Abstract
Background
There is increased interest in the study of cognitive deficits as possible endophenotypic markers for schizophrenia. The main goal of this study was to determine how familiality and schizophrenia spectrum personality symptomatology are related to performance of auditory and visuospatial delayed recognition memory tasks.
Methods
The study sample consisted of 162 subjects divided into five groups. The groups included 39 patients with a DSM-IV diagnosis of schizophrenia or schizophreniform disorder, first-degree relatives of schizophrenia patients, 22 with and 31 without schizophrenia spectrum personality traits, and healthy controls with no family history of psychosis, 22 with and 48 without schizophrenia spectrum traits. Auditory and visuospatial delayed recognition memory performance was assessed.
Results
Significant differences were observed between patients and healthy controls in both auditory [F1,79=7.358 p=.008] and visual [F1,47=34.67, p<.001] delayed recognition tasks. When comparing the four non-patient groups, auditory and visuospatial discriminability decreased as a function of familiality of schizophrenia (p<0.05). Deficits were more pronounced in relatives with schizophrenia spectrum traits [auditory d=0.7114; visual d = .0199].
Conclusions
A biological relationship to schizophrenia increases the likelihood of impaired delayed recognition memory. Likewise, poorer performance is associated with schizophrenia spectrum phenotype only when combined with familiality.
Keywords: Schizophrenia Spectrum Personality, Endophenotype, Cognition, Recognition Memory, Risk Factors, First-degree Relatives
INTRODUCTION
Bolstered by evidence that family members share many of the cognitive deficits found in patients, cognitive measures are increasingly used as potential tools to aid in the discovery of schizophrenia risk genes. It is thought that the genetic architecture of discrete cognitive functions may be simpler than the clinical phenotype, increasing the power to detect genetic effects.
Neurocognitive studies have observed similar, although less severe deficits in healthy relatives of schizophrenia patients, with impairments of attention, working memory, executive functions, language and spatial ability tasks in the small to medium effect size range, when compared to controls (1).
Deficits in working and episodic memory have been well documented in patients and a substantial portion of relatives, involving dysfunction in the prefrontal and medial temporal lobe systems (respectively), which are thought to play a critical role in these functions. In relatives, working memory has most frequently been studied using spatial stimuli (2–4), whereas episodic memory has been most thoroughly studied using verbal tests (5) (see (6) for a meta-analytic review). In this study, we focus on nonverbal episodic memory, as this area has been less frequently studied. Findings have been inconsistent to date, and performance on common clinical tests may reflect impairments in drawing ability and ability to verbally encode faces or simple geometric forms, thereby providing relatively “impure” measures of nonverbal memory.
Cognitive deficits have also been observed in subjects demonstrating schizophrenia spectrum personality (SSP) traits (7; 8). Because most studies evaluating cognition in SSP did not evaluate family history and did not differentiate subjects with SSP and a family history of psychosis from SSP subjects with no biological relatives with psychosis, it is difficult to determine the extent to which cognitive deficits may reflect genetic risk per se, the presence of symptoms, or a possible interaction of the two.
In this study, we tried to overcome some of the methodological problems observed in the evaluation of non-verbal memory, SSP individuals, and relatives of schizophrenia patients. Therefore, we developed two measures of nonverbal recognition memory (abstract designs and unfamiliar melodies) to test in four groups of non-patients: two groups without SSP symptoms (non-SSP groups) and two groups with SSP. The two non-SSP groups were divided into: i) subjects who had at least one first-degree relative with schizophrenia (non-SSP relatives) and ii) subjects without a family history of psychosis (non-SSP community). Similarly, the SSP subjects were divided into groups that had at least one first-degree relative with schizophrenia (SSP relatives) and subjects without a family history of psychosis (SSP community).
By including SSP and non-SSP groups (both with and without family history of psychosis), we aimed to determine the impact of genetic and symptomatic factors, and their possible interaction, on recognition memory. Thus, if symptoms per se impact memory performance, then a similar level of impairment would be expected in both SSP community and SSP family subjects. If genetic factors alone are responsible for performance, then similar levels of impairment would be expected in the SSP and non-SSP family groups. If, in contrast, it is the interaction of genetic and symptomatic factors that is critical, maximal impairment would be expected among the SSP family group, consistent with our team’s prior findings (9).
We hypothesized that patients with schizophrenia would have impaired performance in delayed recognition of unfamiliar auditory melodies and non-namable visuospatial designs compared to healthy controls. Furthermore, we hypothesized that relatives, as a group, would have impaired ability to discriminate signal from noise in both delayed recognition tasks compared to non-schizophrenia relatives, thereby demonstrating a role for genetic factors. We further predicted that this impairment would be most severe in the family SSP group, as these subjects are considered most likely to be carriers of vulnerability genes.
METHODS AND MATERIALS
Subjects
39 DSM-IV schizophrenia and schizophreniform patients, 70 community subjects with no family history of psychosis (48 without SSP traits and 22 with SSP), and 53 first-degree relatives of patients with schizophrenia (31 without SSP and 22 with SSP traits) participated in our study. Non-patient groups (understood as community individuals and family members, with and without SSP symptoms) were matched for sociodemographics. Patients were recruited from outpatient and inpatient units at the Maryland Psychiatric Research Center (MPRC). First-degree relatives of patients were contacted through letters and family seminars after obtaining patient consent. Community subjects without SSP (normal controls) were recruited via public advertisements. Community subjects with SSP were recruited by advertisements about extrasensory perception, clairvoyance, telepathy, or 6th sense (see (10) for details on recruitment of spectrum community participants). Table 1. shows participant characteristics.
Table 1.
Participant characteristics
| Task | Schizophrenia Patients n=39 | Community Subjects n=70 | Family Members n=53 | Comparison of Patients vs. Normal Controls | Comparison of Non-patient Groupsa | ||
|---|---|---|---|---|---|---|---|
| Controlsb (Non-SSP) n=48 |
SSP n=22 |
Non-SSPc n=31 |
SSP n=22 |
Sig. | Sig. | ||
| Age | 35.7(12.25) | 40.7(14.69) | 37.1(9.17) | 46.5(15.42) | 41.14(14.3) | 0.098 | 0.1 |
| Education | 11.9(2.5) | 14.6(2.6) | 15.1(2.1) | 14.2(2.8) | 14.2(2.9) | <0.01 | 0.646 |
| Gender (F/M) |
13/26 | 26/22 | 10/12 | 23/8 | 13/9 | 0.042 | 0.167 |
| Parental SES (1/2/3/4/5) |
0/0/4/11/17 | 1/16/1610/3 | 1/8/6/5/1 | 0/8/14/5/0 | 1/6/6/4/4 | <0.01 | 0.523 |
| Race (C/AA/O) |
25/10/4 | 32/13/3 | 12/7/3 | 23/5/3 | 14/6/2 | 0.791 | 0.780 |
| Paranoid Traits | - | .12 ± .40 | 2.6 ± 1.9 | .14 ± .47 | 1.53 ± 2.13 | - | <0.01 |
| Schizoid Traits | - | .29 ± .67 | 2.9 ± 2.12 | .45 ± .67 | 2.73 ± 2.15 | - | <0.01 |
| Schizotypal Traits | - | .29 ± .60 | 3.45 ± 1.93 | .27 ± .55 | 4 ± 1.56 | - | <0.01 |
| Total SSP Traits | - | .69 ± 1.35 | 8.95 ± 3 | .86 ± 1.36 | 8.27±3.09 | - | <0.01 |
Post hoc Bonferroni analyses indicate significant differences between groups (relatives and community) and non-SSP groups (relatives and community). No statistical differences were observed between SSP relatives and SSP community, and non-SSP relatives and non-SSP community subjects in paranoid, schizoid, schizotypal and total spectrum traits.
14 subjects out of a total of 48 have at least 1 spectrum trait.
9 subjects out of a total of 31 participants have any spectrum traits
Exclusion criteria were: individuals younger than 18, pregnant or breast-feeding women, comorbidity with any other Axis I or II diagnoses, any major medical or neurological illness that may preclude psychometric assessment. Change in drug dosage two weeks prior to the enrollment in the study was an exclusion criterion for the schizophrenia group.
Following a thorough explanation of the study, participants gave informed consent in accordance with procedures approved by the Institutional Review Board of the University of Maryland School of Medicine. Patients were interviewed by a non-investigator clinician (using a standardized form) to assess their ability to understand the procedures and provide informed consent. Subjects received $15/hr. for participating in the study.
Clinical Scales
Psychiatrists or Masters level social workers at the MPRC performed all clinical assessments.
All subjects were initially screened through a semi-structured telephone questionnaire (11) to obtain basic demographic information. For individuals responding to advertisements targeting SSP, probe questions regarding DSM-IV SSP traits were asked. Axis I and II disorders were assessed using the Structured Clinical Interview for DSM- IV (SCID-IV) (12), and Semi-Structured Interview for DSM-IV Personality Disorders (SIDP-R) (13), respectively. The SIDP-R was supplemented with questions borrowed from the Structured Interview for Schizotypy (SIS) (14). Additional information was obtained using the Magical Ideation Scale (15) and the Social Anhedonia Scale (16). Information collected using these interviews and self-rated scales was discussed in a consensus meeting to reach DSM-IV Axis I and II diagnoses. We reduced the threshold of SSP group assignment to one trait less than necessary for meeting DSM criteria for cluster A disorders. Previous studies have used this same threshold (17–20) in order to increase the sensitivity of the instrument to identify affected individuals who do not exhibit functional impairment (9). Family history was obtained using the modified Family History Research Diagnostic Criteria (FH-RDC) (21).
In case of disagreement about the inclusion of individual subjects, further informants were consulted (SIDP-R Informant Interview). Subjects were excluded if disagreement persisted. The interrater reliability estimates (κ) exceeded 0.81 for all instruments.
With regard to psychotropic medication, 29 (74.3%) schizophrenia patients were on antipsychotics (12.8% first generation and 66.6% second generation), 7 (17.9%) on antidepressants, 5 (12.8%) on mood stabilizers, and 3 (7.6%) on anticholinergics. No individuals in the non-patient groups were on antipsychotic medication. Two (6.45%) normal relatives and one (4.54%) SSP relative were on antidepressants.
Of the total sample of 162 subjects, some but not all were assessed on both auditory and visuospatial delayed recognition tasks. Table 2. shows the sample distribution completing each nonverbal delayed recognition task.
Table 2.
Sample distribution across auditory and visuospatial delayed recognition tasks.
| Schizophrenia Patients n=39 |
Controls n=48 |
SSP Community n=22 |
Non-SSP Relatives n=31 |
SSP Relatives n=22 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Auditory | Visual | Auditory | Visual | Auditory | Visual | Auditory | Visual | Auditory | Visual |
| 36 | 19 | 46 | 30 | 21 | 15 | 28 | 15 | 18 | 17 |
Not all participants completed both the auditory and visuospatial task. Most subjects performed the auditory task, but only a subgroup performed the visuospatial task
Experimental Tasks
Delayed recognition of nonverbal auditory material was assessed using yes/no recognition of unfamiliar melodies. During the encoding phase, participants heard a tape containing a series of 5-note melodies that were unfamiliar to them. Each melody was repeated two times. Participants were asked to decide whether the melody ended with a higher note than it began to ensure that they paid attention to the stimuli during the presentation. After a period of about 5–7 minutes, during which individuals spoke with the examiner about everyday issues, the test phase was administered. A tape with a series of 40 5-note melodies (the 20 melodies studied during the encoding phase intermixed with 20 new melodies intended as lures) was played to the participants. Subjects responded “yes” when they believed the melody had been previously presented, and “no” if they thought the melody was new.
Delayed recognition of nonverbal visuospatial material was assessed using yes/no recognition of visuospatial designs. During the encoding phase, the examiner presented 35 designs, one at a time, for five seconds each, to the examinees. They were asked to decide whether the design had more than one horizontal line, to ensure that they paid attention to the items. After a 5–7 minute delay (no rehearsal was allowed; individuals asked about everyday activities), recognition was examined using a test comprising 70 designs. The examinees were presented with a test booklet containing the 35 studied items intermixed with 35 new designs, and were asked to make a yes/no decision whether they had previously seen each design.
Visuospatial figures were created using a 3 by 3 point grid as a framework. For each figure, a series of random numbers were drawn from 1 to 9 (corresponding to the dots on the grid). Dots were connected in the order of the random drawing, with a maximum of 5 lines drawn for any one figure. Figures were drawn with the restriction that no one line traverse more than one vertical level of the grid at a time. Figures were eliminated from the pool of study materials if they resembled namable items such as letters, numbers, or shapes that would have been familiar to subjects prior to testing. Examples of figures used in the studies are provided in Figure 1.
Figure 1.
Sample of an abstract, non-namable figure
Auditory and visuospatial delayed recognition tasks were administered randomly in order to avoid potential order effects.
Data analysis
Data were analyzed separately for each nonverbal task.
We used parametric analyses, insofar as skewness and kurtosis estimates for the dependent measures were within acceptable limits (e.g., skewness/stand error < 2.00). Comparisons of both auditory and visuospatial delayed recognition tasks were first performed between schizophrenia patients and controls to determine the nature of patients’ performance. Comparisons were then performed among the four non-patient groups. Participant characteristics were analyzed using one-way ANOVAs and chi-square analyses.
For the delayed recognition task, ANOVAs were performed on memory discriminability (A′) and response bias (β), as the dependent measures (22). We chose A′ as the discriminability measure because it does not assume (1) normally distributed signal and noise distributions or (2) equality of signal and noise distribution standard deviations.
To ascertain the main effects of familiality alone, SSP alone, and the interaction between family and SSP, for discriminability measures of delayed recognition, we used a 2 (SSP/non-SSP) by 2 (family/non-family) factorial analysis. Given the moderate sample sizes we calculated effect sizes (ES), Cohen’s d (23), for the critical variable of discriminability (A′) among all non-patient groups.
Following the statistical approach used by Egan (24), we estimated relative risk (λ) using prevalence ratios calculated after a cut-off score of 2 sd below the mean discriminability index value (A′) of the normal comparison group in both visuospatial and auditory delayed recognition conditions.
95% relative risk confidence intervals (CI) were calculated following Kleimbaum’s formula (25).
Finally, to determine how the number of SSP traits affected delayed recognition performance and whether associations were similar for both family members and community subjects, Spearman correlations were performed between: a) number of schizoid, schizotypal, and paranoid symptoms and the discriminability measure of A′ and b) total score on the SSP scale and the discriminability measure of A′. Even subjects in the non-SSP groups are likely to have some SSP symptoms, although not enough to pass the inclusion subthreshold for SSP grouping. They then vary continuously on the dimension of number of SSP symptoms.
Statistical significance levels were set at p<.05.
RESULTS
Delayed recognition for nonverbal auditory material
Patients vs. controls
Schizophrenia patients and normal controls who completed the nonverbal auditory task differed in number of years of schooling [F1,81=21.129, p<.001], parental socioeconomic status (SES) [χ24=29.871, p<.001], and ratio of females/males evaluated between both groups [χ21=5.499, p=.019]. No differences were observed in age or race for either task.
Patients demonstrated moderate to severe deficits in ability to discriminate studied unfamiliar melodies from non-studied unfamiliar melodies compared to healthy controls, as assessed by the discriminability measure A′ [F1,79=7.358, p=0.008]. When age, sex, parental SES, and years of schooling were taken into account, A′ values [F1,73=6.008, p=0.02] remained significant. Schizophrenia patients did not differ from non-SSP community participants in the response bias measure (β) [F 1,78=0.146, p=0.704].
Non-patient groups
No differences were observed for any of the sociodemographic variables.
The two (family/non-family) by two (SSP/non-SSP) factorial analyses with A′ as the dependent variable demonstrated a main effect of family [F1,110=12.232, p=.001]. Overall, family members had poorer memory performance than community subjects. No interactions [F1,110=0.153, p=0.696] were observed for A′ (see Figure 2). No main effect of Family [F1,109=.105, p=.746]; SSP [F1,109=.133, p=.716], or interactions [F1,109=1.049, p=.308] were observed for β.
Figure 2.
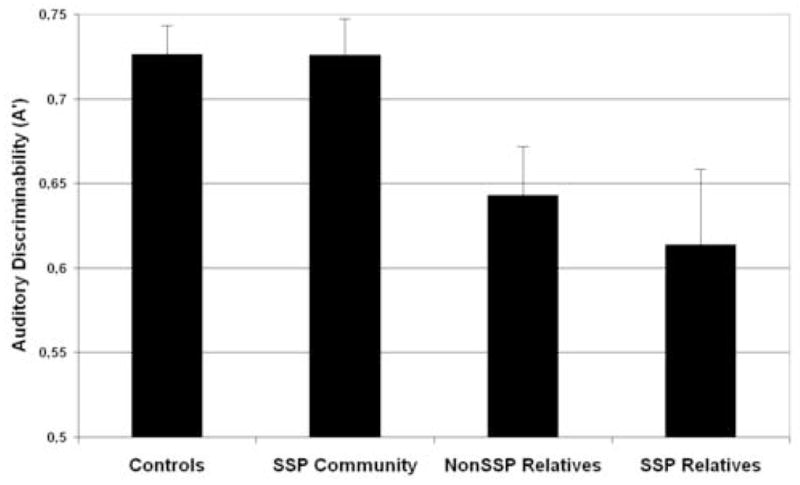
Mean discriminability (A′) and standard error for auditory nonverbal recognition in non-clinical groups. With an A′ mean of (0.6139±.187) the group of SSP relatives demonstrated the poorest nonverbal auditory discriminability. Cohen’s d calculations reflect moderate effect of both groups of family members without (d=0.6056) and with SSP (d=0.7114) traits when compared to controls, thus supporting the main effect of family reported by the ANOVA analysis. Moderate effect sizes were also observed between SSP community subjects and family members with (d=0.6418) and without (d=0.7429) SSP traits. The standardized difference between both groups from the community (with and without SSP) was close to zero (d=0.0062).
Furthermore, we analyzed data regarding the number of standard deviations from the normal control mean. At 2sd below the control mean, we observed that the research paradigm of delayed recognition for auditory designs detected a biologically linked population 16.66% of the time, with a false positive rate of 1.49%. Thus, the likelihood of scoring 2sd below the control mean is 11 times (95% CI [9.28–12.07]) higher in first-degree relatives than in community individuals, independently of presence of symptoms.
Finally, significant negative correlations were observed between delayed recognition A′ values and total number of spectrum traits [rho= −0.360, p=0.040] in the group of relatives. That is, memory performance decreased as the number of spectrum traits increased for relatives. In contrast, no significant symptom-memory correlations were found in the community group (all rho’s>0.05) between the total number of SSP traits [rho= −0.096] or the number of paranoid [rho= 0.151], schizoid [rho= 0.064], and schizotypal [rho= −0.039] traits and A′ scores.
Delayed recognition for nonverbal visuospatial material
Patients vs. controls
Schizophrenia patients completing the nonverbal visuospatial task differed from controls in years of schooling [F1,48=26.339, p<.001] and parental socioeconomic status [χ24=22.268, p<.001]. No differences were observed in gender, age, or race for either task.
Controls obtained significantly higher A′ scores than patients on the visuospatial design test, (controls 0.7314±.076; patients .5755±.107) [F1,47=34.668, p<.001]). When age, sex, parental SES, and years of schooling were taken into account, A′ values [F1,47=8.508, p=0.007] remained significant. β analysis did not result in significant differences between the two groups [F 1,46= 0, p=.995].
Non-patient groups
Non-patient groups were matched for all sociodemographic variables.
Results showed main effects of family [F1,73=4.340, p=.041] and SSP status [F1,73=4.724, p=.033], but no interactions for the discriminability measure A′. These effects were examined more closely using post-hoc tests (Figure 3). No main effect of Family [F1,73=.001, p=.982]; SSP [F1,73=.149, p=.700], or interactions [F1,73=3.591 p=.062] were observed for β.
Figure 3.
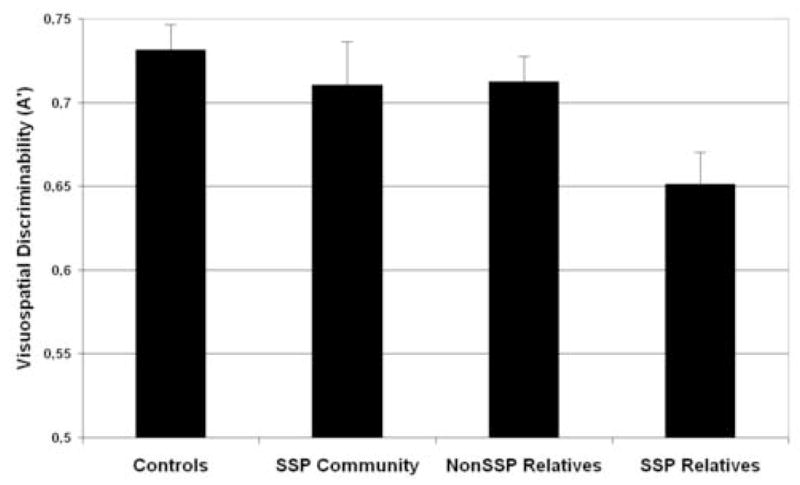
Mean discriminability (A′) and standard error for visuospatial nonverbal recognition in non-clinical groups. With a mean A′ score of (0.6513±.081) SSP relatives demonstrated the most marked impairment among the 4 non-patient groups. Cohen’s d calculations revealed the largest effect size between healthy controls and SSP relatives (d=1.0199). In addition, there were large and moderate effect sizes when comparing SSP relatives to non-SSP relatives (d=0.8370), and to community SSP subjects (d=0.6688), respectively. When compared to healthy controls, SSP community subjects (d=0.239) and non-SSP relatives (d=0.2704) had small effect sizes.
With regard to relative risk calculation, the likelihood of scoring ≥ 2sd below the healthy control mean was 5.6 (95% CI [4.55–6.7]) times higher in first-degree relatives as a group (12.5%) than in community individuals (2.22%), independently of presence of spectrum symptoms, and 10.6 (95% CI [9.48–11.7]) times higher in relatives with SSP traits than in community individuals (with or without SSP traits).
Finally, Spearman correlations revealed that poor recognition of visuospatial designs was associated with an increased number of SSP traits in relatives but not in community subjects. Significant negative correlations were found between delayed recognition A′ values and number of schizotypal traits [rho= −0.478, p=0.021], and between A′ values and total number of spectrum personality traits [rho= −0.450, p=0.031] among familial cases, but not among community individuals (rho values = −0.033; −0.117; −0.049; −0.052 for paranoid, schizoid, schizotypal, and total number of SSP traits, respectively, were non-significant at p >.05).
DISCUSSION
The results for unfamiliar auditory melodies and non-namable visuospatial patterns suggest that schizophrenia patients and their first-degree relatives, especially those who have a diagnosis of SSP, are impaired in their ability to discriminate between previously studied targets and new lures. This converging evidence using different stimulus types and task designs that minimize the role of strategic retrieval processes suggests that recognition memory for non-verbal materials may be studied as an intermediate phenotype of use in genetic studies. These results are consistent with several prior reports using similar non-namable visuospatial patterns that found deficits in nonverbal retention in patients with schizophrenia and their first-degree relatives (26; 27). However, conflicting findings have been documented in nonverbal delayed recognition in schizophrenia (28; 29). These inconsistencies may be due to methodological problems and the fact that face-recognition and visual-reproduction tasks are not appropriate to examine this cognitive domain. With the design of our tasks, we aimed to assess true nonverbal delayed recognition in order to help clarify some of the inconsistencies observed in the literature. The use of very difficult to verbalize (nonverbal) material may increase the demand on precise encoding of to be remembered items. Insofar as memory deficits in schizophrenia stem from inefficient strategies for memorizing target items (encoding problems) (30; 31), these tasks may put a premium on these processes. However, to the extent that episodic memory and working memory are supported by somewhat overlapping networks (32), our tasks are not able to discriminate the role of encoding deficits that could potentially impact WM as well as LTM performance. Finally, by using recognition testing measures, it is likely that retrieval-related demands are minimized, as individuals are asked to distinguish the studied items from the lures, thus relying more on familiarity processes (generalized feeling of prior occurrence of the item) than on conscious recollection of qualitative information of an occurrence. This may offer the advantage of minimizing the role of strategic effects that are likely to differ among subjects.
The second important contribution of this study is the inclusion of four non-patient groups representing the four possible combinations among biological relationship to schizophrenia and SSP expression. Contrary to studies that have not thoroughly examined familiality to schizophrenia and SSP diagnosis in their samples, our study design allowed us to examine how these two conditions affect delayed recognition separately and when they coexist within the same individual. In our study, the inclusion of the SSP community group without a family history of psychosis provides evidence of the role that the spectrum phenotype plays in cognition, concretely, in measures of nonverbal delayed recognition.
We observed a decrease in delayed recognition performance associated with biological relationship to schizophrenia, supporting reports of deficient memory abilities in biological relatives of probands (28; 33). Although we could not prove an interaction among familiality to schizophrenia, SSP, and impaired delayed nonverbal recognition on the basis of the ANOVA, effect size calculations and Spearman correlations suggest that those relatives who had been diagnosed with SSP have greater impairment in distinguishing previously studied designs from new lures than non-SSP relatives and both groups of non-schizophrenia related individuals. This may suggest that relatives with symptomatic (SSP) expression of risk have higher genetic loading than those without it. Therefore, heterogeneity in risk among relatives could be reduced by taking into account expressions of schizotypy. These results support previous evidence showing that when the schizotypy construct is expressed as a dimension, quantitative variation in schizotypy is more strongly related to deficits in visuospatial memory among first-degree relatives of schizophrenia patients than in the general population (34)
Similar studies have demonstrated that genetic risk for schizophrenia and schizotypy symptoms interact for visuospatial memory deficits (34), language performance (35; 36), and attention. Relatives with probable or definite schizotypal, schizoid, and/or paranoid personality disorders had lower adjusted z scores of d′ than those of non-SSP relatives when evaluated with the CPT (37; 38). Using four scales developed by Chapman and colleagues to assess schizotypy, Laurent et al. (2001) observed that, among all relatives, the subgroup with high scores in negative dimension of schizotypy showed worse performance on the WCST. In contrast, no correlations were observed between schizotypy symptomatology and performance among schizophrenia non-related controls (39).
Although there is evidence supporting neurocognitive impairments in schizophrenia spectrum personalities (40; 41) in the areas of executive functioning and attention, results in verbal learning and memory have been inconsistent (40; 42), and deficits in nonverbal delayed recognition have not been previously reported. The subtle detrimental effect of the SSP phenotype (main effect of SSP [F1,73=4.724, p=.033]) when performing the visuospatial delayed recognition task, and the small effect size (d=0.2390) when comparing SSP community vs. controls, supports those studies that suggest a mild decrement in memory performance in individuals with symptom expression of risk (39). Spearman correlations did not show an association between the load of SSP traits and visuospatial delayed recognition in individuals unrelated to schizophrenia. Therefore, we could be observing a different functional relationship between delayed recognition performance and SSP symptoms in familial and non-familial cases.
Our findings suggest that among first-degree relatives of schizophrenia, the presence of even mild SSP identifies individuals at risk for schizophrenia-related cognitive deficits. Mild SSP symptoms alone, in the absence of family history of psychosis, are not efficient in identifying at-risk individuals.
One limitation of the study is the relatively small sample size of groups, which may limit the study’s ability to detect main effects of SSP, as well as interactions between family and SSP (Type II error). The recruitment of individuals with SSP is difficult, especially when suspiciousness traits are present. We tried to analyze our data using statistical analyses that do not rely so intensely on sample size; however, the results need to be confirmed in larger populations. Secondly, our SSP sample is not representative of SSP community subjects since we excluded individuals with a family history of psychosis. In addition, we recruited subjects on the basis of self-recognition of schizotypal symptomatology. Thirdly, it is virtually impossible to design a large series of stimuli that fully avoids verbal description and semantic labeling. We were careful that stimuli should be difficult to describe within the first five seconds of presentation. Fourthly, we did not examine the effect of antipsychotic medication on delayed recognition memory in schizophrenia patients. However, deficits observed in both groups of unmedicated relatives strongly suggest that impaired performance in patients is not caused by antipsychotic medication. Fifthly, we were not able to examine subsamples of performance patterns within members of the same family (patients and their relatives) because only 26% of our family members were biologically related to any of the patients assessed (a total of 11 families were assessed). However, results indicated that deficits in delayed recognition for nonverbal memory are transmitted across families. Finally, not having studied the same subjects in both experimental tasks, we are unable to state whether deficits represent independent vulnerability markers or whether they reflect different expressions of a single cognitive endophenotype.
With the caveat that a family study design can suggest but not confirm a genetic relationship, we argue that our data suggest that SSP symptomatology in the presence of familial relationship is likely to be genetically informative- i.e., including this clinical phenotype in molecular genetic analyses is likely to increase the power to detect significant associations by increasing the density of likely genetic carriers. This is likely to be true for associations between genes and disease itself, as well as associations between genes and a number of neurocognitive and neurophysiological endophenotypes. Although results suggest that impaired nonverbal delayed recognition of auditory melodies and visuospatial designs are familial traits that could be vulnerability markers for schizophrenia, we can not conclude that they are valid endophenotypes of schizophrenia.
Acknowledgments
Supported by NIMH grants MH049826, and 40279, Spanish Ministry of Health, Instituto de Salud Carlos III, RETICS RD06/0011(REM-TAP Network), and by a predoctoral scholarship “Beca para formación de investigadores, modalidad: extranjero, Gobierno Vasco,” from the Basque Government, Spain.
Footnotes
FINANCIAL DISCLOSURES
Our knowledge the author and coauthors have no conflict of interests, financial or otherwise, to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M, et al. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Pirkola T, Tuulio-Henriksson A, Glahn D, Kieseppa T, Haukka J, Kaprio J, et al. Spatial working memory function in twins with schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58:930–936. doi: 10.1016/j.biopsych.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Saperstein AM, Fuller RL, Avila MT, Adami H, McMahon RP, Thaker GK, Gold JM. Spatial Working Memory as a Cognitive Endophenotype of Schizophrenia: Assessing Risk for Pathophysiological Dysfunction. Schizophr Bull. 2006;32(3):498–506. doi: 10.1093/schbul/sbj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Faraone SV. Neuropsychological risk indicators for schizophrenia: a review of family studies. Schizophr Bull. 1994;20:103–119. doi: 10.1093/schbul/20.1.103. [DOI] [PubMed] [Google Scholar]
- 6.Sitskoorn MM, Ebisch SJ, Appels M, Nuyen J, Kahn RS. Memory profiles in parents of patients with schizophrenia. Psychiatry Res. 2004;128:27–37. doi: 10.1016/j.psychres.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Kirrane RM, Mitropoulou V, Nunn M, New AS, Harvey PD, Schopick F, et al. Effects of amphetamine on visuospatial working memory performance in schizophrenia spectrum personality disorder. Neuropsychopharmacology. 2000;22:14–18. doi: 10.1016/S0893-133X(99)00075-5. [DOI] [PubMed] [Google Scholar]
- 8.Avissar S, Roitman G, Schreiber G. Differential effects of the antipsychotics haloperidol and clozapine on G protein measures in mononuclear leukocytes of patients with schizophrenia. Cell Mol Neurobiol. 2001;21:799–811. doi: 10.1023/A:1015164423918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avila MT, Sherr J, Valentine LE, Blaxton TA, Thaker GK. Neurodevelopmental interactions conferring risk for schizophrenia: a study of dermatoglyphic markers in patients and relatives. Schizophr Bull. 2003;29:595–605. doi: 10.1093/oxfordjournals.schbul.a007031. [DOI] [PubMed] [Google Scholar]
- 10.Kunkel R, Adami H, Zetlmeisl M, Ross D, Thaker G. Recruitment of non-patient volunteers with schizophrenia spectrum personality symptoms. Schizophr Res. 1998;34:181–186. doi: 10.1016/s0920-9964(98)00102-9. [DOI] [PubMed] [Google Scholar]
- 11.Strauss ME, Brandt J, McSorley P. Visual vigilance and psychopathology. Psychiatry Res. 1986;18:285–287. doi: 10.1016/0165-1781(86)90115-0. [DOI] [PubMed] [Google Scholar]
- 12.Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality. American Psychiatric Association; 1997. [Google Scholar]
- 13.Spitzer RLWJ, Gibbon M, First MB. Structural Clinical Interview for DSM III-R (SCID) New York: New York State Psychiatric Institute, Biometrics Research Department; 1990. [Google Scholar]
- 14.Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15:559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- 15.Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. J Consult Clin Psychol. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- 16.Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- 17.Siever LJ, Kalus OF, Keefe RS. The boundaries of schizophrenia. Psychiatr Clin North Am. 1993;16:217–244. [PubMed] [Google Scholar]
- 18.Moran MJ, Thaker GK, Laporte DJ, Cassady SL, Ross DE. Covert visual attention in schizophrenia spectrum personality disordered subjects: visuospatial cuing and alerting effects. J Psychiatr Res. 1996;30:261–275. doi: 10.1016/0022-3956(96)00004-0. [DOI] [PubMed] [Google Scholar]
- 19.Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, Lahti A. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55:830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia M, Bernardeschi L, Franchini L, Bellodi L, Smeraldi E. A family study of schizotypal disorder. Schizophr Bull. 1995;21:33–45. doi: 10.1093/schbul/21.1.33. [DOI] [PubMed] [Google Scholar]
- 21.Adami H. Improved Diagnosis of Schizophrenia Spectrum Disorders Using a Modified FH-RDC. Society for Biological Psychiatry. 1990;97A [Google Scholar]
- 22.Stanislaw HTN. Calculation of Signal Detection Theory Measures. Behavior Research Methods, Instrument, & Computer. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- 23.Cohen BM, Buonanno F, Keck PE, Jr, Finklestein SP, Benes FM. Comparison of MRI and CT scans in a group of psychiatric patients. Am J Psychiatry. 1988;145:1084–1088. doi: 10.1176/ajp.145.9.1084. [DOI] [PubMed] [Google Scholar]
- 24.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- 25.Kleinbaum DGKLaMH. Epidemiologic Research: Principles and Quantitative Methods. New York: Van Reinhold Nostrand Co; 1982. [Google Scholar]
- 26.Wood SJ, Proffitt T, Mahony K, Smith DJ, Buchanan JA, Brewer W, et al. Visuospatial memory and learning in first-episode schizophreniform psychosis and established schizophrenia: a functional correlate of hippocampal pathology? Psychol Med. 2002;32:429–438. doi: 10.1017/s0033291702005275. [DOI] [PubMed] [Google Scholar]
- 27.Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? J Abnorm Psychol. 2002;111:478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- 28.Conklin HM, Calkins ME, Anderson CW, Dinzeo TJ, Iacono WG. Recognition memory for faces in schizophrenia patients and their first-degree relatives. Neuropsychologia. 2002;40:2314–2324. doi: 10.1016/s0028-3932(02)00091-x. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg TE, Torrey EF, Gold JM, Bigelow LB, Ragland RD, Taylor E, Weinberger DR. Genetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder. Schizophr Res. 1995;17:77–84. doi: 10.1016/0920-9964(95)00032-h. [DOI] [PubMed] [Google Scholar]
- 30.Gold JM, Rehkemper G, Binks SW, 3rd, Carpenter CJ, Fleming K, Goldberg TE, Weinberger DR. Learning and forgetting in schizophrenia. J Abnorm Psychol. 2000;109:534–538. [PubMed] [Google Scholar]
- 31.Bacon E, Izaute M, Danion JM. Preserved memory monitoring but impaired memory control during episodic encoding in patients with schizophrenia. J Int Neuropsychol Soc. 2007;13:219–227. doi: 10.1017/S1355617707070245. [DOI] [PubMed] [Google Scholar]
- 32.Krause J, Taylor J, Schmidt D, Hautzel H, Mottaghy F, Muller-Gartner H. Imaging and neural modelling in episodic and working memory processes. Neural Netw. 2000;13(8–9):847–59. doi: 10.1016/s0893-6080(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 33.Toomey R, Faraone SV, Simpson JC, Tsuang MT. Negative, positive, and disorganized symptom dimensions in schizophrenia, major depression, and bipolar disorder. J Nerv Ment Dis. 1998;186:470–476. doi: 10.1097/00005053-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JK, Tuulio-Henriksson A, Pirkola T, Huttunen MO, Lonnqvist J, Kaprio J, Cannon TD. Do schizotypal symptoms mediate the relationship between genetic risk for schizophrenia and impaired neuropsychological performance in co-twins of schizophrenic patients? Biol Psychiatry. 2003;54:1200–1204. doi: 10.1016/s0006-3223(03)00637-1. [DOI] [PubMed] [Google Scholar]
- 35.Condray R, Steinhauer SR. Schizotypal personality disorder in individuals with and without schizophrenic relatives: similarities and contrasts in neurocognitive and clinical functioning. Schizophr Res. 1992;7:33–41. doi: 10.1016/0920-9964(92)90071-c. [DOI] [PubMed] [Google Scholar]
- 36.Condray R, Steinhauer SR, Goldstein G. Language comprehension in schizophrenics and their brothers. Biol Psychiatry. 1992;32:790–802. doi: 10.1016/0006-3223(92)90082-b. [DOI] [PubMed] [Google Scholar]
- 37.Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry. 1998;155:1214–1220. doi: 10.1176/ajp.155.9.1214. [DOI] [PubMed] [Google Scholar]
- 38.FitzGerald M, MacDonald D, Krainer M, Hoover I, O’Neil E, Unsal H, et al. Germ-line BRCA1 mutations in Jewish women with early breast cancer. N Engl J Med. 1996;334:143–149. doi: 10.1056/NEJM199601183340302. [DOI] [PubMed] [Google Scholar]
- 39.Laurent A, Duly D, Murry P, Foussard N, Boccara S, Mingat F, et al. WCST performance and schizotypal features in the first-degree relatives of patients with schizophrenia. Psychiatry Res. 2001;104:133–144. doi: 10.1016/s0165-1781(01)00306-7. [DOI] [PubMed] [Google Scholar]
- 40.Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Demeo S, et al. An MRI study of superior temporal gyrus volume in women with schizotypal personality disorder. Am J Psychiatry. 2003;160:2198–2201. doi: 10.1176/appi.ajp.160.12.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenzenweger MF. Psychometric high-risk paradigm, perceptual aberrations, and schizotypy: an update. Schizophr Bull. 1994;20:121–135. doi: 10.1093/schbul/20.1.121. [DOI] [PubMed] [Google Scholar]
- 42.Lenzenweger MF, Gold JM. Auditory working memory and verbal recall memory in schizotypy. Schizophr Res. 2000;42:101–110. doi: 10.1016/s0920-9964(99)00121-8. [DOI] [PubMed] [Google Scholar]



