Abstract
Previously, we reported the development of two in vitro time-resolved Föster energy transfer (tr-FRET) based assays for evaluating the potency and efficacy of different ligands of thyroid hormone receptor (TR) for regulating the recruitment of coregulators; we could measure independently, in separate assays, both the recruitment of SRC3, a transcriptional coactivator, and the dissociation of NCoR, a transcriptional corepressor, from a TR•retinoid × receptor (RXR) heterodimer bound to a DR+4 thyroid hormone response element (TRE). Here, by utilizing the distinct emission peaks of Tb+3, the donor fluorophore used to label the TRE-bound TR•RXR heterodimers, and selecting two distinct acceptor fluorophores, fluorescein and cyanin5 to label of NCoR and SRC3, respectively, we have integrated our previous two assay formats into a single assay. Thus, we can measure the potency of TR ligands simultaneously for NCoR dissociation and SRC3 recruitment activities in a system that mimics many features of the cellular context of TR action. The performance of this dual assay was tested with a known, highly potent physiological TR ligand, T3, and with a synthetic TR antagonist NH-3. Measured potencies and efficacies of these two TR ligands from this dual assay are highly comparable to those obtained from the two independent assays. Thus, this dual-acceptor tr-FRET assay further simplifies the measurement of ligand-modulated TR-coregulator interactions and should improve the overall efficiency of the screening process of TR drug discovery programs.
Keywords: Dual time-resolved fluorescence energy transfer assay, Thyroid hormone receptor, Thyroid hormone ligand screening, High throughput assay
Introduction
Thyroid hormones, acting through nuclear thyroid hormone receptors (TR) [1], are important drug targets for the commonly occurring conditions of hypo- and hyper-thyroidisms, as well as to lower hyperlipidemia [2–4]. As a transcription factor, TR displays dual transcriptional regulatory properties: In the unliganded state, promoter-bound TR, typically, as a heterodimer with retinoid X receptor (RXR), represses target gene transcription by recruiting corepressor proteins such as nuclear receptor corepressor (NCoR). The binding of agonist ligands induces a conformational change in TR that causes the release of corepressors and the recruitment of coactivator proteins such as steroid receptor coactivator 3 (SRC3), thereby promoting transcriptional activation. Both NCoR and SRC3, respectively, target ligand free- or agonist ligand-bound TR through their specific nuclear receptor interaction domains (NRIDs) [5–6]. The potency of a TR ligand is thought to derive, in part, by its ability to control the affinity with which these coregulators interact with TR.
To evaluate how different TR ligands regulate the distinct processes of corepressor dissociation and coactivator recruitment, we recently reported two independent in vitro time-resolved Föster energy transfer (tr-FRET) based assays, an NCoR dissociation assay and an SRC3 recruitment assay, through which ligand-specific coregulator interactions with thyroid hormone response element (TRE)-bound TR•RXR can be measured in a convenient and quantitative manner. These assays involved using TR•RXR heterodimer indirectly labeled with a Tb+3 (fluorescence donor) via a biotinylated TRE (direct repeat with 4bp space, DR+4 response element) that is linked to a streptavidin-Tb (SA-Tb) conjugate which acts as a donor to NRID fragments of NCoR or SRC3, labeled with fluorescein, the fluorescence acceptor [7]. Our assay design replicates many aspects of the cellular context in which TR normally interacts with these coregulators, namely, as a heterodimer with RXR, bound to a TRE, interacting with the complete NRID of these coregulators.
Recently, Kupcho et al., reported that Tb+3, with its multiple distinct emission peaks, can be used as a donor fluorophore simultaneously to two acceptor fluorophores in dual-acceptor FRET technology. It is possible to monitor two distinct binding events with a single protein target using this system, and they demonstrated the ligand-specific orthogonal binding events of corepressor and coactivator-derived peptides with the ligand binding domain of peroxisome proliferator receptors [8].
In this methodology, the distinct emission peaks of Tb+3 are used as an energy source to excite two spectrally different acceptor fluorophores, and the resulting FRET between donor and either of the two acceptors is measured concurrently and without spectral interferences. Using this principle, we further simplified our previous two, independent tr-FRET assay formats into a single tr-FRET assay with which we can assess simultaneously the interactions of both corepressors and coactivators with TRE-bound TR•RXR. For this assay, we labeled the TR-RXR with Tb+3 donor as before, and NCoR and SRC3 with the acceptor fluorophores fluorescein and cyanin5, respectively. Because emission from these two acceptor fluorophores is distinct, we can monitor independently the ligand-specific interactions both of TR with NCoR and of TR with SRC3 by monitoring the distinct FRET signals arising from the Tb-fluorescein and Tb-Cy5 FRET pairs.
As with our prior single-acceptor tr-FRET assays with these components, this dual-acceptor tr-FRET assay can be set up in two formats: a coregulator titration assay (which measures the affinities of NCoR and SRC3 for TR complexed with different ligands) and a ligand titration assay (which measures the potency of ligands for their effects both in displacing NCoR and in recruiting SRC3). Using triiodothyronine (T3), a potent natural TR agonist, and NH-3 [9], a synthetic TR antagonist, as candidate TR ligands, we validate this single assay format and show that the measured potency of thyroid hormones from this single assay method is very similar to the values obtained from the dual assay formats.
Experimental Procedures
Reagents
Terbium-labeled streptavidin and 5-iodoacetamidofluorescein were obtained from Molecular Probes/Invitrogen (Eugene, CA). Cy5-maleimide was from Amersham Biosciences (Piscataway, NJ). T3 (3,5,3’-triiodo-L-thyronine) was purchased from Sigma (St.Louis, MO). NH-3 ({4-[4-hydroxy-3’-isopropyl-5-(4-nitrophenylethynyl)benzyl]-3,5-dimethylphenoxy}acetic acid) has been described previously [9]. A 49 bp DR+4 TRE sequence (5’/5Bio/GAACAGATCTCCTTGGCTCTGGAGGTCACAGGAGGTCAGCGGATCCAT; core half-sites are underlined) was derived from the rat α-myosin heavy chain promoter and was synthesized by Integrated DNA Technologies (Coralville, IA). The sense strand contained biotin covalently attached at its 5’ end. The core DR+4 sequence from the natural promoter, AGGTGACAGGAGGACA, was changed to the consensus DR+4 sequence AGGTCACAGGAGGTCA. Stock solutions of T3 and NH-3 were made in DMSO at a concentration of 5 mM.
Plasmids and Protein Expression
Human TRβ (amino acids 82–456 containing both the DNA and ligand binding domain), human RXRα (full length) and the NRID fragments of mouse NCoR (residues 2057–2453) and human SRC3 (residues 627–829) were expressed in bacteria as six-His fusion proteins and purified as described previously [10–12]. The NRID fragments of NCoR and SRC3 were fluorescently labeled with 5-iodoacetamidofluorescein (Fl) and cyanin5-maleimide (Cy5), respectively, according to our previously published procedure [13].
Simultaneous Coregulator (NCoR and SRC3) Titration Assays
The following 3x concentrated mixtures were prepared. 1). SA-Tb, TRE, TR and RXR in buffer A [50 mM Tris (pH 8) containing 10% glycerol, 0.05% Nonidet P-40, 50 mM KCl, 2mM β-mercaptoethanol, 0.3 mg/mL ovalbumin and 2% DMSO] with T3 or NH-3 or solvent DMSO (Apo). 2). Fl-NCoR and 3). Cy5-SRC3 NRID protein fragments were diluted individually in buffer A. Aliquots (5 µl) of each of these three solutions were added to the wells of a dark microplate (Molecular Devices, Sunnyvale CA), mixed gently and incubated for 1 hr at room temperature in the dark before measurement for tr-FRET. The final concentrations of assay components were SA-Tb (2.5 nM), TRE (10 nM), TR and RXR (15 nM), T3 (3 µM) and NH-3 (25 µM) plus indicated concentrations of fluorphore-labeled NCoR and SRC3. Each assay was performed with a corresponding set of negative control wells that contained all the components and an equivalent volume of the solvent DMSO but without the biotinylated TRE (control); this data was used to correct for diffusion-enhanced FRET.
Simultaneous Ligand Titration Assays
The following 4x reaction components were individually prepared: 1). A mixture of SA-Tb, TRE, TR and RXR in buffer A. 2). Ligand or solvent (DMSO) dilutions in buffer B [20 mM Tris (pH 8), 150 mM NaCl and 2% DMSO]. 3). Fl-NCoR and 4). Cy5-SRC3 in buffer A. Five µl of each of these four solutions was added to wells and tr-FRET was measured as before. In these assays the concentrations of SA-Tb, TRE, TR and RXR remained the same as in the previous experiment, but the fluorophore-labeled NCoR and SRC3 were 25 and 150 nM, respectively. Control wells used to correct for diffusion-enhanced FRET contained all the components minus TRE and appropriately diluted DMSO.
tr-FRET Measurements
tr-FRET was measured on a Wallac Victor II plate reader (Molecular Devices, Sunny Vale, CA). tr-FRET between Tb-Fl (TR-NCoR) and Tb-Cy5 (TR-SRC3) were measured from the same assay plate after excitation of the Tb donor at 340 nm, in both cases. After each pulse of excitation light, the emissions from Tb3+, fluorescein or Cy5 were measured beginning 50 µs post excitation and extending to 1000 µs. This gating or time resolution allows prompt (nanosecond lifetime) emissions from fluorescent contaminants in the assay, as well as from the acceptor fluorophores, fluorescein and Cy5, due to direct excitation by the pulsed excitation light, to fully decay. Thereafter, in the time window 50–1000 µs,[comments] the long lived (millisecond) emissions of the Tb3+ donor fluorophore and also from the acceptor fluorophores, fluorescein and Cy5, excited by FRET from the Tb3+ donor, can be measured on a very low background. Donor (Tb+3) emission intensity was measured using a filter centered at 495 nm (20 nm band pass), acceptor fluorescein emission using a filter centered at 520 nm (25 nm bandwidth), and Cy5 emission with a 670 nm filter (10 nm bandwidth). FRET values are calculated as the ratio of acceptor emission intensity (A) to donor emission intensity (D) and expressed as A/D × 1000 values.
Results
Principle of Dual Acceptor Time-Resolved Föster Resonance Energy Transfer Assay Technology (Dual Acceptor tr-FRET)
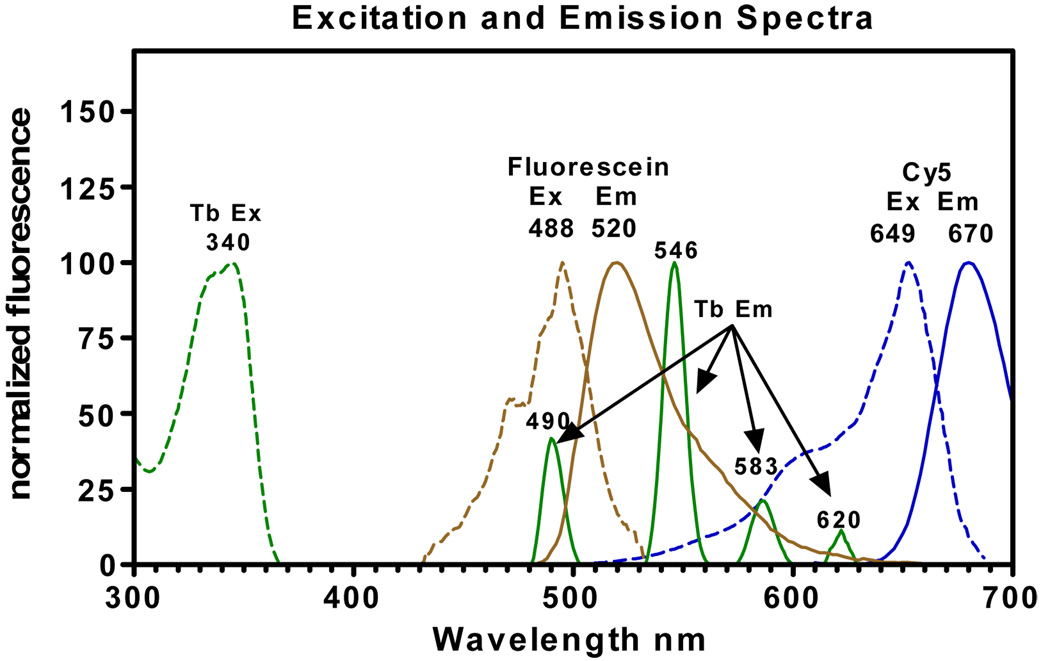
Lanthanide ions such as Tb+3 and Eu+3, when sensitized with light-harvesting organic moieties such as CS124, display unique excitation and emission characteristics that make them ideal donor species for tr-FRET-based methods to monitor multiple interacting events. Excitation of Tb+3 at 340 nm gives an emission spectrum that is characterized by four distinct bands, centered at 490, 546, 583 and 620 nm (Fig. 1). Because these sharp emission bands span a wide range of spectral wavelengths and emission is negligible between and beyond these peaks, Tb+3 emission can be potentially paired with multiple acceptors having excitation spectra that overlap with the Tb emission peaks and that have emission spectra that are distinct and emit where the donor emission from Tb+3 is negligible. By selecting such acceptors and appropriate filters detecting emission of both donor and acceptors at their maxima, two resonance energy transfer processes can be monitored independently, without spectral interferences. Thus, we selected fluorescein having an excitation spectrum overlapping with the first emission band of Tb+3 and Cy5 overlapping with the third and fourth emission peaks of Tb+3 to label NCoR and SRC3 fragments, respectively, to monitor simultaneously their interactions with TR•RXR bound to a SA-Tb labeled TRE (Fig 1). In this format, when TR associates with Fl-NCoR, there is an increase in emission at 520 nm, and with Cy5-SRC3, there is an increase in emission at 670 nm. Additionally, the extended excited state lifetime of Tb+3 (up to several milliseconds) permits measurements of FRET in a time-resolved mode (FRET detection 50 µs post excitation) that minimizes non-specific interferences from short-lifetime assay components and assay plate plastics, and from direct excitation of fluorescein and Cy5 (Fig. 2).
Figure 1.
Excitation (Ex) and emission spectra of Tb+3 labeled streptavidin, fluorescein labeled NCoR and Cy5 labeled SRC3.
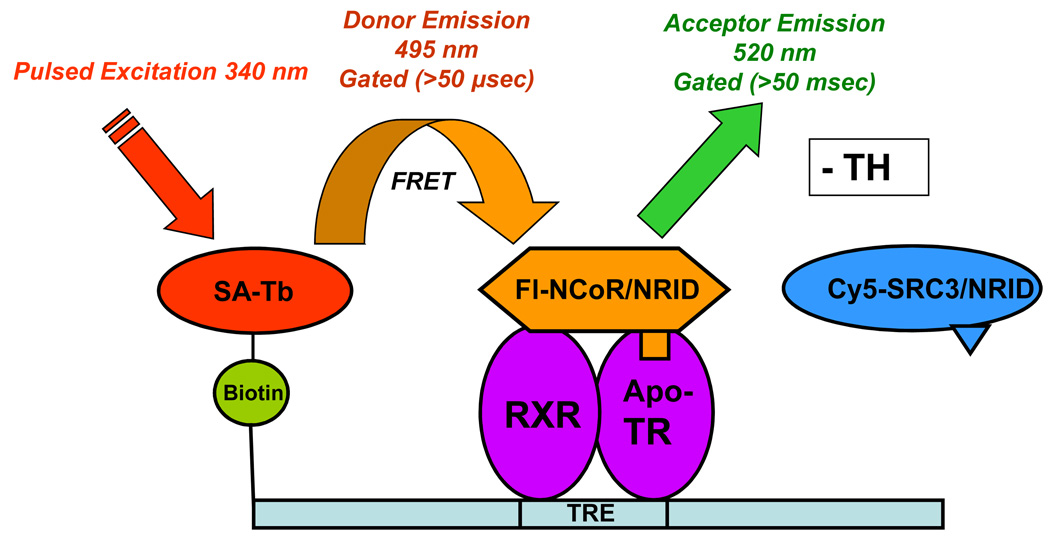
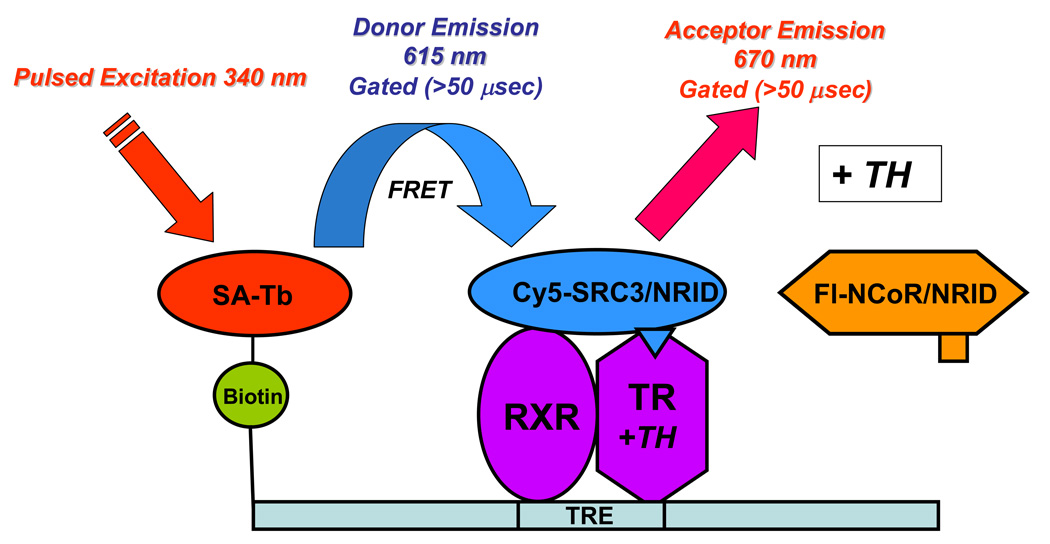
Figure 2.
Principle of the dual tr-FRET assays. A heterodimer of hTRβ (residues 82–456) and hRXRα (full length) was assembled onto a terbium-labeled streptavidin-bound biotinylated DR+4 TRE (49 bp from the rat myosin heavy chain promoter). (A) Unliganded (− TH) TR•RXR bound to a TRE interacts with NCoR fluorescein-labeled NRID fragment of mNCoR, residues 2057–2453. The Tb donor is excited at 340 nm, and the tr-FRET is assessed after 50-µs delay at 495 nm for terbium (donor) and at 520 nm for fluorescein (acceptor). (B) Liganded (+ TH) TR•RXR bound to a TRE interacts with Cy5-labeled hSRC3, residues 627–829. The Tb donor is excited at 340 nm, and the tr-FRET is assessed after 50-µs delay at 495 nm for terbium (donor) and at 670 nm for Cy5 (acceptor).
Simultaneous Measurement of Coregulator (NCoR and SRC3) Binding Affinities for Various TR-Liganded Complexes: Coregulator Titration
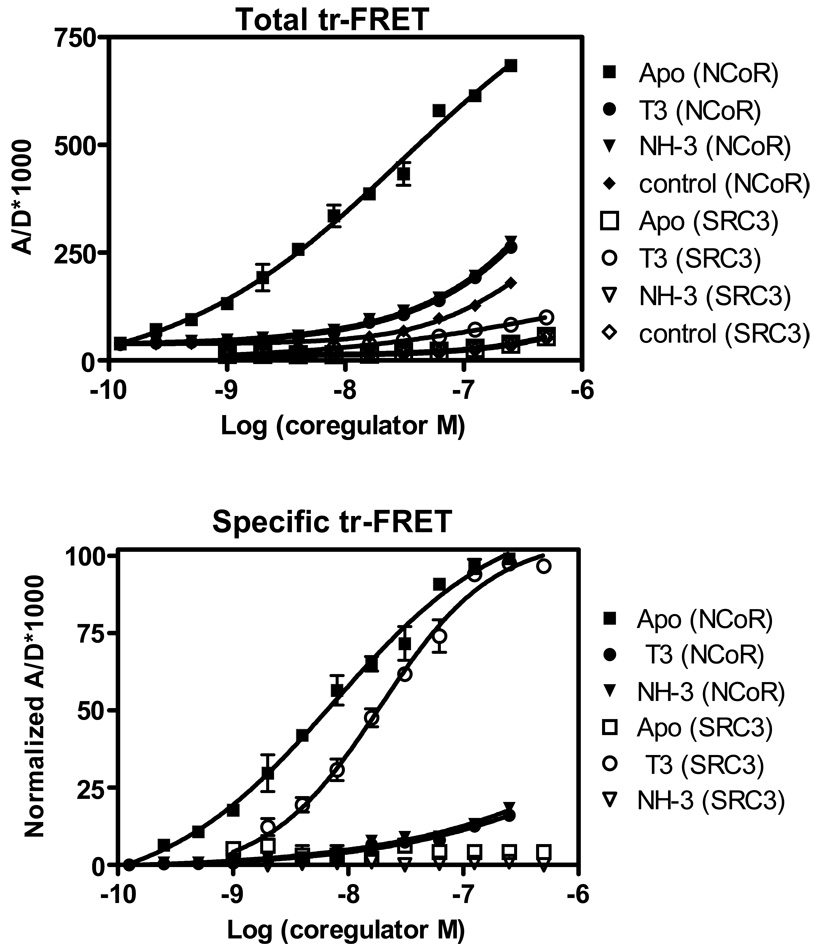
We first applied the Tb+3-based dual-acceptor strategy in a coregulator titration mode of ligand screening. Different TR ligands can be assayed for their abilities to recruit both a corepressor and a coactivator for DR+4 TRE-bound TR•RXR. In this assay, a fixed concentration of Tb+3-labeled-TRE bound with TR•RXR (15 nM) is incubated with no ligand (Apo), or with a fixed saturating concentration of the agonist ligand T3 (3 µM) or the antagonist ligand NH-3 (25 µM) and is then titrated with increasing concentrations of both Fl-NCoR and Cy5-SRC3. The resulting tr-FRET values are shown in Fig. 3 A.
Figure 3.
Simultaneous coregulator titration assay – measurement of coregulator binding affinity to TR complexes. Measurement NCoR and SRC3 binding affinities for unliganded and liganded TRE-bound TR•RXR. (A) A fixed amount of TR•RXR (15 nM) bound to a biotinylated DR+4 TRE was incubated with increasing concentrations of both fluorescein-labeled NCoR and Cy5-labeled SRC3 in the absence of (Apo) and in the presence of 3 µM T3 and 25 µM NH-3, and in the absence of biotinylated TRE (control). Measured tr-FRET values are plotted as the ratio of acceptor to donor × 1000 vs. the log of coregulator concentrations. Note, the binding of SRC3 with TR unliganded [Apo (SRC3)], or treated with NH-3 [NH-3 (SRC3)] or the control are similar and appear superimposed. (B) The tr-FRET values in panel A were corrected for the corresponding diffusion-enhanced FRET values (control). The control corrected Bmax values of SRC3 (T3-TR) and NCoR (Apo TR) were set 100%, and the resulting normalized tr-FRET values are shown. Three independent sets of experiments were performed in replicate, and each assay point in the binding curves represents the mean ± standard deviation of six measurements. Results in panel B were analyzed by nonlinear regression with an equation for the sigmoidal dose response (variable slope) in GraphPad Prism, and the concentrations of fluorophore-labeled NCoR and SRC3 at 50% (EC50) of maximal binding in the presence of T3 and NH-3 were obtained and expressed as mean ± standard deviation of three different experiments.
The diffusion-enhanced background FRET, obtained in the absence of TRE, gave a very low, basically flat line (Fig. 3A, control curves), and these curves were subsequently subtracted from the total tr-FRET values to obtain specific tr-FRET values. Because the efficiency of tr-FRET between the Tb-Cy5 FRET pair is ∼15 times lower than that from the Tb- fluorescein pair, for clarity, the maximal specific tr-FRET values from the NCoR and SRC binding were set equal to 100%; the resulting normalized binding curves are shown in Fig. 3B.
In the absence of ligand (Apo), there was a strong, concentration-dependent saturable FRET between Tb-TR and Fl-NCoR, but the signal with Cy5-SRC3 (Fig. 3B) was only that of the background. This indicates that the unliganded TR interacts with NCoR with high binding affinity (EC50: 8.3±0.8 nM) but has no binding affinity for SRC3. Conversely, T3-bound TR binds SRC3 with strong affinity (EC50: 19.1±2.2 nM), but has FRET only slightly above background with NCoR.
We have shown previously that the residual NCoR binding with the T3-TR complex is probably non-specific, because increasing the already saturating concentration of 3 µM T3 to a very high concentration (100 µM), did not decrease the background [7]. NH-3 at 25 µM, completely prevented both SRC3 and NCoR binding to TR (Fig. 3B). As in our previous two independent tr-FRET assays, NH3 at 3 µM concentration only partially inhibited NCoR-TR interaction (data not shown), confirming the weak NCoR dissociating activity of NH-3 observed in cell-based assays [9].
The ligand specific binding patterns of NCoR and SRC3 from this dual tr-FRET method are in good agreement not only with previous in vitro GST-pull down assays [9–14] but with the coregulator binding affinity and binding capacity also determined in our previous individual tr-FRET assay formats (EC50 13.6±0.41 nM for NCoR with apo TR and 24.6±2.7 nM for SRC3-TR-T3 complex [7]. The dual-acceptor FRET method, however, quantifies these ligand-specific effects simultaneously.
Simultaneous Measurement of TR-NCoR Dissociation and TR-SRC3 Recruitment with Ligand Titration
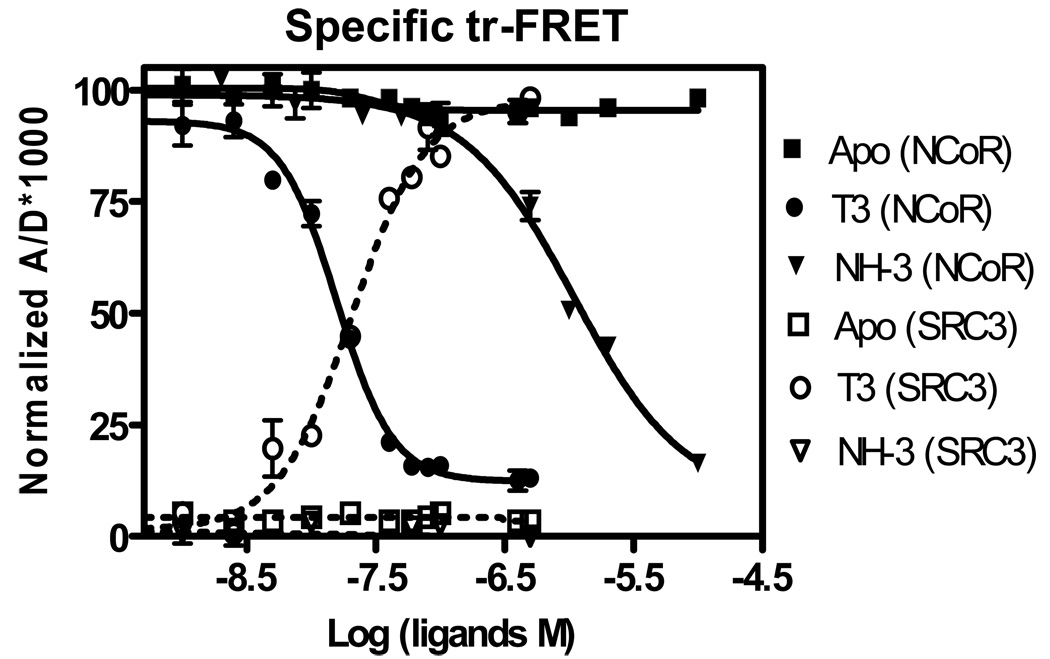
A ligand titration assay was used to simultaneously quantify the relative potencies of T3 and NH-3 in displacing NCoR from and in recruiting SRC3 to TRE-bound TR•RXR heterodimer. For this, we chose 15 nM of TRE-bound TR•RXR and concentrations of Fl-NCoR (25 nM) and Cy5-SRC3 (150 nM), which gave good specific and minimal nonspecific tr-FRET. Increasing concentrations of T3 or NH-3 were titrated into this protein-coregulator mix. The diffusion-enhanced control FRET-corrected, normalized binding curves for both SRC3 recruitment and NCoR dissociation are given in Fig. 4. This shows clearly the simultaneous dissociation of NCoR and recruitment of SRC3 by TR as the concentrations of T3 is increased. Respective EC50 values are 15.7 ± 1.3 nM for NCoR dissociation and 20.7 ± 3.2 nM for SRC recruitment. The TR antagonist, NH-3, while having no effect on SRC3 recruitment, showed partial NCoR dissociation activity with an IC50 of 1090 ± 80 nM. The measured potencies of T3 and NH-3 by this dual-acceptor FRET method is comparable to our earlier individual assay methods where the potency of T3 for SRC3 recruitment was 16.2±1.2 nM, and of T3 and NH-3 for NCoR dissociations were 16.2±1.8 and 874±23 nM [7].
Figure 4.
Simultaneous ligand titration – measurement of TR ligand potency for NCoR dissociation and SRC3 recruitment. Increasing concentrations of T3 and NH-3 were tested for their ability to dissociate fluorescein-NCoR (25 nM) and to recruit Cy5-SRC3 (150 nM) (both at submaximal concentrations) to a fixed concentration of TRE-bound TR•RXR R (15 nM). Diffusion-enhanced control corrected and normalized binding curves are shown. Data were analyzed as described in the previous experiment. Three different sets of experiments were performed in replicate, and each assay point in the binding curves represents the mean ± standard deviation of six measurements. The concentrations of T3 and NH-3 to effect 50% of NCoR dissociation and 50% of SRC3 recruitment were determined and presented as mean ± standard deviation of three independent experiments.
Discussion
In the present study, we integrated our previous two independent in vitro tr-FRET assays of ligand-dependent dissociation of corepressor (NCoR) and coactivator (SRC3) recruitment with a TRE bound TR•RXR [7] into a single in vitro double tr-FRET based assay, in which the activities of a TR ligand in regulating the interaction of TR with these two coregulators can be evaluated simultaneously.
As described in the result section, the favorable emission properties of the donor Tb+3 and the selection of appropriate acceptors exhibiting non-overlapping emission spectrum, as well as performing measurements in a gated detection mode starting 50 µs after pulsed excitation, afforded an effective way to monitor such binding events in a single assay. Consequently, the measured affinities of SRC3 and NCoR for TR-T3 or TR-NH3 complexes and the relative potencies of these two ligands in regulating coregulator interaction are very similar to those quantified by our previous individual NCoR dissociation or SRC3 recruitment tr-FRET assays [7], thus saving half of investigator time. Such dual measurements are difficult to obtain when conventional organic dyes are used as donor flurophores, as has been done in studies of DNA-protein and protein-protein interactions, because they require mathematical deconvolution analysis to separate spectral overlaps [15–19].
Similar to our previous two separate assays, the dual-FRET assay can be set up in two different screening modes: a coregulator titration mode with which screening can be based on differences in SRC3 or NCoR binding affinity for TR complexed with the compounds of interest, and a ligand titration mode in which ligands can be evaluated for their potencies in regulating the magnitude of SRC3 recruitment and NCoR release.
Although tr-FRET based assays measuring the single binding event of coactivator interaction with several NRs have been used previously [20–25], the application of double tr-FRET technology has been recognized only recently with the demonstration of simultaneous assay of the binding of corepressor and coactivator peptides with PPARs [8]. However, unlike the assay for PPARs, the design of our tr-FRET format recapitulates many of the cellular events of NR actions as it monitors TR-coregulator interaction when TR is complexed with its usual heterodimer partner, RXR, and is also bound to a natural TRE. Thus, an assay of our design is likely to provide read-outs that are biologically relevant.
The assay format reported here is simple and convenient (mix and measure mode), rapid (1hr) and of good quality (high specific/non-specific ratios), and it is highly adaptable for high-throughput screening (HTS) format. The highlight of our dual method is that it provides both potency as well as functional information on whether a compound of interest is an agonist, antagonist or mixed agonist/antagonist in the very first round of testing. Such features are particularly important for the development of novel TR therapeutics intended for specific functions: antagonistic ligands having no agonistic activities (for hyperthyroidism), and TR agonists with no antagonistic activities (for obesity and hyperlipidimia). Thus, assays of this type should find application in studies of both drug discovery and ligand pharmacology, not only in the field of TR, but also with other members of the NR superfamily.
Acknowledgments
We thank Dr. Milan K. Bagchi for providing the constructs for TR, RXR and NCoR and Dr. Thomas S. Scanlan for NH-3. We also thank Jillian R. Gunther, Kathryn E. Carlson, and Dr. Sung Hoon Kim for their helpful inputs and discussion. This work was supported by a grant from the National Institutes of Health (PHS R37 DK15556).
Abbreviations used
- TR
thyroid hormone receptor
- tr-FRET
time-resolved fluorescence energy transfer
- Cy5
Cyanin5
- Fl
Fluorescein
References
- 1.Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr. Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 2.Baxter JD, Webb P, Grover G, Scanlan TS. Selective activation of thyroid hormone signaling pathways by GC-1: a new approach to controlling cholesterol and body weight. Trends Endocrinol. Metab. 2004;15:154–157. doi: 10.1016/j.tem.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Grover GJ, Mellstrom K, Malm J. Therapeutic potential for thyroid hormone receptor-beta selective agonists for treating obesity, hyperlipidemia and diabetes. Curr. Vasc. Pharmacol. 2007;5:141–154. doi: 10.2174/157016107780368271. [DOI] [PubMed] [Google Scholar]
- 4.Koehler K, Gordon S, Brandt P, Carlsson B, Backsbro-Saeidi A, Apelqvist T, Agback P, Grover GJ, Nelson W, Grynfarb M, Farnegardh M, Rehnmark S, Malm J. Thyroid receptor ligands. 6. A high affinity "direct antagonist" selective for the thyroid hormone receptor. J. Med. Chem. 2006;49:6635–6637. doi: 10.1021/jm060521i. [DOI] [PubMed] [Google Scholar]
- 5.Privalsky ML. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu. Rev. Physiol. 2004;66:315–360. doi: 10.1146/annurev.physiol.66.032802.155556. [DOI] [PubMed] [Google Scholar]
- 6.Yen PM, Ando S, Feng X, Liu Y, Maruvada P, Xia X. Thyroid hormone action at the cellular, genomic and target gene levels. Mol. Cell Endocrinol. 2006;246:121–127. doi: 10.1016/j.mce.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Jeyakumar M, Webb P, Baxter JD, Scanlan TS, Katzenellenbogen JA. Quantification of ligand-regulated nuclear receptor corepressor and coactivator binding, key interactions determining ligand potency and efficacy for the thyroid hormone receptor. Biochemistry. 2008;47:7465–7476. doi: 10.1021/bi800393u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kupcho KR, Stafslien DK, DeRosier T, Hallis TM, Ozers MS, Vogel KW. Simultaneous monitoring of discrete binding events using dual-acceptor terbium-based LRET. J. Am. Chem. Soc. 2007;129:13372–13373. doi: 10.1021/ja074791h. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen NH, Apriletti JW, ST Cunha Lima, Webb P, Baxter JD, Scanlan TS. Rational design and synthesis of a novel thyroid hormone antagonist that blocks coactivator recruitment. J. Med. Chem. 2002;45:3310–3320. doi: 10.1021/jm0201013. [DOI] [PubMed] [Google Scholar]
- 10.Jeyakumar M, Liu XF, Erdjument-Bromage H, Tempst P, Bagchi MK. Phosphorylation of thyroid hormone receptor-associated nuclear receptor corepressor holocomplex by the DNA-dependent protein kinase enhances its histone deacetylase activity. J. Biol. Chem. 2007;282:9312–9322. doi: 10.1074/jbc.M609009200. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Tamrazi A, Carlson KE, Katzenellenbogen JA. A proteomic microarray approach for exploring ligand-initiated nuclear hormone receptor pharmacology, receptor selectivity, and heterodimer functionality. Mol. Cell Proteomics. 2005;4:267–277. doi: 10.1074/mcp.M400192-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Jeyakumar M, Petukhov S, Bagchi MK. A nuclear receptor corepressor modulates transcriptional activity of antagonist-occupied steroid hormone receptor. Mol. Endocrinol. 1998;12:513–524. doi: 10.1210/mend.12.4.0089. [DOI] [PubMed] [Google Scholar]
- 13.Tamrazi A, Katzenellenbogen JA. Site-specific fluorescent labeling of estrogen receptors and structure-activity relationships of ligands in terms of receptor dimer stability. Methods Enzymol. 2003;364:37–53. doi: 10.1016/s0076-6879(03)64003-6. [DOI] [PubMed] [Google Scholar]
- 14.Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW. TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule exhibits distinct properties from steroid receptor coactivator-1. J. Biol. Chem. 1997;272:27629–27634. doi: 10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- 15.Clamme JP, Deniz AA. Three-color single-molecule fluorescence resonance energy transfer. Chemphyschem. 2005;6:74–77. doi: 10.1002/cphc.200400261. [DOI] [PubMed] [Google Scholar]
- 16.Hohng S, Joo C, Ha T. Single-molecule three-color FRET. Biophys. J. 2004;87:1328–1337. doi: 10.1529/biophysj.104.043935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klostermeier D, Sears P, Wong CH, Millar DP, Williamson JR. A three-fluorophore FRET assay for high-throughput screening of small-molecule inhibitors of ribosome assembly. Nucleic Acids Res. 2004;32:2707–2715. doi: 10.1093/nar/gkh588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Lu Y. FRET study of a trifluorophore-labeled DNAzyme. J. Am. Chem. Soc. 2002;124:15208–15216. doi: 10.1021/ja027647z. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez-Carrozzi VR, Kerppola TK. Dynamics of Fos-Jun-NFAT1 complexes. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4893–4898. doi: 10.1073/pnas.091095998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bramlett KS, Wu Y, Burris TP. Ligands specify coactivator nuclear receptor (NR) box affinity for estrogen receptor subtypes. Mol. Endocrinol. 2001;15:909–922. doi: 10.1210/mend.15.6.0649. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Knappenberger KS, Kack H, Andersson G, Nilsson E, Dartsch C, Scott CW. A homogeneous in vitro functional assay for estrogen receptors: coactivator recruitment. Mol. Endocrinol. 2003;17:346–355. doi: 10.1210/me.2002-0331. [DOI] [PubMed] [Google Scholar]
- 22.Margeat E, Poujol N, Boulahtouf A, Chen Y, Muller JD, Gratton E, Cavailles V, Royer CA. The human estrogen receptor alpha dimer binds a single SRC-1 coactivator molecule with an affinity dictated by agonist structure. J. Mol. Biol. 2001;306:433–442. doi: 10.1006/jmbi.2000.4418. [DOI] [PubMed] [Google Scholar]
- 23.Ozers MS, Marks BD, Gowda K, Kupcho KR, Ervin KM, De Rosier T, Qadir N, Eliason HC, Riddle SM, Shekhani MS. The androgen receptor T877A mutant recruits LXXLL and FXXLF peptides differently than wild-type androgen receptor in a time-resolved fluorescence resonance energy transfer assay. Biochemistry. 2007;46:683–695. doi: 10.1021/bi061321b. [DOI] [PubMed] [Google Scholar]
- 24.Stafslien DK, Vedvik KL, De Rosier T, Ozers MS. Analysis of ligand-dependent recruitment of coactivator peptides to RXRbeta in a time-resolved fluorescence resonance energy transfer assay. Mol. Cell Endocrinol. 2007;264:82–89. doi: 10.1016/j.mce.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Zhou G, Cummings R, Li Y, Mitra S, Wilkinson HA, Elbrecht A, Hermes JD, Schaeffer JM, Smith RG, Moller DE. Nuclear receptors have distinct affinities for coactivators: characterization by fluorescence resonance energy transfer. Mol. Endocrinol. 1998;12:1594–1604. doi: 10.1210/mend.12.10.0176. [DOI] [PubMed] [Google Scholar]