycle. 2004;3:41–45. [PubMed] [Google Scholar]
- Hutchins JRA, Dikovskaya D, Clarke PR. Regulation of Cdc2/Cyclin B Activation in Xenopus Egg Extracts via Inhibitory Phosphorylation of Cdc25C Phosphatase by Ca2+/Calmodium-dependent Kinase II. Mol. Biol. Cell. 2003;14:4003–4014. doi: 10.1091/mbc.E03-02-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrien O, Marheineke K, Goldar A. Paradoxes of eukaryotic DNA replication: MCM proteins and the random completion problem. Bioessays. 2003;25:116–125. doi: 10.1002/bies.10208. [DOI] [PubMed] [Google Scholar]
- Jares P, Blow JJ. Xenopus Cdc7 function is dependent on licensing but not on XORC, XCdc6, or CDK activity and is required for XCdc45 loading. Genes Dev. 2000;14:1528–1540. [PMC free article] [PubMed] [Google Scholar]
- Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- Komander D, Kular GS, Bain J, Elliott M, Alessi DR, Van Aalten DM. Structural basis for UCN-01 (7-hydroxystaurosporine) specificity and PDK1 (3-phosphoinositide-dependent protein kinase-1) inhibition. Biochem. J. 2003;375:255–262. doi: 10.1042/BJ20031119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif C, Harvey SH, O CJ. Ensuring the Stability of the Genome: DNA Damage Checkpoints. Scientific World J. 2001;1:684–702. doi: 10.1100/tsw.2001.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon CS, Foiani M. The DNA replication checkpoint response stabilizes stalled replication forks. Nature. 2001;412:557–561. doi: 10.1038/35087613. [DOI] [PubMed] [Google Scholar]
- Lucas I, Chevrier-Miller M, Sogo JM, Hyrien O. Mechanisms ensuring rapid and complete DNA replication despite random initiation in Xenopus early embryos. J. Mol Biol. 2000;296:769–786. doi: 10.1006/jmbi.2000.3500. [DOI] [PubMed] [Google Scholar]
- Lupardus PJ, Byun T, Yee MC, Hekmat-Nejad M, Cimprich KA. A requirement for replication in activation of the ATR-dependent DNA damage checkpoint. Genes Dev. 2002;16:2327–2332. doi: 10.1101/gad.1013502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbubani HM, Chong JP, Chevalier S, Thömmes P, Blow JJ. Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J. Cell Biol. 1997;136:125–135. doi: 10.1083/jcb.136.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbubani HM, Paull T, Elder JK, Blow JJ. DNA replication initiates at multiple sites on plasmid DNA in Xenopus egg extracts. Nucleic Acids Res. 1992;20:1457–1462. doi: 10.1093/nar/20.7.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marheineke K, Hyrien O. Aphidicolin triggers a block to replication origin firing in Xenopus egg extracts. J. Biol. Chem. 2001;276:17092–17100. doi: 10.1074/jbc.M100271200. [DOI] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Michael WM, Ott R, Fanning E, Newport J. Activation of the DNA replication checkpoint through RNA synthesis by primase. Science. 2000;289:2133–2137. doi: 10.1126/science.289.5487.2133. [DOI] [PubMed] [Google Scholar]
- Mimura S, Takisawa H. Xenopus Cdc45-dependent loading of DNA polymerase alpha onto chromatin under the control of S-phase Cdk. EMBO J. 1998;17:5699–5707. doi: 10.1093/emboj/17.19.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani H, Lygerou Z. Control of DNA replication licensing in a cell cycle. Genes Cells. 2002;7:523–534. doi: 10.1046/j.1365-2443.2002.00544.x. [DOI] [PubMed] [Google Scholar]
- Osborn AJ, Elledge SJ, Zou L. Checking on the fork: the DNA-replication stress-response pathway. Trends Cell Biol. 2002;12:509–516. doi: 10.1016/s0962-8924(02)02380-2. [DOI] [PubMed] [Google Scholar]
- Pasero P, Duncker BP, Schwob E, Gasser SM. A role for the Cdc7 kinase regulatory subunit Dbf4p in the formation of initiation-competent origins of replication. Genes Dev. 1999;13:2159–2176. doi: 10.1101/gad.13.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post SM, Tomkinson AE, Lee EY. The human checkpoint Rad protein Rad17 is chromatin-associated throughout the cell cycle, localizes to DNA replication sites, and interacts with DNA polymerase epsilon. Nucleic Acids Res. 2003;31:5568–5575. doi: 10.1093/nar/gkg765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santocanale C, Diffley JF. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature. 1998;395:615–618. doi: 10.1038/27001. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58:4375–4382. [PubMed] [Google Scholar]
- Shirahige K, Hori Y, Shiraishi K, Yamashita M, Takahashi K, Obuse C, Tsurimoto T, Yoshikawa H. Regulation of DNA-replication origins during cell-cycle progression. Nature. 1998;395:618–621. doi: 10.1038/27007. [DOI] [PubMed] [Google Scholar]
- Snaith HA, Brown GW, Forsburg SL. Schizosaccharomyces pombe Hsk1p is a potential cds1p target required for genome integrity. Mol. Cell Biol. 2000;20:7922–7932. doi: 10.1128/mcb.20.21.7922-7932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MP, Michael WM. DNA damage-induced replication arrest in Xenopus egg extracts. J. Cell Biol. 2003;163:245–255. doi: 10.1083/jcb.200306006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld UP, Howell M, Descombes P, Chevalier S, Rempel RE, Adamczewski J, Maller JL, Hunt T, Blow JJ. Both cyclin A and cyclin E have S-phase promoting (SPF) activity in Xenopus egg extracts. J. Cell Sci. 1996;109:1555–1563. doi: 10.1242/jcs.109.6.1555. [DOI] [PubMed] [Google Scholar]
- Strausfeld UP, Howell M, Rempel R, Maller JL, Hunt T, Blow JJ. Cip1 blocks the initiation of DNA replication in Xenopus extracts by inhibition of cyclin-dependent kinases. Curr. Biol. 1994;4:876–883. doi: 10.1016/s0960-9822(00)00196-2. [DOI] [PubMed] [Google Scholar]
- Tercero JA, Diffley JF. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature. 2001;412:553–557. doi: 10.1038/35087607. [DOI] [PubMed] [Google Scholar]
- Tercero JA, Longhese MP, Diffley JF. A central role for DNA replication forks in checkpoint activation and response. Mol. Cell. 2003;11:1323–1336. doi: 10.1016/s1097-2765(03)00169-2. [DOI] [PubMed] [Google Scholar]
- Valerie K, Povirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene. 2003;22:5792–5812. doi: 10.1038/sj.onc.1206679. [DOI] [PubMed] [Google Scholar]
- Walter JC. Evidence for sequential action of cdc7 and cdk2 protein kinases during initiation of DNA replication in Xenopus egg extracts. J. Biol. Chem. 2000;275:39773–39778. doi: 10.1074/jbc.M008107200. [DOI] [PubMed] [Google Scholar]
- Weinert TA, Kiser GL, Hartwell LH. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- Weinreich M, Stillman B. Cdc7p-Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 1999;18:5334–5346. doi: 10.1093/emboj/18.19.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JA, Keegan KS, Herendeen DR, Bentley NJ, Carr AM, Hoekstra MF, Concannon P. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proc. Natl. Acad. Sci. USA. 1998;95:7445–7450. doi: 10.1073/pnas.95.13.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanow SK, Gold DA, Yoo HY, Dunphy WG. Xenopus Drf1, a regulator of Cdc7, displays checkpoint-dependent accumulation on chromatin during an S-phase arrest. J. Biol. Chem. 2003;278:41083–41092. doi: 10.1074/jbc.M307144200. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- Zou L, Stillman B. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science. 1998;280:593–596. doi: 10.1126/science.280.5363.593. [DOI] [PubMed] [Google Scholar]