Abstract
The chemical neuroanatomy of breathing must ultimately encompass all the various neuronal elements physiologically identified in brainstem respiratory circuits and their apparent aggregation into “compartments” within the medulla and pons. These functionally defined respiratory compartments in the brainstem provide the major source of input to cranial motoneurons controlling the airways, and to spinal motoneurons activating inspiratory and expiratory pump muscles. This review provides an overview of the neuroanatomy of the major compartments comprising brainstem respiratory circuits, and a synopsis of the transmitters used by their constituent respiratory neurons.
Keywords: preBötzinger, Bötzinger, ventral respiratory column, ventral respiratory group, retrotrapezoid nucleus, parafacial respiratory group, Kölliker-Fuse nucleus, parabrachial nucleus
1. Introduction
This essay provides an overview of the transmitters and, to some extent, the receptors associated with mammalian respiratory circuits within the context of functionally defined brainstem respiratory “compartments.” Respiratory neurons (i.e. neurons phasically-firing in synchrony with the respiratory cycle) are concentrated in three main brainstem areas (Fig. 1): the dorsal respiratory group within the nucleus of the solitary tract, the ventrolateral medulla from the level of the spinal-medullary junction through the level of the facial nucleus (i.e., the ventral respiratory column, VRC), and in the pontine respiratory group within the dorsolateral pons. These aggregates of brainstem respiratory neurons are interconnected, and together with respiratory-related sensory afferents, are collectively responsible for the automatic control of breathing as well as adaptive changes in breathing to homeostatic and environmental challenges.
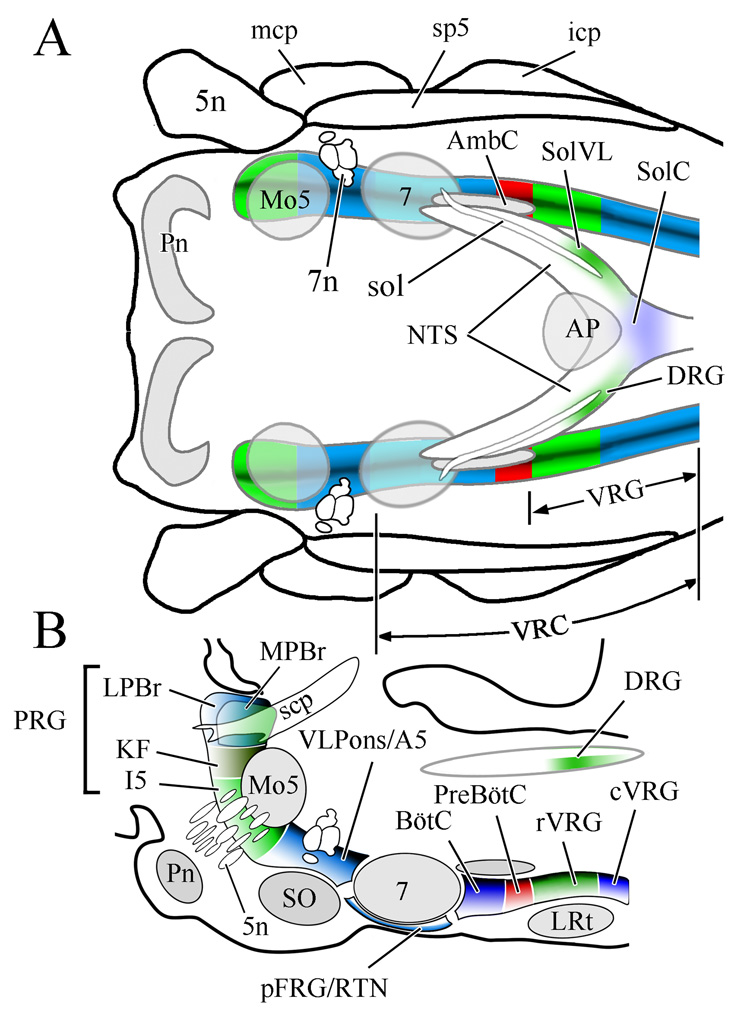
Figure 1.
Respiratory related regions of the rhombencephalon of the rat shown in horizontal (A) and sagittal (B) views. Note that respiratory related regions comprise a nearly continuous column in the lateral rhombencephalon. The boundaries depicted between the various brainstem compartments reflect functional distinctions between adjacent regions relative to their impact on breathing. Reproduced from McCrimmon et al., 2008 with permission; B is redrawn after Fig 1B in Alheid et al., 2004).
Abbreviations, including subsequent figures: 5n, trigeminal nerve; 7, facial nucleus; 7n, facial nerve; A5, A5 noradrenergic neuronal group; AmbC, compact part of nucleus ambiguus; AP, area postrema; BötC, Bötzinger complex; cVRG, caudal division of ventral respiratory group; DRG, dorsal respiratory group; I5, intertrigeminal area; icp, inferior cerebellar peduncle; KF, Kölliker-Fuse nucleus; LPBr, lateral parabrachial region; LRt, lateral reticular nucleus; mcp, medial cerebellar peduncle; Mo5, motor nucleus of the trigeminal nerve; MPBr, medial parabrachial region; NTS, nucleus of the solitary tract; pFRG, parafacial respiratory group; Pn, basilar pontine nuclei; preBötC, preBötzinger complex; PRG, pontine respiratory group; RTN, retrotrapezoid nucleus; rVRG, rostral division of ventral respiratory group; scp, superior cerebellar peduncle; SO, superior olive; sol, solitary tract; SolC, commissural subdivision of the nucleus of the solitary tract; SolVL, ventrolateral subdivision of the nucleus of the solitary tract; sp5, spinal trigeminal tract; vlPons, ventrolateral pontine region; VRC, ventral respiratory column of the medulla; VRG, ventral respiratory group.
A variety of additional neuronal groups in the brainstem and forebrain contribute to the innervation of brainstem respiratory compartments. These include serotonergic and catecholaminergic neurons, neurons in the mesencephalic periaqueductal gray (PAG), and forebrain neurons in hypothalamus, amygdala and cortex. PAG neurons directly target respiratory neurons in caudal medulla but also modulate respiration indirectly via relays in dorsolateral and ventrolateral pons (Hayward et al., 2004). Forebrain neurons targeting respiratory circuits are particularly evident in hypothalamus, with modest contributions from amygdala, and from frontal and insular cortices. These additional respiratory-related regions are not discussed separately but are included, where appropriate, in the context of afferents to respiratory neurons or compartments. Serotonergic and catecholamine influences on respiration are additionally discussed elsewhere in this volume, as are the influence of orexinergic hypothalamic neurons and sex homones.
The present survey is based on data mainly derived from the adult rat, with supplemental observations derived from the mouse, cat, and dog. As with any functional-anatomical system, the neurochemistry of the respiratory network is subject to dramatic developmental changes. This extensive topic is frequently addressed elsewhere in this volume. The neurochemistry of breathing also encompasses the differential expression of specific ion channels, signal transduction pathways, and various transcription factors, as normal components of respiratory circuits and elements modified developmentally, in response to environmental changes, and as mutations in genetic based diseases impacting respiration. These topics have been the target of increasing scrutiny and are addressed by a variety of papers in this volume.
2.0 The nucleus of the solitary tract and the dorsal respiratory group
The caudal third of the nucleus of the solitary tract (cNTS; Fig 1A) is the principal site of termination for sensory afferents conveying respiratory related information from the lungs and peripheral chemoreceptors. The cNTS is also the site of one of three principal concentrations of brainstem respiratory neurons, the “dorsal respiratory group” (see below).
The portion of the cNTS related to breathing includes the areas alongside and extending caudal to the most posterior limits of the area postrema. As the main focus for the mainly topographic sensory terminations of pulmonary and airway afferents traveling in the vagus and glossopharyngeal nerves it provides the first step in the brain’s processing of critical information about the status of lungs and airways (Kubin et al., 2006), as well as sensory input from peripheral chemoreceptors detecting arterial O2 and CO2.
Most sensory afferents to the NTS appear to include glutamate as a transmitter (e.g. Sykes et al., 1997), often together with a monoamine, purine, peptide, or volatile cotransmitter. Included among these are: ATP, substance P, dopamine, calcitonin gene related peptide, brain derived neurotrophic factor (BDNF), histamine, serotonin, and nitric oxide.
Both glutamate and GABA neurons are widespread in the rat cNTS and most GABA cells in the commissural NTS (SolC) are argued to be small interneurons with axons restricted to the NTS (Kawai and Senba, 1999). However, GABAergic projection neurons targeting the VRC are observed rostral to SolC in the interstitial (SolI) and ventral (SolV) subdivisions of the NTS. These represent a substantial proportion of the second order neurons receiving monosynaptic input from pulmonary slowly adapting stretch receptors (Ezure and Tanaka, 2004, see below). Glycinergic neurons are sparse in cNTS but include a subset of the slowly adapting stretch receptor relay neurons some of which colocalize both GABA and glycine (Ezure and Tanaka, 2004).
2.1.1 The dorsal respiratory group
The dorsal respiratory group (DRG) is identified with respiratory neurons (mainly inspiratory) concentrated in the ventrolateral subnucleus of the NTS (SolVL) that fire bursts of action potentials in a fixed phase relationship with breathing that is independent of afferent input. At least in the cat, monosynaptic excitatory projections reach the phrenic nucleus from about 60% of DRG neurons (Fedorko et al., 1983). On the other hand, in the rat, direct projections to the region of the phrenic nucleus are less substantial, representing approximately 14% of DRG neurons (de Castro et al., 1994). Significant projections from the dorsal respiratory group to the C1-2 levels of the spinal cord are additionally observed (which are present in both rat and cat), but their precise functions are only poorly understood. These projections likely target cervical inspiratory neurons that in turn provide propriospinal projections mainly to interneurons in the area of the phrenic nucleus and near thoracic motoneurons innervating intercostal muscles (Lipski and Duffin, 1986). Other than the likely presence of glutamate in excitatory bulbospinal projections of the DRG, their neurochemistry is not well studied. At least in the dog, some bulbospinal projections include axons containing nitric oxide synthase (Marsala et al., 2002).
2.1.2 Slowly and rapidly adapting pulmonary stretch receptor relay neurons
Vagal afferent fibers arising from the lungs and airways are frequently classified on the basis of whether their afferent fibers are myelinated or unmyelinated. Of these, the central pathways arising from myelinated afferents have been more extensively documented. Receptors with myelinated axons are typically separated into two groups, slowly (SAR) and rapidly (RAR) adapting receptors based on their response patterns to maintained lung inflation (e.g. Kubin et al., 2006).
Activation of SARs by lung inflation leads to a series of respiratory reflexes including the classically defined Breuer-Hering reflexes whereby lung inflation terminates an ongoing inspiration, and if inflation is maintained into the expiratory period, expiration is prolonged.
SARs release glutamate that acts via non-NMDA receptors (Bonham et al., 1993) on second order NTS relay neurons (termed pump cells), which in turn relay SAR input to central rhythm and pattern forming neurons in the VRC as well as to the pons (Ezure et al., 2002; Ezure and Tanaka, 2006). Pump cells appear to be mainly GABAergic although a subset (~ 26%) of GABAergic pump cells may also use glycine as a cotransmitter (Ezure and Tanaka, 2004; Takakura et al., 2007). Local axon collaterals of pump cells terminate within the NTS and have been shown to inhibit RAR second order relay neurons (see below). Inhibitory SAR inputs to BötC E-Aug neurons have also been recorded (Manabe and Ezure, 1988), as have inhibitory inputs to chemoreceptive glutamatergic neurons in the retrotrapezoid nucleus (RTN) (Takakura et al., 2007). An additional group of second-order neurons receiving SAR afferent input are distinguished from pump cells by their receipt of a prominent central respiratory drive. These neurons, termed I-β cells are excitatory and have spinal axons that project to the phrenic nucleus (Averill et al., 1985).
Excitation of VRC neurons also results from lung inflation; consequently excitatory pump cells projecting to the ventrolateral medulla are also postulated. One of their principal targets is postulated to be VRC expiratory neurons with a decrementing firing pattern (E-Dec) (Manabe and Ezure, 1988; Hayashi et al., 1996). As yet, specific examples of glutamatergic pump neurons have not been directly demonstrated.
RARs are less sensitive than SARs to lung inflation and respond with only a brief burst of activity. However, a majority of RARs respond with sustained activation to inhaled irritants (e.g. ammonia, cigarette smoke) that evoke airway protective reflexes including: rapid breathing, bronchoconstriction, and mucus secretion. RARs also elicit sighs in response to a decrease in airway compliance (Sant'Ambrogio and Widdicombe, 2001). RAR relay neurons may respond inflation or deflation and may switch phases in which they are active (i.e., from deflation to inflation) depending on lung volume (Lipski et al., 1991). RARs may be silent or weakly active in eupnea.
NTS neurons receiving monosynaptic input from RARs have mainly been demonstrated in lateral portions of SolC just medial to the solitary tract (Lipski et al., 1991; Otake et al., 2001), and they receive inhibitory input from more rostrally located pump neurons (Lipski et al., 1991; Ezure and Tanaka, 2000). NTS RAR relay neurons appear to be excitatory and project widely throughout the VRC and pons; a subset of RAR relay neurons, termed I-γ cells, also receive central respiratory drive and provide descending axon collaterals to the spinal cord (Otake et al., 2001).
2.1.2 Peripheral chemoreceptor relays in caudal NTS
SolC is the primary target of afferents from peripheral chemoreceptors in the carotid body providing important homeostatic feedback on the O2 (as well as the CO2/pH) status of the arterial blood (Lahiri et al., 2006). SolC relay neurons for peripheral chemoreceptors, are an important source of tonic excitation to the DRG and VRC and appear to project to many levels of the VRC including the RTN (Takakura et al., 2006).
SolC afferents from the carotid body run in the carotid sinus nerve (CSN) and appear to release glutamate (Mizusawa et al., 1994), dopamine (Finley et al., 1992; Massari et al., 1996) and potentially substance P, nitric oxide, and BDNF (Gatti et al., 1995; Ichikawa et al., 2007). CSN dopamine afferents to SolC apparently act via presynaptic D2 receptors on excitatory (glutamate) terminals (Kline et al., 2002). Selective elimination of cNTS neurokinin 1 receptors (a.k.a. substance P receptors), on the other hand, via injection into the caudal NTS of substance P conjugated to the metabolic toxin, saporin, reportedly results in little or no change in the respiratory response to chemoreceptor activation (Potts et al., 2007).
2.1.2.1 Tyrosine hydroxylase neurons and hypoxia
Hypoxia, activates tyrosine hydroxylase positive (TH) neurons in caudal NTS (Pequignot et al., 1993); however, afferents from the CSN do not appear to directly synapse on TH positive neurons (Massari et al., 1996), suggesting NTS TH neurons represent third or higher order neurons in peripheral chemoreceptor pathways. TH positive NTS neurons, provide ascending projections to the basal forebrain (including hypothalamus and amygdala), pons, and likely to the rostral ventrolateral medulla (RVLM, Hermes et al., 2006; Reyes and Van Bockstaele, 2006). NTS TH cells, however, do not appear to send axons to caudal portions of the VRC, nor to cardiovascular neurons in the region of the caudal ventrolateral medulla, although such direct projections to these areas do arise from non-TH NTS neurons (Hermes et al., 2006). Whether NTS TH neurons directly target rostral portions of the VRC does not appear to have been directly examined, although the injections made by Reyes and Van Bockstaele (2006) may have included the Bötzinger complex.
2.1.2.2 NMDA receptors and peripheral chemoreceptor pathways
Both NMDA and non-NMDA receptors are argued to participate in the transmission of peripheral chemoafferents in the SolC (Vardhan et al., 1993), NMDA receptor positive neurons account for the majority of NTS neurons activated by hypoxia, as measured by colocalization of c-fos and the NR1 subunit of the NMDA receptor; only few hypoxia activated c-fos expressing NTS neurons also expressed AMPA receptors (Gozal et al., 1999). Consistent with these observations, blockade of NMDA receptors by systemically administered MK801 severely attenuates the increase in frequency normally occurring in the acute response to hypoxia, with little effect on tidal volume. In contrast, MK801 injections caused little change in the respiratory responses to hypercapnia (Ohtake et al., 1998; Reid and Powell, 2005). Beyond direct effects within the NTS, the excitatory effects of peripheral chemoreceptor activation also appear to be mediated almost exclusively by NMDA receptors at expiratory bulbospinal neurons in the caudal ventral respiratory group (Dogas et al., 1995, in dog).
3.0 The ventral respiratory column; serial respiratory compartments in the medulla
The VRC occupies the ventrolateral medulla along its entire length and is populated by various types of respiratory neurons identified by their activation during the inspiratory or expiratory phases of the respiratory cycle (Fig. 2). Respiratory rhythm generation occurs mainly as a result of circuit interactions in the rostral half of the VRC. Bulbospinal neurons in the caudal half of the VRC transmit this rhythm unchanged but their activity modulates the amplitude of respiratory motor output on spinal respiratory nerves (Fig. 3A).
Figure 2.
Discharge patterns of representative types of VRC respiratory neurons in the rat, depicted over three breaths. The abbreviations, glu, gly, and GABA identify the excitatory or inhibitory fast amino acid transmitters (glutamate, glycine, GABA) used by these cells where they have been experimentally established (see text). In instances where the excitatory or inhibitory nature of a particular cell type has been inferred, but where the transmitter has not been established the cells are marked with + or − signs. In the chart higher action potential frequencies are represented by denser color coding in the temporal firing pattern. Note that most current models account for three phases in the respiratory cycle: Inspiration (I-red), early expiration (E1-light blue), late expiration (E2-dark blue). This is supported by the observation of subsets of expiratory neurons whose active phase of firing only occurs during the early or late phase of expiration under normal relaxed breathing (eupnea).
Neuron types include those with decrementing (Dec), constant (Con), or augmenting firing patterns (Aug). Inhibitory E-Dec neurons are included at two levels to reflect the presence of similar inhibitory expiratory neurons rostrally and caudally in the VRC (in the BötC and in the cVRG, respectively). A complete taxonomy of the various neuronal types suggested in different labs, is not encompassed in this single figure, neither have we included alternative terminologies used to designate the neuron types depicted. It is also acknowledged that under various environmental regimens the pattern of firing for individual neuronal types appears to be mutable. Finally, the excitatory and inhibitory nature of the neuronal types depicted represents cells examined in the VRC. While comparable inspiratory and expiratory firing patterns are observed in dorsolateral pontine and NTS neurons, the excitatory and inhibitory transmitters used by these neurons are generally not well defined.
Figure 3.
Comparative distribution and functions of brainstem respiratory neurons. A: Outline of respiratory compartments in the rat VRC. The shaded regions indicate an approximate division of the VRC into a rostral part contributing to rhythm and pattern generation (pink), while more caudal regions appear to contribute only to the pattern of respiratory activity (green). B: Relative distribution of expiratory and inspiratory neuron subtypes in the VRC. Data adapted from plots of propriobulbar neurons recorded in the rat by Ezure et al. (1988; E-Aug, E-Dec, I-Aug, I-Dec, I-Con) & by Sun et al. (1998; E-I and I-E) and normalized to the peak number of expiratory neurons in BötC. Note that the distribution maxima for particular neuron subtypes corresponds to particular VRC compartments, but that the sharp borders shown in the cartoon in A do not adequately represent the actual blending of different cell distributions along the rostral caudal length of the VRC. C. Effect on expiratory duration (Te) of the excitatory amino acid DL-homocysteic acid (DLH) micro-stimulation of the BötC, preBötC, and rVRG. Redrawn with permission after Monnier et al., 2003. Note, stimulation in preBötC caused tachypnea (shortened Te) while stimulation in either the BötC or the anterior part of rVRG produced bradypnea (lengthened Te). Chemical stimulation in posterior portions of the rVRG does not affect respiratory rate (Wang et al., 2002; Monnier et al., 2003).
Within the VRC, respiratory neurons form a sequential series of compartments (Fig. 1B). These were initially identified by relative differences in the peak population frequencies of specific categories of inspiratory or expiratory neurons (Fig 3b, e.g. Ezure et al., 1988; Sun et al., 1998). Respiratory neurons are usually categorized by their characteristic augmenting (I-Aug, E-Aug), decrementing (E-Dec, I-Dec), or relatively constant (E-Con, I-Con) firing pattern, and by firing patterns that either span the boundaries between inspiratory and expiratory phases (E-I, I-E) or which fire only during subportions of the respiratory phases (e.g., pre-I; Fig. 2).
It should be appreciated that within the VRC a given phasic firing pattern may not be uniquely associated with a given excitatory or inhibitory transmitter (Fig. 2). This is particularly evident when comparing inhibitory E-Aug neurons at the rostral end of the VRC (i.e. in the Bötzinger complex; BötC, see below) with presumed excitatory E-Aug neurons at the caudal end of the VRC (in the caudal ventral respiratory group, cVRG; see below). Another pair of contrasting neuron types with similar firing patterns is likely represented by I-Aug neurons. Bulbospinal I-Aug neurons in the VRC are excitatory, using glutamate as a fast amino acid transmitter (Stornetta et al., 2003b). On the other hand, GABAergic propriobulbar I-Aug neurons have been reported (Okazaki et al., 2001). Finally, while E-Dec propriobulbar and bulbospinal neurons were found to be glycinergic (Ezure et al., 2003a), GABAergic propriobulbar E-Dec neurons have also been reported (Okazaki et al., 2001, i.e. their post-I neurons).
3.1 Caudal VRC: Rostral and caudal divisions of the ventral respiratory group
In the caudal half of the medulla a “ventral respiratory group” (VRG) was initially identified and subdivided into rostral (rVRG) and caudal (cVRG) portions based on the prominence of inspiratory (I) neurons in rVRG, and expiratory (E) neurons in cVRG (Fig. 3B).
The rVRG contains the main aggregate of excitatory (glutamatergic) bulbospinal inspiratory neurons (I-Aug). These project monosynaptically to inspiratory motoneurons in the phrenic nucleus in the cervical spinal cord that innervate the diaphragm, and to external intercostal motoneurons in the thoracic spinal cord (Dobbins and Feldman, 1994; Iscoe, 1998; Guyenet et al., 2002; Stornetta et al., 2003b). E-Aug neurons in the cVRG represent excitatory bulbospinal neurons (E-Aug) innervating abdominal and expiratory internal intercostal motoneurons (Iscoe, 1998; Ezure et al., 2003b). Accordingly, rVRG and cVRG neurons are responsible for activation of these inspiratory and expiratory pump muscles, respectively, and at least for bulbospinal I-Aug neurons, glutamate and enkephalin are likely co-transmitters (Stornetta et al., 2003b). Phrenic projecting rVRG axons also appear to contain nitric oxide synthase and release nitric oxide as a cotransmitter with glutamate (Marsala et al., 2002, in dog). Bulbospinal projections in the rat also include inhibitory projections from glycinergic E-Dec neurons in cVRG, and to some extent from glycinergic E-Aug neurons in BötC (Schreihofer et al., 1999; Saito et al., 2002; Ezure et al., 2003a).
The anterior portion of the rVRG also harbors a unique population of NK1 receptor positive I-Aug glutamatergic bulbospinal neurons. These neurons are distinguished from other I-Aug rVRG neurons by their expression of NK1 receptors and by modest differences in their firing pattern (Guyenet et al., 2002). Bulbospinal NK1 receptor positive neurons are, in turn, discriminated from nearby NK1 receptor positive propriobulbar neurons in the preBötzinger complex (preBötC, see below), by their spinal projections and larger size (Alheid et al., 2002; Guyenet et al., 2002), as well as by their I-Aug firing pattern as compared to the E-I pattern common in preBötzinger neurons.
The presence of this neurochemically distinct population of NK1 receptive neurons in the anterior part of rVRG suggests that the rVRG might be further subdivided into anterior and posterior parts (Alheid et al., 2002). This possibility is reinforced by the observation that neuronal activation by small injections of excitatory amino acids into the anterior part of the rVRG slow respiratory frequency, while similar injections placed in the posterior part of rVRG do not change the respiratory rhythm (Wang et al., 2002; Monnier et al., 2003). This difference coincides with the observation that in the rat the highest concentration of VRC bulbospinal neurons is found within the posterior portion of the rVRG, with much fewer propriobulbar respiratory neurons (Dobbins and Feldman, 1994). Only the latter are likely to contribute significantly to the rhythmogenic circuits in the rostral part of the VRC. While bulbospinal neurons in the rVRG and cVRG are importantly involved in determining the pattern of activity on the nerves serving the muscles of the respiratory pump, they appear to have little influence on the frequency of respiratory rhythm. This is consistent with the observations that the spinal projecting VRG premotor neurons either lack medullary collaterals, such as cVRG E-Aug neurons (Ezure et al., 2003b), or for neurons such as bulbospinal I-Aug neurons, have medullary collaterals generally targeting other bulbospinal neurons or cranial motoneurons, (Lipski et al., 1994).
Individual E-Dec neurons within the cVRG of the rat, however, have been shown to project to the spinal cord, and profusely to more rostral portions of the VRC (Saito et al., 2002); their specific respiratory neuronal targets in the medulla are unclear but at least may serve to inhibit inspiratory bulbospinal neurons. While most E-Dec interneurons appear to be inhibitory (Okazaki et al., 2001; Ezure et al., 2003a), rostrally directed projections from the cVRG apparently include excitatory terminations on laryngeal (cricothyroid) motoneurons (Boers et al., Ezure 2002). The specific respiratory neuronal types providing this rostrally directed excitatory input have not been specified.
3.2 Rostral VRC: Bötzinger, preBötzinger, retrotrapezoid nucleus, and the parafacial respiratory group
The rostral half of the VRC, while participating in determining the pattern or envelope of activity on respiratory nerves, additionally encompasses neuronal populations that are the main source of respiratory rhythm generation (i.e., breathing frequency) (Onimaru and Homma, 1987; Smith et al., 1991; Onimaru and Homma, 2003;Feldman and Del Negro, 2006). Rostral VRC neurons related to respiratory rhythm formation are located in several adjacent compartments, including the most anterior portions of the rVRG (Fig. 3A,C), the preBötC, the BötC, and possibly elements of the RTN (see section 3.2.3). The rostral VRC includes substantial populations of respiratory neurons that project locally within the brainstem and particularly within the medulla (i.e. propriobulbar neurons). Inhibitory propriobulbar respiratory neurons include glycinergic E-Aug (Schreihofer et al., 1999) and E-Dec neurons (Ezure et al., 2003a), and inhibitory E-Con, I-E neurons, and I-Dec neurons whose transmitter(s) have not been directly specified. Possible GABAergic propriobulbar I-Dec, I-Aug, and E-Dec neurons have also been reported (Okazaki et al., 2001). Excitatory propriobulbar respiratory neurons include glutamatergic E-I neurons concentrated in the preBötzinger complex (Guyenet et al., 2002), and E-Con neurons. Pre-I neurons, for the most part, have only been observed in the RTN/parafacial region of neonatal rat brainstems in vitro; it is argued that both excitatory and inhibitory pre-I neurons exist (Onimaru and Homma, 1992). The existence of pre-I neurons in the adult rat parafacial regions remains controversial. (Fortuna et al., 2008) argue, for example, that the pre-I pattern of activity seen in neonatal parafacial neurons may be mirrored by BötC E-Aug neurons in the adult rat, under conditions of combined hypoxia and hypercapnea.
3.2.1 The Bötzinger complex
The BötC was initially discriminated by the presence of a prominent population of expiratory neurons in the region immediately caudal to the facial nucleus providing afferents to the NTS of the cat. BötC expiratory neurons (Fig. 3B) have subsequently been intensively examined and shown to provide widespread inhibitory projections within the VRC with E-Aug neurons targeting both inspiratory and expiratory bulbospinal neurons as well as substantial numbers of respiratory related cranial motoneurons (Jiang and Lipski, 1990). Some of the E-Aug BötC neurons also project via axon collaterals to the spinal cord, reaching at least as far as the phrenic nucleus (Tian et al., 1998). A subset of BötC neurons send axons rostrally, targeting the facial nucleus and the RTN, and appear to target further respiratory related areas of the pons (Ezure et al., 2003b; Rosin et al., 2006). In the rat, both E-Aug and E-Dec BötC neurons have been shown to use glycine as a transmitter (Schreihofer et al., 1999; Ezure et al., 2003a). In the cat, on the other hand, GABAergic neurons from the region of the BötC project to the NTS (Livingston and Berger, 1989), however, the respiratory nature of NTS projecting BötC GABAergic neurons to NTS has not been demonstrated. In the dog, GABAergic inputs to inspiratory bulbospinal neurons appear to predominate over glycinergic afferents during the expiratory phase of breathing (Krolo et al., 2000); the BötC is one of the most likely sources of such expiratory inhibitory afferents.
Expiratory neurons in the BötC are particularly relevant as a target of SAR efferents; E-Aug neurons are inhibited, while BötC E-Dec neurons are excited by lung inflation (Manabe and Ezure, 1988). While NK1 receptor positive neurons are prominent in the preBötzinger complex (see below), NK1 agonists injected into the BötC also modify respiration (Fong and Potts, 2006). NK1 agonists in the BotC mimic the effects of local glutamate stimulation (e.g. Wang et al., 2002; Monnier et al., 2003) lengthening the expiratory interval and cause bradypnea. Blockade of BötC NK1 receptors, on the other hand, attenuates the expiratory lengthening response to vagal stimulation, leading Fong and Potts (2006) to suggest that the BötC mediates the expiratory lengthening aspect of the Breuer-Hering reflex.
3.2.2 The preBötzinger complex
The preBötC was identified in vitro as a medullary region essential for respiratory rhythm generation (Smith et al., 1991). Situated between the BötC and rVRG (Fig. 1), the preBötC demonstrates peak populations for neurons whose in vivo firing patterns span the temporal boundaries between the expiratory and inspiratory phases of the respiratory cycle (e.g., “E-I neurons,” Fig 2, Fig 3B) (Sun et al., 1998; Guyenet and Wang, 2001).
From the standpoint of the chemical neuroanatomy of respiratory circuits, the identification of small, NK1 receptor positive respiratory neurons clustered in the region of the preBötC (Gray et al., 1999) and their identification as E-I neurons (Guyenet and Wang, 2001) has provided a focal point for anatomical, pharmacological, developmental, and physiological studies. This has accelerated the pace of research examining this important population of VRC respiratory neurons. As mentioned above, propriobulbar NK1 receptor positive neurons in the preBötC are discriminated from nearby NK1 receptor positive bulbospinal neurons by the larger size of the latter. In addition, at least in the rat, the near absence of bulbospinal neurons in the preBötC also helps to discriminate its general location within the VRC (Dobbins and Feldman, 1994; Sun et al., 1998; Guyenet et al., 2002).
Substance P, the endogenous agonist for NK1 receptors, increases “fictive” respiratory frequency measured on cranial nerves, when applied in vitro in mouse brainstem slices containing the preBötC, while µ-opiate and GABAB agonists decrease respiratory frequency. All of these agonists appear to activate receptors on a similar population of preBötC neurons (Gray et al., 1999), the type 1 neurons described by (Rekling et al., 1996). Accordingly, both µ-opioid receptors and GABAB receptors are colocalized on preBötC neurons that also express the NK1 receptor (Gray et al., 1999). In adult rats, selective destruction of NK1 receptor positive neurons in the region of the preBötC, using the metabolic poison saporin coupled to substance P, leads to moderate to severe disruption of breathing (Gray et al., 2001; Wang et al., 2002). Not surprisingly, such disruptions lead to increased mortality in experimental animals, particularly during the sleep phase of the day-night cycle (McKay et al., 2005). It should be acknowledged, however, that while NK1 receptive neurons are prominent in the preBötC, they are also prevalent in numerous other structures in ventrolateral medulla (Fig. 4) including the BötC (see above), and on a subset (~ 5%) of the sympathoexcitatory C1 bulbospinal neurons in the ventrolateral medulla (Makeham et al., 2001). NK1 receptors are also colocalized on chemosensitive glutamatergic neurons in the RTN (see below) and a loss of these NK1 receptor positive RTN neurons appears to result from induced mutations in the Phox2B transcription factors that mimic those occurring in patients with congenital central hypoventilation syndrome (CCHS).
Figure 4.
NK1 receptors in the rostral VRC of the mouse. Pseudocolored sagittal section through the rhombencephalon of the mouse with immunolabeled NK1 receptors (cyan). Note the dense labeling at the level of the preBötC and also at the ventral and caudal portions of the facial nucleus. Nevertheless, in comparison to areas such as the superior olive (SO) and lateral reticular nucleus (LRt), which are essentially unstained for NK1 receptors, few areas of the VRC (i.e. the RTN, BötC, preBötC, or rVRG) can be considered devoid of NK1 receptors.
Following immunolabeling with diaminobenzidine as the chromogen, the section was subsequently counterstained with ethidium bromide (red) as a fluorescent Nissl stain. Grayscale images of the NK1 receptors were inverted with respect to black and white and copied to the green and blue channels of an RGB image. A grayscale image of the Nissl staining was copied to the red channel of the same image to produce the final composite image.
In addition to µ-opiate and GABAB receptors (see above), preBötC NK1 receptor positive neurons also express the type 2 vesicular glutamate transporter (VGlut2; Guyenet et al., 2002), indicating that these neurons mainly provide excitatory efferents to the remainder of the VRC. Somatostatin is also found in preBötC, colocalized in NK1 receptor positive neurons (Stornetta et al., 2003a), and the a-variant of the 5-HT4 serotonin receptor (Manzke et al., 2003) is colocalized with a subset (~30%) of NK1 receptor positive respiratory neurons in the preBötC as well as in about 50% of µ-opioid positive preBötC neurons. The excitatory action of the specific 5-HT4 agonist BIMU8 stimulates respiration and countered fentanyl induced respiratory depression without blocking the analgesia induced by this µ-opioid agonist (Manzke et al., 2003).
In vitro, thyrotropin (TRH) increases respiratory frequency and the firing rate of “type 1” preBötC neurons (Rekling et al., 1996), which are NK1 receptor positive (Gray et al., 1999). However, while the TRH binding appears to be widespread in the ventrolateral medulla (Bayliss et al., 1994), colocalization of thyrotropin receptors and NK1 receptors does not appear to have been directly demonstrated. The most likely source of TRH afferents to the preBötzinger complex are the nearby TRH positive serotonergic neurons of the caudal raphe nuclei.
3.2.3 The retrotrapezoid nucleus
The retrotrapezoid nucleus (RTN) was initially identified by retrograde labeling from the VRC in cats (Smith et al., 1989). RTN neurons in cats, rats, and mice are found along the ventral surface of the brain just below the facial nucleus and also extending slightly caudally beneath the BötC (Fig. 1B). Because of its location and proximity to the surface of the brain it was readily appreciated (Smith et al., 1989; Nattie et al., 1991) that the RTN approximated at least one of the areas along the ventral surface of the medulla that was earlier identified as chemosensitive. That is to say, increasing CO2 or decreasing pH stimulates breathing, presumably through the action of chemosensitive cells (neurons and/or glia) located at or near the ventral surface of the brain (Li et al., 1999; Mulkey et al., 2004).
RTN neurons appear to be particularly relevant as central chemoreceptors. Specifically, they monotonically increase their firing rate in response to local acidification and provide an extensive excitatory (glutamatergic) output to neurons in the VRC (Mulkey et al., 2004; Rosin et al., 2006). Integration of central and peripheral chemoreceptor afferents also appears to occur to some extent at the RTN since second or higher order relay neurons for peripheral chemosensory afferents located in the caudal commissural NTS (SolC) also send excitatory (glutamatergic) projections to the RTN (Rosin et al., 2006; Takakura et al., 2006).
Afferents from both medullary and pontine serotonergic neurons target the RTN and serotonin injected in the RTN stimulates respiratory activity (Mulkey et al., 2007). Serotonergic activation of the RTN does not appear to be the source of the pH sensitivity in RTN neurons since the effects of serotonin and pH appear to be additive at RTN neurons (Mulkey et al., 2007). Serotonergic neurons, on the other hand, are also argued to be central chemosensors (Richerson, 2004). Interestingly, the peak response of RTN neurons to infused CO2 appears during the waking part of the day-night cycle in rats, while the peak response of CO2 infused into the raphe appears during sleep, suggesting a complementary role for these two structures in central chemoreception (Li et al., 1999; Nattie and Li, 2001).
Finally, NK1 receptors are also colocalized in RTN neurons and selective lesioning of RTN neurons with substance P conjugatged to saporin has been shown to attenuate the central response to CO2 (Nattie and Li, 2002).
Substantial interest in the RTN is sustained by the likely disruption of central chemosensitivity in a variety of clinical settings including sudden infant death syndrome (SIDS) and congenital central hypoventilation syndrome (CCHS). In the latter, a life threatening disruption of central and peripheral chemosensitivity appears to be a critical element amongst a general disregulation of the autonomic outflow. CCHS has been related to mutations in the Phox2B transcription factor (Amiel et al., 2003; Weese-Mayer et al., 2003, 2008). Phox2B is normally expressed in glutamatergic neurons of the RTN (Stornetta et al., 2006), and a knock-out of this gene or induced mutations disrupt the development of the RTN (Dubreuil et al., 2008), as well as the carotid body (the principal site of peripheral chemoreception).
Interestingly, in the experiments reported by Dubreuil et al. (2008) the polyalanine repeats induced in the Phox2B gene resulted in a loss of RTN neurons coexpressing the Phox2B gene and the NK1 receptor. In the report by McKay et al (2005) the increased mortality during the sleep phase of the day-night cycle following lesions of NK1 receptor positive neurons by substance P-saporin injections in the region of the preBötC, was suggested to be relevant to increased human mortality during sleep, consequent to the loss of medullary NK1 receptive neurons in the medulla known to occur neuropathologically and/or in conjunction with aging. Consistent with this view, both patients with CCHS and victims of SIDS have an increased liability of dying during their sleep, and both have demonstrated neuropathology in the region of the acuate nucleus which may encompass the human homolog of the RTN (Weese-Meyer et al., 2008).
3.2.4 The parafacial respiratory group
At about the same time as the discovery of the RTN (Smith et al., 1989), Onimaru and Homma (1987) described a similarly located group of neurons using in vitro brainstem preparations from neonatal rats, with a “pre-I” discharge pattern. Their pre-I neurons were identified beneath the caudal end of the facial nucleus and extended caudally beneath the BötC (Fig. 1, 2). In their initial report, and over the subsequent two decades (Onimaru and Homma, 2003; Onimaru et al., 2006), these authors have argued that pre-I neurons were important for the generation of respiratory rhythm. This functionally defined group of neurons has subsequently been termed the “parafacial respiratory group” (pFRG) (Onimaru and Homma, 2003; Feldman and Del Negro, 2006). Competing with the attention given to the subsequent discovery of respiratory rhythm generation by the preBötC (Smith et al., 1991) and the persisting difficulty in identifying pre-I neurons in vivo in the adult brain, the role of pre-I neurons has only slowly been incorporated within theoretical perspectives on respiratory circuits. This topic, moreover, remains controversial (e.g. Fortuna et al., 2008). A theoretical position, however, has evolved advancing the proposition that the circuits formed by pFRG neurons, represent an expiratory rhythm generator, which is coupled (by reciprocal inhibition) to an inspiratory rhythm generator centered within the preBötC (Feldman et al., 2003; Feldman and Del Negro, 2006).
4.0 Pontine respiratory regions
In a sense, the VRC extends rostrally into the lateral pons as a nearly uninterrupted corridor of neurons interconnected with the VRC (Fig. 1B) (Alheid et al., 2004). As with the medullary VRC, segments of the neuronal populations traversing the pons appear to form functionally distinct groups. Just rostral to the RTN/pFRG, these aggregates include neurons of the ventrolateral pons, and the paratrigeminal area, including the laterally located intertrigeminal area (I5; Fig 1) that is sandwiched between the motor trigeminal nucleus (Mo5) and the principal sensory trigeminal nucleus. Dorsally, the I5 region merges with the Kölliker-Fuse nucleus, which in turn merges with respiratory related neurons in the lateral, and to a lesser extent in the medial parabrachial complex.
Retrograde tracers injected in the VRC (Smith et al., 1989; Alheid et al., 2002, 2004), or transsynaptic neuronal labeling after pseudorabies virus injections in the phrenic nerve (Dobbins and Feldman, 1994) identify the overall topography of respiratory-related neurons throughout the medulla and lateral pontine regions. Respiratory-related neurons extend rostrally from the BötC, and almost surround the facial nucleus, but are most prevalent ventrally at the RTN/parafacial area. More rostrally, respiratory related neurons continue in the ventrolateral pontine area, and are located mainly dorsal to the A5 norepinephrine neurons, but ventrally are intermingled with the latter. Retrogradely labeled pontine neurons continue dorsally as an arc of cells adjacent to the trigeminal motor nucleus (Mo5), lying laterally to Mo5 in the I5 region, as well as rostral (to Mo5). More dorsally, the largest population of VRC projecting pontine neurons is found within the Kölliker-Fuse nucleus (KF) and in the ventrolateral portions of the parabrachial complex (PBr; Fig. 1B).
4.1 The ventrolateral pons/A5 area
Ventral to the respiratory related neurons in the KF and I5 regions is an ill defined area of the ventrolateral pontine reticular formation often identified with the A5 noradrenergic cell group located in the most ventral portions of this area. The ventrolateral pons contains a loosely packed and somewhat scattered field of neurons, with projections to the various compartments of the VRC. Only a small proportion of the VRC projecting neurons are catecholaminergic. The larger part of the VRC projecting cell population consists of smaller-sized neurons situated dorsally to the A5 cells (Alheid, unpublished observations). This loosely organized group of neurons merges caudally with the rostral-most portions of the RTN and to some extent with a larger aggregate of VRC projecting neurons in the rostral division of the lateral paragigantocellular nucleus.
A specific role of the ventrolateral pons with respect to respiration remains somewhat clouded. Chemical inhibition within this region, by injection of the GABA-A receptor agonist muscimol, results in apneusis in vagotomized rats but not in vagus intact animals; chemical stimulation with glutamate, on the other hand, causes prolonged expiration (Jodkowski et al., 1997). The ventrolateral pons has also been implicated in shaping the hypoxic ventilatory response. Specifically, during acute moderate hypoxia in rats (and other small mammals) there is an immediate increase in breathing frequency that, despite maintained hypoxia, then declines back toward, but not to, pre-hypoxic levels (Hayashi et al., 1993). This secondary “hypoxic respiratory depression” appears to require both the dorsal and ventral aspects of the pons in the neonatal rat (Okada et al., 1998). Hypoxia activates expiratory neurons in the ventrolateral pons and chemical blockade in this area also blocks a post-hypoxic depression of respiration (Dick and Coles, 2000).
4.2 The dorsolateral pons and the pontine respiratory group
Collectively, respiratory neurons in PBr and KF are the main constituents of what has been termed the “pontine respiratory group”. It should be appreciated, however, that the cell populations identified with the pontine respiratory group (subsets of PBr and KF neurons) do not represent a homogeneous group of neurons either physiologically or neurochemically. A variety of respiratory neuronal types are found in this region including phasic inspiratory (I, E-I, I-E) and expiratory neurons (E, E-Dec, E-Aug), in adult rats in vivo (Ezure and Tanaka, 2006; Song et al., 2006). Neurons with tonic activity modulated by the respiratory cycle are also observed (Jiang et al., 2004; Song et al., 2006). For some of these, respiratory modulation increases with arousal and in response to painful stimuli (Jiang et al., 2004; Ezure and Tanaka, 2006). It should be noted that the identification of respiratory neurons and respiratory modulated neurons in the pons provides no guarantee that the transmitters used by these cells will match those used by neurons with similar firing patterns in the VRC.
The KF is the source of the most massive projections to the VRC, and additionally projections to respiratory related areas of the NTS (particularly ventrolateral NTS), as well as to the hypoglossal and facial nuclei (Ezure and Tanaka, 2006; Yokota et al., 2007). Spinal projections also originate in the KF and include glutamatergic projections to the phrenic nucleus and it appears that axon collaterals of individual KF neurons target both the phrenic nucleus and bulbospinal neurons in the rVRG (Yokota et al., 2007). Enkephalinergic neurons in the KF also project to the spinal cord and ventrolateral medulla, however, whether enkephalin is also colocalized in glutamatergic KF axons projecting to the VRC or in KF axons targeting the phrenic nucleus (Yokota et al., 2007) is not clear at present.
5.0 Conclusions
The CNS network controlling respiration is a complex array of neurons stretching from the cortex to the lower thoracic/upper lumbar spinal cord. Respiratory neurons in the NTS and in a column formed by a series of brainstem compartments in the medulla represent the core structures responsible for the automatic control of breathing.
It is evident that the neurochemistry of respiration has made great strides over the past decade. It is worth noting that the germinal observation of the preferential colocalization of NK1 receptor positive neurons in the preBötC has provided an important anchor for in vitro and in vivo analyses of respiratory circuits in ventrolateral medulla. Similarly, identification of the RTN as a potential site of pathology in SIDS and CCHS and the discovery of some of the neurochemical correlates of central chemosensory areas provide a simultaneous promise of significant progress in understanding the structural basis of these diseases and for a rational development of therapeutic approaches to their treatment. In this era of rapidly expanding information databases cataloging the molecular biology and genetics of the brain, we can expect an accelerated pace of discovery using combined physiological and neurochemical analyses of respiratory brainstem circuits in health and disease.
Acknowledgements
This work was supported by NIH grants, HL 72415, HL 73474, HL 80208.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143:105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Averill DB, Cameron WE, Berger AJ. Neural elements subserving pulmonary stretch receptor-mediated facilitation of phrenic motoneurons. Brain Res. 1985;346:378–382. doi: 10.1016/0006-8993(85)90874-1. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Kanter RK, Szymeczek-Seay CL, Berger AJ, Millhorn DE. Early postnatal development of thyrotropin-releasing hormone (TRH) expression, TRH receptor binding, and TRH responses in neurons of rat brainstem. J Neurosci. 1994;14:821–833. doi: 10.1523/JNEUROSCI.14-02-00821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers J, Klop EM, Hulshoff AC, de Weerd H, Holstege G. Direct projections from the nucleus retroambiguus to cricothyroid motoneurons in the cat. Neurosci Lett. 2002;319:5–8. doi: 10.1016/s0304-3940(01)02395-3. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Coles SK, McCrimmon DR. Pulmonary Stretch-Receptor Afferents Activate Excitatory Amino-Acid Receptors in the Nucleus-Tractus-Solitarii in Rats. J Physiol. 1993;464:725–745. doi: 10.1113/jphysiol.1993.sp019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro D, Lipski J, Kanjhan R. Electrophysiological study of dorsal respiratory neurons in the medulla oblongata of the rat. Brain Res. 1994;639:49–56. doi: 10.1016/0006-8993(94)91763-9. [DOI] [PubMed] [Google Scholar]
- Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir Physiol. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Dogas Z, Stuth EAE, Hopp FA, McCrimmon DR, Zuperku EJ. NMDA Receptor-Mediated Transmission of Carotid-Body Chemoreceptor Input to Expiratory Bulbospinal Neurons in Dogs. J Physiol. 1995;487:639–651. doi: 10.1113/jphysiol.1995.sp020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K, Manabe M, Yamada H. Distribution of medullary respiratory neurons in the rat. Brain Res. 1988;455:262–270. doi: 10.1016/0006-8993(88)90085-6. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Lung inflation inhibits rapidly adapting receptor relay neurons in the rat. NeuroReport. 2000;11:1709–1712. doi: 10.1097/00001756-200006050-00023. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neurosci. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neurosci. 2006;141:1011–1023. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Kondo M. Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J Neurosci. 2003a;23:8941–8948. doi: 10.1523/JNEUROSCI.23-26-08941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y. Brainstem and spinal projections of augmenting expiratory neurons in the rat. Neurosci Res. 2003b;45:41–51. doi: 10.1016/s0168-0102(02)00197-9. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y, Otake K. Axonal projections of pulmonary slowly adapting receptor relay neurons in the rat. J Comp Neurol. 2002;446:81–94. doi: 10.1002/cne.10185. [DOI] [PubMed] [Google Scholar]
- Fedorko L, Merrill EG, Lipski J. Two descending medullary inspiratory pathways to phrenic motoneurones. Neurosci Lett. 1983;43:285–291. doi: 10.1016/0304-3940(83)90202-1. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JC, Polak J, Katz DM. Transmitter diversity in carotid body afferent neurons: dopaminergic and peptidergic phenotypes. Neurosci. 1992;51:973–987. doi: 10.1016/0306-4522(92)90534-9. [DOI] [PubMed] [Google Scholar]
- Fong AY, Potts JT. Neurokinin-1 receptor activation in Botzinger complex evokes bradypnoea. J Physiol. 2006;575:869–885. doi: 10.1113/jphysiol.2006.114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci. 2008;28:2506–2515. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti PJ, Shirahata M, Johnson TA, Massari VJ. Synaptic interactions of substance P immunoreactive nerve terminals in the baro- and chemoreceptor reflexes of the cat. Brain Res. 1995;693:133–147. doi: 10.1016/0006-8993(95)00728-9. [DOI] [PubMed] [Google Scholar]
- Gozal D, Xue YD, Simakajornboon N. Hypoxia induces c-Fos protein expression in NMDA but not AMPA glutamate receptor labeled neurons within the nucleus tractus solitarii of the conscious rat. Neurosci Lett. 1999;262:93–96. doi: 10.1016/s0304-3940(99)00065-8. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Wang H. Pre-Botzinger neurons with preinspiratory discharges "in vivo" express NK1 receptors in the rat. J Neurophysiol. 2001;86:438–446. doi: 10.1152/jn.2001.86.1.438. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LF, Castellanos M, Davenport PW. Parabrachial neurons mediate dorsal periaqueductal gray evoked respiratory responses in the rat. J Appl Physiol. 2004;96:1146–1154. doi: 10.1152/japplphysiol.00903.2003. [DOI] [PubMed] [Google Scholar]
- Hermes SM, et al. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp Neurol. 2006;198:539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Terayama R, Yamaai T, Yan Z, Sugimoto T. Brain-derived neurotrophic factor-immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia; co-expression with other neurochemical substances. Brain Res. 2007 doi: 10.1016/j.brainres.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neruons by augmenting neurons in the Bötzinger complex in the cat. Exp Brain Res. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Jiang M, Alheid GF, Calandriello T, McCrimmon DR. Parabrachial-lateral pontine neruons link nociception and breathing. Respir Physiol Neurobiol. 2004 doi: 10.1016/j.resp.2004.07.019. In press. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Coles SK, Dick TE. Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol. 1997;82:377–381. doi: 10.1152/jappl.1997.82.2.377. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Senba E. Electrophysiological and morphological characterization of cytochemically-defined neurons in the caudal nucleus of tractus solitarius of the rat. Neurosci. 1999;89:1347–1355. doi: 10.1016/s0306-4522(98)00393-5. [DOI] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol. 2002;88:2736–2744. doi: 10.1152/jn.00224.2002. [DOI] [PubMed] [Google Scholar]
- Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Relative magnitude of tonic and phasic synaptic excitation of medullary inspiratory neurons in dogs. Am J Physiol Regul Integr Comp Physiol. 2000;279:R639–R649. doi: 10.1152/ajpregu.2000.279.2.R639. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol. 2006;91:249–286. doi: 10.1016/j.pbiomolbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Li AH, Randall M, Nattie EE. CO2 microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res. 1986;61:625–637. doi: 10.1007/BF00237589. [DOI] [PubMed] [Google Scholar]
- Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol. 1991;443:55–77. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Livingston CA, Berger AJ. Immunocytochemical localization of GABA in neurons projecting to the ventrolateral nucleus of the solitary tract. Brain Res. 1989;494:143–150. doi: 10.1016/0006-8993(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Makeham JM. NK1 receptor and the ventral medulla of the rat: bulbospinal and catecholaminergic neurons. NeuroReport. 2001;12:3663–3667. doi: 10.1097/00001756-200112040-00012. [DOI] [PubMed] [Google Scholar]
- Manabe M, Ezure K. Decrementing expiratory neurons of the Botzinger complex. I. Response to lung inflation and axonal projection. Exp Brain Res. 1988;72:150–158. doi: 10.1007/BF00248510. [DOI] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Marsala J, Lukácová N, Cizková D, Kafka J, Katsube N, Kuchárová K, Marsala M. The case for the bulbospinal respiratory nitric oxide synthase-immunoreactive pathway in the dog. Exp Neurol. 2002;177:115–132. doi: 10.1006/exnr.2002.7978. [DOI] [PubMed] [Google Scholar]
- Massari VJ, Shirahata M, Johnson TA, Gatti PJ. Carotid sinus nerve terminals which are tyrosine hydroxylase immunoreactive are found in the commissural nucleus of the tractus solitarius. J Neurocytol. 1996;25:197–208. doi: 10.1007/BF02284796. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol. 1994;478(Pt 1):55–66. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol. 2003;548:859–874. doi: 10.1113/jphysiol.2002.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, St.John WM. Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol. 1991;71:1364–1375. doi: 10.1152/jappl.1991.71.4.1364. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li AH. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol. 1998;84:853–861. doi: 10.1152/jappl.1998.84.3.853. [DOI] [PubMed] [Google Scholar]
- Okada Y, Kawai A, Muckenhoff K, Scheid P. Role of the pons in hypoxic respiratory depression in the neonatal rat. Respir Physiol. 1998;111:55–63. doi: 10.1016/s0034-5687(97)00105-9. [DOI] [PubMed] [Google Scholar]
- Okazaki M, Takeda R, Haji A, Yamazaki H. Glutamic acid decarboxylase-immunoreactivity of bulbar respiratory neurons identified by intracellular recording and labeling in rats. Brain Res. 2001;914:34–47. doi: 10.1016/s0006-8993(01)02788-3. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1987;403:380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Whole cell recordings from respiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Pflügers Arch. 1992;420:399–406. doi: 10.1007/BF00374476. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;95:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y, Tanaka I, Ezure K. Morphology of pulmonary rapidly adapting receptor relay neurons in the rat. J Comp Neurol. 2001;430:458–470. doi: 10.1002/1096-9861(20010219)430:4<458::aid-cne1043>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Pequignot JM, Soulier V, Cottet-Emard JM, Dalmaz Y, Borghini N, Peyrin L. Stimulatory effect of long-term hypoxia on the posterior part of A2 noradrenergic cell group in nucleus tractus solitarius of rat. Adv Exp Med Biol. 1993;337:429–434. doi: 10.1007/978-1-4615-2966-8_60. [DOI] [PubMed] [Google Scholar]
- Potts JT, Fong AY, Anguelov PI, Lee S, McGovern D, Grias I. Targeted deletion of neurokinin-1 receptor expressing nucleus tractus solitarii neurons precludes somatosensory depression of arterial baroreceptor-heart rate reflex. Neurosci. 2007;145:1168–1181. doi: 10.1016/j.neuroscience.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SG, Powell FL. Effects of chronic hypoxia on MK-801-induced changes in the acute hypoxic ventilatory response. J Appl Physiol. 2005;99:2108–2114. doi: 10.1152/japplphysiol.01205.2004. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubié M. Thyrotropin-releasing hormone (TRH) depolarizes a subset of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996;75:811–819. doi: 10.1152/jn.1996.75.2.811. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Van Bockstaele EJ. Divergent projections of catecholaminergic neurons in the nucleus of the solitary tract to limbic forebrain and medullary autonomic brain regions. Brain Res. 2006;1117:69–79. doi: 10.1016/j.brainres.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Saito Y, Tanaka I, Ezure K. Morphology of the decrementing expiratory neurons in the brainstem of the rat. Neurosci Res. 2002;44:141–153. doi: 10.1016/s0168-0102(02)00095-0. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Widdicombe J. Reflexes from airway rapidly adapting receptors. Respir Physiol. 2001;125:33–45. doi: 10.1016/s0034-5687(00)00203-6. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Botzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Botzinger complex. J Comp Neurol. 2003a;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Inspiratory augmenting bulbospinal neurons express both glutamatergic and enkephalinergic phenotypes. J Comp Neurol. 2003b;455:113–124. doi: 10.1002/cne.10486. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Goodchild AK, Chalmers JP, Pilowsky PM. The pre-Botzinger complex and phase-spanning neurons in the adult rat. Brain Res. 1998;809:204–213. doi: 10.1016/s0006-8993(98)00872-5. [DOI] [PubMed] [Google Scholar]
- Sykes RM, Spyer KM, Izzo PN. Demonstration of glutamate immunoreactivity in vagal sensory afferents in the nucleus tractus solitarius of the rat. Brain Res. 1997;762:1–11. doi: 10.1016/s0006-8993(97)00368-5. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, West GH, Gwilt JM, Colombari E, Stornetta RL, Guyenet PG. GABAergic pump cells of solitary tract nucleus innervate retrotrapezoid nucleus chemoreceptors. J Neurophysiol. 2007;98:374–381. doi: 10.1152/jn.00322.2007. [DOI] [PubMed] [Google Scholar]
- Tian GF, Peever JH, Duffin J. Bötzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Exp Brain Res. 1998;122:149–156. doi: 10.1007/s002210050502. [DOI] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol. 1993;264:R41–R50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- Wang H, Germanson TP, Guyenet PG. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci. 2002;22:3755–3764. doi: 10.1523/JNEUROSCI.22-09-03755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME, Marazita ML. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med Genet A. 2003;123:267–278. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- Weese-Meyer DE, Berry-Kravis EM, Ceccherini I. Congenital Central Hypoventilation Syndrome (CCHS) and Sudden Infant Death Sydrome (SIDS): Kindred disorders of autonomic regulation. Respir Physiol Neurobiol. 2008 doi: 10.1016/j.resp.2008.05.011. In press. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y. Glutamatergic neurons in the Kolliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: a combined retrograde tracing and in situ hybridization study in the rat. Neurosci Res. 2007;59:341–346. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]