Abstract
We have undertaken a series of experiments to examine the behavior of individual components of cell membranes. Here we report an initial stage of these experiments, in which the properties of a chemically simple lipid mixture are carefully mapped onto a phase diagram. Four different experimental methods were used to establish the phase behavior of the 3-component mixture DSPC/DOPC/chol: (1) confocal fluorescence microscopy observation of giant unilamellar vesicles, GUVs; (2) FRET from perylene to C20:0-DiI; (3) fluorescence of dilute dyes C18:2-DiO and C20:0-DiI; and (4) wide angle x-ray diffraction. This particular 3-component mixture was chosen, in part, for a high level of immiscibility of the components in order to facilitate solving the phase behavior at all compositions. At 23 °C, a large fraction of the possible compositions for this mixture give rise to a solid phase. A region of 3-phase coexistence of {Lα + Lβ + Lo} was detected and defined based on a combination of fluorescence microscopy of GUVs, FRET, and dilute C20:0-DiI fluorescence. At very low cholesterol concentrations, the solid phase is the tilted-chain phase Lβ′. Most of the phase boundaries have been determined to within a few percent of the composition. Measurements of the perturbations of the boundaries of this accurate phase diagram could serve as a means to understand the behaviors of a range of added lipids and proteins.
Keywords: ternary phase diagram, bilayer phase separation, liquid-disordered phase, liquid-ordered phase, lipid rafts
1. Introduction
Biological membranes are involved in many aspects of the life of a cell, and for this reason warrant intense study. Moreover, these mixtures of proteins and lipids are interesting in their own right, as an incompletely characterized 2-dimensional state of matter. A fundamental property of a biological membrane is its phase behavior, by which we mean the number and type of phases that exist, as well as the nature of the nonrandom mixing of the lipid and protein components. Various events in the life of a living cell might be directly connected to the phase behavior of membrane components, for example, virus entry and exit, sorting of proteins to various cellular locations, membrane fusion and fission, and transmission of signals [1–4]. We seek a better understanding of membrane phase behavior by determining thermodynamic phase diagrams of chemically well-defined lipid mixtures.
The first high-quality images of phase coexistence in GUVs showed the power of optical microscopy for examining the phase state of lipid bilayers [5,6]. Those first images showed intriguing phase behaviors, such as the precise coupling of phase state in each leaflet, and the linear features of the solid domains. This direct observation of coexisting phases lifted some of the uncertainty about the nature of the phases. Yet, a major question soon emerged from those studies: When images from GUVs of particular compositions show uniform fluorescence, why would pyrene-PC excimer/monomer ratio and FRET imply complex phase behavior at those same compositions [6]? One possible explanation was that phases had indeed separated, but the domain size was so much smaller than the wavelength of the light that a fluorescently labeled GUV appeared uniform; in this case the phases could be described as “nanodomains” [6]. A second possibility was that no phase separation had occurred, and that the probes were sensitive to the very local compositional heterogeneity that is termed nonideal or nonrandom mixing (the usual situation for mixing of dissimilar molecules within a single phase).
Distinguishing between small “nanodomains” that are actual phases and the much smaller and more transient clusters occurring in nonrandom mixing is not mere semantics. For a cell membrane the distinction could be as sharp as two distinct membrane states versus a multitude of intermediate states, a biological on/off switch of membrane properties versus a gradual conversion. Ultimately, mixing and phase behavior are determined by the interaction energies among all of the components, and any phase transition—whether true first-order phase separation or else degrees of nonrandom mixing—is expected to be characteristic of the types and magnitudes of the interaction energies between the membrane components. Such a description of a lipid bilayer in terms of “microscopic interaction energies” would provide useful physical insight into biological membrane structure [7]. But the chemically simple 3-component mixture that we examined in detail in earlier studies, DPPC/DLPC/chol, is not the best choice for elucidating these underlying physical interactions because of the as yet unknown reasons for images of GUVs appearing to be uniform while spectroscopic methods show lipid compositional heterogeneities [6].
The study of model membranes that contain a significant concentration of cholesterol requires at least two additional phospholipid species in order to detect rich phase behaviors. Reasonable choices for these components based upon natural occurrence are the sphingomyelins, together with glycerophospholipids having one saturated and one unsaturated acyl chain. However, as with DPPC/DLPC/chol, bilayer mixtures containing such natural lipids show phase behaviors that are difficult to interpret. For example, sphingomyelins mixed with glycerophospholipids can exhibit melting detected by calorimetry [8], even though macroscopic phase separations are not visible by optical microscopy [9]. Glycerophospholipids having one saturated and one unsaturated acyl chain, mixed with a higher melting lipid and cholesterol, can display lateral inhomogeneity when probed by FRET, yet also without micron-scale phase separation [6,10]. Whether or not such inhomogeneities are actual phase separations is an unsolved problem. Given our goal to provide a firm thermodynamic basis for understanding membrane phase behavior, in the studies reported here we have chosen to avoid the ambiguity of data whose origin might be in separated nanoscopic phases, or else in the smaller clusters of nonrandomly mixed components. We are indeed interested in why some mixtures do not exhibit compositionally distinct domains on a size scale greater than a few hundred nanometers but do show evidence of distinct clusters; however, the possibility of incorrect assignment of phase transitions leads away from choosing such mixtures as ideal models, at least until more is known.
In addition to choosing a system with interpretable phase behavior, an abundance of high quality data is a key to solving phase diagrams. Some experimental methods have especially rich data content, for example, NMR, ESR, x-ray diffraction, and calorimetry, and have contributed greatly to our understanding of lipid phase behavior [11–22]. Methods such as x-ray diffraction, calorimetry, and microscopy have the particular value for phase studies that the signals arise from phase properties, in contrast to spectroscopic signals from individual molecules, which detect local environment and generally require a model or additional information in order to be interpreted as phase behavior. Data that arise from mechanisms that are not fully understood or that have many steps between the measurement and the phase determination are typically less useful [see for example 23]. Measurements that require short times for sample preparation and data collection provide an advantage when dealing with large numbers of samples. Furthermore, different parts of a phase diagram are best detected by different methods.
In sum, in order to resolve questions of phase behavior in multicomponent lipid bilayer mixtures, consideration must be given to both the choice of the lipid components of the mixture, and also to the methods used to examine the phase behavior. The strategy taken in this study is twofold: (i) choose a 3-component bilayer mixture in which “nanodomains” do not dominate the composition space; and (ii) use different techniques and different probe molecules, and demand explanations that are in harmony. To these ends we chose DSPC/DOPC/chol, and we used four different methods and four different probe molecules. One technique, wide-angle x-ray diffraction, required no probe. Moreover, we used four different means of sample preparation, because of known problems of artifactual de-mixing during sample preparation.
2. Materials and methods
2.1. Materials
Phospholipids were purchased from Avanti Polar Lipids, Inc (Alabaster, AL) and cholesterol from Nu Chek Prep (Elysian, MN). The fluorescent dyes 1,1′-dieicosanyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (C20:0-DiI), 3,3′-dilinoleyloxacarbocyanine perchlorate (C18:2-DiO), and 1-hexadecanoyl-2-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-sn-glycero-3-phosphocholine (16:0, Bodipy)-PC were obtained from Invitrogen (Eugene, OR). Naphthopyrene and perylene were from Sigma-Aldrich Corp. (St. Louis, MO). Purity to 99.9% was confirmed by thin-layer chromatography on washed, activated Adsorbosil TLC plates (Alltech, Deerfield, IL), developed with chloroform/methanol/water (65/25/4) for all phospholipids, chloroform/methanol (9/1) for C20:0-DiI and C18:2-DiO, and with petroleum ether/diethyl ether/chloroform (7/3/3) for cholesterol analysis. All solvents used were HPLC grade. Phospholipid stocks were quantitated by phosphate assay [24], fluorescent dye stocks by absorption spectroscopy using an HP 8452A spectrophotometer (Hewlett-Packard, Palo Alto, CA). Extinction coefficients used are 143,000 M−1cm−1 at 549 nm for C20:0-DiI; 138,000 M−1cm−1 at 484 nm for C18:2-DiO; 91,800 M−1cm−1 at 504 nm for (16:0, Bodipy)-PC (all from Invitrogen, Eugene, OR), 23,700 M−1cm−1 at 460 nm for naphthopyrene (from Sigma-Aldrich, Saint Louis, MO), and 38,500 M−1cm−1 at 436 nm for perylene [25]. Cholesterol stocks were prepared analytically.
In the text below we use the term “trajectory” to mean the 1-dimensional composition dependence of a measurement such as fluorescence. For units of concentration we generally use the mole fraction of the total lipid that is the specified species, for example the mole fraction of the total lipid composition that is DOPC is shown as χDOPC.
2.2. Confocal fluorescence microscopy of giant unilamellar vesicles (GUVs)
2.2.1. Gentle hydration
Most of the GUV samples described here were prepared by the method of “gentle hydration” as described by Akashi et al. [26] except for higher preparation temperatures as noted. Stock chloroform solutions of DSPC and DOPC contained 10 mol% DSPG or DOPG respectively, because charged phospholipids are necessary to obtain GUVs by this method. PG was chosen to serve as the charged lipid because gel-fluid transition temperatures are nearly identical for PG and PC having the same acyl chains [27]. 500 nmol lipid mixtures, together with 0.1 mol% of each fluorescent dye, were dried from organic solvent into a thin film on rotary vacuum at ~ 60 °C, followed by 1 h at high vacuum to remove residual solvent. The thin dry film was then hydrated by introducing wet N2 gas to the lipid film at ~ 60 °C for 45 min. Prewarmed buffer (10 mM KCl, 2.0 mM PIPES, 1.0 mM EDTA, pH 7.0) was added, and the film incubated 20 – 24 h at 65 °C in an argon atmosphere to produce GUVs. The suspension was then cooled over 6 – 8 h to ambient temperature of 23 °C.
2.2.2. Electroswelling
In order to provide a second method of sample preparation for comparison with results from the gentle hydration method, GUVs along several specific compositional trajectories were prepared by the method of “electroswelling” [28], which does not require PG in the system, but requires ~ 0 ionic strength in the aqueous phase during sample preparation. Each sample contained 300 nmol lipid mixture with 0.1 mol% of each fluorescent dye in 200 μL chloroform, evenly deposited onto two pieces of polished, indium tin oxide (ITO)- coated microscope slides (Delta Technologies, Stillwater, MN) on a hotplate set at 60 °C, followed by 1 h at high vacuum to remove residual solvent. A vesicle generation chamber was formed by the two conductive slides and one Buna o-ring, and filled with 100 mM sucrose solution. The films were incubated 2 h at 65 °C with an AC field of 5 Hz, ± 1V (pulse/function generator, WAVETEK, San Diego, CA) to produce GUVs. The suspension was then cooled slowly over 6 – 8 h to 23 °C.
GUVs were examined at ambient temperature of ~ 23 °C. Harvested GUVs were placed on a glass microscope slide within a ring of silicone high-vacuum grease (Dow Corning, Midland, Mich), then enclosed by a no. 1 coverslip, and allowed to settle for ~10 min prior to observation.
2.2.3. Fluorescent probes
The probe pair used for most of these studies, (16:0, Bodipy)-PC and C20:0-DiI, was previously found to have complementary partitioning between coexisting Lα and Lβ phases [5,6]. Confocal fluorescence microscopy images were obtained with an LCS SP2 confocal microscope (Leica, Bannockburn, IL) with a 63× 1.2 NA water-immersion objective and a 488/543 nm beam splitter filter. (16:0, Bodipy)-PC was observed at 488 nm excitation and 496 – 536 nm emission, C20:0-DiI at 543 nm excitation and 550 – 660 nm emission. Non-uniform fluorescence of a bilayer-bound dye over a GUV surface can provide information about size, shape, and phase identity of bilayer domains. Use of a complementary dye pair provides additional information for phase identification, and also enables recognition and avoidance of some common artifacts, in particular, light-induced domains, but also bound small vesicles mistaken for a separate phase, and non-equilibrium dark domains mistaken for an equilibrium phase. Unilamellarity of GUVs was judged following Akashi et al. [26] and the relatively lowest brightness vesicles were used. All images shown here are color-merged (16:0, Bodipy)-PC green fluorescence emission and DiI-C20:0 red with Leica Confocal Software, v. 2.61 Build 1537 (Leica, Bannockburn, IL), unless specified.
2.3. Fluorescence resonance energy transfer (FRET)
Liposomes of DSPC/DOPC/chol were prepared by the method of Rapid Solvent Exchange (RSE) in order to minimize de-mixing artifacts, as described [29]. Samples contained 0.2 mol% perylene as FRET donor and 0.05 mol% C20:0-DiI as FRET acceptor. In brief, the chloroform solution of lipids and dyes was warmed, followed by addition of 0.5 mL aqueous buffer (200 mM KCl, 5 mM PIPES, 1 mM EDTA, pH 7.0). Samples were vortexed under vacuum for 1 min then sealed under argon immediately after the RSE procedure and placed in a water bath at 65 °C. Samples were held at this temperature for 2 h, then cooled at 2 °C/h to 23 °C, and held at this final temperature for 2 – 7 days in the dark before measurement. Sample were made with 0 ≤ χCHOL ≤ 0.6, with χCHOL = 0.025 stepwise increments, and 0 ≤ χDSPC ≤ 1.0. The relatively low dye concentrations yielded adequate S/N with negligible self-quenching distortion of the FRET signals. A total of 725 samples were examined.
Fluorescence was measured with a Hitachi 3010 spectrofluorimeter at 23 °C, exciting perylene at 420 nm using a 5-nm bandpass interference filter. Emission was measured at 470 nm for perylene and 565 nm for C20:0-DiI, with a 10-nm bandpass interference filter. F565 was used as a measure of the FRET efficiency. For 3D visualization in a triangular coordinate system, computer code was written in Mathematica (Wolfram Research, Champagne, IL) and plots were visualized in JavaView v3.95 [30] to enable facile examination of the large data sets from a variety of viewpoints. The fluorescence surface was displayed in 3 dimensions over the entire sample compositional range by use of TableCurve 3D, v 4.0.01 (SYSTAT, CA) or Sigma Plot (SYSTAT, CA).
2.4. Dilute dye fluorescence spectroscopy of liposomes
We also used the direct measurement of the fluorescence of dilute membrane-bound dyes that exhibit significantly different fluorescence intensity in different phases in order to find phase boundaries, as previously described [see Fig. 9 of ref. 31]. A benefit of this especially simple type of measurement is that the interpretation of the shapes of the fluorescence surfaces from large data sets is constrained by the lever-arm rule along tielines and the partition coefficient between phases; without additional variables, the extraction of the phase boundaries is straightforward.
In order to choose conditions that would bring samples close to equilibrium, we explored the influence of some combinations of incubation time and temperature (see Supplementary Material 1). We found that samples with high mole fraction χDSPC > ~ 0.9 gave poorly reproducible data under all conditions examined, perhaps because of insufficient equilibration time when most of the sample is solid. Taking into consideration the large number of samples to be prepared, ~ 1000, our heating/cooling protocol was adequate for most, but not all, compositions.
Liposomes of DSPC/DOPC/chol were prepared by RSE as described above, except that samples contained 250 nmol of the lipid mixture and especially low concentrations of the dyes, 0.01 mol% C18:2-DiO and C20:0-DiI each. This dye concentration is low enough that self-quenching of fluorescence can be neglected (Heberle, unpublished), removing a serious complication to interpreting the fluorescence trajectories. 979 samples were made with 0 ≤ χCHOL ≤ 0.5, with χCHOL = 0.02 stepwise changes and 0 ≤ χDSPC ≤ 1.0. Compositional points were thus evenly distributed in mole fraction increments of 0.02 in the entire compositional space below χCHOL = 0.5. Some samples were prepared at a slower cooling rate of 0.5 °C/h in order to test equilibration. The aqueous lipid concentration of the samples after RSE was 500 μM.
C18:2-DiO and C20:0-DiI were excited at 480 and 540 nm respectively, with a 10-nm bandpass. Emission was measured at 505 nm for C18:2-DiO and 565 nm for C20:0-DiI with a 10-nm bandpass. Just prior to measurement, each sample was diluted with buffer to a final lipid concentration of 3.75 μM. Typical S/N of each sample is higher than 50. 3D views of fluorescence vs composition were plotted with TableCurve 3D or Sigma Plot.
Additional information about equilibrating these samples, as well as treating the large data sets, is in the Supplementary Materials.
2.5. X-ray diffraction
Stock solutions of DOPC and DSPC in chloroform were mixed to yield a total lipid mass of ~ 5 mg. Chloroform was evaporated under high vacuum, then cyclohexane and methanol were added to ~ 15/1 cyclohexane/lipid with no more than 10% methanol. The lipid in cyclohexane/methanol was frozen at −80 °C, and solvent removed by lyophilization at −10 °C for 0.5 – 2 h. Then 6 μL of water were added to the lipid, yielding a final concentration of ~ 1/1 water/lipid. In order to equilibrate water across the bilayers, samples were then cycled three times between −196 °C (liquid nitrogen temperature) and 75 °C, mechanically mixing during each cycle using a 10 μL Drummond Microdispenser (Broomall, PA). The hydrated lipid was then centrifuged into a 1 mm diameter glass x-ray capillary (Charles Supper, Natick, MA), and 3 μL of excess water added before sealing the capillary with a plug of vacuum grease and epoxy.
For comparison by a different means of preparation, samples were also made by RSE from chloroform to the buffer described above for RSE preparations, at a final concentration of 20 mg/mL. The lipid dispersion was then loaded into glass x-ray capillaries and centrifuged at 5000 × g to concentrate the lipid at the bottom of the capillary. The final lipid concentration of these dispersions was ~ 20/1 buffer/lipid. The wide angle x-ray scattering (WAXS) data for these mixtures are not presented, but these samples, with a great excess of water, had the same signature WAXS pattern as the 1/1 water/lipid samples described above.
X-ray measurements used a Ni-filtered Cu Kα line (λ=1.5418 Å) from a Rigaku RU300 rotating anode x-ray source operated at 38 kV and 50 mA. X-rays were focused using orthogonal Franks mirrors. Tantalum slits at the sample stage trimmed the beam to a 1mm square, with an intensity of ~ 3 × 107 rays/s. Sample temperature was controlled with a water-cooled Peltier controller (Melcor Inc., Trenton, NJ) and monitored with a 100Ω platinum RTD sensor (Omega Inc., Stamford, CT). WAXS images were collected on a homebuilt 1 K × 1 K pixel CCD detector [32]. The sample-detector distance was calibrated with p-bromo-benzoic acid, converting detector pixels into reciprocal space, q = 4π/λsinθ, where θ is the scattering angle. Exposure time was 40 minutes for the x-ray data shown.
3. Results
3.1. GUV images
A summary of the images observed for GUVs of different compositions is shown in Fig. 1. Sample compositions ranged over most of the composition-space, except for two regions that did not yield useful data: (i) At the highest concentrations of DSPC, χDSPC > ~ 0.9, GUVs could be formed, but images often showed patterns of light and dark regions that we could not interpret, sometimes even for 1-component pure DSPC vesicles; (ii) No samples were examined having χCHOL > ~ 0.5, because of our experience with artifactual cholesterol precipitation from the dried films that are a necessary intermediate state of the sample during the GUV preparation. Most experiments used gentle hydration to prepare GUVs, but no difference in phase behavior was found for GUVs prepared by electroswelling.
Fig. 1.
Images of giant unilamellar vesicles (GUVs) of DSPC/DOPC/chol reveal compositions where immiscible phases coexist, and other regions where no phase separations are apparent. GUVs were labeled with fluorescent dyes (16:0, Bodipy)-PC (green) and C20:0-DiI (red), both at 0.1 mol%, and observed at 23°C. Examples of GUV appearance are shown in images A, B, C. (A) Open circles indicate uniform fluorescence over the entire GUV observed for both dyes; (B) Filled circles, round domains that show complementary brightness of the two dyes; (C) Filled squares, red domains with clearly angular and linear features are surrounded by green, or else small islands of green are observed within a red background. GUVs with separated domains that show combinations of round and linear features are indicated by triangles. Most experiments used gentle hydration to prepare GUVs, which thus contain 10 mol% PG. But no difference in phase behavior was found for GUVs without PG prepared by electroswelling. Each image is color-merged from the simultaneously collected fluorescence emission from C20:0-DiI and (16:0, Bodipy)-PC using the Leica Confocal software. Images were constructed from confocal microscopy z-scans in 1-μm increments. Scale bars are 5μm.
Regarding the interpretation of image data, individual vesicles in a field of GUVs exhibit somewhat different domain sizes and domain area fractions. For this reason, our criterion for identification of the phase state at a given bilayer composition was that the great majority of vesicles observed show a particular behavior, i.e. uniform or coexisting gel + fluid, or coexisting fluids. Therefore, as expected, determination of a phase boundary was sometimes problematic when detecting the appearance/disappearance of the minor phase. The boundary that is marked most clearly by GUV observations is the upper boundary of {Lα + Lo} coexistence, because large fractions of separated domains abruptly appear/disappear near a critical point, unlike the gradual appearance/disappearance of tiny domains at the end of a tieline. As a typical example of the sharpness of this upper boundary, one such uniform GUV is shown in Fig. 1 that has only 0.5 mol% more cholesterol than do GUVs that show pronounced phase coexistence. Within the {Lα + Lo} region, GUV compositions range from mostly Lα near the left boundary to mostly Lo near the right boundary, as shown in Fig. 2.
Fig. 2.
GUV images reveal the upper and side boundaries of {Lα + Lo}. Samples range from mostly Lα (green, A-C) to mostly Lβ (red, G and H). Vesicles close to the top nearly horizontal boundary (D, E and G) change abruptly to uniform (e.g. F) with a small addition of cholesterol (0.5 mol% more cholesterol from G to F). GUVs were prepared by gentle hydration. Labeling and microscopy conditions as in Fig. 1. Temperature 23°C, scale bar 5μm.
Both domain shape and domain color reveal the simultaneous presence of three phases: Figs. 1 and 2 indicate a region of 3-phase coexistence, which showed up in the hands of three different investigators during these studies, as GUV domains having mixed rounded and linear features (see below). In order to better define the transition from a 2-phase to a 3-phase region, a series of four experiments was done with especially fine compositional increments, shown in Fig. 3. A nearly straight line can be drawn through the points where rounded domains first appear upon exit from the 2-phase region of {Lα + Lβ(β′)}. However, no upper boundary of this putative 3-phase region was detected by these GUV observations. Fig. 3 A2, B2 and C2 show some examples of these mixed rounded and linear features.
Fig. 3.
The lower boundary of the 3-phase coexistence region {Lα + Lo + Lβ} is marked by appearance of rounded as well as linear domain features. Vesicles are labeled as in Fig. 1. In the 2-phase region samples range from mostly Lα (green, series A) to mostly Lβ (red, series D). As cholesterol concentration increases in samples that showed strictly angular and linear red domains of Lβ (image 3 in all series), round features appear (image 2) together with linear features. As cholesterol concentration increases further, linear features disappear (image 1 in all series). Arrows in image D2 point out the dim red-orange Lo phase (dashed arrow) and bright red-orange Lβ (solid arrow). GUVs were prepared by gentle hydration. Labeling and microscopy conditions as in Fig. 1. Temperature 23°C, scale bar 5μm.
Round bright green domains, enriched in (16:0, Bodipy)-PC, are from Lα phase. Other rounded domains in this compositional region contain no measurable green fluorescence and thus are not Lα phase. These rounded domains without green fluorescence exhibit only dim red-orange fluorescence, consistent with not being Lβ phase. Some examples of these rounded domains that have very low levels of green (16:0, Bodipy)-PC are shown by the arrow in Fig. 3 D2. We ascribe these domains to the Lo phase. Bright red-orange domains that appear linear or branched are the Lβ phase, shown by the arrow in Fig. 3 D2.
3.2. Spectroscopic studies
3.2.1. Fluorescence intensity of dilute dyes
The fluorescence intensity of a probe measured at a particular emission wavelength is a function of its extinction coefficient at the excitation wavelength, its quantum yield, and the fraction of total fluorescence intensity at the emission wavelength; each of these factors can be affected by the local environment of the probe [33]. For an infinitely dilute probe exhibiting different fluorescence intensities in different phases, the fluorescence intensity along a tieline of phase coexistence is given by
| (1) |
where Fφ1(Fφ2) is the fluorescence intensity in phase φ1 (φ2), is the probe partition coefficient, and fφ1 is the fraction of phase φ1 given by the lever rule [34].
We emphasize the following important features of the dilute-dye model for a tieline. First, there is an abrupt change in slope of fluorescence at the boundary marked by the appearance of the dye-enriched phase; the stronger the partition, the more abrupt the change. Second, the use of two probes with complementary partitioning behavior increases the likelihood of seeing abrupt changes at both boundaries. Finally, fluorescence trajectories along a tieline are simple convex or concave curves, or else are linear when .
The trajectories of raw data for C18:2-DiO are shown in Fig. S4A, B and those for C20:0-DiI in Fig. S4C, D of the Supplementary Materials. These dilute dye data sets have very different shapes for the different dyes because C18:2-DiO and C20:0-DiI exhibit such different sensitivities to the various phase boundaries: Each dye has characteristically higher or lower fluorescence in each phase, as well as characteristic partitioning between coexisting phases. These factors determine whether fluorescence goes up or down and how steeply in composition-space.
3.2.2 Contour plots of dilute dye fluorescence
The large data sets must be viewed along any possible compositional direction in order to find the phase boundaries: We studied 3-dimensional plots of the data, viewed from many different directions, in order to find all of the phase boundaries. A concise way to examine the fluorescence is to view the entire data set at once as a surface in composition-space.
In a ternary mixture, a series of non-intersecting tielines define the 2-phase coexistence regions. We can extend the important features of the simple 1D fluorescence trajectories to the more complex 2D fluorescence height surface, and display the heights in a contour plot: The “kinks” marking the phase boundaries in 1D fluorescence trajectories of individual tielines line up to form edges in 2D fluorescence surfaces. Contour plots of the fluorescence in Fig. 4 provide a means to follow the path of fluorescence changes along any arbitrary direction in composition-space. We used these contour plots, along with slices (not shown) through the 2D fluorescence surfaces in order to identify the paths of the abrupt fluorescence changes.
Fig. 4.
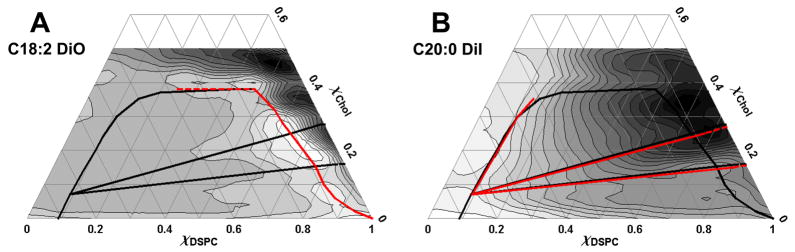
Dilute dye fluorescence of C18:2-DiO and C20:0-DiI, and FRET from perylene donor to C20:0-DiI acceptor reveal several phase boundaries. All phase boundaries are shown by the solid black lines, and the boundaries that are revealed by a particular method are shown in red. (A) Contour plot of dilute C18:2-DiO fluorescence shows the phase boundaries in red to follow the locus of low fluorescence values. A deep valley appears on the right side where fluorescence is low in Lβ or Lo phase but increases as Lα phase forms or as DOPC concentration drops toward 0. Another drop in fluorescence occurs at the upper boundary where coexistence of {Lα + Lo} disappears. (B) Contour plot of dilute C20:0-DiI shows the phase boundaries revealed by C20:0-DiI in red are detected as abrupt changes in fluorescence. There is a steep rise where fluorescence is high in the Lo phase but drops as Lα or Lβ phases form. Along nearly straight lines at the 3-phase boundaries, fluorescence changes abruptly as Lo or Lβ appear/disappear. Fluorescence increases at the left boundary {Lα + Lo} as Lo separates from Lα. Contour plots are linearly grayscaled, with white as lowest value and black as highest. Temperature 23°C.
C18:2-DiO has lower fluorescence in the solid phase, intermediate fluorescence in the Lα phase, and higher fluorescence at higher χCHOL. The partition of this dye strongly favors the Lα phase over either the Lβ(β′) or the Lo phase. This behavior leads to a pronounced increase in fluorescence when Lα phase first separates from either the solid phase or from the lower χCHOL Lo phase. The result is a “valley” in the fluorescence from dilute C18:2-DiO, the light region of Fig. 4A, that traces out the right-hand (solidus) boundary of {Lα + Lβ(β′)}, the right-hand side of the triangle of 3-phase coexistence, and a large part of the right side of the boundary of {Lα + Lo} 2-phase coexistence. An edge of the fluorescence surface corresponds to a sharp increase in contour line density on the right-hand side of Fig. 4A, showing the onset of ordered-disordered phase coexistence regions along a valley marked by most of the red line in Fig. 4A. The sharpness of the edge (shown in Fig. S4A) implies that the probe strongly partitions into the minor, disordered Lα phase, a result that is expected from the structure of the probe and confirmed by microscopy of GUVs. Because of its strong partitioning out of Lβ or Lo, the corresponding boundaries on the left-hand side of Fig. 4A, which mark the appearance of ordered Lo or Lβ(β′) phase, do not show up in the C18:2-DiO surface. In fact, the entire left side of the fluorescence surface for this dye is dominated by a featureless plane.
As with C18:2-DiO, C20:0-DiI has lower fluorescence in the solid phase, intermediate fluorescence in the Lα phase, and high fluorescence at higher χCHOL. But quite unlike C18:2-DiO, C20:0-DiI favors the Lβ(β′) phase or the Lo phase over Lα. This behavior leads to a modest increase in fluorescence when Lβ(β′) separates from Lα and a large increase in fluorescence when Lo phase separates from Lα. We note that the steep increase in fluorescence at high concentration of DSPC within the Lo phase region as a function of composition is not explained by the studies reported here. But the observation of these fluorescence changes within the 1-phase region implies that some properties of the Lo phase are strongly concentration-dependent.
An edge marking the appearance of ordered phase is seen in the C20:0-DiI surface, marked as the solid line on the left side of Fig. 4B. To the right of this edge, a hill emerges that can be divided into three distinct regions which join at the point χDSPC = 0.09, χCHOL = 0.07: (1) Below χCHOL = 0.07, the contours are initially closely spaced but become more spread out upon moving through the phase coexistence region, indicating a steep initial rise in fluorescence and implying strong partition of the probe into the ordered Lβ(β′) phase; (2) Above χCHOL = 0.07, the contours are evenly spaced across the phase coexistence region, indicating weaker probe partition into the ordered Lo phase. These partitioning behaviors are confirmed by microscopy of GUVs; (3) Between the {Lα + Lo} and {Lα + Lβ(β′)} regions is a steep cliff face, which we identify as a boundary of the {Lα + Lo + Lβ} coexistence region. The edges of the cliff face, marked by abrupt change in density and direction of the contour lines, form remarkably straight lines in composition-space, shown as solid red lines in Fig. 4B.
3.2.3 Contour plots of FRET
A contour plot of the FRET experiments over all of composition space is shown in Fig. 5A. One feature of Fig. 5A is the dip in FRET trajectories at lower χCHOL, which appears in the graph as the light region. This FRET behavior is caused by separation of the donor dye from the acceptor dye when each partitions favorably into a different phase. In this case, perylene has a slight preference for the Lα phase whereas C20:0-DiI has a pronounced preference for the Lβ(β′) phase, as shown by GUV images in this region of coexistence. We have previously termed the reduced FRET caused by dye separation a region of reduced efficiency, or RRE [6]. The RRE traces out the left-hand (liquidus) boundary of the 2-phase coexistence region of {Lα + Lβ(β′)}.
Fig. 5.
FRET from a perylene donor to a C20:0-DiI acceptor reveals several phase boundaries. (A) FRET contour plots, with the phase boundaries in red that are reliably detected by FRET. A steep rise occurs where FRET is high in the Lo phase but drops as Lα or Lβ phases form. Along nearly straight lines, FRET contours change abruptly as Lo or Lβ appear/disappear. FRET increases at the left boundary of {Lα + Lo} as Lo separates from Lα. Contour plots are linearly grayscaled, with white as lowest value and black as highest. Temperature 23°C. Blue dashed lines indicate nine individual FRET trajectories shown in (B), in order to illustrate how phase boundaries are determined; (B) Nine FRET trajectories along fixed ratios of with cholesterol mole fractions shown from 0 – 0.4. The compositions that were determined (by use of all of the methods reported in this study) to fall along boundaries of the triangle of 3-phase coexistence are marked on each trajectory with red circles.
Another prominent feature of Fig. 5A is a hill of increased FRET values, which appears in the contour plot as the darker region. Over a large range of compositions, FRET is enhanced over that in neighboring phase regions, because both dyes partition into the same phase, Lo, over coexisting Lα phase. We term the enhanced FRET caused by dye co-localization, a composition-space region of enhanced efficiency, or REE.
Remarkably linear features appear along certain compositional directions. As we discuss later, these are boundaries of a triangle of 3-phase coexistence. These boundaries show up as sharp changes in the FRET trajectories, for example along fixed ratios of , as shown in Fig. 5B.
At intermediate cholesterol concentrations, the FRET surface is dominated by a steeply sloping face connecting the high-cholesterol REE and the low-cholesterol RRE, these two regions meeting at a straight line in composition-space. We interpret the composition-space occupied by this cliff face as an {Lα + Lo + Lβ} coexistence region, as follows: beginning with the lower cholesterol {Lα + Lβ} edge, addition of a small amount of cholesterol at any composition along the edge results in an increase in FRET. This increase results from the sudden appearance of Lo, which is forming at the expense of the Lβ phase and in which FRET is enhanced compared with the Lβ phase. As more cholesterol is added, ultimately all of the Lβ is replaced with Lo, and the increase in FRET abruptly ends, as shown by change in density and direction of contour lines along a straight line in composition space. This change also occurs along a straight-line edge on the FRET surface at the first {Lα + Lo} tieline.
3.3. Wide-angle x-ray diffraction
Fluorescence microscopy of labeled GUVs can identify coexisting lamellar gel and fluid phases if domains are larger than ~ 0.3 nm, but this technique cannot distinguish between Lβ and Lβ′ phases. Therefore, we used wide-angle x-ray diffraction to identify the type of gel phase coexisting with Lα phase in the binary DSPC/DOPC mixtures. Fig. 6 shows wide-angle x-ray diffraction results for pure DSPC, pure DOPC, and a 1/1 mixture of DOPC/DSPC.
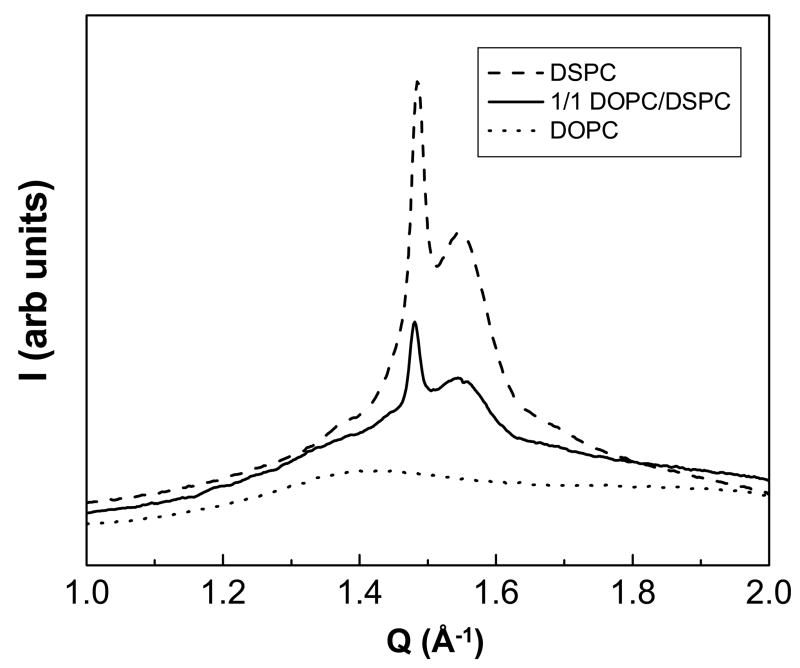
Fig. 6.
X-ray scattering intensity I vs. reciprocal space distance Q in the wide-angle region for fully hydrated bilayer samples of pure DOPC, pure DSPC, and a 1/1 mixture of DOPC/DSPC. The pure DSPC sample shows a sharp reflection at d-spacing 4.2 Å and a broader reflection at 4.05 Å, characteristic of Lβ′. The wide-angle diffraction for a 1/1 mixture of DOPC and DSPC has a very sharp and a broader peak with the same d-spacings as pure DSPC. Temperature 23°C.
The pure DSPC sample shows a sharp reflection at a d-spacing of 4.2 Å and a broader reflection at 4.05 Å, consistent with a distorted hexagonal lattice, and indicative of the Lβ′ phase as previously described by McIntosh [35] and by Tristram-Nagle, et. al [18]. The pure DOPC sample has a very broad wide-angle peak centered at 4.5 Å, consistent with an Lα phase.
The wide-angle diffraction for a 1/1 mixture of DOPC and DSPC has a very sharp peak at 4.2 Å and a broader peak at 4.05 Å, as in pure DSPC. Because the wide-angle peak for the Lα phase is so broad, it is difficult to identify the presence of Lα phase in these x-ray data. By combining evidence from the fluorescence microscopy of GUVs with the x-ray data, we conclude for 1/1 DOPC/DSPC that Lα is in coexistence with an Lβ′ phase that has morphology identical to that of pure hydrated DSPC (and all binary DOPC/DSPC mixtures in the gel-fluid coexistence region).
3.4. Compositional resolution
Sample composition increments of 2 mol% in these studies set a lower limit on our ability to detect any small phase regions. We also have approximately 2 mol% compositional resolution in drawing certain boundaries, especially the straight-line boundaries of the triangle of 3-phase coexistence. Had we used much less than 2 mol% compositional resolution, we could not have recognized the hallmark straight-line nature of the boundaries of the triangle of 3-phase coexistence.
3.5. Experimental conditions
Our temperature of observation was 23 °C, chosen for convenience. We are indeed interested in the phase behavior of bilayer mixtures at other temperatures including 37 °C, and plan to examine temperature dependence in future experiments. We used a range of ionic strengths in the aqueous phases of these lipid mixtures, but found no effect on the phase boundaries with buffers ranging from zero ionic strength for GUV samples formed by electroswelling, to ~ 200 mM ionic strength for samples prepared by RSE.
4. Discussion
4.1. Strategy for solving the phase diagram
The system DSPC/DOPC/chol is suitable as a basis for future studies of microscopic interaction energies, and of additional lipids and proteins as 4th components. We chose DOPC because of its relatively unfavorable interactions with cholesterol and with DSPC, which result in macroscopic liquid/liquid separation over a large region of composition-space, in contrast to the low-melting lipids POPC or SOPC, which do not seem to exhibit such behavior [36]. We chose DSPC because unlike sphingomyelins, DSPC-rich solid domains are readily observed by fluorescence microscopy, making possible a more completely determined phase diagram, and also because DSPC is so stable and readily obtained. Each of these choices involves a trade of biological relevance for more clearly interpretable phase behavior.
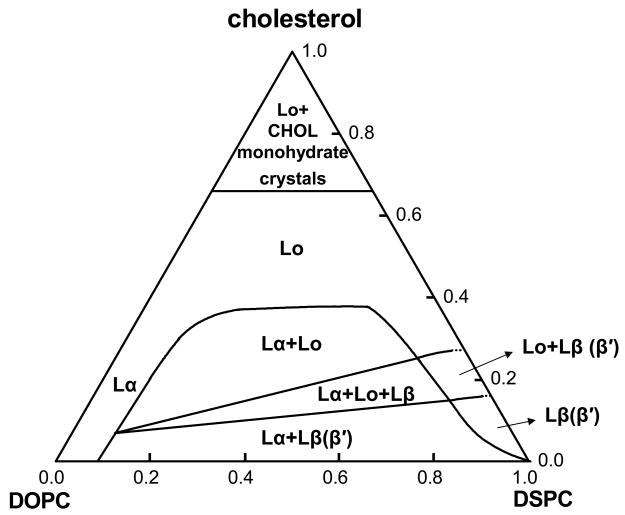
A combination of four different types of experiments provided sufficient information to construct a relatively complete phase diagram of DSPC/DOPC/chol in excess water at a constant temperature of 23 °C. Our findings are organized into a pseudo-ternary phase diagram†, Fig. 7. In summary of the steps leading to this phase diagram, and discussed below in more detail: (a) GUV images of coexisting domains provided sufficient information to identify two 2-phase coexistence regions {Lα + Lβ(β′)} and {Lα + Lo}. A 3-phase coexistence region {Lα + Lo + Lβ} could be strongly inferred: (i) We observed a nearly linear boundary for appearance of rounded domains bordering the region {Lα + Lβ}; and (ii) The rounded domains contain no measurable green fluorescence and thus are not Lα phase, and only dim red-orange fluorescence, consistent with not being Lβ phase. In certain regions of composition the GUV images revealed the location of phase boundaries with some precision, in other regions boundaries were not at all apparent from GUV experiments; (b) Dilute dye fluorescence was sufficiently sensitive to phase behavior that several boundaries could be located with precision; (c) FRET revealed some boundaries with high precision, including boundaries of the 3-phase coexistence region; and (d) Wide-angle x-ray diffraction identified the Lβ′ phase at low χCHOL.
Fig. 7.
Ternary phase diagram for DSPC/DOPC/chol at 23°C, based upon the 4 methods described in the text. Numbers on the DOPC-DSPC axis correspond to χDSPC, numbers on the PC-cholesterol axes to χCHOL. Each side of the 3-phase region is a tieline of the adjacent 2-phase region. For the region {Lα + Lβ(β′)}, the boundary of χCHOL = 0 is also a tieline.
4.2. Lipid mixing behavior
4.2.1. Fluid/gel coexistence region
The pure DSPC vertex of the phase diagram is also the solidus boundary for the binary mixtures having χCHOL = 0. This identification comes from the dilute C18:2-DiO data (essentially, the path of the right side dip in fluorescence at low χCHOL in Fig. 4A), and is supported by the x-ray diffraction finding of the identical pattern and location of sharp diffraction peaks, hence the same morphology, for the DSPC/DOPC = 1/1 binary mixture as observed for the Lβ′ phase of pure DSPC.
We chose the fluidus boundary at χCHOL = 0 to be at χDSPC ~ 0.09 based upon GUV images, and because this choice leads to boundaries of the 3-phase coexistence region that are consistent with Shreinemakers’ Rules [37]. However, a fluidus boundary closer to χDSPC ~ 0.2 is found from both the dilute C20:0-DiI fluorescence trajectories and from the FRET measurements. The spectrophotometric experiments have the benefit of avoiding the dry film stage of sample preparation needed to make GUVs, which tends to produce compositional heterogeneity compared with the RSE method, but this is known to be a problem only at χCHOL greater than ~ 0.4 [7, 29]
The upper boundary of the region {Lα + Lβ(β′)} is a straight line from χCHOL = 0.07 to χCHOL = 0.15 – 0.16. This means that cholesterol mixes less favorably in the DOPC-rich Lα liquid phase than it does in the DSPC-rich Lβ solid phase: the chemical potential of cholesterol, μCHOL, is more than 2-fold higher in the Lα phase than in the Lβ phase with which it is in equilibrium.
4.2.2. 3-phase coexistence triangle
GUV images in Fig. 3 show remarkable shape changes of the coexisting domains as sample compositions cross the lower boundary of the triangle with increasing χCHOL. Red-orange domains that were strictly angular in the 2-phase region {Lα + Lβ(β′)} (see Fig. 3 A3, B3, C3 and D3) start to become rounded while some domain segments are still visibly straight (see Fig 3 A2, B2, C2 and D2). As cholesterol concentration increases in this region, GUV images show more and more rounded domains (see Fig. 3 A1, B1, C1 and D1).
3-phase coexistence is also inferred by the three colors observed exclusively in this region of the phase diagram. Lα is green from strong partitioning of (16:0, Bodipy)-PC; Lβ is bright red-orange from strong partitioning of C20:0-DiI; and Lo is dim red-orange from weak partitioning of C20:0-DiI.
The contour plots of both FRET and C20:0-DiI show pronounced change in contour magnitude and direction along straight lines. The lower of these straight lines, from χCHOL = 0.07 to χCHOL = 0.15 – 0.16, corresponds precisely to the straight-line feature in the image data: the appearance of both rounded plus linear domains along with three distinct domain colors. The right-hand boundary of the triangle boundary appeared clearly in the dilute C18:2-DiO surface in Fig. 4A.
4.2.3. Liquid-liquid coexistence
The lower boundary of {Lα + Lo} shows up in the FRET contour plot of Fig. 5A as a straight line that marks the change in direction of closely spaced contour lines running from χCHOL = 0.07 to χCHOL = 0.25. The left boundary, at low χDSPC, appears most clearly in the GUV images: onset of macroscopic liquid-liquid domains, as shown in Fig. 1. The relatively linear upper boundary has a maximum at χCHOL ~ 0.38. This upper boundary is reliably determined by GUV imaging (Fig. 1) and by the locus of low values of C18:2-DiO fluorescence (Fig. 4A). The flatness of the upper boundary near χCHOL ~ 0.38 might indicate a significant change in the structure of the lattice at this particular cholesterol concentration (see below).
4.2.4. Regular arrays of cholesterol (“superlattices”) might exist
The solid Lβ phase becomes saturated with cholesterol at χCHOL = 0.15 – 0.16, and with more cholesterol, precipitates out an Lo phase having χCHOL = 0.25. Both of these cholesterol concentrations might be significant: χCHOL = 0.154 has been identified for many years to be a cholesterol superlattice [38]. More recently, MC simulations show that it represents a cholesterol concentration that retains a highly favorable chol-PC interaction with minimal unfavorable multibody interactions [39]. Other MC simulations reveal that χCHOL = 0.25, another well-known cholesterol superlattice composition, also involves a favorable chol-PC interaction, also with minimally unfavorable multibody interactions [39]. Finally, the nearly horizontal upper boundary of {Lα + Lo} that we determine to occur at χCHOL = ~ 0.38, is remarkably close to χCHOL = 0.40, yet another cholesterol superlattice composition that has been described in detail by MC simulations of DOPC/chol as a cholesterol concentration at which the lattice energy dramatically increases. The microscopic interaction energies in the ternary mixture in this study have not yet been examined in detail, but we note that binary mixtures of DSPC/chol do not show the “superlattice compositions” because of the small size of the multibody interaction that effectively acts as a long-range repulsive force.
4.3. Difficulty of finding tielines
In principle, relative mole fractions of coexisting phases in a given GUV could be found from the relative area fractions of coexisting domains [40]. However, we have not yet been successful in this analysis. Shape distortion of domains, and therefore area distortion over the field of view can be corrected in part, as perhaps can the very different molecular areas in the different phases. But other problems do not yet have a satisfactory solution. Confocal fluorescence microscopy reconstructions of an entire 3D vesicle cannot provide interpretable information for a several micron wide strip around the equator because of poor z-axis resolution. And domains can pinch off from the GUV and disappear into the medium during the many hours of sample preparation and even during observations with the microscope. Although FRET and single dye data can in principle be fit quantitatively to determine both the phase boundaries and the dye partition coefficients along a tieline, in practice this is quite difficult to implement in a ternary system and is the subject of ongoing work in our lab. As for our x-ray diffraction data, we did not attempt to calculate relative areas under the peak for the 1/1 DSPC/DOPC sample to obtain phase fractions because of the difficulty in separating the peak into a sharp and a very broad component. Moreover, water scatters strongly in the wide-angle region, introducing inaccuracy in baseline subtraction and scaling
The only tielines that we have directly determined are those of the sides of the triangle of 3-phase coexistence. Each of these sides is a tieline that bounds a 2-phase region.
4.4. Comparison with other multi-component lipid bilayer mixtures
Several 3-component lipid bilayer mixtures containing cholesterol have been studied in sufficient detail for useful comparison with the results presented here for DSPC/DOPC/chol. DPPC/DLPC/chol is quite different in several regions [6]. At low χCHOL both mixtures show macroscopic separation of Lα and Lβ phases. One difference between these mixtures is that the solid phase of DPPC can accommodate DLPC up to χDLPC ~ 0.15, whereas the Lβ′ phase of DSPC seems to accept < 2 mol% DOPC, precipitating out a DOPC-rich Lα phase. The most striking difference between the two mixtures is the absence of macroscopic coexisting liquid domains of {Lα + Lo} in DPPC/DLPC/chol, which are such a prominent feature in DSPC/DOPC/chol. In the former mixtures, measurements of FRET as well as of di-pyrene-PC excimer/monomer ratios are consistent with either extremely nonideal mixing, or with domains of {Lα + Lo}, but with the domains being smaller than ~ 300 nm, because they do not show up in optical microscopy imaging of GUVs. We have observed similar behavior for DPPC/POPC/chol and DPPC/SOPC/chol as for DPPC/DLPC/chol [36].
So far, the most detailed ternary bilayer mixture phase diagram that has been published is that of DPPC/DOPC/chol. GUV imaging [41] and 2HNMR [42] were used to map out much of the phase behavior at temperatures from 15 to 50 °C. In those studies the general features of coexisting {Lα + Lβ} and {Lα + Lo} phases were found, but several boundaries could not be detected. Moreover, the boundary of {Lα + Lo} determined by 2HNMR agreed qualitatively, but not quantitatively, with that from GUV imaging, most likely because of light-induced domains in the latter. We note that these investigators report that the phase boundaries depend upon the dye concentration [41]. Within our compositional resolution of ~ 2 mole %, we found no such dependence of phase boundaries on dye concentration over the range of dye/lipid from 1/1000 to 1/10,000.
By imaging of GUVs, behavior similar to that of DPPC/DOPC/chol was reported for 3-component mixtures of cholesterol with palmitoyl-SM/DOPC or palmitoyl-SM/POPC [43]. However, as Ayuyan & Cohen [44] have described and we have confirmed, separated domains in mixtures containing POPC seem to be artifacts (see accompanying paper [36]).
A report from one lab describes rich phase behavior of the mixture palm-SM/POPC/chol [45]. A more recent study [23] of the mixture brain-SM/POPC/chol also reports rich phase behavior. These two reports are discussed in more detail in the accompanying paper [36]. Here we mention simply that these studies required correct determination of tielines, which was not done.
4.5. Interpretation of lipid composition of biological membranes
If our phase diagram in Fig. 7 is relevant to biological membrane phase behavior, the question arises as to which parts of our phase diagram can offer insight, given the measured lipid compositions of natural membranes. This question is complicated by several factors, not least the scarcity of lipid compositional data for individual leaflets of biological membranes. Even when we have reliable lipid compositional data, and even if for each leaflet, such a measured overall composition should not be considered to lead inevitably to a particular phase behavior in a real biological membrane--instead, overall membrane composition at a given moment might be thought of as an ensemble average of different compositional regions having potentially different phase behaviors, over different areas of the membrane: (i) Relatively small neighboring areas of a biological membrane might have their phase behaviors uncoupled from each other, for example because of an area’s enclosure by a ring of proteins [46]. Whether or not phase uncoupling occurs, membrane proteins are almost certain to decrease any lipid-lipid cooperativity in their immediate vicinity. (ii) Patches of selected lipid and protein composition are fusing with, or pinching off from a biological membrane during the normal life of the cell [47]. Moreover (iii) protein activity can change the local lipid composition, either catalytically to produce chemical changes in membrane lipids, or by aggregation/disaggregation to release/bind selected local membrane lipids. With such active membrane compositional changes in mind, we might view a lipid bilayer phase diagram as indicating the various phase regions that can possibly occur-- that is, there could be different phase states over the membrane surface depending upon the local compositional heterogeneities [48]. The phase diagram of Fig. 7 would then be interpreted to show possible states rather than a static description of a given biological membrane, because composition could vary on the size scale of phase domains. The experimentally determined composition (of a given membrane leaflet) would thus correspond to an ensemble average over the entire membrane area.
4.5.1. phases in biological membranes
At χCHOL values found in the plasma membrane, liquids Lα and Lo, as well as solids Lβ and crystals of cholesterol monohydrate, could possibly occur [49]. If so, then any Lβ phase and Lo phase that form might well have very different properties from those of the more simple 1- or 2-component systems such as DPPC or cholesterol-rich DPPC mixtures that have been so thoroughly studied [50]. The manner in which coexisting phases differ from their neighboring 1-phase regions is to be saturated with the very lipid components that are enriched in the coexisting phase--thus any Lβ phase or Lo phase in a biological membrane could not accommodate any more of some other components that are rich in the coexisting phase.
Finally, we point out the significant effort required to map the boundaries, which benefited greatly from examination of so many different samples by several different methods. Without sufficient data, phase diagrams can only be crudely sketched [23,45]. A corollary is the great difficulty of establishing the phase behavior of one particular composition of an unexplored mixture, however carefully chosen, without the information contained in the compositional dependence of the measurements.
5. Summary
Four different experimental methods were used to establish the phase behavior of the 3-component mixture DSPC/DOPC/chol: (1) confocal fluorescence microscopy observation of GUVs; (2) FRET; (3) fluorescence of dilute dyes C18:2-DiO and C20:0-DiI; and (4) x-ray diffraction.
Most of the phase boundaries have been determined to within a few percent of the composition.
The boundaries of a region of 3-phase coexistence are defined for {Lα + Lo + Lβ} from GUV images, from FRET, and from C20:0-DiI fluorescence.
A large fraction of the possible compositions for this mixture give rise to a solid phase at 23 °C.
At very low cholesterol concentrations, the solid phase is the tilted-chain phase Lβ′. This phase appears to accommodate little or no cholesterol.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Science Foundation (MCB-0315330) and the American Chemical Society (PRF-38464-AC7) to G.W.F. T.M. and F.A.H. were supported in part by an NIH Research Service Award (1-T32-GM08267). We thank Gilman E. Toombes and Mark W. Tate for help with the x-ray measurements and we acknowledge the DOE for support of the x-ray equipment (DE-FG02-97ER62443).
Abbreviations
- DSPC
1,2-distearoyl-sn-glycero-3-phosphocholine
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DSPG
1,2-distearoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (sodium salt)
- DOPG
1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (sodium salt)
- TLC
thin-layer chromatography
- GUV
giant unilamellar vesicle
- RSE
rapid solvent exchange
- FRET
fluorescence resonance energy transfer
- RRE
region of reduced efficiency
- REE
region of enhanced efficiency; (16:0, Bodipy)-PC, 2-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine
- C20:0-DiI
1,1′-dieicosanyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- C18:2-DiO
3,3′-dilinoleyloxacarbocyanine perchlorate
- WAXS
Wide-angle x-ray scattering; MC, Monte Carlo
Footnotes
The mixture we study is “pseudo-ternary” because we neglect all components of the aqueous buffer. Most important, we neglect water, on the basis that its chemical potential is constant at every lipid composition we examined because of the presence of excess water. However, we emphasize that the water composition of each phase varies over the phase diagram (and we do not measure what it is).
When cutting across tielines in a 2-phase region, the compositions of the coexisting phases change. As a result, fluorescence intensities in the coexisting phases can change, as can the partition coefficient of the probe between the phases, and therefore we cannot make general statements about whether the 2-phase fluorescence surface is concave, convex, or monotonic in composition-space.
Because the upper boundary is so flat, we cannot completely rule out that this 2-phase region has been interrupted by the appearance of a third phase that we have not detected, or that one of the phases, Lα or Lo, begins to undergo a continuous transition as cholesterol fraction increases making coexisting domains too small to detect.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. PNAS. 2006;103:11364–11369. doi: 10.1073/pnas.0602818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meder D, Moreno MJ, Verkade P, Vaz WL, Simons K. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc Natl Acad Sci U S A. 2006;103:329–334. doi: 10.1073/pnas.0509885103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fullekrug J, Simons K. Lipid rafts and apical membrane traffic. Ann N Y Acad Sci. 2004;1014:164–169. doi: 10.1196/annals.1294.017. [DOI] [PubMed] [Google Scholar]
- 4.Holowka D, Gosse JA, Hammond AT, Han X, Sengupta P, Smith NL, Wagenknecht-Wiesner A, Wu M, Young RM, Baird B. Lipid segregation and IgE receptor signaling: a decade of progress. Biochim Biophys Acta. 2005;1746:252–259. doi: 10.1016/j.bbamcr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Korlach J, Schwille P, Webb WW, Feigenson GW. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc Natl Acad Sci U S A. 1999;96:8461–8466. doi: 10.1073/pnas.96.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigenson GW, Buboltz JT. Ternary phase diagram of dipalmitoyl-PC/dilauroyl-PC/cholesterol: nanoscopic domain formation driven by cholesterol. Biophys J. 2001;80:2775–2788. doi: 10.1016/S0006-3495(01)76245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, Buboltz JT, Feigenson GW. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim Biophys Acta. 1999;1417:89–100. doi: 10.1016/s0005-2736(98)00260-0. [DOI] [PubMed] [Google Scholar]
- 8.Maulik PR, Shipley GG. X-ray diffraction and calorimetric study of N-lignoceryl sphingomyelin membranes. Biophys J. 1995;69:1909–1916. doi: 10.1016/S0006-3495(95)80061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feigenson, unpublished observations
- 10.Silvius JR. Fluorescence energy transfer reveals microdomain formation at physiological temperatures in lipid mixtures modeling the outer leaflet of the plasma membrane. Biophys J. 2003;85:1034–1045. doi: 10.1016/S0006-3495(03)74542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vist MR, Davis JH. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990;29:451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- 12.Chan SI, Seiter CHA, Feigenson GW. Anisotropic and Restricted Molecular Motion in Lecithin Bilayers. Biochem Biophys Res Comm. 1972;46:1488–1493. doi: 10.1016/0006-291x(72)90775-9. [DOI] [PubMed] [Google Scholar]
- 13.Polozov IV, Gawrisch K. Characterization of the liquid-ordered state by proton MAS NMR. Biophys J. 2006;90:2051–2061. doi: 10.1529/biophysj.105.070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge M, Gidwani A, Brown HA, Holowka D, Baird B, Freed JH. Ordered and disordered phases coexist in plasma membrane vesicles of RBL-2H3 mast cells. An ESR study. Biophys J. 2003;85:1278–1288. doi: 10.1016/S0006-3495(03)74563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimburg T, Wurz U, Marsh D. Binary phase diagram of hydrated dimyristoylglycerol-dimyristoylphosphatidylcholine mixtures. Biophys J. 1992;63:1369–1378. doi: 10.1016/S0006-3495(92)81714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimshick EJ, McConnell HM. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973;53:446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- 17.Luzzati V, Husson F. The structure of the liquid-crystalline phases of lipid-water systems. J Cell Biol. 1962;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tristram-Nagle S, Zhang R, Suter RM, Worthington CRM, Sun W-J, Nagle JF. Measurement of chain tilt angle in fully hydrated bilayers of gel phase lecithins. Biophys J. 1993;64:1097–1109. doi: 10.1016/S0006-3495(93)81475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladbrooke BD, Chapman D. Thermal analysis of lipids, proteins, and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969;3:304–357. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- 20.Hinz HJ, Sturtevant JM. Calorimetric investigation of the influence of cholesterol on the transition properties of bilayers formed from synthetic L-α-lecithins in aqueous suspension. J Biol Chem. 1972;247:3697–3700. [PubMed] [Google Scholar]
- 21.Mannock DA, Lewis RN, McElhaney RN. Comparative calorimetric and spectroscopic studies of the effects of lanosterol and cholesterol on the thermotropic phase behavior and organization of dipalmitoylphosphatidylcholine bilayer membranes. Biophys J. 2006;91:3327–3340. doi: 10.1529/biophysj.106.084368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heerklotz H, Tsamaloukas A. Gradual change or phase transition: characterizing fluid lipid-cholesterol membranes on the basis of thermal volume changes. Biophys J. 2006;91:600–607. doi: 10.1529/biophysj.106.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pokorny A, Yandek LE, Elegbede AI, Hinderliter A, Almeida PF. Temperature and composition dependence of the interaction of delta-lysin with ternary mixtures of sphingomyelin/cholesterol/POPC. Biophys J. 2006;91:2184–2197. doi: 10.1529/biophysj.106.085027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingsley PB, Feigenson GW. The synthesis of a perdeuterated phospholipid:1,2-dimyristoyl-sn-glycero-3-phosphocholine-d72. Chem Phys Lipids. 1979;24:135–147. [Google Scholar]
- 25.Berlman IB. Handbook of Fluorescence Spectra of Aromatic Molecules. Academic Press. 1971:399–400. [Google Scholar]
- 26.Akashi K-i, Miyata H, Itoh H, Jr, Kinosita K. Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys J. 1996;71:3242–3250. doi: 10.1016/S0006-3495(96)79517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Findlay EJ, Barton PG. Phase behavior of synthetic phosphatidylglycerols and binary mixtures with phosphatidylcholines in presence and absence of calcium ions. Biochemistry. 1978;17:2400–2405. doi: 10.1021/bi00605a023. [DOI] [PubMed] [Google Scholar]
- 28.Angelova MI, Soleau S, Meleard P, Faucon JF, Bothorel P. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Progr Colloid Polym Sci. 1992;89:127–131. [Google Scholar]
- 29.Buboltz JT, Feigenson GW. A novel strategy for the preparation of liposomes: rapid solvent exchange. Biochim Biophys Acta. 1999;1417:232–245. doi: 10.1016/s0005-2736(99)00006-1. [DOI] [PubMed] [Google Scholar]
- 30.JavaView v3.95 . www.javaview.de.
- 31.Heberle FA, Buboltz JT, Stringer D, Feigenson GW. Fluorescence methods to detect phase boundaries in lipid bilayer mixtures. Biochim Biophys Acta. 2005;1746:186–192. doi: 10.1016/j.bbamcr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tate MW, Gruner SM, Eikenberry EF. Coupling format variations in x-ray detectors based on charge coupled devices. Rev Sci Instr. 1997;68:47–54. [Google Scholar]
- 33.Vaz WLC, Melo E. Fluorescence spectroscopic studies on phase heterogeneity in lipid bilayer membranes. J Fluorescence. 2001;11:255–271. [Google Scholar]
- 34.Florine-Casteel K, Feigenson GW. On the use of partition coefficients to characterize the distribution of fluorescent membrane probes between coexisting gel and fluid lipid phase: an analysis of the partition behavior of 1,6-diphenyl-1,3,5-hexatriene. Biochim Biophys Acta. 1988;941:102–106. doi: 10.1016/0005-2736(88)90218-0. [DOI] [PubMed] [Google Scholar]
- 35.McIntosh TJ. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta. 1978;513:43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Wu J, Shao H, Kong F, Jain N, Hunt G, Feigenson GW. Phase studies of model biomembranes: Macroscopic coexistence of Lα + Lβ, with light-induced coexistence of Lα + Lo phases. doi: 10.1016/j.bbamem.2007.07.009. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsh JS. Principles of Phase Diagrams. McGraw-Hill; New York: 1935. pp. 122–170. [Google Scholar]
- 38.Cannon B, Heath G, Huang J, Somerharju P, Virtanen JA, Cheng KH. Time-resolved fluorescence and fourier transform infrared spectroscopic investigations of lateral packing defects and superlattice domains in compositionally uniform cholesterol/phosphatidylcholine bilayers. Biophys J. 2003;84:3777–3791. doi: 10.1016/S0006-3495(03)75106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang J. Exploration of molecular interactions in cholesterol superlattices: effect of multibody interactions. Biophys J. 2002;83:1014–1025. doi: 10.1016/S0006-3495(02)75227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veatch SL, Polozov IV, Gawrisch K, Keller SL. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys J. 2004;86:2910–2922. doi: 10.1016/S0006-3495(04)74342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veatch SL, Leung SS, Hancock RE, Thewalt JL. Fluorescent probes alter miscibility phase boundaries in ternary vesicles. J Phys Chem B. 2007;111:502– 504. doi: 10.1021/jp067636i. [DOI] [PubMed] [Google Scholar]
- 42.Veatch SL, Polozov IV, Gawrisch K, Keller SL. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys J. 2004;86:2910–2922. doi: 10.1016/S0006-3495(04)74342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veatch SL, Keller SL. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys Rev Lett. 2005;94:148101. doi: 10.1103/PhysRevLett.94.148101. [DOI] [PubMed] [Google Scholar]
- 44.Ayuyan AG, Cohen FS. Lipid peroxides promote large rafts: Effects of excitation of probes in fluorescence microscopy and electrochemical reactions during vesicle formation. Biophys J. 2006;91:2172–2183. doi: 10.1529/biophysj.106.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Almeida RFM, Fedorov A, Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746:234–251. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee S, Maxfield FR. Membrane domains. Annu Rev Cell Dev Biol. 2004;20:839–866. doi: 10.1146/annurev.cellbio.20.010403.095451. [DOI] [PubMed] [Google Scholar]
- 48.Swamy MJ, Ciani L, Ge MT, Smith AK, Holowka D, Baird B, Freed JH. Coexisting domains in the plasma membranes of live cells characterized by spin-label ESR spectroscopy. Biophys J. 2006;90:4452–4465. doi: 10.1529/biophysj.105.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishimura SY, Vrljic M, Klein LO, McConnell HM, Moerner WE. Cholesterol depletion induces solid-like regions in the plasma membrane. Biophys J. 2006;90:927–938. doi: 10.1529/biophysj.105.070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke JA, Heron AJ, Seddon JM, Law RV. The diversity of the liquid ordered (Lo) phase of phosphatidylcholine/cholesterol membranes: A variable temperature multinuclear solid-state NMR and X-Ray diffraction study. Biophys J. 2006;90:2383–2393. doi: 10.1529/biophysj.104.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.