Abstract
Objective
To determine which of the classic modifiable coronary heart disease (CHD) risk factors, measured in midlife, are associated with subclinical coronary atherosclerosis in older age.
Design
Prospective study
Setting
Community based
Participants
Participants were 400 community-dwelling middle-aged adults who had no history of CHD at baseline (1972–4) when CHD risk factors were measured, and who were still free of known CHD in 2000–2002.
Measurements
Coronary artery plaque burden was assessed by coronary artery calcium (CAC) score using computed tomography in 2000–2002.
Results
Ordinal logistic regression analysis was used to compare baseline risk factors with severity of CAC. Mean age was 42 years at baseline and 69 years at the time of CAC assessment; 46.5% were male. In analyses adjusted for age, sex, and all other risk factors, one standard deviation increase in body mass index [OR 1.24 (CI 95% 1.02–1.51) P = 0.03], cholesterol [OR 1.28 (CI 95% 1.03–1.58) P = 0.02], pulse pressure [OR 1.24 (CI 95% 1.03–1.50) P = 0.03], and log triglycerides [OR1.22 (CI 95% 0.99–1.50 P = 0.06] each independently predicted the presence and severity of coronary artery atherosclerosis.
Conclusions
Plaque burden in elderly survivors without clinical heart disease is influenced by modifiable risk factors measured more than 25 years earlier.
Keywords: coronary artery calcium, midlife risk factors
Introduction
Coronary artery atherosclerosis is a chronic disease that typically progresses for decades without symptoms. Autopsy studies have demonstrated early coronary artery lesions in adolescents in association with cigarette smoking and high cholesterol levels (1). In the Bogalusa Heart Study increasing numbers of heart disease risk factors in youth were associated with increasing severity of asymptomatic coronary atherosclerosis (2). In the Muscatine Study (3) heart disease risk factors measured in childhood were associated with quantitative increments of subclinical coronary atherosclerosis assessed by coronary artery calcium (CAC) years later.
Age is the strongest predictor of cardiovascular disease in adult men and women (4). Cohort studies (5; 6) show that high levels of potentially modifiable heart disease risk factors measured in midlife still predict an increased risk of clinical coronary heart disease (CHD) in old age. In the Framingham Heart Study, for example, hypertension in middle age contributed consistently to future cardiovascular events (5).
Subclinical plaque burden measured using electron-beam computed tomography (CT) to quantify CAC has been shown to correlate well with coronary artery plaque burden and to predict cardiovascular events (7–9). However, the effect of midlife risk factors on CAC development later in life and the main risk factors involved have not been sufficiently evaluated (8).
The purpose of this paper was to determine which of the classic potentially modifiable CHD risk factors, measured in midlife, are associated with moderate or advanced subclinical coronary atherosclerosis two to three decades later in men and women without symptomatic heart disease.
Methods
Between 1972 and 1974, 82% of community-dwelling adult residents of Rancho Bernardo, a southern California community, enrolled in a Lipid Research Clinic Prevalence Study of heart disease risk factors. Most participants were aged 30 to 79 years, white, middle or upper-middle class, and had at least a high school education. The baseline evaluation included a brief medical history, a cigarette smoking questionnaire, and measurement of height, weight, blood pressure, fasting plasma glucose, cholesterol, and triglyceride levels. HDL cholesterol and waist girth were not measured in 1972–4.
Using a standard protocol, systolic and diastolic blood pressure were measured in seated, rested subjects. Height and weight were measured with participants wearing light clothing and no shoes. Morning blood samples were obtained. Fasting plasma cholesterol and triglycerides were measured in a Center for Disease Control-certified Lipid Research Clinic Laboratory, using an Auto Analyzer I (Technicon Instruments, Tarrytown, New York). Fasting plasma glucose was measured by a hexokinase method in a clinical laboratory. Body mass index (BMI) (kg/m2) was calculated as an estimate of obesity. Pulse pressure was calculated as the difference between systolic and diastolic blood pressure.
Surviving members of this cohort study, renamed the Rancho Bernardo Study, have been followed periodically to the present. Between 1997 and 1999 approximately 70% of surviving local, community-dwelling, ambulatory members of the cohort attended a follow-up clinic visit when a new cardiovascular disease and medication history were obtained. Between 2000 and 2002, 422 participants who had attended the 1997–1999 clinic visits, and who had no history of myocardial infarction, angina pectoris, or coronary artery revascularization, were invited for coronary artery scan with an Imatron C-150 ultrafast electron-beam CT scanner (GE Imatron, San Francisco, California). For this analysis we included the 400 participants with both CAC and all risk factor measurements done in 1972–1974. Heart images were obtained with 100-ms scan time using 3-mm slices starting at the level of the carina and proceeding to the level of the diaphragm, approximately 40 to 45 “slices” were obtained. Tomographic imaging was electrocardiographically triggered at 40% or 65% of the RR interval, depending on the participant’s heart rate. The Agatston method (10) was used to quantify a total CAC score defined as the product of the area of calcification per coronary tomography segment and a factor rated 1 though 4 depending on the maximal calcium x-ray density in that segment. The reported inter-scan variability of the Agatston method is 15% or less (11), however, the volumetric scores have somewhat better inter-scan variability (less than 10%) (12; 13).
Statistical Analysis
CAC scores were categorized according to the Rumberger criteria, which define a calcium score of 0 to 10 Hounsfield units (HU) as little/none, 11 to 100 HU as mild, 101 to 399 HU as moderate, and greater than 400 HU as severe (14). In univariate analyses, CHD risk factors were compared by CAC score category using analysis of variance for continuous variables and chi-squared analysis for categorical variables. Values for systolic and diastolic blood pressure, triglycerides, and fasting plasma glucose were skewed and therefore log-transformed for analysis.
The association of midlife classical heart disease risk factors with the CAC scores measured 26 to 30 years later was analyzed using ordinal logistic regression with little or no CAC (0–10 HU) as the reference category. Sex-specific standardized values of BMI, pulse pressure, cholesterol, and log-transformed triglycerides were included in age- and sex- adjusted ordinal logistic models. All analyses were performed using SAS (version 9.1, SAS Institute, Inc., Cary, NC).
Results
Participants were 27 to 60 years old (mean age 41.8 yrs) at baseline, and 55 to 88 years (mean age 69.4 yrs) at the CT scan visit; 46.5% were male. Thirty-five percent (n =141) had little or no CAC (CAC score 0–10 HU), 21% (n = 83) had mild CAC (score 11–100 HU), 22% (n = 86) had moderate CAC (score 101–399 HU), and nearly 23% (n = 90) had severe CAC (score ≥400 HU). The odds of increasingly severe future CAC scores were 5.43-fold (3.63–8.12) greater for men than for women, and 2.51-fold (1.90–3.32) greater for each 10-year increase in age greater than 40 years.
Age- and sex-adjusted levels of baseline risk factors by CAC scores are shown in Table 1. Those with more severe CAC were more likely to be male (P <0.001), to be older (P <0.001), and to have higher baseline BMI (P = 0.007), cholesterol (P = 0.002), and triglyceride levels (P <0.001). Systolic and diastolic blood pressure and fasting plasma glucose did not differ significantly by CAC (P = 0.14 and 0.51).
Table 1.
Age- and sex-adjusted baseline characteristics.
| CHD Risk Factor (measured in 1972–74) |
Coronary Artery Calcium Score Categories (measured in 2000–02) | P | |||
|---|---|---|---|---|---|
| 0–10 HU little or none (n = 141) |
11–100 HU mild (n = 83) |
101–399 HU moderate (n = 86) |
≥400 HU severe (n = 90) |
||
| Male sex (%) 1 | 24.1 (16.2; 32.1) | 40.9 (31.1; 50.8) | 56.7 (47.0; 66.4) | 76.9 (67.0; 86.8) | <0.001 |
| Age (years) 2 | 38.1 (36.9; 39.3) | 41.9 (40.4; 43.4) | 43.2 (41.8; 44.6) | 46.0 (44.6; 47.5) | <0.001 |
| Body mass index (kg/m2) 2 | 23.0 (22.5; 23.4) | 23.7 (23.2; 24.3) | 23.8 (23.2; 24.3) | 24.3 (23.8; 24.9) | 0.007 |
| Systolic blood pressure (mm Hg) 3 | 112.2 (109.9; 114.4) | 113.3 (111.1; 116.7) | 115.6 (113.3; 117.9) | 116.7 (113.3; 119.1) | 0.146 |
| Diastolic blood pressure(mm Hg) 3 | 73.0 (71.5; 75.2) | 73.0 (70.8; 75.2) | 75.2 (73.0; 77.5) | 74.4 (71.5; 76.7) | 0.518 |
| Pulse pressure (mm Hg) 2 | 39.6 (37.8; 41.3) | 40.1 (37.9; 42.2) | 40.5 (38.4; 42.6) | 43.1 (40.9; 45.4) | 0.115 |
| Total cholesterol (mg/dL) 2 | 182.9 (177.2; 188.5) | 194.2 (187.4; 200.9) | 194.4 (187.7; 201.0) | 201.3 (194.3; 208.3) | 0.002 |
| Triglycerides (mg/dL) 3 | 79.0 (73.0; 85.6) | 86.5 (78.3; 94.6) | 102.5 (92.8; 112.2) | 99.5 (90.0; 109.9) | <0.001 |
| Fasting glucose (mg/dL) 3,4 | 97.5 (94.6; 99.5) | 97.5 (94.6; 101.5) | 101.5 (97.5; 104.6) | 103.5 (99.5; 106.7) | 0.077 |
| Smoking history 1 | 49.5 (40.5; 58.6) | 54.2 (43.4; 65.0) | 56.4 (47.1; 69.8) | 58.7 (47.4; 70.1) | 0.693 |
proportions are reported; p-values represent Wald chi-square tests for differences
means (95% confidence interval) reported; p-values represent F-test for differences
geometric means (95% confidence interval) reported; p-values represent F-test for differences
Fasting plasma glucose available for 369 individuals (N=126,77,83,83)
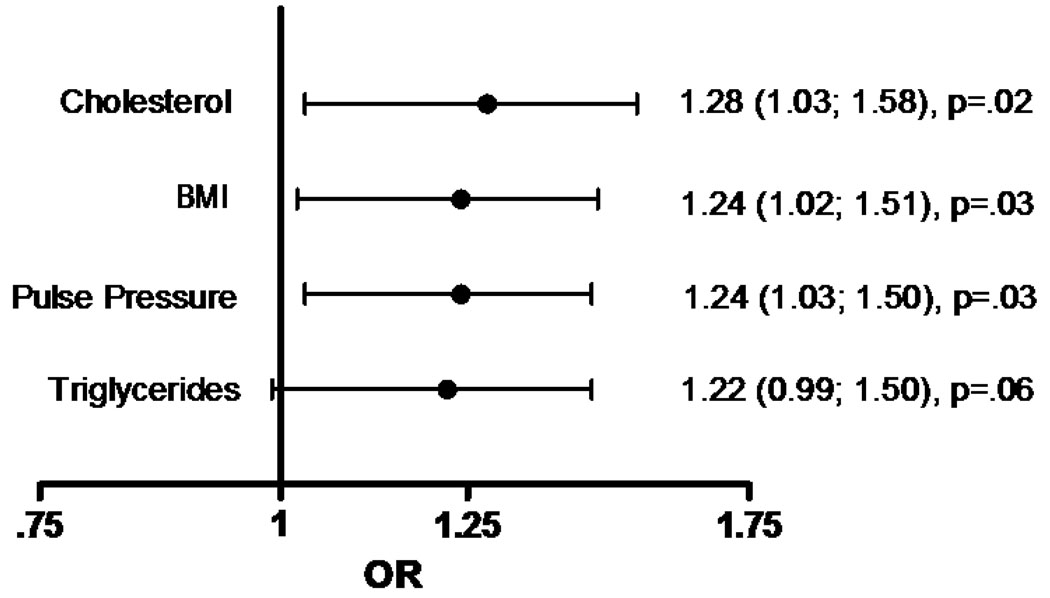
To evaluate the independent effect of each risk factor on severe CAC, age, sex, BMI, cholesterol, triglycerides, and pulse pressure were included in multivariate adjusted logistic regression models, such that all risk factors in the model are adjusted for each other. Figure 1 shows the multiply adjusted odds of increasingly severe CAC calculated for the sex-specific standard deviation of the four independent risk factors. As shown, the odds of increasingly severe future CAC scores were similar for each risk factor: 1.28 (1.03; 1.58) times higher for a standard deviation increase in cholesterol, 1.24 (1.02; 1.51) for a standard deviation increase in BMI, 1.24 (1.03; 1.50) for a standard deviation increase in pulse pressure, and 1.22 (0.99; 1.50) for a standard deviation increase in log triglycerides. None of these associations differed significantly by sex (P = 0.74, P = 0.45, P = 0.27, P = 0.37, respectively). Baseline fasting plasma glucose and tobacco smoking were not associated with increased risk of higher CAC scores in this model and did not alter results when included in the complete model. Although increasing pulse pressure was associated with significantly higher CAC scores, in separate age and BMI-adjusted models, neither systolic nor diastolic blood pressure were independently predictive of CAC (data not shown).
Figure 1.
Multiply adjusted (additionally adjusted for age and sex) odds ratios [OR (95% C.I.), p-value] of sex-specific standardized classical risk factors1 and increasingly severe coronary artery calcification.
Discussion
In this community-dwelling cohort of middle-aged Caucasians who were free of known CHD, age and male sex were, as expected, the strongest predictors of plaque burden 26–30 years later. Independent of age, sex, and each of the other risk factors, there remained a 22 to 28% increased odds of more severe coronary artery plaque burden with each standard deviation increase in plasma cholesterol, body mass index, pulse pressure, and log triglycerides.
Childhood or young adult CHD risk factors have been shown to predict subclinical atherosclerosis in middle age. For example, in a study of 2229 Finns, the presence of cardiovascular risk factors [smoking, low density lipoprotein (LDL), BMI and systolic blood pressure] during adolescence predicted adult carotid intima-media thickness (15). In both the Bogalusa Heart Study and the Muscatine Study, the presence of early-onset obesity was associated with carotid atherosclerosis (16) or CAC (16; 17) in young adults. Clearly exposure to risk factors in childhood or youth promotes atherosclerosis in younger adults. Less attention has been paid to the role of midlife heart disease risk factors and subclinical CHD in older adults. To our knowledge, this is the first report highlighting the association between midlife heart disease risk factors and subclinical coronary calcium assessed decades later in survivors who remained free of clinical heart disease.
The CARDIA study analyzed the role of risk factors in young adults aged 18 to 30 for CAC 15 years later; in that study higher levels of total cholesterol, fasting blood glucose, and systolic blood pressure predicted future CAC (18). The magnitude of the associations with risk factors was comparable to the present longer study of older adults. Unlike Rancho Bernardo, however, the CARDIA cohort did not show an independent association between BMI and CAC. Systolic blood pressure was a strong predictor of CAC in CARDIA, while in Rancho Bernardo only pulse pressure predicted CAC. It is likely that these differences reflect the younger age or shorter duration of follow up in CARDIA. Also reflecting the age differences, only 9.6% of the CARDIA population had detectable CAC at baseline versus 64% in the Rancho Bernardo Study cohort. Unlike CARDIA, fasting blood glucose was not a predictor of CAC in the Rancho Bernardo Study. This difference is compatible with the observation that post-challenge glucose is a stronger predictor of clinical cardiovascular disease than fasting hyperglycemia in older adults (19). In the Muscatine study, diastolic blood pressure, BMI, and the ratio of total cholesterol to HDL cholesterol measured in youth predicted CAC measured in young adults (ages 20–34 years) independent of other risk factors (20).
Pulse pressure, which also represents arterial stiffness, was the only blood pressure parameter that independently predicted CAC in the Rancho Bernardo cohort. The association of pulse pressure but not systolic or diastolic blood pressure with CAC is compatible with the observation that pulse pressure is the better predictor of cardiovascular disease mainly in older adults (21).
The associations of midlife modifiable risk factors with the severity of CAC in old age reported here support the current emphasis on long-term risk as well as short-term risk for CHD. Recent calculations show that a 50-year-old man with a 7% 10-year risk for “hard” CHD events actually has an average lifetime CHD risk of nearly 70%, and his median survival is more than 11 years shorter than the calculated survival for a man of the same age with optimal risk factors (22). Another group calculated that asymptomatic seniors aged 70 years and older with very low CAC scores (<10 HU) would have a reduced cardiovascular risk comparable to that of individuals 10 years younger (23). A recent study of CAC and all-cause mortality in 3570 elderly people (>70 years) found that CAC continues to discriminate risk mortality in old age (24).
In summary, midlife CHD risk factors predict subclinical CHD in old age, and suggest that treatment risk factors in midlife are likely to improve prognosis even in asymptomatic older adults.
Acknowledgments
Funding
This work was supported by the National Institute on Aging [grant number AG07181]; and the National Institute of Diabetes and Digestive and Kidney Diseases [grant number DK31801]. C.K.K was a recipient of a grant from CAPES/Brazil (PDEE sandwich program).
Footnotes
Conflicts of Interest: None declared.
Values of standard deviations (male; female): Cholesterol (mg/dL) (32.3; 32.7), BMI (kg/m2) (2.5; 2.7), Pulse Pressure (mmHg) (8.87; 10.65), log Triglycerides (mg/dL) (0.5; 0.4).
References
- 1.Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA. 1990;264:3018–3024. doi: 10.1001/jama.1990.03450230054029. [DOI] [PubMed] [Google Scholar]
- 2.Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 3.Davis PH, Dawson JD, Mahoney LT, et al. Increased carotid intimal-medial thickness and coronary calcification are related in young and middle-aged adults. The Muscatine study. Circulation. 1999;100:838–842. doi: 10.1161/01.cir.100.8.838. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 5.Stokes J, 3rd, Kannel WB, Wolf PA, et al. The relative importance of selected risk factors for various manifestations of cardiovascular disease among men and women from 35 to 64 years old: 30 years of follow-up in the Framingham Study. Circulation. 1987;75:V65–V73. [PubMed] [Google Scholar]
- 6.Semenciw RM, Morrison HI, Mao Y, et al. Major risk factors for cardiovascular disease mortality in adults: results from the Nutrition Canada Survey cohort. Int J Epidemiol. 1988;17:317–324. doi: 10.1093/ije/17.2.317. [DOI] [PubMed] [Google Scholar]
- 7.Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 8.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Guerci AD, Spadaro LA, Popma JJ, et al. Relation of coronary calcium score by electron beam computed tomography to arteriographic findings in asymptomatic and symptomatic adults. Am J Cardiol. 1997;79:128–133. doi: 10.1016/s0002-9149(96)00698-4. [DOI] [PubMed] [Google Scholar]
- 10.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 11.Mao S, Bakhsheshi H, Lu B, et al. Effect of electrocardiogram triggering on reproducibility of coronary artery calcium scoring. Radiology. 2001;220:707–711. doi: 10.1148/radiol.2203001129. [DOI] [PubMed] [Google Scholar]
- 12.Budoff MJ, Kessler P, Gao YL, et al. The interscan variation of CT coronary artery calcification score: analysis of the Calcium Acetate Renagel Comparison (CARE)-2 study. Acad Radiol. 2008;15:58–61. doi: 10.1016/j.acra.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Callister TQ, Cooil B, Raya SP, et al. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208:807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 14.Rumberger JA, Sheedy PF, 2nd, Breen JF, et al. Electron beam computed tomography and coronary artery disease: scanning for coronary artery calcification. Mayo Clin Proc. 1996;71:369–377. doi: 10.4065/71.4.369. [DOI] [PubMed] [Google Scholar]
- 15.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 16.Freedman DS, Dietz WH, Tang R, et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:159–166. doi: 10.1038/sj.ijo.0802515. [DOI] [PubMed] [Google Scholar]
- 17.Mahoney LT, Burns TL, Stanford W, et al. Usefulness of the Framingham risk score and body mass index to predict early coronary artery calcium in young adults (Muscatine Study) Am J Cardiol. 2001;88:509–515. doi: 10.1016/s0002-9149(01)01728-3. [DOI] [PubMed] [Google Scholar]
- 18.Loria CM, Liu K, Lewis CE, et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49:2013–2020. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Gao W, Qiao Q, Tuomilehto J. Post-challenge hyperglycaemia rather than fasting hyperglycaemia is an independent risk factor of cardiovascular disease events. Clin Lab. 2004;50:609–615. [PubMed] [Google Scholar]
- 20.Mahoney LT, Burns TL, Stanford W, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27:277–284. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]
- 21.Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–1089. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 23.Shaw LJ, Raggi P, Berman DS, et al. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188:112–119. doi: 10.1016/j.atherosclerosis.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Raggi P, Gongora MC, Gopal A, et al. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52:17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]