Abstract
Background
LCIS is known to be a risk factor for the development of invasive breast cancer. Debate continues as to whether LCIS is also a precursor lesion. We hypothesized that if LCIS were a precursor, its presence in the lumpectomy specimen, particularly at the margin, could increase LR after BCT.
Methods
2894 patients treated with BCT for DCIS, Stage I or II breast cancer between 1/80–5/07 were identified. Patients with DCIS or invasive cancer at the margins or those receiving neoadjuvant therapy were excluded. Group A had 290 patients with LCIS in the lumpectomy; 84 had LCIS at the final margin. Group B included 2604 patients with no evidence of LCIS.
Results
Median patient age in Gr.A and Gr.B was 57 years and 58 years (p=.05); 12% and 13% of patients in Gr.A and B had margins <2mm (p=NS). The histologic distribution of tumor types in Gr.A was lobular 47.2%, ductal 34.5%, DCIS 11.4%, and other invasive histologies 6.9%, compared with 4.1%, 76.3%,13.6%, and 6.0% for Gr.B (p<0.0001). There was no significant difference between the groups in TNM stage. The crude rate of LR was 4.5% (Gr.A) and 3.8% (Gr.B) (p=NS). Five- and 10-year actuarial LR rates for LCIS at the margin were 6% and 6%; 1% and 15% for LCIS present but not at the margin, and 2% and 6% for no LCIS (p=NS). In multivariate analysis, menopausal status and adjuvant therapy use were significant predictors of LR. LCIS, either in the specimen or at the margin, was not significantly associated with LR.
Conclusions
The presence of LCIS, even at the margin, in BCT specimens does not have an impact on LR. Re-excision is not indicated if LCIS is present or close to margin surfaces. These findings do not support consideration of LCIS as a precursor to the development of invasive lesions.
Keywords: Breast Cancer, Lobular Carcinoma in Situ, Breast-Conserving Therapy, Local Recurrence
Introduction
Since the original description by of lobular carcinoma in situ (LCIS) as “a proliferation of small, uniform, discohesive cells filling and distending the acinar units within a lobule” by Foote and Stewart in 19411, confusion has existed about its management. LCIS was initially thought to be a favorable form of malignancy, and mastectomy was the recommended treatment. Subsequent work demonstrating a bilateral risk of breast cancer and a predominance of infiltrating ductal carcinomas resulted in LCIS being considered as a risk factor for, rather than a precursor for invasive lesions2, 3, and treatment with unilateral mastectomy was largely abandoned. However, recent studies comparing the molecular signatures of LCIS and co-existing invasive lesions4–6 have re-opened the debate regarding the true significance of LCIS and its possible malignant potential. This has resulted in confusion regarding the proper management of LCIS when it is found in conjunction with invasive disease or ductal carcinoma in situ (DCIS). Studies examining this question have been small, have often included patients with invasive carcinoma or DCIS at resection margins, and have had discordant results.7–11 The lack of consensus on this topic is illustrated by the results of a recent online survey by the American Society of Breast Disease which posed the question of appropriate management of a patient with LCIS at the margin of a lumpectomy for invasive cancer; 40% of those who replied stated that they consider re-excision in this circumstance, and 8% always performed a re-excision.12 If LCIS is truly a precursor lesion, than its presence in a lumpectomy specimen, particularly at the margin, should increase local recurrence (LR) in patients treated with breast-conserving therapy (BCT). In an attempt to test this hypothesis, we compared local recurrence rates in patients with and without LCIS who underwent BCT for the treatment of invasive carcinoma or DCIS.
Methods and Materials
The study population consisted of women with American Joint Committee on Cancer (AJCC) stages 0-II13 breast cancer treated with BCT between January 1980 and May 2007. Exclusion criteria included the presence of DCIS or invasive carcinoma at the final resection margin and the receipt of neoadjuvant therapy. All patients received whole breast irradiation (RT).
Patients were identified in a database housed in the Fox Chase Cancer Center Department of Radiation Oncology, which is maintained by a single data manager. The protocol for data collection, storage, and retrieval is compliant with the Hospital Institutional Review Board (IRB) and Federal Health Insurance Portability and Accountability Act (HIPAA) regulations. Patients had surgery at both the Fox Chase Cancer Center and other hospitals. If patients belonged to the latter group, their pathology slides and imaging studies were reviewed prior to the initiation of RT to confirm eligibility for breast-conserving therapy and to verify tumor stage, histology, and margin status. Details of the radiation treatment policy during the period of this study have been previously described.14 In brief, all patients were treated with RT using breast tangents to a median dose of 46 Gy. Most patients were treated by a 6 MV linear accelerator. The primary tumor bed was boosted in 99% of patients, almost all with electrons. Some patients in the early years of the study period received an interstitial implant or photon breast boost, while some patients in the later years of the study period received higher 10 MV or 18 MV beams with a beam spoiler. The total radiation dose to the tumor bed was generally determined by the extent of surgery and the final margin status, and ranged from 44–66 Gy with a median dose of 60 Gy. Axillary staging was performed by axillary lymph node dissection or sentinel lymph node biopsy depending on the era in which the patient was treated and the presence of lymph node involvement. Patients received adjuvant chemotherapy and/or hormonal therapy as recommended by medical oncology based on tumor characteristics, hormone receptor status, and the overall health of the patient.
Follow-up information was obtained from the medical records of the Fox Chase Cancer Center, outside correspondence, or, if there had been no contact for 12 months, by contacting the patients’ last known treating physician. The overall lost to follow-up rate (defined as no follow-up for greater than 5 years) is 593 of the 3487 patients in the database (17%). If patients experienced a recurrence and were treated at an outside institution, pathology reports and relevant clinical data were obtained from the institution at which the patient was treated. There were 2894 consecutive patients meeting the inclusion criteria for this study, including 2722 who received their primary breast RT at Fox Chase Cancer Center and 172 who received their RT at the Hospital of the University of Pennsylvania but who are now patients of the Department of Radiation Oncology at Fox Chase Cancer Center. The database was used to identify patients who had LCIS present in their pathology specimens as defined on the original pathology report. No attempt was made to retrieve and/or re-review slides for the entire study population. When LCIS was present in the specimen, it was defined as being present at the final margin if LCIS was described as touching an inked surface. Margins were defined as close for invasive carcinoma or DCIS if tumor cells were within 2 mm of an inked margin. The primary endpoint of the study was LR in the ipsilateral breast. A true recurrence/ marginal miss (TR/MM) was defined as a recurrence of the index lesion within the same quadrant of the breast, in the region of the lumpectomy cavity or the boost field (if given).15 Regional nodal recurrences were not considered local recurrences for the purpose of this study.
Univariate analysis was performed using the Kaplan-Meier estimation model. The multivariate analysis was carried out using a Cox proportional hazard model to look for impendent predictors of LR. A stepwise data reduction procedure was used to look for the most parsimonious model.
Results
The study population consisted of 2894 women with a median age of 58 years (range, 20–94 years). Seventy percent of patients were post-menopausal. Median follow-up was 72 months (range, 1–296 months). Median tumor size was 1.5 cm. The diagnosis of carcinoma was made by mammography alone in 54% of patients; 12% of tumors were only evident on physical exam, and the remaining 34% were evident on both mammography and physical exam. Invasive ductal carcinoma (IDC) was present in 2087 (72%) patients, 245 (9%) had invasive lobular carcinoma (ILC), 386 (13%) had pure DCIS, and 176 (6.0%) patients had other invasive histologies. Axillary nodal metastases were present in 605 (21%) patients. Adjuvant systemic therapy was given to 1738 (60%) patients; 14% received chemotherapy alone, 31% received tamoxifen alone, and 16% received both chemotherapy and tamoxifen. The characteristics of the entire patient population are summarized in Table 1. LR as the first site of failure occurred in 112 patients, a crude rate of local recurrence of 3.9 %. LR was observed in 8 patients concurrent with a regional recurrence, and in 11 patients at the time of distant recurrence. Isolated LR occurred at the site of the original tumor or adjacent to it (true recurrence or marginal miss) in 56% of cases, elsewhere in the breast in 35%, and as skin or diffuse inflammatory recurrence in 7%. The site of LR was unknown in 2%. The median time to LR was 70 months (range, 6–260 months). The 5- and 10-year actuarial LR rates for the entire population were 2% and 6%, respectively.
TABLE 1.
Characteristics of entire population
| Entire population n = 2894 |
|||
|---|---|---|---|
| n | % | ||
| Age | |||
| ≤40 | 186 | 6.4% | |
| 41–50 | 1096 | 37.9 % | |
| 51–60 | 1046 | 36.2 % | |
| 61+ | 566 | 19.5% | |
| Date of diagnosis | |||
| 1980–1990 | 599 | 20.7% | |
| 1991–2000 | 1449 | 50.0% | |
| After 2000 | 846 | 29.3% | |
| Tumor size | |||
| TIS | 391 | 13.4% | |
| T1 | 1991 | 68.7% | |
| T2 | 506 | 17.5% | |
| T3 | 6 | < 1% | |
| Histology | |||
| DCIS | 386 | 13.3% | |
| Invasive ductal | 2087 | 72.1% | |
| Invasive lobular | 245 | 8.5% | |
| Other | 176 | 6.1% | |
| EIC | |||
| Absent | 1770 | 61.2% | |
| Present | 294 | 10.2% | |
| Unknown | 830 | 28.6% | |
| Tumor grade | |||
| Grade 1 | 269 | 9.3% | |
| Grade 2 | 871 | 30.1% | |
| Grade 3 | 897 | 31.1% | |
| Unknown | 857 | 29.5% | |
| LVI | |||
| Absent | 1391 | 48.0% | |
| Present | 364 | 12.6% | |
| Unknown | 1139 | 39.4% | |
| Nodal status | |||
| Negative | 2289 | 79.0% | |
| Positive | 605 | 21% | |
| Margin status | |||
| Negative | 2437 | 84.1% | |
| Close | 373 | 12.9% | |
| LCIS only at margin | 84 | 3% | |
| Adjuvant therapy | |||
| Chemotherapy alone | 399 | 13.8% | |
| Tamoxifen alone | 888 | 30.7% | |
| Both | 451 | 15.6% | |
| None | 1156 | 40% | |
| ER/PR status | |||
| Positive | 1905 | 65.8% | |
| Negative | 482 | 16.7% | |
| Unknown | 507 | 17.5% | |
| Menopausal status | |||
| Pre-menopausal | 743 | 25.7% | |
| Peri-menopausal | 136 | 4.7% | |
| Post-menopausal | 2015 | 69.6% | |
| Method of detection | |||
| Physical exam Only | 336 | 11.6% | |
| Mammography Only | 1553 | 53.6% | |
| Both | 1003 | 34.7% | |
| Other (MRI/US) | 2 | > 1% | |
| Follow-up | |||
| Median | 72 months (1–296 mos) | ||
| Follow-up ≥ 5 years | 1657 | 57.2% | |
| Follow-up ≤ 5 years | 1237 | 42.8% | |
LCIS, lobular carcinoma in situ; T, tumor; DCIS, ductal carcinoma in situ; ER, estrogen receptor; US, ultrasound; PR, progesterone receptor; MRI, magnetic resonance imaging; EIC, extensive intraductal component; LVI, lymphovascular invasion
LCIS was present in 290 of the 2894 (10%) patients studied. The characteristics of patients with and without LCIS are compared in Table 2. The median age of patients with LCIS in the specimen was 57 years, 1 year younger than that of patients without LCIS (p = .05), but the percentage of premenopausal patients was slightly higher in the no LCIS group (p = 0.003). The diagnosis of LCIS occurred more frequently in recent years, with 43% of cases with LCIS diagnosed in 2001 or later, compared with only 28% of cases without LCIS (p < 0.0001) diagnosed during this time period. The median tumor size of the invasive cancers seen in association with LCIS was slightly smaller than the tumor size seen without LCIS; (1.2 vs. 1.4 cm, p = 0.005), but there was no difference in nodal status or TNM stage groups between patients with and without LCIS. Consistent with this size difference, patients with an LCIS component were more likely to have tumors detected by mammography alone than their counterparts without an LCIS component (p = 0.01). Significant differences in tumor histology were noted between groups. Infiltrating lobular carcinoma was present in 47% of patients with LCIS compared with only 4% of those with no LCIS (p < 0.0001). This difference in histology also resulted in significantly more patients in the LCIS group having estrogen or progesterone receptor-positive tumors, and significantly fewer patients having grade 3 tumors or lymphovascular invasion (Table 2). An extensive intraductal component was present in 13% of LCIS patients and 10% of those without LCIS, and was not reported for 23% and 29% of patients in these groups, respectively. The proportion of patients with invasive carcinoma or intraductal carcinoma within 2 mm of the margin surface did not differ between groups.
TABLE 2.
Characteristics of patients with LCIS present in specimen vs. no LCIS
| LCIS + (n = 290) |
LCIS − (n = 2604) |
p | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age | 0.01 | |||||
| < 40 | 8 | 2.8% | 178 | 6.8% | ||
| 41–50 | 129 | 44.4% | 967 | 37.1% | ||
| 51–60 | 97 | 33.5% | 949 | 36.5% | ||
| 61+ | 56 | 19.3% | 510 | 19.6% | ||
| Date of diagnosis | <0.01 | |||||
| 1980–1990 | 23 | 7.9% | 576 | 22.2% | ||
| 1991–2000 | 142 | 48.9% | 1307 | 50.1% | ||
| After 2000 | 125 | 43.2% | 721 | 27.7% | ||
| Tumor size | NS | |||||
| TIS | 33 | 11.4% | 358 | 13.8% | ||
| T1 | 211 | 72.8% | 1780 | 68.3% | ||
| T2 | 45 | 15.5% | 461 | 17.7% | ||
| T3 | 1 | 0.3% | 5 | < 1% | ||
| Histology | <0.01 | |||||
| DCIS | 33 | 11.4% | 353 | 13.5% | ||
| Invasive ductal | 100 | 34.5% | 1987 | 76.3% | ||
| Invasive lobular | 137 | 47.2% | 108 | 4.1% | ||
| Other | 20 | 6.9% | 156 | 6.1% | ||
| EIC | NS | |||||
| Absent | 186 | 64.1% | 1584 | 60.8% | ||
| Present | 38 | 13.1% | 256 | 9.9% | ||
| Unknown | 66 | 22.8% | 764 | 29.3% | ||
| Tumor grade | ||||||
| Grade 1 | 29 | 10.0% | 240 | 9.2% | <0.01 | |
| Grade 2 | 108 | 37.2% | 763 | 29.3% | ||
| Grade 3 | 40 | 13.8% | 857 | 33.0% | ||
| Unknown | 113 | 39.0% | 744 | 28.5% | ||
| LVI | <0.01 | |||||
| Absent | 172 | 59.3% | 1219 | 46.8% | ||
| Present | 22 | 7.6% | 342 | 13.2% | ||
| Unknown | 96 | 33.1% | 1043 | 40.1% | ||
| Nodal status | NS | |||||
| Negative | 227 | 78.3% | 2062 | 79.1% | ||
| Positive | 63 | 21.7% | 542 | 20.9% | ||
| Margin status | NS | |||||
| Negative | 256 | 88.3% | 2265 | 87.0% | ||
| Close | 34 | 11.7% | 339 | 13.0% | ||
| Adjuvant therapy | <0.01 | |||||
| Chemotherapy alone | 20 | 6.9% | 379 | 14.5% | ||
| Tamoxifen alone | 118 | 40.7% | 770 | 29.8% | ||
| Both | 66 | 22.8% | 385 | 14.8% | ||
| None | 86 | 29.6% | 1070 | 40.9% | ||
| ER/PR status | <0.01 | |||||
| Positive | 224 | 77.2% | 1681 | 64.5% | ||
| Negative | 21 | 7% | 461 | 18% | ||
| Unknown | 45 | 15.8% | 462 | 17.5% | ||
| Menopausal status | <0.01 | |||||
| Pre-menopausal | 65 | 22.4% | 678 | 26.0% | ||
| Peri-menopausal | 25 | 8.6% | 111 | 4.3% | ||
| Post-menopausal | 200 | 69.0% | 1815 | 69.7% | ||
| Method of detection | 0.01 | |||||
| Mammo only | 171 | 59.0% | 1382 | 53.0% | ||
| Physical exam only | 40 | 13.9% | 296 | 11.4% | ||
| Both | 78 | 26.9% | 925 | 35.5% | ||
| Other (MRI/US) | 1 | < 1% | 1 | < 1% | ||
| Follow-up | <0.01 | |||||
| Median | 59 months (1–284 mos) | 73 months (1–296 mos) | ||||
| Follow-up ≥ 5 years | 142 | 48.9% | 1515 | 58.1% | ||
| Follow-up ≤ 5 years | 148 | 51.1% | 1089 | 41.9% | ||
LCIS, lobular carcinoma in situ; T, tumor; DCIS, ductal carcinoma in situ; ER, estrogen receptor; US, ultrasound; PR, progesterone receptor; MRI, magnetic resonance imaging; EIC, extensive intraductal component; LVI, lymphovascular invasion
The crude rate of local recurrence in the patients with LCIS in the specimen was 4.4% compared with 3.8% for those without LCIS (p = 0.39). The median time to LR in the LCIS group was 58 months (range, 7–284 months) compared with 71 months (range, 6–286 months) for the groups without LCIS (p ≤ 0.0001). For the patients with LCIS, 69% of the recurrences were TR/MM compared with 55% for those without LCIS (p = NS). The location of the local recurrences in the patients with and without LCIS is summarized in Table 3.
TABLE 3.
Location of local recurrences
| No LCIS present | LCIS present | |
|---|---|---|
| True recurrence (TR) | 24 | 3 |
| Marginal miss (MM) | 30 | 6 |
| Elsewhere | 35 | 4 |
| Skin | 4 | 0 |
| Diffuse | 4 | 0 |
| Unknown | 2 | 0 |
LCIS, lobular carcinoma in situ; TR, true recurrence; MM, marginal miss
In 84 of the patients with LCIS, the LCIS was present at the specimen margin. These patients did not differ significantly from the entire group of patients with LCIS in demographics, tumor characteristics, or treatment. The 5-year actuarial rate of LR for patients with and without LCIS was 2% for both groups (p = NS). When the LCIS group was divided according to the presence of LCIS at the margin, the 5-year actuarial rate of LR for patients with margins free of LCIS was 1%, compared with 6% for patients with LCIS present at the margin (p = NS). The median time to LR for the patients with LCIS at the final margin was 61 months, which did not differ significantly from the median time to LR of 58 months for patients with LCIS present, but not at the margin (p = NS). All of the recurrences (n = 3) in the group of patients with LCIS at the final margin occurred as TR/MM, a crude LR rate of 3.6% for the 84 patients in this subset. The 10-year actuarial rate of local recurrence for patients with no LCIS was 6%, the rate for those with LCIS present but not at the margin was 15%, and the rate for those with LCIS at the margin was 6%. These differences were not statistically significant. Actuarial freedom from local recurrence curves are shown in Figure 1 and Figure 2.
FIG. 1.
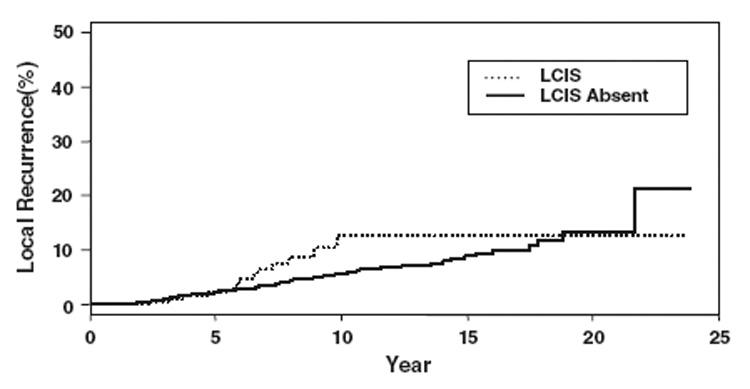
Actuarial freedom from local recurrence shown in terms of local recurrence and LCIS.
FIG. 2.
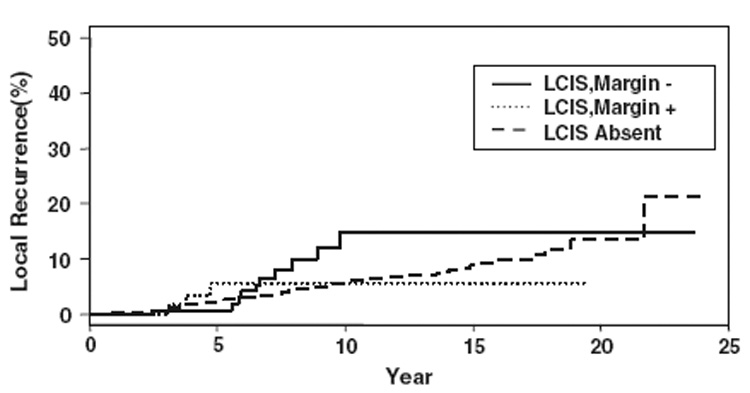
Actuarial freedom from local recurrence curves shown in terms of local recurrence, LCIS, and margin status.
A univariate analysis examining significant predictors of local recurrence was carried out for all the variables included in Table 2. Patient age, menopausal status, and the use of adjuvant therapy were highly significant predictors of local recurrence. The presence of an extensive intraductal component also significantly predicted for local recurrence, while method of tumor detection and nodal status approached statistical significance. The presence of LCIS in the specimen or at the specimen margin was not a significant predictor of local recurrence in univariate analysis. A multivariate analysis incorporating LCIS was carried out to adjust for differences among the cohorts with and without LCIS and is shown in Table 4. No significant effect of LCIS on local recurrence was observed in this model. The presence of LCIS in the specimen, but not at the margin (Hazard Ratio, 1.66; 95% CI 0.86–3.18) and LCIS at the margin (Hazard Ratio, 1.52; 95% CI 0.48–4.83) were not significantly associated with LR.
TABLE 4.
Multivariate analysis
| Multivariate analysis | ||||
|---|---|---|---|---|
| HR | 95% CI | p | ||
| LCIS | Absent | Ref | - | - |
| Present | 1.5 | 0.9–2.8 | 0.14 | |
| Present, but not at margin |
1.66 | 0.86–3.18 | 0.13 | |
| Present at margin | 1.52 | 0.48–4.83 | 0.47 | |
|
Adjuvant therapy |
None | Ref | ||
| Chemotherapy only | 0.752 | 0.443, 1.279 |
0.2927 | |
| Chemotherapy + Tamoxifen |
0.313 | 0.135, 0.727 |
0.0069 | |
| Tamoxifen only | 0.537 | 0.317, 0.911 |
0.0211 | |
|
Menopausal status |
Pre-menopausal | Ref | - | - |
| Peri-menopausal | 0.488 | 0.208, 1.149 |
0.1006 | |
| Post-menopausal | 0.410 | 0.273, 0.615 |
<0.0001 | |
HR, hazard ratio; LCIS, lobular carcinoma in situ
Discussion
Although LCIS was first described by Foote and Stewart in 19411, management of the patient with LCIS, both alone or in conjunction with invasive carcinoma, has remained controversial. Until relatively recently, LCIS was accepted as a risk factor for the development of invasive breast carcinoma in both the affected and non-affected breast.2, 3 Recently, several lines of evidence have suggested that LCIS may be a precursor of invasive carcinoma, resulting in the need to reevaluate its clinical management when it occurs in conjunction with invasive carcinoma or DCIS. Evidence suggesting that LCIS may be a precursor lesion includes the significantly greater risk of developing cancer in the ipsilateral breast after a diagnosis of LCIS reported in recent studies16, 17, the high proportion of infiltrating lobular carcinomas that occur following a diagnosis of LCIS, usually seen in the same quadrant of the breast where LCIS was identified17, the presence of shared molecular alterations in LCIS and co-existing ILC6, 18, and the recognition of histologic variants of LCIS, such as pleomorphic LCIS with molecular profiles suggestive of a more aggressive biology.18 If LCIS is a precursor lesion, its presence in the lumpectomy specimen, particularly at the specimen margin, would be expected to be associated with an increased risk of local recurrence. In this study, which included 290 patients with LCIS, we found no evidence that the presence of LCIS at the margin or in the lumpectomy specimen increased the incidence of local failure after treatment with excision and radiotherapy. The presence of LCIS in the specimen, but not at the margin, is relevant to the question of the biology of LCIS as a precursor, since studies of mastectomy specimens have demonstrated that more than 50% of patients diagnosed with LCIS have multiple foci in the ipsilateral breast.3 If LCIS is a precursor lesion, patients with LCIS at the specimen margin would be anticipated to have a higher incidence of TR/MM recurrences, while those with LCIS in the specimen but not at a margin might be expected to have an increase in recurrences in other areas of the breast (i.e. “elsewhere” recurrences). No trend toward either of these outcomes was observed.
Other studies which have examined the impact of LCIS on local control after breast conserving therapy are summarized in Table 5.7–11 The majority of these studies have found no relationship between LCIS and local recurrence. Our study confirms these results in a large group of patients all known to have margins free of invasive carcinoma or DCIS. Prior studies, including an earlier report from the Fox Chase Cancer Center that found LCIS to be associated with an increased risk of local recurrence11, have included patients with positive or unknown margin status.
TABLE 5.
Summary of other studies examining impact of LCIS on local control after breast-conserving therapy
| % Local recurrence | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Years (19xx - ) | Stage | Control # | LCIS # | Median follow-up (y) | LCIS + | LCIS − | p value |
| Sasson et al.11 | 79–95 | I, II | 1208 | 65 | 6.3 | 29 | 6/10y | .0003 |
| Moran et al.10 | prior 1992 | 0 – II | 1045 | 51 | 10.6 | 23 | 17/10y | NS |
| Abner et al.7 | 68–86 | I – II | 1062 | 137 | 13.4 | 13 | 12/8y | NS |
| Jolly et al.9 | 80–96 | I – II | 551 | 56 | 8.7 | 14 | 7/10y | .04 |
| Ben-David et al.8 | 89–03 | 0 – II | 121 | 66 | 3.9 | 0 | 0.9/5y | NS |
| Current study | 74–07 | 0 – II | 2607 | 290 | 5.2 | 6 | 6/10y | NS |
LCIS, lobular carcinoma in situ; NS, not significant
Approximately 22% of patients in the initial Fox Chase Cancer Center study had positive or unknown margins, a finding known to be associated with LR, making interpretation of the positive results of the study difficult. Margins free of invasive or intraductal carcinoma were a requirement for entry into our study, and the proportion of patients with margins of 2 mm or less did not differ between groups. In addition, with the exception of the report of Ben-David et al.8, which was a matched pair analysis, other studies that have examined this question have not taken into account the increased frequency of the diagnosis of LCIS in more recent time periods. This is important since rates of local recurrence have decreased steadily over time19, 20, and differences in the dates of treatment of patients with and without LCIS have the potential to mask the effect of the lesion on local control. Local recurrence rates have decreased over time due to improved techniques in both surgery and RT, more detailed pathologic evaluation of margin status, and increased use of adjuvant therapies such as tamoxifen. The decline in LR in more recent time periods, coupled with the increased frequency of the diagnosis of LCIS in the same time period, has the potential to obscure the effect of LCIS on local control if not taken into account. The studies of Moran and Hafty10 and Abner et al.7 report considerably higher rates of local recurrence in the non-LCIS control groups than are observed today, and may not be relevant to current practice. In common with the study of Jolly et al.9, we found that the presence of LCIS was associated with a higher incidence of infiltrating lobular carcinomas, a lower grade of invasive carcinomas, a higher likelihood of estrogen receptor positivity, and a greater use of tamoxifen. The 10-year local failure rate of 7% reported for their control group is very similar to the 6% reported in our study, but in contrast to the doubling of the risk of local recurrence that they observed in the LCIS group, we did not see a significant difference in LR. The reasons for this are not clear. One difference between our study and theirs is that we did not review the pathology slides, but based our classification of the presence or absence of LCIS on the initial diagnosis, while Jolly et al. conducted a review of the pathology specimens of both cases and controls. The number of cases initially classified as having LCIS who were found not to have LCIS on re-review is not stated, nor is the number of cases in the control group found to have LCIS. However, we believe that it is extremely unlikely that there are significant numbers of patients in our study whose LCIS status was misclassified. All of the pathology was read by a group of pathologists whose practice was limited to cancer, and all specimens from outside institutions were reviewed at the Fox Chase Cancer Center.
While our study and the bulk of the published literature provides reassurance that the presence of LCIS in association with invasive or in situ carcinoma should not be considered when assessing patient suitability for BCT or the need for re-excision, studies of pure LCIS indicate that the time to development of cancer after a diagnosis of LCIS may be extremely prolonged. In the study of Rosen et al. the average interval to the development of cancer was 20.4 years after biopsy3, although in the study of Page et al. 75% of cancers developed within 15 years of biopsy.21 The median follow-up of our study was 5.2 years, so we cannot exclude the possibility that with a longer follow-up duration, an impact of LCIS on local recurrence might be observed. However, the time to LR in patients with LCIS (58 months) was significantly shorter than the time to LR in those without LCIS (71 months; p < 0.0001), making it unlikely that further follow-up would change the study outcome. In addition, other published studies have median follow-up periods ranging from 3.9 years to 13.4 years, and those with the longest follow-up periods do not demonstrate an association between LCIS and local recurrence. Our study included patients who were recognized as having LCIS prior to the advent of e-cadherin staining, and the conclusions cannot be extrapolated to patients with uncommon morphologic variants of LCIS, such as pleomorphic LCIS which may not have been classified as LCIS during the time period of our study. Further information on the natural history of these lesions is needed to determine their impact on surgical therapy.
In conclusion, our study did not find a statistically significant increase in LR in patients found to have LCIS co-existent with invasive carcinoma or DCIS treated with breast-conserving surgery and RT. Therefore, we believe that re-excision is not indicated if LCIS is present or close to margin surfaces. As long as final margins are negative for invasive carcinoma or DCIS, patients with LCIS should receive post-operative RT and adjuvant therapy in same manner as those without LCIS, and are appropriate candidates for breast conservation.
Acknowledgments
The authors thank Cindy Rosser for her collection and management of the data for the study population.
Footnotes
Abstract presentation at the Scientific Session of the 61st Annual Cancer Symposium of the Society of Surgical Oncology, Chicago, IL March 13–16, 2008
References
- 1.Foote F, Stewart F. Lobular carcinoma in situ: A rare form of mammary carcinoma. Am J Pathol. 1941;17:491–496. doi: 10.3322/canjclin.32.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haagensen CD, Lane N, Lattes R, Bodian C. Lobular neoplasia (so-called lobular carcinoma in situ) of the breast. Cancer. 1978;42(2):737–769. doi: 10.1002/1097-0142(197808)42:2<737::aid-cncr2820420247>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Rosen PP, Kosloff C, Lieberman PH, et al. Lobular carcinoma in situ of the breast. Detailed analysis of 99 patients with average follow-up of 24 years. Am J Surg Pathol. 1978;2(3):225–251. doi: 10.1097/00000478-197809000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Shelley Hwang E, Nyante SJ, Yi Chen Y, et al. Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer. 2004;100(12):2562–2572. doi: 10.1002/cncr.20273. [DOI] [PubMed] [Google Scholar]
- 5.Berx G, Cleton-Jansen AM, Strumane K, et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996;13(9):1919–1925. [PubMed] [Google Scholar]
- 6.Lakhani SR, Collins N, Sloane JP, Stratton MR. Loss of heterozygosity in lobular carcinoma in situ of the breast. Clin Mol Pathol. 1995;48(2):M74–M78. doi: 10.1136/mp.48.2.m74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abner AL, Connolly JL, Recht A, et al. The relation between the presence and extent of lobular carcinoma in situ and the risk of local recurrence for patients with infiltrating carcinoma of the breast treated with conservative surgery and radiation therapy. Cancer. 2000;88(5):1072–1077. doi: 10.1002/(sici)1097-0142(20000301)88:5<1072::aid-cncr18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Ben-David MA, Kleer CG, Paramagul C, et al. Is lobular carcinoma in situ as a component of breast carcinoma a risk factor for local failure after breast-conserving therapy? Results of a matched pair analysis. Cancer. 2006;106(1):28–34. doi: 10.1002/cncr.21555. [DOI] [PubMed] [Google Scholar]
- 9.Jolly S, Kestin LL, Goldstein NS, Vicini FA. The impact of lobular carcinoma in situ in association with invasive breast cancer on the rate of local recurrence in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2006;66(2):365–371. doi: 10.1016/j.ijrobp.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 10.Moran M, Haffty BG. Lobular carcinoma in situ as a component of breast cancer: the long-term outcome in patients treated with breast-conservation therapy. Int J Radiat Oncol Biol Phys. 1998;40(2):353–358. doi: 10.1016/s0360-3016(97)00573-7. [DOI] [PubMed] [Google Scholar]
- 11.Sasson AR, Fowble B, Hanlon AL, et al. Lobular carcinoma in situ increases the risk of local recurrence in selected patients with stages I and II breast carcinoma treated with conservative surgery and radiation. Cancer. 2001;91(10):1862–1869. doi: 10.1002/1097-0142(20010515)91:10<1862::aid-cncr1207>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.American Society of Breast Disease. ASBD Advisor. 2008 Feb 17 [Google Scholar]
- 13.American Joint Committee on Cancer. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002. [Google Scholar]
- 14.Fowble B, Freedman G. Cancer of the Breast. In: Wang C, editor. Clinical Radiation Oncology: Indications, Techniques and Results. New York: Wiley-Liss, Inc; 2000. pp. 243–251. [Google Scholar]
- 15.Recht A, Silver B, Schnitt S, et al. Breast relapse following primary radiation therapy for early breast cancer. I. Classification, frequency and salvage. Int J Radiat Oncol Biol Phys. 1985;11(7):1271–1276. doi: 10.1016/0360-3016(85)90241-x. [DOI] [PubMed] [Google Scholar]
- 16.Lakhani SR, Audretsch W, Cleton-Jensen AM, et al. The management of lobular carcinoma in situ (LCIS). Is LCIS the same as ductal carcinoma in situ (DCIS)? Eur J Cancer. 2006;42(14):2205–2211. doi: 10.1016/j.ejca.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Fisher ER, Land SR, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: twelve-year observations concerning lobular carcinoma in situ. Cancer. 2004;100(2):238–244. doi: 10.1002/cncr.11883. [DOI] [PubMed] [Google Scholar]
- 18.Bratthauer GL, Moinfar F, Stamatakos MD, et al. Combined E-cadherin and high molecular weight cytokeratin immunoprofile differentiates lobular, ductal, and hybrid mammary intraepithelial neoplasias. Hum Pathol. 2002;33(6):620–627. doi: 10.1053/hupa.2002.124789. [DOI] [PubMed] [Google Scholar]
- 19.Cabioglu N, Hunt KK, Buchholz TA, et al. Improving local control with breast-conserving therapy: a 27-year single-institution experience. Cancer. 2005;104(1):20–29. doi: 10.1002/cncr.21121. [DOI] [PubMed] [Google Scholar]
- 20.Pass H, Vicini FA, Kestin LL, et al. Changes in management techniques and patterns of disease recurrence over time in patients with breast carcinoma treated with breast-conserving therapy at a single institution. Cancer. 2004;101(4):713–720. doi: 10.1002/cncr.20410. [DOI] [PubMed] [Google Scholar]
- 21.Page DL, Kidd TE, Jr, Dupont WD, et al. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22(12):1232–1239. doi: 10.1016/0046-8177(91)90105-x. [DOI] [PubMed] [Google Scholar]


