Abstract
Introduction
Erectile dysfunction (ED) as well as cardiovascular diseases (CVD) are associated with endothelial dysfunction and increased levels of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α).
Aim
We hypothesized that increased TNF-α levels impair cavernosal function.
Methods
In vitro organ bath studies were used to measure cavernosal reactivity in mice infused with vehicle or TNF-α (220 ng.Kg.−1min.−1) for 14 days. Gene expression of NOS isoforms was evaluated by real time PCR.
Main Outcome Measures
Corpora cavernosa from TNF-α-infused mice exhibited decreased NO-dependent relaxation, which was associated with decreased endothelial nitric oxide synthase (eNOS) and neuronal (nNOS) NOS cavernosal expression.
Results
Cavernosal strips from TNF-α-infused mice displayed decreased nonadrenergic-noncholinergic (NANC)-induced relaxation [59.4±6.2 vs Control: 76.2±4.7; 16 Hz] compared to control animals. These responses were associated with decreased gene expression of eNOS and nNOS (p<0.05). Sympathetic-mediated as well as phenylephrine (PE)-induced contractile responses [PE-induced contraction; 1.32±0.06 vs Control: 0.9±0.09, mN] were increased in cavernosal strips from TNF-α-infused mice. Additionally, infusion of TNF-α increased cavernosal responses to ET-1 and ETA receptor expression (p<0.05) and slightly decreased TNF-α receptor 1 (TNFR1) expression (p=0.063).
Conclusion
Corpora cavernosa from TNF-α-infused mice display increased contractile responses and decreased NANC nerve-mediated relaxation associated with decreased eNOS and nNOS gene expression. These changes may trigger ED and indicate that TNF-α plays a detrimental role in erectile function. Blockade of TNF-α actions may represent an alternative therapeutic approach for ED, especially in pathological conditions associated with increased levels of this cytokine.
Keywords: tumor necrosis factor alpha, corpus cavernosum, endothelial nitric oxide synthase, neuronal nitric oxide synthase, mouse
INTRODUCTION
Epidemiological data demonstrate a close relationship between erectile dysfunction (ED) and cardiovascular diseases (CVD).1−6 A clear connection between vasculogenic ED and CVD is evidenced by common risk factors such as hypertension7, smoking,8 dislipidemia,9, 10 obesity,11, 12 and diabetes.13, 14 Moreover, the clustering of these factors increases the risk for ED even further, as observed in metabolic syndrome.13, 15−17 ED is considered the early clinical manifestation of generalized vascular disease and carries an independent risk factor for cardiovascular events.18, 19 In this sense, the penile vasculature may be seen as an early sentinel that predicts CVD and especially the atherosclerotic process observed in coronary artery diseases.2, 18 In addition, ED is associated with endothelial dysfunction and increased levels of pro-inflammatory cytokines.3, 11
Tumor necrosis factor-alpha (TNF-α) is a pro-inflammatory cytokine with a broad range of actions and contributes to many cardiovascular diseases, including myocardial ischemia-reperfusion injury, chronic heart failure, atherosclerosis, and sepsis-associated cardiovascular disorders.20−22 The vascular endothelium is a major target for the actions of TNF-α and administration of TNF-α induces impairment of endothelium-dependent vasorelaxation in a variety of vascular beds and decreases the release of nitric oxide (NO).23
Vlachopoulos and co-workers3 have demonstrated increased levels of TNF-α in patients with ED and a negative correlation between sexual performance and TNF-α levels.3 In addition, transgenic animals that overexpress human TNF-α (hTNF-α) display fewer erections and decreased mounting behavior.24 Although TNF-α effects in the vasculature have been demonstrated, no information exists about its actions in the cavernosal tissue. Therefore, we hypothesized that TNF-α impairs cavernosal smooth muscle reactivity and thus leads to erectile dysfunction. To test our hypothesis we have used various pharmacological tools to evaluate relaxant and contractile responses of cavernosal segments from control and TNF-α—infused animals. In addition, corporal NOS expression was determined.
METHODS
Animals
Male C57BL/6 mice (12 weeks-old, from Jackson Laboratories, Bar Harbor, ME) were used in these studies. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Medical College of Georgia Committee on the Use of Animals in Research and Education. The animals were housed four per cage on a 12-h light/dark cycle and fed a standard chow diet with water ad libitum.
Human recombinant TNF-αinfusion
After anesthesia with ketamine (100 mg.Kg−1) and xylazine (2.9 mg.Kg−1), mini-pumps were implanted subcutaneously to deliver hTNF-α (220 ng.Kg−1.min−1, Sigma Chemical Co. St. Louis, MO).
Blood pressure measurements
At the end of treatment (14 days), mice were anesthetized with ketamine (100 mg.Kg−1) and xylazine (2.9 mg.Kg−1). Following anesthesia a sterile catheter was inserted into the left carotid artery. The incision was closed with sterile 6−0 Ethicon Opthalmic suture. The catheter was secured on the back of the mouse. All surgeries were conducted under aseptic and sterile conditions. After the animals recovered from anesthesia, the catheter was connected to a transducer and mean arterial pressure (MAP) was recorded for 3−4 hours. Subsequently, the animals were euthanized and cavernosal tissues were isolated for further studies.
Functional Studies in Cavernosal Strips
After carbon dioxide (CO2) euthanasia, the penis was excised, transferred into ice-cold physiological salt solution (PSS), and dissected to remove the tunica albuginea. One crural strip preparation (1×1×10 mm) was obtained from each corpus cavernosum (two crural strips from each penis). Changes in isometric force were recorded using a PowerLab/8SP data acquisition system (Chart software, version 5.0; ADInstruments, Colorado Springs, CO), as previously shown elsewhere.25 Cumulative concentration-response curves to acetylcholine (ACh; endothelium-dependent vasodilator; 10−9 to 3×10−5 M) were obtained in cavernosal strips contracted with phenylephrine (PE; 10−5 M). Cavernosum contractility was evaluated with cumulative concentration-response curves to PE (10−9 to 10−4 M) and endothelin-1 (ET-1, 10−10 to 10−6). Electrical field stimulation (EFS) to determine sympathetic-mediated contractions and NANC-mediated relaxations were performed as previously described.25
Quantitative Real-Time Reverse-Transcriptase Polymerase Chain Reaction
Total RNA was extracted from cavernosal strips using the RNeasy kit (Qiagen Sciences, Maryland, USA). The quantity, purity and integrity of all RNA samples were determined by NanoDrop spectrophotometers (NanoDrop Technologies, Wilmington, DE). One microgram of total RNA was reverse transcribed. Quantitative real-time reverse-transcriptase polymerase chain reaction (qPCR) reactions were performed as previously described in detail.26 Primers for preproendothelin-1 (preproET-1) (No Mm00438656_m1), ETA receptor (Rn00561137_m1), eNOS (Mm01164908_m1), nNOS (Mm00435175) and TNF-α receptor 1(TNFR1) (Rn01492348_m1) mRNA were obtained from Applied Biosystems.
Drugs and Solutions
Physiological salt solution of the following composition was used: 130 mM NaCl, 14.9 mM NaHCO3, 5.5 mM dextrose, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4.7H2O, 1.6 mM CaCl2.2H2O, and 0.026 mM EDTA. Acetylcholine chloride, atropine, Nω-nitro-L-arginine methyl ester (L-NAME), bretylium tosylate and phenylephrine hydrochloride were purchased from Sigma Chemical Co. (St. Louis, MO). Endothelin-1 was obtained from Tocris (Ellisville, MO).
Statistical Analysis
Contractions were recorded as changes in the displacement (mN) from baseline and are represented as mN for n experiments. Relaxation was expressed as percentage change from the PE contracted levels. Agonist concentration–response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 4.0; GraphPad Software Inc., San Diego, CA, USA). Agonist potencies and maximum response are expressed as pD2 (negative logarithm of the molar concentration of agonist producing 50% of the maximum response) and Emax (maximum effect elicited by the agonist), respectively. Statistically significant differences were calculated by one-way analysis of variance (ANOVA) plus Bonferroni`s post hoc test or Student's t-test. P<0.05 was considered as statistically significant.
RESULTS
The body weight of the TNF-α-infused mice used in these studies was not statistically different (27.6 ± 1.4; n=13) compared to the control group (27.5 ± 1.0; n=11). In addition, no differences were observed in the mean arterial blood pressure between the groups [(mmHg) control, 105±1.7 vs. TNF-α-infused, 110±3.8].
Functional responses of corpora cavernosa
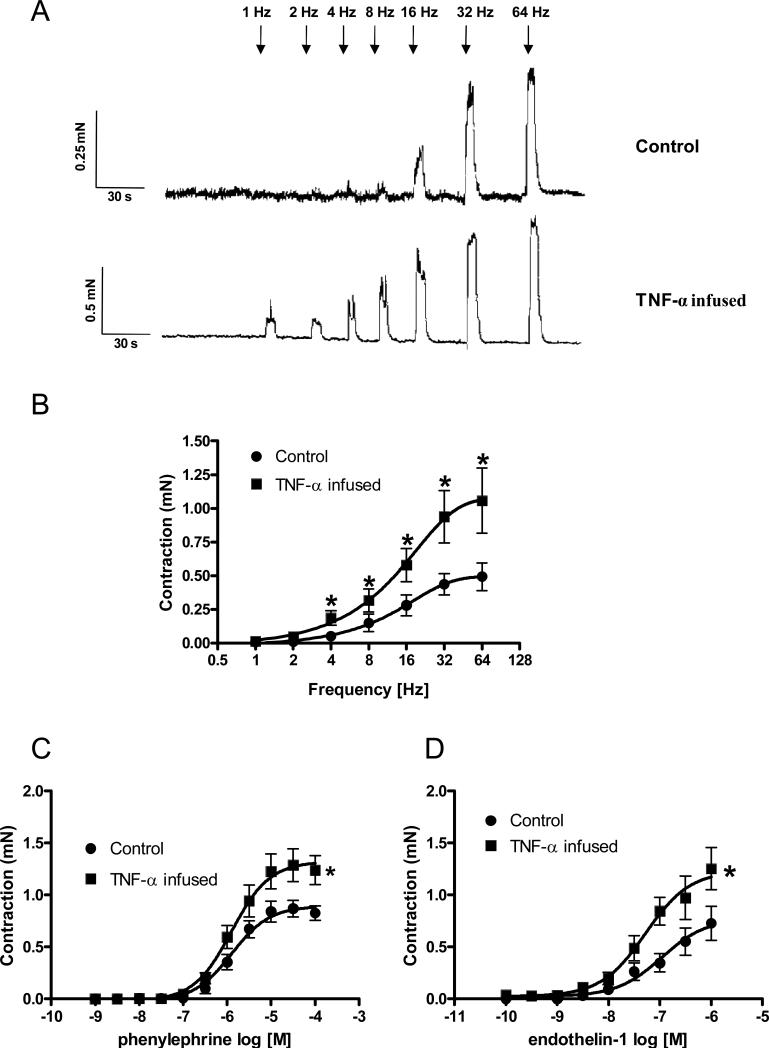
In order to determine the presence of changes in sympathetic-induced contractile responses in cavernosal strips, frequency-dependent EFS curves were performed. After incubation with atropine 10−6 M plus L-NAME 10−4 M, EFS (1−64 Hz) produced increased sympathetic-induced contractions in cavernosal strips from TNF-α–infused mice compared to the control group (Figures 1A, 1B). These responses were completely abolished by the sympathetic nerve blocking agent bretylium tosylate (3×10−5 M) or by the alpha-adrenergic antagonist terazosin (10−6 M), confirming that these responses are neuronal in origin and adrenergic in nature.
Figure 1. Contractile responses upon stimulation of adrenergic nerves, α1-adrenergic and ET-1 receptor activation are augmented in cavernosal segments from TNF-α-infused mice.
(A) Representative traces of the frequency-response curves elicited by EFS (1−64 Hz). (B) Frequency-response curves elicited by EFS (1−64 Hz) were performed in the presence of L-NAME 10−4 M plus atropine 10−6 M in cavernosal strips from control and TNF-α-infused mice (n=5 and 6, respectively). (C) Phenylephrine concentration-response curves (n=8 in all groups). (D) Endothelin-1-induced contractile responses (n=7 in all groups). Experimental values of contraction of cavernosal strips from control (●) and TNF-α-infused (■) mice are in mN and data represent the mean ± SEM of n experiments. *, p < 0.05 compared with values from control segments.
Cavernosal segments from TNF-α-infused mice also exhibited increased responses to PE (Figures 1C) and ET-1 (Figure 1D) compared to those in control strips. The addition of ETA receptor antagonist nearly abolished the contractile response to ET-1 in all groups (data not shown).
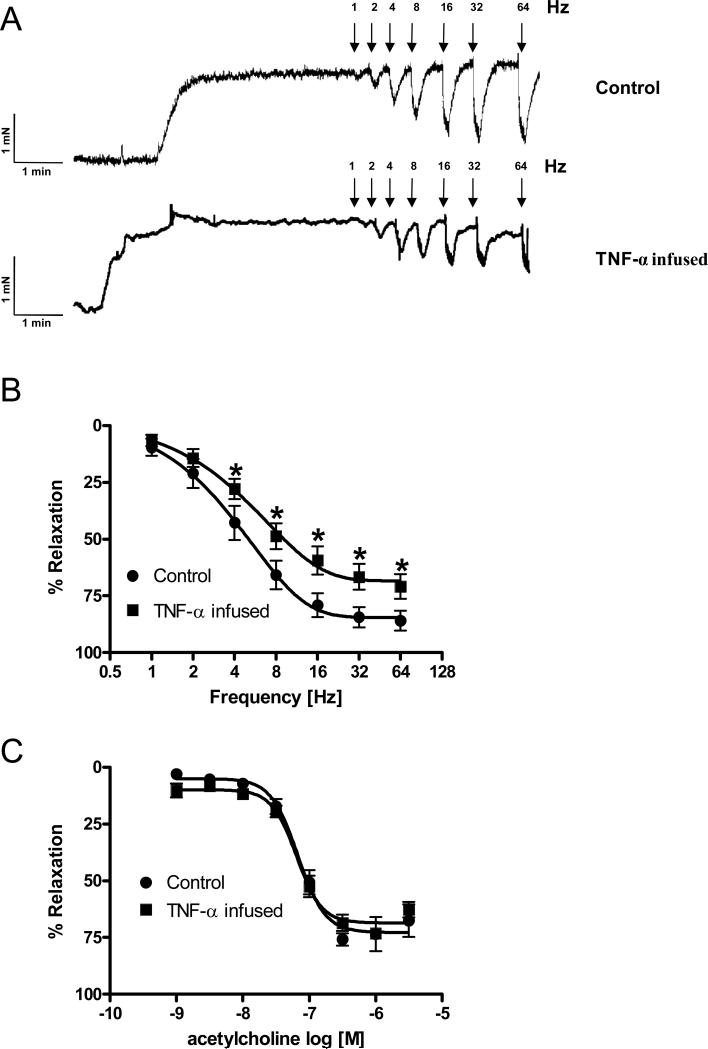
Whereas eNOS activation seems to account for ACh-induced relaxation, NANC-induced relaxation of cavernosal segments is mediated via NO derived from nNOS. Accordingly, ACh-induced relaxation is completely blocked in the presence of 10−6 M atropine or 10−4 M L-NAME. However, relaxation induced by EFS is minimally altered in the presence of atropine, but it is completely blocked by incubation with L-NAME 10−4 M. To evaluate the effects of NANC nerve stimulation, cavernosal smooth muscle strips were incubated with bretylium tosylate (3×10−5 M; sympathetic nerve blocking agent) and atropine 10−6 M. After 45 min of incubation, strips were contracted with PE (10−5 M) and frequency-dependent responses to EFS (1−64 Hz) were evaluated. EFS-induced relaxations were significantly decreased in cavernosal segments from TNF-α-infused mice in comparison to strips from control mice (Figures 2A and 2B).
Figure 2. Relaxant responses due to NANC nerve stimulation and endothelium-dependent relaxation in cavernosal segments from control and TNF-α-infused mice.
(A) Representative traces showing NANC-induced relaxation in cavernosal segments from control (top) and TNF-α-infused (bottom) mice; (B) Frequency-responses curves elicited by EFS (NANC nerves stimulation) and (C) acetylcholine concentration-response curves in cavernosal strips pre-contracted with PE (10−5 M), from control (●) and TNF-α-infused (■) mice. Experimental values of the relaxations induced by EFS (n=9 and 10, respectively), and acetylcholine (n=7 and 10, respectively) were calculated relatively to the maximal changes from the contraction produced by PE in each tissue, which was taken as 100%. Data represent the mean ± SEM of n experiments. *, P<0.05 vs. control.
The cumulative addition of ACh (10−9 to 3×10−5 M) produced concentration-dependent relaxations of PE-contracted tissues in both groups (Figure 2C). However, there was no difference in ACh-induced relaxation between the groups (Figure 2C).
TNF-α effects on nNOS, eNOS, ET-1 and TNFR1 receptor expression
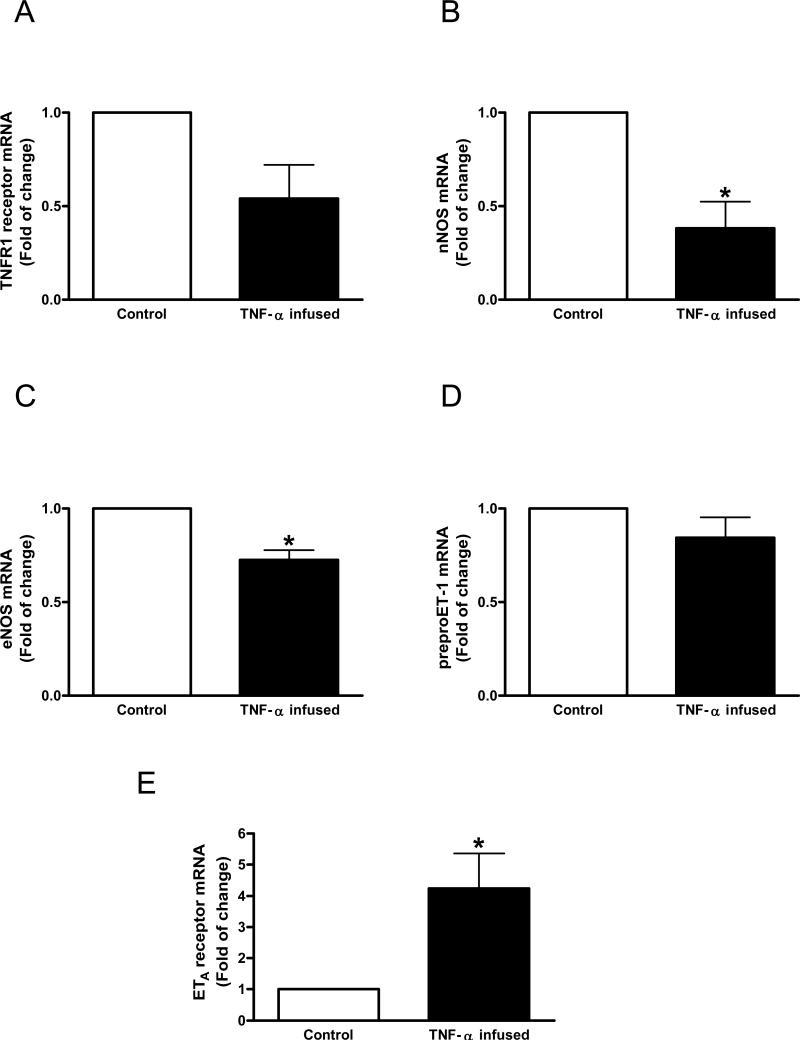
In the second set of experiments we first determined whether corpora cavernosa express TNFR1 receptor, the main receptor that mediates TNF-α effects. Cavernosal strips from mice display TNFR1 receptor expression (Figure 3A). There was a tendency of reduced TNFR1 expression in cavernosal tissue from TNF-α-infused mice (p=0.0638), indicating down-regulation due to TNF-α infusion. Consistent with the changes observed in functional data, TNF-α infusion decreased nNOS gene expression (Figure 3B). Despite the fact that cavernosal strips from TNF-α-infused mice displayed no changes to ACh-induced relaxation, decreased eNOS gene expression was observed with TNF-α infusion (Figure 3C). TNF-α infusion did not change preproET-1 gene expression in mouse corpora cavernosa (Figure 3D). In contrast, TNF-α infusion induced a 4 fold increase in ETA receptor gene expression in cavernosal tissue.
Figure 3.
Messenger RNA expression of (A) TNF-α receptor 1 (TNFR1), (B) nNOS, (C) eNOS (D) preproET-1 and (E) ETA receptor determined by qPCR using total RNA extracted from cavernosal tissue of control (open bar) or TNF-α-infused (filled bar) mice. Bar graphs show fold of change in gene expression. Values were normalized by the correspondent 18s rRNA of each sample. Results are mean±SEM (n=5 to 7). *p<0.05, vs control.
DISCUSSION
A role for cytokines in ED has emerged.11 Cytokines induce genes that synthesize other peptides in the cytokine family and several mediators, such as prostanoids, leukotrienes, NO, bradykinin (BK), reactive oxygen species, and platelet-activating factor, all of which can affect vascular function. The vascular responses appear to be related to the balance between all the vasoactive factors released under the influence of cytokines, and regional differences in release and responsiveness to these factors appear to contribute to the dilator or constrictor response observed within a specific vascular bed.27 Consistent with the fact that the cavernosal tissue is a complex extension of the vascular bed, risk factors that affect the vasculature have been shown to affect cavernosal function as well. Accordingly, the penile tissue has been recognized as an early sentinel for atherosclerosis that underlines coronary artery disease and CVD.18, 19
Vlachopoulos and collaborators3 have demonstrated the association between low grade inflammation, altered endothelial-prothrombotic state and ED. In addition, high levels of TNF-α were demonstrated in patients with ED.3 Several studies show that TNF-α plays a key role in inducing endothelial dysfunction,22, 23, 28−30 thus causing changes in vascular reactivity. In addition, a mouse model that overexpresses hTNF-α not only exhibits decreased induced erections, but also decreased mounting behavior and number of intromissions.24 “One should consider that these altered behavioral responses may be due to the central actions of TNF-α. Although the TNF-α transport system in blood-brain barrier is saturable,31 circulating levels of TNF-α can reach the central nervous system.” Taken together, these studies suggest that TNF-α may play an important role on erectile function and be a link between ED and CVD.
Herein we present the novelty that isolated corpora cavernosa from TNF-α-infused mice display decreased NANC-dependent relaxation and increased sympathetic-mediated contractions that would favor penile detumescence to occur. This study also shows that gene expression of key enzymes involved in erectile responses, such as eNOS and nNOS, are decreased in cavernosal strips from TNF-α-infused mice. Additionally, this study corroborates the notion that ED is a systemic arterial defect not only confined to the penile vasculature, and offers a humoral basis for this association.
Our results are consistent with previous studies showing that TNF-α caused increased contractile responses to PE in vessels from pregnant rats.32 Although the exact mechanisms remain unclear for this effect, Parris and co-workers33 demonstrated that TNF-α increases Ca2+ sensitivity of the myofilaments by increasing myosin light chain (MLC20) phosphorylation. In addition, these effects seem to be mediated by the RhoA/Rho-kinase signaling pathway.34
While nNOS-derived NO is considered the major mediator of NANC relaxation in the penis, eNOS is mainly involved in endothelium-dependent relaxation.35 In the present study cavernosal strips from TNF-α-infused mice displayed decreased NANC relaxation associated with decreased nNOS gene expression. Although there is no report about TNF-α actions on nNOS expression in corpora cavernosa, it has been demonstrated that TNF-α infusion significantly induced an increase in MAP and reduction in renal nNOS protein expression, within both the cortex and medulla from pregnant rats.36 Consistent with our results, TNF-α suppresses eNOS expression by inhibiting gene promoter activity 37, 38, predominantly through destabilization of eNOS mRNA in endothelial cells.38 Despite this fact, cavernosal tissue from TNF-α-infused mice did not have any changes in ACh-induced relaxation. Although counter-regulatory mechanisms may account for this response, time-dependent changes seem more likely to be involved in this apparent discrepancy. Accordingly, ED is frequently associated with CVD, where TNF-α levels are increased chronically. Similarly, although cavernosal tissue from TNF-α-infused mice displayed increased contraction to ET-1, no differences were observed in ET-1 gene expression between the groups. Interestingly, TNF-α has been shown to enhance ET-1 secretion in endothelial cells.39 Increased ETA receptor gene expression demonstrated in this study may account for the increased contractile response to ET-1 in cavernosal tissue from TNF-α-infused mice. Although real-time PCR is a reliable quantitative technique, several post-translational changes in gene expression may occur. Thus, additional studies to determine protein expression levels are necessary.
TNF-α plays a substantial proatherogenic role. 40 However, the presence of tumor necrosis factor receptor-1 (TNFR1) in the vascular wall causes atherosclerosis independently of macrophage infiltration.40 In the present study we demonstrated that cavernosal tissues from mice express TNFR1, which substantiates TNF-α action in the penis. Infusion of TNF-α tended to decrease the expression of TNFR1 in corporal tissue, which may be a negative feedback to limit TNF-α-induced changes in cavernosal tissue.
Finally, a recent study shows that cavernosal tissue from TNF-α knockout mice displays increased NANC and endothelium-dependent relaxation associated with increased nNOS and eNOS expression.35 Moreover, sympathetic and PE-induced contractile responses were decreased in corpora cavernosa from TNF-α knockout mice, which display increased number of spontaneous erections additionally supporting our findings.35
In summary, we have shown that TNF-α infusion causes changes in cavernosal reactivity that would facilitate erectile dysfunction: increased responses to adrenergic nerve stimulation and decreased NANC-dependent relaxation that are associated with decreased corporal eNOS and nNOS gene expression. Blockade of TNF-α actions may represent an alternative therapeutic approach for erectile dysfunction, especially in pathological conditions associated with increased levels of this cytokine.
Acknowledgement
This study was supported by grants from the National Institutes of Health (HL71138 and HL74167) and Fundacao de Amparo a Pesquisa do Estado de Sao Paulo – FAPESP, Brazil.
ABBREVIATIONS
- ACh
acetylcholine
- ANOVA
one-way analysis of variance
- ED
erectile dysfunction
- EFS
electrical field stimulation
- Emax
maximum effect elicited by the agonist
- eNOS
endothelial nitric oxide synthase
- KCl
potassium chloride
- L-NAME
Nω-nitro-L-arginine methyl ester
- MAP
mean arterial pressure
- mN
millinewton
- NANC
nonadrenergic-noncholinergic
- nNOS
neuronal NO synthase
- NO
nitric oxide
- PBS
phosphate buffer saline
- pD2
negative logarithm of the molar concentration of agonist producing 50% of the maximum response
- PDE-5
phosphodiesterase-5
- PE
phenylephrine
- ROS
reactive oxygen species
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TNF-α
tumor necrosis factor-alpha
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Muller A, Mulhall JP. Cardiovascular disease, metabolic syndrome and erectile dysfunction. Curr Opin Urol. 2006;16:435–43. doi: 10.1097/01.mou.0000250284.83108.a6. [DOI] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Rokkas K, Ioakeimidis N, Stefanadis C. Inflammation, metabolic syndrome, erectile dysfunction, and coronary artery disease: common links. Eur Urol. 2007;52:1590–600. doi: 10.1016/j.eururo.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Vlachopoulos C, Aznaouridis K, Ioakeimidis N, et al. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur Heart J. 2006;27:2640–8. doi: 10.1093/eurheartj/ehl341. [DOI] [PubMed] [Google Scholar]
- 4.Guay AT. ED2: erectile dysfunction = endothelial dysfunction. Endocrinol Metab Clin North Am. 2007;36:453–63. doi: 10.1016/j.ecl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Jensen J, Lendorf A, Stimpel H, Frost J, Ibsen H, Rosenkilde P. The prevalence and etiology of impotence in 101 male hypertensive outpatients. Am J Hypertens. 1999;12:271–5. doi: 10.1016/s0895-7061(98)00225-8. [DOI] [PubMed] [Google Scholar]
- 6.Karadag F, Ozcan H, Karul AB, Ceylan E, Cildag O. Correlates of erectile dysfunction in moderate-to-severe chronic obstructive pulmonary disease patients. Respirology. 2007;12:248–53. doi: 10.1111/j.1440-1843.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- 7.Moulik PK, Hardy KJ. Hypertension, anti-hypertensive drug therapy and erectile dysfunction in diabetes. Diabet Med. 2003;20:290–3. doi: 10.1046/j.1464-5491.2003.00911.x. [DOI] [PubMed] [Google Scholar]
- 8.Tostes RC, Carneiro FS, Lee AJ, et al. Cigarette smoking and erectile dysfunction: focus on NO bioavailability and ROS generation. J Sex Med. 2008;5:1284–95. doi: 10.1111/j.1743-6109.2008.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei M, Macera CA, Davis DR, Hornung CA, Nankin HR, Blair SN. Total cholesterol and high density lipoprotein cholesterol as important predictors of erectile dysfunction. Am J Epidemiol. 1994;140:930–7. doi: 10.1093/oxfordjournals.aje.a117181. [DOI] [PubMed] [Google Scholar]
- 10.Miner M, Billups KL. Erectile dysfunction and dyslipidemia: relevance and role of phosphodiesterase type-5 inhibitors and statins. J Sex Med. 2008;5:1066–78. doi: 10.1111/j.1743-6109.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 11.Giugliano F, Esposito K, Di Palo C, et al. Erectile dysfunction associates with endothelial dysfunction and raised proinflammatory cytokine levels in obese men. J Endocrinol Invest. 2004;27:665–9. doi: 10.1007/BF03347500. [DOI] [PubMed] [Google Scholar]
- 12.Corona G, Mannucci E, Fisher AD, et al. Low Levels of Androgens in Men with Erectile Dysfunction and Obesity. J Sex Med. 2008 doi: 10.1111/j.1743-6109.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 13.Heidler S, Temml C, Broessner C, et al. Is the metabolic syndrome an independent risk factor for erectile dysfunction? J Urol. 2007;177:651–4. doi: 10.1016/j.juro.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Esposito K, Ciotola M, Giugliano F, et al. Phenotypic assessment of endothelial microparticles in diabetic and nondiabetic men with erectile dysfunction. J Sex Med. 2008;5:1436–42. doi: 10.1111/j.1743-6109.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- 15.Jackson G. The metabolic syndrome and erectile dysfunction: multiple vascular risk factors and hypogonadism. Eur Urol. 2006;50:426–7. doi: 10.1016/j.eururo.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Corona G, Mannucci E, Petrone L, et al. NCEP-ATPIII-defined metabolic syndrome, type 2 diabetes mellitus, and prevalence of hypogonadism in male patients with sexual dysfunction. J Sex Med. 2007;4:1038–45. doi: 10.1111/j.1743-6109.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 17.Guay A, Jacobson J. The relationship between testosterone levels, the metabolic syndrome (by two criteria), and insulin resistance in a population of men with organic erectile dysfunction. J Sex Med. 2007;4:1046–55. doi: 10.1111/j.1743-6109.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 18.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 19.Ponholzer A, Temml C, Obermayr R, Wehrberger C, Madersbacher S. Is erectile dysfunction an indicator for increased risk of coronary heart disease and stroke? Eur Urol. 2005;48:512–8. doi: 10.1016/j.eururo.2005.05.014. discussion 17−8. [DOI] [PubMed] [Google Scholar]
- 20.Berk BC, Abe JI, Min W, Surapisitchat J, Yan C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann N Y Acad Sci. 2001;947:93–109. doi: 10.1111/j.1749-6632.2001.tb03932.x. discussion 09−11. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari R. Tumor necrosis factor in CHF: a double facet cytokine. Cardiovasc Res. 1998;37:554–9. doi: 10.1016/s0008-6363(97)00309-x. [DOI] [PubMed] [Google Scholar]
- 22.Meldrum DR, Meng X, Dinarello CA, et al. Human myocardial tissue TNFalpha expression following acute global ischemia in vivo. J Mol Cell Cardiol. 1998;30:1683–9. doi: 10.1006/jmcc.1998.0776. [DOI] [PubMed] [Google Scholar]
- 23.Chia S, Qadan M, Newton R, Ludlam CA, Fox KA, Newby DE. Intra-arterial tumor necrosis factor-alpha impairs endothelium-dependent vasodilatation and stimulates local tissue plasminogen activator release in humans. Arterioscler Thromb Vasc Biol. 2003;23:695–701. doi: 10.1161/01.ATV.0000065195.22904.FA. [DOI] [PubMed] [Google Scholar]
- 24.Hayward MD, Jones BK, Saparov A, et al. An extensive phenotypic characterization of the hTNFalpha transgenic mice. BMC Physiol. 2007;7:13. doi: 10.1186/1472-6793-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carneiro FS, Giachini FR, Lima VV, et al. Adenosine actions are preserved in corpus cavernosum from obese and type II diabetic db/db mouse. J Sex Med. 2008;5:1156–66. doi: 10.1111/j.1743-6109.2007.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carneiro FS, Giachini FR, Lima VV, et al. DOCA-salt treatment enhances responses to endothelin-1 in murine corpus cavernosum. Can J Physiol Pharmacol. 2008;86:320–8. doi: 10.1139/Y08-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vila E, Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1016–21. doi: 10.1152/ajpheart.00779.2004. [DOI] [PubMed] [Google Scholar]
- 28.Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation. 1997;96:3042–7. doi: 10.1161/01.cir.96.9.3042. [DOI] [PubMed] [Google Scholar]
- 29.Holm T, Aukrust P, Andreassen AK, et al. Peripheral endothelial dysfunction in heart transplant recipients: possible role of proinflammatory cytokines. Clin Transplant. 2000;14:218–25. doi: 10.1034/j.1399-0012.2000.140307.x. [DOI] [PubMed] [Google Scholar]
- 30.Gao X, Belmadani S, Picchi A, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–54. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–76. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 32.Giardina JB, Green GM, Cockrell KL, Granger JP, Khalil RA. TNF-alpha enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R130–43. doi: 10.1152/ajpregu.00704.2001. [DOI] [PubMed] [Google Scholar]
- 33.Parris JR, Cobban HJ, Littlejohn AF, MacEwan DJ, Nixon GF. Tumour necrosis factor-alpha activates a calcium sensitization pathway in guinea-pig bronchial smooth muscle. J Physiol. 1999;(Pt 2):518, 561–9. doi: 10.1111/j.1469-7793.1999.0561p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter I, Cobban HJ, Vandenabeele P, MacEwan DJ, Nixon GF. Tumor necrosis factor-alpha-induced activation of RhoA in airway smooth muscle cells: role in the Ca2+ sensitization of myosin light chain20 phosphorylation. Mol Pharmacol. 2003;63:714–21. doi: 10.1124/mol.63.3.714. [DOI] [PubMed] [Google Scholar]
- 35.Musicki B, Burnett AL. eNOS function and dysfunction in the penis. Exp Biol Med (Maywood) 2006;231:154–65. doi: 10.1177/153537020623100205. [DOI] [PubMed] [Google Scholar]
- 36.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002;15:170–5. doi: 10.1016/s0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- 37.Neumann P, Gertzberg N, Johnson A. TNF-alpha induces a decrease in eNOS promoter activity. Am J Physiol Lung Cell Mol Physiol. 2004;286:L452–9. doi: 10.1152/ajplung.00378.2002. [DOI] [PubMed] [Google Scholar]
- 38.Yoshizumi M, Perrella MA, Burnett JC, Jr., Lee ME. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. 1993;73:205–9. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- 39.Corder R, Carrier M, Khan N, Klemm P, Vane JR. Cytokine regulation of endothelin-1 release from bovine aortic endothelial cells. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S56–8. [PubMed] [Google Scholar]
- 40.Zhang L, Peppel K, Sivashanmugam P, et al. Expression of Tumor Necrosis Factor Receptor-1 in Arterial Wall Cells Promotes Atherosclerosis. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/ATVBAHA.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]