Abstract
Abnormalities in DC function are implicated in defective immune regulation that leads to type-1 diabetes (T1D) in NOD mice and humans. In this study, we used GM-CSF and Flt3-L to modulate DC function in NOD mice and observed the effects on T1D development. Treatment with either ligand at earlier stages of insulitis suppressed the development of T1D. Unlike Flt3-L, GM-CSF was more effective in suppressing T1D, even when administered at later stages of insulitis. In vitro studies and in vivo adoptive transfer experiments revealed that CD4+CD25+ T cells from GM-CSF-treated mice could suppress effector T cell response and T1D. This suppression is likely mediated through enhanced IL-10 and TGF-β1 production. Adoptive transfer of GM-CSF exposed DCs to naive mice resulted in an expansion of Foxp3+ T cells and a significant delay in T1D onset. Our results indicate that GM-CSF acted primarily on DCs and caused an expansion of Foxp3+ Tregs which delayed the onset of T1D in NOD mice.
Keywords: GM-CSF, dendritic cells, regulatory T cells, tolerance, Type 1 diabetes, NOD
Introduction
Type 1 diabetes (T1D) is a T cell-mediated disease in which insulin-producing pancreatic β-cells are selectively destroyed. Studies in T1D patients and using NOD mice show an essential role for CD4+ T cells in β-cell destruction [1; 2; 3]. Thus, these pathogenic T cells most likely encounter the β cell antigens on APCs because the target cells do not express MHC class II molecules under normal conditions [4; 5; 6]. Dendritic cells (DC) are a major type of APC constituent of islet infiltrates. It has long been recognized that DCs are the most potent APCs and the only population capable of activating primary T cell response to self-antigens [7; 8]. A number of studies suggest a critical role for DCs in the development and progression of T1D in the NOD mouse because they are the first leukocytes to infiltrate islets during insulitis [1; 9]. Therefore, the importance of DCs in initiating and promoting autoimmunity through β-cell antigen presentation is well-recognized.
On the other hand, a growing body of evidence indicates that DCs play a key role in maintaining peripheral tolerance to self-antigens [10; 11; 12]. This notion has been further substantiated by the ability of immature and non-activated DCs to induce and/or stimulate regulatory T cell populations (Tregs) under normal circumstances [13]. Maintenance of natural Tregs function in both mice and humans are linked to their direct interactions with DCs [12; 14]. Therefore, abnormalities in the functions of DCs and Tregs in T1D patients and animal models have been implicated in promoting autoimmunity in T1D. Phenotypic, maturation and functional abnormalities in DCs have been noted in both humans with T1D and NOD mice [15; 16; 17]. Waning of naturally-existing Treg function during the progression of T1D has also been reported and implicated in the development of T1D [18]. It has been shown that DCs in NOD mice fail to efficiently activate Tregs, due in part to maturation defects, thus allowing pathogenic T cells to cause autoimmune destruction of β cells [19]. Therefore, restoration of tolerogenic DC and Treg functions has been the focus of recent immunotherapeutic studies aimed at preventing and suppressing autoimmune responses against β-cell antigens [20; 21].
In recent studies, we examined GM-CSF and Flt3-L for their ability to modulate DC function and induce T cell tolerance in different immunization induced-autoimmune models [22; 23; 24; 25]. These studies demonstrated the ability of GM-CSF to induce maturation-resistant tolerogenic CD8a- DCs in vivo and suppress autoimmune response by generating Tregs. On the other hand, Flt3-L induced both CD8a+ and CD8a- DCs and skewed T cell response against inoculated antigens predominantly towards Th1 type and aggravated the disease [22; 23; 24]. However, studies using NOD mice in which T1D develops spontaneously without the requirement for exogenous antigen inoculation have shown that both GM-CSF and Flt3-L could delay the onset of diabetes [26; 27; 28; 29]. Therefore it is likely that the ability of these DC modulators to repair and/or restore tolerogenic function of DCs and suppress autoimmunity in T1D might depend on the dose and/or time of initiation of the treatment relative to the development of insulitis.
In the current study, we examined the ability of GM-CSF or Flt3-L treatment, initiated at different times, to modulate the function of CD8a+ and CD8a- DC sub-populations and affect the disease progression in NOD mice. Treatment of NOD mice with Flt3-L or GM-CSF at very early stages of insulitis resulted in an overall increase in the number of DCs and CD4+CD25+ Tregs, and caused significant delay in the onset of T1D. However, treatment with GM-CSF, not Flt3-L, at later stages of insulitis significantly delayed the onset of hyperglycemia until long after the cessation of treatment. The protection was mediated through IL-10 and TGF-β1 produced by CD4+CD25+ Tregs. Furthermore, adoptive transfer of GM-CSF-modulated DCs into naïve recipient NOD mice was sufficient to restore natural Treg function and cause delay in the disease onset.
Research Design and Methods
Mice
Female NOD/Ltj and NOD SCID mice (Jackson Laboratories Bar Harbor, ME) were housed in the Biological Resources Laboratory facility at the University of Illinois-Chicago and cared for in accordance with the guidelines set forth by the University of Illinois animal care and use committee.
Cytokines and Antibodies
Recombinant mouse GM-CSF and Flt3-L were purchased from either Cell Sciences or Biosource. FITC-conjugated anti-CD11c and PE-conjugated anti-H-2kd (MHC II), anti-CD4, anti-CD8a, anti-CD25, anti-CD80, anti-CD86, and anti-CD40 were obtained from BD Pharmingen. APC conjugated anti-Foxp3 was obtained from eBiosciences.
Treatment with DC Modulators
NOD mice were given i.p. injections of GM-CSF (2 μg/mouse/day) or Flt3-L (5 μg/mouse/day) for 5 consecutive days. In some experiments, mice were given more than one course of treatment as described in the respective figure legend. Blood samples were collected using tail vein incision and glucose levels examined weekly for hyperglycemia using an “accu-chek complete” glucometer. Mice were considered diabetic when glucose level was maintained >250 mg/dl for two consecutive weeks.
Analysis of DCs
Spleen cells were stained with FITC-conjugated anti-mouse CD11c in combination with PE-conjugated anti-mouse B7.1, B7.2, CD40, CD8a, or MHC class II, and analyzed in a FACS analyzer (BD Biosciences). RNA and mRNA were isolated from enriched splenic CD11c+, CD8a+CD11c+, CD8a-CD11c+ DCs using Trizol and mRNA isolation kit (Miltenyi Biotec), respectively. cDNAs were synthesized and used for PCR to detect the levels of IL-10, IL-6, TNF-α, IL-1, and IL-12 transcripts (Maxim Biotec).
Tregs Analysis
Cells were blocked with anti-CD16/CD32 Fc block antibody on ice for 15 minutes. Cells were surface-stained with FITC-labeled anti-CD4 and PE-labeled CD25 antibodies on ice for 30 minutes. These cells were fixed, permeabelized using fixation/permeabelization kit (eBiosciences) and stained using APC labeled anti-Foxp3 or isotype control antibody. Stained cells were analyzed using BD Facs Calibur or CyAn analyzer (DAKO-Cytomation), and the data were analyzed using Summit or WinMdi applications.
DC and T cell Enrichment
For CD4+CD25+ enrichment, splenic CD4+ T cells were first negatively selected using magnetic beads and CD25+ T cells were then positively selected using magnetic beads (Miltenyi Biotec). We consistently obtained >90% pure CD4+CD25+ T cells. For CD11c DC isolation, splenocytes were positively selected by magnetic bead separation (Miltenyi Biotec). DC purity was around 85%. For the isolation of CD8a+ and CD8a- DCs, whole splencoytes were labeled with fluorchrome-conjugated CD11c and CD8a antibodies, and CD11c+CD8a+ and CD11c+CD8a- DCs were isolated by fluorescence-activated cell sorting (FACS) using a MoFlo high-speed sorter. This enabled us to obtain >95% pure CD8a+ and CD8a- DC sub-populations.
In Vitro Inhibition Assays
CD4+CD25- T cells were labeled with CFSE and placed in the lower wells of 24 well trans-well plates (Corning) along with irradiated (5000 gray) APCs (generated from pan T cell depleted splenocytes) and soluble anti-CD3 antibody. Increasing numbers of CD4+CD25+ T cells were added to the upper well. After 4 days of incubation, CD4+CD25- T cell proliferation was determined by FACS. In some assays, anti-IL-10R and anti-TGF-β (20 μg/mL) were added either separately or together to the trans-wells.
Adoptive Transfer of T cells
Splenocytes (5 × 106) from recently diabetic mice were adoptively transferred either alone or with purified CD4+CD25+ T cells (1× 106) from GM-CSF treated or untreated mice into 6-week-old female NOD SCID mice and blood glucose levels monitored.
Measurement of Cytokine Production
To examine the spontaneous cytokine release by DCs from naïve and GM-CSF or Flt3-L treated mice, FACS-enriched DC subpopulations were cultured ex vivo for 48 h. Cell free supernatants from these cultures were assayed for pro-inflammatory cytokines, IL-1β and IL-12. Similarly, T cell culture supernatants were tested for the production of the Th1 and Th2 cytokines by ELISA following the manufacturer’s instructions (eBioscience) and the OD450 was recorded using a Microplate reader (Bio-Rad).
In Vivo DC Function
Effect of GM-CSF-induced modulation of DC function on T cells and the disease development was tested in two separate experiments. In one set of experiments, we adoptively transferred either purified CD4+ or CD4+CD25- T cells (3 × 106) into NOD SCID mice (n=5) that were either treated or left untreated with GM-CSF. In another experiment, DCs (2 × 106 cells) from GM-CSF-treated or untreated female NOD SCID mice were adoptively transferred into 7-week-old (n=5) female NOD mice and monitored for diabetes by measuring glucose levels.
Histochemistry
Pancreata were fixed in 10% formaldehyde, 5-μm paraffin sections were prepared and stained with hematoxylin and eosin (H&E). Stained sections were analyzed in a blinded fashion, using a grading system, in which 0 = no evidence of infiltration, 1 = peri-islet infiltration, 2 = <25%, 3 = 25–50%, 4 = >50% infiltration of each islet, and 5 = complete loss, or only remnants, of islets seen. Approximately 100 islets were examined for every group.
Statistical analysis
Mean, SD, and statistical significance were calculated using an SPSS application. Statistical significance was determined using student’s T-test, or Log rank test. In most cases, values of individual treated groups were compared with those of untreated groups. A value of p≤0.05 was considered significant.
Results
GM-CSF and Flt3-L Induce Modulation of DC Phenotype
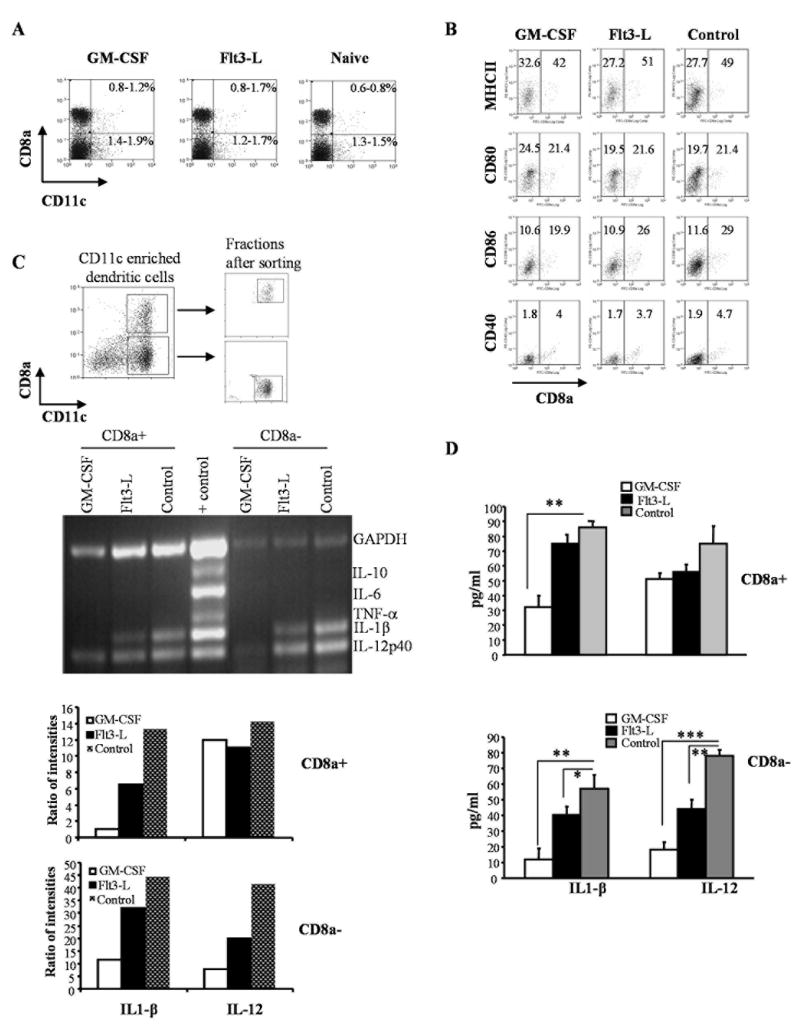
DCs in NOD mice exhibit phenotypic, maturation and functional defects [6; 16; 17]. To examine the ability of GM-CSF and Flt3-L to modulate DC phenotype, pre-diabetic NOD mice were treated with these cytokines and examined for DC surface markers and cytokine transcript levels. Unlike our previous reports using other mouse strains [22; 23; 24], the frequencies of peripheral myeloid (CD8a-) and lymphoid (CD8a+) DCs were not significantly different than in cytokine-treated mice (Fig. 1A). However, the total number of cells in the spleen from GM-CSF and Flt3-L-treated mice were 2-3 times higher compared to controls (not shown). Examination of surface activation markers revealed that DCs from cytokine-treated and untreated mice expressed comparable levels of B7.1, B7.2, CD40 and MHC-II (Fig. 1B). Cells were sorted to contain either CD11C+CD8a+ or CD11C+CD8a- DCs (Figure-1C upper panel) and RNA obtained from these two distinct populations were subjected to RT-PCR to determine the relative levels of relevant cytokine transcripts. The level of IL-12 transcripts was comparable in CD8a+ DCs obtained from all three groups; however, the IL1β levels were reduced in both treatment groups relative to the untreated control (Figure-1C). In contrast, the levels of both IL1β and IL-12 transcripts were lower in CD8a- DCS from Flt3-L treated mice. However, the levels of these two cytokine transcripts were even lower in CD8a- DCs from GM-CSF treated groups (Figure-1C).
Figure 1. GM-CSF and Flt3-L treatments suppress inflammatory cytokines in both CD8a+ and CD8a- DC subsets of NOD mice.
Euglycemic female NOD mice were treated with either GM-CSF (2μg/mouse/day), Flt3-L (5μg/mouse/day) or PBS (naïve group) for 5 consecutive days starting 11 and 13 weeks of age intra-peritoneally (i.p.). A) Mice were sacrificed 48 hours after the last treatment, single cell suspension of splenocytes were stained with anti-CD8a and anti-CD11c Abs conjugated to fluorescent probes to examine for changes in the frequency of DC subsets. Percentage values of CD8a+ and CD8a- populations among total splenocytes are shown. B) Purified CD11c+ DCs were stained with fluorochrome labeled anti-CD8a and MHC-II, CD80, CD86, or CD40 Abs and analyzed by FACS. Mean fluorescence intensity values of activation marker specific staining on CD8a+ and CD8a- populations are shown. C) Purified CD11c+ DCs were stained using fluorochrome labeled anti-CD8a Ab and sorted into CD8a+ and CD8a- DCs using a cell sorter. Dot plots show the gating pattern for the isolation of CD8a+ and CD8a- DCs from enriched CD11c+ DCs (upper panels). RNA samples prepared from CD8a+ and CD8a- DC subsets were used in a multiplex RT-PCR assay to detect relative levels of cytokine transcripts (middle). Relative densitometry values of individual bands are plotted as bar diagrams (lower panels). D) Purified CD8a+ or CD8a- DCs were cultured (1×106 cells/ml) for 48h and supernatants were tested for secreted levels of IL-1β and IL-12 by ELISA. Mean + SD values from three independent assays carried out in triplicate are shown. *** represents p<0.001, ** represents p < 0.01. * represents p<0.05.
To further substantiate these findings, we measured the cytokine levels in supernatants from these DC cultures. The amounts of IL-12 and IL1β secreted by CD8a- DCs from GM-CSF mice were significantly lower compared to CD8a- DCs from either control or Flt3-L treated mice. CD8a- DCs from Flt3-L also showed lower secreted cytokines relative to untreated controls. However, the levels of suppression in CD8a- DCs from Flt3-L mice were not as significant as they were in CD8a- DCs from GM-CSF treated mice (Figure-1D). These data show that both Flt3-L and GM-CSF have the ability to suppress inflammatory cytokine production by DCs of NOD mice and therefore, may exert a suppressive effect on ongoing β cell specific effector T cell response.
Later Treatment with GM-CSF, not Flt3-L, Delays the Onset of T1D in NOD Mice
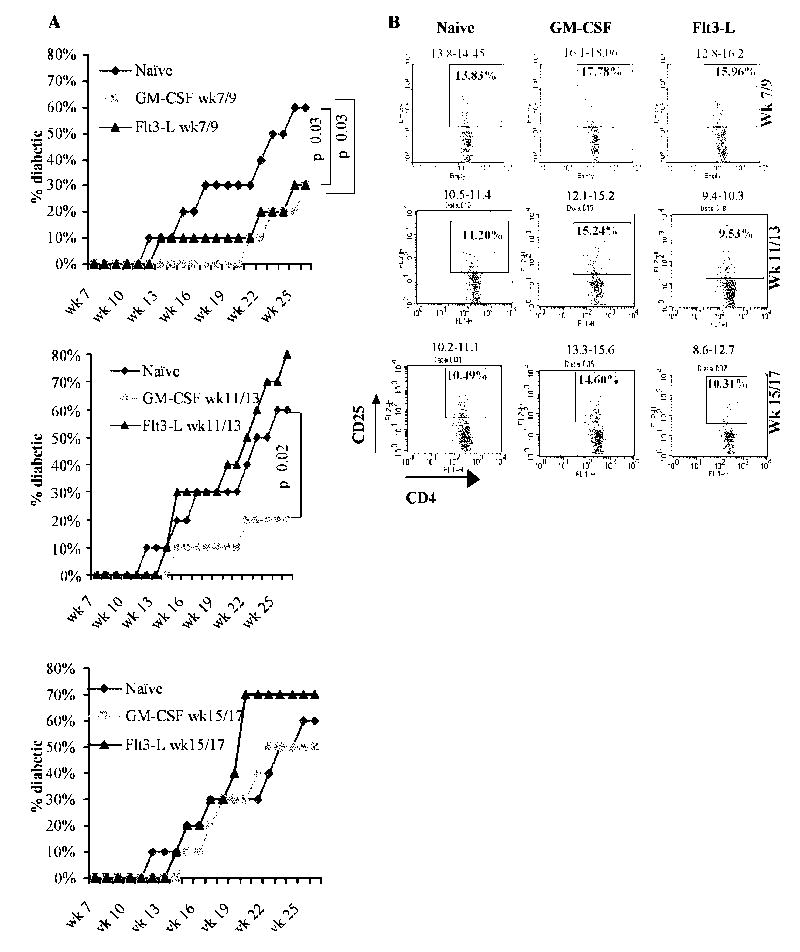
To determine the effects of treatment with GM-CSF and Flt3-L on T1D development, female NOD mice were either left untreated, treated with 2μg/mouse/day of GM-CSF or 5μg/mouse/day of Flt3-L for 5 consecutive days starting week 7 (stage 1), 11 (stage 2), or 15 (stage 3) and monitored for up to 30 weeks of age. While treatment with GM-CSF and Flt3-L at stage 1 (Fig. 2A, left panel) significantly delayed the onset of diabetes by up to 20 weeks of age, only GM-CSF could delay hyperglycemia significantly when the treatment was initiated at week 11 (Fig. 2A, middle panel). However, both GM-CSF and Flt3-L failed to show significant protective effect when the treatment was initiated at an hyperglycemic stage (week 15 of age). Although Flt3-L showed protection during stage 1, a similar treatment at stage 2 and stage 3 appeared to exacerbate the disease. While GM-CSF treatment at different ages resulted in a significant increase in the frequency of CD4+CD25+ T cells, Flt3-L caused an increase in these cell numbers only when the treatment was initiated at week 7 compared to control mice (Fig. 2B). These results suggest that GM-CSF is more effective than Flt3-L in suppressing the disease progression by enhancing CD4+CD25+ T cell numbers even when the treatment is initiated at a later stage. Since GM-CSF was better able to inhibit inflammatory cytokines and induce protection from diabetes, only this cytokine was used in further studies.
Figure 2. Diabetes incidence in Flt3-L and GM-CSF treated NOD mice.
Groups of 10 NOD mice were treated with GM-CSF (2μg/mouse/day), Flt3-L (5μg/mouse/day), or PBS (naïve) for 5 consecutive days starting 7 and 9 weeks (upper panel), 11 and 13 weeks (middle panel), or 15 and 17 weeks (lower panel) of age i.p, and blood glucose levels monitored weekly. A) Mice were considered diabetic when glucose levels remained above 250 mg/dL for 2 consecutive weeks. Experiment was terminated when >70% of untreated NOD mice became diabetic or mice reached 30 weeks of age. Log-rank test was carried out to determine statistical significance and the corresponding P values are shown in the figure. B) Sets of mice from parallel experiment were euthanized 48 hours after the last treatment, splenocytes from treated and untreated mice were stained using fluorochrome labeled anti-CD4 and CD25 Abs and analyzed by FACS. Plots represent splenocytes gated on CD4+ population and the representative percentage values of CD25+ cells (inside the scatter plot) and the range of percentage values from 3 mice analyzed individually (above the scatter plot) are shown.
Repeated Treatment with GM-CSF Leads to Prolonged Suppression of T1D
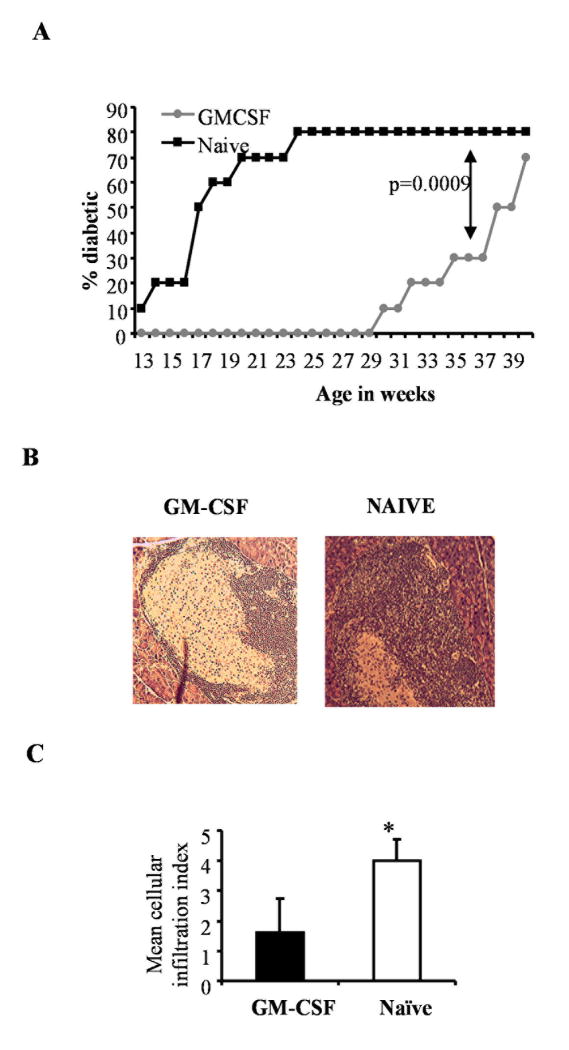
Although GM-CSF showed the potential to delay hyperglycemia significantly, therapeutic effect was diminished at later stages. Therefore, we reasoned that repeated intermittent treatment initiated before the onset of destructive insulitis may result in a longer lasting protection from the disease. Therefore, we treated NOD mice bi-weekly from 7 to 19 weeks of age with GM-CSF and monitored for hyperglycemia. As shown in Fig. 3, 80% of the untreated mice developed disease by 22 weeks of age compared to none of the GM-CSF-treated mice. To determine the duration of protection, we monitored the animals until 40 weeks of age without further GM-CSF treatment. Treated mice remained disease-free through 28 weeks of age, and even as late as week 34 the disease incidence was only one-half of that noted in the untreated controls by 22 weeks of age. Eventually, by 39 weeks of age, 70% of the treated mice developed hyperglycemia (Fig. 3A). This result indicated that intermittent treatment with GM-CSF initiated at pre-hyperglycemic stages could provide sustained protection against T1D.
Figure 3. Therapeutic potential of GM-CSF.
Groups of 10 NOD mice were treated with 2μg/mL/mouse of GM-CSF or PBS (naïve) for five consecutive days starting 7, 9, 11, 13, 15 and 19 weeks of age and monitored for T1D. A) Blood glucose levels monitored weekly and the mice were considered diabetic when glucose levels remained above 250 mg/dL for 2 consecutive weeks. Log-rank test was carried out to determine statistical significance. B) Pancreatic sections from a set of mice were stained with hematoxylin and eosin. Representative images of islets from GM-CSF-treated and naive animals are shown. C) Stained sections were examined, insulitis was scored in a blinded fashion as described in research design and methods, and the mean±SD of insulitis grades plotted as bar-diagram. * indicates a p value of <0.05.
Histopathological examination of pancreatic sections from cytokine-treated and untreated mice showed that while the untreated mice had severe insulitis, treated mice had moderate peri-insulitis and/or insulitis (fig. 3B and 3C). These results indicated that relatively early (e.g. when animals might have insulitis but with sufficient residual functional β cells) treatment with GM-CSF can delay the onset of hyperglycemia, perhaps by suppressing ongoing autoimmune response and β cell destruction.
GM-CSF Treatment expands CD4+CD25+ T cells that Express Fox3 and Secrete IL-10 and TGF-β
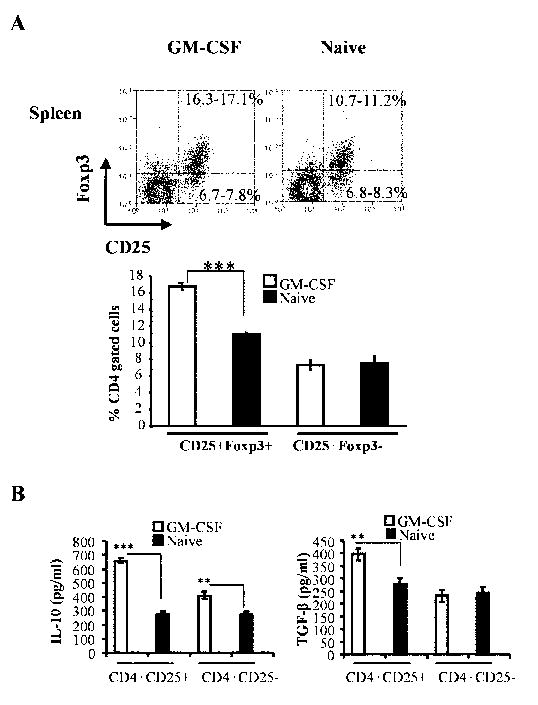
To understand the effect of GM-CSF exposed DCs on T cell function, we examined T cells from treated mice for their phenotype and cytokine profiles. First, CD4+ T cells of GM-CSF-treated pre-diabetic (12 weeks old) NOD mice were examined for CD25 and Foxp3 expression. As shown in Fig. 4A, about 10% of the splenic CD4+ T cells from untreated control mice were CD25+Foxp3+, whereas approximately 17% from the treated mice showed this phenotype. These results showed that GM-CSF treatment resulted in an expansion of CD25+Foxp3+ Tregs, which may have contributed to disease suppression.
Figure 4. GM-CSF treatment induces CD4+CD25+Foxp3+ T cells.
A) Splenocytes from pre-diabetic GM-CSF and PBS (naïve)-treated NOD mice were stained using fluorochrome labeled anti-CD4, CD25, and Foxp3 Abs and analyzed by FACS. Representative scatter plots and range of percentage values of CD25+Foxp3+ and CD25-Foxp3+ cells from 4 mice tested individually are shown. Mean + SD values of CD25+Foxp3+ and CD25+Foxp3- T cells (lower panel) are shown. B) CD4+CD25+ and CD4+CD25- cells were isolated from GM-CSF-treated and untreated naïve mice, cultured for 48 hours in anti- CD3/ anti-CD28 Ab (5μg each/mL) coated wells. Cell free supernatants were harvested and analyzed for cytokine production by ELISA. Bars represent Mean±SD of values obtained from 4 mice tested in triplicates. (*** p<0.001, ** p<0.01)
Next, we tested the cytokine profiles of both CD4+CD25+ and CD4+CD25- T cell populations. While CD4+CD25- T cells from GM-CSF-treated mice produced higher amounts of IL-10, CD4+CD25+ T cells from these mice produced substantially higher amounts of both IL-10 and TGF-β1 compared to untreated controls upon activation (Fig. 4B). In contrast, both CD4+CD25+ and CD4+CD25- T cells from untreated mice produced significantly lower, but comparable, levels of IL-10 and TGF-β1. These results indicated that GM-CSF-mediated suppression of diabetes in NOD mice may occur through induction of IL-10 and TGF-β producing T cells.
CD4+CD25+ T cells from GM-CSF-Treated Mice Suppress Induction of T1D by Adoptively Transferred Splenocytes
To examine whether CD4+CD25+ T cells from GM-CSF-treated NOD mice can suppress diabetes, NOD-SCID mice were adoptively transferred with splenocytes from diabetic NOD mice alone or along with CD4+CD25+ T cells from GM-CSF-treated and untreated mice. Co-adaptive transfer of CD4+CD25+ T cells from either treated or untreated mice delayed the onset of diabetes compared to mice that received only the diabetogenic splenocytes. However, the onset of hyperglycemia was delayed in animals that received CD4+CD25+ T cells from GM-CSF-treated mice by at least 4 weeks, compared to those that received CD4+CD25+ T cells from untreated controls (Fig. 5; p=0.04). Fifty percent of control CD4+CD25+ recipients developed diabetes 2 weeks later than the untreated control group. However, in mice that received CD4+CD25+ T cells from GM-CSF-treated donors the onset of diabetes was delayed in 60% of animals until 15 weeks post cell transfer. This suggested a higher efficiency of suppression by CD4+CD25+ T cells from GM-CSF-treated donors relative to CD4+CD25+ cells from control mice.
Figure 5. CD4+CD25+ T cells from GM-CSF-treated mice delay splenocyte-adoptive transfer induced hyperglycemia in NOD-Scid mice.
Splenocytes from 16-20-week-old diabetic mice were transferred i.v into 6-week-old NOD scid mice (n=5; 5×106 cells/mouse) either alone (control) or with CD4+CD25+ T cells (1×106/mouse) from pre-diabetic GM-CSF-treated or untreated (naïve) non-diabetic female NOD mice. Blood glucose levels were monitored weekly. Recipient mice were considered diabetic when glucose levels remained above 250 mg/dL for 2 consecutive weeks. Log-rank test was carried out to determine statistical significance.
GM-CSF-Exposed DCs Cause Expansion of CD4+CD25+Foxp3+ T cells In Vivo
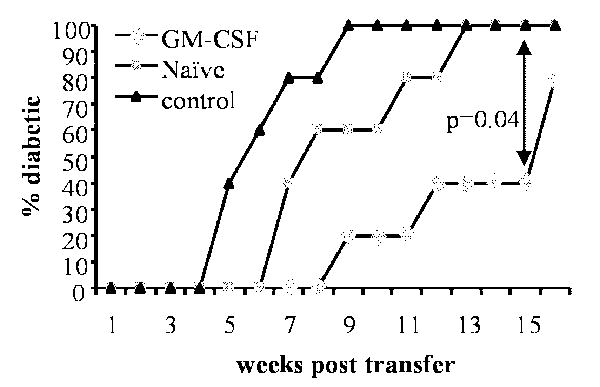
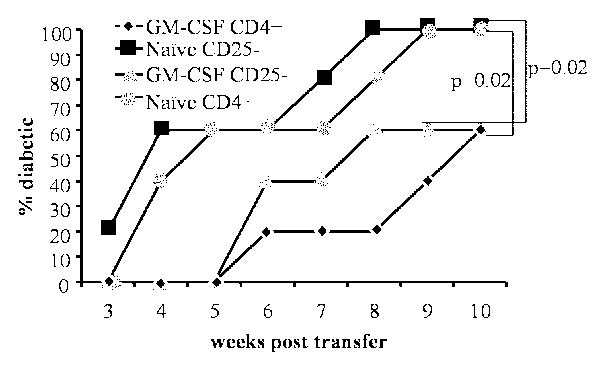
Although GM-CSF treatment led to the expansion of CD25+Foxp3+ Tregs and sustained DCs in a semi-matured status, it was not clear whether the primary effect was on DCs. To test this, we adoptively transferred total CD4+ or CD4+CD25- T cells from non-diabetic mice into GM-CSF-treated and untreated NOD-SCID mice that are devoid of a functional adaptive immune system and monitored them for hyperglycemia. As shown in Fig. 6, there was a considerable delay in the disease onset in GM-CSF-treated NOD-SCID mice that received either total CD4+ T cells or CD4+CD25- T cells relative to untreated control mice. This suggested that direct exposure of T cells to GM-CSF may not be necessary for the GM-CSF-induced suppression of their diabetogenic ability. These results combined with the ability of GM-CSF to suppress inflammatory cytokine production by DCs (Figure-1) and delay the onset of diabetes in NOD-SCID mice (Figure-6) further indicate that GM-CSF acts on DCs, which in turn exert tolerogenic effect on adoptively transferred T cells in recipient mice.
Figure 6. Diabetogenicity of adoptively transferred T cells is inhibited in GM-CSF-treated NOD-Scid mice.
Six-week-old female NOD scid mice (n=5/group) were either treated with GM-CSF as indicated in methods or left untreated (naïve). These mice received CD4+CD25- or CD4+ T cells (2×106/mouse) from 8-week-old female NOD mice. T cell recipients were bled weekly for monitoring blood glucose levels and considered diabetic when it remained above 250 mg/dL for 2 consecutive weeks. Log-rank test was carried out to determine statistical significance.
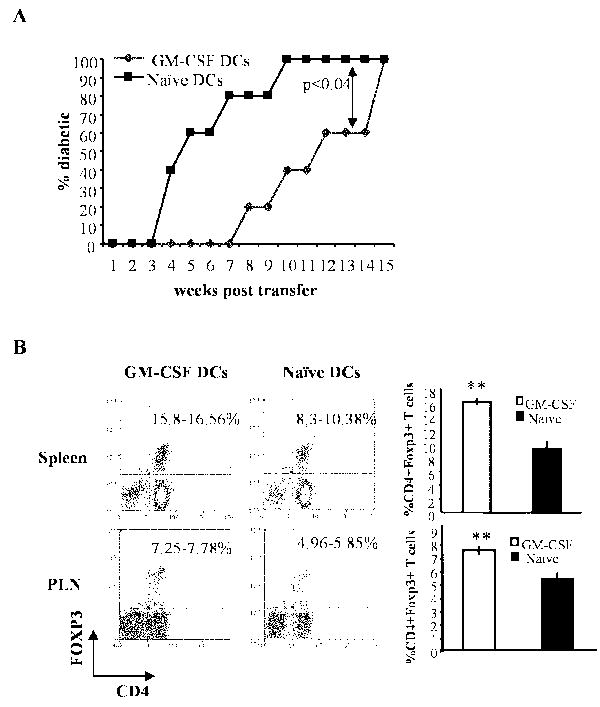
To unequivocally demonstrate the role of DCs in inducing Tregs purified DCs from GM-CSF-treated and untreated NOD mice were adoptively transferred into pre-diabetic NOD mice, and the recipients were monitored for hyperglycemia. Mice that received DCs from GM-CSF -treated donors showed a significant delay (by at least 4 weeks) in disease onset relative to recipients of DCs from controls (Fig. 7A). Further, the percentage of Foxp3+ cells was significantly higher in GM-CSF-exposed DC recipient spleen and pancreatic lymph nodes (Fig. 7B). These results indicated that GM-CSF-exposed DCs facilitate expansion of Foxp3+ Tregs and promote their tolerogenic function leading to disease suppression.
Figure 7. Tolerogenic effect of GM-CSF on DCs alone is sufficient to induce Foxp3+ T cells and suppress T1D.
A) DCs from female NOD scid mice treated with GM-CSF for 5 consecutive days or left untreated (naïve) were isolated and transferred i.v. into 7-week-old NOD mice (n=5/group) and the recipient mice were monitored weekly for blood glucose levels. B) Two weeks post-DC transfer, mice from a parallel experiment (n=3/group) were sacrificed and analyzed for Foxp3+ expression in both spleen and pancreatic lymph node (PLN) cells. Shown are percentage ranges of CD4+Foxp3+ T cells from 3 mice analyzed individually. Bars in the right panel represent mean ±SD of percentage of Foxp3+ T cells among CD4+ cells. ** represents p < 0.01.
Discussion
In this study, we demonstrated that treatment with GM-CSF can significantly delay the onset of T1D in NOD mice and that the protective effect can persist long after cessation of the treatment. The protection was mediated through IL-10 and TGF-β1 produced by an expanded population of CD4+CD25+ Tregs. The Treg expansion was facilitated by tolerogenic DCs induced by GM-CSF.
GM-CSF and Flt3-L are potent DC growth factors that can profoundly affect expansion, maturation and function of different subsets of DCs [30; 31]. We showed earlier that GM-CSF can suppress ongoing EAT and experimental myasthenia gravis [22; 23; 32], and that suppression was associated with an increase in CD4+CD25+ T cell frequencies and was mediated through IL-10 produced by these cells. In contrast, Flt3-L treatment exacerbated the disease [22; 23].
Unlike our earlier studies using other strains of mice, neither GM-CSF nor Flt3-L treatment induced an increase in the overall percentage of CD8a- DC subset in NOD mice; perhaps due to inherent defects in myeloid DC differentiation in NOD mice [33]. However, both treatments resulted in reduced levels of pro-inflammatory cytokine transcripts and significant reduction in secreted IL-1β/ IL-12 in CD8a- and IL-1β in CD8a+ subsets of DCs, features often noted in tolerogenic DCs.
Recent studies have shown that DCs which produce lower levels of pro-inflammatory cytokines, irrespective of the levels of activation markers on the surface, can promote T cell tolerance [24; 34]. Importantly, pro-inflammatory cytokines play a key role in breaking tolerance by antagonizing Treg expansion and development. For example, while TNF-α can negatively regulate Treg numbers and function [35], IL-1β can break tolerance by directly expanding effector T cell populations in NOD mice [36]. Interestingly, GM-CSF can influence DC maturation by increasing IL1Ra, an IL1-β inhibitor, production by monocytes and PBMCs in humans, and activating NF-kB which is a key regulator of DC maturation [37; 38]. Therefore, down-modulation of IL-12 and IL-1β levels in DCs induced by GM-CSF and Flt3-L suggests that these cytokines might protect NOD mice from T1D through the induction of tolerogenic DCs and by promoting Treg function.
As anticipated, treatment using both GM-CSF and Flt3-L resulted in an increase in the overall number of CD4+CD25+ Treg frequencies. However, the efficacy of GM-CSF and Flt3-L treatments in suppressing the disease progression in NOD mice was dependent on the time of treatment initiation. In a very recent study, treatment of NOD mice with GM-CSF was initiated at 3 weeks of age and was repeated 3 times per week for the first 3 weeks, followed by 2 injections per week, up to 52 weeks of age. This study showed that prolonged treatment with GM-CSF starting at an age as early as 3 weeks could help maintain the DCs in a tolerogenic state, induce Tregs and abrogate T1D in NOD mice [27]. Similarly, early treatment with Flt3-L can overcome DC deficiency in NOD mice and protect against diabetes development [26]. These observations are consistent with the current findings which show that early treatment with either GM-CSF or Flt3-L can confer protection. However, none of these earlier studies evaluated the efficacy of initiating the treatment at later stages of the disease development process, which would be the likely scenario in humans. Therefore, we examined the ability of GM-CSF and Flt3-L to modulate DC function and influence T1D outcome in NOD mice at different stages of disease development. While GM-CSF caused a delay in the disease onset in 100% and 80% of mice that received the treatment starting on week 7 and 11, respectively, Flt3-L could induce a significant delay in the onset of hyperglycemia only when the treatment was initiated at an earlier age (i.e. week 7 of age). It is important to note that in this study, intermittent treatment with GM-CSF starting as late as week 7 (at a time when insulitis has likely ensued) and only through week 19 of age was sufficient to cause a prolonged delay in the onset of hyperglycemia. These results indicate that GM-CSF is more efficient in suppressing the development of hyperglycemia.
Suppression of T1D in GM-CSF-treated NOD mice appeared to be mediated by CD4+CD25+FoxP3+ T cells. The frequency and the overall number of these T cells were significantly higher in GM-CSF treated mice compared to control mice. Further, the dominant regulatory function of CD4+CD25+ T cells was evident from their ability to produce significantly higher amounts of IL-10 and TGF-β1 compared to control CD4+CD25+ T cells. The superior ability of CD4+CD25+ T cells from GM-CSF-treated mice to suppress diabetogenic T cell function compared to control CD4+CD25+ T cells was evident when they delayed the onset of diabetes in an adoptive splenocyte transfer model using NOD-SCID mice. It has been clearly demonstrated that CD4+CD25+ T cells from diabetic patients are less effective than their normal counterparts in suppressing effector T cells [18]. Defective APC function, as evidenced by their inability to activate CD4+CD25+ T cells effectively, has been implicated in the loss of natural Treg function in T1D patients and NOD mice over time [19]. Our observations show that GM-CSF can enhance tolerogenic T cell function.
Although GM-CSF is known to act primarily on DCs, it is not clear whether the enhancement of Treg function in NOD mice upon treatment with this cytokine was a consequence of the direct effects on T cells or indirect effects mediated through DCs. The delayed onset of hyperglycemia in GM-CSF treated NOD-SCID recipients of diabetogenic T cells (Fig. 6) and in pre-diabetic NOD mice that received GM-CSF-exposed DCs (Fig. 7) supported the notion that GM-CSF primarily acted on DCs and rendered them tolerogenic. Therefore, an increase in the CD4+Foxp3+ T cell numbers and their enhanced suppressor function observed in GM-CSF-treated mice are most likely the result of tolerogenic DC-T cell interaction. Earlier studies have shown that GM-CSF-exposed DCs induce weaker activation of diabetogenic TCR transgenic CD8+ T cells, and tolerance to auto- and allo-antigens [27; 39]. Our observation that GM-CSF-exposed adoptively transferred DCs could significantly delay hyperglycemia in NOD mice with a concomitant significant increase in the frequency of CD4+Foxp3+ T cells in recipient mice further support this notion.
In summary, our results clearly show that the DC defect in NOD mice may be contributing significantly to the disease development, and GM-CSF treatment ameliorates this defect and delays the onset of T1D. This inherent defect in DC function may also explain the disease onset approximately 10 weeks after cessation of GM-CSF treatment, perhaps due to replenishment with new DCs that were not exposed to exogenous GM-CSF. Therefore, long-term, but intermittent, treatment with GM-CSF might be necessary to “educate” newly generated DCs and sustain them in a tolerogenic state for a longer term protection from T1D.
Acknowledgments
This work was supported by The National Institutes of Health grant RO1 AI058190 to BSP and NIH R21 AI069848 to CV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12:580–91. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 2.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med. 2002;196:369–77. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabien N, Bergerot I, Maguer-Satta V, Orgiazzi J, Thivolet C. Pancreatic lymph nodes are early targets of T cells during adoptive transfer of diabetes in NOD mice. J Autoimmun. 1995;8:323–34. doi: 10.1006/jaut.1994.0025. [DOI] [PubMed] [Google Scholar]
- 4.Rosmalen JG, Leenen PJ, Katz JD, Voerman JS, Drexhage HA. Dendritic cells in the autoimmune insulitis in NOD mouse models of diabetes. Adv Exp Med Biol. 1997;417:291–4. doi: 10.1007/978-1-4757-9966-8_47. [DOI] [PubMed] [Google Scholar]
- 5.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7:727–38. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 6.Serreze DV, Gaskins HR, Leiter EH. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol. 1993;150:2534–43. [PubMed] [Google Scholar]
- 7.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 9.Summers KL, Behme MT, Mahon JL, Singh B. Characterization of dendritic cells in humans with type 1 diabetes. Ann N Y Acad Sci. 2003;1005:226–9. doi: 10.1196/annals.1288.032. [DOI] [PubMed] [Google Scholar]
- 10.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–40. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 11.Groux H, Fournier N, Cottrez F. Role of dendritic cells in the generation of regulatory T cells. Semin Immunol. 2004;16:99–106. doi: 10.1016/j.smim.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 13.Mahnke K, Johnson TS, Ring S, Enk AH. Tolerogenic dendritic cells and regulatory T cells: a two-way relationship. J Dermatol Sci. 2007;46:159–67. doi: 10.1016/j.jdermsci.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Wilczynski JR, Radwan M, Kalinka J. The characterization and role of regulatory T cells in immune reactions. Front Biosci. 2008;13:2266–74. doi: 10.2741/2840. [DOI] [PubMed] [Google Scholar]
- 15.Dahlen E, Hedlund G, Dawe K. Low CD86 expression in the nonobese diabetic mouse results in the impairment of both T cell activation and CTLA-4 up-regulation. J Immunol. 2000;164:2444–56. doi: 10.4049/jimmunol.164.5.2444. [DOI] [PubMed] [Google Scholar]
- 16.Alard P, Manirarora JN, Parnell SA, Hudkins JL, Clark SL, Kosiewicz MM. Deficiency in NOD antigen-presenting cell function may be responsible for suboptimal CD4+CD25+ T-cell-mediated regulation and type 1 diabetes development in NOD mice. Diabetes. 2006;55:2098–105. doi: 10.2337/db05-0810. [DOI] [PubMed] [Google Scholar]
- 17.Jansen A, van Hagen M, Drexhage HA. Defective maturation and function of antigen-presenting cells in type 1 diabetes. Lancet. 1995;345:491–2. doi: 10.1016/s0140-6736(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 18.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 19.Boudaly S, Morin J, Berthier R, Marche P, Boitard C. Altered dendritic cells (DC) might be responsible for regulatory T cell imbalance and autoimmunity in nonobese diabetic (NOD) mice. Eur Cytokine Netw. 2002;13:29–37. [PubMed] [Google Scholar]
- 20.You S, Leforban B, Garcia C, Bach JF, Bluestone JA, Chatenoud L. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci U S A. 2007;104:6335–40. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–37. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 22.Vasu C, Dogan RN, Holterman MJ, Prabhakar BS. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol. 2003;170:5511–22. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- 23.Sheng JR, Li L, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN. Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J Immunol. 2006;177:5296–306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- 24.Gangi E, Vasu C, Cheatem D, Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol. 2005;174:7006–13. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 25.Dogan RN, Vasu C, Holterman MJ, Prabhakar BS. Absence of IL-4, and not suppression of the Th2 response, prevents development of experimental autoimmune Graves’ disease. J Immunol. 2003;170:2195–204. doi: 10.4049/jimmunol.170.4.2195. [DOI] [PubMed] [Google Scholar]
- 26.O’Keeffe M, Brodnicki TC, Fancke B, Vremec D, Morahan G, Maraskovsky E, Steptoe R, Harrison LC, Shortman K. Fms-like tyrosine kinase 3 ligand administration overcomes a genetically determined dendritic cell deficiency in NOD mice and protects against diabetes development. Int Immunol. 2005;17:307–14. doi: 10.1093/intimm/dxh210. [DOI] [PubMed] [Google Scholar]
- 27.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:3638–47. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 28.Morin J, Faideau B, Gagnerault MC, Lepault F, Boitard C, Boudaly S. Passive transfer of flt-3L-derived dendritic cells delays diabetes development in NOD mice and associates with early production of interleukin (IL)-4 and IL-10 in the spleen of recipient mice. Clin Exp Immunol. 2003;134:388–95. doi: 10.1111/j.1365-2249.2003.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chilton PM, Rezzoug F, Fugier-Vivier I, Weeter LA, Xu H, Huang Y, Ray MB, Ildstad ST. Flt3-ligand treatment prevents diabetes in NOD mice. Diabetes. 2004;53:1995–2002. doi: 10.2337/diabetes.53.8.1995. [DOI] [PubMed] [Google Scholar]
- 30.O’Keeffe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, Shortman K. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–30. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 31.Curti A, Fogli M, Ratta M, Tura S, Lemoli RM. Stem cell factor and FLT3-ligand are strictly required to sustain the long-term expansion of primitive CD34+DR- dendritic cell precursors. J Immunol. 2001;166:848–54. doi: 10.4049/jimmunol.166.2.848. [DOI] [PubMed] [Google Scholar]
- 32.Vasu C, Holterman MJ, Prabhakar BS. Modulation of dendritic cell function and cytokine production to prevent thyroid autoimmunity. Autoimmunity. 2003;36:389–96. doi: 10.1080/08916930310001603073. [DOI] [PubMed] [Google Scholar]
- 33.Peng R, Bathjat K, Li Y, Clare-Salzler MJ. Defective maturation of myeloid dendritic cell (DC) in NOD mice is controlled by IDD10/17/18. Ann N Y Acad Sci. 2003;1005:184–6. doi: 10.1196/annals.1288.023. [DOI] [PubMed] [Google Scholar]
- 34.Enk AH. Dendritic cells in tolerance induction. Immunol Lett. 2005;99:8–11. doi: 10.1016/j.imlet.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Morris GP, Kong YC. Interference with CD4+CD25+ T-cell-mediated tolerance to experimental autoimmune thyroiditis by glucocorticoid-induced tumor necrosis factor receptor monoclonal antibody. J Autoimmun. 2006;26:24–31. doi: 10.1016/j.jaut.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan BJ, Thomas HE, Pai S, Santamaria P, Iwakura Y, Steptoe RJ, Kay TW, Thomas R. IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J Immunol. 2006;176:7278–87. doi: 10.4049/jimmunol.176.12.7278. [DOI] [PubMed] [Google Scholar]
- 37.Janson RW, Hance KR, Arend WP. Production of IL-1 receptor antagonist by human in vitro-derived macrophages. Effects of lipopolysaccharide and granulocyte-macrophage colony-stimulating factor. J Immunol. 1991;147:4218–23. [PubMed] [Google Scholar]
- 38.Poutsiaka DD, Clark BD, Vannier E, Dinarello CA. Production of interleukin-1 receptor antagonist and interleukin-1 beta by peripheral blood mononuclear cells is differentially regulated. Blood. 1991;78:1275–81. [PubMed] [Google Scholar]
- 39.Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]