Summary
The NLR (nucleotide-binding domain leucine-rich repeat containing) family of intracellular sensors is a critical component of the innate immune system. A number of NLR family members can form multiprotein complexes, called inflammasomes, and are capable of activating the cysteine protease caspase-1 in response to a wide range of stimuli including both microbial and self molecules. Caspase-1 activation leads to processing and secretion of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18, which play crucial roles in host defense to infectious insults. Dysregulation of the inflammasome has also been linked to a number of autoinflammatory and autoimmune disorders. Recent advances in the inflammasome field will be discussed in this review.
Introduction
The innate immune system is the first line of defense against microbial infections. This initial non-specific innate immune response activates the adaptive immune system leading to specific pathogen directed humoral and cellular immune responses. The innate immune system possesses germline-encoded pattern recognition receptors (PRRs) that are capable of recognizing highly conserved markers that are specific to microbes, called pathogen-associated molecular patterns (PAMPs). These receptors include Toll-like receptors (TLRs), nucleotide-binding domain leucine-rich repeat containing receptors (NLRs), RIG-I-like RNA helicases (RLHs) and C-type lectin receptors (CLRs) [1]. The innate immune system serves to monitor for more than just the presence of microbes; it also contains PRRs that recognize danger signals produced by cells in response to pathogenic conditions. These danger signals or danger-associated molecular patterns (DAMPs) are released in conditions of cellular damage or stress or formed by pathogen-mediated modification of host proteins and can be recognized by the PRRs of the innate immune system. Thus the innate immune system can identify pathology occurring independently of infectious causes. In fact the recognition of DAMPs is known to be a critical factor in sterile inflammatory responses, in which the innate immune system responds to tissue damage mediated by non-microbial insults such as ischemia or trauma [2]. It has been postulated that the sensing of DAMPs associated with pathogenic organisms may provide the immune system the information needed to discriminate between these and commensal organisms.
The inflammasome: a multiprotein complex that activates caspase-1
The human NLR family of cytoplasmic proteins contains 22 members including 14 NLRP members, 5 members of the NLRC subfamily, NAIP, NLRX, and CIITA [3-5]. The field has been complicated by multiple names used for the same molecule. A standardized nomenclature has recently been proposed for the NLR family [6], which we will use throughout this review, wherein the structure of the molecule dictates its name. The NLR family has a unique structure composed of a central nucleotide-binding domain called NACHT, which is located between an N-terminal protein-binding domain (CARD (caspase-recruitment domain), PYD (pyrin domain) or BIR (Baculovirus IAP repeat)) and a C-terminal LRR (Leucine-rich repeat) domain. The molecules are named NLR with a suffix of P or C referring to the N terminal moiety, PYD or CARD respectively, followed by a number.
A number of the NLR molecules have been shown to form a complex with caspase-1 and the adaptor molecule ASC (apoptosis-associated speck-like protein containing a CARD) termed an inflammasome, with the specific name stemming from the individual NLR molecule. The central effector molecule of the inflammasome is the cysteine protease caspase-1 that, upon activation cleaves cytosolic pro-IL-1β, pro-IL-18 and pro-IL-33 to their active forms IL-1β, IL-18 and IL-33, which are then secreted. Recent studies have suggested caspase-1 may have additional functions including regulation of glycolysis pathways [7] and unconventional protein secretion [8]; however, the role of NLR inflammasomes in these processes has not been examined. In addition to NLRP1, NLRP3, and NLRC4, a number of other NLR molecules including, NLRP2, NLRP6, NLRP7, NLRP10 and NLRP12 have been demonstrated to modulate caspase-1 activity in vitro [5]. However, to date only the NLRP1, NLRP3 and NLRC4 inflammasomes have been shown to have clear physiologic roles.
NLR proteins as immune sensors are not unique to humans and mice; plants contain similar molecules called R proteins that recognize, directly or indirectly, pathogen-derived molecules [9,10]. Upon pathogen recognition, conformational changes and/or translocation of these sensors activate the downstream signaling pathways triggering innate immune responses in the plant. R proteins associate with the ubiquitin ligase-associated protein SGT1, and the chaperone protein heat-shock protein 90 (HSP90), both of which are structurally conserved in eukaryotes. Similarly, SGT1 and HSP90 were recently shown to play a role in inflammasome formation in humans. Mayor and colleagues showed an association between SGT1 and HSP90 with NLRP2, NLRP4, NLRP12, NOD1, NOD2, and NLRC4 [11]. SGT1 and HSP90 are also required for NLR function including NLRP3 inflammasome activation, Nod1-induced NF-κB activation and NLRP12-induced NF-κB inducing kinase (NIK) degradation [12,13].
Mutations within both the NLRP1 and NLRP3 genes have been associated with human disease; sequence variants in the NLRP1 gene have been linked to autoimmune and autoinflammatory diseases associated with vitiligo [14] and mutations in NLRP3 are responsible for the autoinflammatory syndromes, Muckle-Wells syndrome, familial cold autoinflammatory syndrome, and neonatal-onset multisystem inflammatory disease known collectively as cryopyrin-associated periodic syndrome (CAPS) [15-17]. Over 40 mutations within the NLRP3 gene have been identified that are associated with CAPS, which result in a constitutively active form of NLRP3 causing increased activation of the inflammasome with resultant increased secretion of IL-1β [18]. Inhibition of the actions of this exacerbated IL-1β production with the recombinant human IL-1 receptor antagonist (IL-1Ra) Anakinra has proven to be highly successful in abrogating disease severity in CAPS [19,20].
The NLRP3 inflammasome
The NLRP3 (also known as Nalp3, cryopyrin, CIAS1, PYPAF1 and CLR1.1) inflammasome is the best studied inflammasome and is activated by chemically and structurally diverse stimuli (Figure 1). Biochemical analysis demonstrated that human NLRP3 can associate with ASC, Cardinal and caspase-1 to form an inflammasome [21,22]. The functional role for Cardinal in this complex is still unclear; in addition no murine homologue for Cardinal has been identified and the murine NLRP3 inflammasome is thought to be composed of NLRP3, ASC and caspase-1 alone. Numerous studies have now demonstrated that NLRP3 plays a crucial role in caspase-1 activation in response to both microbial and non-microbial stimuli. LPS and extracellular ATP activate caspase-1 in a NLRP3 dependent fashion [23,24]. Binding of ATP to the P2X7 receptor recruits the channel pannexin-1 and leads to the formation of a membrane pore, which is required for caspase-1 activation [25,26]. NLRP3 inflammasome activation also occurs following stimulation with the bacterial potassium ionophore nigericin, the marine toxin maitotoxin, and bacterial pore forming toxins such as listeriolysin O from Listeria monocytogenes, aerolysin from Aeromonas hydrophila and Staphylococcus aureus hemolysins [23,27]. Mycobacterium tuberculosis, through its ESX-1 secretion system, has also been shown to activate the NLRP3 inflammasome [28]. Master and colleagues have also demonstrated that the M. tuberculosis gene, zmp1, which encodes a putative Zn2+ metalloprotease, can inhibit inflammasome activation [29]. DNA, bacterial RNA and the antiviral imidazoquinoline compounds R837 and R848 also induce the NLRP3 inflammasome activation independently of TLR and RIG-I [30-32].
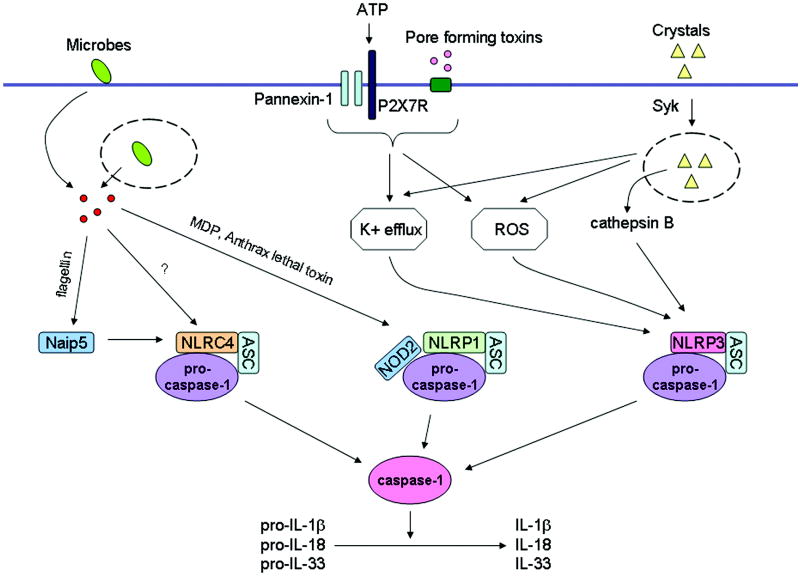
Figure 1.
Inflammasome activation by microbes and danger signals. Several NLRs can form multiprotein complexes called inflammasomes. Activation of the inflammasome results in activation of the cysteine protease caspase-1 and the resultant processing of pro-IL-1β, pro-IL-18 and pro-IL-33 into biologically active IL-1β, IL-18 and IL-33 respectively. Activation of the NLRC4 inflammasome following infection of macrophages with S. typhimurium, P. aeruginosa, S. flexneri or L. pneumophila requires a functional type III or type IV secretion system. Bacterial-derived cytosolic flagellin augments caspase-1 activation following infection with L. pneumophila, S. typhimurium, and P. aeruginosa possibly through a Naip5-dependent pathway. Anthrax lethal toxin and MDP are capable of activating the NLRP1 inflammasome in a manner that may also require NOD2. A wide variety of stimuli including bacterial pore-forming toxins, ATP, DNA, bacterial RNA and crystals such as silica, asbestos, uric acid, alum and amyloid-β can activate the NLRP3 inflammasome. NLRP3 activating PAMPs and DAMPs induce a K+ efflux and the generation of mitochondrial-derived ROS that play a role in NLRP3 inflammasome activation by an unknown mechanism. Crystal induced lysosomal damage, and the resultant release of cathepsin B, are also postulated to play a role in NLRP3 inflammasome activation by an unknown mechanism.
Interestingly, a number of crystalline molecules have been demonstrated to activate the NLRP3 inflammasome. The first study to demonstrate this showed uric acid crystals and calcium pyrophosphate dihydrate, the causative agents of gout and pseudogout respectively, are capable of activating the NLRP3 inflammasome [33]. Subsequently, crystalline silica and asbestos, responsible for causing the fibrotic lung disorders silicosis and asbestosis, have also been demonstrated to activate a similar pathway [34-36]. In addition, the adjuvant properties of aluminum hydroxide (alum) have been shown to be dependent upon its ability to activate the NLRP3 inflammasome [37-40]. Fibrillar amyloid-β, involved in the pathogenesis of Alzheimer’s disease, can also activate the NLRP3 inflammasome in a seemingly similar manner [41].
A number of mechanisms have been shown to play a role in activation of the NLRP3 inflammasome; however, the direct ligand for NLRP3 has yet to be identified. Pore formation is a common step in NLRP3 activation shared by a number of stimuli such as the various bacterial pore-forming toxins as well as by ATP through pannexin-1. Two main hypotheses exist for how pore-formation can lead to NLRP3 inflammasome activation. The first is that membrane disruption results in the modification or release of an endogenous molecule that is recognized by NLRP3 [5,42,43]. The second theory is that microbial molecules gain entry into the cytosol through the pores where they interact with NLRP3 [44]. It has recently been shown that silica, alum and amyloid-β cause lysosomal damage resulting in the release of cathepsin B, which leads to NLRP3 inflammasome activation [36,41]. This model still implicates membrane damage, in particular lysosomal membrane damage, as the initiating event in NLRP3 inflammasome activation, however it does not provide an explanation for NLRP3 activation by non-crystalline molecules such as ATP.
Two events that appear to be required for both toxin- and crystal-mediated NLRP3 inflammasome activation are efflux of intracellular potassium and the generation of reactive oxygen species (ROS). A decrease in intracellular potassium concentrations may be required for assembly of the NLRP3 inflammasome, however the precise role of potassium in this process is unknown [45]. Similarly blockade of ROS production using chemical inhibitors has been shown to mitigate the ability of silica, asbestos and ATP to activate the NLRP3 inflammasome [34,35,46]. The source of ROS involved in NLRP3 inflammasome activation may be mitochondrial derived as mice deficient in specific components of the NADPH oxidase system are still capable of activating the NLRP3 inflammasome [36,47]. An intriguing study by Ng and colleagues demonstrated that uric acid crystals could directly engage cholesterol rich cellular membranes resulting in the activation of a Syk kinase-dependent signaling cascade [48]. Further studies to determine if this pathway is also involved in NLRP3 inflammasome activation and how these various signaling pathways interact will be very revealing.
The NLRC4 inflammasome
The NLRC4 (also known as IPAF, CARD12, CLR2.1 and CLAN) inflammasome is also capable of regulating caspase-1 activation and IL-1β processing. Activation of the NLRC4 inflammasome also leads to rapid cell death. NLRC4 contains an N-terminal CARD, a central NACHT domain and C-terminal LRRs. Recent studies have helped reveal in part the mechanism by which the NLRC4 inflammasome is activated. The NLRC4 inflammasome activators identified to date are Gram-negative bacteria that possess either a type III or type IV secretion system and include Salmonella, Shigella, Legionella, and Pseudomonas (Figure 1) [49-54].
Infection of ASC-deficient macrophages with Salmonella, Shigella and Pseudomonas resulted in defective caspase-1 activation and IL-1β secretion [49,51,52]. However cell death in response to infection proceeded with similar kinetics compared to WT macrophages. These observations suggest caspase-1 activation and cytotoxicity are under separate control. ASC is a crucial component of the NLRC4 inflammasome and is necessary for the production of IL-1β, but in the absence of ASC, NLRC4 may partner with another caspase in order to mediate cytotoxicity. Further studies are needed to elucidate these NLRC4-dependent but caspase-1-independent cell death pathways.
The mechanism by which the NLRC4-inflammasome is activated is beginning to be elucidated. It was initially found that S. typhimurium and L. pneumophila strains deficient in flagellin were defective in their ability to activate macrophage caspase-1 [55-58]. In addition the delivery of purified flagellin into the macrophage cytosol by transfection was capable of activating caspase-1 in an NLRC4-dependent manner [57,58]. Pathogens, such as Salmonella, Legionella, Shigella and Pseudomonas, also require a functional bacterial type III (T3SS) or type IV (T4SS) secretion system in order to activate the NLRC4 inflammasome [50,52,59,60]. These findings have led to the hypothesis that flagellin monomers inadvertently gain entry into the cytosol of infected cells through T3SS or T4SS [57,58]. This accidental delivery of flagellin is detected by the NLRC4 inflammasome and leads to the activation of caspase-1.
In an elegant study performed by Lightfield and colleagues retroviral transduction of L. pneumophila flagellin, in the absence of transfection agents, secretion systems or other bacterial components, was sufficient to trigger NLRC4-dependent macrophage death [61]. Activation of the NLRC4 inflammasome in response to a C-terminal 35 amino acid fragment of L. pneumophila flagellin was dependent on Naip5 [61]. L. pneumophila is unique in that Naip5 in addition to NLRC4 is involved in susceptibility to infection with L. pneumophila. Naip5 and NLRC4 have been shown to physically interact suggesting that the C-terminus of flagellin may trigger the formation of an inflammasome composed of NLRC4 and Naip5 [50,61]. Expression of full-length flagellin and infection with S. typhimurium, and P. aeruginosa were still capable of inducing pyroptosis in a manner that is dependent on NLRC4 but only partially Naip5 dependent [61]. These data suggest that regions of flagellin outside of the C-terminal 35 amino acids can activate NLRC4 in the absence of Naip5. It is tempting to postulate that another related Naip protein may serve as an adaptor for these functions.
The NLRC4 inflammasome can also be activated independently of flagellin. The non-flagellated bacterium S. flexneri has been shown to activate capase-1 in an NLRC4-dependent manner [51]. In addition, P. aeruginosa mutant PAKΔfliC, which is deficient in flagellin, is still capable of activating caspase-1 in an NLRC4-dependent manner [52]. This activation of the NLRC4 inflammasome by organisms deficient in flagellin was still dependent on the T3SS. Of note the groups of Aderem and Núñez did not observe caspase-1 activation using flagellin-deficient P. aeruginosa strains [53,54]. This was perhaps due to differences in multiplicities of infection and infection times between these studies suggesting that both flagellin-dependent and –independent pathways to activate the NLRC4 inflammasome exist.
The NLRP1 Inflammasome
The NLRP1 (also known as Nalp1, DEFCAP, NAC, CARD7 and CLR17.1) inflammasome is a complex composed of caspase-1, caspase-5, and the adaptor molecule ASC [21]. More recently Faustin and colleagues utilized a cell free system to demonstrate that the minimum components required to activate caspase-1 were NLRP1 and pro-caspase-1 [62]. They propose a two-step mechanism for NLRP1 inflammasome activation, whereby the bacterial cell wall component muramyl dipeptide (MDP) causes a conformational change in NLRP1, which then allows the protein to bind ribonucleotide triphosphates and oligomerize [62]. The physiologic role of MDP activation of the NLRP1 inflammasome has yet to be addressed. The adaptor molecule ASC was not required for NLRP1 inflammasome activation although its presence did enhance caspase-1 activation. The anti-apoptotic proteins Bcl-2 and Bcl-XL may also interact with NLRP1 and suppress caspase-1 activation and IL-1β production. Macrophages deficient in Bcl-2, exposed to MDP, exhibit more caspase-1 activity and IL-1β processing, whereas Bcl-2 overexpression led to less caspase-1 activation and IL-1β secretion [63].
Bacillus anthracis lethal toxin (LT) can induce caspase-1-dependent cell death of macrophages. Boyden and Dietrich revealed that the Nlrp1b gene, one of three paralogues of NLRP1 in mice, is responsible for macrophage susceptibility to LT [64]. Hsu et al. have also shown that NLRP1 plays a role in LT-induced caspase-1-dependent IL-1β secretion in response to intact B. anthracis [65]. This study also demonstrated that NOD2 was also required for B. anthracis-induced IL-1β secretion suggesting that NOD2 may interact with the NLRP1 inflammasome [65].
Conclusions
Great strides have been made over the past few years into understanding the immune receptors and pathways critical for the rapid innate response to intracellular pathogens and cellular perturbations. It is apparent that the NLR family of molecules and their functional complex called the inflammasome are central to the generation and regulation of this response. It is equally clear that this field is in its infancy with many key questions unresolved and others as yet unasked, including the fundamental issue of whether NLR and PAMP/DAMP interaction is direct or indirect. In addition, the vast majority of studies have focused on NLRP3, NLRP1 and NLRC4 in macrophages. The wide expression pattern of these molecules across immune cells suggests there are many roles for NLR molecules yet to be examined. The wider significance of the NLR family and the inflammasome lies in their critical role in the initiation of immune responses rendering them an attractive target for therapeutic intervention in a wide range of inflammatory diseases.
Acknowledgments
This work was supported by National Institutes of Health grants K08 AI065517 (F.S.S.) and K08 AI067736 (S.L.C.) and cooperative agreement K01 CK000101 from the Centers for Disease Control and Prevention (J.H.F.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• of outstanding interest
- 1.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 2.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Z, Ting JP. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol. 2008;20:3–9. doi: 10.1016/j.coi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Dostert C, Meylan E, Tschopp J. Intracellular pattern-recognition receptors. Adv Drug Deliv Rev. 2008;60:830–840. doi: 10.1016/j.addr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 6.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. This study identifies novel caspase-1 substrates involved in the glycolysis pathway.
- 8••.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. This study demonstrates that the secretion of numerous leaderless proteins depends on caspase-1 activity.
- 9.He P, Shan L, Sheen J. Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant-microbe interactions. Cell Microbiol. 2007;9:1385–1396. doi: 10.1111/j.1462-5822.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 10.Altenbach D, Robatzek S. Pattern recognition receptors: from the cell surface to intracellular dynamics. Mol Plant Microbe Interact. 2007;20:1031–1039. doi: 10.1094/MPMI-20-9-1031. [DOI] [PubMed] [Google Scholar]
- 11.Mayor A, Martinon F, De Smedt T, Petrilli V, Tschopp J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. 2007;8:497–503. doi: 10.1038/ni1459. [DOI] [PubMed] [Google Scholar]
- 12.da Silva Correia J, Miranda Y, Leonard N, Ulevitch R. SGT1 is essential for Nod1 activation. Proc Natl Acad Sci U S A. 2007;104:6764–6769. doi: 10.1073/pnas.0610926104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur JC, Lich JD, Aziz RK, Kotb M, Ting JP. Heat shock protein 90 associates with monarch-1 and regulates its ability to promote degradation of NF-kappaB-inducing kinase. J Immunol. 2007;179:6291–6296. doi: 10.4049/jimmunol.179.9.6291. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dode C, Le Du N, Cuisset L, Letourneur F, Berthelot JM, Vaudour G, Meyrier A, Watts RA, Scott DG, Nicholls A, et al. New mutations of CIAS1 that are responsible for Muckle-Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. Am J Hum Genet. 2002;70:1498–1506. doi: 10.1086/340786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, de Saint Basile G. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins PN, Lachmann HJ, McDermott MF. Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N Engl J Med. 2003;348:2583–2584. doi: 10.1056/NEJM200306193482523. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, Anderson JP, Wanderer AA, Firestein GS. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 22.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 23.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 24.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 27.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 28.Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol. 2008;10:1866–1878. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, Timmins GS, Sander P, Deretic V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–232. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 31.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 32.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 33.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 34.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. Along with ref 38 and 39 these studies demonstrate the role for NLRP3 in driving adaptive immune responses by the vaccine adjuvant alum.
- 38••.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 40.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol. 2007;82:259–264. doi: 10.1189/jlb.1206755. [DOI] [PubMed] [Google Scholar]
- 44.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 46.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 48.Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 50.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nunez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 54.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci U S A. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 58.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 59.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hilbi H, Moss JE, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell RA, Yuan J, Sansonetti PJ, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 61•.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008 doi: 10.1038/ni.1646. This study shows a crucial role for Naip5 in the activation of caspase-1 in response to flagellin.
- 62••.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. This study is the first to reconstitute a functional NLRP1 inflammasome.
- 63.Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 64.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 65.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]