Dr Lewis K. Dahl identified many of the relationships that underlie our current thinking and focus of research on hypertension. Among the first to establish the interactions between salt and hypertension, the realization that genetic factors played a major role in how the kidney responded to salt loads1 led to development of the genetically predisposed Dahl salt-sensitive rat. His research, although not specifically directed toward aging, recognized that the effects of high-salt diets were not necessarily immediate. In fact, he suggested that at least one third of the life span was required for salt-induced changes in systolic blood pressure (SBP).
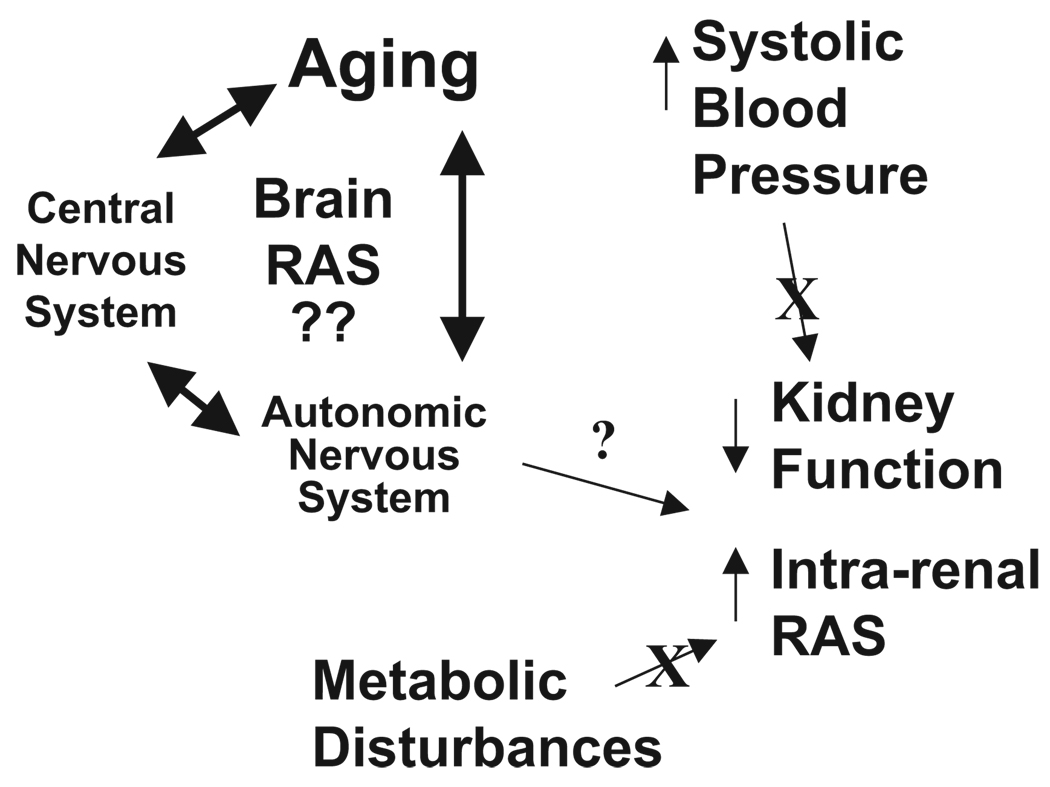
I am very grateful for the honor of presenting the Dahl lectureship on work, which, over the past several years, has had aging as the focus. The emphasis has been on the autonomic nervous system, as influenced by the renin-angiotensin system (RAS), specifically, the balance between angiotensin (Ang) II and Ang-(1–7) and the contributions of brain cardiovascular areas to the constellation of changes occurring with advancing age. The overall message derived is that, although the brain RAS plays a major role in all of the age-related changes in cardiovascular and metabolic function, neither the SBP nor the metabolic changes appear to be initiating factors in the activation of the intrarenal RAS or the decline in kidney function during aging (Figure 1).
Figure 1.
Diagram depicting the proposed relationships among SBP and metabolic dysfunction in the regulation of the intrarenal RAS and renal function during aging.
Differential Regulation of the Circulating and Intrarenal RAS During Aging
Components of the classical circulating RAS, in particular, renin release from the juxtaglomerular cells of the kidney, undergo a decline in older animals. This includes reductions in renal tissue renin mRNA, juxtaglomerular cell renin content, responsiveness of renin release to various challenges, and plasma renin and angiotensin (Ang) II.2–9 The renal vasoconstrictor responses to exogenously administered Ang II are increased in older animals,6 perhaps resulting from the reductions in the circulating system. In striking contrast, however, kidney Ang II content increases in older animals.6 This latter finding is not surprising, because all of the components of the RAS are synthesized locally within the kidney,10–12 and local production of Ang peptides is regulated independent of the circulating system.11,13–16 Thus, whereas the juxtaglomerular cell renin and its release into the circulation are considered representative of the circulating/hormonal RAS, renal tissue content, tubular fluid, or urinary RAS components are considered representative of the intrarenal RAS.
The concept of differential regulation of the circulating and intrarenal or other tissue RAS is not new.12 One possibility for these differences relate to the increase in SBP. An increase in arterial pressure during aging may lead to a baromediated reduction in renin release from the kidney, contributing to the decline in the circulating RAS. In contrast, for the intrarenal system, there is evidence that the regulation of tubular renin may be opposite to or at least different from that of the circulation.12 Although kidney Ang II content may reflect receptor-mediated uptake of the peptide, as well as local synthesis, urinary components representing the intrarenal RAS likely reflect a tubular site of synthesis and regulation.17 In Ang II–dependent hypertension, Ang peptides increase in the kidney18 but not in hypertension caused by high salt.19 For the intrarenal RAS, changes in glomerular filtration rate, either hyperfiltration or loss of nephrons, and reduced glomerular filtration rate may contribute to the independent regulation. In addition, the extent to which the metabolic dysfunction accompanying aging contributes to changes in the kidney is not completely known. The role of each of the above potential contributors to the regulation of the intrarenal RAS are emphasized in this review.
Differential Regulation of Circulating and Intrarenal RAS During Long-Term RAS Blockade
Our attempts to dissect factors contributing to age-related regulation of the intrarenal RAS and reductions in renal function arose from studies of long-term systemic RAS blockade. Angiotensin-converting enzyme (ACE) inhibition or Ang II type 1 (AT1) receptor blockade in hypertension improves kidney function in part by reducing arterial pressure, but in normotensive rats, long-term RAS inhibition is also protective. RAS blockade is associated with improvements in kidney function, including decreases in proteinuria, glomerulosclerosis, collagen deposition, and reduced intraglomerular pressure during aging.20–23
However, the decline in the circulating RAS9 raises questions about mechanisms underlying the beneficial effects of RAS blockade to reduce age-related impairments. We assessed effects of long-term RAS blockade on components of the intrarenal RAS in Wistar rats, a well-characterized model of aging with a life span of ≈24 months of age.20,21,24,25 When treated for 22 months starting at 2 months of age with either losartan or enalapril, these rats have lower SBPs than vehicle-treated older control rats.26 Treated rats also have a higher mitochondrial number, better learning and memory, less cardiac and renal fibrosis, and an extended life span.27 Accompanying the cardiovascular and renal improvements in the older animals, plasma Ang I and Ang II are elevated in the losartan-treated group and Ang I in the enalapril group (Figure 2), as expected from shorter-term studies with these treatments and consistent with interruption of the feedback inhibition of renin release.26 The urinary Ang peptides, in contrast, are lower in both treatment groups, illustrating markedly different regulation within these 2 compartments. The fact that plasma Ang peptides increase dramatically with long-term RAS blockade and urinary levels of the peptides decline renders filtration as the predominant source of urinary peptides unlikely. Although suppression of the intrarenal RAS may be responsible for the renoprotective effects,21 treated rats had lower pressure and improved overall renal function28; thus, it is not clear to what extent lower pressure contributes to improved renal function and secondarily to lower levels of urinary RAS peptides or vice versa. Because there was no young control group, it is not possible to determine whether lower intrarenal RAS in treated animals reflects the maintenance of normal young urinary peptide levels or lowering of RAS components below levels seen in young adult rats.
Figure 2.
Urinary and plasma Ang peptides at 24 months of age in control Wistar rats (Con; n=7) or after 22 months treatment with enalapril (Enal; 10 mg/kg per day; n=7) or losartan (Los; 30 mg/kg per day; n=6). Ang peptides in the urine in all of the treatment groups were suppressed in comparison with plasma. *P<0.01 vs losartan; **P<0.01 and ÔP<0.001 vs control. Data obtained from Reference 26.
Intrarenal RAS Regulation in Animals Without Age-Related Hypertension and Effects of Long-Term RAS Blockade
In the human population, as well as in a variety of rat strains, the numerous age-related pathologies commonly develop in association but are not entirely linked. In general, Wistar and Sprague-Dawley (SD) rats exhibit a more rapid aging process and shorter life span than other strains and tend to exhibit age-related impairments in cardiovascular, metabolic, and renal function, including elevations in SBP. The Fischer 344 rat represents an interesting model of aging, because insulin resistance and kidney damage occur without an increase in SBP.29,30 The Fischer 344 X Brown Norway rat expresses greater longevity than Fischer 344 animals with rescue from many of their age-related deficits, including the decline in renal function.31,32
In an attempt to rule out elevated SBP during aging as a contributor to activation of the intrarenal RAS, we studied male Fischer 344 rats between 3 and 15 months of age33 (Figure 3A). As expected, SBP did not increase and is actually lower in older than in younger rats. Contrary to what occurs in older rats and human subjects with an age-related increase in SBP, there is no decline in plasma Ang II. In terms of the intrarenal RAS, urinary Ang II is higher in older rats compared with young animals (Figure 3B) and is accompanied by marked increases in protein excretion. Serum leptin increases in older control rats relative to young rats with a similar trend for serum insulin (Figure 4). Serum glucose is higher in older than in younger rats, and older rats exhibit indices of reduced insulin sensitivity.33 Long-term (1-year) AT1 receptor blockade with L-158,809, a potent and selective antagonist, prevents increases in insulin, leptin, and glucose in older F344 rats, associated with reduced weight gain.33 There are no significant differences in SBPs between the older control and L-158,809-treated groups, and proteinuria, a marker of renal damage, is also completely prevented. Ang II excretion is maintained at levels similar to those of younger animals.33 Plasma Ang II is higher in treated than in untreated young or older rats, as evidence of effective AT1 receptor blockade for loss of AT1-mediated feedback control of renin release. Again, the differential regulation of systemic and intrarenal (urinary) Ang systems during long-term RAS blockade is clear.
Figure 3.
A, SBP and plasma Ang II in young (3 months old) and older (15 months old) Fischer 344 rats. B, Ang II and protein excretion in young and older Fischer 344 rats. *P<0.05 vs young rats. Data from Reference 33.
Figure 4.
Serum insulin, glucose, and leptin levels in young (3 months old) and older (15 months old) Fischer 344 rats. *P<0.01 vs young rats. Data from Reference 33.
The Fischer X Brown Norway rat is considered a model of “healthy aging,” because these animals have a ≈36-month life span and less cardiovascular decline than SD or Wistar rats.31 A preliminary study illustrates that urinary Ang II and protein are maintained at low levels in a comparison of 9-month-old versus 30-month-old animals.34 Treatment of these rats for 6 months with an ACE inhibitor or AT1 receptor blocker beginning at 24 months of age decreased fat mass without lowering blood pressure.35 However, RAS blockade does not lower insulin or leptin relative to older control rats and does not alter the excretion of Ang II or protein. Thus, a different pattern of protein and Ang II excretion is evident in healthy aging as opposed to Fischer 344 rats, although neither rat exhibits elevated SBP as part of the aging process. From these studies, however, SBP appears to be independent of the intrarenal RAS activation (Figure 1).
Clinical trials show that RAS blockade, either by ACE inhibitors36 or AT1 receptor blockers,37,38 may substantially lower the risk for cardiac hypertrophy39 and new-onset type 2 diabetes in hypertensive subjects. However, the exact mechanism underlying this effect is unknown. Beneficial effects are reported most often in studies of hypertensive subjects, and certainly blocking activation of the intrarenal system might contribute to lowering of pressure systemically via less salt and water reabsorption at the tubular level or may prevent renal injury at the glomerulus by reductions in intraglomerular pressure. However, our study in Fischer 344 rats demonstrates the advantageous effects of RAS blockade on metabolic function and renal injury independent of SBP-lowering actions.40,41 With systemic treatments, improvements in metabolism and renal function may involve actions at the skeletal muscle,41,42 brain and autonomic nervous system,43–45 or other organs, such as the liver and pancreas. 21,46,47 In addition, because RAS blockade prevents increases in leptin, glucose, and insulin, as well as activation of the intrarenal RAS, these studies do not illustrate which of the defects comes first, metabolic or renal, or which is directly or secondarily prevented by RAS blockade. Other studies presented below shed greater light on this point.
Contribution of the Brain RAS to Regulation of the Intrarenal RAS During Aging
The rationale for considering brain-kidney interactions in regulation of the intrarenal RAS was stimulated by a longstanding interest in the role of the renal nerves in control of renal function. An interesting report used an innovative molecular approach to address this interaction. A fusion protein leading to cellular generation of Ang II directly, without the need for processing enzymes,48,49 was placed behind a glial-fibrillary acid protein promoter for the generation of transgenic mice.50 The mice with targeted expression of Ang II generation and secretion to glial cells when backcrossed with angiotensinogen-deficient mice corrected the renal deficits characteristic of total angiotensinogen-deficient animals. This intriguing observation supports the concept that brain mechanisms involving the RAS could exert powerful effects on renal development and function.
Transgenic rats deficient in brain, or more precisely, glial angiotensinogen (ASrAogen), developed by Drs Ganten and Bader by insertion of angiotensinogen antisense driven by a glial-fibrillary acid protein promote in to the Hannover SD rat genome provide a unique tool to assess effects of the brain RAS on aging.51–53 The targeted disruption of glial angiotensinogen appears to preserve neuronal Ang peptides,43,44,54–58 but ASrAogen rats have lower Ang I in brain tissue. We compared ASrAogen rats with Hannover SD rats at what would be young adult (≈15 weeks), middle age (≈48 weeks), and early aging (≈68 weeks) time points, given the life span of ≈70 to 80 weeks of age for the Hannover SD substrain.56 SBP is lower in young adult ASrAogen rats than in control Hannover SD, and they maintain low SBP (Figure 5). Older ASrAogen rats have some baroreflex impairment, but the gain or sensitivity of reflex control of the heart rate in older animals is comparable with that in younger SD rats,56 and heart rate remains low, as evidence of reduced cardiac sympathetic and/or enhanced parasympathetic nervous system activity.43 The life span is ≈30% longer in ASrAogen rats,56 and they maintain lower leptin, glucose, and insulin levels compared with age-matched SD rats or (mRen2)27 hypertensive rats.43,44 Body weight is lower at all of the time points in the face of increased food intake relative to age-matched SD rats, suggesting that absence of glial RAS leads to central nervous system–mediated improved energy metabolism.43,44 Thus, glial-derived Ang II appears to participate in the age-related impairments in autonomic function. Because older ASrAogen rats resemble animals on long-term systemic RAS blockade, we speculate that the beneficial effects of systemic treatment on the wide array of age-related deficits in function in Fischer 344, Wistar, or SD rats could involve blockade of the brain RAS. RAS blockers are thought to gain access to the brain with long-term treatment, but whether differences in the extent of brain RAS blockade track with effectiveness of therapy is under investigation. A recent study suggests that, in human subjects, cognitive decline is slowed to a greater extent, with RAS blockers having li-pophilic properties.59.
Figure 5.
Differences in systolic blood pressure, insulin, and glucose at 15, 48, and 68 weeks of age in SD and ASrAogen rats. The SD animals have an increase in pressure at the latter time point. In contrast, the ASrAogen animals have a lower pressure at 68 weeks compared with the earlier time points. Insulin and glucose are also higher in the SD versus the ASrAogen rats at the 68-week time point. *P<0.05 or **P<0.001 vs ASrAogen rats. ÔP<0.01 vs 16 and 48 weeks of age. Data from Reference 43.
The concept that targeted disruption of the glial RAS in ASrAogen rats influences activation of the intrarenal RAS during aging is being tested. Plasma Ang II declines in older SD rats coincident with higher SBP but not in age-matched ASrAogen rats that do not exhibit elevations in SBP.45 Urinary Ang II and protein are higher in SD rats at 48 weeks and 68 weeks as compared with 16 weeks of age, with little change in ASrAogen animals over this time frame.45 Insulin increases in SD rats at 68 weeks but not at the 48-week time point. This puts activation of the intrarenal RAS and development of proteinuria45 at time points preceding elevated SBP or development of insulin resistance in the SD rats (Figure 5). The SD rats are heavier, however, at all of the time points relative to ASrAogen rats.43,44 Thus, the contribution of body weight, a factor known to play a role in renal injury,60 cannot be excluded from potential age-related causes of impairments in renal function.
In conclusion, the use of transgenic rats provides additional insight into the independent regulation of circulating and intrarenal RAS. Plasma Ang II and kidney renin mRNA decline significantly when SBP increases. The intrarenal RAS increases independently of hypertension and markers of insulin resistance. An increase in urinary Ang II occurs along with the proteinuria at the times tested, but precise determination of the time course of changes is lacking. Nonetheless, the ASrAogen rats are protected from age-related decline in cardiovascular, renal, and metabolic function and in this manner resemble animals treated long-term with RAS blockade.
Time Course of Changes in Renal Function and Activation of the Intrarenal RAS
The reduction in circulating levels of Ang II in SD rats during aging appears to occur coincident with increases in SBP45 rather than with the onset of kidney damage, as assessed by proteinuria or activation of the intrarenal RAS. Because Fischer 344 rats and ASrAogen rats do not have an increase in SBP, maintenance of normal circulating levels of RAS peptides may reflect the absence of pressure-mediated inhibition of renin release. On the other hand, intrarenal RAS elevation in SD and Fischer 344 rats occurs without increases in SBP. In SD rats, development of insulin resistance also occurs at a later time point than increases in proteinuria and the intrarenal RAS, suggesting that these age-related changes are not hypertension or hyperglycemia-related (Figure 1). In both Fischer 344 and SD rats, increases in Ang II and protein excretion over those of younger animals occur at 45 to 65 weeks of age, but whether activation of the intrarenal RAS is secondary to progressive renal damage or is an initiating factor is not known from the above studies. A more detailed assessment of the time course of activation of the intrarenal RAS relative to the elevation in proteinuria in aging SD rats is in progress.61 Ang II excretion assessed weekly from 24 to 40 weeks of age is stable or tends to decline in male SD rats, before the increase at >45 weeks of age. In contrast, protein excretion increases significantly at 34 weeks of age and continues to increase steadily thereafter,61 subsequent to a modest, transient increase in creatinine excretion between 24 and 27 weeks of age.61 Although we do not yet have serum creatinine data in these animals, the transient increase in urinary creatinine may be indicative of increased glomerular filtration, because it is not corrected by factoring for body weight. Interestingly, the increase in intrarenal Ang II excretion follows rather than precedes increases in urinary protein, which may place it secondary to a transient episode of hyperfiltration. Therefore, at present, increases in urinary protein would appear to be the best indicators of renal functional changes during the aging process, rather than markers of the intrarenal RAS, such as Ang II. Long-term RAS blockade prevents increases in both urinary protein and Ang peptide excretion in older animals; thus, the renoprotective effects may result at least in part from preventing the hyperfiltration and/or the increase in tubular protein as the first step, which secondarily prevents activation of the intrarenal RAS.
Hyperfiltration plays a role in early renal changes in diabetes,62 and hyperfiltration is prevented in streptozotocin-induced diabetic rats in response to bilateral renal denervation. 63 However, there are reports that renal nerves are protective against the progression of diabetic nephropathy.64 Increases in the glomerular filtration rate in response to amino acid load are also reduced by sectioning renal nerves.65 Because the glial angiotensinogen deficiency in ASrAogen rats is associated with preservation of renal function and a lack of change in protein or Ang II excretion during the aging process, hyperfiltration initiated by changes in renal nerve activity for control of glomerular hemodynamics is an attractive hypothesis. Indeed, in normotensive Wistar rats there is an increase in renal norepinephrine content during middle age that could be interpreted as consistent with increased renal nerve activity.66 This potential mechanism may provide a link between the age-related functional changes in the kidney and the central nervous system.
Is Activation of the Intrarenal RAS Characteristic of Other Tissue RAS During Aging?
From the comparison of ASrAogen and SD rats, it is inferred that the brain RAS (elevated or not) contributes to age-related elevations in pressure, changes in metabolism, and activation of the intrarenal RAS. There is a reduction in the endogenous Ang-(1–7) tone within the solitary tract nucleus in brain medulla oblongata for facilitation of reflex control of heart rate and a reduction in neprilysin mRNA67 in older SD rats. Preliminary studies show a trend for a reduction of neprilysin activity in brain medullary tissue of older rats.68 A decline in neprilysin activity in the plasma and kidney of older rats is reported.5 Thus, a deficit in Ang-(1–7) by reduced formation or enhanced production/reduced metabolism of Ang II may underlie age-related changes and serve as the substrate for impairments in autonomic control mechanisms.
There are reports of an increase in Ang II in cardiac tissue in older rats,69 but data on regulation of the RAS in other tissues during the aging process are minimal. It would be interesting to know whether long-term treatments with RAS blockers are associated with changes in the levels of Ang peptides in tissues such as heart and brain, in addition to suppressing the age-related increase in urinary Ang II. Knowledge of whether the protective benefits of RAS inhibitors are related to tissue penetrability70 might help resolve this issue. Finally, our preliminary studies in Fischer 344 X Brown Norway rats reveal that these animals maintain low levels of Ang II excretion to a late age, consistent with the concept of health aging. Understanding the mechanisms affording cardiovascular and renal protection in these animals would aid the identification of common features of impairment at earlier ages in Fischer 344, SD, and Wistar animals.
Overall Conclusions
Together, the accumulated data indicate that aging is associated with activation of the intrarenal RAS in several normotensive strains of rats. The renal RAS activation is independent of age-related increases in SBP. However, the decline in circulating RAS during aging may be a consequence of the age-related increase in pressure, because plasma Ang II does not decline in rats without increased SBP during aging. Given the differences in timing of the onset and development of insulin resistance, renal injury as indicated by proteinuria, and activation of the intrarenal RAS observed with increasing age, all 3 of the age-related changes appear to be independent of each other (Figure 1). The intrarenal RAS increase may track with other markers of metabolic dysfunction, such as elevated weight gain, but not insulin resistance. Whether other indices of impaired autonomic function including baroreflex dysfunction and elevated renal sympathetic nerve activity contribute to changes in renal function or activation of the renal RAS is yet to be demonstrated. There appears to be an age-related decrease in Ang-(1–7) relative to Ang I or Ang II in urine, plasma, and brain, which may be restored with a long-term AT1 receptor or Ang-converting enzyme blockade. Systemic RAS blockade or glial angiotensinogen deficiency prevents other age-related changes, arguing strongly for mechanisms involving the blockade of local tissue RAS, brain and kidney in particular, as contributors to protective effects of systemic RAS blockade during aging.
Acknowledgments
I express sincerest appreciation to my long-time colleagues for their constant support, critical technical and intellectual input, and friendship. Their contributions are reflected in numerous aspects of the work presented in this review.
Sources of Funding
This work was supported by grants HL-51952 and HL-51952S1 from the National Heart, Lung, and Blood Institute and National Cancer Institute grant CA-122318. The American Heart Association-MidAtlantic Affiliate pre-doctoral fellowship grant 0215151U (S.O. Kasper), Unifi, Inc (Greensboro, NC), and the Farley-Hudson Foundation (Jacksonville, NC) also provided partial support for this work.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Dahl LK. Possible role of salt intake in the development of essential hypertension. Int J Epidemiol. 2005;34:967–972. doi: 10.1093/ije/dyh317. [DOI] [PubMed] [Google Scholar]
- 2.Baylis C. Renal responses to acute angiotensin II inhibition and administered angiotensin II in the aging, conscious, chronically catheterized rat. Am J Kidney Dis. 1993;22:842–850. doi: 10.1016/s0272-6386(12)70344-x. [DOI] [PubMed] [Google Scholar]
- 3.Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol. 2004:699–700. doi: 10.1681/ASN.V94699. [DOI] [PubMed] [Google Scholar]
- 4.Masilamani S, Zhang XZ, Baylis C. Blunted pressure natriuretic response in the old rat: participation of the renal nerves. Am J Kidney Dis. 1998;32:605–610. doi: 10.1016/s0272-6386(98)70024-1. [DOI] [PubMed] [Google Scholar]
- 5.Reckelhoff JF, Baylis C. Proximal tubular metalloprotease activity is decreased in the senescent rat kidney. Life Sci. 1992;50:959–963. doi: 10.1016/0024-3205(92)90174-n. [DOI] [PubMed] [Google Scholar]
- 6.Thompson MM, Oyama TT, Kelly FJ, Kennefick TM, Anderson S. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am J Physiol. 2000;279:R1787–R1794. doi: 10.1152/ajpregu.2000.279.5.R1787. [DOI] [PubMed] [Google Scholar]
- 7.Anderson S, Rennke HG, Zatz R. Glomerular adaptations with normal aging and with long-term converting enzyme inhibition in rats. Am J Physiol. 1994;267:F35–F43. doi: 10.1152/ajprenal.1994.267.1.F35. [DOI] [PubMed] [Google Scholar]
- 8.Anderson S. Ageing and the renin-angiotensin system. Nephrol Dial Transplant. 1997;12:1093–1094. doi: 10.1093/ndt/12.6.1093. [DOI] [PubMed] [Google Scholar]
- 9.Jung FF, Kennefick TM, Ingelfinger JR, Vora JP, Anderson S S. Down-Regulation of the intrarenal renin-angiotensin system in the aging rat. J Am Soc Nephrol. 1994;5:1573–1580. doi: 10.1681/ASN.V581573. [DOI] [PubMed] [Google Scholar]
- 10.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 11.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412–422. [PubMed] [Google Scholar]
- 12.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal reninangiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 13.Allred AJ, Chappell MC, Ferrario CM, Diz DI. Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol. 2000;279:F636–F645. doi: 10.1152/ajprenal.2000.279.4.F636. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DJ, Lawrence AC, Towrie A, Kladis A, Valentijn AJ. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension. 1991;18:763–773. doi: 10.1161/01.hyp.18.6.763. [DOI] [PubMed] [Google Scholar]
- 15.Modrall JG, Sadjadi J, Brosnihan KB, Gallagher PE, Yu C-H, Kramer GL, Bernstein KE, Chappell MC. Depletion of tissue angiotensinconverting enzyme differentially influences the intrarenal and urinary expression of angiotensin peptides. Hypertension. 2004;43:849–853. doi: 10.1161/01.HYP.0000121462.27393.f6. [DOI] [PubMed] [Google Scholar]
- 16.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mREN2.Lewis rat. Am J Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 17.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 18.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, Arnaiz MdRL, Inserra F. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept. 2005;128:247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Ferder LF, Inserra F, Basso N. Effects of renin-angiotensin system blockade in the aging kidney. Exp Gerontol. 2003;38:237–244. doi: 10.1016/s0531-5565(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 22.Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1996;76:612–619. doi: 10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferder L, Inserra F, Romano L, Ercole L, Pszenny V. Decreased glomerulosclerosis in aging by angiotensin-converting enzyme inhibitors. J Am Soc Nephrol. 1994;5:1147–1152. doi: 10.1681/ASN.V541147. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez Bosc LV, Kurnjek ML, Muller A, Terragno NA, Basso N. Effect of chronic angiotensin II inhibition on the nitric oxide synthase in the normal rat during aging. J Hypertens. 2001;19:1403–1409. doi: 10.1097/00004872-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 25.GonzalezX BL, Kurnjek ML, Muller A, Basso N. Effect of chronic angiotensin II inhibition on the cardiovascular system of the normal rat. Am J Hypertens. 2000;13:1301–1307. doi: 10.1016/s0895-7061(00)01209-7. [DOI] [PubMed] [Google Scholar]
- 26.Kasper SO, Basso N, Kurnjek ML, Paglia N, Ferrario CM, Ferder LF, Diz DI. Divergent regulation of circulating and intra-renal renin-angiotensin systems in response to long-term blockade. Am J Nephrol. 2005;25:335–341. doi: 10.1159/000086571. [DOI] [PubMed] [Google Scholar]
- 27.Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol. 2007;293:H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 28.Ferder LF, Inserra F, Basso N. Advances in our understanding of aging: role of the renin-angiotensin system. Curr Opin Pharmacol. 2002;2:189–194. doi: 10.1016/s1471-4892(02)00139-x. [DOI] [PubMed] [Google Scholar]
- 29.Wei JY, Mendelowitz D, Anastasi N, Rowe JW. Influence of age on cardiovascular reflex response in anesthetized rats. Am J Physiol. 1985;249:R31–R38. doi: 10.1152/ajpregu.1985.249.1.R31. [DOI] [PubMed] [Google Scholar]
- 30.Wei JY, Mendelowitz D, Anastasi N, Rowe JW. Maintenance of carotid baroreflex function in advanced age in the rat. Am J Physiol. 1986;250:R1047–R1051. doi: 10.1152/ajpregu.1986.250.6.R1047. [DOI] [PubMed] [Google Scholar]
- 31.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway X Fischer 344, and Fischer 344 X brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eiam-Ong S, Sabatini S. Age-related changes in renal function, membrane protein metabolism, and Na,K-ATPase activity and abundance in hypokalemic F344 X BNF[sub 1] rats. Gerontology. 1999;45:254–264. doi: 10.1159/000022098. [DOI] [PubMed] [Google Scholar]
- 33.Gilliam-Davis S, Payne VS, Kasper SO, Tommasi EN, Robbins ME, Diz DI. Long-term AT1 receptor blockade improves metabolic function and provides renoprotection in Fischer-344 rats. Am J Physiol. 2007;293:H1327–H1333. doi: 10.1152/ajpheart.00457.2007. [DOI] [PubMed] [Google Scholar]
- 34.Kasper SO, Gilliam-Davis S, Groban L, Carter CS, Sonntag WE, Chappell MC, Diz DI. Effects of aging and renin-angiotensin system (RAS) blockade on the intra-renal RAS in older Fischer 344 X Brown Norway rats [abstract] FASEB J. 2008;22:735.11. [Google Scholar]
- 35.Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, Sonntag WE, Pahor M. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59:416–423. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH, Zinman B. Ramipril and the development of diabetes. JAMA. 2001;286:1882–1885. doi: 10.1001/jama.286.15.1882. [DOI] [PubMed] [Google Scholar]
- 37.Dahlof B, Devereux R, Kjeldsen S, Julius S, Beevers G, Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm L, Nieminen M, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 38.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 39.Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Lindholm LH, Dahlof B for the LIFE Study Investigators. In-treatment resolution or absence of electrocardiographic left ventricular hypertrophy is associated with decreased incidence of new-onset diabetes mellitus in hypertensive patients: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study. Hypertension. 2007;50:984–990. doi: 10.1161/HYPERTENSIONAHA.107.096818. [DOI] [PubMed] [Google Scholar]
- 40.Sola S, Mir MQS, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) Study. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 41.Shiuchi T, Iwai M, Li HS, Wu L, Min LJ, Li JM, Okumura M, Cui TX, Horiuchi M. Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension. 2004;43:1003–1010. doi: 10.1161/01.HYP.0000125142.41703.64. [DOI] [PubMed] [Google Scholar]
- 42.Henriksen EJ, Jacob S. Angiotensin converting enzyme inhibitors and modulation of skeletal muscle insulin resistance. Diabetes Obes Metab. 2003;5:214–222. doi: 10.1046/j.1463-1326.2003.00265.x. [DOI] [PubMed] [Google Scholar]
- 43.Kasper SO, Carter CS, Ferrario CM, Ganten D, Ferder LF, Sonntag WE, Gallagher PE, Diz DI. Growth and metabolism disturbances in transgenic rats with altered renin-angiotensin system expression. Physiol Genomics. 2005;23:311–317. doi: 10.1152/physiolgenomics.00163.2005. [DOI] [PubMed] [Google Scholar]
- 44.Kasper SO, Ferrario CM, Ganten D, Diz DI. Rats with low brain angiotensinogen do not exhibit insulin resistance during early aging. Endocrine. 2006;30:167–174. doi: 10.1385/ENDO:30:2:167. [DOI] [PubMed] [Google Scholar]
- 45.Oden SD, Ganten D, Ferrario CM, Chappell MC, Diz DI. Rats with low brain angiotensinogen maintain normal levels of insulin and components of the circulating and intra-renal renin-angiotensin systems during aging [abstract] FASEB J. 2004;18:A738. [Google Scholar]
- 46.Ferder L, Inserra F, Romano L, Ercole L, Pszenny V. Effects of angiotensin-converting enzyme inhibition on mitochondrial number in the aging mouse. Am J Physiol Cell Physiol. 1993;34:C15–C18. doi: 10.1152/ajpcell.1993.265.1.C15. [DOI] [PubMed] [Google Scholar]
- 47.Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin-an-giotensin system in the ZDF rat. Diabetes. 2004;53:989–997. doi: 10.2337/diabetes.53.4.989. [DOI] [PubMed] [Google Scholar]
- 48.Methot D, vanKats JP, Lochard N, Tremblay F, Silversides DW, Reudelhuber TL. Development and application of a biological peptide pump for the study of the in vivo actions of angiotensin peptides. Am J Hypertens. 2001;6:38S–43S. doi: 10.1016/s0895-7061(01)02068-4. [DOI] [PubMed] [Google Scholar]
- 49.van Kats JP, Methot D, Paradis P, Silversides DW, Reudelhuber TL. Use of a biological peptide pump to study chronic peptide hormone action in transgenic mice. Direct and indirect effects of angiotensin II on the heart. J Biol Chem. 2001;276:44012–44017. doi: 10.1074/jbc.M106132200. [DOI] [PubMed] [Google Scholar]
- 50.Lochard N, Silversides DW, van Kats JP, Mercure C, Reudelhuber TL. Brain-specific restoration of angiotensin II corrects renal defects seen in angiotensinogen-deficient mice. J Biol Chem. 2003;278:2184–2189. doi: 10.1074/jbc.M209933200. [DOI] [PubMed] [Google Scholar]
- 51.Monti J, Schinke M, Bohm M, Ganten D, Bader M, Bricca G. Glial angiotensinogen regulates brain angiotensin II receptors in transgenic rats TGR(ASrAOGEN) Am J Physiol. 2001;280:R233–R240. doi: 10.1152/ajpregu.2001.280.1.R233. [DOI] [PubMed] [Google Scholar]
- 52.Schinke M, Bohm M, Bricca G, Ganten D, Bader M. Permanent inhibition of angiotensinogen synthesis by antisense RNA expression. Hypertension. 1996;27:508–513. doi: 10.1161/01.hyp.27.3.508. [DOI] [PubMed] [Google Scholar]
- 53.Schinke M, Baltatu O, Bohm M, Peters J, Rascher W, Bricca G, Lippoldt A, Ganten D, Bader M. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci U S A. 1999;96:3975–3980. doi: 10.1073/pnas.96.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinsant S, Chappell MC, Ferrario CM, Ganten D, Diz DI. Low glial angiotensinogen is not associated with deficits in angiotensin peptides in neuronal pathways in transgenic ASrAogen rats [abstract] FASEB J. 2005;19:A1188. [Google Scholar]
- 55.Diz DI, Jessup JA, Westwood BM, Bosch SM, Vinsant S, Gallagher PE, Averill DB. Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla. Clin Exper Pharmacol Physiol. 2001;29:473–482. doi: 10.1046/j.1440-1681.2002.03659.x. [DOI] [PubMed] [Google Scholar]
- 56.Diz DI, Kasper SO, Sakima A, Ferrario CM. Aging and the brain reninangiotensin system: insights from studies in transgenic rats. Cleve Clin J Med. 2007;74 suppl 1:S95–S98. doi: 10.3949/ccjm.74.suppl_1.s95. [DOI] [PubMed] [Google Scholar]
- 57.Kasper SO, Ferrario CM, Ganten D, Diz DI. Central depletion of angiotensinogen is associated with elevated AT1 receptors in the SFO and PVN. Neurotoxicity Res. 2004;6:259–265. doi: 10.1007/BF03033436. [DOI] [PubMed] [Google Scholar]
- 58.Sakima A, Averill DB, Kasper SO, Jackson L, Ganten D, Ferrario CM, Gallagher PE, Diz DI. Baroreceptor reflex regulation in anesthetized transgenic rats with low glial-derived angiotensinogen. Am J Physiol. 2007;92:H1412–H1419. doi: 10.1152/ajpheart.00984.2006. [DOI] [PubMed] [Google Scholar]
- 59.Sink KM, Leng X, Williamson J, Kritchevsky S, Psaty B, kuller L, Yasar S, Atkinson H, Robbins M, Goff D. Centrally active ACE inhibitors may slow cognitive decline: the Cardiovascular Health Study [abstract] J Am Geriatr Soc. 2007;55:s14. [Google Scholar]
- 60.Hall JE, Louis K. Dahl Memorial Lecture. Renal and cardiovascular mechanisms of hypertension in obesity. Hypertension. 1994;23:381–394. doi: 10.1161/01.hyp.23.3.381. [DOI] [PubMed] [Google Scholar]
- 61.Gilliam-Davis S, Chappell MC, Diz DI. Elevated angiotensin I and creatinine excretion precede the increase in proteinuria during aging in Sprague-Dawley rats [abstract] Hypertension. 2007;50:E121. [Google Scholar]
- 62.Hostetter TH. Hypertrophy and hyperfunction of the diabetic kidney. J Clin Invest. 2001;107:161–162. doi: 10.1172/JCI12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luippold G, Beilharz M, Muhlbauer B. Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant. 2004;19:342–347. doi: 10.1093/ndt/gfg584. [DOI] [PubMed] [Google Scholar]
- 64.Matsuoka H. Protective role of renal nerves in the development of diabetic nephropathy. Diabetes Res. 1993;23:19–29. [PubMed] [Google Scholar]
- 65.Mendez RE, Lopez R, Lopez G, Marti MS, Martinez-Maldonado M. Effects of dopamine-receptor antagonists and renal denervation on amino acid-induced hyperfiltration. Am J Physiol. 1991;261:F70–F75. doi: 10.1152/ajprenal.1991.261.1.F70. [DOI] [PubMed] [Google Scholar]
- 66.Diz DI, Baer PG, Nasjletti A. Effect of norepinephrine and renal denervation on renal PGE2 and kallikrein in rats. Am J Physiol. 1981;241:F477–F481. doi: 10.1152/ajprenal.1981.241.5.F477. [DOI] [PubMed] [Google Scholar]
- 67.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats. Role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 68.Gegick S, Garcia-Espinosa MA, Gallagher PE, Westwood BM, Ferrario CM, Ganten D, Chappell MC, Diz DI. Reduced formation of Ang-(1–7) by ACE2 in dorsal medulla oblongata of Sprague-Dawley (SD) and ASrAogen rats during aging [abstract] FASEB J. 2006;20:A1209. [Google Scholar]
- 69.Groban L, Pailes NA, Bennett CDL, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 70.Pilote L, Abrahamowicz M, Rodrigues E, Eisenberg MJ, Rahme E. Mortality rates in elderly patients who take different angiotensin-converting enzyme inhibitors after acute myocardial infarction: a class effect? Ann Intern Med. 2004;141:102–112. doi: 10.7326/0003-4819-141-2-200407200-00008. [DOI] [PubMed] [Google Scholar]