Abstract
Histone deacetylase (HDAC) inhibitors reactivate epigenetically-silenced genes in cancer cells, triggering cell cycle arrest and apoptosis. Recent evidence suggests that dietary constituents can act as HDAC inhibitors, such as the isothiocyanates found in cruciferous vegetables and the allyl compounds present in garlic. Broccoli sprouts are a rich source of sulforaphane (SFN), an isothiocyanate that is metabolized via the mercapturic acid pathway and inhibits HDAC activity in human colon, prostate, and breast cancer cells. In mouse preclinical models, SFN inhibited HDAC activity and induced histone hyperacetylation coincident with tumor suppression. Inhibition of HDAC activity also was observed in circulating peripheral blood mononuclear cells obtained from people who consumed a single serving of broccoli sprouts. Garlic organosulfur compounds can be metabolized to allyl mercaptan (AM), a competitive HDAC inhibitor that induced rapid and sustained histone hyperacetylation in human colon cancer cells. Inhibition of HDAC activity by AM was associated with increased histone acetylation and Sp3 transcription factor binding to the promoter region of the P21WAF1 gene, resulting in elevated p21 protein expression and cell cycle arrest. Collectively, the results from these studies, and others reviewed herein, provide new insights into the relationships between reversible histone modifications, diet, and cancer chemoprevention.
Keywords: Brassica, cruciferous vegetables, phenylbutyrate, butyrate, valproic acid
INTRODUCTION
There is much interest in the study of isothiocyanates and allyl sulfides, and the foods from which they are derived [Clarke et al., 2008; El-Bayoumy et al., 2006; Higdon et al., 2007; Myzak and Dashwood; Pinto et al., 2006; Powolny and Singh 2008]. For instance, entering the terms “isothiocyanates” and “allyl sulfides” into PubMed resulted in 10282 and 600 citations, respectively. This journal lists several papers on the topic, describing the antimutagenic effects of garlic extract in the Salmonella assay and in Chinese hamster ovary cells [Knasmuller et al., 1989]; the anti-clastogenic properties of garlic extract in mice given mitomycin C, cyclophosphamide, or sodium arsenite [Das et al., 1993]; the protection by Brassica campestris mustard leaf towards chromosomal damage and oxidative stress induced by γ-radiation, cyclophosphamide, and urethane in mice [Tiku et al., 2008]; the inhibitory effects of diallyl sulfide in Chinese hamster V79 cells treated with dimethylnitrosamine [Fiorio and Bronzetti 1995]; and the anti-genotoxic activity of sulforaphane (SFN) in cultured human lymphocytes treated with ethyl methanesulfonate, vincristrine, H2O2, and mitomycin C [Fimognari et al., 2005].
SFN is an isothiocyanate, derived from glucoraphanin in broccoli and broccoli sprouts, that was first identified as a potent inducer of phase 2 detoxification enzymes [Zhang et al., 1992; Fahey et al., 1997]. Enzyme induction occurs via the Kelch-like ECH-associated protein 1–nuclear factor E2-related factor-2 (Keap1-Nrf2) pathway, although other mechanisms have been implicated in the chemoprotective effects of SFN (see [Clarke et al., 2008; Fimognari and Hrelia 2007; Juge Myzak and Dashwood 2006) for recent reviews). A phase I clinical study of broccoli sprout extracts examined the safety, tolerance, and metabolism of constituent glucosinolates and isothiocyanates in human volunteers [Shapiro et al., 2006].
Similarly, organosulfur compounds from Allium vegetables have garnered significant interest due to their reported health benefits, including anti-cancer properties [El-Bayoumy et al., 2006; Liu et al., 2007; Milner 2006; Myzak and Dashwood 2006; Nagini 2008; Pittler and Ernst 2007; Sener et al., 2007]. A recent study, for example, found odds ratios among persons with high versus low intakes of garlic and onions that were associated with a significantly reduced risk of colorectal adenoma [Millen et al., 2007].
Our interest in dietary isothiocyanates and allyl sulfides evolved out of the growing body of evidence connecting their cancer chemopreventive effects with epigenetic mechanisms, and in particular the modulation of histone acetylation status and histone deacetylase (HDAC) activity. These findings are reviewed in the following sections.
HDAC INHIBITORS AND CANCER THERAPY
The term “epigenetics” refers to heritable changes in gene function that occur without a change in DNA sequence [Delage and Dashwood 2008a; Delage and Dashwood 2008b]. Epigenetic changes have been implicated in the deregulation of gene expression during cancer development [Gal-Yam et al., 2008; Gronbaek et al., 2007; Kondo and Issa 2004]. There is much excitement in this area because, in contrast to genetic alterations, epigenetic changes are potentially modifiable. Epigenetic abnormalities can affect both the DNA methylation status and the pattern of histone “marks” in cancer cells, resulting in inappropriate gene silencing [Clarke et al., 2008; Gal-Yam et al., 2008]. For example, loss of monoacetylation and trimethylation of histone H4 lysine 20 is a common hallmark of human tumor cells [Fraga et al., 2005], and the risk of prostate cancer recurrence is predicted by altered patterns of histone acetylation and methylation [Seligson et al., 2005]. Human gastric adenomas and carcinomas have reduced levels of acetylated histone H4 [Ono et al., 2002], and in human colon cancer cells expression of the cell cycle regulator p21WAF1 (p21) is influenced by the acetylation status of histone H3 [Chen et al., 2007].
Histone acetylation typically results in an ‘open’ chromatin configuration that facilitates transcription factor access to DNA and gene transcription, but the reverse scenario can silence tumor suppressor genes in cancer cells [Wade 2001]. The acetylation and deacetylation of histones is catalyzed, respectively, by histone acetyltransferases (HATs) and histone deacetylases (HDACs). Over-expression and/or increased activity of HDACs occurs in many malignancies, and the repression of transcription can result in dysregulated cell cycle kinetics, apoptosis, and differentiation [Dokmanovic and Marks 2005; Mariadason 2008; McLaughlin and La Thangue 2004]. HDAC inhibitors are a current ‘hot topic’, and in the past year alone over 300 publications mentioning HDAC inhibitors were cited in PubMed.
In cancer cells, HDAC inhibitors have the ability to de-repress epigenetically-silenced genes, resulting in the re-expression of cell cycle regulators that trigger growth arrest, apoptosis, or differentiation [Marks et al., 2001]. This is not a genome-wide “shotgun” approach to gene activation, since only a select cadre of genes appears to be affected. For example, about 2–5% of silenced genes were reactivated within the initial hours of HDAC inhibitor treatment [Butler et al., 2002; Glaser et al., 2003], and p21 was an early target for upregulation [Kumagai et al., 2007; Okamoto et al., 2006; Rocchi et al., 2005].
Much of the work in this area has focused on competitive HDAC inhibitors with a hydroxamic acid functional group, such as trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA) [Donadelli et al., 2003; Komatsu et al., 2006; Kumagai et al., 2007; Sowa et al., 1997]. SAHA is marketed as Vorinostat (Zolinza®), and has shown promise in the treatment of patients with advanced cutaneous T-cell lymphoma [Marks 2007]. However, resistance to HDAC inhibitor drugs is of clinical concern in many patients, as well as toxicity, due to factors such as pharmacokinetics and the tumor micro-environment [Fantin and Richon 2007]. As a consequence, there are ongoing efforts to develop newer class- and isoform-selective HDAC inhibitors [Itoh et al., 2008; Jones and Steinkuhler 2008].
DIETARY HDAC INHIBITORS – A CHEMOPREVENTION PARADIGM
Based on some of the issues and concerns about HDAC inhibitor drugs currently used in the clinical setting, we turned our attention to dietary agents with structural features that might be compatible with HDAC inhibition. A simple working hypothesis was that the clinical response to HDAC inhibitor drugs, including pharmacokinetic variations, might be influenced by other HDAC inhibitors in the patient's diet.
As a starting point, we focused on food constituents with chemical structures that contained a spacer ‘arm’ that might fit the HDAC active site, and a functional group that could interact with the buried catalytic zinc atom [Dokmanovic and Marks 2005; McLaughlin and La Thangue 2004; Xu et al., 2007]. We were guided by prior work indicating that a carboxylate group can substitute for the hydroxamic acid moiety in binding to zinc within the HDAC pocket. Over three decades ago, the short-chain fatty acid butyrate was observed to cause histone modifications in HeLa and Friend erythroleukemia cells [Riggs et al., 1977]. Butyrate is generated during the fermentation of dietary fiber in the large intestine, and serves as the primary metabolic fuel for the colonocytes [Cummings and Englyst 1987; Cummings et al., 1987]. Recent studies identified butyrate as a competitive HDAC inhibitor, with an apparent inhibition constant (Ki) on the order of 46 µM [Sekhavat et al., 2007].
Interestingly, carboxylate-based HDAC inhibitors are gaining interest in the treatment of a wide range of maladies besides cancer. The antiepileptic agent valproic acid (Depakene®, Convulex®) and the hyperammonemia drug phenylbutyrate (Buphenyl®) are clinically-used compounds with HDAC inhibitory activity [Gottlicher 2004; Jung 2001]. Other applications may be found in treating bipolar disorder, Parkinson’s disease, rheumatoid arthritis, amyotrophic lateral sclerosis, and Huntington’s disease [Gottlicher 2004; Gardian et al., 2004; Chung et al., 2003; Ryu et al., 2005; Hogarth et al., 2007; Gardian et al., 2005], and this list is likely to grow in the future.
ISOTHIOCYANATES AS HDAC INHIBITORS
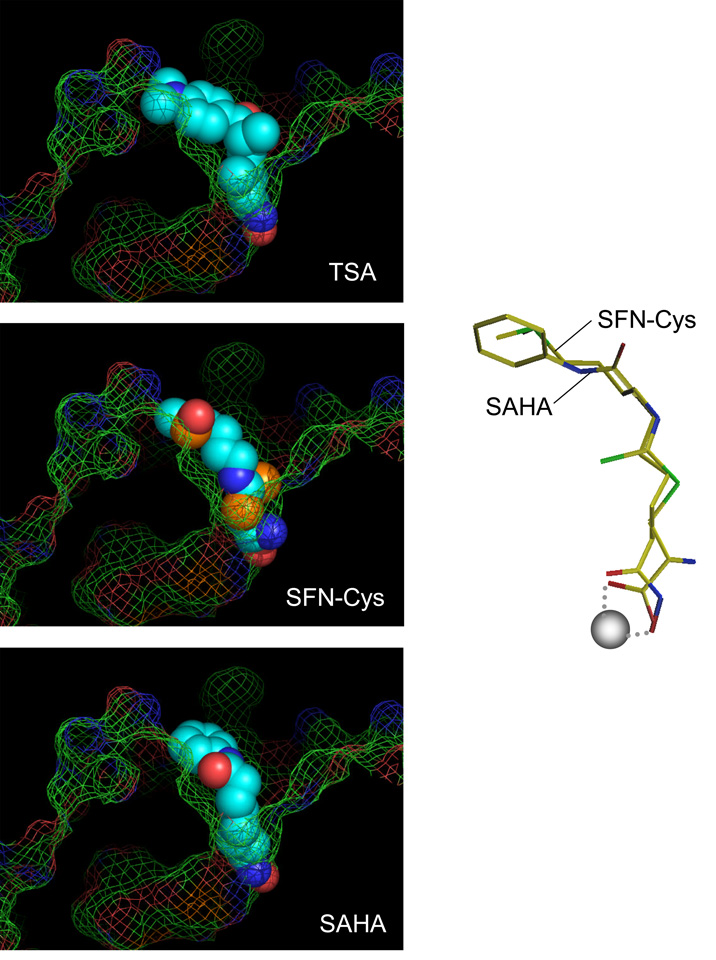
In addition to butyrate, what other dietary constituents might contain a ‘spacer-carboxylate’ arrangement in their chemical structure? We hypothesized that SFN might act as an HDAC inhibitor, based on the published literature describing p21 induction and cell cycle arrest/apoptosis in various human cancer cell lines [Fimognari et al., 2002; Gamet-Payrastre et al., 2000; Parnaud et al., 2004; Shen et al., 2006; Singh et al., 2004]. Like other isothiocyanates, SFN is metabolized via the mercapturic acid pathway, and computer modeling predicted that SFN-cysteine (SFN-Cys) was a good fit for the HDAC active site (Fig. 1). HDAC inhibition was not observed in a cell-free assay with parent SFN, or when HeLa cells were treated prior to SFN exposure with a chemical that blocked the mercapturic acid pathway. However, when HeLa cells were incubated with 3–15 µM SFN, the surrounding media contained metabolite(s) that inhibited HDAC activity in the cell-free assay [Myzak et al., 2004]. Subsequent studies confirmed the HDAC inhibitory effects of SFN in human colon and prostate cancer cells [Myzak et al., 2006b; Myzak et al., 2004], as well as in human breast cancer cell lines [Pledgie-Tracy et al., 2007]. HDAC inhibition in prostate BPH-1, LnCaP, and PC3 cells was associated with increased global histone acetylation, along with localized histone hyperacetylation on the promoter regions of P21WAF1 and BAX.
Fig. 1.
Inhibitors in the HDAC pocket. Binding of TSA (top) and SAHA (bottom) were from structural studies [Finnin et al., 1999]. Accelrys Insight II software was used to model interactions of putative inhibitors, with the following parameters: bidentate binding of the ligand to the zinc atom; H-bond partners for buried polar atoms; avoiding steric conflicts between ligand and enzyme based on a fixed protein; maintaining favorable torsion angles; following the favored positions of TSA and SAHA. SFN-Cys fit the HDAC pocket (center) and had comparable geometry to SAHA in the active site (right), with the α-carboxyl group of the cysteine moiety forming a bidentate ligand with the buried zinc atom (gray sphere). For full details, see [Myzak et al., 2004].
In vivo, dietary SFN retarded the growth of PC3 prostate cancer xenografts and spontaneous intestinal polyps in mouse preclinical models [Myzak et al., 2006a; Myzak et al., 2007], with evidence for HDAC inhibition and increased histone acetylation in tissues such as the gastrointestinal tract, prostate, and peripheral blood mononuclear cells (PBMCs). PBMCs have been used in human clinical trials with HDAC inhibitor drugs, serving as a surrogate for the changes that might be anticipated in other tissues with respect to HDAC activity and histone status [Marks et al., 2001; Marks 2007; Warrell et al., 1998]. Thus, we performed a pilot study with SFN-rich broccoli sprouts in human volunteers [Myzak et al., 2007]. In brief, healthy volunteers in the age range 18–55 yrs, with no history of non-nutritional supplement use, refrained from cruciferous vegetable intake for 48 hours, and each person then consumed 68 g of broccoli sprouts with a bagel and cream cheese. Blood was drawn at 0, 3, 6, 24, and 48 hours, and PBMCs were assayed using a commercial HDAC activity kit. HDAC activity was inhibited as early as 3 hour after broccoli sprout intake, it returned to normal by 24 hours, and there was a concomitant induction of histone acetylation [Dashwood and Ho 2007; Myzak et al., 2007]. This was the first study to show that a naturally consumed food, broccoli sprouts, had such a marked effect on HDAC activity and histone acetylation in humans.
Importantly, the pilot study in human volunteers addressed, in part, the question of whether concentrations that inhibit HDAC activity in vitro might ever be achievable in vivo; by operational definition, the consumption of broccoli sprouts in human volunteers provided sufficiently high concentrations in PBMCs to affect HDAC activity and histone acetylation status. Because this might be due to SFN and/or other phytochemicals in broccoli sprouts, we are repeating the studies to determine the specific concentrations of SFN and its metabolites achieved in human plasma and urine, following single and multiple ingestions of broccoli sprouts. Once the range of SFN concentrations in human PBMCs is established after broccoli sprout consumption, these data will be used to advance in vitro mechanistic studies. The latter will include “loss of function” experiments to define the relative importance of HDAC inhibition versus other potential mechanisms of chemoprevention. It is noteworthy that under conditions in the Apcmin mouse in which the development of spontaneous intestinal polyps was inhibited, tissue concentrations of SFN were in the range 3–30 µM (Hu et al., 2006), which is comparable to doses that inhibited HDAC activity in human colon cancer cells [Myzak et al., 2004].
ALLYL COMPOUNDS AS HDAC INHIBITORS
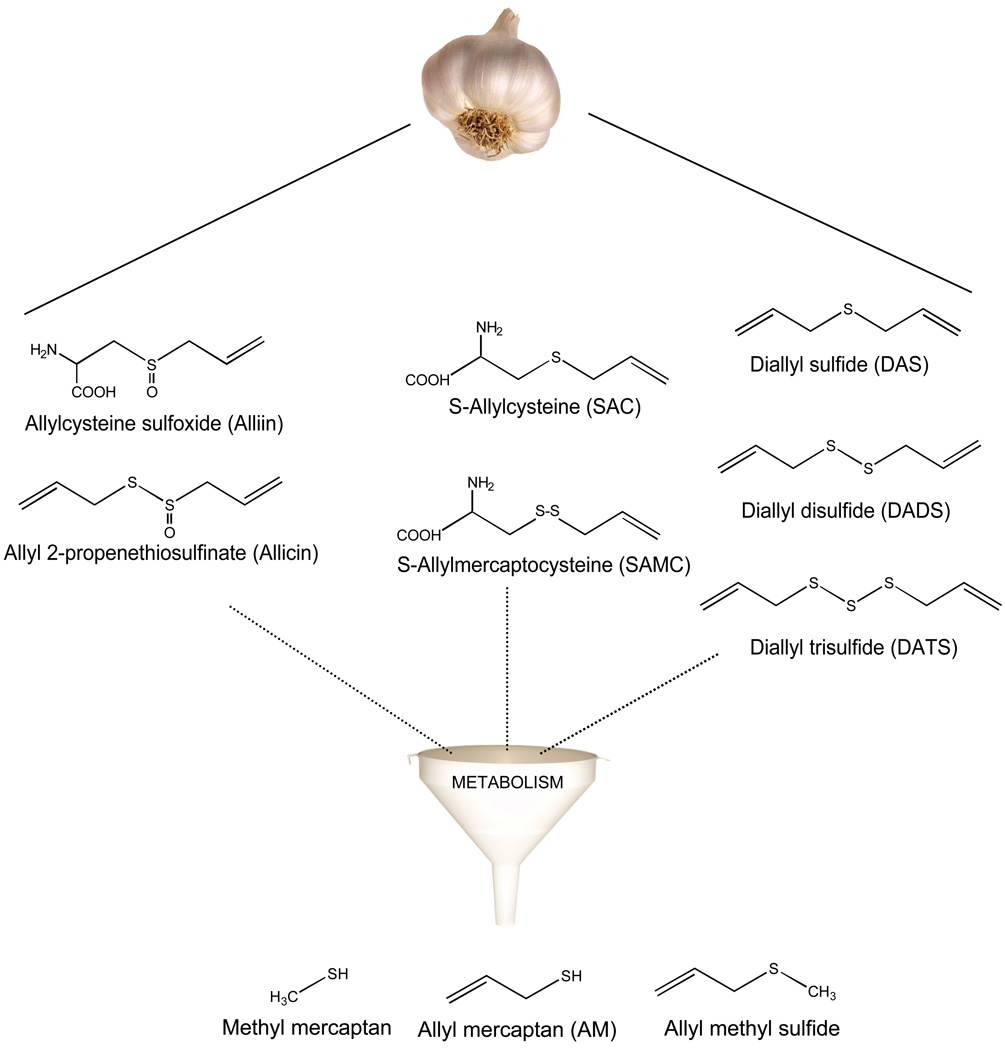
We also became interested in the inhibition of HDAC activity by dietary organosulfur compounds, such as those found in garlic (Fig. 2). Induction of histone acetylation was reported previously in cancer cells treated with the garlic compounds diallyl disulfide (DADS) and S-allyl mercaptocysteine (SAMC) [Lea et al., 2001; Lea et al., 2002], suggesting that these compounds may alter HDAC enzymes. In primary rat hepatocytes, DADS is metabolized to allyl mercaptan (AM) within 30 min [Sheen et al., 1999], which is noteworthy given that AM was more effective than its precursors (DADS, SAMC) at inhibiting HDAC activity in cell-free conditions [Druesne et al., 2004].
Fig. 2.
Organosulfur compounds in garlic. Garlic compounds such as Alliin, Allicin, S-allylcysteine (SAC), S-allyl mercaptocysteine (SAMC), diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) are metabolized to allyl mercaptan (AM), allyl methyl sulfide, and methyl mercaptan. AM was identified as a competitive HDAC inhibitor (Ki = 24 µM with human HDAC8) and induced histone acetylation in colon cancer cells [Nian et al., 2008].
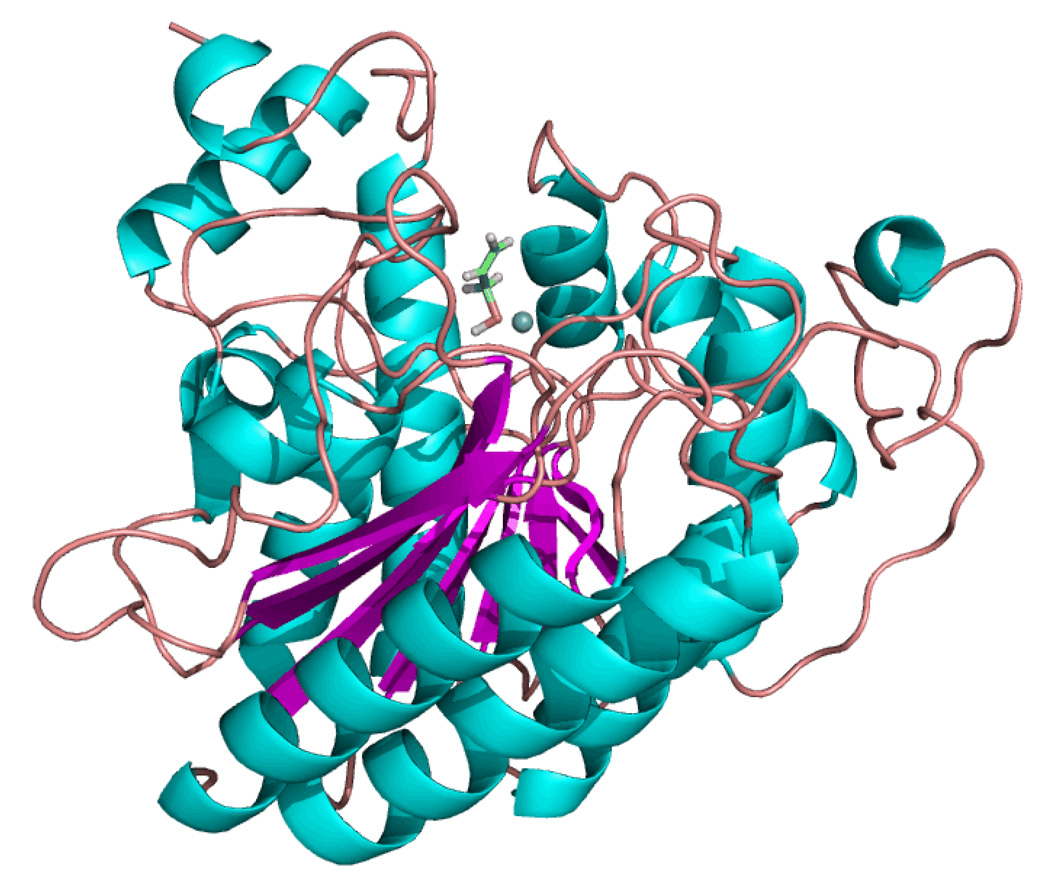
We screened several garlic organosulfur compounds and identified AM as the most potent HDAC inhibitor in assays with HeLa nuclear extracts, lysates from human colon cancer cells, or purified human HDAC8 [Nian et al., 2008]. Using MacroModel® software v8.5 (Schrödinger Inc.) to execute iterative docking simulations with human HDAC8 (Protein Databank entry 1T67), AM was found to be a good fit for the enzyme active site (Fig. 3). In an optimized truncated model, a strong interaction was predicted (−120 kcal/mol) between the buried zinc atom in the enzyme pocket and the sulfur atom of AM. Structure-activity studies confirmed the loss of HDAC inhibition after replacement of the –SH group in AM with an –OH moiety. Enzyme kinetics assays with a purified human HDAC provided evidence for a competitive mechanism (Ki = 24 µM AM).
Fig. 3.
Allyl mercaptan docked in the HDAC pocket. The interaction of AM with human HDAC8 was simulated using MacroModel v8.5 and Jaguar v5.5 software (Schrödinger), as reported elsewhere [Nian et al., 2008]. AM fit into the enzyme pocket with the sulfhydryl group (yellow) presumably ligated to the catalytic zinc atom (blue sphere).
Inhibition of HDAC activity by AM in human colon cancer cells was accompanied by a rapid, sustained accumulation of acetylated histones. Chromatin immunoprecipitation assays revealed an increase in acetylated histone H3 on the P21WAF1 gene promoter within 4 hours of AM exposure, along with increased binding of the transcription factor Sp3. Twenty-four hours after AM treatment there was enhanced binding of p53 in the distal enhancer region of the P21WAF1 gene promoter. The expression of p21(Waf1) protein was increased at time-points between 3 and 72 hours after AM treatment, and coincided with G1 growth arrest.
The working hypothesis is that metabolic conversion of organosulfur compounds to HDAC inhibitors in situ may contribute to the overall cancer chemoprotective properties of garlic and other Allium foods [Milner 2006; Wargovich 2006]. Support for this concept in vivo comes from studies demonstrating increased histone acetylation in colonocytes from rats treated with DADS [Druesne-Pecollo et al., 2007], and in the liver of mice 6 h after oral exposure to AM, DADS, or garlic oil (H. Nian and R.H. Dashwood, unpublished data). It remains to be determined whether garlic organosulfur compounds can affect HDAC (or HAT) activities and histone acetylation in human volunteers, and without any associated toxicity [Bianchini and Vainio 2001]. This is an important consideration, because dietary “chemopreventive” HDAC inhibitors typically are effective in the micromolar to millimolar range [Dashwood et al., 2006; Dashwood and Ho 2007; Delage and Dashwood 2008a; Delage and Dashwood 2008b], whereas HDAC inhibitors used therapeutically are thought to be effective at nM concentrations, but not without some toxicity and drug resistance [Fantin and Richon 2007].
FUTURE PERSPECTIVES
The specific focus here has been on the HDAC inhibitory properties of dietary isothiocyanates and allyl compounds, but other compounds with the ‘spacer-carboxylate’ arrangement exist in the human diet and are worthy of further study [Dashwood and Ho 2007; Dashwood et al., 2006; Delage and Dashwood 2008]. An evolving theme from this work is that metabolism plays a pivotal role in generating intermediates with HDAC inhibitory activity. We speculate that metabolic conversion of SFN to SFN-Cys might generate the ‘ultimate’ HDAC inhibitor, but this could hold true for several other dietary anticarcinogenic isothiocyanates, including those found in glucosinolate-containing plants such as mustard, radish, horseradish, wasabi, and daikon [Higdon et al., 2007]. Interestingly, the cysteine moiety in SFN-Cys occupied most of the HDAC active site in modeling simulations (Fig. 1), but cysteine itself lacked appreciable HDAC inhibitory activity in vitro [Dashwood et al., 2006]. This suggests that the Cys-conjugated intermediate is preferred, and that the isothiocyanate ‘cap’ group influences docking to the HDAC enzyme, perhaps helping to orient the inhibitor to the pocket region. Similar findings have been reported for hydroxamate-based HDAC inhibitors, where the ‘cap’ group influences enzyme specificity among various class I and class II HDACs [Furumai et al., 2001; McLaughlin and La Thangue 2004; Mork et al., 2005; Rosato and Grant 2003].
In the case of garlic and other Allium vegetables, water- and oil-soluble organosulfur compounds might be ‘funneled’ via metabolism to generate small molecule HDAC inhibitors, such as AM (Fig. 2). AM exhibited competitive kinetics with purified human HDAC8, but this metabolite almost certainly reacts with other thiol-containing proteins, such as those in the microtubule network [Hosono et al., 2008]. An important avenue for future work will be to examine the relative importance of HDACs compared with other cellular targets of allyl compounds, under normal physiological conditions and food intake levels, bearing in mind that garlic supplements are popular in the US (http://lpi.oregonstate.edu/infocenter/phytochemicals/garlic/).
Finally, there is evidence that with oxidative stress, HDAC inhibition might result in genes becoming activated that further exacerbate the underlying pathological condition, such as in chronic obstructive pulmonary disease [Yang et al., 2006]. Additional caveats were discussed elsewhere, such as the potential double-edge sword of targeting multiple HDAC enzymes [Dashwood et al., 2006]. These considerations add to the growing fascination surrounding the study of diet, epigenetics, and cancer chemoprevention, and the possibility that HDAC inhibitors in food might help reverse aberrant patterns of histone changes in cancer cells. Broccoli with garlic sauce, anyone?
ACKNOWLEDGEMENTS
Studies with SFN and AM were supported by NIH grants CA090890, CA065525, CA122906, and CA122959 from the U.S. National Cancer Institute, and by Environmental Health Sciences Center grant P30 ES00210, from the National Institute of Environmental Health Sciences. We are grateful to all the co-authors and collaborators on the original publications cited in this review, as well as Nihal Gooneratne for modeling studies presented in Fig. 1.
REFERENCES
- Bianchini F, Vainio H. Allium vegetables and organosulfur compounds: do they help prevent cancer? Environ Health Perspect. 2001;109(9):893–902. doi: 10.1289/ehp.01109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci U S A. 2002;99(18):11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YX, Fang JY, Lu R, Qiu DK. Expression of p21(WAF1) is related to acetylation of histone H3 in total chromatin in human colorectal cancer. World J Gastroenterol. 2007;13(15):2209–2213. doi: 10.3748/wjg.v13.i15.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YL, Lee MY, Wang AJ, Yao LF. A therapeutic strategy uses histone deacetylase inhibitors to modulate the expression of genes involved in the pathogenesis of rheumatoid arthritis. Mol Ther. 2003;8(5):707–717. doi: 10.1016/s1525-0016(03)00235-1. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH, Englyst HN. Fermentation in the human large intestine and the available substrates. Am J Clin Nutr. 1987;45(5 Suppl):1243–1255. doi: 10.1093/ajcn/45.5.1243. [DOI] [PubMed] [Google Scholar]
- Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Roychoudhury A, Sharma A, Talukder G. Modification of clastogenicity of three known clastogens by garlic extract in mice in vivo. Environ Mol Mutagen. 1993;21(4):383–388. doi: 10.1002/em.2850210410. [DOI] [PubMed] [Google Scholar]
- Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol. 2007;17(5):363–369. doi: 10.1016/j.semcancer.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage B, Dashwood RH. Dietary Manipulation of Histone Structure and Function. Annu Rev Nutr. 2008a;28:347–366. doi: 10.1146/annurev.nutr.28.061807.155354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage B, Dashwood RH. Targeting the epigenome with dietary agents. In: Surh Y-J, Dong Z, Cadenas E, Packer L, editors. Dietary Modulation of Cell Signaling Pathways. Boca Raton: CRC Press; 2008b. pp. 337–369. [Google Scholar]
- Dashwood RH, Myzak MC, Ho E. Dietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention? Carcinogenesis. 2006;27(2):344–349. doi: 10.1093/carcin/bgi253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokmanovic M, Marks PA. Prospects: histone deacetylase inhibitors. J Cell Biochem. 2005;96(2):293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- Donadelli M, Costanzo C, Faggioli L, Scupoli MT, Moore PS, Bassi C, Scarpa A, Palmieri M. Trichostatin A, an inhibitor of histone deacetylases, strongly suppresses growth of pancreatic adenocarcinoma cells. Mol Carcinog. 2003;38(2):59–69. doi: 10.1002/mc.10145. [DOI] [PubMed] [Google Scholar]
- Druesne N, Pagniez A, Mayeur C, Thomas M, Cherbuy C, Duee PH, Martel P, Chaumontet C. Diallyl disulfide (DADS) increases histone acetylation and p21(waf1/cip1) expression in human colon tumor cell lines. Carcinogenesis. 2004;25(7):1227–1236. doi: 10.1093/carcin/bgh123. [DOI] [PubMed] [Google Scholar]
- Druesne-Pecollo N, Chaumontet C, Pagniez A, Vaugelade P, Bruneau A, Thomas M, Cherbuy C, Duee PH, Martel P. In vivo treatment by diallyl disulfide increases histone acetylation in rat colonocytes. Biochem Biophys Res Commun. 2007;354(1):140–147. doi: 10.1016/j.bbrc.2006.12.158. [DOI] [PubMed] [Google Scholar]
- El-Bayoumy K, Sinha R, Pinto JT, Rivlin RS. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J Nutr. 2006;136(3 Suppl):864S–869S. doi: 10.1093/jn/136.3.864S. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94(19):10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin VR, Richon VM. Mechanisms of resistance to histone deacetylase inhibitors and their therapeutic implications. Clin Cancer Res. 2007;13(24):7237–7242. doi: 10.1158/1078-0432.CCR-07-2114. [DOI] [PubMed] [Google Scholar]
- Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat Res. 2007;635(2–3):90–104. doi: 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Fimognari C, Nusse M, Cesari R, Iori R, Cantelli-Forti G, Hrelia P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis. 2002;23(4):581–586. doi: 10.1093/carcin/23.4.581. [DOI] [PubMed] [Google Scholar]
- Fimognari C, Berti F, Cantelli-Forti G, Hrelia P. Effect of sulforaphane on micronucleus induction in cultured human lymphocytes by four different mutagens. Environ Mol Mutagen. 2005;46(4):260–267. doi: 10.1002/em.20156. [DOI] [PubMed] [Google Scholar]
- Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Breslow R, Pavletich NP. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401(6749):188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- Fiorio R, Bronzetti G. Diallyl sulfide inhibits the induction of HPRT-deficient mutants in Chinese hamster V79 cells treated with dimethylnitrosoamine in the presence of S-9 of rats induced with acetone. Environ Mol Mutagen. 1995;25(4):344–346. doi: 10.1002/em.2850250413. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37(4):391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- Furumai R, Komatsu Y, Nishino N, Khochbin S, Yoshida M, Horinouchi S. Potent histone deacetylase inhibitors built from trichostatin A and cyclic tetrapeptide antibiotics including trapoxin. Proc Natl Acad Sci U S A. 2001;98(1):87–92. doi: 10.1073/pnas.011405598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-Yam EN, Saito Y, Egger G, Jones PA. Cancer epigenetics: modifications, screening, and therapy. Annu Rev Med. 2008;59:267–280. doi: 10.1146/annurev.med.59.061606.095816. [DOI] [PubMed] [Google Scholar]
- Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60(5):1426–1433. [PubMed] [Google Scholar]
- Gardian G, Yang L, Cleren C, Calingasan NY, Klivenyi P, Beal MF. Neuroprotective effects of phenylbutyrate against MPTP neurotoxicity. Neuromolecular Med. 2004;5(3):235–241. doi: 10.1385/NMM:5:3:235. [DOI] [PubMed] [Google Scholar]
- Gardian G, Browne SE, Choi DK, Klivenyi P, Gregorio J, Kubilus JK, Ryu H, Langley B, Ratan RR, Ferrante RJ, et al. Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington's disease. J Biol Chem. 2005;280(1):556–563. doi: 10.1074/jbc.M410210200. [DOI] [PubMed] [Google Scholar]
- Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther. 2003;2(2):151–163. [PubMed] [Google Scholar]
- Gottlicher M. Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann Hematol. 2004;83(Suppl 1):S91–S92. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- Gronbaek K, Hother C, Jones PA. Epigenetic changes in cancer. APMIS. 2007;115(10):1039–1059. doi: 10.1111/j.1600-0463.2007.apm_636.xml.x. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth P, Lovrecic L, Krainc D. Sodium phenylbutyrate in Huntington's disease: a dose-finding study. Mov Disord. 2007;22(13):1962–1964. doi: 10.1002/mds.21632. [DOI] [PubMed] [Google Scholar]
- Hosono T, Hosono-Fukao T, Inada K, Tanaka R, Yamada H, Iitsuka Y, Seki T, Hasegawa I, Ariga T. Alkenyl group is responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Khor TO, Shen G, Jeong WS, Hebber V, Chen C, Xu C, Reddy B, Chada K, Kong AN. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetables. Carcinogenesis. 2006;27(10):2038–2046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Suzuki T, Miyata N. Isoform-selective histone deacetylase inhibitors. Curr Pharm Des. 2008;14(6):529–544. doi: 10.2174/138161208783885335. [DOI] [PubMed] [Google Scholar]
- Jones P, Steinkuhler C. From natural products to small molecule ketone histone deacetylase inhibitors: development of new class specific agents. Curr Pharm Des. 2008;14(6):545–561. doi: 10.2174/138161208783885317. [DOI] [PubMed] [Google Scholar]
- Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64(9):1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. Inhibitors of histone deacetylase as new anticancer agents. Curr Med Chem. 2001;8(12):1505–1511. doi: 10.2174/0929867013372058. [DOI] [PubMed] [Google Scholar]
- Knasmuller S, de Martin R, Domjan G, Szakmary A. Studies on the antimutagenic activities of garlic extract. Environ Mol Mutagen. 1989;13(4):357–365. doi: 10.1002/em.2850130413. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Kawamata N, Takeuchi S, Yin D, Chien W, Miller CW, Koeffler HP. SAHA, a HDAC inhibitor, has profound anti-growth activity against non-small cell lung cancer cells. Oncol Rep. 2006;15(1):187–191. [PubMed] [Google Scholar]
- Kondo Y, Issa JP. Epigenetic changes in colorectal cancer. Cancer Metastasis Rev. 2004;23(1–2):29–39. doi: 10.1023/a:1025806911782. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Wakimoto N, Yin D, Gery S, Kawamata N, Takai N, Komatsu N, Chumakov A, Imai Y, Koeffler HP. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (Vorinostat, SAHA) profoundly inhibits the growth of human pancreatic cancer cells. Int J Cancer. 2007;121(3):656–665. doi: 10.1002/ijc.22558. [DOI] [PubMed] [Google Scholar]
- Lea MA, Randolph VM, Lee JE, desBordes C. Induction of histone acetylation in mouse erythroleukemia cells by some organosulfur compounds including allyl isothiocyanate. Int J Cancer. 2001;92(6):784–789. doi: 10.1002/ijc.1277. [DOI] [PubMed] [Google Scholar]
- Lea MA, Rasheed M, Randolph VM, Khan F, Shareef A, desBordes C. Induction of histone acetylation and inhibition of growth of mouse erythroleukemia cells by S-allylmercaptocysteine. Nutr Cancer. 2002;43(1):90–102. doi: 10.1207/S15327914NC431_11. [DOI] [PubMed] [Google Scholar]
- Liu CT, Sheen LY, Lii CK. Does garlic have a role as an antidiabetic agent? Mol Nutr Food Res. 2007;51(11):1353–1364. doi: 10.1002/mnfr.200700082. [DOI] [PubMed] [Google Scholar]
- Mariadason JM. HDACs and HDAC inhibitors in colon cancer. Epigenetics. 2008;3(1):28–37. doi: 10.4161/epi.3.1.5736. [DOI] [PubMed] [Google Scholar]
- Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26(9):1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- McLaughlin F, La Thangue NB. Histone deacetylase inhibitors open new doors in cancer therapy. Biochem Pharmacol. 2004;68(6):1139–1144. doi: 10.1016/j.bcp.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Millen AE, Subar AF, Graubard BI, Peters U, Hayes RB, Weissfeld JL, Yokochi LA, Ziegler RG. Fruit and vegetable intake and prevalence of colorectal adenoma in a cancer screening trial. Am J Clin Nutr. 2007;86(6):1754–1764. doi: 10.1093/ajcn/86.5.1754. [DOI] [PubMed] [Google Scholar]
- Milner JA. Preclinical perspectives on garlic and cancer. J Nutr. 2006;136(3 Suppl):827S–831S. doi: 10.1093/jn/136.3.727S. [DOI] [PubMed] [Google Scholar]
- Mork CN, Faller DV, Spanjaard RA. A mechanistic approach to anticancer therapy: targeting the cell cycle with histone deacetylase inhibitors. Curr Pharm Des. 2005;11(9):1091–1104. doi: 10.2174/1381612053507567. [DOI] [PubMed] [Google Scholar]
- Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64(16):5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- Myzak MC, Dashwood RH. Chemoprotection by sulforaphane: keep one eye beyond Keap1. Cancer Lett. 2006a;233(2):208–218. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzak MC, Dashwood RH. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr Drug Targets. 2006b;7(4):443–452. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006a;20(3):506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006b;27(4):811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood) 2007;232(2):227–234. [PMC free article] [PubMed] [Google Scholar]
- Nagini S. Cancer chemoprevention by garlic and its organosulfur compounds-panacea or promise? Anticancer Agents Med Chem. 2008;8(3):313–321. doi: 10.2174/187152008783961879. [DOI] [PubMed] [Google Scholar]
- Nian H, Delage B, Pinto JT, Dashwood RH. Allyl mercaptan, a garlic-derived organosulfur compound, inhibits histone deacetylase and enhances Sp3 binding on the P21WAF1 promoter. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Fujioka Y, Takahashi A, Takahashi T, Taniguchi T, Ishikawa Y, Yokoyama M. Trichostatin A, an inhibitor of histone deacetylase, inhibits smooth muscle cell proliferation via induction of p21(WAF1) J Atheroscler Thromb. 2006;13(4):183–191. doi: 10.5551/jat.13.183. [DOI] [PubMed] [Google Scholar]
- Ono S, Oue N, Kuniyasu H, Suzuki T, Ito R, Matsusaki K, Ishikawa T, Tahara E, Yasui W. Acetylated histone H4 is reduced in human gastric adenomas and carcinomas. J Exp Clin Cancer Res. 2002;21(3):377–382. [PubMed] [Google Scholar]
- Parnaud G, Li P, Cassar G, Rouimi P, Tulliez J, Combaret L, Gamet-Payrastre L. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer. 2004;48(2):198–206. doi: 10.1207/s15327914nc4802_10. [DOI] [PubMed] [Google Scholar]
- Pinto JT, Krasnikov BF, Cooper AJ. Redox-sensitive proteins are potential targets of garlic-derived mercaptocysteine derivatives. J Nutr. 2006;136(3 Suppl):835S–841S. doi: 10.1093/jn/136.3.835S. [DOI] [PubMed] [Google Scholar]
- Pittler MH, Ernst E. Clinical effectiveness of garlic (Allium sativum) Mol Nutr Food Res. 2007;51(11):1382–1385. doi: 10.1002/mnfr.200700073. [DOI] [PubMed] [Google Scholar]
- Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6(3):1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs MG, Whittaker RG, Neumann JR, Ingram VM. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Rocchi P, Tonelli R, Camerin C, Purgato S, Fronza R, Bianucci F, Guerra F, Pession A, Ferreri AM. p21Waf1/Cip1 is a common target induced by short-chain fatty acid HDAC inhibitors (valproic acid, tributyrin and sodium butyrate) in neuroblastoma cells. Oncol Rep. 2005;13(6):1139–1144. [PubMed] [Google Scholar]
- Rosato RR, Grant S. Histone deacetylase inhibitors in cancer therapy. Cancer Biol Ther. 2003;2(1):30–37. doi: 10.4161/cbt.190. [DOI] [PubMed] [Google Scholar]
- Ryu H, Smith K, Camelo SI, Carreras I, Lee J, Iglesias AH, Dangond F, Cormier KA, Cudkowicz ME, Brown RH, Jr, et al. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J Neurochem. 2005;93(5):1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x. [DOI] [PubMed] [Google Scholar]
- Sekhavat A, Sun JM, Davie JR. Competitive inhibition of histone deacetylase activity by trichostatin A and butyrate. Biochem Cell Biol. 2007;85(6):751–758. doi: 10.1139/o07-145. [DOI] [PubMed] [Google Scholar]
- Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435(7046):1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- Sener G, Sakarcan A, Yegen BC. Role of garlic in the prevention of ischemia-reperfusion injury. Mol Nutr Food Res. 2007;51(11):1345–1352. doi: 10.1002/mnfr.200700078. [DOI] [PubMed] [Google Scholar]
- Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55(1):53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- Sheen LY, Wu CC, Lii CK, Tsai SJ. Metabolites of diallyl disulfide and diallyl sulfide in primary rat hepatocytes. Food Chem Toxicol. 1999;37(12):1139–1146. doi: 10.1016/s0278-6915(99)00108-8. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Chen C, Hebbar V, Kong AN. p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother Pharmacol. 2006;57(3):317–327. doi: 10.1007/s00280-005-0050-3. [DOI] [PubMed] [Google Scholar]
- Singh SV, Herman-Antosiewicz A, Singh AV, Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L, Baskaran R. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J Biol Chem. 2004;279(24):25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- Sowa Y, Orita T, Minamikawa S, Nakano K, Mizuno T, Nomura H, Sakai T. Histone deacetylase inhibitor activates the WAF1/Cip1 gene promoter through the Sp1 sites. Biochem Biophys Res Commun. 1997;241(1):142–150. doi: 10.1006/bbrc.1997.7786. [DOI] [PubMed] [Google Scholar]
- Tiku AB, Abraham SK, Kale RK. Protective effect of the cruciferous vegetable mustard leaf (Brassica campestris) against in vivo chromosomal damage and oxidative stress induced by gamma-radiation and genotoxic chemicals. Environ Mol Mutagen. 2008;49(5):335–342. doi: 10.1002/em.20383. [DOI] [PubMed] [Google Scholar]
- Wade PA. Transcriptional control at regulatory checkpoints by histone deacetylases: molecular connections between cancer and chromatin. Hum Mol Genet. 2001;10(7):693–698. doi: 10.1093/hmg/10.7.693. [DOI] [PubMed] [Google Scholar]
- Wargovich MJ. Diallylsulfide and allylmethylsulfide are uniquely effective among organosulfur compounds in inhibiting CYP2E1 protein in animal models. J Nutr. 2006;136(3 Suppl):832S–834S. doi: 10.1093/jn/136.3.832S. [DOI] [PubMed] [Google Scholar]
- Warrell RP, Jr, He LZ, Richon V, Calleja E, Pandolfi PP. Therapeutic targeting of transcription in acute promyelocytic leukemia by use of an inhibitor of histone deacetylase. J Natl Cancer Inst. 1998;90(21):1621–1625. doi: 10.1093/jnci/90.21.1621. [DOI] [PubMed] [Google Scholar]
- Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89(6):2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]