Abstract
Nitric oxide (NO) is a gas messenger with diverse physiological roles in the nervous system, from modulation of synaptic plasticity and neurogenesis to the mediation of neuronal death. NO production in the brain is catalyzed by three isoforms of NO synthase (NOS) including neuronal NOS (nNOS), inducible NOS and endothelial NOS. In this report, we demonstrate a method for in vitro and in vivo silencing of nNOS using RNAi strategies. Because of their efficiency in infecting postmitotic cells like neurons, lentiviral vectors were used as nNOS shRNA carriers. Of the siRNA sequences screened, one corresponding to exon 10 of the rat nNOS specifically and efficiently inhibited nNOS expression at the mRNA and protein level. In vitro experiments using rat cortical neurons showed the general efficacy of shRNA vectors in silencing constitutively expressed nNOS. To demonstrate the anatomical specificity of nNOS silencing in vivo, vectors were used to selectively knock down the endogenous nNOS expression in cortical GABAergic interneurons of rat piriform cortex. Our findings show that the method reported here can achieve stable and highly effective nNOS suppression in an anatomically defined brain region. The ability of our nNOS silencing vectors to effectively and precisely silence nNOS expression shows their value as research tools for further studies of the role of nNOS in specific brain circuits. Furthermore, our findings raise the possibility for future considerations of lentiviral strategies as therapies for diseases of the nervous system involving NO neurotoxic cascades.
Keywords: neuronal NOS, RNAi, lentivirus, piriform cortex
Introduction
Nitric oxide (NO) is a molecular messenger with prominent roles in neuronal signaling (Garthwaite et al., 1988;Gally et al., 1990;Edelman and Gally, 1992;Schuman and Madison, 1994;West and Grace, 2000) but may also initiate or contribute to neuronal cell death (Nowicki et al., 1991;Zhou et al., 2007). As a diffusible gas that can cross membrane barriers within 100-micrometer distance (Wood and Garthwaite, 1994;Philippides et al., 2000), NO is ideally suited to modulate synaptic plasticity in a spatially constrained fashion (Dawson and Snyder, 1994;Schuman, 1994;Esplugues, 2002;Sunico et al., 2005). NO is synthesized from the terminal guanidino nitrogen of arginine by the action of nitric oxide synthase (NOS). In the nervous system, NOS exists in three isoforms, i.e. neuronal, endothelial and inducible NOS with distinct cellular localizations and roles (Marletta, 1993;Bredt and Snyder, 1994;Yun et al., 1996). Under conditions of excessive activation of the NMDA receptor, NO synthesized in the cytosol by the action of nNOS may initiate both cytosolic and nuclear apoptotic signaling (Garthwaite et al., 1989;Dawson et al., 1991;Bonfoco et al., 1996). As recently shown by us (Koliatsos et al., 2004;Zhou et al., 2006;Zhou et al., 2007) and others (Corso et al., 1997;Parathath et al., 2007), similar processes can be signaled between neurons, i.e. nNOS-expressing interneurons can provide the source of NO that can diffuse and initiate apoptotic cascades in adjacent projection neurons. Excessive intra- or intercellular release of NO may partake in neurotoxic or degenerative insults associated with stroke (Love, 1999;Keynes and Garthwaite, 2004), traumatic brain injury (Rink et al., 1995;Takeuchi et al., 2000;Bell, 2007), Huntington's (Norris et al., 1996;Deckel, 2001;Deckel et al., 2002) and Alzheimer's disease (Gomez-Isla et al., 1996; Kordower et al., 2001;Koliatsos et al., 2006).
The spatially constrained mode of NO toxicity requires a great deal of anatomical specificity in animal models involving manipulations of NOS expression and NO release. Although genetic deletion and pharmacological inhibition of nNOS are powerful strategies that have been successfully used to show a role for nNOS/NO in neuronal injury and apoptosis (Koliatsos et al., 2004;Zhou et al., 2006;Zhou et al., 2007), the effects of these manipulations are not regionally selective. At present, even the most sophisticated genetic designs affect entire populations of neurons, typically in multiple brain regions (Huang et al., 1993;O'Dell et al., 1994;Huang et al., 1995). Thus, pharmacological approaches and transgenic knockout technologies do not allow targeting nNOS expression-NO release in small neuronal networks or individual neurons in the intact brain.
One of the best studied in vivo models of nNOS induction linked to neuronal cell death is the transsynaptic degeneration of pyramidal neurons in the primary olfactory (piriform) cortex after their disconnection from the olfactory bulb (Koliatsos et al., 2004;Zhou et al., 2006). In this model, the transsynaptic apoptosis of >53×103 pyramidal neurons within one day post-injury is preceded by induction of nNOS and release of NO in adjacent GABAergic cortical interneurons (Capurso et al., 1997;Koliatsos et al., 2004). Here, we report a method for an anatomically specific knock-down of nNOS expression in the rat piriform cortex using an RNA interference (RNAi) silencing strategy. RNAi is a natural process of sequence-specific, post-transcriptional gene silencing initiated by double-stranded RNA (dsRNA) homologous to the target gene (Hannon, 2002;McManus and Sharp, 2002). The mechanism of gene silencing by RNAi is known to proceed via a highly conserved two-step process (Zamore et al., 2000). First, long dsRNAs are cleaved by the ribonuclease Dicer, generating small interfering RNAs (siRNAs) 21–23 nucleotides in length (Ketting et al., 2001;Bernstein et al., 2001). Subsequently, the single-stranded antisense siRNA associates with a nuclease complex the RNA interference silencing ribonucleoprotein complex (RISC) and guides target RNA cleavage (Hammond et al., 2000;Uryu et al., 2002). For long-lasting gene silencing, current methodologies take advantage of short-hairpin RNAs (shRNAs) expressed in plasmids or, in the case of cells that are hard to transfect, viral vectors; these RNA substrates are then converted into siRNA in vivo (Brummelkamp et al., 2002;Paddison et al., 2002;Miyagishi and Taira, 2002). Viral vectors founded on lentiviruses can transduce non-dividing cells and, thus, have advantages for applications involving neurons (Naldini et al., 1996;Van den et al., 2003;Dittgen et al., 2004). In the present report, we show that an nNOS shRNA driven by RNA polymerase III (Pol III) promoter in the context of a lentiviral vector can selectively silence nNOS expression in the superficial layer I of the rat piriform cortex.
Materials and Methods
Short Hairpin RNA (shRNA) Design and Vector Production
A series of 21 nucleotide siRNA duplexes against the rat nNOS consensus coding sequence (GenBank Accession No.NM_052799) were designed using the generally available Web software provided by Dharmacon RNA Technologies (Dharmacon,Inc.,Chicago,http://www.dharmacon.com/DesignCenter/DesignCenterPage.aspx). Sequences were determined to be unique to the rat gene by Basic Local Alignment Search Tool (BLAST) searches of the GenBank database. A total of four siRNA duplexes were screened for nNOS knock-down by Western blot analysis in co-transfection experiments with nNOS expression plasmid in HEK293 T cells. The most successful sequence and one non-silencing Luciferase sequence were designed into a shRNA oligonucleotide template consisting of sense, hairpin loop, antisense and terminator sequences, all of which were flanked by restriction enzyme sites to facilitate directional subcloning. The shRNAs were subcloned into the lentiviral vector pLL3.7, generously provided by Dr. Parijs (Massachusetts Institute of Technology, Cambridge, MA) (Rubinson et al., 2003). The resulting vectors encoded eGFP under the transcriptional control of the CMV promoter and either shRNA against nNOS or a nonsilencing-Luciferase shRNA under the U6 promoter. The silencing activity of the shRNAs was tested using heterologous transfection as described in the next section. To produce viral vectors, HEK293 T cells were transiently transfected with the four plasmids as described (Zufferey et al., 1999;Fleming et al., 2001). Briefly,the pLL3.7 shRNA plasmid was transfected into HEK293 T cells together with three packaging plasmids encoding gag-pol (PLP-1) (Invitrogen, Carlsbad, CA), rev (PLP-2) (Invitrogen, Carlsbad, CA), and VSV-G env (Clontech, Mountain View, CA) using the Lipofectamine Plus reagent (Invitrogen). The supernatant was collected 48-72 hours after transfection, passed through 0.2um filters and concentrated by ultracentrifugation (50,000g for 120 minutes at 10 °C). The viral pellet was resuspended in PBS. Transduction unit (TU) titer was assessed on HEK293 T cells in the presence of polybrene 8μg/mL (Sigma-Aldrich, St. Louis, MO). Titers of 2-5×108 TU/ml were routinely achieved.
Cell transfection with nNOS and shRNA plasmids
A full-length nNOS plasmid containing the rat nNOS cDNA in Bluescript SK(-) was generously provided by Dr. Solomon Snyder at Johns Hopkins Medical School, Baltimore, MD. The full length gene was subcloned into the XhoI/SacII site of pIRES2 expression vector (Clontech). In order to establish the knockdown efficacy of the nNOS shRNA sequences, HEK293 T cells were co-transfected with the nNOS expression plasmid and equimolar ratios of the vector plasmids encoding shRNA sequence targeting nNOS (shnNOS) or Luciferase (shLuc) gene. Transfections were performed using Lipofectamine 2000 reagent as per manufacturer's instructions (Invitrogen). Forty eight hours after transfection, nNOS expression was established by Western blot analysis.
Lentiviral Transduction of Cortical Neurons In Vitro
Embryonic day 18 rat cortical cells (E18RC) were isolated from tissues extracted from rat brain (BrainBits, Springfield, IL) as described (Brewer and Torricelli, 2007). Cells were resuspended in Neurobasal medium with 2% w/v B27 supplement (NB-B27, Gibco BRL, Gaithersburg, MD) and 0.5 mM L-glutamine (Gibco) and counted by trypan blue exclusion. A typical cell concentration obtained under these conditions was ∼7 × 106 cells/ml. Dissociated cells were then plated on p35 plates coated with poly-L-lysine (2×106 cells/plate) in the previous medium and incubated at 37 °C with 5% CO2 and 9% oxygen. Cells were transduced with the lentiviral vector at a multiplicity of infection (MOI) = 5 in the presence of 8 μg/ml polybrene (Sigma-Aldrich).
Western blot analysis
Cultured cortical neurons were lysed in M-PER Mammalian Protein Extraction (Pierce Biotechnology, Inc., Rockford, IL) supplemented with Complete Protease Inhibitor Tablets (Roche). The supernatant was collected after centrifugation and protein concentration was determined using a BCA Protein Assay Kit (Pierce). Equal amounts of protein (15μg) were loaded per lane and separated on a 4-20% sodium dodecyl sulfate–polyacrylamide gels and electro-blotted onto nitrocellulose membranes (Schleicher and Schuell, Keene, NH). The rabbit polyclonal anti-nNOS IgG (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:1000 dilution, and the mouse mAb against GAPDH (Abcam Inc., Cambridge, MA) at 1: 5.000 dilution. Secondary antibodies were peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA), respectively, at 1:3000 dilution. Primary and secondary antibodies were diluted in Tris-buffered saline with 0.1% Tween 20 and 4% nonfat milk. After incubation with the secondary antibody for one hour, immunoreactive protein species were detected with an enhanced chemiluminescence kit (Pierce). For quantitation of immunoreactive nNOS bands, blots were scanned and the pixel count and intensity of immunoreactive bands were quantified using the QuantityOne Software (BioRad). Neuronal NOS signal intensity in non-transduced and both silencing- and control-vector transduced neurons was normalized using GAPDH signals as loading controls. Neuronal NOS signals in transduced neurons were expressed as percentages of signal intensity in non-transduced uninfected neurons.
RNA extraction and real-time RT-PCR
Total RNA was isolated from cultured cells using the Qiagen Rneasy kit according to the manufacturer's protocol (Qiagen, Valencia, CA). Template cDNA was reverse transcribed from 1μg of RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) and SYBR green-based real-time PCR was performed using the iCycler (Bio-Rad). Primers were synthesized by Integrated DNA Technologies (Coralville, IA). GAPDH was used for normalization of expression levels. 25 μl PCR reactions, run in duplicate for each sample, contained diluted cDNA (1:4, 2μl), 0.5μl of 10μM sense and antisense primer stocks, 12.5 μl iQ SYBR green super mix (Bio-Rad), and 9.5 μl nuclease free water. PCR cycling conditions were 95°C for 5 min followed by 35 cycles of 95°C for 30 sec, 63°C for 1min. Immediately following amplification, melt curve analysis was carried out by heating the amplicon from 63 to 95°C in 66 0.5 °C increments. Reactions without template and reactions containing 25ng of total RNA served as non-template and genomic DNA contamination controls, respectively. Cycle number was obtained when amplification exceeded threshold (Ct) values (Livak, 1997). All target gene Ct values in each parameter were normalized to a reference Ct value to determine the ΔCt value (target gene Ct - reference gene Ct). Relative gene expression was shown as fold change in gene expression using the comparative Ct method (ΔΔCt method) (Livak, 1997). The amount of nNOS transcript in controls was designated as 100%, to which cells infected with the silencing lentivirus were compared to produce % change in nNOS mRNA expression. The sequences of the rat nNOS primers were: forward primer, 5′GACAACGTTCCTGTGGTCCT; reverse primer, 5′ TCCAGTGTGCTCTTCAGGTG.
Animals and Surgical Procedures
Subjects were pathogen-free Sprague–Dawley rats (2-3 months of age; Charles River Laboratories, Inc., Wilmington, MA). Animals were maintained in a 12h light–dark cycle, had free access to food and water and were handled based on protocols approved by the Institutional Animal Care and Use Committee of the Johns Hopkins Medical Institutions according to National Institutes of Health guidelines (NIH Publications No. 8023, 1978 revision). Surgical procedures were carried out using gas anesthesia (enflurane: oxygen: nitrous oxide = 1: 33: 66) and aseptic methods. For the injection of virus vectors, animals were mounted on a small-animal Kopf stereotaxic unit (Kopf Instruments, Tujunga, CA). Injections were made under microscopic guidance via pulled glass micropipettes controlled by a Nanoinjector device (World Precision Instruments, Sarasota, FL). Animals received 1.5 μl of the silencing (n= 6) or control (n = 6) vector over 10 min into the piriform cortex targeted at 1.6 mm anterior to bregma and 4.0 mm lateral to midline using the flat skull position (Dottori et al., 1998). The injector was left in place for 3 min post-infusion to allow sufficient vector diffusion.
Animals were subjected to unilateral bulbotomy about 20 days after vector injection. Lesions were used to induce nNOS expression in interneurons of piriform cortex (Koliatsos et al., 2004) and, thus, allow us to explore whether pretreatment with nNOS silencing vectors prevents the induced upregulation of the enzyme. Rats were anesthetized, adjusted on the small-animal Kopf stereotaxic device and subjected to unilateral transection of the olfactory peduncle on the right, 0.5 mm rostral to the frontal pole, under direct visualization.
Preparation of tissues and Immunocytochemistry (ICC)
Twenty-four hours after bulbotomy all animals were deeply anesthetized and perfused with 4% freshly depolymerized paraformaldehyde based on protocols approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. Brain tissues were further fixed by immersion in the same fixative for an additional 4 hr at 4°C. Brains were cryoprotected by transferring to 30% sucrose in PBS at 4°C until submersed and frozen for further processing. Serial coronal sections (40 μm) through the forebrain were prepared on a sliding microtome. Sections through the entire anterior piriform cortex, beginning at the first appearance of piriform cortex ventral to the anterior olfactory nucleus and ending at the decussation of the anterior commissure, were saved in antifreeze buffer in - 20°C. Every 4th section starting at a random level through the major island of Calleja rostrally and ending at the decussation of the anterior commissure caudally was processed for ICC detection of nNOS expression with a mouse monoclonal antibody (1:500; Zymed laboratories, South San Francisco, CA). Immunoreactivity was indirectly visualized with Cy3 conjugated goat anti-mouse IgG (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA) emitting at the red range. Sections were first blocked with 5% normal goat serum in PBS with 0.1% Triton X-100 for 1 h and then incubated in the primary antibody solution on a shaker for 24 h (4°C). Secondary incubations were performed for 2-4 h at RT. Sections were counterstained with the nuclear dye DAPI, dehydrated, and coverslipped with DPX (Fluka, Hauppauge, New York). Sections were then studied with a Zeiss Axiophot microscope equipped for epifluorescence and images were captured with a Spot RT Slider digital camera (Diagnostic Instruments Inc., Sterling Heights, MI) using software provided by the manufacturer.
A total of 9 sections (every 4th from a series centered around the vector injection site) were entered for cell counts per case (n = 6 per nNOS or luciferase vector). Neuronal NOS immunoreactive profiles were counted at 40× magnification in layer I of piriform cortex on the side of bulbotomy. These numbers were multiplied by sampling factor 4 to generate numbers of nNOS (+) neurons per case and the resulting numbers were corrected for differences in cell size by applying Abercrombie's adjustment for split-cell error (Abercrombie, 1946) (Ni = ni.t/t+d, where ni = estimated cell number, t = section thickness, and d = average profile diameter). Cases were grouped per experimental history (nNOS versus luciferase vector) and differences between the two groups were studied with a two-tailed unpaired Students't test.
Results
Design of shRNAs, preparation and in vitro validation of nNOS shRNA lentiviral vectors
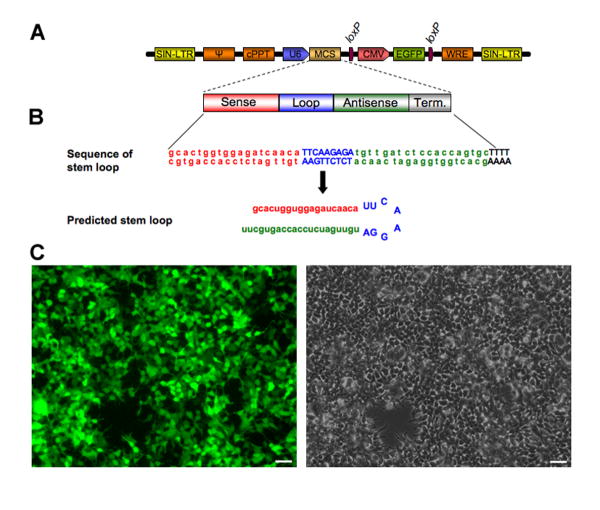
Putative short interfering RNA (siRNA) target sequences were designed against the rat nNOS gene using the Web software provided by Dharmacon RNA Technologies. Four siRNA sequences were screened with Western blot analysis after co-transfection of siRNAs with nNOS expression plasmid in HEK293 T cells (data not shown). The best performing sequence was GCACUGGUGGAGAUCAACA and corresponded to exon 10 of the rat nNOS (Bredt et al., 1991). This sequence was used to generate shRNA for both in vitro and in vivo experiments. Oligonucleotides were synthesized that contained the sense and antisense sequences of the siRNA target of interest flanking a standard hairpin loop sequence (Fig.1A-B). Sense and antisense oligonucleotides were then annealed and cloned into the pLL3.7 lentiviral vector system to express short hairpin RNA (shRNA) directed against nNOS under the control of the U6 promoter (Fig. 1A-B). The transduction efficiency of these vectors was then tested in HEK293 T cells using an MOI equal to 1. Based on EGFP expression, more than 90% of cells were transduced with no apparent cytotoxicity (Fig. 1C).
Figure 1.
Neuronal NOS shRNA design and expression from viral vectors.
(A) Schematic drawing of the pLL3.7 silencing vector for knocking down nNOS. The vector encodes a stem loop sequence for nNOS shRNA driven by mouse U6 promoter and CMV-driven EGFP (a fluorescent reporter gene) donwnstream. SIN-LTR, self-inactivating long term repeat; Ψ, HIV packaging signal; cPPT, central polypurine track; MCS, multiple cloning site; CMV, cytomegalovirus promoter; WRE, Woodchuck hepatitis virus response element.
(B) Sequence of the shRNA used in our study. The rat nNOS stem loop sequence is on top, with sense bases identical to the target gene sequence indicated in red, sequence forming the loop structure in blue, and antisense bases complementary to the sense bases in green; polyT termination sequence for RNA Polymerase III is in black. The predicted stem-loop structure (shRNA duplex) is at the bottom.
(C) Transduction efficiency of our nNOS silencing vector. HEK293 T cells were transduced with the nNOS shRNA vector (MOI=1). The EGFP fluorescence (left panel) and the corresponding phase-contrast image (right panel) are shown. GFP expression reveals high transduction efficiency, with more than 90% of cells being transduced.
Scale bars : 50 μm
Sequences of shRNA form stem-loop structures that are processed to functional siRNAs in target cells. To ensure that reduction in the expression of the target gene is specific and does not reflect a potential global suppression of gene expression by high levels of double stranded RNA, we used unrelated shRNA sequences targeted to firefly luciferase as controls.
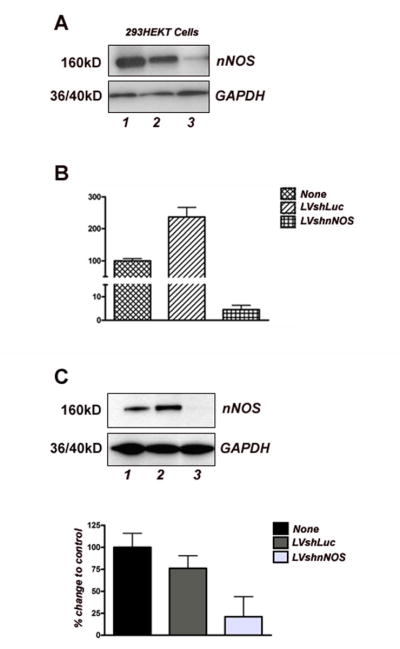
We next tested the gene-silencing capacity of nNOS hairpin in a transient transfection assay using HEK293 T cells. Cells were transfected with a rat nNOS cDNA expression plasmid alone or in combination with equimolar ratios of vector plasmids encoding shRNA sequences for nNOS or luciferase genes. Forty eight hours after transfection, nNOS silencing was established at the protein level with Western blot analysis that showed a higher than 90% reduction in nNOS expression in nNOS shRNA-transfected cells. In contrast, infection of HEK293 T cells with the control luciferase shRNA plasmid had no effect on nNOS expression (Fig. 2A).
Figure 2.
Validation of the nNOS shRNA (LVshnNOS) vector using a transient transfection assay (A) and transduction of primary neuronal cultures (B-C).
(A) Western blot analysis of protein lysates from HEK293 T cells transfected with an expression vector encoding rat nNOS alone (Lane 1) or in combination with a Lucipherase shRNA plasmid (Lane 2) and an nNOS shRNA plasmid (Lane 3). Lane 3 shows a substantial decrease in nNOS expression.
(B-C) Silencing of endogenous nNOS in primary rat neuronal cultures as demonstrated by real-time PCR (B) and Western blot analysis (C). Control vectors (LVshLuc) contained an unrelated Luciferase shRNA sequence. In (B), RNA was isolated 48 h after transduction; real-time PCR used primers for both GAPDH (internal control) and nNOS transcripts; expression in non-transduced neurons was set as 100% and expression using silencing and control vectors was configured accordingly (n=3 experiments). In (C); nNOS protein is nearly undetectable on lysates from rat neurons transduced with the silencing vector (lane 3). Lanes 1 and 2 represent luciferase-vector transduced and non-transduced neurons, respectively. Densitometric analysis is presented as a histogram of relative density, with immunoreactive nNOS band density in non-transduced neurons set as 100%. (n=3 experiments).
Lentivirus-mediated downregulation of endogenous nNOS expression in primary neuronal cultures
To analyze the effect of lentiviral vector-mediated RNAi in primary neuronal cells, cortical neurons were prepared from dissociated E18 rat cortex and after 6 days in culture they were transduced with the silencing or control vectors (MOI = 5 for both). Transduction efficiency was ≥ 90% as determined by the number of neurons expressing GFP. Neuronal NOS silencing was ascertained with real-time RT-PCR and Western blot analysis (Fig. 2B-C).
To explore the knockdown effect of our lentivirus silencing vector on nNOS gene transcription, neuronal RNA was analyzed 48 hrs after transduction with the lentiviral shRNA vectors. Using an MOI of 5, we achieved a reduction of nNOS expression by more than 90% based on quantitative RT-PCR analysis (Fig. 2B). Higher MOI did not result in further reduction of nNOS expression (data not shown).
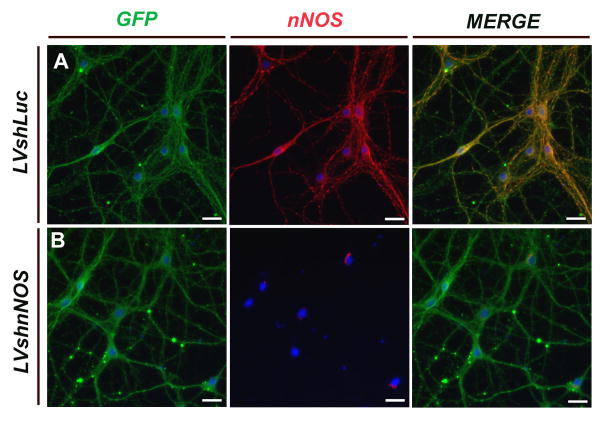
To explore the effects of the silencing vector on nNOS protein levels, neurons were transduced with the silencing or control vector as described above and Western blot analysis was performed on protein samples from cell lysates. Treatment of neurons with the silencing vector resulted in a significant reduction (∼ 75%) of nNOS protein levels compared with those in uninfected neurons or neurons infected with the control vector (Fig. 2C). ICC was used to confirm the absence of nNOS protein expression in rat neurons transduced with nNOS silencing vectors. Although nNOS was detected by specific antibodies in neurons transduced with control vectors (Fig. 3A), no nNOS immunoreactivity was observed in neurons transduced with the silencing vector (Fig. 3B).
Figure 3.
Lentiviral-mediating silencing of nNOS immunoreactivity in rat cortical neurons by ICC. Primary rat E18 cortical neurons were transduced with shRNA vectors targeting either Luciferase (A; LVshLuc) or nNOS (B; LVshnNOS). Transduction was assessed by EGFP expression and nNOS immunoreactivity (red). Cultures were counterstained with DAPI (blue). All neurons in both control and silencing cell preparations show robust transduction with lentiviral vectors, but neurons infected with the silencing vector do not have detectable nNOS immunoreactivity.
Scale bars : 25 μm
In situ silencing of nNOS expression in piriform cortex with shRNA lentiviral vectors
To investigate the efficiency of the nNOS shRNA vector in vivo, we targeted nNOS-expressing interneurons in the outer piriform cortex of adult rats (Koliatsos et al., 2004). Silencing or control vectors were stereotaxically injected in the anterior piriform cortex. Twenty days later, i.e. time sufficient to allow for efficient transduction of neurons and lentiviral-mediated expression of the shRNAs, we performed unilateral bulbotomy on the side of vector injection to maximize the expression of nNOS in interneurons of layer I of piriform cortex as described (Koliatsos et al., 2004). Animals were sacrificed 24 hours post-bulbotomy and coronal sections through the forebrain were processed for nNOS ICC.
Examination of nNOS-stained sections through the anterior piriform cortex shows a significant reduction in nNOS immunoreactivity in interneurons of subjects that received the nNOS shRNA vector injection (Fig.4A, lower panel, arrows), as compared to subjects injected with the control luciferase shRNA virus (Fig. 4A, upper panel,arrows). Adjacent sections visualized with a green emission filter show many infected neurons in the piriform cortex of injected animals (Fig. 4A, inset in lower panel). We assessed levels of nNOS silencing by counting numbers of nNOS-immunoreactive neurons in layer I of piriform cortex. We found that nNOS-positive cells in animals injected with the nNOS shRNA vector are reduced by 23% (p < 0.05) compared to control group injected with the luciferase shRNA virus (Fig. 4B).
Figure 4.
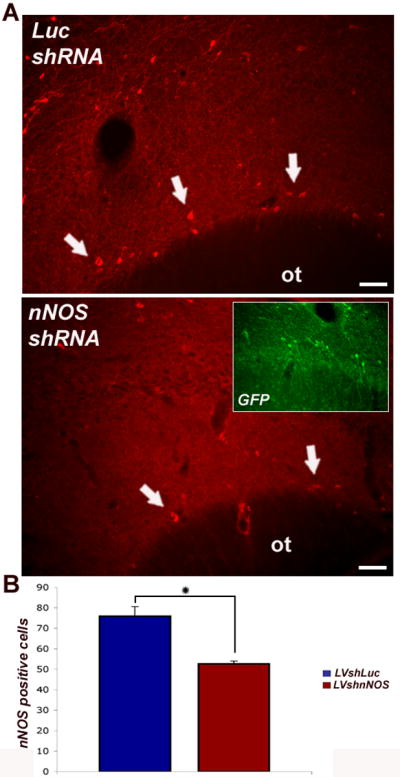
Lentiviral-mediated silencing of nNOS in vivo.
Piriform cortex was targeted because of the abundance of nNOS expression in layer I interneurons (arrows), especially after bulb lesions. Silencing (nNOS shRNA) or control (Luciferase shRNA) vectors that coexpress reporter GFP were stereotaxically injected into the superficial piriform cortex. Prior to sacrifice, all rats were subjected to bulbotomy. Coronal sections through the forebrain were processed for nNOS immunocytochemistry (red) and numbers of nNOS (+) interneurons in layer I of piriform cortex were counted in nine serial sections ipsilateral to vector injection.
(A) Examination of nNOS-stained sections shows a substantial reduction of nNOS immunoreactivity after nNOS shRNA vector injections (lower panel, arrows) compared to control ones (upper panel, arrows). Adjacent sections visualized with a green emission filter combination show numerous neurons in the piriform cortex that were GFP positive, i.e. evidence of local infection with lentivirus (inset in lower panel). Scale bars : 25 μm
(B) Numbers of nNOS positive neurons on the injection side were compared between animals injected with control vectors (LVshLuc, n = 6) and subjects injected with silencing vectors (LVshnNOS, n = 6). Difference in cell numbers was significant by Student's t test (p < 0.05).
Discussion
In this report, we demonstrate a method for in vitro and in vivo silencing of nNOS using RNAi strategies. Of the four sequences that were designed and screened only one siRNA, i.e. the one corresponding to exon 10 of the rat nNOS, specifically and efficiently inhibited expression of the target gene at the mRNA and protein level. The low activity of the other siRNAs may be related to limited accessibility of the corresponding mRNA segments for cleavage due to their secondary structure (Elbashir et al., 2002;Holen et al., 2002). The selection of the target gene sequence for siRNA synthesis is based, for the most part, on trial and error and there are no reliable ways to predict an ideal siRNA sequence for knocking down the expression of a particular gene.
The active nNOS siRNA sequence was used to construct an shRNA expression cassette that was then incorporated into a lentiviral vector system. A key advantage of lentiviral vectors over other gene delivery systems is that they can efficiently transduce post-mitotic cells like neurons. Because lentiviral vectors allow transduction of non-dividing cells and have also been shown to result in long-term gene expression in neurons (Blomer et al., 1997;Zala et al., 2004;Watson et al., 2004), we tested whether shRNA expression from this vector was functional in cultured neurons and in neurons expressing nNOS in vivo. Our in vitro experiments proved the efficacy of this vector with respect to both high transduction efficiency (≥ 90%) and nNOS silencing in cultured primary cortical neurons. Our in vivo experiments targeting nNOS-expressing interneurons in the piriform cortex of adult rats showed that, even though the vectors may perform somewhat different in vivo compared to in vitro (based on the main measure of in vivo efficacy, i.e. numbers of nNOS-immunoreactive neurons, silencing effect was at 23%), these vectors can be used to deliver shRNAs to the rat piriform cortex and provide persistent knockdown of endogenous nNOS expression. The main reason for the apparent attenuated efficiency of our lentiviral vectors in vivo may be the difficulty to achieve, in vivo, the same MOI as in vitro, due to the complex spatial distribution of target cells and limits in the amount of vector that can be delivered into any single brain site. Diffusion of the injected virus to brain structures that surround the neurons targeted for silencing is also inevitable in live applications.
The selection of piriform cortex as the target brain region for nNOS silencing vectors was motivated by a need to show the selective knock- down of nNOS expression in an anatomically defined cortical region and our general familiarity with this cortical target (Koliatsos et al., 2004;Zhou et al., 2006;Zhou et al., 2007). In piriform cortex, target cells are cortical GABAergic interneurons that constitutively express nNOS and this expression is further upregulated with surgical (Koliatsos et al., 2004;Zhou et al., 2006) or pharmacological (Zhou et al., 2007) lesions and contributes to the transsynaptic apoptosis of a subset of pyramidal neurons (Koliatsos et al., 2004). Inhibitory cortical interneurons have recently received a great deal of attention for their role in a number of neurodevelopmental disorders, presumably because of their regulatory influences in the establishment of mature cortical circuits (Xu et al., 2003;Levitt et al., 2004). Therefore, experiments using our lentiviral RNAi methodology may shed light into broader physiological roles of a subset of cortical interneurons, especially with respect to the development and repair of cortical circuitry.
In addition to studies of cortical plasticity, the permanence of the silencing effect observed with our nNOS shRNA vectors allows for long-term investigations into the role of NO signaling in late neurogenic events including the generation and, possibly, differentiation of newborn neurons. For example, it has been proposed that endogenous NO, produced in proximity to neuronal precursors in the subventricular zone ( SVZ), rostral migratory stream (RMS), and the olfactory bulb (OB), plays a regulatory role in adult neurogenesis by suppressing the proliferation rate of undifferentiated precursors and by promoting neuronal differentiation (Moreno-Lopez et al., 2000;Packer et al., 2003;Moreno-Lopez et al., 2004;Torroglosa et al., 2007). In other studies, especially in the PNS, NO produced by nNOS may play protective roles for peripheral sensory neurons (Thippeswamy et al., 2001) as well as interneurons and motor neurons in the spinal cord after sciatic transections (Keilhoff et al., 2004).
One of the greatest potential applications of RNAi strategies is their use as therapies for neurological diseases (Xia et al., 2004;Raoul et al., 2005;Harper et al., 2005;Singer et al., 2005). The complex anatomical organization of the nervous system and the selective vulnerability of specific subsets of neurons to particular pathogenic mechanisms and diseases argue for regionally precise therapeutic interventions. Such interventions may include the silencing of genes whose expression may have toxic effects in one site, but beneficial or neutral effects in other sites. Retinal diseases may be prime examples of pathological events in small neuronal networks accessible to RNAi nNOS interventions. For example, it has been recently shown that both in the case of animal models of chronic glaucoma (Park et al., 2007) and retinitis pigmentosa (Komeima et al., 2008) blocking nNOS may have protective effects on injured retinal ganglion and photoreceptor cells, respectively. RNAi nNOS technologies that allow effective and precisely targeted nNOS gene silencing may hold promise as therapeutic strategies for retinal diseases and possibly other disorders of the nervous system with significant involvement of nNOS-NO neurotoxic cascades.
Acknowledgments
This work was supported by NIH grants RO1 AG16263 and 3 P50 AG05146.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Bell JD. Molecular cross talk in traumatic brain injury. J Neurosci. 2007;27:2153–2154. doi: 10.1523/JNEUROSCI.4929-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage F. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. Journal of Virology. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfoco E, Leist M, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Cytoskeletal breakdown and apoptosis elicited by NO donors in cerebellar granule cells require NMDA receptor activation. J Neurochem. 1996;67:2484–2493. doi: 10.1046/j.1471-4159.1996.67062484.x. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Brummelkamp T, Bernards R, Agani F. A system for stable expression of short interfering RNAs in mammalian cells. Science (New York, N Y) 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Capurso SA, Calhoun ME, Sukhov RR, Mouton PR, Price DL, Koliatsos VE. Deafferentation causes apoptosis in cortical sensory neurons in the adult rat. J Neurosci. 1997;17:7372–7384. doi: 10.1523/JNEUROSCI.17-19-07372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso TD, Sesma MA, Tenkova TI, Der TC, Wozniak DF, Farber NB, Olney JW. Multifocal brain damage induced by phencyclidine is augmented by pilocarpine. Brain Research. 1997;752:1–14. doi: 10.1016/s0006-8993(96)01347-9. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci USA. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckel AW. Nitric oxide and nitric oxide synthase in Huntington's disease. J Neurosci Res. 2001;64:99–107. doi: 10.1002/jnr.1057. [DOI] [PubMed] [Google Scholar]
- Deckel AW, Tang V, Nuttal D, Gary K, Elder R. Altered neuronal nitric oxide synthase expression contributes to disease progression in Huntington's disease transgenic mice. Brain Res. 2002;939:76–86. doi: 10.1016/s0006-8993(02)02550-7. [DOI] [PubMed] [Google Scholar]
- Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, Helmchen F, Denk W, Brecht M, Osten P. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci U S A. 2004;101:18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottori M, Hartley L, Galea M, Paxinos G, Polizzotto M, Kilpatrick T, Bartlett PF, Murphy M, Kontgen F, Boyd AW. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci U S A. 1998;95:13248–13253. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykxhoorn DM, Novina CD, Sharp PA. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol. 2003;4:457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Gally JA. Nitric oxide: linking space and time in the brain. Proc Natl Acad Sci U S A. 1992;89:11651–11652. doi: 10.1073/pnas.89.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- Esplugues JV. NO as a signalling molecule in the nervous system. Br J Pharmacol. 2002;135:1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J, Ginn SL, Weinberger RP, Trahair TN, Smythe JA, Alexander IE. Adeno-associated virus and lentivirus vectors mediate efficient and sustained transduction of cultured mouse and human dorsal root ganglia sensory neurons. Hum Gene Ther. 2001;12:77–86. doi: 10.1089/104303401450997. [DOI] [PubMed] [Google Scholar]
- Gally JA, Montague PR, Reeke GN, Jr, Edelman GM. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990;87:3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Garthwaite G, Palmer RMJ, Moncada S. Nmda Receptor Activation Induces Nitric-Oxide Synthesis from Arginine in Rat-Brain Slices. European Journal of Pharmacology-Molecular Pharmacology Section. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, Yang L, Kotin RM, Paulson HL, Davidson BL. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holen T, Amarzguioui M, Wiiger MT, Babaie E, Prydz H. Positional effects of short interfering RNAs targeting the human coagulation trigger Tissue Factor. Nucleic Acids Res. 2002;30:1757–1766. doi: 10.1093/nar/30.8.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Fansa H, Wolf G. Neuronal NOS deficiency promotes apoptotic cell death of spinal cord neurons after peripheral nerve transection. Nitric Oxide. 2004;10:101–111. doi: 10.1016/j.niox.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes RG, Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Current Molecular Medicine. 2004;4:179–191. doi: 10.2174/1566524043479176. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Dawson TM, Kecojevic A, Zhou Y, Wang YF, Huang KX. Cortical interneurons become activated by deafferentation and instruct the apoptosis of pyramidal neurons. Proc Natl Acad Sci U S A. 2004;101:14264–14269. doi: 10.1073/pnas.0404364101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, Kecojevic A, Troncoso JC, Gastard MC, Bennett DA, Schneider JA. Early involvement of small inhibitory cortical interneurons in Alzheimer's disease. Acta Neuropathol. 2006;112:147–162. doi: 10.1007/s00401-006-0068-6. [DOI] [PubMed] [Google Scholar]
- Komeima K, Usui S, Shen J, Rogers BS, Campochiaro PA. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2008.06.020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends in Neurosciences. 2004;27:400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Livak. Relative quantification of gene expression. ABI Prism 7700 Sequence Detection System, 1997;Applied Biosystems User Bulletin #2.
- Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta MA. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993;268:12231–12234. [PubMed] [Google Scholar]
- McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- Miyagishi M, Taira K. U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol. 2002;20:497–500. doi: 10.1038/nbt0502-497. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Noval JA, Gonzalez-Bonet LG, Estrada C. Morphological bases for a role of nitric oxide in adult neurogenesis. Brain Research. 2000;869:244–250. doi: 10.1016/s0006-8993(00)02474-4. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. Journal of Neuroscience. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. see comments. [DOI] [PubMed] [Google Scholar]
- Norris PJ, Waldvogel HJ, Faull RL, Love DR, Emson PC. Decreased neuronal nitric oxide synthase messenger RNA and somatostatin messenger RNA in the striatum of Huntington's disease. Neuroscience. 1996;72:1037–1047. doi: 10.1016/0306-4522(95)00596-x. [DOI] [PubMed] [Google Scholar]
- Nowicki JP, Duval D, Poignet H, Scatton B. Nitric oxide mediates neuronal death after focal cerebral ischemia in the mous. Eur J Pharmacol. 1991;204:339–340. doi: 10.1016/0014-2999(91)90862-k. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, Fishman MC. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science (New York), NY. 1994;265:542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison PJ, Hannon GJ. RNA interference: the new somatic cell genetics? Cancer Cell. 2002;2:17–23. doi: 10.1016/s1535-6108(02)00092-2. [DOI] [PubMed] [Google Scholar]
- Parathath SR, Gravanis I, Tsirka SE. Nitric oxide synthase isoforms undertake unique roles during excitotoxicity. Stroke. 2007;38:1938–1945. doi: 10.1161/STROKEAHA.106.478826. [DOI] [PubMed] [Google Scholar]
- Park SH, Kim JH, Kim YH, Park CK. Expression of neuronal nitric oxide synthase in the retina of a rat model of chronic glaucoma. Vision Res. 2007;47:2732–2740. doi: 10.1016/j.visres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Philippides A, Husbands P, O'Shea M. Four-dimensional neuronal signaling by nitric oxide: a computational analysis. J Neurosci. 2000;20:1199–1207. doi: 10.1523/JNEUROSCI.20-03-01199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- Rink A, Fung KM, Trojanowski JQ, Lee VM, Neugebauer E, McIntosh TK. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol. 1995;147:1575–1583. [PMC free article] [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Schuman EM. Molecular consequences of diffusible signaling: locally distributed synaptic enhancement in hippocampal neurons. Semin Cell Biol. 1994;5:251–261. doi: 10.1006/scel.1994.1031. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. Nitric oxide and synaptic function. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- Singer O, Marr RA, Rockenstein E, Crews L, Coufal NG, Gage FH, Verma IM, Masliah E. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- Sunico CR, Portillo F, Gonzalez-Forero D, Moreno-Lopez B. Nitric-oxide-directed synaptic remodeling in the adult mammal CNS. J Neurosci. 2005;25:1448–1458. doi: 10.1523/JNEUROSCI.4600-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A, Miyaishi O, Kiuchi K, Isobe K. Cu/Zn- and Mn-superoxide dismutases are specifically up-regulated in neurons after focal brain injury. J Neurobiol. 2000;45:39–46. doi: 10.1002/1097-4695(200010)45:1<39::aid-neu4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Thippeswamy T, Jain RK, Mumtaz N, Morris R. Inhibition of neuronal nitric oxide synthase results in neurodegenerative changes in the axotomised dorsal root ganglion neurons: evidence for a neuroprotective role of nitric oxide in vivo. Neurosci Res. 2001;40:37–44. doi: 10.1016/s0168-0102(01)00205-x. [DOI] [PubMed] [Google Scholar]
- Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C, Matarredona ER, Campos-Caro A, Estrada C. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25:88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S, Lee VM, Trojanowski JQ. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci. 2002;22:446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den HC, Eggermont K, Nuttin B, Debyser Z, Baekelandt V. Lentiviral vector-mediated delivery of short hairpin RNA results in persistent knockdown of gene expression in mouse brain. Hum Gene Ther. 2003;14:1799–1807. doi: 10.1089/104303403322611809. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Karolewski BA, Wolfe JH. Stable gene delivery to CNS cells using lentiviral vectors. Methods Mol Biol. 2004;246:413–428. doi: 10.1385/1-59259-650-9:413. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Striatal nitric oxide signaling regulates the neuronal activity of midbrain dopamine neurons in vivo. J Neurophysiol. 2000;83:1796–1808. doi: 10.1152/jn.2000.83.4.1796. [DOI] [PubMed] [Google Scholar]
- Wood J, Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994;33:1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM, Davidson BL. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- Xu Q, de la CE, Anderson SA. Cortical interneuron fate determination: diverse sources for distinct subtypes? Cereb Cortex. 2003;13:670–676. doi: 10.1093/cercor/13.6.670. [DOI] [PubMed] [Google Scholar]
- Yun HY, Dawson VL, Dawson TM. Neurobiology of nitric oxide. Crit Rev Neurobiol. 1996;10:291–316. doi: 10.1615/critrevneurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- Zala D, Bensadoun JC, Pereira dA, Leavitt BR, Gutekunst CA, Aebischer P, Hayden MR, Deglon N. Long-term lentiviral-mediated expression of ciliary neurotrophic factor in the striatum of Huntington's disease transgenic mice. Exp Neurol. 2004;185:26–35. doi: 10.1016/j.expneurol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Zhou L, Welsh AM, Chen D, Koliatsos VE. NMDA inhibitors cause apoptosis of pyramidal neurons in mature piriform cortex: Evidence for a nitric oxide-mediated effect involving inhibitory interneurons. Neuropharmacology. 2007;52:1528–1537. doi: 10.1016/j.neuropharm.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou L, Chen H, Koliatsos VE. An AMPA glutamatergic receptor activation-nitric oxide synthesis step signals transsynaptic apoptosis in limbic cortex. Neuropharmacology. 2006;51:67–76. doi: 10.1016/j.neuropharm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]