Abstract
Background: The dose intensity of chemotherapy has been described as affecting the outcome of the treatment of a number of different types of tumors. A delay in the resumption of chemotherapy after definitive surgery for the treatment of osteosarcoma can decrease the overall dose intensity. The goal of this study was to assess the prognostic significance of the time to resumption of chemotherapy after definitive surgery in patients with localized osteosarcoma in an extremity.
Methods: The relationships of the time between definitive surgery and resumption of chemotherapy with death and adverse events in 703 patients with a localized resectable osteosarcoma in an extremity (556 treated in the Children's Oncology Group [COG] Study [INT 0133] and 147 treated at five tertiary care cancer centers) were assessed with use of Cox proportional hazards models.
Results: The twenty-fifth, fiftieth, and seventy-fifth percentiles of time from definitive surgery to resumption of chemotherapy were twelve, sixteen, and twenty-one days, respectively. Overall survival was poorer for patients who had had a delay of greater than twenty-one days before the resumption of chemotherapy compared with those who had had a shorter delay (hazard ratio = 1.57 [95% confidence interval = 1.04 to 2.36]; p = 0.03). Of seventy-one COG-study patients with postoperative complications, 32% (twenty-three) had a delay of more than twenty-one days before resumption of chemotherapy, but 20% (eighty-nine) of 444 patients with no complications had a similar delay.
Conclusions: In this retrospective analysis, increased time from the definitive surgery to the resumption of chemotherapy was found to be associated with an increased risk of death of patients with localized osteosarcoma in an extremity. Within the limitations of a retrospective study, the data indicate that it is best to resume chemotherapy within twenty-one days after definitive surgery. Surgeons, oncologists, patients, and those responsible for scheduling need to work together to ensure timely resumption of chemotherapy after surgery.
Level of Evidence: Prognostic Level II. See Instructions to Authors for a complete description of levels of evidence.
Osteosarcoma is the most common primary malignant bone tumor in children and adolescents less than twenty years of age, with approximately 400 new cases each year in the United States1. The majority of the cases are confined to the extremities, making them amenable to surgical resection1,2. Preoperative chemotherapy reduces peritumor edema and vascularity, thus facilitating eventual limb-salvage procedures. With combined modality treatment, 50% to 80% of patients with localized osteosarcoma in an extremity can be expected to survive long term (five years or longer)3-6.
Several dose-intensity analyses have supported the contention that the actual dose intensity delivered in adjuvant chemotherapy for osteosarcoma determines the treatment outcome7-9. A lengthy delay before the resumption of chemotherapy after definitive surgery could compromise the overall dose intensity. The appropriate interval from the definitive surgery to the resumption of chemotherapy is, in part, dictated by concerns about wound-healing and the risk of infection. Such considerations make deciding when to resume chemotherapy a challenge. In a previously published study, investigators at Memorial Sloan-Kettering Cancer Center found a trend toward poorer disease-free survival when chemotherapy had been delayed more than twenty-four days after surgery in patients with a poor histological response to preoperative chemotherapy10. The aim of the present retrospective review was to assess the impact of the time until the resumption of contemporary chemotherapy after definitive surgery on the outcome in patients with newly diagnosed localized resectable osteosarcoma in an extremity.
Materials and Methods
Patients and Data Collection
This retrospective study was a collaborative effort of a cooperative group (the Children's Oncology Group [COG]) and five tertiary care centers: the Mayo Clinic (Rochester, Minnesota), Children's Hospital and Regional Medical Center (Seattle, Washington), Children's Hospital and Clinics of Minnesota (Minneapolis, Minnesota), University of Minnesota Cancer Center (Minneapolis, Minnesota), and Children's Hospital of Philadelphia (Philadelphia, Pennsylvania).
The tertiary care centers were chosen on the basis of previous collaboration with pilot studies, a common philosophy regarding the treatment of osteosarcoma, the presence of orthopaedic oncologists, and a previous record of outstanding collaboration on sarcoma projects involving retrospective reviews.
Information regarding patients treated in the cooperative group trial was provided by the COG Statistics and Data Center. Local institutional review boards approved the study at all of the other institutions, where local investigators abstracted the data. Variables that were collected included age, sex, race, the bone in which the tumor was located, the size of the primary tumor, serum levels of lactic dehydrogenase and alkaline phosphatase, the type of surgery, the surgical margins, the extent of tumor necrosis at the time of the surgery, and the number of days to the resumption of chemotherapy after the surgery. Information regarding surgical complications was collected prospectively only for the patients in the COG study. The percentages of patients for whom lactic dehydrogenase, alkaline phosphatase, and tumor-size values were missing differed between the group in the COG study (1.3% [lactic dehydrogenase], 1.4% [alkaline phosphatase], and 10.4% [tumor size]) and the group not in the COG study (44.2%, 39.5%, and 37.4%, respectively) because this information was not routinely obtained for the patients who were not in the COG study whereas the data were required for entry of patients into the COG study. For the remaining variables, data points were missing for, at most, 12% of the patients in either group.
Eligibility criteria included an age of less than thirty years, a new diagnosis of localized conventional high-grade osteosarcoma in an extremity between 1988 and 2002, resumption of chemotherapy postoperatively, and no disease progression prior to surgery. In addition, the patient had to have received neoadjuvant chemotherapy with (1) doxorubicin, cisplatin, and high-dose methotrexate with or without ifosfamide and with or without muramyl tripeptide phosphatidyl ethanolamine either in, or according to the previously published protocol of, the COG Phase-III Intergroup Study INT-0133 (protocol identification numbers, CCG7921 and POG 9351)5; (2) doxorubicin, ifosfamide, and high-dose methotrexate with or without cisplatin either in, or according to previously reported protocols in, pilot studies11,12; or (3) doxorubicin, cisplatin, and high-dose methotrexate with or without ifosfamide in Pediatric Oncology Group (POG) Study 9754.
Statistical Methods
We analyzed the time from the definitive surgery to the resumption of chemotherapy as a continuous variable (one-week increases) as well as a categorical variable (dichotomized at the fiftieth and seventy-fifth percentiles). We used the Cox proportional hazards regression model13 to assess the relationship of this interval with death and adverse events and calculated hazard ratios with 95% confidence intervals. Cox-model coefficients were based on their asymptotic distribution in the proportional hazards model. Patients were included in the analysis as long as they had begun the intended postoperative chemotherapy regimen.
Univariate regression analyses with use of multiplicative interaction terms between the surgery-chemotherapy interval and tumor necrosis were conducted to assess effect modification within categories of this variable. Patients who had tumor necrosis of >95% were considered to have had a good response to the preoperative chemotherapy whereas those with necrosis of ≤95% were considered to have had a poor response. In the multivariate model, we adjusted for the two well-recognized prognostic variables, tumor size and necrosis.
The median time to resumption of chemotherapy was compared between various categories of variables potentially associated with definitive surgery. These included the location of the tumor within the extremity (distal [to the knee or elbow] or proximal [to the knee or elbow]), tumor size (maximum tumor size in any dimension based on the imaging studies at diagnosis), type of surgical procedure (limb salvage or amputation, with rotationplasty classified as a limb salvage procedure because the complication rates associated with rotationplasty are more like those associated with limb salvage than those associated with amputation), and type of surgical margins (classified according to the musculoskeletal sarcoma grading system of Enneking et al.14).
Overall and event-free survival rates were estimated with use of the Kaplan-Meier method13. Event-free survival was defined as the time from the definitive surgery until an adverse event or the last patient contact. Adverse events were defined as disease progression (local or distant recurrence), a secondary malignant tumor, or death from any cause (i.e., disease, toxicity related to the chemotherapy, or an accident). Overall survival was defined as the time from the definitive surgery until death from any cause or the last patient contact.
Finally, we examined survival after recurrence segregated by a delay of more than 21 days compared with twenty-one days or less before the resumption of chemotherapy after the definitive surgery. To do this, we used Kaplan-Meier curves and compared censoring patterns for those who did and those who did not resume chemotherapy within twenty-one days after the surgery. To compare the censoring patterns, we used a chi-square goodness-of-fit test assessing the null hypothesis that the proportion censored with less than two years of follow-up (during the time when most recurrences are expected to occur) was the same for both groups. All tests were two-sided, and p values of <0.05 were considered significant.
Source of Funding
Limited funding for statistical support was provided by discretionary funds from the Mayo Clinic Department of Pediatric and Adolescent Medicine. The Children's Oncology Group (COG) trial INT-0133, from which the majority of the patients analyzed in this trial originated, was supported by COG Grant CA 98543. A complete listing of grant support for research conducted by COG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm.
Results
Seven hundred and three patients—556 patients from the COG study and 147 patients from the other five collaborating institutions—met the eligibility criteria. Table I shows patient demographics and tumor characteristics.
TABLE I.
Patient and Tumor Characteristics
| No. (%)
|
||
|---|---|---|
| Characteristics | Patients in COG Study | Patients Not in COG Study |
| Age at diagnosis | ||
| 0-11 yr | 177 (31.8) | 38 (25.9) |
| 12-14 yr | 183 (32.9) | 47 (32.0) |
| 15-30 yr | 196 (35.3) | 62 (42.2) |
| Sex | ||
| Male | 316 (56.8) | 87 (59.2) |
| Female | 240 (43.2) | 60 (40.8) |
| Race | ||
| White | 379 (68.2) | 111 (86.0) |
| Black | 75 (13.5) | 5 (3.9) |
| Other | 102 (18.3) | 13 (10.1) |
| Site of primary tumor | ||
| Femur | 316 (56.8) | 84 (57.1) |
| Tibia | 149 (26.8) | 31 (21.1) |
| Humerus | 66 (11.9) | 22 (15.0) |
| Fibula | 13 (2.3) | 9 (6.1) |
| Radius or ulna | 8 (1.4) | 1 (0.7) |
| Other | 4 (0.7) | 0 (0) |
| Primary tumor size* | ||
| <9 cm | 218 (39.2) | 39 (26.5) |
| ≥9 cm | 338 (60.8) | 108 (73.5) |
| Lactic dehydrogenase level at or above institutional limit | ||
| No | 359 (65.4) | 69 (84.1) |
| Yes | 190 (34.6) | 13 (15.9) |
| Alkaline phosphatase level at or above institutional limit | ||
| No | 330 (60.2) | 68 (76.4) |
| Yes | 218 (39.8) | 21 (23.6) |
| Type of surgery | ||
| Limb salvage | 385 (76.8) | 124 (84.4) |
| Amputation | 116 (23.2) | 23 (15.6) |
| Surgical margins† | ||
| Wide | 445 (87.4) | 108 (74.0) |
| Intralesional | 6 (1.2) | 1 (0.7) |
| Marginal | 16 (3.1) | 29 (19.9) |
| Radical | 42 (8.3) | 8 (5.5) |
| Tumor necrosis | ||
| ≤95% | 279 (52.8) | 86 (60.6) |
| >95% | 249 (47.2) | 56 (39.4) |
Maximum tumor size in any dimension based on the imaging studies at the time of diagnosis.
Classified according to the musculoskeletal sarcoma grading system of Enneking et al.14.
Survival
At the time of the last follow-up, 140 of the 703 patients had died after an average of three years (median, 2.7 years; range, 0.1 to 10.3 years) following the definitive surgery and 226 patients had experienced at least one adverse event at a mean of 1.8 years (median, 1.5 years; range, 0.04 to 7.1 years) following the definitive surgery. No patient died as a result of complications at the time of the definitive surgery. The overall survival and event-free survival rates at five years following the surgery were 78.8% and 66.1%, respectively.
Relationship of Time to Resumption of Chemotherapy After Definitive Surgery with Outcome
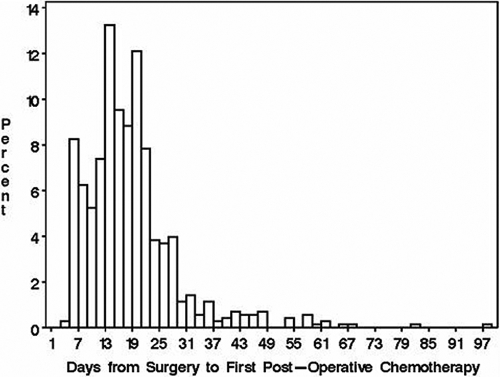
The time to resumption of chemotherapy ranged from three to ninety-seven days (twenty-fifth, fiftieth, and seventy-fifth percentiles, twelve, sixteen, and twenty-one days, respectively) (Fig. 1). Table II summarizes the median time and the range of times to resumption of chemotherapy analyzed according to characteristics related to the definitive surgery. The median times to resumption of chemotherapy differed significantly between the types of surgery (p = 0.005) and between the types of surgical margins (p = 0.01). Overall, 13.8% (seventy-one) of 515 COG-study patients had postoperative complications of hemorrhage, infection, hematoma, arterial thrombosis, and/or wound slough recorded on the data-capture forms. The median time to resumption of chemotherapy for the COG-study patients who had complications was fifteen days compared with eighteen days for those who did not have complications. The delay in the resumption of chemotherapy was more than twenty-one days for 20% (eighty-nine) of the 444 COG-study patients who had no surgical complications compared with 32% (twenty-three) of the seventy-one COG-study patients who had surgical complications.
Fig. 1.
Histogram of time to resumption of chemotherapy after surgery in days for all patients.
TABLE II.
Time to Resumption of Chemotherapy According to Characteristics Related to Definitive Surgery
| Characteristic* | Median Time (Range) to Resumption of Chemotherapy (days) | P Value |
|---|---|---|
| Location of the tumor† | 0.59 | |
| Distal extremity | 16 (3-67) | |
| Proximal extremity | 17 (5-97) | |
| Primary tumor size‡ | 0.54 | |
| <9 cm | 16 (5-82) | |
| ≥9 cm | 16 (3-97) | |
| Type of surgery | 0.005 | |
| Limb salvage | 17 (5-97) | |
| Amputation | 16 (3-61) | |
| Surgical margins§ | 0.01 | |
| Wide | 16 (3-97) | |
| Not wide | 19 (5-66) | |
| Tumor necrosis | 0.06 | |
| ≤95% | 16 (3-66) | |
| >95% | 17 (4-97) |
The n value for each characteristic is the total number of patients with available data for that particular characteristic.
Below-the-knee and below-the-elbow tumors were considered distal, and above-the-knee and above-the-elbow tumors were considered proximal.
Maximum tumor size in any dimension based on the imaging studies at the time of diagnosis.
Classified according to the musculoskeletal sarcoma grading system of Enneking et al.14.
Table III summarizes the results of the univariate analyses. All comparisons showed a nonsignificant increase in the risk of death and adverse events with an increase in the delay in the resumption of chemotherapy after definitive surgery. Table IV summarizes the results of the multivariate model with adjustment for tumor size and necrosis. The increase in the risk of death and adverse events with each one-week increase in the delay in the resumption of chemotherapy was not significant when the interval was analyzed as a continuous variable. However, when it was analyzed as a categorical variable, there was a significant increase in the risk of death of patients who had a delay of more than twenty-one days compared with those who resumed chemotherapy within twenty-one days after the definitive surgery (hazard ratio = 1.57 [95% confidence interval = 1.04 to 2.36]; p = 0.03). Even a delay of more than sixteen days, as compared with resumption of chemotherapy within sixteen days, was associated with a trend toward poorer overall survival (hazard ratio = 1.46 [95% confidence interval = 0.99 to 2.14]; p = 0.06). The corresponding hazard ratios for adverse events were increased with an increase in the delay, but not to the same extent as those for the risk of death. Patients with a delay of more than twenty-one days tended to have lower event-free survival rates than those who had resumed chemotherapy within twenty-one days after the definitive surgery (hazard ratio = 1.33 [95% confidence interval = 0.96 to 1.86]; p = 0.09), but this result was not significant.
TABLE III.
Univariate Analyses of the Relationship Between the Delay in Resumption of Chemotherapy After Definitive Surgery and the Risk of Death and Adverse Events
| Death
|
Adverse Events
|
|||
|---|---|---|---|---|
| Delay | Hazard Ratio (95% Confidence Interval) | P Value | Hazard Ratio (95% Confidence Interval) | P Value |
| 1-wk increase* | 1.06 (0.97-1.17) | 0.21 | 1.04 (0.96-1.13) | 0.30 |
| ≤16 days† | 1.0 (reference) | 0.28 | 1.0 (reference) | 0.41 |
| >16 days† | 1.20 (0.86-1.68) | 1.12 (0.86-1.45) | ||
| ≤21 days† | 1.0 (reference) | 0.11 | 1.0 (reference) | 0.20 |
| >21 days† | 1.34 (0.94-1.93) | 1.21 (0.91-1.62) | ||
Time to resumption of chemotherapy analyzed as a continuous variable (in weeks).
Time to resumption of chemotherapy analyzed as a binary variable dichotomized at either less than or equal to sixteen or less than or equal to twenty-one days.
TABLE IV.
Multivariate* Analyses of the Relationship Between the Delay in Resumption of Chemotherapy After Definitive Surgery and the Risk of Death and Adverse Events
| Death
|
Adverse Events
|
|||
|---|---|---|---|---|
| Delay | Hazard Ratio (95% Confidence Interval) | P Value | Hazard Ratio (95% Confidence Interval) | P Value |
| 1-wk increase† | 1.08 (0.97-1.21) | 0.16 | 1.05 (0.96-1.15) | 0.32 |
| ≤16 days‡ | 1.0 (reference) | 0.06 | 1.0 (reference) | 0.19 |
| >16 days‡ | 1.46 (0.99-2.14) | 1.22 (0.91-1.64) | ||
| ≤21 days‡ | 1.0 (reference) | 0.03 | 1.0 (reference) | 0.09 |
| >21 days‡ | 1.57 (1.04-2.36) | 1.33 (0.96-1.86) | ||
All models were adjusted for tumor size and necrosis. The model included 561 patients with complete data.
Time to resumption of chemotherapy analyzed as a continuous variable (in weeks).
Time to resumption of chemotherapy analyzed as a binary variable dichotomized at either less than or equal to sixteen or less than or equal to twenty-one days.
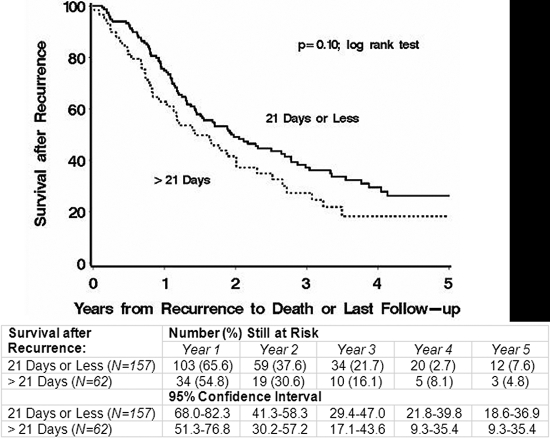
There is no clear explanation for the observation that the risk of adverse events was not increased to the same extent as the risk of death. We thought that there were two possible reasons for this discrepancy: (1) there was a difference in postrecurrence survival between patients who did and those who did not resume chemotherapy within twenty-one days after the definitive surgery or (2) there was a differential early censoring pattern among patients who did and those who did not resume chemotherapy within twenty-one days after the definitive surgery. To investigate the first possibility, we used Kaplan-Meier analysis to examine survival after recurrence segregated by a delay of more than 21 days compared with twenty-one days or less, but we found no significant difference between the two groups (p = 0.10) (Fig. 2). To investigate the second possibility, we used a chi-square test to examine the proportion of patients censored during the first two years following the surgery (the time when most recurrences are expected to occur); again, we found no significant difference between the two groups.
Fig. 2.
Kaplan-Meier curves comparing the survival after recurrence according to whether the delay in the resumption of chemotherapy after the definitive surgery was greater than twenty-one days as opposed to twenty-one days or less.
Assessment of Effect Modification
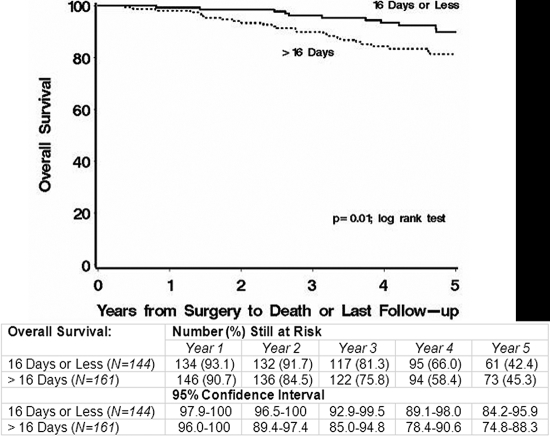
Table V summarizes the results of the regression analyses assessing effect modification. When the time to resumption of chemotherapy was analyzed as a categorical variable in the group with a good response to the preoperative chemotherapy, the hazard ratio for death was found to be 2.45 (95% confidence interval = 1.26 to 4.76; p = 0.01) for patients who had a delay of more than sixteen days compared with those who resumed chemotherapy within sixteen days after the definitive surgery. In the group with a poor response to the preoperative chemotherapy, the corresponding hazard ratio was 0.93 (95% confidence interval = 0.61 to 1.42; p = 0.75, p for interaction = 0.02). With a similar delay, there was an increased risk of adverse events among patients with a good response; however, the hazard ratio was not significantly different from that of patients with a poor response. Figure 3 shows a comparison of the Kaplan-Meier curves for patients with a good response and a delay either of more than sixteen days or of sixteen days or less. No other time interval of delay showed a significant or clinically relevant interaction with the extent of tumor necrosis.
TABLE V.
Analyses of the Relationship Between the Delay in Resumption of Chemotherapy After Definitive Surgery and the Risk of Death and Adverse Events Stratified by Grades of Tumor Necrosis
| Delay
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Each 1-Wk Increase*
|
≤16 Days vs. >16 Days†
|
≤21 Days vs. >21 Days†
|
||||||||||
| Hazard Ratio | 95% Confidence Interval | P Value | P Value for Interaction | Hazard Ratio | 95% Confidence Interval | P Value | P Value for Interaction | Hazard Ratio | 95% Confidence Interval | P Value | P Value for Interaction | |
| Death | ||||||||||||
| Good response to preop. chemotherapy (>95% tumor necrosis) | 1.10 | 0.97-1.26 | 0.15 | 2.45 | 1.26-4.76 | 0.01 | 1.72 | 0.95-3.13 | 0.07 | |||
| Poor response to preop. chemotherapy (≤95% tumor necrosis) | 1.08 | 0.92-1.26 | 0.34 | 0.80 | 0.93 | 0.61-1.42 | 0.75 | 0.02 | 1.38 | 0.86-2.23 | 0.18 | 0.55 |
| Adverse events | ||||||||||||
| Good response to preop. chemotherapy (>95% tumor necrosis) | 1.03 | 0.91-1.17 | 0.61 | 1.47 | 0.92-2.35 | 0.11 | 1.23 | 0.76-1.99 | 0.41 | |||
| Poor response to preop. chemotherapy (≤95% tumor necrosis) | 1.09 | 0.96-1.24 | 0.17 | 0.53 | 1.00 | 0.71-1.39 | 0.98 | 0.18 | 1.36 | 0.93-1.99 | 0.12 | 0.74 |
Time to resumption of chemotherapy analyzed as a continuous variable (in weeks).
Time to resumption of chemotherapy analyzed as a binary variable dichotomized at either less than or equal to sixteen or less than or equal to twenty-one days.
Fig. 3.
Kaplan-Meier curves comparing overall survival according to whether the delay in the resumption of chemotherapy after the definitive surgery was greater than sixteen days as opposed to sixteen days or less in the group of patients with a good response to the preoperative therapy.
Discussion
Delays in chemotherapy reduce the overall dose intensity. Although recently some investigators did not find a clear survival benefit from increasing the received dose or dose intensity15-17, the results of dose-intensity analyses performed by other investigators support the hypothesis that the actual dose intensity delivered determines the outcome of treatment of osteosarcoma8,9. However, it is important to realize that there have been no prospective dose-intensity analyses of patients with osteosarcoma. Furthermore, the various methods used to calculate dose intensity make comparisons difficult.
In this large cohort of patients with osteosarcoma treated with contemporary chemotherapy, we found that a delay of more than twenty-one days in the resumption of chemotherapy after definitive surgery was associated with a decrease in overall survival. After controlling for the two prognostic factors that are most predictive of survival of patients with osteosarcoma, tumor necrosis and size, we found a 57% increase in the risk of death when resumption of chemotherapy was delayed for more than twenty-one days compared with when it was resumed within twenty-one days after the definitive surgery.
In addition, we analyzed the time to resumption of chemotherapy according to various characteristics related to surgery (Table II). We found the median time to resumption of chemotherapy to differ significantly between the types of surgery and the types of surgical margins; however, the difference was not clinically meaningful (one day and three days, respectively). The observation that 32% of the patients with surgical complications had a delay in the resumption of chemotherapy of more than twenty-one days was not unexpected, but perhaps what it is most surprising and troubling is that 20% of the patients with no reported complications still had a delay of more than twenty-one days. We also found that the risk of adverse events was not increased to the same extent as the risk of death in the group with delayed resumption of chemotherapy after definitive surgery. We attempted to explore this discrepancy further, but we could not find a clear explanation for it.
In a previously published study from the Memorial Sloan-Kettering Cancer Center10, investigators found that a delay in resumption of chemotherapy of more than twenty-four days resulted in poorer disease-free survival only when the patients had had a poor histological response to preoperative chemotherapy, but the difference was not significant (p = 0.15). Delay apparently did not have an effect on the outcome for patients who had had a good response to preoperative chemotherapy in that study. In our study, however, patients with a good response to the preoperative chemotherapy fared worse when the postoperative chemotherapy was delayed for more than sixteen days after the definitive surgery whereas the impact of delayed resumption of chemotherapy on the patients with a poor response was not significant. Not only is our study larger but the chemotherapy combinations received by our study participants were different from the regimens utilized in the study at the Memorial Sloan-Kettering Cancer Center. These differences make a direct comparison of the two studies difficult but could account for the different observations.
There were limitations to our study, including the fact that it was a retrospective review. Most importantly, the study cohort was a combination of patients treated in a randomized, national multi-institutional trial (n = 556) and patients who were not part of that study but were treated according to the same protocol or with relatively similar contemporary chemotherapy regimens at a limited number of institutions (n = 147). In addition to the inherent heterogeneity of these chemotherapy regimens (although they were all considered contemporary), the proportions of missing data for certain variables were significantly different between the COG-study and non-COG-study patients, as already described. Therefore, we elected not to adjust for those variables in the multivariate model, and it is possible that one of them might be related to both the delay in the resumption of chemotherapy and the underlying risk of death or recurrence. The choice to assess two time delays (sixteen and twenty-one days) and to assess interactions was exploratory in nature, and we hope that future studies will repeat these analyses to verify the results. Finally, we did not have complete information regarding whether all patients received all of the planned chemotherapy cycles as specified by the protocol or if there were other delays between chemotherapy courses including a delay in the performance of definitive surgery, either of which may have negatively impacted survival. However, our approach was consistent with the primary analysis of the largest cohort of patients in this study (the COG cohort), in which treatment assignment was used to perform the outcome analysis irrespective of whether patients completed the planned chemotherapy.
Lastly, it goes without saying that when it comes to resumption of chemotherapy after surgery, oncologists who favor minimal interruptions between chemotherapy cycles almost always defer the decision to the surgeon because of the presumed risks of delayed wound-healing and wound infection. The fact that 20% of the patients with no record of complications still had a delay in the resumption of chemotherapy beyond twenty-one days suggests several possibilities. The first is that complications were not being reported accurately, but this is unlikely given that the data-capture forms for the COG-study patients asked a direct yes-or-no question about surgical complications. The second possibility is that there are logistical issues or issues regarding communication among the surgeon, patient, pediatric oncologist, and individuals responsible for scheduling that interfere with the patient returning for chemotherapy in a timely manner. The third possibility is that patients have “hospital fatigue” and do not want to return to the hospital so soon after surgery.
In conclusion, the results of our multicenter study suggest that a delay of more than twenty-one days in the resumption of chemotherapy after definitive surgery is associated with an increased risk of death of patients with localized osteosarcoma in an extremity, although no association with event-free survival was found. Thirty-two percent of the COG-study patients with surgical complications had such a delay, which was presumed to be due to the surgical complications; however, there was no apparent reason why 20% of the patients without complications had a similar delay. To address these two separate issues, we suggest that surgeons carefully weigh the risks of surgical complications from limb salvage procedures, which may result in a delay in resumption of chemotherapy and a decrease in survival, and that surgeons and pediatric oncologists work very closely together to ensure that the patient returns to the pediatric oncology clinic in a timely fashion after the surgery to resume chemotherapy. If resistance from the patient is encountered, an explanation of the importance of dose intensity should be offered even before the surgical procedure. We believe that the results of this study are important and should be evaluated prospectively in future studies. 
Acknowledgments
Note: The authors thank Christine Lohse, MS (Mayo Clinic, Rochester, Minnesota), for statistical assistance; Casey Hooke, RN (Children's Health Care, Minneapolis, Minnesota), for assistance in obtaining information about the patients; Shantae Ockimey, Research Associate (Children's Hospital of Philadelphia, Pennsylvania), for extracting and compiling the data; and the Children's Oncology Group for their cooperation.
Presented, in part, at the Annual Meeting of the Musculoskeletal Tumor Society, St. Louis, Missouri, May 11, 2007.
Disclosure: The authors did not receive any outside funding or grants in support of their research for or preparation of this work. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
References
- 1.Gurney JG, Swensen AR, Bulterys M. Malignant bone tumors. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. Cancer incidence and survival among children and adolescents: United States SEER program 1975-1995. NIH pub. no. 99-4649. Bethesda, MD: National Cancer Institute; 1999. p 99-110.
- 2.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2002;20:776-90. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, Müller C, Tienghi A, Wiebe T, Comandone A, Böhling T, Del Prever AB, Brosjö O, Bacci G, Saeter G; Italian and Scandinavian Sarcoma Groups. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845-52. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Ferrari S, Longhi A, Picci P, Mercuri M, Alvegard TA, Saeter G, Donati D, Manfrini M, Lari S, Briccoli A, Forni C; Italian Sarcoma Group/Scandinavian Sarcoma Group. High dose ifosfamide in combination with high dose methotrexate, Adriamycin and cisplatin in the neoadjuvant treatment of extremity osteosarcoma: preliminary results of an Italian Sarcoma Group/Scandinavian Sarcoma Group pilot study. J Chemother. 2002;14:198-206. [DOI] [PubMed] [Google Scholar]
- 5.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, Harris MB, Healey J, Huvos A, Link M, Montebello J, Nadel H, Nieder M, Sato J, Siegal G, Weiner M, Wells R, Wold L, Womer R, Grier H. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004-11. [DOI] [PubMed] [Google Scholar]
- 6.Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41:2836-45. [DOI] [PubMed] [Google Scholar]
- 7.Delepine N, Delepine G, Bacci G, Rosen G, Desbois JC. Influence of methotrexate dose intensity on outcome of patients with high grade osteogenic osteosarcoma. Analysis of the literature. Cancer. 1996;78:2127-35. [PubMed] [Google Scholar]
- 8.Smith MA, Ungerleider RS, Horowitz ME, Simon R. Influence of doxorubicin dose intensity on response and outcome for patients with osteogenic sarcoma and Ewing's sarcoma. J Natl Cancer Inst. 1991;83:1460-70. [DOI] [PubMed] [Google Scholar]
- 9.Bacci G, Picci P, Avella M, Dallari D, Ferrari S, Prasad R, Di Scioscio M, Malaguti C, Caldora P. The importance of dose-intensity in neoadjuvant chemotherapy of osteosarcoma: a retrospective analysis of high-dose methotrexate, cisplatinum and Adriamycin used preoperatively. J Chemother. 1990;2:127-35. [DOI] [PubMed] [Google Scholar]
- 10.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10:5-15. [DOI] [PubMed] [Google Scholar]
- 11.Arndt C, Miser J, Pritchard D, Sim F, Rock M, Shives T, Neglia J, Bostrom B, Nachman J, Gaynon P, Sato J, Ettinger L, Hutchinson R, Wold L. Treatment of high-grade osteosarcoma (OGS) with ifosfamide (IFOS), mesna (MES), Adriamycin (ADR), high-dose methotrexate (HDMTX), and cisplatin (CDDP) [abstract]. Med Pediatr Oncol. 1996;27:227a. [Google Scholar]
- 12.Miser J, Arndt C, Smithson W, Gilchrist G, Edmonson J, Sim F, Rock M, Pritchard D, Shives T, Wold L, Schaid D, Woods W, Neglia J, Thompson R, Nachman J, Gaynon P, Wiersma S, Hutchinson R, Sato J, Bostrom B, Thatcher G, Nickerson J, Ettinger L, Pendergrass T, Mason J, Swenson B, Conrad E. Treatment of high-grade osteosarcoma (OGS) with ifosfamide (IFOS), mesna (MES), Adriamycin (ADR), high-dose methotrexate (HDMTX), with or without cisplatin (CDDP); results of two pilot trials [abstract]. Proc Am Soc Clin Oncol. 1994;13:A1442. [Google Scholar]
- 13.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000.
- 14.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. 1980. Clin Orthop Relat Res. 2003;415:4-18. [DOI] [PubMed] [Google Scholar]
- 15.Eselgrim M, Grunert H, Kühne T, Zoubek A, Kevric M, Bürger H, Jürgens H, Mayer-Steinacker R, Gosheger G, Bielack SS. Dose intensity of chemotherapy for osteosarcoma and outcome in the Cooperative Osteosarcoma Study Group (COSS) trials. Pediatr Blood Cancer. 2006;47:42-50. [DOI] [PubMed] [Google Scholar]
- 16.Lewis IJ, Weeden S, Machin D, Stark D, Craft AW. Received dose and dose-intensity of chemotherapy and outcome in nonmetastatic extremity osteosarcoma. European Osteosarcoma Intergroup. J Clin Oncol. 2000;18:4028-37. [DOI] [PubMed] [Google Scholar]
- 17.Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van Glabbeke M, Kirkpatrick A, Hauben EI, Craft AW, Taminiau AH; MRC BO06 and EORTC 80931 collaborators; European Osteosarcoma Intergroup. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:112-28. [DOI] [PubMed] [Google Scholar]