Abstract
Boys at risk for alcoholism show deviant P300 amplitude development. Genetic influences on P300, however, are related to a range of externalizing disorders. This study examined whether P300 development from adolescence to early adulthood differed between groups varying in severity of paternal externalizing. Parietal P300 was assessed during the “rotated heads” task on up to 3 times between the ages of 17 and 24 years. Participants were divided into 3 paternal externalizing groups: (a) severe (father has adult antisocial behavior), (b) intermediate (father has alcohol dependence but not a more severe disorder), and (c) low (father has no externalizing disorders or substance treatment and is not extreme in alcohol use). Mixed models were used to evaluate linear change in amplitude. P300 decreased with age. The severe-risk group had smaller P300 initially and changed less with time than did the low-risk group. The intermediate-risk group did not differ significantly from the low-risk group, but differed marginally from the severe-risk males. Externalizing and early-onset substance disorders in the sons were associated with smaller initial values of P300. Measures of deviant P300 development may be vulnerability markers for externalizing psychopathology.
Keywords: P300 amplitude, externalizing, growth model, development
Reduced P300 amplitude has been proposed as an endophenotype reflecting genetic influences on alcohol dependence (Begleiter, Porjesz, Bihari, & Kissin, 1984; Hesselbrock, Begleiter, Porjesz, O’Connor, & Bauer, 2001; Hill, Steinhauer, & Zubin, 1987; Polich, Pollock, & Bloom, 1994; Steinhauer, Hill, & Zubin, 1987) or a broader range of disinhibited psychopathology of which alcoholism is one possible manifestation (Iacono, Malone, & McGue, 2003; Patrick et al., 2006; Porjesz et al., 1998, 2005). An endophenotype is a measurable characteristic of an individual that is influenced by the same genes contributing to risk for a disorder but that is expressed at a level closer to gene transcription than are clinical symptoms (Gottesman & Gould, 2003). An assumption is often made that candidate endophenotypes are stable characteristics, that is, that people with a high level of a dimensional endophenotype at one time point tend to be high scorers at later times and people with a low level tend to remain the low scorers.
Visual P300 amplitude decreases with age in the normal population (Carlson & Iacono, 2006; Courchesne, 1978; Friedman, Putnam, & Sutton, 1989; Hill et al., 1999; Katsanis, Iacono, & McGue, 1996). The assumption of rank stability is not violated if people at low and high genetic risk change at similar rates so that there is a mean decrease but a preserved ranking. However, this does not appear to be the case for the P300 of males at risk for alcoholism. Hill et al. (1999) reported that males with a family history of alcoholism showed less decrease in visual amplitude during childhood and adolescence than did peers at low familial risk. Their growth models predicted that the mean amplitudes of young men with and without a family history of alcoholism would converge at age 22. These findings are consistent with the results of a meta-analysis of P300 amplitude in the sons of alcoholic men that indicated a greater difference between high- and low-risk boys under the age of 18 than between those at later ages (Polich et al., 1994). If the P300 amplitude development differs across those with and without vulnerability and the mean amplitudes of high- and low-risk groups converge, then the stable ordering of individuals will be compromised. If this is the case, cross-sectional measures of P300 at later ages will no longer discriminate risk groups and will no longer qualify as endophenotypes. P300 reductions reported in older alcoholic or drug-abusing populations may be because of the cumulative effects of substance use or comorbid psychopathology. As yet, no studies have compared P300 development in high- and low-risk individuals with measures taken up to the time of predicted convergence.
A further unknown concerns whether unusual P300 development in childhood and adolescence is best conceptualized as marking a vulnerability for alcoholism or for a broader phenotype represented by a range of disinhibited disorders. Two independent twin studies of covariance among disinhibited phenotypes suggested that the bulk of genetic influences on alcohol involvement (Young, Stallings, Corley, Krauter, & Hewitt, 2000) and alcohol dependence (Krueger et al., 2002) in childhood and adolescence are mediated by a latent “externalizing” phenotype that also contributes to risk for other substance disorders, antisocial behavior, and disinhibited personality traits. Externalizing disorders include attention deficit/hyperactivity, oppositional defiant, and conduct disorders at younger ages and the adult symptoms of antisocial personality disorder and alcohol and drug dependence at later ages. A twin family study of transmission of risk from parents to offspring suggested that a general vulnerability for externalizing disorders rather than risk for specific disorders is transmitted genetically (Hicks, Krueger, Iacono, McGue, & Patrick, 2004).
This is consistent with a recent investigation of comorbidity that contrasted a substance disorder typology that included an antisocial subtype with a dimensional latent trait model (Krueger, Markon, Patrick, & Iacono, 2005). The results supported a single externalizing dimension underlying conduct disorder before age 15, antisocial personality disorder symptoms occurring after age 15 (i.e., the adult antisocial behavior syndrome), alcohol dependence, and drug dependence. In this model, individual disorders require different levels of severity on the externalizing dimension for people to pass a diagnostic threshold. At least in adulthood, a less severe level of externalizing is necessary for the occurrence of alcohol dependence, and a relatively greater severity level of externalizing is required for drug dependence and adult antisocial behavior. Adult antisocial behavior was a relatively better indicator of severe externalizing than were the other disorders. High comorbidity of antisocial behavior and alcohol and drug dependence occurs in this model because those with adult antisocial behavior have severe enough levels of externalizing to pass the thresholds for each of these disorders.
P300 amplitude appears to reflect vulnerability for the externalizing dimension. Amplitude in adolescent males is related to the shared variance across externalizing disorders rather than to variance unique to individual disorders (Patrick et al., 2006), and this relationship appears to be entirely because of a common set of genes influencing P300 and externalizing (Hicks et al., 2007). Consistent with this notion is the finding that the relationship between P300 amplitude and early-onset alcohol problems is mediated by individual differences in measures strongly related to externalizing characteristics (Habeych, Charles, Sclabassi, Kirisci, & Tarter, 2005; Justus, Finn, & Steinmetz, 2001).
It is presently unclear whether the unusual developmental trajectory in boys with a paternal family history of alcoholism reported by Hill and her colleagues (Hill et al., 1999; Hill & Shen, 2002) would be related to varying levels of paternal externalizing. If so, P300 amplitude may not distinguish those with and without vulnerability for externalizing disorders past early adulthood. A deviant developmental trajectory, however, may provide clues regarding the nature of genetic influences on the etiology of externalizing.
Consistent with this notion, we previously reported that both the initial value (i.e., intercept) and the rate of change (i.e., slope) of P300 amplitude trajectories in a linear latent growth model were significantly heritable for males assessed from adolescence to adulthood (Carlson & Iacono, 2006). Visual P300 amplitude was highly stable from ages 17 to 24 (median test–retest r = .72 across 3-year assessment intervals), and 87% of the stable variance represented by the intercept was because of additive genetic influences. Amplitude decreased during this range at a rate of 0.61 µ V/year, and 15% of the variability in slope was because of genetic variance. A perfect inverse genetic correlation between intercept and slope was found in the best-fitting growth model, suggesting that to the extent that change in P300 amplitude is genetically influenced, it is likely influenced by the same genes contributing to the stable variance in P300 reflected by intercept.
The present study uses the same sample of young men from which the twin pairs in the Carlson and Iacono (2006) report were drawn. This study investigates how P300 amplitude trajectory differs across young men varying in familial risk for externalizing. These young men were administered a challenging visual task of the kind associated with larger differences between high- and low-risk males (Polich et al., 1994) at roughly 3-year intervals starting at approximately age 17. This time frame covers an important period of development in frontal cortical areas (Gogtay et al., 2004; Sowell, Thompson, Tessner, & Toga, 2001; Spear, 2000), some of which are thought to underlie cognitive risk factors associated with disinhibited behavior (Fuster, 2002; Giancola & Tarter, 1999; Pihl, Peterson, & Finn, 1990). This period also encompasses the point of convergence in the growth curves of young men varying in family history of alcoholism as predicted by Hill et al. (1999). This enables an evaluation of whether the trajectories of those at high and low risk do converge in the early 20s.
The young men examined were grouped on the basis of severity of paternal externalizing psychopathology. A low-risk group was composed of young men whose fathers had no history of alcohol, drug, or antisocial behavior disorders. To reduce false negatives in this group, we also excluded young men whose fathers were extreme on measures of alcohol use or who had a history of substance disorder treatment. A group of participants at risk for severe externalizing consisted of all the young men whose fathers had the adult antisocial behavior syndrome. Consistent with Krueger et al.’s (2005) externalizing severity conceptualization, a group with fathers at an intermediate level of externalizing severity was formed from all participants whose fathers had alcohol dependence but not drug dependence or adult antisocial behavior. Besides reflecting an intermediate level of risk, the inclusion of this group provides a link to many past P300 studies in which the focus has been on the offspring of fathers with alcoholism. It extends this research by providing an opportunity to examine the effects of paternal alcoholism per se, in the absence of other comorbid externalizing disorders.
We hypothesized that the trajectory of P300 amplitude development would be related to familial risk for externalizing psychopathology. Specifically, consistent with Hill et al.’s (1999) findings of different trajectories in families differing in alcoholism risk, we hypothesized that greater paternal externalizing would be associated with smaller intercept and less change with age. Previous work found a stronger association of both P300 amplitude and externalizing disorders with earlier onset substance use disorders (i.e., onset by the age 20 assessment) than with later onset substance use disorders (Carlson, McLarnon, & Iacono, 2007). We, therefore, further predicted that those young men who develop an externalizing or earlier onset substance use disorder will have a high-risk P300 amplitude trajectory compared with those who do not have these disorders.
Method
Participants
Participants were drawn from the 578 young men who were in the 17-year-old age cohort of the Minnesota Twin Family Study (MTFS; Iacono, Carlson, Taylor, Elkins, & McGue, 1999). The total number of participants per paternal risk group was 161 men in the low-risk group (from 87 fathers), 126 men in the intermediate-risk group (from 70 fathers), and 82 men in the severe-risk group (from 44 fathers). Participants were identified from birth records of the Minnesota Department of Health as being twins born in the state of Minnesota during the years 1972–1978. They were invited to come to the laboratories of the MTFS by phone or by mail during their senior year of high school. Details regarding the sampling approach and representativeness of the sample recruited were provided in Iacono et al. (1999) and Holdcraft and Iacono (2004). Table 1 depicts demographic data for the participants in the externalizing risk groups. In general, the data in Table 1 indicate that compared with the low-risk fathers, both the intermediate- and severe-risk fathers were less well educated and had lower levels of occupational status. Ethnicity did not differ significantly across risk groups. Regarding offspring diagnoses, prevalence rates were substantially higher in the severe- versus the low-risk group. As expected, those in the intermediate-risk group generally tended to fall between these other two groups. Offspring IQ did not differentiate the groups. Data were available for the first visit and two subsequent follow-ups at roughly 3-year intervals. Informed consent was obtained from all adult participants. Informed assent and parental consent were obtained when participants were under 18 years. All procedures were approved by the appropriate institutional ethics review board.
Table 1.
Demographic and Clinical Information for the Paternal Externalizing Risk Groups
| Paternal externalizing risk group (%) |
||||||
|---|---|---|---|---|---|---|
| Demographic and clinical information | Low (n = 87) | Intermediate (n = 70) | Severe (n = 44) | χ2 | df | p |
| Fathers | ||||||
| Education | 17.19 | 8 | .028 | |||
| Less than high school degree | 2.3 | 1.4 | 0 | |||
| High school degree/GED | 50.0a,b | 75.4a | 75.0b | |||
| Associate’s degree | 11.6a | 2.9a | 2.3 | |||
| BA/BS | 29.1 | 18.8 | 20.5 | |||
| Professional degree | 7.0 | 1.4 | 2.3 | |||
| Occupation | 25.12 | 12 | .014 | |||
| Major professional | 18.4a | 7.1a | 6.8 | |||
| Lesser professional | 23.0 | 12.9 | 11.4 | |||
| Minor professional | 18.4 | 22.9 | 20.5 | |||
| Clerical, sales, technician, etc. | 9.2 | 10.0 | 11.4 | |||
| Skilled manual | 25.3 | 34.3 | 20.5 | |||
| Semiskilled | 4.6b | 10.0 | 18.2b | |||
| Unskilled | 1.1b | 2.9 | 11.4b | |||
| Ethnicity | 1.34 | 2 | .518 | |||
| African American | 1.1 | 0 | 0 | |||
| European American | 98.9 | 100 | 100 | |||
| Diagnoses (lifetime) | ||||||
| Conduct disorder | NA | 37.1c | 61.4c | NA | ||
| Adult antisocial behavior | NA | NA | 100 | NA | ||
| Alcohol dependence | NA | 100c | 77.3c | NA | ||
| Illicit drug dependence | NA | NA | 15.9 | NA | ||
| Nicotine dependence | 22.1a,b | 68.1a | 70.5b | 43.75 | 2 | <.001 |
| Offspring | ||||||
|
N = 161 |
N = 126 |
N = 82 |
||||
| Diagnoses | ||||||
| Lifetime attention deficit/hyperactivity disorder | 2.3a,b | 11.7a | 20.5b | 23.48 | 2 | <.001 |
| Lifetime oppositional defiant disorder | 10.9a,b | 21.2a | 27.3b | 11.94 | 2 | .003 |
| Lifetime conduct disorder | 37.7a,b | 50.9a,c | 73.8b,c | 27.45 | 2 | <.001 |
| Lifetime adult antisocial behavior | 14.1a,b | 29.0a,c | 47.2b,c | 28.16 | 2 | <.001 |
| Early-onset alcohol dependence | 35.2b | 39.0 | 50.6b | 5.42 | 2 | .066 |
| Early-onset illicit drug dependence | 11.9b | 11.7c | 25.3b,c | 8.61 | 2 | .014 |
| Early-onset nicotine dependence | 25.5b | 32.3c | 50.6b,c | 15.58 | 2 | <.001 |
| Any disorder | 60.2a,b | 76.4a,c | 91.5b,c | 27.97 | 2 | <.001 |
|
M (SD) |
M (SD) |
M (SD) |
F |
df |
p |
|
| IQ | 104.01 (13.45) | 101.66 (13.94) | 104.01 (14.60) | 1.31 | 2, 399 | .272 |
Note. Paternal externalizing groups were low risk (no evidence of significant paternal substance misuse or antisociality), intermediate risk (paternal alcohol dependence, but no paternal drug dependence or adult antisociality), and severe risk (significant paternal adult antisociality). Diagnoses are at the probable (all but one symptom present) or definite (all Diagnostic and Statistical Manual of Mental Disorders [3rd ed., revised; American Psychiatric Association, 1987] criteria met) level of certainty. Early-onset substance disorders are thus those that occurred before the age 20 assessment. IQ is prorated from the Wechsler Adult Intelligence Scale—Revised Wechsler, 1981) Vocabulary, Information, Block Design, and Picture Arrangement subtests administered at Time 1. NA = not applicable because of group definitions.
Low-risk group ≠ intermediate-risk group.
Low-risk group ≠ severe-risk group.
Intermediate-risk group ≠ severe-risk group. All ps for group contrasts < .05.
Rotated Heads Task
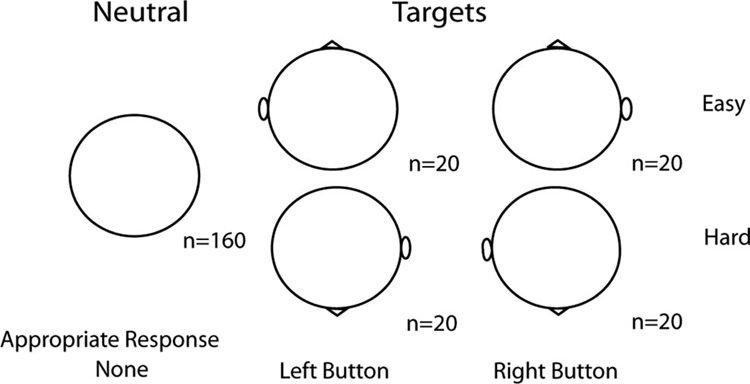
At each assessment, participants performed the “rotated heads” task of Begleiter et al. (1984). A series of stimuli representing the superior view of a schematic head were presented on a computer monitor positioned approximately 48 cm from the participant. Figure 1 illustrates the stimuli used in this task. On 80 of the trials, stimuli were presented with a cartoon nose and a single ear. Participants were instructed to press one of two buttons corresponding to the side of the head the ear was on as quickly but as accurately as they could. On half of these target trials, the nose was pointed toward the top of the screen. In these trials, an ear on the right side of the screen was on the right side of the head and an ear on the left side of the screen was on the left side of the head. This was designated the easy condition. On the remaining target trials, the nose pointed to the bottom of the screen. In this hard condition, an ear on the right side of the screen corresponded to the left side of the head and vice versa. Correct responses on these trials entailed pressing a button with the hand opposite to the side of the screen the ear appeared on. There were an equal number of left and right ears in both the easy and the hard conditions. These target stimuli were presented pseudorandomly within a series of 160 neutral stimuli consisting of a simple oval. Participants were instructed not to respond to these nontargets. Stimuli appeared with a duration of 98 ms and an intertrial interval that varied randomly between 1 s and 2 s.
Figure 1.
Stimuli used in the Rotated Heads task of Begleiter et al. (1984); n = the number of each stimulus presented.
Psychophysiological Assessment
Electroencephalographic (EEG) and electrooculographic (EOG) data were filtered and amplified with a Grass Model 12c Neurodata Acquisition System (Grass Instruments, Quincy, MA). Signals were collected through AC amplifiers with 1/2 amplitude low-and high-frequency filter settings at .01 and 30 Hz (18 dB/octave roll-off), respectively. EEG signals were recorded from the Pz, P3, and P4 scalp sites using an electrode cap with a linked ears reference. A ground electrode was affixed to the right shin, and EOG sensors were placed above and to the temporal side of an eye. Electrode site impedance was kept below 5 kΩ for the scalp sites and the ears and below 10 kΩ for the ground and EOG sites. Data were sampled during a 2,000-ms epoch with a 500-ms prestimulus baseline at a rate of 256 Hz. If a participant failed to respond within the epoch or if the A–D converter became saturated, the trial was rejected and repeated after the presentation of two additional neutral stimuli. This was done to preserve the global probability of target versus neutral stimuli while ensuring that participants had the same number of trials contributing to the event-related potentials.
EEG signals were subjected offline to a 7.5-Hz low-pass digital filter to attenuate high-frequency noise and to the blink-correction algorithm of Gratton, Coles, and Donchin (1983). As in past studies from the MTFS that used this task (e.g., Carlson & Iacono, 2006; Carlson, Iacono, & McGue, 2002, Carlson, Iacono, & McGue, 2004; Carlson, Katsanis, Iacono, & Mertz, 1999; Carlson et al., 2007; Hicks et al., 2007; Iacono, Carlson, Malone, & McGue, 2002; Katsanis, Iacono, McGue, & Carlson, 1997; Patrick et al., 2006), a rater unaware of participant diagnosis or family history used an interactive computer algorithm to identify P300 at the Pz site. The algorithm identified the maximum voltage in a window from 200 to 800 ms after stimulus onset. This liberal latency range was chosen so as not to artificially constrain P300 latency. The minimum and maximum observed latencies in this study were 300.78 and 617.19 ms, respectively. In cases in which there was more than one peak, the rater chose the second peak. The peak selected was also checked against the waveforms from P3 and P4 and corrected if needed. Amplitude was the difference in voltage between the mean of the 500-ms baseline and the selected peak.
Psychiatric Assessment
At the initial assessment, participants and their biological fathers underwent structured clinical interviews. Responses were subsequently reviewed in case conferences of at least two graduate students with advanced training in psychiatric diagnosis. Diagnostic and Statistical Manual for Mental Disorders (3rd ed., revised, or DSM–III–R; American Psychiatric Association, 1987) criteria were used because this was the nosology at study inception.
Table 2 summarizes the semistructured clinical interviews used to assess disorders. Fathers were interviewed about pre–age 15 symptoms of conduct disorder (CD), post–age 15 symptoms of antisocial personality disorder (i.e., the adult antisocial behavior or AAB syndrome), and nicotine, alcohol, and drug use disorders. Fathers were also asked about their age at first drink without parental permission and the maximum number of alcoholic drinks consumed in a 24-hr period. A drink was defined as being equivalent to a glass of wine, a bottle or can of beer, a shot glass of liquor, or a mixed drink.
At the initial assessment, adolescents were interviewed about their symptoms of attention deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), CD, AAB, and substance disorders. At Time 1 only, their mothers also reported on their symptoms of ADHD, ODD, CD, and substance disorders. A symptom was considered present if either informant reported it present and absent if they agreed on its absence. At the two follow-up assessments, the young men were interviewed about lifetime substance use disorders, CD, and AAB. At the second follow-up, they reported on substance disorders since the previous assessment and lifetime CD and AAB. The fathers’ interviewers were unaware of offspring diagnoses and vice versa. Kappa coefficients for the diagnoses used in this study ranged from 0.71 for ODD to 0.91 for substance use disorders (Iacono et al., 1999), suggesting good to excellent reliability.
Consistent with past P300 studies from the MTFS (Carlson et al., 2002, 2004; Carlson et al., 1999, 2007; Iacono, Carlson, & Malone, 2000; Iacono et al., 2002, 1999), a disorder was considered present if it was at the definite (i.e., minimum DSM–III–R criteria are met) or probable (i.e., all but one DSM–III–R criterion is met) level of diagnostic certainty and absent otherwise. Collapsing across the definite and probable levels of certainty, an approach for diagnosing lifetime psychopathology that was introduced with the Research Diagnostic Criteria (Spitzer, Endicott, & Robins, 1978), is important for assessing disorders in individuals who are not currently symptomatic and who may have difficulty recalling symptoms experienced years or decades earlier. Earlier onset substance disorder was defined as in Carlson et al. (2007) as the presence of alcohol abuse or dependence, illicit drug abuse or dependence, or nicotine dependence with onset by the first follow-up (e.g., onset by approximately age 20).
Externalizing Risk Group Definitions
An externalizing low-risk group consisted of those young men whose fathers had no history of CD, AAB, alcohol abuse or dependence, illicit drug abuse or dependence, or alcohol or drug treatment. Earlier age at first alcohol use has been associated with higher rates of externalizing characteristics such as antisocial behavior, illicit substance use and abuse, and disinhibited personality characteristics (Clark, 2004; McGue, Iacono, Legrand, Malone, & Elkins, 2001; Schuckit & Russell, 1983). The maximum number of drinks in a 24-hr period has been described as a promising quantitative endophenotype for alcoholism risk (Malone, Iacono, & McGue, 2002). Both of these paternal alcohol use measures have been associated with reduced P300 amplitude in sons even in the absence of paternal substance disorder diagnoses (Iacono et al., 2002). As in Iacono et al. (2002), to reduce false negatives young men whose fathers were in the upper decile of the maximum consumption measure or in the lower decile of age at first drink were excluded from the low-risk group. A severe externalizing risk group consisted of young men whose fathers met criteria for the AAB syndrome. An intermediate externalizing risk group consisted of young men whose fathers had alcohol dependence, but not drug dependence or AAB.
Young men were said to have an externalizing disorder if they had lifetime ADHD, ODD, CD, AAB, or earlier onset alcohol abuse or dependence, drug abuse or dependence, or nicotine dependence. The inclusion of nicotine dependence reflected its emergence as a strongly heritable indicator of externalizing in MTFS youths (McGue, Iacono, & Krueger, 2006), coupled with the fact that when included as part of the externalizing composite, the composite was genetically related to P300 amplitude (Hicks et al., 2007). The inclusion of substance abuse in this definition was intended to reduce the number of false negatives in the disorder-free group.
Diagnostic status was unknown for a small number of participants because they were disorder free at earlier assessments and did not participate at later occasions. Excluded were 36.2% of the 578 young men in this sample who had fathers who did not meet criteria for one of our externalizing groups and whose externalizing status would thus have been ambiguous. P300 was missing on some occasions because participants did not participate, because of computer or equipment problems, or because of poor task performance (i.e., >5 errors). Across the three paternal risk groups there were 1,107 potential P300 measurements (i.e., 3 assessments × [161 + 126 + 82 participants]). Of these, 30.4% were missing for one of the aforementioned reasons. The number of participants in each paternal risk group with usable P300 amplitude at each time point is presented in Table 3.
Table 3.
Descriptive Statistics for Observed P300 Amplitude and Age by Each Assessment Occasion and Subgrouping of Participants by Paternal Externalizing and Participant Externalizing Disorder
| Paternal externalizing risk | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | Severe | All participants | |||||||||
| Assessment occasion and subgrouping | N | M | SD | N | M | SD | N | M | SD | N | M | SD |
| P300 amplitude (µV) | ||||||||||||
| Assessment 1 | ||||||||||||
| Offspring disorder absent | 58 | 28.62 | 8.27 | 28 | 25.95 | 8.27 | 6 | 30.04 | 8.29 | 92 | 27.90 | 8.29 |
| Offspring disorder present | 76 | 22.38 | 7.79 | 73 | 23.64 | 7.74 | 65 | 21.13 | 6.77 | 216 | 22.43 | 7.51 |
| Total | 134 | 25.08 | 8.55 | 101 | 24.28 | 7.92 | 71 | 21.88 | 7.29 | 306 | 24.08 | 8.14 |
| Assessment 2 | ||||||||||||
| Offspring disorder absent | 53 | 24.10 | 7.68 | 28 | 24.92 | 7.47 | 3 | 22.58 | 5.22 | 84 | 24.32 | 7.49 |
| Offspring disorder present | 58 | 21.66 | 6.45 | 61 | 21.38 | 7.78 | 44 | 18.93 | 5.96 | 163 | 20.82 | 6.92 |
| Total | 111 | 22.82 | 7.14 | 89 | 22.49 | 7.82 | 47 | 19.16 | 5.94 | 247 | 22.01 | 7.29 |
| Assessment 3 | ||||||||||||
| Offspring disorder absent | 46 | 21.73 | 5.92 | 22 | 22.93 | 7.30 | 5 | 26.50 | 3.47 | 73 | 22.41 | 6.29 |
| Offspring disorder present | 48 | 18.83 | 7.71 | 54 | 20.21 | 6.55 | 38 | 18.61 | 5.91 | 140 | 19.30 | 6.80 |
| Total | 94 | 20.25 | 7.00 | 78 | 21.00 | 6.84 | 43 | 19.53 | 6.20 | 213 | 20.37 | 6.78 |
| Age (years) | ||||||||||||
| Assessment 1 | ||||||||||||
| Offspring disorder absent | 58 | 17.45 | 0.42 | 28 | 17.45 | 0.43 | 6 | 17.26 | 0.49 | 92 | 17.44 | 0.43 |
| Offspring disorder present | 76 | 17.59 | 0.39 | 73 | 17.47 | 0.36 | 65 | 17.47 | 0.41 | 214 | 17.51 | 0.39 |
| Total | 134 | 17.53 | 0.41 | 101 | 17.47 | 0.38 | 71 | 17.46 | 0.41 | 306 | 17.49 | 0.40 |
| Assessment 2 | ||||||||||||
| Offspring disorder absent | 53 | 20.65 | 0.52 | 28 | 20.59 | 0.59 | 3 | 20.85 | 0.49 | 84 | 20.64 | 0.54 |
| Offspring disorder present | 58 | 20.72 | 0.41 | 61 | 20.55 | 0.47 | 44 | 20.57 | 0.52 | 163 | 20.61 | 0.52 |
| Total | 111 | 20.69 | 0.47 | 89 | 20.56 | 0.51 | 47 | 20.59 | 0.52 | 247 | 20.62 | 0.49 |
| Assessment 3 | ||||||||||||
| Offspring disorder absent | 46 | 23.83 | 0.73 | 22 | 24.03 | 0.76 | 5 | 24.33 | 1.14 | 73 | 23.92 | 0.77 |
| Offspring disorder present | 48 | 23.97 | 0.73 | 54 | 24.17 | 1.02 | 38 | 24.30 | 1.22 | 140 | 24.14 | 0.99 |
| Total | 94 | 23.90 | 0.73 | 76 | 24.13 | 0.95 | 43 | 24.30 | 1.20 | 213 | 24.06 | 0.93 |
Note. Paternal externalizing groups were low risk (no evidence of significant paternal substance misuse or antisociality), intermediate risk (paternal alcohol dependence but no paternal drug dependence or adult antisociality), and severe risk (significant paternal adult antisociality). Offspring disorders were attention deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, adult antisocial behavior, alcohol abuse or dependence by age 20, drug abuse or dependence by age 20, and nicotine dependence by age 20.
Growth Model Analysis
Provisional visual inspection of individual growth plots of P300 amplitude by age suggested a linear model of change for this age range (Carlson & Iacono, 2006). SAS PROC MIXED (SAS Institute, 1998) with full maximum likelihood estimation, unstructured residual variance matrices, and the “between– within” method for determining degrees of freedom was used to fit mixed models as suggested by Singer and Willet (2003). SAS PROC MIXED assumes that data are missing at random. The models fit to P300 amplitude were “three-level” linear mixed models appropriate for use with multiple participants from the same family (Khoo & Muthén, 2000). As such, random effects were allowed for intercept, slope, and their covariance at the level of both the individual participant and the family.
The measure of age at each time point was the participant’s age (X) minus the mean age at the initial assessment (17.49 years). Centering at the mean age makes our P300 intercept interpretable as the mean amplitude at this wave of assessment. This makes our intercept directly comparable to that of our past published studies reporting on P300 amplitude at this wave (Carlson et al., 2002, 2004; Carlson et al., 1999; Carlson et al., 2007; Iacono et al., 2000; Iacono et al., 2002; Iacono et al., 1999), as well as being consistent with our behavior genetic growth model using these participants (Carlson & Iacono, 2006). Although individuals were targeted for assessment at ages 17, 20, and 23, to optimize participation we allowed some flexibility in scheduling around the targeted age, especially as participants became older and more independent. A continuous measure of age rather than a dummy variable coded for assessment wave was used because of the nontrivial variability in age at some assessment points (particularly at Time 3). This also allowed for more precise estimates of growth parameters by using the relatively greater amount of information contained in a continuous measure. Paternal risk group and offspring externalizing disorder were treated as categorical variables. The genetically mediated covariance between intercept and slope previously reported (Carlson & Iacono, 2006) makes separate interpretations of intercept and slope differences problematic. As such, we have adopted the approach of Hill et al. (1999) and used Wald tests of joint hypotheses regarding intercept and slope to test differences between groups in P300 amplitude trajectories. Such an approach tests the joint null hypotheses that groups do not differ in intercept and slope. Follow-up tests to determine age ranges in which groups differed significantly in P300 amplitude were conducted using the approach of D. J. Bauer and Curran (2005; see also Preacher, Curran, & Bauer, 2006). This approach identifies what the difference in intercept between two risk groups would be at each age and a 95% confidence interval for this value.1 We further used the approach of D. J. Bauer and Curran (2005) to identify the precise ages that bracket the range in which these group differences are statistically significant.
Results
Attrition
We examined several patterns of missing data to see whether paternal risk groups differed in rates of attrition. About a quarter of all families (24.8%) in these three groups had complete P300 data at all assessments for both sons. This rate did not differ significantly across paternal risk groups (27.6% of low paternal risk, 30.0% of intermediate paternal risk, and 18.2% of severe paternal risk groups), χ2(2, N = 201) = 2.06, p= .357. Furthermore, 42.6% of families had P300 data at all three assessments from one offspring and no more than one missing P300 measure from the other (48.3 % of low paternal risk, 44.3% of intermediate paternal risk, and 36.4 % of paternal AAB groups). The paternal risk groups did not differ significantly in this pattern, χ2(2, N = 201) =1.68, p= .432. Finally, the majority (61.2%) of families had at least two P300 measures from both offspring (66.7% of low paternal risk, 57.1 % of intermediate paternal risk, and 52.3 % of paternal AAB groups, a nonsignificant difference), χ2(2, N = 201) = 2.95, p= .229). In addition to all of these analyses of the effects of missing data being nonsignificant, they all resulted in effect sizes that are conventionally regarded as being in the small effect range (J. Cohen, 1988; .039 ≤ Cohen’s w ≤.121). Given these patterns of missing data and the small effect sizes represented, it appears that there were no important systematic biases in attrition related to paternal history. This is consistent with the data missing at random.
Paternal Externalizing and P300 Amplitude Change
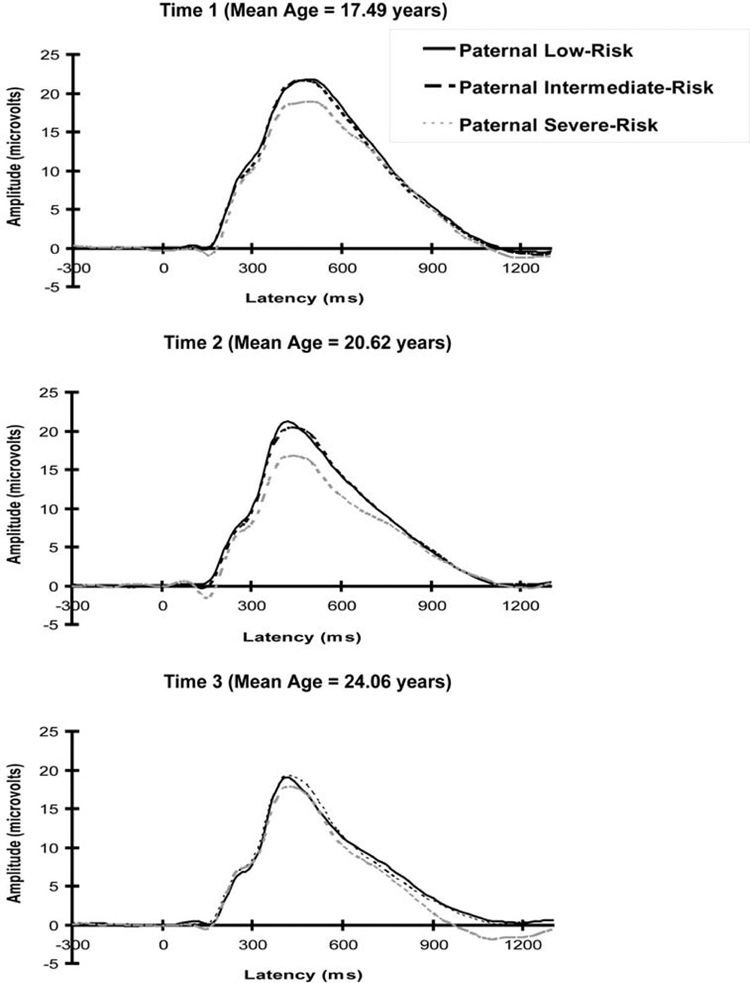
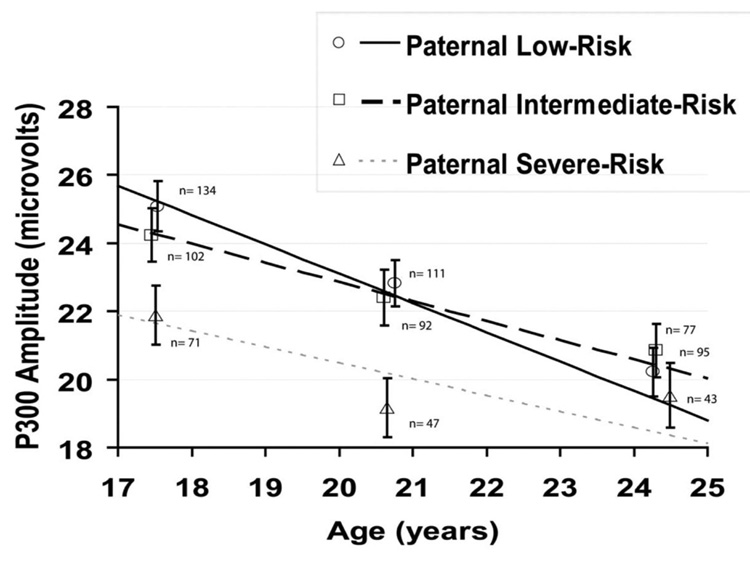
Grand mean event-related potential waveforms for each paternal risk group at each assessment are presented in Figure 2. Table 3 presents descriptive statistics for the observed values of P300 amplitude and age at each measurement for those differing in diagnostic status and paternal risk. In the growth model, P300 decreased significantly with age, F(1, 573) = 96.36, p <.001. There was a significant difference across paternal risk groups in the mean intercept, F(2, 187) = 3.77, p= .025, and slope, F(2, 573) = 3.59, p= .028, of the developmental trajectories. The P300 amplitude trajectories predicted by the model are presented in Figure 3. The paternal AAB group had a significantly different P300 amplitude trajectory from the low paternal risk group, χ2(1, N = 243) = 6.61, p= .010.2 The young men whose fathers had AAB had a trajectory characterized by a smaller intercept (M = 21.66 µV, SE = 1.07) and slope (M = −0.47 µV/year, SE = 0.13) than the low paternal risk group (mean intercept = 25.26 µV; SE = 1.31; mean slope = −0.86 µV/year, SE = 0.16).
Figure 2.
Grand mean P300 amplitude waveforms at Pz for target stimuli by paternal externalizing risk group at each assessment. Paternal externalizing groups were low risk (no evidence of significant paternal substance misuse or antisociality), intermediate risk (paternal alcohol dependence but no paternal drug dependence or adult antisociality), and severe risk (significant paternal adult antisociality).
Figure 3.
P300 amplitude growth trajectories based on mixed models analysis and observed mean P300 amplitude presented separately by paternal externalizing risk group. Observed mean amplitude (±1 SE) is presented separately for each assessment wave and risk group. Means are plotted at the mean age at assessment for the respective paternal risk group. Trajectories are mean group intercept + mean group slope * (age – mean age at first assessment). Paternal externalizing groups were low risk (no evidence of significant paternal substance misuse or antisociality), intermediate risk (paternal alcohol dependence but no paternal drug dependence or adult antisociality), and severe risk (significant paternal adult antisociality).
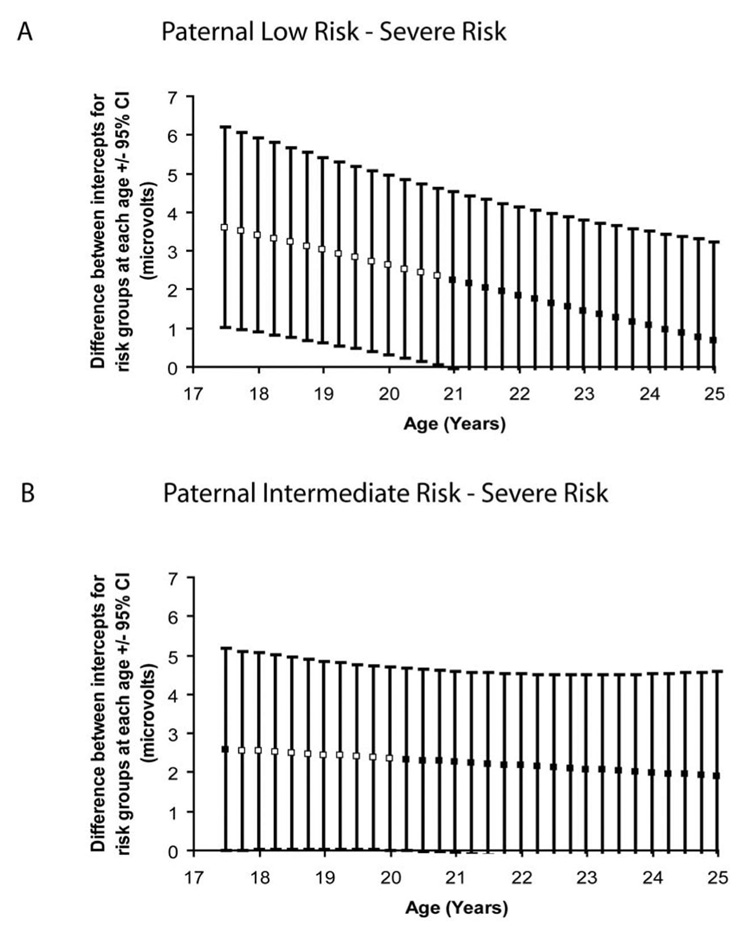
Figure 4a illustrates what the difference in intercept between the paternal AAB and low paternal risk groups would be at different ages in the range observed if age had been centered at that point. Age is incremented by 0.25-year intervals, and 95% confidence intervals are provided for each estimate. This approach (D. J. Bauer & Curran, 2005) for probing interactions in mixed models identifies the age at which these two groups would no longer differ significantly in P300 amplitude. The projected age range at which the paternal AAB and low-risk groups would no longer significantly differ was 20.87 years (in Figure 4a, the confidence intervals overlap 0 at this age). These two groups differed significantly at all earlier ages.
Figure 4.
Mean difference in intercept between paternal risk groups as a function of age. Estimates and 95% confidence intervals are derived by the method of D. J. Bauer and Curran (2005) at 0.25-year increments during the observed age range. Black squares indicate points at which the groups do not significantly differ, and white squares indicate a statistically significant group difference at α = .05. A: Paternal low-risk group – severe risk group. B: Paternal intermediate-risk group – severe risk group. Paternal externalizing groups were low risk (no evidence of significant paternal substance misuse or antisociality), intermediate risk (paternal alcohol dependence but no paternal drug dependence or adult antisociality), and severe risk (significant paternal adult antisociality).
There was a marginal difference between the developmental trajectories of the intermediate paternal risk group and the paternal AAB risk group, χ2(1, N = 208) =3.66, p= .056. The trajectory for the intermediate paternal risk group was characterized by a higher intercept (M = 24.26 µV, SE = 1.38) and slope (M= −0.57 µV/year, SE = 0.17) than that of the paternal AAB risk group. Figure 4b illustrates the differences in intercept between these groups in the range observed. These differences were statistically significant between the ages of 17.63 and 20.15 years. The low- and intermediate-risk groups did not have significantly different trajectories, χ2(1, N = 287) = 0.41, p= .521.3
Offspring Externalizing and P300 Amplitude Change
A conditional growth model with sons’ externalizing status added as a predictor of intercept and slope was fit.4 As before, P300 decreased significantly with age, F(1, 571) = 86.08, p < .001. Once the externalizing disorder status of the sons was added to the model, there was only a trend for the trajectories of the paternal AAB group to differ from the paternal low-risk group, χ2(1, N = 243) = 3.31, p= .069. The developmental trajectories of young men who developed an externalizing or early-onset substance disorder differed significantly from those who did not, χ2(1, N = 369) = 12.20, p < .001. The trajectory for the men with a disorder had a smaller intercept (M = 21.39 µV, SE = 1.03) than that of the men who did not develop a disorder (M = 24.33 µV, SE = 0.90), F(1, 41) = 10.94, p = .002, although the difference in slope between the groups was relatively small (mean difference = −.063, SE = .135), F(1, 571) = 0.22, p = .640.
P300 Amplitude Differences on Neutral Trials
The young men with a paternal history of AAB had lower intercepts and less change over time than the low-risk participants. It is possible that the reduced slope characterizing this trajectory may be an artifact of the participants at highest risk having P300 amplitude at the start of the study close to an absolute psycho-physiological lower limit. The reduced change over time may reflect a floor effect consistent with Wilder’s (1967) law of initial values. One way to examine this possibility is to assume that the amplitude of the response to the neutral nontarget stimuli represents this lower limit and to then determine to what degree the target amplitude approximates this value at the time of the earliest assessment. A mixed model with task condition (neutral or target) as a fixed factor, amplitude measures nested within participants, and participants nested within families was fit to the Time 1 P300 amplitude of the paternal AAB group using PROC MIXED. This analysis was restricted to only the 40 men with data from the first and last assessments. In this group target, amplitude (M = 22.72 µV, SE = 1.17) was significantly greater than neutral P300 amplitude (M = 11.94 µV, SE = 1.03) at Time 1, F(1, 24) = 109.58, p <.001. This 10.78 µV (SE = 1.03) difference was substantially greater than the observed decline of 3.72 µV (SE = 0.90) in target P300 amplitude from Time 1 to Time 3 in this group, suggesting that their reduced slope for target amplitude cannot be readily attributed to a floor effect.
Discussion
Consistent with past findings (Hill et al., 1999), visual P300 amplitude decreased significantly with age from 17 to 24 years in young men from a community sample. This finding is consistent with the results of studies using the same task as used here (Hill et al., 1999; Katsanis et al., 1996), as well as with a variety of other visual tasks (Courchesne, 1978; Friedman et al., 1989; Johnson, 1989; Mullis, Holcomb, Diner, & Dykman, 1985; Taylor, 1993). As we had predicted, developmental trajectories differed significantly across groups of young men varying in paternal history of externalizing problems. On the basis of findings of Hill and colleagues (Hill & Shen, 2002; Hill et al., 1999), we had predicted that sons with heightened risk would have developmental trajectories characterized by lower intercepts and less change with age relative to those at low risk. Consistent with P300 amplitude being associated with risk for an externalizing dimension underlying vulnerability for substance use disorders (Hicks et al., 2007; Patrick et al., 2006) and findings that paternal AAB is a more severe indicator of externalizing than of alcohol dependence (Krueger et al., 2005), our hypothesis would predict the greatest difference between the sons of fathers with AAB and a low-risk group. This was the case. Men with a paternal history of alcohol dependence but no AAB or drug dependence would be predicted as being intermediate between the low-risk group and the paternal AAB group. Although there was a trend for a difference in trajectory between this intermediate paternal externalizing risk group and the paternal AAB group, this was not statistically significant by conventional standards. This intermediate-risk group also did not differ significantly from the low-risk group. The ability to detect externalizing risk group differences may require a fairly large difference in the level of paternal externalizing severity.
Those who had developed an externalizing or earlier onset substance disorder had significantly different trajectories from those who did not have such a disorder. To a large extent, diagnostic status of the offspring is confounded with risk in this sample. Almost 92% of the paternal AAB group and 76.4% of the intermediate-risk group had at least one earlier onset substance disorder or other externalizing disorder. There are several reasons why an interpretation of trajectory differences as reflecting a vulnerability contributing to these disorders rather than an effect of having a disorder is reasonable.
P300 amplitude reductions in those with early-onset substance problems appear to preexist the development of heavy substance involvement or other externalizing psychopathology (Carlson et al., 2007) rather than reflect a potentially more severe effect of substance use at younger ages influencing neurodevelopment. A study of genetic and environmental influences on P300 amplitude development in twin pairs from the same sample reported on herein (Carlson & Iacono, 2006) found that 87% of the variability in the stable variance associated with intercept was because of individual differences in genetic effects. All of the correlations between slope and intercept appeared to be because of a common set of genes influencing both growth parameters. Furthermore, all of the heritable influence on P300 amplitude measured at each age could be attributed to genetic effects that were shared across age. As the relationship between externalizing and P300 amplitude in adolescence appears to be entirely genetically mediated (Hicks et al., 2007) and all of the genetic influence on amplitude in this age range is mediated by intercept and slope, it is likely that the reduced intercept of the affected participants seen in the present study reflects a genetically influenced vulnerability rather than the effects of having a disorder.
Generalizability of Findings
The present study was focused only on men, and potential gender effects on risk and P300 change could not be examined. Results with high-risk young women are less consistent than those seen with high-risk young men. Although some support has accrued for a possible Gender × Risk Status interaction (e.g., Hill, Muka, Steinhauer, & Locke, 1995; Steinhauer & Hill, 1993; Hill & Steinhauer, 1993), amplitude reduction may also be present in high-risk girls (e.g., Carlson et al., 2002; Reese & Polich, 2003; van der Stelt, Geesken, Gunning, Snel, & Kok, 1998; Yoon, Iacono, Malone, & McGue, 2006). The results of the present study should not be generalized to young women.
These findings may not generalize to studies of P300 at different ages or across a wider number of measurements. Examination of growth curves in past studies of visual P300 amplitude change suggested that a linear model is reasonable for the three time points examined here, and unless it is of a fairly large effect size, a quadratic term would be difficult to detect with three time points. A quadratic component of change was supported by Hill et al. (1999) using the same task as we did but with a wider and largely earlier age range (7–18 years) measured on up to five occasions. Nonlinear influences on P300 change may be important across a broader range of ages than was assessed here, and quadratic change in particular may differentiate at-risk groups. Whether such effects exist for MTFS participants will become clear as subsequent assessments are completed in this longitudinal investigation.
P300 Amplitude and Risk-Group Differences at Later Ages
Hill et al. (1999) predicted that the growth curves of their male participants at risk for alcoholism would converge with those of low-risk participants at age 22. In the present study, P300 amplitude of sons with high and low paternal risk differed significantly until age 20.87 years (although projected P300 values had not converged by this age or even by age 25; see Figure 3 and Figure 4a). The high- and intermediate-risk groups differed significantly between the ages of 17.63 and 20.15 years. This being the case, it is unclear why reductions are seen in abstinent adults with alcoholism as a function of familial risk at later ages (H. L. Cohen, Wang, Porjesz, & Begleiter, 1995; Patterson, Williams, McLean, Smith, Schaeffer, 1987; Pfefferbaum, Ford, White, & Mathalon, 1991; although see contrary findings in Glenn, Parsons, & Sinha, 1994, and Keenan, Freeman, & Harrell, 1997). Similarly, if the deviant trajectory is related to risk for antisocial personality disorder, it is unclear why P300 reductions are observed in adult antisocial men (e.g., L. O. Bauer, O’Connor, & Hesselbrock, 1994; Costa et al., 2000; O’Connor, Bauer, Tasman, & Hesselbrock, 1994).
This latter set of findings may be because of developmental differences in the activity of P300 generators leading to changes in the topography of P300, as suggested by L. O. Bauer and Hesselbrock (2003). They found cross-sectional evidence consistent with a shift to greater involvement of frontal P300 generators with increasing age in young people without conduct problems, but no such shift in those with conduct problems. Reduced amplitude at parietal sites might normalize with adulthood, but frontal reductions would become more apparent. Intriguingly, in a study of twins assessed longitudinally at ages 16 and 18, genetic influences on P300 amplitude in young men tended to decrease with age for posterior sites but to increase for frontal sites (van Beijsterveldt, van Baal, Molenaar, Boomsma, & de Geus, 2001). This is consistent with a genetically influenced anterior shift in P300 generators. Furthermore, although differences in topography with age are not universally supported, Mullis et al. (1985) suggested that late adolescence to early adulthood is associated with a visual P300 amplitude shift from posterior to anterior sites in a nonpsychiatric sample. The present study and the studies by Hill and colleagues (Hill et al., 1999; Hill & Shen, 2002) that support a decreasing difference between high-and low-risk children into adulthood focused exclusively on posterior sites. Future work looking longitudinally at shifts in the contribution of different neural generators in high-and low-risk participants is needed to clarify this issue.
Speculation on Developmental Significance
Differences in the trajectory of P300 amplitude development related to familial externalizing may provide important clues to developmental processes in the etiology of disinhibited behavior disorders. Given that the intercept and slope of P300 growth trajectories in adolescence and early adulthood are significantly influenced by a common set of genes, these trajectories may provide clues about how genetic influences on individual differences in the development of the central nervous system lead to vulnerability for a disorder. For example, past research involving functional MRI (Rangaswamy et al., 2004) and current source localization (Chen et al., 2007) has suggested a link between P300 amplitude reduction related to alcoholism risk and frontal cortex abnormalities, including areas involved in working memory systems (McCarthy, Luby, Gore, & Goldman-Rakic, 1997). The genetic risk indexed by P300 amplitude development may be mediated by developmental differences in such brain systems.
Antisocial men appear to have reduced gray matter in the prefrontal cortex (Raine, Lencz, Bihrle, LaCasse, & Colletti, 2000), and it is unclear when this difference first manifests. Adolescence is a crucial period when the prefrontal cortex is thought to mature through the processes of targeted cell body loss, synaptic pruning, and increased axonal myelination of pathways connecting it to other brain structures (Lewis, 1997; Sowell et al., 2001; Spear, 2000). Normatively larger visual P300 might be seen at early ages because more neurons are recruited in the stimulus processing involved in the updating of memory, stimulus categorization, or inhibition of inappropriate responses required to perform a task like the one used in the present study. Fewer neurons may be required with increasing maturity because of greater refinement and efficiency of the cortical areas activated (e.g., Durston et al., 2006; Tamm, Menon, & Reiss, 2002). If P300 amplitude reflects genetic influences on the development of frontally influenced cognitive processes, a more restrictive limit on the degrees of freedom available for neuronal and synaptic pruning in vulnerable children could limit the rate of decrease possible for P300 amplitude over adolescence and into early adulthood. The limited number of relevant neurons activated could thus be reflected by both the lower intercept (fewer neurons) and the smaller slope (fewer cells to lose) seen in the young men at risk for externalizing problems reported here.
On one hand, such a limitation could reflect an etiologically important functional restriction reflected by the trajectory of the high-risk young men. On the other hand, the inverse correlation of intercept with slope might reflect a psychophysiological floor effect consistent with the law of initial values (Raykov, 1995; Wilder, 1967). If the difference between target and nontarget P300 amplitude is largely a function of the extent to which relevant neural systems are engaged rather than different systems being activated, this floor does not appear to have been met for the target P300 amplitude of the high-risk men during the age range examined. The difference between target and nontarget P300 (M = 10.78 µV) was significant and sizable for this group relative to the differences in target amplitude from age 17 to age 24 (M = 3.72 µV). It is unlikely that the trajectory of P300 development observed in these young men merely reflects an artifact of having a low initial intercept.
Conclusion
P300 amplitude decreased with age from mid-adolescence to early adulthood. Rather than reflecting a specific vulnerability to alcohol dependence, a deviant trajectory of P300 change was associated with severe paternal externalizing psychopathology, consistent with prior findings suggesting a general vulnerability for externalizing disorder. By age 21, P300 amplitude no longer significantly distinguished the risk groups, with the low- and severe-risk trajectory groups projected to converge later in young adulthood, potentially limiting the utility of P300 amplitude as an endophenotype with older samples. Future studies are needed to confirm these findings in independent samples. Given past support indicating that the heritable influences on amplitude during this age range can be attributed to the effects of common genetic influences on the intercept and slope of amplitude growth trajectories, future research may profit by searching for genes that influence P300 change rather than cross-sectional amplitude. Our understanding of what puts people at risk for the various externalizing disorders might be advanced by use of P300 amplitude trajectory as a source of hypotheses regarding relevant neural and cognitive processes that change over the same time course.
Table 2.
Psychiatric Assessment Protocols for Fathers and Sons at Each Assessment
| Time 1 (mean age = 17.49 years) |
Time 2 (mean age = 20.62 years) |
Time 3 (mean age = 24.06 years) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| DSM–III–R diagnoses | Subject interview |
Mother interview |
Time coverage |
Subject interview |
Mother interview |
Time coverage |
Subject interview |
Mother interview |
Time coverage |
| Fathers | |||||||||
| Conduct disorder | SCID-II | — | LT | — | — | — | — | — | — |
| Adult antisocial behavior | SCID-II | — | LT | — | — | — | — | — | — |
| Alcohol abuse/dependence | SAM | — | LT | — | — | — | — | — | — |
| Drug abuse/dependence | SAM | — | LT | — | — | — | — | — | — |
| Nicotine dependence | SAM | — | LT | — | — | — | — | — | — |
| Offspring | |||||||||
| Attention deficit/hyperactivity disorder | DICA–R | DICA–P | LT | — | — | — | — | — | — |
| Oppositional defiant disorder | DICA–R | DICA–P | LT | — | — | — | — | — | — |
| Conduct disorder | SCID-II | DICA–P | LT | SCID-II | — | LT | SCID-II | — | LT |
| Adult antisocial behavior | SCID-II | — | LT | SCID-II | — | LT | SCID-II | — | LT |
| Alcohol abuse/dependence | SAM | DICA–P | LT | SAM | — | LT | SAM | — | SPA |
| Drug abuse/dependence | SAM | DICA–P | LT | SAM | — | LT | SAM | — | SPA |
| Nicotine dependence | SAM | DICA–P | LT | SAM | — | LT | SAM | — | SPA |
Note. Drugs considered for abuse and dependence diagnoses were amphetamines, cannabis, cocaine, hallucinogens, inhalants, opiates (including heroin), phencyclidine, and sedatives. DSM–III–R = Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.;American Psychiatric Association, 1987); SCID-II = Structured Clinical Interview for DSM–III–R Personality Disorders (Spitzer, Williams, Gibbon, & First, 1990); SAM = Substance Abuse Module of the Composite International Diagnostic Interview (Robins, Baber, & Cottler, 1987; Robins et al., 1988); DICA–R = Diagnostic Interview for Children and Adolescents—Revised (Reich, 2000); DICA–P = Diagnostic Interview for Children and Adolescents—Parent Version; LT = Lifetime Assessment; SPA = since previous assessment.
Acknowledgments
This research was supported by National Institute on Drug Abuse Grant DA 05147 and National Institute of Alcohol Abuse and Alcoholism Grants AA 00175 and AA 09367.
Footnotes
For the contrast between paternal risk groups, paternal risk was dummy coded as 0 and 1 corresponding to the two risk groups being compared. As in D. J. Bauer and Curran (2005), the fixed effects portion of the growth equation is µy|x1, y1 = γ00 + γ10x1 + γ01w1 + γ11x1w1, where y = P300 amplitude, γ00 = the model intercept, γ10 = the fixed effect for age, x1 = age – the mean age at the first assessment, γ01 = fixed effect for paternal risk group, and w1 = the dummy code for paternal risk group. The equation for the paternal risk group coded 0 is γ00 + γ10x1, whereas the equation for the paternal risk group coded 1 is γ00 + γ10x1 + γ01 + γ11x1. The difference between the trajectories of these two risk groups is therefore γ01 + γ11x1. This value is the difference in P300 at age x1 related to paternal risk. The 95% confidence interval for this value is ±1.9602 * [variance of γ01 + 2 * x1 * (covariance between γ01 and γ11) + (x12 * variance of γ11) ]1/2. The fixed effects, variances, and covariances are estimated in the mixed model.
We eliminated relatively extreme participants and refit our growth models. The same basic pattern of findings was observed in the trimmed sample. The trajectories for the low- and severe-risk groups still differed significantly, χ2(1) = 5.74, p= .017; there was a nonsignificant trend for the intermediate- and severe-risk groups to differ, χ2(1) = 3.46, p= .063; and the low- and intermediate-risk groups did not differ significantly, χ2(1) = 0.27, p= .606. The trajectories for the low- and severe-risk groups still differed significantly, χ2(1, N = 233) = 5.74, p= .017, there was a non-significant trend for the intermediate and severe risk groups to differ, χ2(1, N = 199) = 3.46, p= .063, and the low and intermediate risk groups did not differ significantly, χ2(1, N = 278) = 0.27, p= .606.
The presence of a lifetime maternal substance disorder increases the chances that group differences may be because of fetal exposure to alcohol or drugs or risk transmitted from affected mothers to their offspring. We eliminated all participants whose mother had a definite or probable lifetime diagnosis of alcohol abuse or dependence or drug abuse or dependence and refit the growth models. The same pattern of results was found.
A model was also fit with interactions between paternal externalizing and offspring diagnosis influencing intercept and slope. There were no trends for significant fixed effects for these interactions ( ps ≥ 0.151), the same main effects on intercept and slope reported were significant, the models did not differ significantly by likelihood ratio test, χ2(2, N = 369) = 5.2, p = .075, and elimination improved model fit as indicated by differences in the Akaike Information Criterion (Akaike, 1987) and the Bayesian Information Criterion (Schwartz, 1978). The model without either interaction reported on here fit better than the model with both interaction terms (Akaike Information Criterion difference= −2.8, Bayesian Information Criterion difference= −15.8). Given these findings and the further fact that only 7 (8.5%) of the sons of men with AAB did not have a relevant diagnosis, these underpowered interaction models were not considered further.
Contributor Information
Scott R. Carlson, Department of Psychology, University of British Columbia, Vancouver, British Columbia, Canada
William G. Iacono, Department of Psychology, University of Minnesota.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed., rev. Washington, DC: Author; 1987. [Google Scholar]
- Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research. 2005;40:373–400. doi: 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: Interactive effects on P300 amplitude and topography in male adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Bauer LO, O’Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcoholism: Clinical and Experimental Research. 1994;18:1300–1305. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984 September 24;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43:470–480. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in adolescent twins discordant and concordant for alcohol use disorders. Biological Psychology. 2002;61:203–227. doi: 10.1016/s0301-0511(02)00059-5. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in nonalcoholic adolescent twin pairs who become discordant for alcoholism as adults. Psychophysiology. 2004;41:841–844. doi: 10.1111/j.1469-8986.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Katsanis J, Iacono WG, Mertz AK. Substance dependence and externalizing psychopathology in adolescent boys with small, average, or large P300 event-related potential amplitude. Psychophysiology. 1999;36:583–590. [PubMed] [Google Scholar]
- Carlson SR, McLarnon ME, Iacono WG. P300 amplitude, externalizing psychopathology, and “earlier” vs. “later” onset substance use disorder. Journal of Abnormal Psychology. 2007;116:565–577. doi: 10.1037/0021-843X.116.3.565. [DOI] [PubMed] [Google Scholar]
- Chen AC, Porjesz B, Ranaswamy M, Kamarajan C, Tang Y, Jones KA, et al. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcoholism: Clinical and Experimental Research. 2007;31:156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Clark DB. The natural history of adolescent alcohol use disorders. Addiction. 2004;99:5–22. doi: 10.1111/j.1360-0443.2004.00851.x. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Wang W, Porjesz B, Begleiter H. Auditory P300 in young alcoholics: Regional response characteristics. Alcoholism: Clinical and Experimental Research. 1995;19:469–475. doi: 10.1111/j.1530-0277.1995.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, et al. Frontal P300 decrements: Alcohol dependence and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: Changes in long-latency event-related potentials from childhood to adulthood. Electroencephalography and Clinical Neurophysiology. 1978;45:468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Putnam L, Sutton S. Cognitive brain potentials in children, young adults, and senior citizens: Homologous components and changes in scalp distribution. Developmental Neuropsychology. 1989;5:33–60. [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. Journal of Neurocytology. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Tarter RE. Executive cognitive functioning and risk for substance abuse. Psychological Science. 1999;10:203–205. [Google Scholar]
- Glenn S, Parsons OA, Sinha R. Assessment of recovery of electrophysiological and neuropsychological functions in chronic alcoholics. Biological Psychiatry. 1994;36:443–452. doi: 10.1016/0006-3223(94)90639-4. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Gledd JN, Lusk L, Hayashi KM, Greenstein D, Valtuzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Habeych ME, Charles PJ, Sclabassi RJ, Kirisci L, Tarter RE. Direct and mediated associations between P300 amplitude in childhood and substance use disorders outcome in young adulthood. Biological Psychiatry. 2005;57:76–82. doi: 10.1016/j.biopsych.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Hesselbrock V, Begleiter H, Porjesz B, O’Connor S, Bauer L. P300 event-related potential amplitude as an endophenotype of alcoholism-evidence from the Collaborative Study of the Genetics of Alcoholism. Journal of Biomedical Science. 2001;8:77–82. doi: 10.1007/BF02255974. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: A twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muka D, Steinhauer S, Locke J. P300 amplitude decrements in children from families of alcoholic female probands. Biological Psychiatry. 1995;38:622–632. doi: 10.1016/0006-3223(94)00384-7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biological Psychiatry. 2002;51:621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biological Psychiatry. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Event-related potentials in women at risk for alcoholism. Alcohol. 1993;10:349–354. doi: 10.1016/0741-8329(93)90019-k. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Zubin J. Biological markers for alcoholism: A vulnerability model conceptualization. In: Rivers PC, editor. Nebraska Symposium on Motivation. Vol. 34: Alcohol and addictive behavior; University of Nebraska Press; Lincoln. 1987. pp. 207–256. [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cross-generational effects on gender differences in psychoactive drug abuse and dependence. Drug and Alcohol Dependence. 2004;74:147–158. doi: 10.1016/j.drugalcdep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM. Identifying a multivariate endophenotype for substance use disorders using psychophysiological measures. International Journal of Psychophysiology. 2000;38:81–96. doi: 10.1016/s0167-8760(00)00132-x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IR, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr Developmental evidence for modality-dependent P300 generators: A normative study. Psychophysiology. 1989;26:651–667. doi: 10.1111/j.1469-8986.1989.tb03167.x. [DOI] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300, disinhibited personality, and early-onset alcohol problems. Alcoholism: Clinical and Experimental Research. 2001;25:1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK. The association between P300 and age from preadolescence to early adulthood. International Journal of Psychophysiology. 1996;24:213–221. doi: 10.1016/s0167-8760(96)00063-3. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34:47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Freeman PR, Harrell R. The effects of family history, sobriety length, and drinking history in younger alcoholics on P300 auditory-evoked potentials. Alcohol and Alcoholism. 1997;32:233–239. doi: 10.1093/oxfordjournals.alcalc.a008262. [DOI] [PubMed] [Google Scholar]
- Khoo ST, Muthén BO. Longitudinal data on families: Growth modeling alternatives. In: Rose JS, Chassin L, Chassin CC, Sherman SJ, editors. Multivariate applications in substance use research: New methods for new questions. Mahwah, NJ: Erlbaum; 2000. pp. 43–68. [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: Insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Malone SM, Iacono WG, McGue M. Drinks of the father: Father’s maximum number of drinks consumed predicts externalizing disorders, substance use, and substance use disorders in preadolescent and adolescent offspring. Alcoholism: Clinical and Experimental Research. 2002;26:1823–1832. doi: 10.1097/01.ALC.0000042222.59908.F9. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. Journal of Neurophysiology. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Krueger RF. The association of early adolescent problem behaviour and adult psychopathology: A multivariate behavioural genetic perspective. Behavior Genetics. 2006;36:591–602. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I: Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcoholism: Clinical and Experimental Research. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Mullis RJ, Holcomb PJ, Diner BC, Dykman RA. The effects of aging on the P3 component of the visual event-related potential. Electroencephalography and Clinical Neurophysiology. 1985;62:141–149. doi: 10.1016/0168-5597(85)90026-7. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Bauer L, Tasman A, Hesselbrock V. Reduced P3 amplitudes are associated with both a family history of alcoholism and antisocial personality disorder. Progress in Neuropsychopharmacology and Biological Psychiatry. 1994;18:1307–1321. doi: 10.1016/0278-5846(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson BW, Williams HL, McLean GA, Smith LT, Schaeffer KW. Alcoholism and family history of alcoholism: Effects on visual and auditory event-related potentials. Alcohol. 1987;4:265–274. doi: 10.1016/0741-8329(87)90022-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcoholism: Clinical and Experimental Research. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Peterson JB, Finn P. An heuristic model for the inherited predisposition to alcoholism. Psychology of Addictive Behaviors. 1990;4:12–25. [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, et al. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: Preliminary results from the COGA project. Alcoholism: Clinical and Experimental Research. 1998;22:1317–1323. (Erratum appears in Alcoholism: Clinical and Experimental Research, 22, 1854) [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmenabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neurophysiology. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional MRI study of visual oddball: Evidence for frontoparietal dysfunction in subjects at risk for alcoholism. NeuroImage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Raykov T. On statistical approaches to the study of the “Law of Initial Values.”. Quality & Quantity. 1995;29:251–271. [Google Scholar]
- Reese C, Polich J. Alcoholism risk and the P300 event-related brain potential: Modality, task, and gender effects. Brain and Cognition. 2003;53:46–57. doi: 10.1016/s0278-2626(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic Interview for Children and Adolescents (DICA) Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Robins LN, Baber T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis: Author; 1987. [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The composite international diagnostic interview. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- SAS Institute. Changes and enhancements to SAS/STAT software in V7 and V8. Cary, NC: Author; 1998. [Google Scholar]
- Schuckit MA, Russell JW. Clinical importance of age at first drink in a group of young men. American Journal of Psychiatry. 1983;140:1221–1223. doi: 10.1176/ajp.140.9.1221. [DOI] [PubMed] [Google Scholar]
- Schwartz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: Rationale and reliability. Archives of General Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R, patient version (with psychotic screen; personality disorders) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- Steinhauer SR, Hill SY. Auditory event-related potentials in children at high risk for alcoholism. Journal of Studies on Alcohol. 1993;54:408–421. doi: 10.15288/jsa.1993.54.408. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hill SY, Zubin J. Event-related potentials in alcoholics and their first-degree relatives. Alcohol. 1987;4:307–314. doi: 10.1016/0741-8329(87)90028-0. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Taylor MJ. Maturational changes in ERPs to orthographic and phonological tasks. Electroencephalography and Clinical Neurophysiology. 1993;88:494–507. doi: 10.1016/0168-5597(93)90038-q. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, van Baal GCM, Molenaar PCM, Boomsma DI, de Geus EJC. Stability of genetic and environmental influences on P300 amplitude: A longitudinal study in adolescent twins. Behavior Genetics. 2001;31:533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Geesken R, Gunning WB, Snel J, Kok A. P3 scalp topography to target and novel visual stimuli in children of alcoholics. Alcohol. 1998;15:119–136. doi: 10.1016/s0741-8329(97)00106-7. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised (WAIS-R) New York: Psychological Corporation; 1981. [Google Scholar]
- Wilder J. Stimulus and response: The law of initial value. Bristol, England: Wright; 1967. [Google Scholar]
- Yoon HH, Iacono WG, Malone SM, McGue M. Using the brain P300 response to identify novel phenotypes reflecting genetic vulnerability for adolescent substance misuse. Addictive Behaviors. 2006;31:1067–1087. doi: 10.1016/j.addbeh.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96:684–695. [PubMed] [Google Scholar]