Abstract
Two different attentional networks have been associated with visuospatial attention and conflict resolution. In most situations either one of the two networks is active or both are increased in activity together. By using functional magnetic resonance imaging and a flanker task, we show conditions in which one network (anterior attention system) is increased in activity whereas the other (visuospatial attention system) is reduced, showing that attentional conflict and selection are separate aspects of attention. Further, we distinguish between neural systems involved in different forms of conflict. Specifically, we dissociate patterns of activity in the basal ganglia and insula cortex during simple violations in expectancies (i.e., sudden changes in the frequency of an event) from patterns of activity in the anterior attention system specifically correlated with response conflict as evidenced by longer response latencies and more errors. These data provide a systems-level approach in understanding integrated attentional networks.
Imaging studies of attention have systematically activated two quite different neural networks. When subjects orient to locations in the visual field, either covertly or by moving their eyes, cortical areas including superior parietal and superior frontal regions systematically are shown to be active (1, 2). Thus, this network involving the parietal cortex has been linked to control of spatial attention (1–4). However, when subjects have to respond to one aspect of a stimulus and ignore competing aspects, as in the Stroop effect, a different set of brain areas including the anterior cingulate cortex and the dorsolateral prefrontal cortex are active (5–8). This latter system involving prefrontal regions presumably plays a top-down role in biasing the system toward behaviorally relevant information over competing information (9–12). Most conditions that manipulate task difficulty either activate only one of the two networks or increase activity in both networks at the same time, thus providing little evidence of dissociation between the networks. However, our study shows there are conditions that can produce increased activity in one network and reduced activity in the other.
At a superficial level, these systems have been described as being situated in anterior and posterior regions of the brain (13, 14). According to Fuster (15), the areas posterior to the central sulcus in the neocortex of the primate are primarily involved in the representation and processing of sensation, whereas those areas anterior to the central sulcus, the frontal areas, are primarily devoted to the representation and processing of action. Consistent with this view is a model of attention developed by Posner and Petersen (14) that incorporates both an anterior and posterior attentional system. The posterior system has been implicated in the deployment of attention to a relevant spatial location (1, 16). This system involves regions of the parietal cortex as well as subcortical structures of the pulvinar and superior colliculi (14, 16) and more recently has been suggested to involve superior frontal regions (e.g., frontal eye fields) (1). This “posterior” or visuospatial attention system is involved in orienting of attention to a target location. Presumably, processing of the target is enhanced by giving priority to it by attending to its location. Hence, attention can function to guide the eyes to an appropriate area of the visual field (17, 18). Single-cell recording studies have shown a greater discharge rate in neurons in the parietal cortex when a monkey attends to the location of a stimulus (19, 20). Similar effects have been show in humans by using positron emission tomography (PET) (21, 22) and with functional magnetic resonance imaging (fMRI) (23, 24).
A second more anterior attention system involves regions of the prefrontal cortex including the anterior cingulate cortex and dorsolateral prefrontal cortex. These regions have been associated with the deployment of attentional control in overriding rare, highly salient, and often well learned responses, perhaps best typified by performance on Strooplike tasks (5–8). The anterior attention system is thought to involve top-down processing or biasing of the system in favor of objects or locations with high behavioral relevance (9–12). This description is analogous to a “central executive” that is responsible for coordinating attentional responses by activating compatible responses and inhibiting incompatible systems or responses (25–27), including the visuospatial attention system (14).
Although there is some agreement as to the general nature of these previously described systems, details regarding the unique contributions of regions within these subsystems in attentional control have not been resolved. For example, the visuospatial attention system appears to involve anterior regions as well as posterior regions (e.g., supplementary and frontal eye fields). It has been demonstrated that lesions to frontal regions can give rise to deficits in the deployment of attention by this posterior system (28), and Corbetta and colleagues (1) have shown superior frontal and parietal activity during visual attention paradigms by using positron emission tomography. Further, the unique contributions of regions within these systems have not been characterized fully. For example, the anterior cingulate cortex has been proposed to have a number of functions, including error monitoring (29), executive attention (14), and, more recently, conflict monitoring (30, 31). Likewise, the posterior parietal cortex has been hypothesized to have a number of functions including both orienting and target detection that has only recently been dissociated (24). In general, these attentional systems typically have been examined in isolation of one another, although presumably they reflect one distributed attentional network (32).
The current study examines these attentional systems within a single task design using the classic flanker paradigm (33) with fMRI. Compatible and incompatible flankers are presented on either side of a target stimulus (e.g., >>> > >>> or <<< > <<<) and subjects press the left or right key that corresponds to the direction of the center arrow. This task provides an excellent means for examining the previously described attentional subsystems. By simply varying the congruency of flanker stimuli, we can examine attentional control during transient or persistent interference from peripheral stimuli (i.e., conflict resolution vs. selective attention). On incompatible trials, the combined influence of the target and flankers lead to conflict in the form of competition between correct and incorrect responses as evidenced behaviorally by longer reaction times (33–35). With consecutive incompatible trials, attention can be directed to the target and not to the flankers, resulting in increased selected attention or, as Allport (36) describes, “selection for action.” Selective attention suggests that the attentional system can selectively designate a subset of relevant sensory information. Gratton et al. (37) have shown that the balance between conflict from flankers and their suppression with selective attention is determined by the compatibility of the flankers with the target in preceding trials. If the preceding trial has compatible flankers, then the current trial of incompatible flankers results in increased conflict; if the preceding trial has incompatible flankers, then selective attention is increased. Accordingly, conflict and selective attention can be manipulated by varying the predictive value of preceding flankers such that 70% of the trials are compatible and 30% are incompatible and vice versa, similar to the cost-benefit analysis used by Posner and Snyder (38) to examine facilitation and inhibition with attentional control. Paquet and Craig (39) have demonstrated this phenomenon behaviorally by using the flanker paradigm and, more recently, this phenomenon has been related to the function of the anterior cingulate cortex in an event-related fMRI study (31). The current study in this paper examines this phenomenon by using whole-brain echo-planar imaging.
Methods
Eight right-handed young adults (4 female and 4 male) between the ages of 20 and 36 years were scanned during performance of a flanker task. All subjects were screened for any contraindication for an MRI, and their consent was acquired before the procedure. Subjects were presented with arrows that pointed to the left (<) or right (>) displayed in the center of a rear projection screen placed in the bore of the magnet. Compatible and incompatible flankers were presented on either side of the target stimulus (e.g., >>> > >>> or <<< > <<<). Subjects were instructed to press the left key if the center stimulus was pointing left (<) and the right key if the center stimulus was pointing right (>). The predictive value of the flankers was manipulated by block with valid blocks consisting of 70% compatible and 30% incompatible flankers (i.e., valid condition) and invalid blocks consisting of 30% compatible and 70% incompatible flankers (i.e., invalid condition). This manipulation of flanker validity is similar to the cost benefit analysis described by Posner and Snyder (38). The stimulus duration was 1,000 msec with a 500-msec interstimulus interval. Blocks consisted of 40 randomly presented trials of either the valid or invalid condition and were presented in an ABBA order (6) during each of four runs where A was a valid block and B was an invalid block. There were a total of 320 behavioral trials per condition.
All subjects were acclimated to the scanner environment in a simulated scanner that looked and sounded like the actual scanner and then positioned in a 1.5-T General Electric scanner. A sagittal localizer of 15 images was acquired (multiecho multiphase: time of repetition (TR) 400; time to echo (TE) 12; 5-mm skip 1). A T1-weighted volumetric scan using a spin-echo sequence (spoiled-gradient sequence: TR 500; TE 12; 5-mm skip 0; 124 images) was acquired in the coronal plane. Twenty-six coronal T2*-weighted blood oxygenation level-dependent (BOLD) images were acquired with echo-planar imaging (gradient echo sequence: TR 6,000; TE 40; flip 90; 40 repetitions). Four runs of 40 repetitions were acquired across the experiment.
Image data were corrected for head motion with 3-dimensional automated image registration (40) and then registered to a reference brain. One subject (a male) had more than 0.5 voxels of movement in the z plane and was eliminated from further analysis. To examine the effect of the flanker congruency manipulation, a 7 × 2 (subjects × conditions) analysis of variance with contiguity threshold of 5 contiguous pixels and F ≥ 8.00 was performed on the subjects' pooled data. There were 80 data points per condition per subject. The resulting F-maps were aligned to the anatomical images and then registered in stereotactic space using afni software (41).
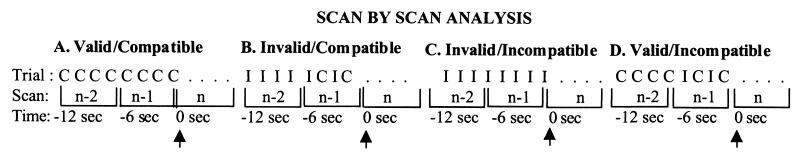
We hypothesized that incompatible trials preceded by consecutive compatible trials would result in a different pattern of results than would incompatible trials preceded by consecutive incompatible trials. Although this study did not incorporate an event-related design a priori (42), a post hoc scan by scan analysis was performed on brain regions identified as having significant magnetic resonance (MR) signal change with the omnibus ANOVA. Scans for correct trials only were analyzed to determine whether we could approximate the temporal resolution of such a design. Each 6-sec scan consisted of four 1.5-sec behavioral trials, so the MR signal intensity reflects the signal associated with four behavioral trials as illustrated in Fig. 1. The behavioral trials consisted of (i) all compatible trials in the valid condition, (ii) two compatible trials in the invalid condition, (iii) all incompatible trials in the invalid condition, or (iv) two incompatible trials in the valid condition. We focused on scans consisting only of two compatible trials in the invalid condition and two incompatible trials in the valid condition because of the low frequency of these trial types in these respective conditions. However, these scans are identical to one another in every way except for the scan that precedes them (i.e., preceding context), which consists of all compatible or all incompatible trials—the manipulation of interest. A total of 74 data points (scans) were analyzed for the four trial types (valid condition/compatible trials, invalid condition/compatible trials, invalid condition/incompatible trials, and valid condition/incompatible trials) that are illustrated in Fig. 1.
Figure 1.
Post hoc scan-by-scan analyses were performed on brain regions identified as having significant MR signal change with the omnibus ANOVA for correct trials. Each 6-sec scan consisted of four behavioral trials, so the four trials were collapsed and averaged. In order for the MR signal to stabilize within an experimental condition (valid vs. invalid condition), scans containing the trial type of interest [compatible (C) or incompatible (I) trials] were preceded by at least one scan of all four compatible trials (valid condition) or all four incompatible trials (invalid condition). Thus the scans of interest consisted of four compatible trials preceded by four compatible trials (A); two compatible trials preceded by four incompatible trials (B); four incompatible trials preceded by four incompatible trials (C); and two incompatible trials preceded by four compatible trials (D). Scans that were analyzed (indicated by the arrows) consisted of those occurring 6 sec after the scan of interest to adjust for the typical 5- to 6-sec delay in peak of the hemodynamic response.
Results
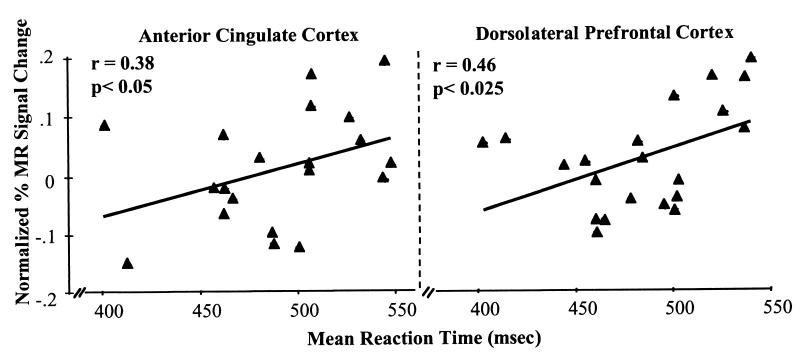
Overall, behavioral performance during the flanker task was worse for incompatible trials as compared with compatible trials (P < 0.05). Mean reaction times were slower and mean accuracies were lower for the incompatible trials (508 msec and 94% correct) as compared with compatible trials (464 msec and 98% correct). As predicted, performance was worse for incompatible trials in the valid condition (514 msec and 91% correct) than that during the invalid condition (505 msec and 96% correct). Activity in regions of the anterior cingulate cortex and dorsolateral prefrontal cortex correlated with this pattern of behavioral performance such that activity (percentage change in MR signal intensity) increased in these areas as a function of increased mean reaction time (refer to Fig. 2).¶
Figure 2.
Percentage change in normalized MR signal intensity for the anterior cingulate cortex and dorsolateral prefrontal cortex and plotted as a function of mean reaction times for each subject on correct compatible and incompatible trials for the valid and invalid conditions.
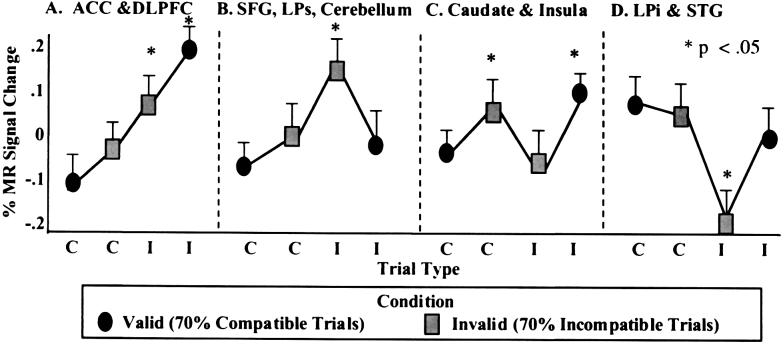
Our imaging results showed four distinct patterns of change in MR signal intensity as a function of trial type (compatibility of the flankers) and condition type (valid vs. invalid) for specific brain regions. The normalized percentage change in MR signal intensity for these regions is plotted in Fig. 3 as a function of trial type (compatible or incompatible) and condition (valid and invalid) and the location of activation is depicted in Fig. 4 and Table 1. Post hoc Student's t tests were performed to determine differences in signal change for each of the four trial types for the four resulting patterns of data. The first pattern was observed in the anterior cingulate cortex and dorsolateral prefrontal cortex. Activity in these regions increased as a function of interference or conflict from the flanker stimuli such that the greatest signal change was observed for incompatible trials (P < 0.05), particularly when they followed consecutive compatible trials (valid condition). A second pattern of results involved the right superior frontal gyrus, superior parietal lobule, and portions of the right cerebellum. These regions increased in activity during incompatible trials after consecutive incompatible trials (invalid condition), and the extent of MR signal change was significantly different from the other three trial types. A third pattern involving the basal ganglia and left insula showed specificity to violations of expectancy (i.e., sudden changes in the expected trial type) such that increases occurred in these regions to either compatible trials after consecutive incompatible trials or incompatible trials after consecutive compatible trials. Finally, inferior parietal and superior temporal regions showed an inverse relation in activity to that observed in the superior parietal and frontal regions. Activity in these regions decreased during incompatible trials, particularly when following consecutive incompatible trials (invalid condition), which was significantly different from all other trial types.
Figure 3.
Depiction of the four patterns of percentage change in normalized MR signal intensity as a function of compatible (C) and incompatible (I) trial type within the valid (70% compatible trials) and invalid (70% incompatible trials) conditions for correct trials across subjects. (A) The first pattern was observed in the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC). Activity in these regions increased as a function of increasing interference from the flanker stimuli and were evidenced by increasing response latencies. (B) A second pattern of results involved the right superior frontal gyrus (SFG), superior parietal lobule (LPs) and portions of the right cerebellum. These regions increased in activity during incompatible trials after consecutive incompatible trials (invalid condition). (C) A third pattern involving the basal ganglia and left insula showed increases in activity to compatible trials after consecutive incompatible trials or incompatible trials embedded in mostly compatible trials. (D) Finally, an inferior parietal (LPi) region and the superior temporal gyrus (STG) showed an inverse relation in activity to that observed in LPs and SFG. Activity in this region decreased during incompatible trials, particularly when embedded among incompatible trials (invalid condition).
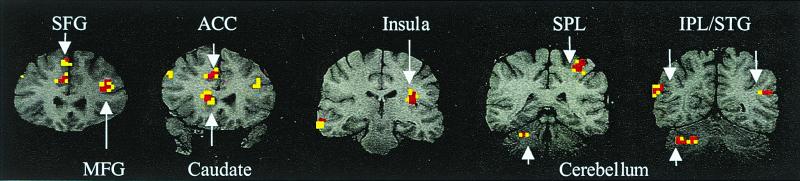
Figure 4.
Location of brain activity by gyrus and Brodmann's areas in regions demonstrating the four different patterns of percentage change in MR signal intensity depicted in Fig. 3 and illustrated here in the coronal plane.
Table 1.
Anatomical location of maximum F ratios and volume of activations for brain regions with significant change in MR signal intensity during performance of the flanker task
| Region of interest | BA | X | Y | Z | Max. F | Size in voxels |
|---|---|---|---|---|---|---|
| Superior frontal gyrus | 8 | −5 | 18 | 57 | 11.50 | 5 |
| Middle frontal gyrus | 46 | 31 | 18 | 23 | 19.73 | 16 |
| Anterior cingulate gyrus | 32 | −8 | 22 | 32 | 22.19 | 14 |
| Caudate nucleus | −15 | 16 | 10 | 23.02 | 10 | |
| Insular cortex | 34 | −20 | 14 | 29.95 | 17 | |
| Superior parietal lobule | 7 | 33 | −43 | 49 | 29.95 | 27 |
| Inferior parietal lobule | 40 | −54 | −53 | 23 | 34.52 | 19 |
| Superior temporal gyrus* | 41/42 | 50 | −47 | 21 | 53.43 | 9 |
| Cerebellum | −30 | −62 | −25 | 29.95 | 10 |
X, right to left; Y, anterior to posterior; Z, superior to inferior.
Region of interest extended into supramarginal gyrus.
Discussion
This study examined independent contributions of neural systems of a distributed attentional network by using a flanker task with fMRI. By manipulating the predictive validity of the flankers, we were able to dissociate attentional subsystems both behaviorally and neuroanatomically. Consistent with Gratton et al. (37) and others (31), greater interference was demonstrated by longer reaction times and lower accuracy when flanker stimuli were incompatible with the target stimulus. This interference increased if the incompatible trial followed consecutive compatible trials as in the valid condition.
Activity in two brain regions, the anterior cingulate cortex and dorsolateral prefrontal cortex, was correlated with behavioral performance. Therefore it may be assumed that these brain regions play an important role in detecting or resolving conflict. Recently, Botvinick et al. (31) showed the involvement of the anterior cingulate cortex in conflict during performance of a similar flanker task. That study did not address the role of prefrontal cortex in that task or its role in conflict. Whether activity in the anterior cingulate cortex and dorsolateral prefrontal cortex is associated with detecting conflict or resolving the conflict cannot be determined in our current study. Duncan and Owen (43), in fact, suggest that these regions may serve quite similar roles in cognition given their coactivation in a number of neuroimaging studies. However, in a previous developmental fMRI study involving conflict from prepotent response tendencies (44), we observed a dissociation in function of prefrontal and anterior cingulate regions.‖ In that study, we showed that greater activity in the anterior cingulate cortex correlated with more errors, whereas greater activity in ventral prefrontal cortex correlated with better performance (i.e., fewer errors). These findings suggest that the anterior cingulate cortex may index or monitor conflict, but that the prefrontal cortex may be more involved in resolving the conflict perhaps by maintaining the relevant task information. Such dissociation may not be possible in the current study, given the lack of variance in behavioral performance (91% or more correct) as compared with the previous study (73% or more correct).
Overall, four distinct neural subsystems seemed to be differentially involved in the performance of the flanker task, including the previously described “anterior system” that involves the anterior cingulate cortex and dorsolateral prefrontal cortex. Activity in these regions increased as a function of interference or conflict from the flanker stimuli such that the greatest signal change was observed for incompatible trials, particularly when following consecutive compatible trials (valid condition). These results are consistent with the notion of an anterior attention system being involved in the deployment of attentional control in overriding, rare, highly salient events with high behavioral importance analogous to a central executive (9–12, 25–27). Accordingly, this system appears responsible for coordinating attentional responses by activating compatible responses and inhibiting incompatible systems or responses.
A second system that may be analogous to the visuospatial attention system was involved in selective attention. This system was activated during persistent interference from peripheral information as in the case of an incompatible trial after consecutive incompatible trials. The brain regions involved in this neural system were the right superior frontal gyrus [Brodmann's area (BA) 8], superior parietal cortex (BA 7), and portions of the right cerebellum. These regions fit with the role of the visuospatial attention system in orienting of attention to the relevant target location and guiding the eye to an appropriate area of the visual field.
Another system including the caudate nucleus and insula was active when the congruency of the trial contradicted the preceding trials in that block. When the probability of a specific flanker type was high (whether compatible or incompatible), a trial of the opposite type activated these regions, suggesting sensitivity of these regions to violations in expectancy or sudden changes in the frequency of an event. These findings are consistent with reports of basal ganglia- and insula-related activity with changes in the probability of events or sequences as shown in previous neuroimaging studies of serial reaction time on implicit learning tasks (45–48).
Finally, an inferior parietal region (BA 40) and also portions of auditory cortex in BA 41/42 showed significant decreases in activity during incompatible trials. This effect is the inverse of that observed in the superior parietal and frontal regions. The inverse relation between neural subsystems may reflect competing attentional or perceptual processes and their subsequent suppression as suggested by Haxby et al. (49) and Shulman et al. (50). Competing processes may explain these results in that the superior parietal region may be more involved in narrowing of attentional focus, whereas the inferior parietal region may be more involved in broadening of attention beyond the fovea to include the periphery. Clearly such processes are incompatible and cannot cooccur. Thus, there is suppression of one system over the other. Whether the superior parietal region is the source of this suppression or some other brain system is responsible for this suppression cannot be determined in the current study.
In sum, these results provide an important dissociation of systems involved in conflict, expectancy, and attentional selection within a single task design. Conflict associated with overriding, highly salient events, as in the case of incompatible trials after compatible trials, activated the anterior cingulate and dorsolateral prefrontal cortex, whereas conflict caused by simple violations in expectations activated the regions of the basal ganglia and insula. Furthermore, brain regions (dorsolateral prefrontal cortex and anterior cingulate cortex) involved in conflict detection or control were dissociated from brain regions (superior frontal gyrus and superior parietal cortex) involved in selective attention. This latter finding is consistent with that of Botvinick et al. (31), suggesting that conflict and selective attention involve distinct neural systems. Finally, our results suggest the importance of examining behavioral phenomena at a systems level in parallel with studies focusing on single subsystems or even single brain regions with functional neuroimaging.
Acknowledgments
This work was supported in part by a 5-K01 MH01297–03 award to B.J.C.
Abbreviations
- fMRI
functional magnetic resonance imaging
- MR
magnetic resonance
- BA
Brodmann's area
Footnotes
Behavioral data from two of the subjects were lost because of technical software problems. Therefore, the correlations in Fig. 2 are provided for those subjects with behavioral data.
References
- 1.Corbetta M, Miezin F M, Shulman G L, Petersen S E. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nobre A C, Sebestyen G N, Gitelman D R, Mesulam M M, Frackowiak R S, Frith C D. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- 3.Bushnell M C, Goldberg M E, Robinson D L. J Neurophysiol. 1981;46:755–771. doi: 10.1152/jn.1981.46.4.755. [DOI] [PubMed] [Google Scholar]
- 4.Coull J T, Nobre A C. J Neurosci. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardo J V, Pardo P, Janer K W, Raichle M E. Proc Natl Acad Sci USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bench C J, Frith C D, Grasby P M, Friston K J, Paulesu E, Frackowiak R S, Dolan R J. Neuropsychologia. 1993;35:1373–1380. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- 7.George M S, Ketter T A, Parekh P I, Rosinsky N, Ring H, Casey B J, Trimble M R, Horwitz B, Herscovitch P. Hum Brain Mapp. 1994;1:194–209. doi: 10.1002/hbm.460010305. [DOI] [PubMed] [Google Scholar]
- 8.Carter C S, Mintun M, Cohen J D. Neuroimage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- 9.Desimone R, Duncan J. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J D, Servan-Schreiber D. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Awh E, Jonides J. In: The Attentive Brain. Parasuraman R, editor. Cambridge, MA: MIT Press; 1998. pp. 353–380. [Google Scholar]
- 12.Rainer G, Asaad W F, Miller E K. Nature (London) 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- 13.Moscovitch M, Umilta C. In: Modular Deficits in Alzheimer-Type Dementia. Issues in the Biology of Language and Cognition. Schwartz M F, editor. Cambridge, MA: MIT Press; 1990. pp. pp.1–59. [Google Scholar]
- 14.Posner M I, Petersen S E. Annu Rev Neurosci. 1990;13:25–32. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 15.Fuster J M. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. New York: Raven; 1989. [Google Scholar]
- 16.Posner M I, Cohen Y. In: Attention and Performance. Bouma H, Bouhuis D, editors. X. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. pp. 55–66. [Google Scholar]
- 17.Goldberg M E, Wurtz R H. J Neurophysiol. 1972;35:560–574. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- 18.Wurtz R H, Mohler C W. J Neurophysiol. 1976;39:745–762. doi: 10.1152/jn.1976.39.4.745. [DOI] [PubMed] [Google Scholar]
- 19.Robinson D L, Goldberg M E, Stanton G B. J Neurophysiol. 1978;41:910–932. doi: 10.1152/jn.1978.41.4.910. [DOI] [PubMed] [Google Scholar]
- 20.Wurtz R H, Goldberg M E, Robinson D L. Prog Psychobiol Physiol Psychol. 1980;9:42–83. [Google Scholar]
- 21.Corbetta M, Shulman G L. Philos Trans R Soc London B. 1998;353:1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbetta M, Miezin F M, Dobmeyer S, Shulman G L, Petersen S E. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coull J T, Nobre A C. J Neurosci. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbetta M, Kincade J M, Ollinger J M, McAvoy M P, Shulman G L. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 25.Awh E, Jonides J, Reuter-Lorenz P A. J Exp Psychol Hum Percept Perform. 1998;24:780–790. doi: 10.1037//0096-1523.24.3.780. [DOI] [PubMed] [Google Scholar]
- 26.Kimberg D Y, D'Esposito M, Farah M J. Curr Dir Psychol Sci. 1998;6:185–192. [Google Scholar]
- 27.Baddeley A. Quant J Exp Psychol. 1986;38:527–533. doi: 10.1080/14640748608401613. [DOI] [PubMed] [Google Scholar]
- 28.Swick D, Knight R T. In: The Attentive Brain. Parasuraman R, editor. Cambridge, MA: MIT Press; 1998. pp. pp.143–162. [Google Scholar]
- 29.Gehring W J, Gross B, Coles M G H, Meyer D E, Donchin E. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 30.Carter C S, Braver T S, Barch D M, Botvinick M M, Noll D, Cohen J D. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 31.Botvinick M, Nystrom L, Fissell K, Carter C, Cohen J D. Nature (London) 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 32.Posner M I, DiGirilamo G J. In: The Attentive Brain. Parasuraman R, editor. Cambridge, MA: MIT Press; 1998. pp. 401–423. [Google Scholar]
- 33.Eriksen B A, Eriksen C W. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 34.Coles M G H, Gratton G, Bashore T R, Eriksen C W, Donchin E. J Exp Psychol Hum Percept Perform. 1985;11:529–553. doi: 10.1037//0096-1523.11.5.529. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J D, Servan-Schreiber D, McClelland J L. Am J Psychol. 1992;105:239–269. [PubMed] [Google Scholar]
- 36.Allport A. In: Perspectives on Perception and Action. Heuer H, Sanders A F, editors. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. pp. 395–419. [Google Scholar]
- 37.Gratton G, Coles M G H, Donchin E. J Exp Psychol. 1992;4:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- 38.Posner M I, Snyder C R R. In: Information Processing and Cognition: The Loyola Symposium. Solso R, editor. Hillsdale, NJ: Lawrence Erlbaum Associates; 1975. pp. 669–682. [Google Scholar]
- 39.Paquet L, Craig G L. Memory Cognit. 1997;25:182–189. doi: 10.3758/bf03201111. [DOI] [PubMed] [Google Scholar]
- 40.Woods R P, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 41.Cox R W. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 42.Buckner R L, Bandettini P A, O'Craven K M, Savoy R L, Petersen S E, Raichle M E, Rosen B R. Proc Natl Acad Sci USA. 1996;93:14878–14883. doi: 10.1073/pnas.93.25.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duncan, J. & Owen, A. M. (2000) Trends Neurosci., in press. [DOI] [PubMed]
- 44.Casey B J, Trainor R J, Orendi J L, Schubert A B, Nystrom L N, Giedd J N, Castellanos F X, Haxby J V, Noll D C, Cohen J D, et al. J Cognit Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 45.Grafton S T, Hazeltine E, Ivry R. J Cognit Neurosci. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- 46.Berns G S, Cohen J D, Mintun M A. Science. 1997;276:1272–1275. doi: 10.1126/science.276.5316.1272. [DOI] [PubMed] [Google Scholar]
- 47.Hazeltine E, Grafton S T, Ivry R. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- 48.Rauch S L, Savage C R, Brown H D, Curran T, Alpert N M, Kendrick A, Fischman A J, Kosslyn S M. Hum Brain Mapp. 1995;3:271–286. [Google Scholar]
- 49.Haxby J V, Horwitz B, Ungerleider L G, Maisog J M, Pietrini P, Grady C L. J Neurosci. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shulman G L, Corbetta M, Buckner R L, Raichle M E, Fiez J A, Miezin F M, Petersen S E. Cereb Cortex. 1997;7:193–206. doi: 10.1093/cercor/7.3.193. [DOI] [PubMed] [Google Scholar]